Abstract
The community of bacteria that colonize the urinary tract, the urinary microbiome, is hypothesized to influence a wide variety of urinary tract conditions. Older adults who reside in nursing homes are frequently diagnosed and treated for urinary tract conditions such as urinary tract infection. We investigated the urinary microbiome of older adults residing in a nursing home to determine if there are features of the urinary microbiome that are associated with specific conditions and exposure in this population. We were also interested in the stability of urinary microbiome over time and in similarities between the urinary and gastrointestinal microbiome. Urine samples were prospectively collected over a period of 10 months from a cohort of 26 older adults (aged >65 years) residing in a single nursing home located in Central Massachusetts. Serial samples were obtained from 6 individuals over 10 months and 5 participants were concurrently enrolled in a study of the gastrointestinal microbiome. Information collected on participants included demographics, medical history, duration of residence in the nursing home, frailty, dementia symptoms, urinary symptoms, antibiotic treatment, urinary catheterization, and hospitalizations over a 10-month period. Clean catch, midstream urine samples were collected and stored at −80°C. DNA was extracted and 16S rRNA gene sequencing was performed. The length of stay in the nursing facility and the Clinical Frailty Scale correlated with significant changes in microbiome composition. An increase in the relative abundance of a putative urinary pathogen, Aerococcus urinae, was the largest factor influencing change that occurred over the duration of residence.
Keywords: Infection, Older adult nursing home residents, Urinary microbiome, Urinary tract infections
The human urinary tract is not a sterile environment, even in the absence of infection (1) and microbial presence can be detected, especially as we age, using molecular techniques (2). The bacterial community that colonizes the human urinary tract, the urinary microbiome, may influence a number of urinary tract conditions, including the development of a urinary tract infection (UTI) (3). The use of high-throughput sequencing to investigate common urinary tract colonizers is an emerging field (4,5). There is growing evidence that the makeup of these bacterial communities can influence the health of the urinary tract (1,3,6).
The connection between the urinary microbiome, pathogen colonization, and UTI is an understudied area of research. In the gut, loss of beneficial commensal organisms can allow pathogens access to previously utilized nutrients, leading to infection (7), the same may be true in the urinary tract. It is generally established that urinary pathogens are acquired from the gastrointestinal (8) tract, and we have identified potential urinary pathogens in the gut microbiomes of nursing home residents (9). Changes in the older adult urinary microbiome, including loss of diversity or dysbiosis, may allow potentially pathogenic organisms to colonize the urinary tract and lead to the development of UTI. Study of the nursing home resident urinary microbiome would advance understanding of asymptomatic bacteriuria (ASB) and the development of pathogenic UTIs in this population. Findings may serve to reduce antibiotic utilization, especially if the composition of the urinary microbiome discriminates ASB from UTI, and would indicate potential targets for improved molecular diagnostics for UTI that would have tremendous potential to reduce antibiotic utilization among this population (10).
UTI is a common diagnosis among older adults and is responsible for millions of health care encounters, antibiotic prescriptions (11), and as many as 100 000 hospitalizations (12). In the acute setting, UTI is currently diagnosed on the basis of chemical urinalysis looking for the presence of leukesterase and nitrites and microscopic analysis looking for the presence of bacteria and white blood cells (13). These methods are very sensitive for detection of infection; however, they have very poor specificity, and up to 40% of antibiotic courses given to this population for UTI do not meet clinical criteria for treatment (10). Because of poor specificity, it is recommended that factors such as symptoms (eg, dysuria, flank, or suprapubic pain), examination findings (eg, costovertebral angle tenderness), and lab tests (eg, leukocytosis) be considered in the setting of a urinalysis that is suggestive of infection when making the decision to treat an older adult with antibiotics for UTI (14,15). Unfortunately, in acute care facilities, these recommendations are often not followed, resulting in inappropriate testing and treatment for UTI (16). Additionally, antibiotics are commonly proscribed to older adults for vague or nonspecific symptoms that are often incorrectly attributed to a UTI (17). As a result, overtreatment with antibiotics is common and leads directly to multidrug-resistant organisms (15), antibiotic-associated diarrhea (18), and Clostridiodies difficile infections (19). Urine culture will often show the growth of potential pathogens in older adults who do not have an active infection (20). Upwards of 20% of urine cultures from community-dwelling older adults and 50% from nursing home residents will show the presence of potentially pathogenic bacteria in the absence of symptoms, this is known as ASB (21). Conversely, pathogens may not be isolated with standard culture techniques and treatment may be withheld from patients who would benefit from treatment due to the presence of misclassified (22) or atypical organism (23).
To investigate the urinary microbiome among this population, we carried out a prospective study of older adults residing in a skilled nursing facility. Our goals were to determine if this community was stable over the period of months to years that we conducted our study and to investigate trends in the urinary microbiome that were associated with participant characteristics of age, sex, clinical frailty, dementia symptoms, UTI symptoms, and duration of residence within the nursing home. We also sought to investigate if there were urinary microbiome changes associated with the exposures of recent antibiotic treatment, the specific antibiotic used, recent hospitalizations, or urinary catheterization. Because a concurrent study of the gastrointestinal microbiome was being undertaken among the same study population, we also compared urinary microbiome measurements to gastrointestinal microbiome measurements taken at similar times to determine if these communities share similar features.
