Abstract
Introduction
Initiation of tobacco products typically occurs in adolescence. Adolescence is a critical period in development where the maturation of brain neurocircuitry is vulnerable to nicotine. Nicotine-containing products and psychostimulants, such as methamphetamine (METH), are often coabused. Rodent studies have shown that nicotine exposure in early adolescence increases subsequent drug intake and reward. Given the exponential increase in e-cigarette use among adolescents, there is a pressing need to understand whether adolescent nicotine exposure impacts concurrent increased METH use. The objective of this study is to evaluate age, sex, and longitudinal effects of nicotine pretreatment on METH reinforcement.
Aims and Methods
Male and female Sprague-Dawley rats were pretreated with a subchronic, low-dose nicotine (2×, 30 µg/kg/0.1 mL, intravenous) or saline during early adolescence (postnatal days [PN] 28–31) or adulthood (PN 86–89). Following nicotine pretreatment, on PN 32 or PN 90, animals underwent operant intravenous self-administration for METH (20 µg/kg/inf) over a 2-hour period for five consecutive days.
Results
Early adolescent nicotine exposure enhances intravenous METH self-administration in male, but not female adolescents. Male adult rats self-administer METH over the 5-day testing period, independent of nicotine exposure. In contrast, nicotine exposure increases METH self-administration in female adults during the later sessions of the 5-day testing period.
Conclusions
Taken together, our data highlight age- and sex-dependent effects of low dose, subchronic nicotine pretreatment on subsequent intravenous METH self-administration.
Implications
A majority of polysubstance users begin smoking before the age of 18. Mounting evidence highlights adolescent susceptibility to nicotine exposure on brain and behavior. With the escalation in nicotine-containing products and stimulant use among adolescents, it is important to identify the consequences from adolescent nicotine use, including polysubstance use. Our study provides evidence that adolescent nicotine exposure enhances subsequent METH use, with important sex- and age-dependent effects.
Introduction
Initiation and use of nicotine-containing products typically occur during adolescence. Although there has been a decrease in traditional tobacco cigarette use among middle to high school students, e-cigarettes are currently the most used form of nicotine among teens in the United States.1 Adolescence is critical period of development where the brain undergoes changes in structural and functional reorganization in regions necessary for successful independence in adulthood.2 This maturation process is particularly vulnerable to the effects of adolescent exposure to nicotine, the major constituent in tobacco products. We and others have illustrated in preclinical studies that adolescent nicotine exposure enhances stimulant reinforcement and reward.3–6
In humans, the progression from adolescent tobacco use to heavy, illicit substance use has been observed and described as the “gateway hypothesis.” 7 Nicotine interacts with psychostimulants to influence brain, behavior, and health of users.8 Methamphetamine (METH) is a more potent analog of the stimulant, amphetamine, with similar structure yet longer lasting effects and greater impact on dopamine transporter-mediated cell physiology.9,10 Prevalence of METH use has increased among adolescence from 2019 to 2020.11 Individuals who use METH are typically smokers, most of whom report tobacco initiation during adolescence prior to METH use.8,12–15
With the nation facing a second wave of the METH epidemic16 and given the recent spike in adolescent e-cigarette use,1 it is critical to understand the contributions of nicotine exposure on METH use, including age- and sex-dependent effects. Preclinical studies have consistently demonstrated that early adolescent nicotine pretreatment enhances stimulant reward.17,18 Limited preclinical studies have assessed the consequences of adolescent nicotine exposure effects on subsequent and long-term METH reinforcement, extinction, and reinstatement.19–21 Even fewer studies assess nicotine-induced METH self-administration effects in both males and females. However, sex- and age-dependent effects for amphetamine and METH reward and self-administration alone have been observed.22–27
This study builds on previous work that identified an age-dependent nicotine-induced enhancement of subsequent, 1-day METH self-administration in male rats.19 We use a well-established 4-day intravenous nicotine pretreatment paradigm to model adolescent initiation of nicotine smoking behavior in rats.5,6,19,28 One day following nicotine exposure, animals self-administer METH for a 2-hour period each day for five consecutive days at a dose that previous studies have shown nicotine-induced enhancement of METH reinforcement in adolescent male rats.19 We hypothesize that early adolescent, but not adult, nicotine exposure will enhance METH intake with a higher response in female versus male rats.21 Our work adds to the literature, as we assess both age and sex effects in nicotine-induced METH reinforcement over 5 days.
Methods
Animals
Male and female Sprague-Dawley rats (Charles Rivers) arrived on postnatal day (PN) 17 or PN 74. Juveniles were weaned on PN 21. All animals were group housed in a controlled 12-hour light–dark cycle (lights on at 0700) in an Association for Assessment and Accreditation of Laboratory Animal Care-accredited vivarium. Prior to catheterization, animals were weighed daily and handled for 3 days (PN 21–23 and PN 79–81). Food and water were provided ad libitum except for the day before and during intravenous self-administration where animals were temporally food restricted (15–20 g/rat/day). Animals were provided enough food to maintain normal growth.28 To avoid litter effects, one pup per litter per experimental group was used. Rats were randomly assigned to experimental groups using a random sequence generator. All experiments were performed in accordance and approved by the Institutional Animal Care and Use Committee at the University of California, Irvine.
