Abstract
Purpose
Knee osteoarthritis (KOA) is a degenerative disease with inflammation, becoming persistent as it progresses, resulting in reduced quality of life. Exercise is the recommended treatment for KOA; however, the extent of pain reduction with exercise is heterogeneous and the prognostic implications of baseline factors in patients undergoing exercise are still unknown. This study examined the association between the response to exercise therapy and clinical outcomes, radiologic severity, and pain sensitization, and investigated the optimal predictive value for the effectiveness of exercise.
Patients and Methods
Demographics, radiologic severity, pressure pain threshold (PPT), and temporal summation of pain (TSP) at the knee, tibia, and forearm were assessed at baseline. The pain numeric rating scale (NRS) was assessed before and after 12 weeks of exercise. Patients were divided into responder/non-responder groups according to recommended criteria: responder, ≥30% reduction in pain; non-responder, <30% reduction in pain, and each variable was compared between the groups. The area under the curve (AUC) and cutoff points were determined by receiver operating characteristic curve analysis.
Results
Sixty-five patients were categorized as responders and 26 as non-responders. In the non-responder group, baseline NRS (P<0.01), pain duration (P<0.01), and TSP at the knee (P<0.001) and tibia (P<0.05) were significantly higher, and PPT at the knee (P<0.001), tibia (P<0.001), and forearm (P<0.001) were significantly lower, than those in the responder group; however, no significant differences between groups were found in other demographics and radiologic severity. The variables that showed moderate or better predictive ability (AUC≥0.7) were PPT at the knee (cutoff points: 241.5 kPa), tibia (307.5 kPa), forearm (318.5 kPa), and TSP at the knee (15.5 mm).
Conclusion
Our findings suggest that pain sensitization is associated with the response to exercise therapy. Furthermore, we provide clinically predictive values for PPT and TSP in predicting the outcome to exercise in KOA.
Keywords: pressure pain threshold, temporal summation of pain, cutoff point, prognostic prediction
Introduction
Knee osteoarthritis (KOA) is the most common painful musculoskeletal disorder.1,2 Over the past 20 years, there has been a noticeable increase in the prevalence of KOA, and this trend is predicted to continue.3 In Japan, the Research on Osteoarthritis Against Disability study has reported a high prevalence of KOA.4 Further, in many developed nations, the rising cost of treating KOA is deemed unsustainable; thus, efforts should be made to optimize the algorithm of treatment.
The goal of the management of KOA aims to improve function and the health-related quality of life while managing pain.5 In clinical guidelines, exercise and patient education, which are non-pharmacological treatments, are recommended.5 Participation in an exercise program may improve pain, physical function, depression, self-efficacy, and social function in patients with KOA;6 however, the effect size of exercise in pain reduction is only moderate.7 In clinical practice, patients with KOA are classified into responders and non-responders to exercise therapy, and different pain mechanisms may be related to the difference in treatment response between responders and non-responders. Predicting responses to exercise at baseline is beneficial for selecting exercise modalities and combinations with other treatments in patients with a low response to exercise. It is necessary to examine the characteristics associated with the exercise response to optimize interventions.
Several risk factors are associated with poor treatment outcomes in KOA. Surgical studies have shown that even preoperative radiologic severity is not necessarily associated with postoperative pain after total knee arthroplasty (TKA), and a low Kellgren–Lawrence (KL) grade is a risk factor for poor postoperative outcomes.8 Recently, enhanced pain sensitization is among the risk factors for chronic postsurgical pain after TKA;8–10 this suggests that pain sensitization may contribute to chronic joint pain or response to treatment.9–13
Quantitative sensory testing (QST) is a clinical method for assessing responses to sensory stimuli, and is used as an indicator of changes in the neural function of the somatosensory system.14–16 In QST parameters, the pressure pain threshold (PPT) and temporal summation of pain (TSP) have been specifically used as measures of pain sensitization.17–19 However, the association between the response to exercise therapy and pain sensitization remains unclear in studies conducted on exercise therapy.20,21 Moreover, QST has not been established as a clinical assessment because its predictive value for predicting exercise responsiveness is still unknown.
This study aimed to investigate the association between the response to exercise therapy and clinical outcomes, radiologic severity, and pain sensitization. We also investigated the discriminative ability and optimal predictive value of associated variables for identifying responders and non-responders to exercise therapy.
Materials and Methods
Participants
In this prospective cohort study, patients with KOA, who were newly referred for physiotherapy by an orthopedic surgeon, were identified at the Maehara Orthopedic Rehabilitation Clinic in Obu, Japan, between April 1, 2021 and July 31, 2022.
The inclusion criteria were as follows: age ≥50 years, a diagnosis of KOA confirmed by radiographic findings (KL grade ≥1), and knee pain ≥3/10 on a numeric rating scale (NRS) for at least 6 months. In addition, the exclusion criteria were as follows: systemic inflammatory disease, cognitive impairment, severe medical comorbidities (eg, cardiovascular disease, cerebrovascular disease, and cancer), any type of surgery within the past 6 months, presence of other pain problems (eg, neck and low back pain), and leg pain and numbness referred from the lumbar spine.