Method
After informed consent and enrollment, we collected demographic information, medical history, frailty as measured by the Clinical Frailty Scale (24) (with a score of 1 representing a fit individual and a score of 9 representing a terminally ill individual) as documented by nursing home staff and physicians, dementia symptoms as measured by the Clinical Dementia Rating Scale (25), as well as information on exposures that would potentially affect the urinary microbiome. These exposures included antibiotic courses, hospitalization, and urinary catheterization within the preceding 3 months from sample collection. Participants and caregivers were also asked about any potential UTI symptoms including fevers, chills, suprapubic pain, flank pain, dysuria, new urgency, new incontinence, change in quality of urine (foul smell, turbidity, color change), new or worsening confusion, or change in mental status that participants had at the time of sample collection. Serial samples were obtained from a subset of 6 participants to determine if the urinary microbiome is stable over the time frame of the study, which has been seen in the gastrointestinal microbiome among this population (9).
We collected clean catch, midstream urine samples from participants, which has been shown to be nearly equivalent to sterile urine collection methods for microbiome studies (26), in sterile urine cups and frozen at −4°C on an onsite freezer within 3 hours of collection and subsequently moved to −80°C within 3 days for long-term storage prior to sample analysis. Urine samples were thawed in a cold-water bath and 5 mL aliquots. These were sedimented in a centrifuge (2 600 × g, 5 min) and washed once with phosphate buffered saline. For individuals concurrently enrolled in a study of the gastrointestinal microbiome, stool was collected over the course of normal elimination and stored on an onsite freezer and subsequently moved to −80°C for storage prior to extraction. DNA was extracted using the Powersoil DNA isolation kit (MoBio, Carlsbad CA) on an epMotion 5075 TMX liquid handling workstation, according to manufacturer protocols (Mo Bio Laboratories catalog no. 27100-4-EP). Sequencing libraries for 16S rDNA profiling were constructed following methods previously described (27) using the 341F and 806R universal primers to amplify the V3–V4 region. 300 nt paired-end sequences were generated on the Illumina MiSeq platform. Reads were assembled and clustered, and an Operational Taxonomic Unit (OTU) table was generated using the UPARSE pipeline in USEARCH version v10.0.240 (28). Taxonomic classifications were determined using SINTAX (28) and RDP training set v16 (with species names; https://drive5.com/usearch/manual/sintax_downloads.html).
Table 1.
Participant Demographics
| Variable | Average | Range |
|---|---|---|
| Age (years) | 85 (6) | 79–95 |
| Time residence (months) | 48.5 (32.5) | 6–114 |
| Clinical Dementia Rating (25) | 1.6 (0.6) | 1–3 |
| Clinical Frailty Scale (42) | 6.5 (0.6) | 5–7 |
| Exposures Within 90 Days of Sample Collection | Number of Samples | Percentage |
| Antibiotics | 6 | 18 |
| Hospitalization | 4 | 12 |
| Urinary catheterization | 6 | 18 |
Statistical analysis was performed using R. To evaluate the microbiome relationship between samples, we calculated pairwise Bray–Curtis compositional dissimilarity and performed t-distributed stochastic neighbor embedding (tSNE). Sample similarity according to exposures of interest was assessed by the variability of bacterial abundance using permutation multivariate analysis of variance (PERMANOVA). To control for the effect of serial samples obtained from the same participant, the PERMANOVA was run iteratively by selecting one sample at random for every participant in the study. The PERMANOVA on each variable was then run 1 000 times with different permutations of included samples. For every variable, we then estimated the frequency of significance as the number of times the PERMANOVA-associated p value was less than .05 over the total number of iterations, 1 000. This analysis was performed on duration of residence, antibiotic exposure within 90 days, the specific antibiotic administered, the condition treated, hospitalization within 90 days, urinary catheterization within 90 days, diagnosis of dementia, gender, and concurrent symptoms suggesting of UTI.
To investigate the relationship between the abundance of OTUs and duration of time of residence in the facility, we ran mixed-effect random forest (MERF) regression modeling. Specifically for every identified OTU, we used a MERF to regress its abundance as a function of duration of residence and adjusting for age, sex, and Clinical Frailty Scale (29). MERF is a machine learning approach that is suitable for high-dimensional data such as those generated from microbiome analysis, does not assume any underlying distribution, and allows to deal with multiple samples from the same participant. A permutated importance analysis model (30) was run on the MERF output to determine the significance of the MERF-identified associations between each OTU abundance and the duration of time spent in the facility. To evaluate the relationship between stool and urine microbiome among 5 individuals concurrently enrolled in a study of the gastrointestinal microbiome, we calculated the pairwise Bray-Curtis dissimilarity distance for urine and stool samples. Spearman correlation was run to test for significant differences between the distance of urine samples and associated stool samples.
Results
Cohort Description
We collected samples from 26 older adults residing in a single nursing home over the course of 10 months from March 2018 through January 2019. Participants’ ages ranged from 79 to 95 (average 85) and the majority were female (23/26, 88%), all identified as White and non-Hispanic. There was a wide range of time that the older adults had been residents of the nursing home with an average of 48.5 months but ranging from 6 to 114 months. Overall, the cohort was frail with a mean Clinical Frailty Scale of 6.5, and all had at least some symptoms of dementia (mean CDR of 1.35) while 9/26 (34%) carried a diagnosis of dementia. The demographics of the cohort are representative of the nursing home population in Central Massachusetts, although this group tended to have higher average scores on dementia and frailty rating scales then a cohort recruited from the same population for a study of the gastrointestinal microbiome (31).
Over the course of the study, 6 participants had a course of antibiotics within 90 days and 3 participants received a course of antibiotics during the study period. Hospitalization occurred within 90 days of sample collection for 3 participants. Ureteral catheterization occurred within 90 days for 6 participants, although none had a chronic-indwelling catheter. Samples from 3 individuals were obtained who were tested for UTI within 2 weeks of sample collection for symptoms reported by caregivers (all were for confusion or mental status change); none were treated with antibiotics for the symptoms or findings on urinalysis/culture.