Drugs and Reagents
Nicotine tartrate (Glentham Life Sciences) and METH (National Institute of Drug Abuse) were dissolved in saline and filtered via 0.22 µm sterile filters (VWR). Nicotine was calculated as a base and pH 7.2–7.4.
Surgical Procedure
On PN 24 or 82, catheters were surgically implanted into the right external jugular vein as described.29 Animals were anesthetized with equithesin (0.35 mL/100 g, IP). Immediately after surgery, rats were administered the analgesic carprofen (5 mg/kg, SC). To maintain patency, cannulas were flushed daily with sterile heparinized saline solution. Animals were given 3 days to recover from catheter surgery.
Four-Day Nicotine Pretreatment
Starting on PN 28 or PN 86, rats were administered nicotine (2×, 0.03 mg/kg/0.1 mL, IV) or saline injections spaced one minute apart daily for 4 days.28
METH Self-administration
One day after nicotine pretreatment, animals were tested in self-administration chambers that measured at 28 × 25 × 30 cm high with two nose pokes holes for 5 days (PN 32–36 or PN 90–94). Adolescent and adult animals underwent a 2-hour nose poke session on a fixed ratio 1 schedule to administer METH (0.2 mg/kg/infusion (inf))19 with a time-out period of 20 s signaled by a cue light over the reinforced hole. During this time-out period, animals were restricted from receiving another reinforced response (inf) and the house light was turned off. Nose poke of the nonreinforced hole was scored but did not result in a signal or inf. To determine catheter patency, after the last intravenous self-administration session, rats were administered propofol, a rapid anesthetic (0.05 mL for adolescents, 0.1 mL for adults, IV).
Statistical Analysis
Data were analyzed with JMP (SAS Institute, Cary, NC). Animals were excluded from the analysis if they did not display immediate anesthesia from propofol. Further, animals were excluded if they were outliers on box and whisker plots separated by all groups (sex × age × pretreatment) for mean day 1–5 reinforced and nonreinforced responses (n = 3), and mean day 4–5 reinforced and nonreinforced responses (n = 5), and mean total METH intake (mg/kg, n = 3). For 5-day METH acquisition, mean response data over days were analyzed by a repeated measure four-way analysis of variance (ANOVA) for sex (male or female) × age (adolescent or adult) × pretreatment (saline or nicotine) × day, with a repeated measure on day for reinforced and nonreinforced responses (n = 8–11/group). For mean day 4–5 METH self-administration, mean response data over time were analyzed by a three-way ANOVA for sex × age × pretreatment (n = 8–11/group). For METH intake, mean daily intake (mg/kg) were analyzed by a repeated measure four-way ANOVA for sex × age × day, with a repeated measure on day (n = 8–11/group). Bonferroni corrected post hoc analyses were applied for significant main or interactive effects with one- or two-tailed t-tests, as appropriate.
Results
Early Adolescent, But Not Adult, Nicotine Exposure Sex Dependently Enhances Subsequent Acquisition of METH Self-administration
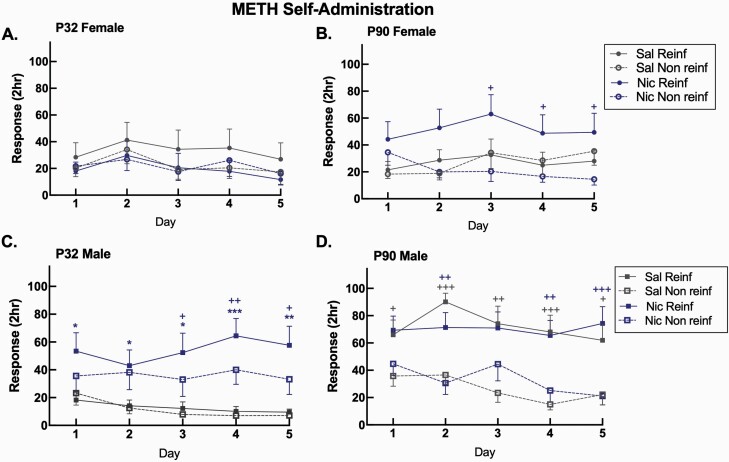
To determine whether age- and sex-dependent nicotine-induced enhancement of METH self-administration was maintained throughout the 5 days of self-administration, we assessed response data over time. Overall ANOVA illustrated significant main effect for sex (F1,69 = 8.20, p = .006), age (F1,69 = 14.07, p = .0004), as well as age × sex × pretreatment interactive effects (F1,69 = 9.99, p = .002) for reinforced responses. For nonreinforced responses, we observe a significant main effect for day (F4,276 = 3.71, p = .006) as well as day × age (F4,276 = 2.71, p = .03), day × age × pretreatment (F4,276 = 2.61, p = .04), and day × pretreatment × sex interactive effects (F4,276 = 2.46, p = .05). Since we identified interactions for every measure, we separated the data out by age, sex, pretreatment, response, and day (Figure 1, A–D). Neither saline- nor nicotine-pretreated female adolescent rats acquire METH self-administration over the 5-day period (Figure 1, A). We define acquisition as significant preference for the METH reinforced over nonreinforced hole (Figure 1, B–D). Nicotine-pretreated male adolescents responded for METH significantly more than saline-pretreated male adolescents on days 1–5 (p ≤ .05), with a preference for reinforced versus nonreinforced responding on days 3–5 (p < .05, Figure 1, C). Nicotine when compared with saline exposure significantly increased nonreinforced responding for adolescent males on days 1–5 (p = .02, data not shown). We further observe the emergence and maintenance of preference for reinforced over nonreinforced responding in male adults, independent of nicotine exposure, throughout acquisition (p < .05, Figure 1, D) and in nicotine-exposed female adults on days 3–5 (p < .05, Figure 1, B).