Approval was obtained from the Institutional Ethics Committees of Kobe Gakuin University in Kobe, Japan (No.: 19–23) and Maehara Orthopedic Rehabilitation Clinic in Aichi, Japan (No.: 19–001), and written informed consent was obtained from all patients prior to the study.
Procedures
Demographic data, clinical pain intensity, radiologic severity, and QST were collected at baseline, and all patients underwent 12 weeks of exercise therapy. Pain intensity was assessed before and after exercise therapy. Patients were divided into responder and non-responder groups according to the criteria recommended by the Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT): responder, ≥30% (moderate) reduction in pain; non-responder, <30% reduction in pain, compared with the pain rating before the exercise therapy.22
Demographic Data and Clinical Pain Intensity
At the baseline visit, data were collected on demographic characteristics (age, sex, body mass index [BMI], NRS, and pain duration). NRS was defined as the peak pain in the past week.
Radiologic Severity
All patients underwent radiographic assessments by an orthopedic surgeon. Radiographic assessments were performed using the KL grade,23 reflecting changes in the entire joint structure.
Quantitative Sensory Testing
QST was conducted by an experienced physiotherapist and performed in a quiet, temperature-controlled room. Patients were placed in the supine position on a bed during QST; they were first accustomed to QST pressure stimulation, followed by PPT and TSP assessment. PPT and TSP were assessed using a hand-held pressure algometer (Algometer Type II, Somedic AB, Sweden) with a 1-cm2 contact probe;24,25 they were also assessed at the knee joint as a local site, the tibialis anterior (tibia, 5 cm distal to the tibial tuberosity), and extensor carpi radialis longus (forearm, 5 cm distal to the lateral epicondyle of the humerus) as control (remote) sites, on the affected side. Four test sites at the knee were assessed as follows: (1) 2 cm distal to the inferior medial edge of the patella; (2) 2 cm distal to the inferior lateral edge of the patella; (3) 3 cm lateral to the midpoint on the lateral edge of the patella; and (4) 3 cm medial to the mid-point on the medial edge of the patella.25 In the case of bilateral symptomatic KOA, the greater symptomatic knee was defined as the affected joint.
Pressure Pain Threshold
The PPT, the minimum necessary force to evoke pain, was assessed at each site by applying pressure.25 The PPT was measured twice at each site, and the mean value was used in the analysis. The knee PPT was defined as the site with the lowest PPT among the four sites at the knee joint.
Temporal Summation of Pain
TSP, increasing pain evoked by sequential pain stimulation, consisted of ten pressure stimuli (1-s in duration, with a 1-s interval).26,27 The pressure intensity was set at the PPT level.25 The skin contact between individual pressure stimulations was maintained at a very low intensity, such that the patients felt no pain. Patients indicated the pressure pain intensity on a visual analog scale (VAS), with “0” indicating “no pain”, and “100” indicating the “worst possible pain.” TSP was calculated by subtracting the pain VAS at the 1st stimulus from that at the 10th.28 The knee TSP was assessed at the site with the lowest PPT.25
Intervention
Patients underwent 12 weeks of exercise therapy, with 40 minutes per session once a week at the clinic, and home exercise, with at least 20 minutes 3 times a week.29,30 Recent studies in KOA have recommended 8–12 weeks of exercise therapy.31 Exercise combined with patient education was individually supervised by five physiotherapists who had completed a workshop on non-pharmacologic treatments recommended in clinical practice guidelines. The exercise programs were individualized and progressive, considering the preferences and abilities of the patient.20,32 The exercise therapy consisted of the following prescription: neuromuscular exercise, aerobic exercise, strength exercises, and stretching.6,32–34 The physiotherapist checked the patient’s home exercise practices based on their self-recorded activity diaries during exercise sessions completed at the clinic. If they complained of increased pain, the intensity of exercise was temporarily decreased, and the physiotherapist instructed them on exercises and lifestyle modifications to reduce the mechanical load on the joints. Adverse events occurring during clinic exercise sessions were recorded. The patient education focused on osteoarthritis disease characteristics and symptoms, etiology of KOA, mechanism of joint pain, risk factors, effects of exercise, coping strategies, and self-management.35
Data Analyses
Data are presented as mean ± standard deviation (SD). The data normality of the study was assessed with the Kolmogorov–Smirnov test. Demographic data and QST parameters were compared between responder and non-responder groups using the Student’s t-test, Mann–Whitney U-test, and Fisher’s exact test, as appropriate. We used “R” as calculated by Z-translation to evaluate the magnitude of the effect size (R=Z/√N).36 Furthermore, receiver operating characteristic (ROC) curve analysis was performed to examine the cutoff point, sensitivity, and specificity for predicting the response to exercise for variables that differed between groups. Additionally, the area under the curve (AUC) was evaluated to assess the discrimination ability; AUC closer to 1 indicates better, whereas AUC closer to 0.5 indicates poorer overall diagnostic performance.37 AUC ≥0.9 indicates high accuracy, 0.7–0.9 indicates moderate accuracy, 0.5–0.7 indicates low accuracy, and 0.5 indicates a chance result.38 The AUC is presented with the associated confidence interval (CI). The Youden Index (Sn+Sp−1) was defined as the cutoff point.39 Statistical analyses were performed using SPSS version 27 (IBM Corporation, Armonk, NY, USA). P-values <0.05 were considered statistically significant.