Urinary Microbiome Varies With Clinical Frailty Scale and Duration of Residence in Nursing Home
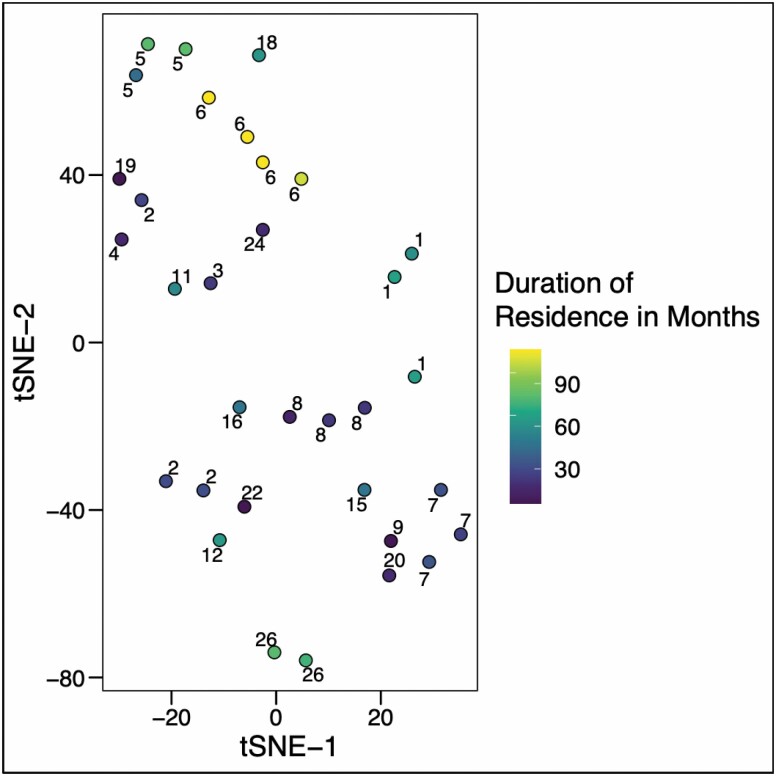
A total of 35 samples were analyzed. We visualized sample dissimilarity (Figure 1) using tSNE based on the Bray-Curtis dissimilarity index according to clinical variables including recent courses of antibiotics, specific antibiotics used, conditions treated, hospitalizations, urinary catheterizations, Clinical Frailty Scale, dementia symptoms, potential UTI symptoms, and duration of nursing home residence. Two factors were determined to result in significant similarity among samples by PERMANOVA. These were the duration of residence within the nursing (p = .039–.74) home and the Clinical Frailty Scale (p = .004–.18). The results of all statistical tests are given in Supplementary Table 1.
Figure 1.
Bray-Curtis dissimilarity distance between samples plotted by t-distributed stochastic neighbor embedding (tSNE). Numbers associated with individual points denote the study participant that the sample was obtained from. Points are color-coded according to the duration the participant had resided in the nursing home.
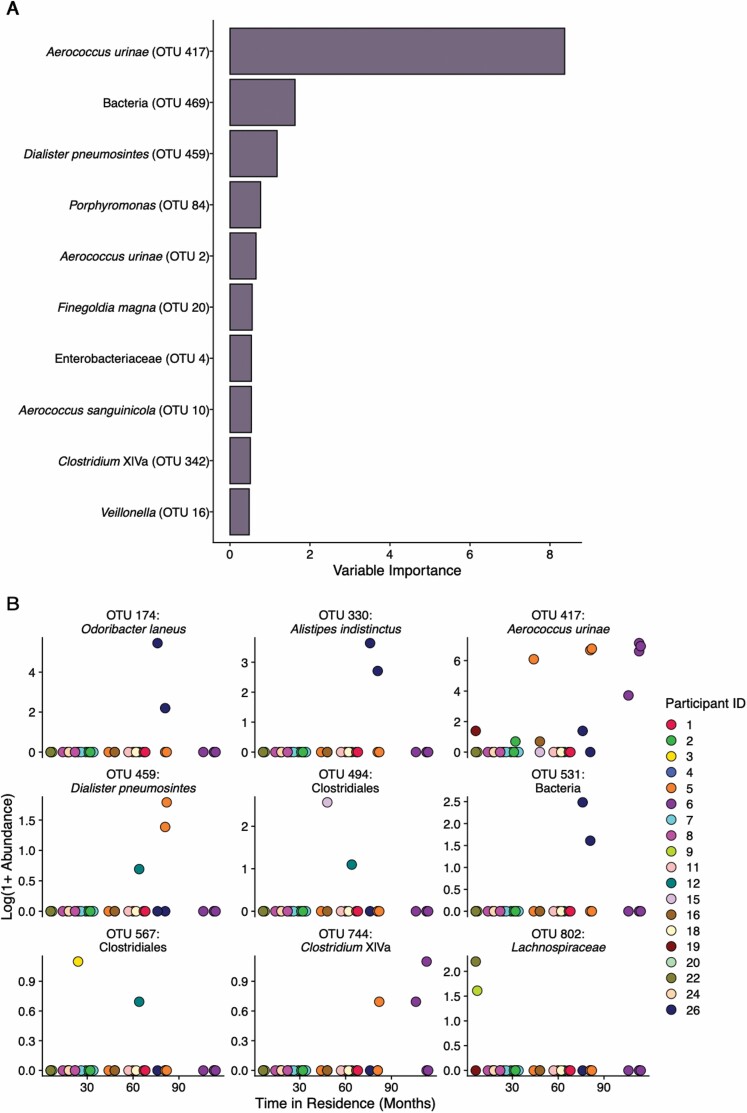
The results of the MERF analysis to determine which OTUs have abundance levels that are associated with duration of residence in the nursing home, while adjusting for age, sex, and Clinical Frailty Scale, are shown in Figure 2. The 10 OTUs with the highest permuted variable importance are shown in Panel A and the 9 OTUs that showed a statistically significant association with duration of residence (p < .1) are shown in Panel B. The relative abundance of the 3 OTUs representing Aerococcus urinae, Dilaster pneumosintes, and Clostridium cluster XIVa was identified as being significantly associated with increasing duration of residence and showed high permuted variable importance within the model. All 3 of these organisms show increased relative abundance with increasing time of residence within the nursing home.