Figure 1.
Nicotine-induced enhancement of 5-day METH self-administration in male adolescent rats. Mean 2-hour response ± SEM in (A) female adolescent, (B) female adult, (C) male adolescent, and (D) male adult Sprague-Dawley rats. *p ≤ .05, **p < .01, ***p < .001 adolescent Nic versus adolescent Sal Reinf responses; +p < .05, ++p < .01, +++p < .001 Reinf versus Non Reinf responses. n = 8–11/group. Open and closed circles represent individual female animals, open and closed squares represent individual male animals. METH = methamphetamine; Nic = nicotine (blue); NR = nonreinforced; Reinf = reinforced; Sal = saline (gray).
To evaluate age effects on METH self-administration, we separated data out by pretreatment and day. Overall, saline-pretreated male adults self-administered more METH than all other saline-pretreated groups across days 1–5, including male adolescents and females (p < .05, data not shown). In nicotine-treated animals, male adults self-administer more METH than female adolescents on days 1 and 5 (p < .05, data not shown) and male adolescents self-administer more METH than female adolescents on day 4 (p < .05, data not shown).
Early Adolescent Nicotine-Induced Enhancement of METH Acquisition Persists Postnicotine Exposure in Male, But Not Female, Rats
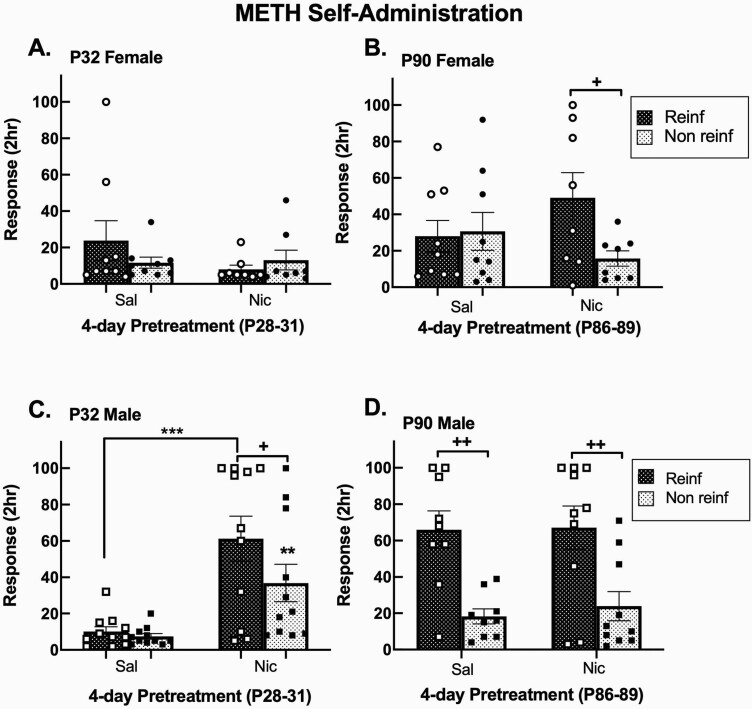
To determine whether nicotine-induced enhancement of METH responding remained after the first day of testing, we assessed mean day 4–5 responses (Figure 2, A–D). Overall ANOVA showed significant main effects for sex (F1,67 = 11.18, p = .01), age (F1,67 = 14.25, p = .0003), and pretreatment (F1,67 = 4.11, p = .05) as well as sex × age x pretreatment interactive effects (F1,67 = 9.29, p = .003) for reinforced responses. We further identified a significant age × pretreatment (F1,67 = 4.50, p = .04) and pretreatment × sex interactions (F1,67 = 6.60, p = .01) for nonreinforced responses. Data were split by age and sex to analyze effects of nicotine pretreatment. Nicotine exposure significantly increased reinforced and nonreinforced responding for male adolescents (p < .001 and p < .01, respectively, Figure 2, C). Adult males discriminated between reinforced and nonreinforced responding regardless of pretreatment, whereas nicotine pretreatment enhanced this discrimination in male adolescents and female adults (p < .05, Figure 2, B–D).