The minimal required sample size per group was determined as 24 participants (power: 80%, AUC: 0.7). Based on the assumption that approximately 30% of participants would be deemed as non-responders by IMMPACT criteria, a total sample size of 100 participants was calculated as necessary to account for a 20% loss to follow up.
Results
The study recruited 108 patients, and 17 patients discontinued outpatient visits because of personal reasons. Therefore, the final number of participants who participated in the analyses was 91. The patients who dropped out were not significantly different from those who completed follow-up assessments in terms of age (P=0.334), sex (P=0.688), BMI (P=0.176), pain duration (P=0.835), and baseline NRS (P=0.871). No patient reported any treatment-specific adverse events.
Demographic Data and Clinical Pain Intensity
Demographic and clinical pain intensity data are shown in Table 1. In total, 65 patients were categorized into the responder group and 26 into the non-responder group. No significant differences between the groups were found in age (P=0.310), sex (P=0.411), and BMI (P=0.160). However, there were significant differences between the groups in pain duration (R=0.274, P<0.01), baseline NRS (R=0.275, P<0.01), and follow-up NRS (R=0.732, P<0.001). The number of patients using non-steroidal anti-inflammatory drugs (NSAIDs), duloxetine, and opioids was 27 (41.5%), 0 (0%), and 0 (0%) in the responder group and 20 (76.9%), 3 (11.5%), and 1 (3.8%) in the non-responder group, respectively. The ratio of patients who used NSAIDs (P<0.001) and duloxetine (P<0.05) was significantly higher in the non-responder group than in the responder group.
Table 1.
Demographic Data in Responder and Non-Responder Groups
| Variables | Responder Group | Non-Responder Group | P |
|---|---|---|---|
| No. patients, n (%) | 65 (71.4) | 26 (28.6) | |
| Age, mean ± SD | 67.6±9.2 | 69.7±10.3 | 0.310 |
| Sex (percentage female) | 75.3 | 84.6 | 0.411 |
| BMI, mean ± SD (kg/m²) | 24.3±3.9 | 25.1±3.3 | 0.160 |
| Pain duration, mean ± SD (months) | 39.4±41.6 | 69.3±56.3 | <0.01 |
| Baseline NRS | 5.0±1.7 | 6.1±1.9 | <0.01 |
| KL grade | |||
| 1 | 26 (40.0) | 9 (34.6) | 0.401 |
| 2 | 22 (33.9) | 6 (23.1) | |
| 3 | 14 (21.5) | 10 (38.4) | |
| 4 | 3 (4.6) | 1 (3.9) |
Abbreviations: SD, standard deviation; BMI, body mass index; NRS, numerical rating scale; KL, Kellgren–Lawrence.
Radiologic Severity
Radiologic severity data are shown in Table 1. No significant differences between the groups were found in the KL grade (P=0.401).
Quantitative Sensory Testing
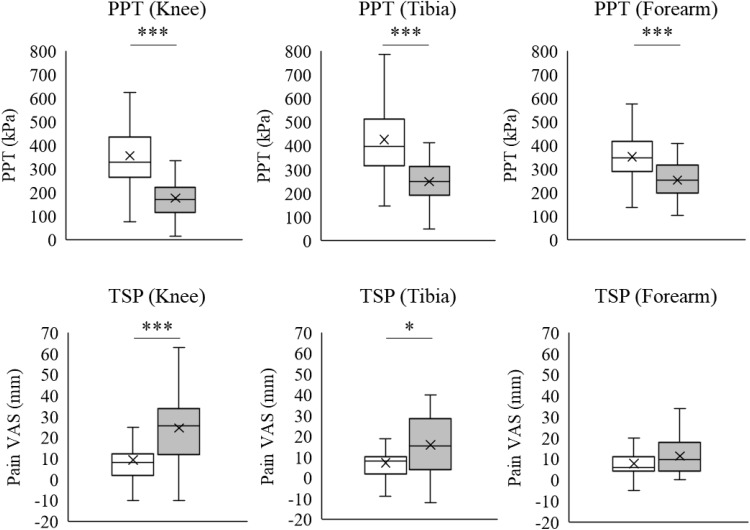
Figure 1 shows the results of the comparisons between the responder and non-responder groups in PPT and TSP. PPT at the knee (responder group: 355.4±140.2; non-responder group: 176.5±89.7; R=0.576, P<0.001), tibia (responder group: 426.6±145.8; non-responder group: 249.2±92.7; R=0.517, P<0.001), and forearm (responder group: 351.9±108.6; non-responder group: 253.2±92.7; R=0.393, P<0.001) was significantly lower in the non-responder group than in the responder group. TSP at the knee (responder group: 9.2±11.4; non-responder group: 24.5±17.6; R=0.425, P<0.001) and tibia (responder group: 7.3±7.6; non-responder group: 15.9±14.7; R=0.255, P<0.05) was significantly higher in the non-responder than in the responder group. TSP at the forearm did not significantly differ between the groups (responder group: 7.8±6.6; non-responder group: 11.3±8.8; P=0.094).