Figure 2.
Mixed-effects random forest model of Operational Taxonomic Unit (OTU) abundance associated with duration of residence in the nursing home. Panel A shows the permuted variable importance of the top 10 OTUs that show changes in relative abundance associated with longer duration of residence in the nursing home with the most important contributor being the potential urinary pathogen Aerococcus urinae. Panel B shows the relative abundance of OTUs that show variation associated with duration of residence in the nursing home in the mixed-effect random forest (MERF) model with a significance of p < .1 plotted against time of residence within the nursing home. Each plotted point represents an individual sample. Three organisms, Aerococcus urinae, Dilaster pneumosinties, and Clostridium cluster XIVa, show high importance in the model and are significantly associated with increasing duration of residence in the nursing home.
Composition and Stability of the Urinary Microbiome Over Time
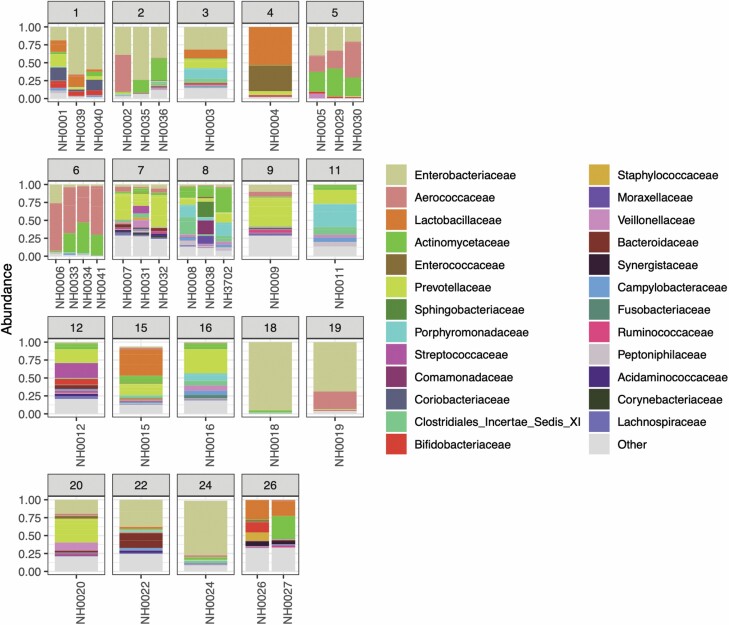
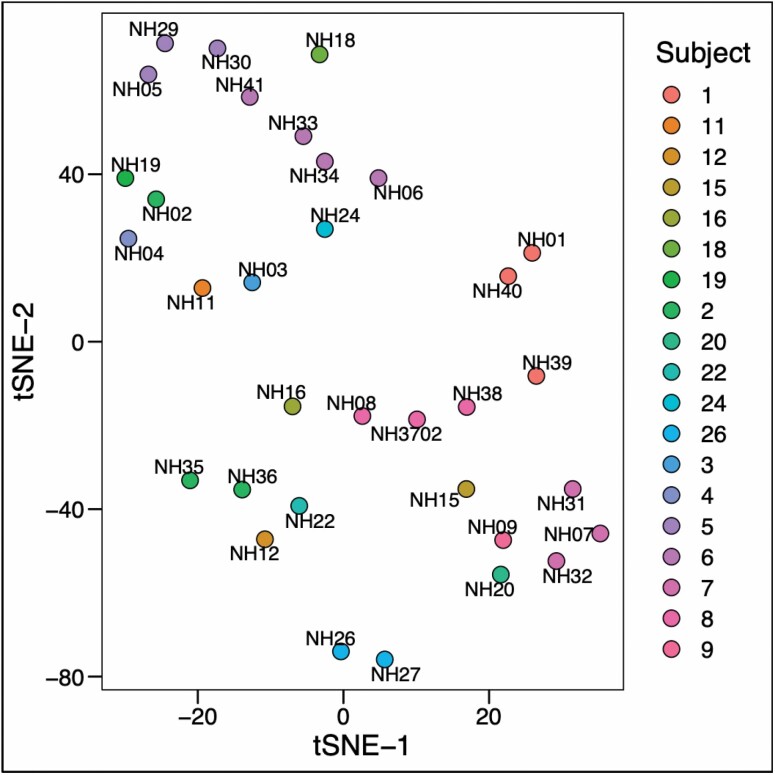
To visualize the intraindividual differences between serial samples obtained from the 6 participants enrolled in the longitudinal portion of the study, we plotted relative abundances of different detected orders of bacteria grouped according to the participant that sample was obtained from (Figure 3). Serial samples obtained from the same participant are shown adjacent to each other. The detected orders of bacteria and their relative abundance visually appear stable across serial samples obtained from the same individual, even though these samples were obtained months apart. We explored intraindividual separation between samples from the same participant over time using tSNE. The results of this analysis are shown in Figure 4. Although samples from the same individual visually appear to cluster, this separation by an individual does not reach statistical significance (p = .54–.99).