Figure 2.
Nicotine-induced enhancement of METH self-administration persists in male adolescent rats. Mean day 4–5 2-hour responses ± SEM in (A) female adolescent, (B) female adult, (C) male adolescent, and (D) male adult Sprague-Dawley rats. **p < .01, ***p < .01 adolescent Nic versus adolescent Sal Reinf or Non-Reinf responses; +p < .05, ++p < .01 Reinf versus Non-Reinf responses. n = 8–11/group. Open and closed circles represent individual female animals, open and closed squares represent individual male animals. METH = methamphetamine; Nic = nicotine; NR = nonreinforced; Reinf = reinforced; Sal = saline.
Nicotine Exposure Age- and Sex Dependently Enhances METH Intake
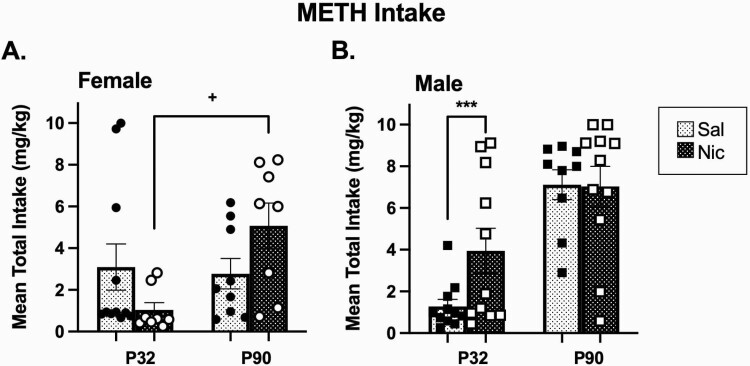
To determine whether nicotine exposure increases METH intake, we assessed mean METH administered (mg/kg) daily. Overall ANOVA illustrated significant main effects for sex (F1,69 = 8.97, p = .004) and age (F1,69 = 15.14, p = .0002) as well as sex × age × pretreatment interactions (F1,69 = 12.89, p = .001). No effects were observed for day, therefore, we collapsed mean intake (mg/kg) by day and separated remaining data by age, sex, and pretreatment (Figure 3, A and B). Our data illustrate nicotine when compared with saline enhanced METH intake in male adolescents (p = .0008, Figure 3, B). Further, nicotine-exposed female adults administer more METH than nicotine-exposed female adolescents (p = .03, Figure 3, A). Lastly, males administer more METH (mg/kg) than females (p = .0038, data not shown) and adults administer more METH (mg/kg) than adolescents (p = .0002, data not shown).
Figure 3.
Nicotine-induced enhancement of total (5 day) METH intake in male adolescent and female adult rats. Mean total METH intake (mg/kg) ± SEM in female (A) and male (B) Sprague-Dawley rats. ***p < .001 Nic versus Sal intake; +p < .05 adult versus adolescent nicotine-exposed animals; n = 8–11/group. Open and closed circles represent individual female animals, open and closed squares represent individual male animals. METH = methamphetamine; Nic = nicotine; Sal = saline.
Discussion
In our current study, we successfully replicate nicotine-induced enhancement of METH acquisition19 and show that nicotine increases METH intake, in adolescent male, but not female, rats. Further, nicotine-treated adolescent males illustrate discrimination for the METH reinforced nose poke on the last 3 days of self-administration and saline-treated adolescents respond less for METH than adult rats. Lastly, adult nicotine exposure enhances METH intake and discrimination for reinforced versus nonreinforced responding for METH in female adults, whereas male adult rats show increased intake and discrimination independent of nicotine exposure. Taken together, our findings illustrate that nicotine effects on METH self-administration persist at least 5 days after exposure and these effects are age- and sex dependent.
This study used a subchronic (4-day) nicotine exposure paradigm that more closely models nicotine initiation or experimentation in adolescent humans.28,30 Using our paradigm, we demonstrate that brief, low-dose nicotine exposure is sufficient to enhance subsequent METH reinforcement in adolescent males and adult females, but not adult males or adolescent female rats. Our intravenous administration model is less stressful than other routes of repeated exposure and separated into two doses spaced a minute apart to model a standard time-out period in nicotine self-administration studies.28 For METH intake, we used intravenous self-administration versus oral intake where taste preference could impact results. Moreover, our study assesses METH self-administration using a stringent paradigm, that does not require prior response (eg, food or sucrose) training.