Figure 1.
PPT and TSP at the knee, tibia, and forearm in the responder (white) and non-responder groups (gray).
Notes: Significant differences between the groups were found for PPT at all measurement sites and TSP at the knee and tibialis anterior. No significant difference is observed in TSP at the extensor carpi radialis longus. *P<0.05; ***P<0.001.
Abbreviations: PPT, pressure pain threshold; TSP, temporal summation of pain; VAS, visual analog scale.
Predictive Value on ROC Curve Analysis
Table 2 shows the AUC, recommended cutoff points, sensitivity, and specificity values from the ROC curve analysis. For pain duration, the AUC distinguishing responder and non-responder groups was 0.674, and the cutoff point was 42.0 months (sensitivity, specificity: 65.4%, 66.2%). Regarding baseline NRS, the AUC was 0.673, and the cutoff point was 4.5 (sensitivity, specificity: 80.8%, 47.7%). For the knee PPT, the AUC was 0.868, and the cutoff point was 241.5 kPa (sensitivity, specificity: 81.5%, 84.6%). Regarding the tibial PPT, the AUC was 0.849, and the cutoff point was 307.5 kPa (sensitivity, specificity: 83.1%, 73.1%). For the forearm PPT, the AUC was 0.754, and the cutoff point was 318.5 kPa (sensitivity, specificity: 64.6%, 76.9%). For the knee TSP, the AUC was 0.771, and the cutoff point was 15.5 mm (sensitivity, specificity: 69.2%, 83.0%). For the tibia TSP, the AUC was 0.662, and the cutoff point was 14.0 mm (sensitivity, specificity: 53.8%, 89.2%). Accordingly, the knee, tibia, and forearm PPT and knee TSP showed moderate accuracy; in contrast, the tibia and forearm TSP showed low accuracy in distinguishing responders from non-responders.
Table 2.
AUC, Sensitivity, and Specificity for Recommended Cutoff Points
| Variables | AUC | 95% CI | Cutoff Point (Sensitivity, Specificity) |
|---|---|---|---|
| Pain duration (months) | 0.674 | 0.550–0.798 | 42.0 (65.4%, 66.2%) |
| Baseline NRS | 0.673 | 0.553–0.793 | 4.5 (80.8%, 47.7%) |
| Knee PPT (kPa) | 0.868 | 0.790–0.947 | 241.5 (81.5%, 84.6%) |
| Tibia PPT (kPa) | 0.849 | 0.768–0.930 | 307.5 (83.1%, 73.1%) |
| Forearm PPT (kPa) | 0.754 | 0.650–0.859 | 318.5 (64.6%, 76.9%) |
| Knee TSP (mm) | 0.771 | 0.653–0.889 | 15.5 (69.2%, 83.0%) |
| Tibia TSP (mm) | 0.662 | 0.522–0.802 | 14.0 (53.8%, 89.2%) |
| Forearm TSP (mm) | 0.613 | 0.472–0.753 | 16.5 (34.6%, 93.8%) |
Abbreviations: AUC, area under the curve; CI, confidence interval; NRS, numerical rating scale; PPT, pressure pain threshold; TSP, temporal summation of pain.
Discussion
The aim of this study was to determine the association between the response to exercise therapy and clinical outcomes, radiologic severity, and pain sensitization, and the discriminative ability and optimal predictive value of associated variables for identifying responders and non-responders to exercise therapy. Although the radiologic severity did not significantly differ between responders and non-responders, non-responders were found to have severe pain intensity, long duration of pain, lower PPT, and facilitated TSP at local and remote sites before treatment. Additionally, variables with a greater predictive ability (AUC≥0.7) comprised the PPT at the knee (cutoff point: 241.5 kPa), tibia (307.5 kPa), and forearm (318.5 kPa), and TSP at the knee (15.5 mm). These recommended cutoff points showed moderate to high sensitivity and specificity. Accordingly, our findings suggest that pain sensitization was associated with pain reduction after 12 weeks of exercise therapy in patients with any severity of KOA.
Exercise therapy is the first line of treatment for KOA.5 However, the effectiveness of exercise therapy varies among individuals,29 and the pain mechanism in the peripheral and central nervous systems may differ between responders and non-responders to exercise therapy. It may be possible to provide tailor-made interventions by identifying factors associated with the prognosis. The pain mechanism in KOA can be assessed by biochemical markers for inflammation,40 spinal cord response,41 and brain activity42 on functional magnetic resonance imaging (MRI); however, these assessments are not useful in clinical practice. Conversely, QST can be used to evaluate pain sensitization in KOA at the bedside. If the characteristics, based on the pain mechanism by QST, in subgroups unresponsive to exercise therapy can be determined, it would be possible to provide tailored treatments to each subgroup. To the best of our knowledge, this is the first study to demonstrate the specific sites for QST assessment and cutoff points clinically useful in KOA.
