Purpose
We quantified lung glycolytic metabolic activity, clinical symptoms and inflammation, coagulation, and endothelial activation biomarkers in 2019 coronavirus disease (COVID-19) pneumonia survivors.
Methods
Adults previously hospitalized with moderate to severe COVID-19 pneumonia were prospectively included. Subjects filled out a questionnaire on clinical consequences, underwent chest CT and 18F-FDG PET/CT, and provided blood samples on the same day. Forty-five volunteers served as control subjects. Analysis of CT images and quantitative voxel-based analysis of PET/CT images were performed for both groups. 18F-FDG uptake in the whole-lung volume and in high- and low-attenuation areas was calculated and normalized to liver values. Quantification of plasma markers of inflammation (interleukin 6), d-dimer, and endothelial cell activation (angiopoietins 1 and 2, vascular cell adhesion molecule 1, and intercellular adhesion molecule 1) was also performed.
Results
We enrolled 53 COVID-19 survivors (62.3% were male; median age, 50 years). All survivors reported at least 1 persistent symptom, and 41.5% reported more than 6 symptoms. The mean lung density was greater in survivors than in control subjects, and more metabolic activity was observed in normal and dense lung areas, even months after symptom onset. Plasma proinflammatory, coagulation, and endothelial activation biomarker concentrations were also significantly higher in survivors.
Conclusion
We observed more metabolic activity in areas of high and normal lung attenuation several months after moderate to severe COVID-19 pneumonia. In addition, plasma markers of thromboinflammation and endothelial activation persisted. These findings may have implications for our understanding of the in vivo pathogenesis and long-lasting effects of COVID-19 pneumonia.
Key Words: COVID-19 pneumonia, endothelial cell activation, immunometabolism, long COVID, PET/CT, pulmonary inflammation
As it enters its third year, with more than 490 million cases worldwide1 the 2019 coronavirus disease (COVID-19) pandemic still poses significant challenges for patients, families and health systems. One such challenge is the estimation of long-term health consequences for survivors. Recent clinical studies suggest that more than 80% of patients who have been hospitalized with COVID-19 pneumonia have at least 1 long-term symptom, and 55% of these patients report 3 or more symptoms.2 Although the mechanisms underlying clinical manifestations in COVID-19 survivors have not been defined fully, impaired tissue repair, persistent inflammatory activation, and a procoagulant state induced by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection may contribute to sequelae and their progression, mainly in the lungs.3
More than 60% of COVID-19 survivors experience breathlessness and other respiratory symptoms several months after hospitalization.4,5 These manifestations evolve to fibrotic-like changes that may reflect permanent pulmonary damage in approximately one-third of these patients.6 In COVID-19 pneumonia survivors, the extent of lung involvement observed on chest CT may correlate with the persistence of symptoms and alteration of pulmonary function parameters.7 However, the visual analysis of CT images has limited sensitivity for the quantification of active pulmonary inflammation or fibrotic activity.
Activated inflammatory cells increase glucose uptake, and 18F-FDG PET/CT is a valuable tool for the detection and quantification of the metabolic activity of inflammatory cells in the lung.8 The finding of increased pulmonary 18F-FDG uptake in areas with radiological abnormalities or even in normal lung parenchyma may have implications for our understanding of the in vivo pathogenesis of COVID-19 that could improve the management of long-term manifestations in survivors. In this prospective study, we quantified lung glycolytic metabolic activity, clinical symptoms, and immune proinflammatory, coagulation, and endothelial activation biomarkers to characterize the impact of moderate to severe COVID-19 pneumonia on lung inflammation and repair.
PATIENTS AND METHODS
Study Design and Data Acquisition
Adult patients with COVID-19 pneumonia who had been admitted to the Rede D'Or Hospitals, Rio de Janeiro, Brazil, were invited after discharge (October 2020–December 2021) to participate in this prospective longitudinal study. Additional inclusion criteria were (a) symptom onset more than 30 days previously, (b) confirmation of the COVID-19 diagnosis by reverse transcription–polymerase chain reaction of a nasopharyngeal swab sample, (c) moderate to severe pneumonia during hospitalization, and (d) performance of unenhanced chest CT examination in the acute phase of the disease. The severity of COVID-19 pneumonia was determined using the World Health Organization's clinical progression scale.9 Briefly, moderate COVID-19 pneumonia was defined as cases requiring hospitalization without (scale 4) or with oxygen supplementation (scale 5), and severe cases were those requiring noninvasive high-flow oxygen supplementation (scale 6) or invasive ventilation (scales 7, 8, and 9). Exclusion criteria were (a) previous chronic pulmonary disease, (b) smoking history of more than 10 pack-years, (c) HIV positivity or immunosuppression, and (d) neoplasms (to exclude other causes of 18F-FDG pulmonary uptake).
Survivors fulfilling the inclusion criteria filled out a standardized questionnaire on the clinical consequences of COVID-19 (the Post-COVID-19 Health and Wellbeing Follow-up Survey),10 submitted blood samples, and underwent chest CT and 18F-FDG PET/CT examinations on the same day. The plasma concentrations of inflammation, coagulation, and endothelial cell activation biomarkers were measured and compared with those in a control group of 26 healthy (COVID-19 reverse transcription–polymerase chain reaction–negative) blood donors. The images were compared with those from 19 healthy volunteers who participated in another study with the same protocol (institutional review board approval no. CAAE 60502816.0.0000.5249).