Figure 3.
Barplots of detected bacterial genera and relative abundance. The numbers above plots denote study participants. Of note, Participants 1, 2, 6, and 7 received courses of antibiotics between the first and second analyzed samples.
Figure 4.
Bray-Curtis dissimilarity distance between samples plotted by t-distributed stochastic neighbor embedding (tSNE) according to the study participant. Numbers associated with individual points denote the individual sample. Points are color-coded according to the participant the sample was obtained from.
Effect of Recent Antibiotic Exposure
Because antibiotics have been documented to have large, and in some cases, long-lasting changes within the gut microbiome (32), we sought to determine if similar effects were seen in the urinary microbiome of our study population. There did not appear to be any statistically significant association by PERMANOVA when samples were grouped according to recent antibiotic exposure, the specific antibiotics that were administered, or the condition that was treated. In addition, Participants 1, 2, 6, and 7 had received courses of antibiotics over the course of the study between sample collections. There did not appear to be large shifts in the relative abundance of detected genera from these participants after being treated with antibiotics.
Comparison With Stool Microbiome
Five participants in this study were concurrently enrolled in a study of the gut microbiome among older adults living in nursing homes and had stool samples that were collected within 7 days of urine samples analyzed in this study (9). To evaluate if similar beta-diversity is seen in urine and stool microbiomes among these individuals, pairwise comparisons of Bray-Curtis dissimilarity distance of stool samples and urine samples among these participants were analyzed using a Spearman correlation test. This demonstrated an inverse correlation (p = .006) between urine and stool sample distance, suggesting that these communities are distinct and do not vary together. The results are shown in Supplementary Figure 1.
Discussion
Here we report an analysis of the urinary microbiome among older adults residing in a nursing home. Our most intriguing finding is that this community appears to vary with respect to the number of months the older adult has been a resident of the nursing home. A. urinae is seen as the strongest contributor to this effect and has been described as a potential cause of UTIs among this population and may be misidentified with conventional culture techniques (22). D. pneumosintes has been most frequently detected in human gingival plaque and may contribute to periodontal disease (33). It has also been detected in gastrointestinal microbiome studies (34) and in the female genitourinary tract (35), but a specific contribution to the urinary microbiome has not been reported. Clostridium cluster XIVa is a butyrate-producing organism that adheres to mucins (36). It has generally been reported as being associated with a healthy gastrointestinal microbiota and a lower abundance has been reported in gastrointestinal disease states such as cystic fibrosis (37) and inflammatory bowel disease (38). Its role in the urinary microbiome has not been previously reported.
It is noteworthy that the organism represented by the OTU that is the strongest driver of this trend is a potential urinary pathogen that has been reported as causing UTIs among this population and may be misidentified using traditional culture techniques (22). The increasing detection of a potential urinary tract pathogen over time among asymptomatic individuals may reflect a changing microbial community more conducive to pathogen colonization. There may be environmental factors within the nursing home, such as diet or specific nursing practices that are contributing to an environment that allows colonization of potential pathogens.
It makes intuitive sense that a homogenous living environment may drive a convergence of microbiome populations, but the trend we detected appeared to have taken a while to occur. Most of the participants in this study (23/26) had resided in this nursing facility for over a year. We did observe that the urinary microbiome among this population appears to be relatively stable in the order of months. Gut microbiome stability over a similar time period has been reported among individuals sharing a similar living environment both in this population (9) and younger healthy individuals (39). This is in some contrast to findings of temporal variability in the gut and cutaneous microbiome of healthy individuals who reside in the community (40). It may be that some factors of the microbiome are more sensitive to perturbations such as diet, as large changes within the gut microbiome among older adults have been observed with diet interventions over the course of months (41). There may be other factors in the urinary microbiome that are slower to change, and these may be what is contributing to the association we observed with respect to duration of residence within the nursing home.
We also report a significant urinary microbiome association with the participant’s Clinical Frailty Scale (42). Associations between the gastrointestinal microbiome and frailty measures in older adults have been previously reported (9,43). Age and frailty have also been reported to affect the urinary microbiome of women and may partly be related to incontinence (44), which is a component of the Clinical Frailty Scale (42) we used in this study and may partly explain this association. Examining the relationship between the urinary microbiome, development of UTI, and frailty in older adults is the subject of planned future studies.
It is generally theorized that UTI is caused by the spread of pathogens from the gastrointestinal tract to the genitourinary tract (8) and as such we did consider that the urinary microbiome may correlate closely with the gastrointestinal microbiome. We did investigate this among 5 participants who had concurrent stool samples for gastrointestinal microbiome analysis obtained within 7 days of providing urine samples for this study. In this small cohort, there did not appear to be similar relative makeup of these communities compared to other participants in the study, and in fact, it showed an inverse correlation. This suggests that similarities seen in gastrointestinal microbiomes were not seen in the urinary microbiome. The urinary microbiome communities may be distinct and not merely a reflection of the gastrointestinal microbiome. This observation is in agreement with previous studies that suggest that the urinary microbiome is a distinct community (44).