Prior studies using this 4-day nicotine pretreatment paradigm have consistently illustrated age-dependent effects of nicotine-induced enhancement of subsequent cocaine, ethanol, METH, and fentanyl reinforcement.5,6,19,28 Of note, the study using this paradigm to identify nicotine-induced enhancement of METH reinforcement only assessed males and for a single day following nicotine treatment.19 Our data successfully replicate this prior finding of age-dependent nicotine-induced enhancement of METH self-administration in male rats. However, we do not show discrimination in our nicotine-treated adolescent males on day 1 of METH self-administration. Further, we observe an expression of sensitization where nicotine, but not saline, pretreated adolescent males have higher nonreinforced responding. Rodent studies have shown that nicotine-exposed male adolescents exhibit behavioral sensitivity to psychostimulant-induced locomotion.31–34 Nonetheless, we do not believe our results are confounded by increased locomotion as preference for reinforced versus nonreinforced nose poke responding for nicotine-treated male adolescents are observed on days 3–5 of self-administration. However, future studies are needed to assess nicotine pretreatment effects on METH-induced behavioral plasticity directly. Future studies could also include a saline self-administration control group to evaluate general cue reactivity among the groups. Overall, our data add to the literature by illustrating these effects are sex dependent and remain beyond one day postexposure. Few studies have delved into nicotine-induced METH self-administration during adolescence and in both sexes.
Although preclinical studies have shown that adolescent nicotine exposure enhances subsequent cocaine reinforcement, independent of sex,5,28 we observe adolescent nicotine-induced enhancement of METH reinforcement in male, but not female rats. In male rodents, adolescent, but not adult, low-dose nicotine exposure has been shown to enhance subsequent METH intravenous self-administration.19,20 Moreover, adolescent nicotine exposure does not affect METH extinction or METH-primed reinstatement in adult male rodents.21 However, nicotine-induced sex differences have been observed in subsequent METH-seeking in adolescent rats.21 Age-, sex-, and nicotine-dependent effects may be expected since studies have demonstrated such effects in amphetamine self-administration and nicotine-induced cocaine and amphetamine locomotor sensitization.27,31,32 In contrast to our data, studies show that at higher doses of nicotine, early adolescent exposure enhances subsequent METH oral intake in female, but not male, rats.21
Rodent studies show nicotine is more rewarding in female adolescents.22 In contrast, we observe enhanced nicotine-induced effects in male adolescents. Nicotine, sex steroids, and steroid metabolites can interact with ligand gated ion channels such as nAChRs, which are critical for the maturation of the brain and enact age-, sex-, and dose-dependent effects.22,35 However, our observed nicotine-induced effects are not likely mediated directly by gonadal hormones.36 Sexual differentiation takes place in the brain during adolescence, mostly independent of puberty influences.30,36 Our adolescent sex-dependent influences may be mediated instead by increased nicotine-stimulated dopamine release and increased striatal dopamine receptors followed by pruning.37–39 Additionally, nicotine potency and efficacy during adolescence, nicotine-induced changes in the dopamine system, and nicotine-induced activation of the hypothalamic–pituitary–adrenal axis and decreased anxiety-like behavior are more prevalent in adolescent male than female rodents.36,40–42 Our saline-treated adolescent results are not in agreement with METH self-administration studies that show no age differences for short access METH self-administration in male rats24 and that females self-administer more METH than males. Conflicting results may be due to differing methods including routes of drug administration (ie, oral vs. intravenous self-administration), lever versus nose pokes, and training sessions.21,24 However, similar to cocaine reinforcement, saline-treated adolescent rats respond less for METH compared to adults.5,6,19,43
For adults, clinical and preclinical studies show that female adults are more sensitive to nicotine, than males.44,45 While studies have shown nicotine conditioning decreases METH self-administration in female rats,45 we observe nicotine-induced discrimination between reinforced and nonreinforced responding after day 3 of self-administration and overall METH intake (mg/kg) in female adults. Conversely, low-dose nicotine exposure had no effect on METH self-administration in male adults.46 In regards to saline-treated adults, data are consistent with previous studies where male adult rats have increased METH response during short access self-administration.26 In contrast to the literature, our saline-treated females responded less for METH and exhibit lower METH intake than males.47 Methodological differences in METH studies described including repeated saline exposure, METH dose, baseline locomotor test prior to self-administration, and the self-administration protocol, may all contribute to the observed differences.