The subgroup of patients with severe pain in KOA was characterized as having widespread pressure hyperalgesia.11,25 Furthermore, several previous studies have investigated the role of pain sensitization in the effectiveness of exercise therapy in KOA. A secondary analysis of a randomized controlled trial in KOA by Henriksen et al reported that PPT and TSP could not predict the response to 12 weeks of exercise.20 Contrarily, the recent study on KOA by O’Leary et al investigated QST parameters and psychosocial factors as predictors of outcomes after 6 months of physiotherapy and found that pain sensitization assessed by PPT and TSP predicted non-response.21 However, the appropriate assessment sites and predictive value for predicting outcomes were unclear because this study analyzed the QST results using principal component analysis. In the present study, the predictive ability, cutoff points, sensitivity, and specificity of PPT and TSP for predicting the response to exercise using ROC analysis were examined. It was found that the knee, tibia, and forearm PPT, including the knee TSP have an AUC≥0.7, and the recommended cutoff points showed moderate to high sensitivity and specificity. Thus, lower PPT and facilitated TSP may predict the response to exercise therapy.
Recent studies indicate that a high prevalence of pain sensitization in patients with KOA, at 20%; further, patients with pain sensitization have poorer quality of life compared to those without sensitization.43,44 Therefore, clarifying the role of sensitization in chronic joint pain has important clinical implications. Local PPT is an indicator of peripheral sensitization, signifying nociceptor activation.11,25 Repeated nociceptive input causes peripheral sensitization,11 which decreases the activation threshold and increases the responsiveness to pain in primary afferent nociceptors.45 Peripheral pain mechanisms in KOA originate in joint components, mainly in the synovium and subchondral bone.46 Neogi et al reported that lower knee PPT was correlated with the degree of synovitis on MRI.47 Our study found that the knee PPT in non-responders was lower than that in responders; thus, peripheral sensitization may have been induced more strongly in non-responders than in responders via joint inflammation. Furthermore, in a large cohort study (n=2126), almost all patients with KOA and chronic peripheral sensitization exhibited evidence of central sensitization.48 The present study also showed lower PPT in remote sites and facilitated TSP in non-responders compared to that in responders, suggesting central sensitization in non-responders. Animal studies have shown that their receptive fields increase when dorsal horn neurons are sensitized.49,50 In clinical practice, secondary hyperalgesia occurring in KOA is thought to reflect similar adaptations at the spinal level.51 Therefore, in patients with KOA who are non-responsive to exercise therapy, the central nervous system may have become hyperexcitable, in addition to peripheral sensitization.
Based on this study, exercise-induced hyperalgesia may be involved in non-response to exercise, although it is unclear why pain sensitization affected the response to exercise in the findings obtained. KOA pain usually worsens with exercise and is better at rest. Wideman et al reported that increased discomfort during walking was associated with facilitated knee TSP in KOA.52 Similarly, patients with hypersensitivity assessed by QST are more likely to have increased pain during walking in chronic low back pain.53 In the results obtained in this study, non-responders presented with lower PPT and facilitated TSP; therefore, pain sensitization may have attenuated the effects of exercise-induced hypoalgesia. Additionally, the non-responders had a higher rate of medication use than the responders; however, the outcomes after exercise therapy were poor. Therefore, individualized approaches that consider pain sensitization (eg, graded exercise, pain neuroscience education, and pharmacotherapy) may be necessary to optimize treatment in patients with pain sensitization.
This study has some limitations. First, we did not include a control group, which did not enable us to evaluate the natural course of pain and placebo response. Second, inflammatory findings were not evaluated. Although a previous study reported that bone marrow lesions were associated with walking pain in patients with KOA undergoing TKA,54 future studies should evaluate synovitis and subchondral bone on MRI. Third, the patients were recruited from a single institution in a primary care setting. Moreover, pain sensitivity may be affected by racial and skeletal differences;55 therefore, it should be taken into consideration that the results were obtained from only Asian patients with KOA. Fourth, although there were no differences in the characteristics at baseline between patients who dropped out and those who were able to complete the 12-week exercise program, we were unable to analyze the factors contributing to dropout, ie, interruption/discontinuation of outpatient visits, and this may have influenced the results of this study. Finally, pain duration and pain intensity at baseline may have been confounders in the results of this study. However, prior studies have shown that pain symptoms and pain duration are not necessarily associated with the response to treatment in KOA.29,56 Thus, the results of this study are interesting in highlighting the role of pain sensitization on poor outcomes after exercise therapy for knee OA.
Conclusion
Despite mild osteoarthritis severity, patients with KOA who were non-responsive to 12 weeks of exercise therapy demonstrated widespread hyperalgesia before treatment. Furthermore, the knee, tibia, forearm PPT, and knee TSP showed more than the moderate predictive ability for response to exercise therapy; we suggest that these QST parameters can be combined to predict treatment response. These findings are beneficial for outcome prediction and treatment selection in clinical practice.
Acknowledgments
The authors want to thank all the subjects examined in this study and the staff at the Department of Rehabilitation, Maehara Orthopedics Rehabilitation Clinic, for their support with data collection.