Some features of the urinary microbiome among this population appear to be consistent with microbiomes from other body sites (skin, oral, and gastrointestinal). There are large variations in the composition of this community between individuals (45) with clear differences seen between participants and variation in the community over a short period of time, suggesting that the urinary microbiome, like the gastrointestinal, oral, and cutaneous microbiome, has a large amount of interindividual and intraindividual variability (45). However, large variations in serial samples taken from older adults even after treatment with antibiotics were not seen. It is well documented that there are large shifts in the gastrointestinal microbiome after a course of antibiotics and the return to baseline varies among individuals, returning to a baseline composition that takes about 2 months (46,47). A caveat to this investigation is that 3 of the 4 participants in this study received antibiotics during the study period had also received antibiotics within 90 days prior to being enrolled. It is possible that the urinary microbiome had not returned to baseline prior to receiving an additional course of antibiotics. None of our other exposures of interest (hospitalizations, urinary catheterization, and urinary symptoms) showed any significant association.
The results presented here represent data from a small number of individuals in a single nursing home located in Central Massachusetts and may not be generalizable to residents of nursing homes that may have different resident demographics, diets, and environmental practices. We performed 16S rRNA sequencing of samples, which allows for evaluation of bacterial populations based on OTUs, or groups of similar sequences (48). This does not always allow us to identify an OTU as representing a specific bacterial species and does not allow for analysis of gene content, so we cannot assess the metabolic or pathogenic potential of the organisms detected. However, we feel there are important implications for future work investigating the urinary microbiome and the development of UTI in this population. In future work, we hope to determine if detection of potential pathogens, such as A. urinae, by microbiome analysis in urine is associated with the development of UTI. If this is the case, then the urinary microbiome may represent a target for interventions, such as probiotics that, can prevent dysbiotic changes or pathogen colonization that may be associated with long-term residence within a nursing home. There is already some evidence that diet or probiotic supplements can prevent recurrent UTIs (49). In addition, changes in the urinary microbiome associated with longer-term residence may explain why some individuals among this population are at particularly high risk for recurrent UTIs (50). Given the very large disease burden from this condition in the older adult population (51), a better understanding of this connect could allow for low-cost interventions that prevent the development of UTI.
Supplementary Material
Contributor Information
Evan S Bradley, Department of Emergency Medicine, UMass Memorial Medical Center and University of Massachusetts Medical School, Worcester, Massachusetts, USA; Program in Microbiome Dynamics, University of Massachusetts Medical School, Worcester, Massachusetts, USA.
Brent Schell, Department of Emergency Medicine, UMass Memorial Medical Center and University of Massachusetts Medical School, Worcester, Massachusetts, USA.
Doyle V Ward, Program in Microbiome Dynamics, University of Massachusetts Medical School, Worcester, Massachusetts, USA; Department of Microbiology and Physiological Systems, Center for Microbiome Research, University of Massachusetts Medical School, Worcester, Massachusetts, USA.
Vanni Bucci, Program in Microbiome Dynamics, University of Massachusetts Medical School, Worcester, Massachusetts, USA; Department of Microbiology and Physiological Systems, Center for Microbiome Research, University of Massachusetts Medical School, Worcester, Massachusetts, USA.
Abigail Zeamer, Department of Microbiology and Physiological Systems, Center for Microbiome Research, University of Massachusetts Medical School, Worcester, Massachusetts, USA.
John P Haran, Department of Emergency Medicine, UMass Memorial Medical Center and University of Massachusetts Medical School, Worcester, Massachusetts, USA; Program in Microbiome Dynamics, University of Massachusetts Medical School, Worcester, Massachusetts, USA.
Funding
This work was supported by the Joseph P. Healy Award to support internal faculty research at the University of Massachusetts Medical School, the sponsor had no role in the conduct of this research or the preparation of this manuscript.
Conflict of Interest
None declared.
Author Contributions
Primary study concept and design by E.S.B., J.P.H., and B.S. Preparation of manuscript by E.S.B. and J.P.H. Data analysis performed by E.S.B., D.V.W., V.B., and A.Z. Data collection was performed by B.S., E.S.B., and J.P.H.
References
- 1. Fouts DE, Pieper R, Szpakowski S, et al. Integrated next-generation sequencing of 16S rDNA and metaproteomics differentiate the healthy urine microbiome from asymptomatic bacteriuria in neuropathic bladder associated with spinal cord injury. J Transl Med. 2012;10:174. doi: 10.1186/1479-5876-10-174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bao Y, Al KF, Chanyi RM, et al. Questions and challenges associated with studying the microbiome of the urinary tract. Ann Transl Med. 2017;5(2):33. doi: 10.21037/atm.2016.12.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Whiteside SA, Razvi H, Dave S, Reid G, Burton JP. The microbiome of the urinary tract—a role beyond infection. Nat Rev Urol. 2015;12(2):81–90. doi: 10.1038/nrurol.2014.361 [DOI] [PubMed] [Google Scholar]