When considering mechanisms for our observed adolescent nicotine-induced effects, nicotinic receptor-induced changes in the dopaminergic system are likely involved. Studies have shown that the nonselective nicotinic acetylcholine receptor antagonist, mecamylamine, inhibits cocaine-induced place preference and self-administration.48,49 Other preclinical studies have demonstrated the role of α7 and α4β2-containing nicotinic receptors in nicotine-induced enhancement of cocaine self-administration19 and attenuation of METH-induced dopaminergic deficits.50 Additional mechanisms mediating age-dependent nicotine-induced effects include 5-HT1A receptor activity in the limbic region for the enhancement of 1-day cocaine and METH reinforcement,19 increased D2 receptor activity,40 and microglia activation5 for the enhancement of cocaine reinforcement as well as increased ΔFosB in the nucleus accumbens in stimulant reward.18 Follow-up studies are necessary to identify whether D2 receptor activity, microglia activation, ΔFosB accumulation, 5-HT1A, and/or additional neural mechanisms underlie our age- and sex-dependent nicotine exposure effects on METH reinforcement. While our study examined nicotine-induced acquisition of METH self-administration, additional studies are necessary to assess nicotine-induced age and sex effects on the motivation for METH using higher schedules of reinforcement and progressive ratio as well as extinction and reinstatement using our subchronic nicotine pretreatment paradigm. Other studies should explore more chronic procedures, and nicotine combined with METH in co-self-administration studies.
Conclusion
Initiation of tobacco typically begins during adolescence and can lead to long‐term consequences, including illicit substance abuse (ie, the “gateway hypothesis”). In this study, we observe early adolescent, but not adult, nicotine exposure enhances the acquisition and maintenance of METH self-administration in male, but not female rats. We further identify differences in nicotine-induced METH response in our adult rat populations. Taken together, our results highlight the complexity and importance of including the biological variable, sex, and age to evaluate substance use in translational animal models. Inclusion of additional groups may assist in the identification of at-risk populations and lead to the development of preventative and/or targeted therapeutic strategies toward each distinctive group.
Supplementary Material
A Contributorship Form detailing each author’s specific involvement with this content, as well as any supplementary data, are available online at https://academic.oup.com/ntr.
Acknowledgments
We thank Drs Frances M. Leslie and James D. Belluzzi for continued support and feedback as well as Emily Castro, Jiaqi Li, and Karen Florely Martinez Regueira for their technical assistance.
Contributor Information
Anjelica Cardenas, Department of Pharmaceutical Sciences, College of Health Sciences, University of California, Irvine, Irvine, CA, USA.
Shahrdad Lotfipour, Department of Pharmaceutical Sciences, College of Health Sciences, University of California, Irvine, Irvine, CA, USA; Department of Pathology and Laboratory Medicine, School of Medicine, University of California, Irvine, Irvine, CA, USA; Department of Emergency Medicine, School of Medicine, University of California, Irvine, Irvine, CA, USA.
Funding
This work was supported by the Tobacco-Related Disease Research Program Project Grant [T31IP1427; 22RT-0103] (SL); University of California, Irvine (UCI) Institute for Clinical and Translational Sciences (ICTS) Pilot Studies Program (National Institute of Health, National Center for Advancing Translational Science (NIH, NCATS)) (SL); UCI Department of Emergency Medicine Prestige Funds (SL); Brain and Behavior Research Grant [21517] (SL); UCI School of Medicine Start Up funds (SL). AC was supported by the Ford Foundation Fellowship and the University of California President’s Pre-Professoriate Fellowship.
Declaration of Interests
None declared.
Authors’ Contributions
AC and SL conceived the study design, conducted data analyses, and contributed to manuscript writing. AC collected data and conducted literature review. All authors contributed to the critical revision of data analysis and approved the final version of the manuscript.
Data Availability
Data will be available, upon reasonable request from the authors.