Data Sharing Statement
The authors confirm that the data underlying the findings described in this manuscript are available from the corresponding author upon reasonable request.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Felson DT. The sources of pain in knee osteoarthritis. Curr Opin Rheumatol. 2005;17(5):624–628. doi: 10.1097/01.bor.0000172800.49120.97 [DOI] [PubMed] [Google Scholar]
- 2.Schaible HG. Mechanisms of chronic pain in osteoarthritis. Curr Rheumatol Rep. 2012;14(6):549–556. doi: 10.1007/s11926-012-0279-x [DOI] [PubMed] [Google Scholar]
- 3.Kingsbury SR, Gross HJ, Isherwood G, et al. Osteoarthritis in Europe: impact on health status, work productivity and use of pharmacotherapies in five European countries. Rheumatology. 2014;53(5):937–947. doi: 10.1093/rheumatology/ket463 [DOI] [PubMed] [Google Scholar]
- 4.Muraki S, Oka H, Akune T, et al. Prevalence of radiographic knee osteoarthritis and its association with knee pain in the elderly of Japanese population-based cohorts: the ROAD study. Osteoarthritis Cartilage. 2009;17(9):1137–1143. doi: 10.1016/j.joca.2009.04.005 [DOI] [PubMed] [Google Scholar]
- 5.Bannuru RR, Osani MC, Vaysbrot EE, et al. OARSI guidelines for the non-surgical management of knee, Hip, and polyarticular osteoarthritis. Osteoarthritis Cartilage. 2019;27(11):1578–1589. doi: 10.1016/j.joca.2019.06.011 [DOI] [PubMed] [Google Scholar]
- 6.Hurley M, Dickson K, Hallett R, et al. Exercise interventions and patient beliefs for people with Hip, knee or Hip and knee osteoarthritis: a mixed methods review. Cochrane Database Syst Rev. 2018;4(4):CD010842. doi: 10.1002/14651858.CD010842.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fransen M, McConnell S, Harmer AR, et al. Exercise for osteoarthritis of the knee: a Cochrane systematic review. Br J Sports Med. 2015;49(24):1554–1557. doi: 10.1136/bjsports-2015-095424 [DOI] [PubMed] [Google Scholar]
- 8.Wylde V, Sayers A, Odutola A, Gooberman-Hill R, Dieppe P, Blom AW. Central sensitization as a determinant of patients’ benefit from total Hip and knee replacement. Eur J Pain. 2017;21(2):357–365. doi: 10.1002/ejp.929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petersen KK, Simonsen O, Laursen MB, Arendt-Nielsen L. The role of preoperative radiologic severity, sensory testing, and temporal summation on chronic postoperative pain following total knee arthroplasty. Clin J Pain. 2018;34(3):193–197. doi: 10.1097/AJP.0000000000000528 [DOI] [PubMed] [Google Scholar]
- 10.Izumi M, Petersen KK, Laursen MB, Arendt-Nielsen L, Graven-Nielsen T. Facilitated temporal summation of pain correlates with clinical pain intensity after Hip arthroplasty. Pain. 2017;158(2):323–332. doi: 10.1097/j.pain.0000000000000764 [DOI] [PubMed] [Google Scholar]
- 11.Thakur M, Dickenson AH, Baron R. Osteoarthritis pain: nociceptive or neuropathic? Nat Rev Rheumatol. 2014;10(6):374–380. doi: 10.1038/nrrheum.2014.47 [DOI] [PubMed] [Google Scholar]
- 12.Arendt-Nielsen L, Yarnitsky D. Experimental and clinical applications of quantitative sensory testing applied to skin, muscles and viscera. J Pain. 2009;10(6):556–572. doi: 10.1016/j.jpain.2009.02.002 [DOI] [PubMed] [Google Scholar]
- 13.Frey-Law LA, Bohr NL, Sluka KA, et al. Pain sensitivity profiles in patients with advanced knee osteoarthritis. Pain. 2016;157(9):1988–1999. doi: 10.1097/j.pain.0000000000000603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pavlaković G, Petzke F. The role of quantitative sensory testing in the evaluation of musculoskeletal pain conditions. Curr Rheumatol Rep. 2010;12(6):455–461. doi: 10.1007/s11926-010-0131-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yarnitsky D, Granot M. Chapter 27 Quantitative sensory testing. Handb Clin Neurol. 2006;81:397–409. [DOI] [PubMed] [Google Scholar]
- 16.Rolke R, Baron R, Maier C, et al. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): standardized protocol and reference values [published correction appears in Pain. Pain. 2006;123(3):231–243. doi: 10.1016/j.pain.2006.01.041 [DOI] [PubMed] [Google Scholar]
- 17.Wylde V, Palmer S, Learmonth ID, Dieppe P. Test-retest reliability of Quantitative Sensory Testing in knee osteoarthritis and healthy participants. Osteoarthritis Cartilage. 2011;19(6):655–658. doi: 10.1016/j.joca.2011.02.009 [DOI] [PubMed] [Google Scholar]
- 18.Uddin Z, MacDermid JC. Quantitative sensory testing in chronic musculoskeletal Pain. Pain Med. 2016;17(9):1694–1703. doi: 10.1093/pm/pnv105 [DOI] [PubMed] [Google Scholar]