- 4. Thomas-White K, Brady M, Wolfe AJ, Mueller ER. The bladder is not sterile: history and current discoveries on the urinary microbiome. Curr Bladder Dysfunct Rep. 2016;11(1):18–24. doi: 10.1007/s11884-016-0345-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bersanelli M, Santoni M, Ticinesi A, Buti S. The urinary microbiome and anticancer immunotherapy: the potentially hidden role of unculturable microbes. Target Oncol. 2019;14(3):247–252. doi: 10.1007/s11523-019-00643-7 [DOI] [PubMed] [Google Scholar]
- 6. Rani A, Ranjan R, McGee HS, et al. Urinary microbiome of kidney transplant patients reveals dysbiosis with potential for antibiotic resistance. Transl Res. 2017;181:59–70. doi: 10.1016/j.trsl.2016.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hooper LV, Gordon JI. Commensal host–bacterial relationships in the gut. Science. 2001;292(5519):1115–1118. doi: 10.1126/science.1058709 [DOI] [PubMed] [Google Scholar]
- 8. Stapelton A. Urinary tract infection pathogenesis: host factors. Infect Dis Clin. 2014;28(1):149–159. doi: 10.1016/j.idc.2013.10.006 [DOI] [PubMed] [Google Scholar]
- 9. Haran JP, Bucci V, Dutta P, Ward D, McCormick B. The nursing home elder microbiome stability and associations with age, frailty, nutrition and physical location. J Med Microbiol. 2018;67(1):40–51. doi: 10.1099/jmm.0.000640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rotjanapan P, Dosa D, Thomas KS. Potentially inappropriate treatment of urinary tract infections in two Rhode Island nursing homes. Arch Intern Med. 2011;171(5):438–443. doi: 10.1001/archinternmed.2011.13 [DOI] [PubMed] [Google Scholar]
- 11. Miller DC, Saigal CS, Litwin MS. The demographic burden of urologic diseases in America. Urol Clin North Am. 2009;36(1):11–27, v. doi: 10.1016/j.ucl.2008.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ruben FL, Dearwater SR, Norden CW, et al. Clinical infections in the noninstitutionalized geriatric age group: methods utilized and incidence of infections. The Pittsburgh Good Health Study. Am J Epidemiol. 1995;141(2):145–157. doi: 10.1093/oxfordjournals.aje.a117402 [DOI] [PubMed] [Google Scholar]
- 13. Rowe TA, Juthani-Mehta M. Diagnosis and management of urinary tract infection in older adults. Infect Dis Clin North Am. 2014;28(1):75–89. doi: 10.1016/j.idc.2013.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cortes-Penfield NW, Trautner BW, Jump RLP. Urinary tract infection and asymptomatic bacteriuria in older adults. Infect Dis Clin North Am. 2017;31(4):673–688. doi: 10.1016/j.idc.2017.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kistler CE, Zimmerman S, Scales K, et al. The antibiotic prescribing pathway for presumed urinary tract infections in nursing home residents. J Am Geriatr Soc. 2017;65(8):1719–1725. doi: 10.1111/jgs.14857 [DOI] [PubMed] [Google Scholar]
- 16. Yin P, Kiss A, Leis JA. Urinalysis orders among patients admitted to the general medicine service. JAMA Intern Med. 2015;175(10):1711–1713. doi: 10.1001/jamainternmed.2015.4036 [DOI] [PubMed] [Google Scholar]
- 17. Crnich CJ, Jump RL, Nace DA. Improving management of urinary tract infections in older adults: a paradigm shift or therapeutic nihilism? J Am Geriatr Soc. 2017;65(8):1661–1663. doi: 10.1111/jgs.14961 [DOI] [PubMed] [Google Scholar]
- 18. Wiström J, Norrby SR, Myhre EB, et al. Frequency of antibiotic-associated diarrhoea in 2462 antibiotic-treated hospitalized patients: a prospective study. J Antimicrob Chemother. 2001;47(1):43–50. doi: 10.1093/jac/47.1.43 [DOI] [PubMed] [Google Scholar]
- 19. Furuya-Kanamori L, Stone JC, Clark J, et al. Comorbidities, exposure to medications, and the risk of community-acquired Clostridium difficile infection: a systematic review and meta-analysis. Infect Control Hosp Epidemiol. 2015;36(2):132–141. doi: 10.1017/ice.2014.39 [DOI] [PubMed] [Google Scholar]
- 20. Nicolle LE, Gupta K, Bradley SF, et al. Clinical practice guideline for the management of asymptomatic bacteriuria: 2019 update by the Infectious Diseases Society of America. Clin Infect Dis. 2019;68(10):e83–e110. doi: 10.1093/cid/ciy1121 [DOI] [PubMed] [Google Scholar]
- 21. Nicolle LE. Urinary tract infections in the older adult. Clin Geriatr Med. 2016;32(3):523–538. doi: 10.1016/j.cger.2016.03.002 [DOI] [PubMed] [Google Scholar]
- 22. Zhang Q, Kwoh C, Attorri S, Clarridge JE 3rd. Aerococcus urinae in urinary tract infections. J Clin Microbiol. 2000;38(4):1703–1705. doi: 10.1128/JCM.38.4.1703-1705.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brubaker L, Wolfe AJ. The female urinary microbiota, urinary health and common urinary disorders. Ann Transl Med. 2017;5(2):34. doi: 10.21037/atm.2016.11.62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Church S, Rogers E, Rockwood K, Theou O. A scoping review of the Clinical Frailty Scale. BMC Geriatr. 2020;20(1):393. doi: 10.1186/s12877-020-01801-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Morris JC, Ernesto C, Schafer K, et al. Clinical dementia rating training and reliability in multicenter studies: the Alzheimer’s Disease Cooperative Study experience. Neurology. 1997;48(6):1508–1510. doi: 10.1212/wnl.48.6.1508 [DOI] [PubMed] [Google Scholar]