References
- 1. NIDA. Electronic Cigarettes (E-cigarettes). Bethesda, MD: NIH; 2018. https://www.drugabuse.gov/publications/research-reports/tobacco-nicotine-e-cigarettes/what-are-electronic-cigarettes. [Google Scholar]
- 2. Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24(4):417–463. [DOI] [PubMed] [Google Scholar]
- 3. Ren M, Lotfipour S. Nicotine gateway effects on adolescent substance use. West J Emerg Med. 2019;20(5):696–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Leslie FM. Unique, long-term effects of nicotine on adolescent brain. Pharmacol Biochem Behav. 2020;197:173010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Linker KE, Gad M, Tawadrous P, et al. Microglial activation increases cocaine self-administration following adolescent nicotine exposure. Nat Commun. 2020;11(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cardenas A, Martinez M, Saenz Mejia A, Lotfipour S. Early adolescent subchronic low-dose nicotine exposure increases subsequent cocaine and fentanyl self-administration in Sprague-Dawley rats. Behav Pharmacol. 2020;32(1):86–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kandel D. Stages in adolescent involvement in drug use. Science. 1975;190(4217):912–914. [DOI] [PubMed] [Google Scholar]
- 8. Cross SJ, Lotfipour S, Leslie FM. Mechanisms and genetic factors underlying co-use of nicotine and alcohol or other drugs of abuse. Am J Drug Alcohol Abuse. 2017;43(2):171–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Goodwin JS, Larson GA, Swant J, et al. Amphetamine and methamphetamine differentially affect dopamine transporters in vitro and in vivo. J Biol Chem. 2009;284(5):2978–2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Abuse TNIoD. Medications Development Research for Treatment of Amphetamine and Methamphetamine Addiction. National Institutes of Health, Department of Health and Human Services; 2005. https://archives.drugabuse.gov/sites/default/files/methmeds.pdf. [Google Scholar]
- 11. Johnson LD, Miech RA, O’Malley PM, Bachman JG, Schulenberg JE, Patrick ME. Monitoring the Future National Survey Results on Drug Use, 1975–2020. Ann Arbor, MI: Institute for Social Research, University of Michigan; National Institute on Drug Abuse; The National Institutes of Health; 2021. [Google Scholar]
- 12. Brecht ML, Greenwell L, Anglin MD. Substance use pathways to methamphetamine use among treated users. Addict Behav. 2007;32(1):24–38. [DOI] [PubMed] [Google Scholar]
- 13. Russell K, Dryden DM, Liang Y, et al. Risk factors for methamphetamine use in youth: a systematic review. BMC Pediatr. 2008;8:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brecht ML, O’Brien A, von Mayrhauser C, Anglin MD. Methamphetamine use behaviors and gender differences. Addict Behav. 2004;29(1):89–106. [DOI] [PubMed] [Google Scholar]
- 15. Kohut SJ. Interactions between nicotine and drugs of abuse: a review of preclinical findings. Am J Drug Alcohol Abuse. 2017;43(2):155–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. The Lancet. Opioids and methamphetamine: a tale of two crises. Lancet. 2018;391(10122):713. [DOI] [PubMed] [Google Scholar]
- 17. Kota D, Robinson SE, Imad Damaj M. Enhanced nicotine reward in adulthood after exposure to nicotine during early adolescence in mice. Biochem Pharmacol. 2009;78(7):873–879. [DOI] [PubMed] [Google Scholar]
- 18. Alajaji M, Lazenka MF, Kota D, et al. Early adolescent nicotine exposure affects later-life cocaine reward in mice. Neuropharmacology. 2016;105:308–317. [DOI] [PubMed] [Google Scholar]
- 19. Dao JM, McQuown SC, Loughlin SE, Belluzzi JD, Leslie FM. Nicotine alters limbic function in adolescent rat by a 5-HT1A receptor mechanism. Neuropsychopharmacology. 2011;36(7):1319–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pipkin JA, Kaplan GJ, Plant CP, et al. Nicotine exposure beginning in adolescence enhances the acquisition of methamphetamine self-administration, but not methamphetamine-primed reinstatement in male rats. Drug Alcohol Depend. 2014;142:341–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Harmony ZR, Alderson EM, Garcia-Carachure I, Bituin LD, Crawford CA. Effects of nicotine exposure on oral methamphetamine self-administration, extinction, and drug-primed reinstatement in adolescent male and female rats. Drug Alcohol Depend. 2020;209:107927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Torres OV, Natividad LA, Tejeda HA, Van Weelden SA, O’Dell LE. Female rats display dose-dependent differences to the rewarding and aversive effects of nicotine in an age-, hormone-, and sex-dependent manner. Psychopharmacology (Berl). 2009;206(2):303–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pittenger ST, Chou S, Barrett ST, Catalano I, Lydiatt M, Bevins RA. Nicotine- and cocaine-triggered methamphetamine reinstatement in female and male Sprague-Dawley rats. Pharmacol Biochem Behav. 2017;159:69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Anker JJ, Baron TR, Zlebnik NE, Carroll ME. Escalation of methamphetamine self-administration in adolescent and adult rats. Drug Alcohol Depend. 2012;124(1–2):149–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cox BM, Young AB, See RE, Reichel CM. Sex differences in methamphetamine seeking in rats: impact of oxytocin. Psychoneuroendocrinology. 2013;38(10):2343–2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Reichel CM, Chan CH, Ghee SM, See RE. Sex differences in escalation of methamphetamine self-administration: cognitive and motivational consequences in rats. Psychopharmacology (Berl). 2012;223(4):371–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shahbazi M, Moffett AM, Williams BF, Frantz KJ. Age- and sex-dependent amphetamine self-administration in rats. Psychopharmacology (Berl). 2008;196(1):71–81. [DOI] [PubMed] [Google Scholar]