- 19.Suokas AK, Walsh DA, McWilliams DF, et al. Quantitative sensory testing in painful osteoarthritis: a systematic review and meta-analysis. Osteoarthritis Cartilage. 2012;20(10):1075–1085. doi: 10.1016/j.joca.2012.06.009 [DOI] [PubMed] [Google Scholar]
- 20.Henriksen M, Klokker L, Graven-Nielsen T, et al. Association of exercise therapy and reduction of pain sensitivity in patients with knee osteoarthritis: a randomized controlled trial. Arthritis Care Res. 2014;66(12):1836–1843. doi: 10.1002/acr.22375 [DOI] [PubMed] [Google Scholar]
- 21.O’Leary H, Smart KM, Moloney NA, Blake C, Doody CM. Pain sensitization associated with nonresponse after physiotherapy in people with knee osteoarthritis. Pain. 2018;159(9):1877–1886. doi: 10.1097/j.pain.0000000000001288 [DOI] [PubMed] [Google Scholar]
- 22.Dworkin RH, Turk DC, Wyrwich KW, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9(2):105–121. doi: 10.1016/j.jpain.2007.09.005 [DOI] [PubMed] [Google Scholar]
- 23.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494–502. doi: 10.1136/ard.16.4.494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Imamura M, Imamura ST, Kaziyama HH, et al. Impact of nervous system hyperalgesia on pain, disability, and quality of life in patients with knee osteoarthritis: a controlled analysis. Arthritis Rheum. 2008;59(10):1424–1431. doi: 10.1002/art.24120 [DOI] [PubMed] [Google Scholar]
- 25.Arendt-Nielsen L, Nie H, Laursen MB, et al. Sensitization in patients with painful knee osteoarthritis. Pain. 2010;149(3):573–581. doi: 10.1016/j.pain.2010.04.003 [DOI] [PubMed] [Google Scholar]
- 26.Staud R, Cannon RC, Mauderli AP, Robinson ME, Price DD, Vierck CJ. Temporal summation of pain from mechanical stimulation of muscle tissue in normal controls and subjects with fibromyalgia syndrome. Pain. 2003;102(1–2):87–95. doi: 10.1016/s0304-3959(02)00344-5 [DOI] [PubMed] [Google Scholar]
- 27.Staud R, Craggs JG, Robinson ME, Perlstein WM, Price DD. Brain activity related to temporal summation of C-fiber evoked pain. Pain. 2007;129(1–2):130–142. doi: 10.1016/j.pain.2006.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hattori T, Shimo K, Niwa Y, Tokiwa Y, Matsubara T. Association of chronic pain with radiologic severity and central sensitization in Hip osteoarthritis patients. J Pain Res. 2021;14:1153–1160. doi: 10.2147/JPR.S296273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee AC, Harvey WF, Han X, et al. Pain and functional trajectories in symptomatic knee osteoarthritis over up to 12 weeks of exercise exposure. Osteoarthritis Cartilage. 2018;26(4):501–512. doi: 10.1016/j.joca.2018.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hansen S, Vaegter HB, Petersen KK. Pretreatment exercise-induced hypoalgesia is associated with change in pain and function after standardized exercise therapy in painful knee osteoarthritis. Clin J Pain. 2020;36(1):16–24. doi: 10.1097/AJP.0000000000000771 [DOI] [PubMed] [Google Scholar]
- 31.Raposo F, Ramos M, Lúcia Cruz A. Effects of exercise on knee osteoarthritis: a systematic review. Musculoskeletal Care. 2021;19(4):399–435. doi: 10.1002/msc.1538 [DOI] [PubMed] [Google Scholar]
- 32.Fernandes L, Hagen KB, Bijlsma JW, et al. EULAR recommendations for the non-pharmacological core management of Hip and knee osteoarthritis. Ann Rheum Dis. 2013;72(7):1125–1135. doi: 10.1136/annrheumdis-2012-202745 [DOI] [PubMed] [Google Scholar]
- 33.Ageberg E, Link A, Roos EM. Feasibility of neuromuscular training in patients with severe Hip or knee OA: the individualized goal-based NEMEX-TJR training program. BMC Musculoskelet Disord. 2010;11:126. doi: 10.1186/1471-2474-11-126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fransen M, McConnell S. Exercise for osteoarthritis of the knee. Cochrane Database Syst Rev. 2008;1(4):CD004376. [DOI] [PubMed] [Google Scholar]
- 35.Skou ST, Roos EM. Good Life with osteoArthritis in Denmark (GLA:D™): evidence-based education and supervised neuromuscular exercise delivered by certified physiotherapists nationwide. BMC Musculoskelet Disord. 2017;18(1):72. doi: 10.1186/s12891-017-1439-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cohen JL. Statistical Power Analysis for the Behavioral Sciences. Hillsday, NJ: Erlbaum; 1988. [Google Scholar]
- 37.Akobeng AK. Understanding diagnostic tests 3: receiver operating characteristic curves. Acta Paediatr. 2007;96(5):644–647. doi: 10.1111/j.1651-2227.2006.00178.x [DOI] [PubMed] [Google Scholar]
- 38.Swets JA. Measuring the accuracy of diagnostic systems. Science. 1988;240(4857):1285–1293. doi: 10.1126/science.3287615 [DOI] [PubMed] [Google Scholar]