- 26. Hourigan SK, Zhu W, S W Wong W, et al. Studying the urine microbiome in superficial bladder cancer: samples obtained by midstream voiding versus cystoscopy. BMC Urol. 2020;20(1):5. doi: 10.1186/s12894-020-0576-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kozich JJ, Westcott SL, Baxter NT, Highlander SK, Schloss PD. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl Environ Microbiol. 2013;79(17):5112–5120. doi: 10.1128/AEM.01043-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Edgar RC. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods. 2013;10(10):996–998. doi: 10.1038/nmeth.2604 [DOI] [PubMed] [Google Scholar]
- 29. Hajjem A, Bellavance F, Larocque D. Mixed-effects random forest for clustered data. J Stat Comput Simul. 2014;84(6):1313–1328. doi: 10.1080/00949655.2012.741599 [DOI] [Google Scholar]
- 30. Wei P, Lu Z, Song J. Variable importance analysis: a comprehensive review. Reliab Eng Syst Saf. 2015;142:399–432. doi: 10.1016/j.ress.2015.05.018 [DOI] [Google Scholar]
- 31. Haran JP, Bhattarai SK, Foley SE, et al. Alzheimer’s disease microbiome is associated with dysregulation of the anti-inflammatory P-glycoprotein pathway. mBio. 2019;10(3):e00632–19. doi: 10.1128/mBio.00632-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Modi SR, Collins JJ, Relman DA. Antibiotics and the gut microbiota. J Clin Invest. 2014;124(10):4212–4218. doi: 10.1172/JCI72333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Contreras A, Doan N, Chen C, Rusitanonta T, Flynn MJ, Slots J. Importance of Dialister pneumosintes in human periodontitis. Oral Microbiol Immunol. 2000;15(4):269–272. doi: 10.1034/j.1399-302x.2000.150410.x [DOI] [PubMed] [Google Scholar]
- 34. Xu Z, Xie Z, Sun J, et al. Gut microbiome reveals specific dysbiosis in primary osteoporosis. Front Cell Infect Microbiol. 2020;10:160. doi: 10.3389/fcimb.2020.00160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Masha SC, Owuor C, Ngoi JM, et al. Comparative analysis of the vaginal microbiome of pregnant women with either Trichomonas vaginalis or Chlamydia trachomatis. PLoS One. 2019;14(12):e0225545. doi: 10.1371/journal.pone.0225545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Van den Abbeele P, Belzer C, Goossens M, et al. Butyrate-producing Clostridium cluster XIVa species specifically colonize mucins in an in vitro gut model. ISME J. 2013;7(5):949–961. doi: 10.1038/ismej.2012.158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Duytschaever G, Huys G, Bekaert M, Boulanger L, De Boeck K, Vandamme P. Dysbiosis of bifidobacteria and Clostridium cluster XIVa in the cystic fibrosis fecal microbiota. J Cyst Fibros. 2013;12(3):206–215. doi: 10.1016/j.jcf.2012.10.003 [DOI] [PubMed] [Google Scholar]
- 38. Takeshita K, Mizuno S, Mikami Y, et al. A single species of Clostridium subcluster XIVa decreased in ulcerative colitis patients. Inflamm Bowel Dis. 2016;22(12):2802–2810. doi: 10.1097/MIB.0000000000000972 [DOI] [PubMed] [Google Scholar]
- 39. Sharma A, Richardson M, Cralle L, et al. Longitudinal homogenization of the microbiome between both occupants and the built environment in a cohort of United States Air Force Cadets. Microbiome. 2019;7(1):70. doi: 10.1186/s40168-019-0686-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Flores GE, Caporaso JG, Henley JB, et al. Temporal variability is a personalized feature of the human microbiome. Genome Biol. 2014;15(12):531. doi: 10.1186/s13059-014-0531-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ghosh TS, Rampelli S, Jeffery IB, et al. Mediterranean diet intervention alters the gut microbiome in older people reducing frailty and improving health status: the NU-AGE 1-year dietary intervention across five European countries. Gut. 2020;69(7):1218–1228. doi: 10.1136/gutjnl-2019-319654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173(5):489–495. doi: 10.1503/cmaj.050051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Claesson MJ, Jeffery IB, Conde S, et al. Gut microbiota composition correlates with diet and health in the elderly. Nature. 2012;488(7410):178–184. doi: 10.1038/nature11319 [DOI] [PubMed] [Google Scholar]
- 44. Adebayo AS, Ackermann G, Bowyer RCE, et al. The urinary tract microbiome in older women exhibits host genetic and environmental influences. Cell Host Microbe. 2020;28(2):298–305.e3. doi: 10.1016/j.chom.2020.06.022 [DOI] [PubMed] [Google Scholar]
- 45. Costello EK, Lauber CL, Hamady M, Fierer N, Gordon JI, Knight R. Bacterial community variation in human body habitats across space and time. Science. 2009;326(5960):1694–1697. doi: 10.1126/science.1177486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Langdon A, Crook N, Dantas G. The effects of antibiotics on the microbiome throughout development and alternative approaches for therapeutic modulation. Genome Med. 2016;8(1):39. doi: 10.1186/s13073-016-0294-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dethlefsen L, Relman DA. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc Natl Acad Sci U S A. 2011;108(Suppl. 1):4554–4561. doi: 10.1073/pnas.1000087107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Caporaso JG, Lauber CL, Walters WA, et al. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc Natl Acad Sci U S A. 2011;108(Suppl. 1):4516–4522. doi: 10.1073/pnas.1000080107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Aragón IM, Herrera-Imbroda B, Queipo-Ortuño MI, et al. The urinary tract microbiome in health and disease. Eur Urol Focus. 2018;4(1):128–138. doi: 10.1016/j.euf.2016.11.001 [DOI] [PubMed] [Google Scholar]
- 50. Molander U, Arvidsson L, Milsom I, Sandberg T. A longitudinal cohort study of elderly women with urinary tract infections. Maturitas. 2000;34(2):127–131. doi: 10.1016/s0378-5122(99)00102-4 [DOI] [PubMed] [Google Scholar]
- 51. Griebling TL. Urinary tract infection in women. In: Urologic Diseases in America. 2007;7:587–619. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.