- 28. McQuown SC, Belluzzi JD, Leslie FM. Low dose nicotine treatment during early adolescence increases subsequent cocaine reward. Neurotoxicol Teratol. 2007;29(1):66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Belluzzi JD, Wang R, Leslie FM. Acetaldehyde enhances acquisition of nicotine self-administration in adolescent rats. Neuropsychopharmacology. 2005;30(4):705–712. [DOI] [PubMed] [Google Scholar]
- 30. Yuan M, Cross SJ, Loughlin SE, Leslie FM. Nicotine and the adolescent brain. J Physiol. 2015;593(16):3397–3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Collins SL, Montano R, Izenwasser S. Nicotine treatment produces persistent increases in amphetamine-stimulated locomotor activity in periadolescent male but not female or adult male rats. Brain Res Dev Brain Res. 2004;153(2):175–187. [DOI] [PubMed] [Google Scholar]
- 32. Collins SL, Izenwasser S. Chronic nicotine differentially alters cocaine-induced locomotor activity in adolescent vs. adult male and female rats. Neuropharmacology. 2004;46(3):349–362. [DOI] [PubMed] [Google Scholar]
- 33. Santos GC, Marin MT, Cruz FC, Delucia R, Planeta CS. Amphetamine- and nicotine-induced cross-sensitization in adolescent rats persists until adulthood. Addict Biol. 2009;14(3):270–275. [DOI] [PubMed] [Google Scholar]
- 34. McQuown SC, Dao JM, Belluzzi JD, Leslie FM. Age-dependent effects of low-dose nicotine treatment on cocaine-induced behavioral plasticity in rats. Psychopharmacology (Berl). 2009;207(1):143–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dwyer JB, McQuown SC, Leslie FM. The dynamic effects of nicotine on the developing brain. Pharmacol Ther. 2009;122(2):125–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cross SJ, Linker KE, Leslie FM. Sex-dependent effects of nicotine on the developing brain. J Neurosci Res. 2017;95(1–2):422–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Azam L, Chen Y, Leslie FM. Developmental regulation of nicotinic acetylcholine receptors within midbrain dopamine neurons. Neuroscience. 2007;144(4):1347–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Andersen SL, Thompson AP, Krenzel E, Teicher MH. Pubertal changes in gonadal hormones do not underlie adolescent dopamine receptor overproduction. Psychoneuroendocrinology. 2002;27(6):683–691. [DOI] [PubMed] [Google Scholar]
- 39. Andersen SL, Rutstein M, Benzo JM, Hostetter JC, Teicher MH. Sex differences in dopamine receptor overproduction and elimination. Neuroreport. 1997;8(6):1495–1498. [DOI] [PubMed] [Google Scholar]
- 40. Cao J, Belluzzi JD, Loughlin SE, Dao JM, Chen Y, Leslie FM. Locomotor and stress responses to nicotine differ in adolescent and adult rats. Pharmacol Biochem Behav. 2010;96(1):82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Damaj MI. Influence of gender and sex hormones on nicotine acute pharmacological effects in mice. J Pharmacol Exp Ther. 2001;296(1):132–140. [PubMed] [Google Scholar]
- 42. al’Absi M. Hypothalamic-pituitary-adrenocortical responses to psychological stress and risk for smoking relapse. Int J Psychophysiol. 2006;59(3):218–227. [DOI] [PubMed] [Google Scholar]
- 43. Cao J, Lotfipour S, Loughlin SE, Leslie FM. Adolescent maturation of cocaine-sensitive neural mechanisms. Neuropsychopharmacology. 2007;32(11):2279–2289. [DOI] [PubMed] [Google Scholar]
- 44. Torres OV, O’Dell LE. Stress is a principal factor that promotes tobacco use in females. Prog Neuropsychopharmacol Biol Psychiatry. 2016;65:260–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Randall PA, Fortino B, Huynh YW, et al. Effects of nicotine conditioning history on alcohol and methamphetamine self-administration in rats. Pharmacol Biochem Behav. 2019;179:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Neugebauer NM, Harrod SB, Bardo MT. Nicotine elicits methamphetamine-seeking in rats previously administered nicotine. Drug Alcohol Depend. 2010;106(1):72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ruda-Kucerova J, Amchova P, Babinska Z, Dusek L, Micale V, Sulcova A. Sex differences in the reinstatement of methamphetamine seeking after forced abstinence in Sprague-Dawley rats. Front Psychiatry. 2015;6:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Levin ED, Mead T, Rezvani AH, Rose JE, Gallivan C, Gross R. The nicotinic antagonist mecamylamine preferentially inhibits cocaine vs. food self-administration in rats. Physiol Behav. 2000;71(5):565–570. [DOI] [PubMed] [Google Scholar]
- 49. Zachariou V, Caldarone BJ, Weathers-Lowin A, et al. Nicotine receptor inactivation decreases sensitivity to cocaine. Neuropsychopharmacology. 2001;24(5):576–589. [DOI] [PubMed] [Google Scholar]
- 50. Vieira-Brock PL, McFadden LM, Nielsen SM, et al. Chronic nicotine exposure attenuates methamphetamine-induced dopaminergic deficits. J Pharmacol Exp Ther. 2015;355(3):463–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be available, upon reasonable request from the authors.