- 39.Greiner M, Pfeiffer D, Smith RD. Principles and practical application of the receiver-operating characteristic analysis for diagnostic tests. Prev Vet Med. 2000;45(1–2):23–41. doi: 10.1016/S0167-5877(00)00115-X [DOI] [PubMed] [Google Scholar]
- 40.Arendt-Nielsen L, Eskehave TN, Egsgaard LL, et al. Association between experimental pain biomarkers and serologic markers in patients with different degrees of painful knee osteoarthritis. Arthritis Rheumatol. 2014;66(12):3317–3326. doi: 10.1002/art.38856 [DOI] [PubMed] [Google Scholar]
- 41.Bosma RL, Ameli Mojarad E, Leung L, Pukall C, Staud R, Stroman PW. Neural correlates of temporal summation of second pain in the human brainstem and spinal cord. Hum Brain Mapp. 2015;36(12):5038–5050. doi: 10.1002/hbm.22993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pujol J, Martínez-Vilavella G, Llorente-Onaindia J, et al. Brain imaging of pain sensitization in patients with knee osteoarthritis. Pain. 2017;158(9):1831–1838. doi: 10.1097/j.pain.0000000000000985 [DOI] [PubMed] [Google Scholar]
- 43.Aw NM, Yeo SJ, Wylde V, et al. Impact of pain sensitisation on the quality of life of patients with knee osteoarthritis. RMD Open. 2022;8(1):e001938. doi: 10.1136/rmdopen-2021-001938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Previtali D, Capone G, Marchettini P, Candrian C, Zaffagnini S, Filardo G. High prevalence of pain sensitization in knee osteoarthritis: a meta-analysis with meta-regression. Cartilage. 2022;13(1):19476035221087698. doi: 10.1177/19476035221087698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hucho T, Levine JD. Signaling pathways in sensitization: toward a nociceptor cell biology. Neuron. 2007;55(3):365–376. doi: 10.1016/j.neuron.2007.07.008 [DOI] [PubMed] [Google Scholar]
- 46.Vincent TL. Peripheral pain mechanisms in osteoarthritis. Pain. 2020;161(1 Suppl 1):S138–S146. doi: 10.1097/j.pain.0000000000001923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Neogi T, Guermazi A, Roemer F, et al. Association of joint inflammation with pain sensitization in knee osteoarthritis: the Multicenter Osteoarthritis Study. Arthritis Rheumatol. 2016;68:654–661. doi: 10.1002/art.39488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Neogi T, Frey-Law L, Scholz J, et al. Sensitivity and sensitisation in relation to pain severity in knee osteoarthritis: trait or state? Ann Rheum Dis. 2015;74(3):682–688. doi: 10.1136/annrheumdis-2013-204191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hoheisel U, Mense S, Simons DG, Yu XM. Appearance of new receptive fields in rat dorsal horn neurons following noxious stimulation of skeletal muscle: a model for referral of muscle pain? Neurosci Lett. 1993;153(1):9–12. doi: 10.1016/0304-3940(93)90064-R [DOI] [PubMed] [Google Scholar]
- 50.Hoheisel U, Koch K, Mense S. Functional reorganization in the rat dorsal horn during an experimental myositis. Pain. 1994;59(1):111–118. doi: 10.1016/0304-3959(94)90054-X [DOI] [PubMed] [Google Scholar]
- 51.Graven-Nielsen T. Fundamentals of muscle pain, referred pain, and deep tissue hyperalgesia. Scand J Rheumatol Suppl. 2006;122:1–43. doi: 10.1080/03009740600865980 [DOI] [PubMed] [Google Scholar]
- 52.Wideman TH, Finan PH, Edwards RR, et al. Increased sensitivity to physical activity among individuals with knee osteoarthritis: relation to pain outcomes, psychological factors, and responses to quantitative sensory testing. Pain. 2014;155(4):703–711. doi: 10.1016/j.pain.2013.12.028 [DOI] [PubMed] [Google Scholar]
- 53.Vaegter HB, Petersen KK, Sjodsholm LV, Schou P, Andersen MB, Graven-Nielsen T. Impaired exercise-induced hypoalgesia in individuals reporting an increase in low back pain during acute exercise. Eur J Pain. 2021;25(5):1053–1063. doi: 10.1002/ejp.1726 [DOI] [PubMed] [Google Scholar]
- 54.Satake Y, Izumi M, Aso K, Igarashi Y, Sasaki N, Ikeuchi M. Comparison of predisposing factors between pain on walking and pain at rest in patients with knee osteoarthritis. J Pain Res. 2021;14:1113–1118. doi: 10.2147/JPR.S298100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Watson PJ, Latif RK, Rowbotham DJ. Ethnic differences in thermal pain responses: a comparison of South Asian and White British healthy males. Pain. 2005;118(1–2):194–200. doi: 10.1016/j.pain.2005.08.010 [DOI] [PubMed] [Google Scholar]
- 56.Tanaka S, Nishigami T, Wand BM, et al. Identifying participants with knee osteoarthritis likely to benefit from physical therapy education and exercise: a hypothesis-generating study. Eur J Pain. 2021;25(2):485–496. doi: 10.1002/ejp.1687 [DOI] [PubMed] [Google Scholar]