Survivors' demographic characteristics and clinical data from the acute phase of COVID-19, defined as the time between symptom onset and hospital discharge, were retrieved from electronic medical records. These data included age, sex, body mass index, comorbidities, symptom onset time, length of hospital stay, and mechanical ventilation requirement.
The ethics committee of our institution approved this study (no. CAAE 5523520.3.0000.5249), and all participants provided written informed consent before all study procedures. The study complies with the Declaration of Helsinki and good clinical practice guidelines.
Chest CT Image Acquisition
During patients' hospitalization, chest CT examinations were performed with helical CT systems from different manufacturers. Follow-up chest CT examinations were performed immediately after PET/CT examinations, both with a Siemens (Erlangen, Germany) Biograph device. The acute-phase and follow-up CT examinations were performed with patients in supine position, during end-inspiration, from the thoracic inlet to the diaphragm, without contrast medium injection. The scanning parameters were as follows: tube voltage, 100–140 kVp; tube current modulation, 100–350 mAs; slice thickness, 1–1.25 mm with 50% superposition; and voxel matrix, 512 × 512, 768 × 768, or 1024 × 1024. Reconstruction algorithms were applied with different convolution kernels, depending on the CT system used.
Visual CT Lung Parenchyma Analysis
A senior thoracic radiologist (M.M.B.) with more than 20 years of experience performed visual analysis of the follow-up CT images. The abnormal CT findings reported were as follows: (a) traction bronchiectasis, parenchymal bands, and/or honeycombing, designated fibrotic-like changes6; (b) linear opacities without parenchymal distortion, designated reticular abnormalities; and (c) ground-glass opacities (GGOs) and consolidation, as defined by the Fleischner Society glossary.11 A visual semiquantitative scoring system was used to evaluate the extent of pulmonary abnormalities in each lobe (0, no involvement; 1, <5% involvement; 2, 6%–25% involvement; 3, 26%–50% involvement; 4, 51%–75% involvement; 5, >75% involvement).12 The total CT score was the sum of the scores for each lobe and ranged from 0 (no involvement) to 25 (maximum involvement).12
Densitometric Analysis
Densitometric analyses of the acute-phase and follow-up chest CT images were performed by G.M.R. and A.R.d.C., and the paired data were compared. The acute-phase examination showing the greatest extent of pulmonary involvement during hospitalization was used for each patient.
The lung parenchyma was segmented automatically using U-net,13 a generic (not pulmonary-specific) deep learning and semantic segmentation architecture. The left and right lungs were labeled individually, and the trachea and main bronchi were excluded, but high-density areas were included. Before segmentation, the images were cropped to the body region using threshold and morphological operations and rescaled to a resolution of 256 × 256 pixels. Before processing, the Hounsfield units (HU) were cropped to the width window ranging from −1024 to +600 (values smaller and greater than these values were assigned the values of −1024 and +600, respectively) and normalized to the range 0 to 1. After lung segmentation, the total lung volume (TLV) was calculated in milliliters.
The densitometric analysis was performed using the protocol recommended by Newell et al.14 Normal-attenuation (<−700 HU) and high-attenuation (>−700 HU) areas were calculated. All densitometry-derived variables were expressed as percentages of the TLV.
Dedicated Chest PET/CT Acquisition
Neck base–upper abdomen 18F-FDG PET/CT examinations were performed approximately 60 minutes after the intravenous injection of 0.12 mCi/kg 18F-FDG. Each scan took approximately 10 minutes. Before 18F-FDG injection, the patients fasted for at least 6 hours, and their serum glucose levels were verified to be ≤200 mg/dL. An identical dedicated chest PET/CT protocol was used to acquire the control images of the 19 control subjects.
PET/CT Image Analysis
Persistent pulmonary inflammation was analyzed by quantitative comparison of the 18F-FDG PET/CT images from COVID-19 survivors and control subjects. The SUV calculated from a single attenuation–corrected PET image was used as a measure of lung inflammation.15 The lungs were segmented from whole attenuation–corrected CT images including all pulmonary vessels. To define the normal- and high-attenuation areas for analysis, the lung masks were reduced to decrease the partial volume effect from the lung periphery, and the masks and CT data were converted to PET resolution using a grid average. An SUV was calculated for each voxel from the count value, patient weight, time of injection, and duration of acquisition, and each region of interest was characterized by the mean SUV from its voxels. The SUVs were normalized using the liver SUV to account for differences in participants' pulmonary metabolic activity.
Thromboinflammatory and Endothelial Activation Biomarker Quantification
The concentrations of interleukin 6 (IL-6), as a marker of inflammation, and human intercellular adhesion molecule 1 (ICAM-1/CD56), vascular cell adhesion molecule 1 (VCAM-1/CD106), and angiopoietins 1 and 2 (Ang-1 and Ang-2), as markers of endothelial cell activation, and the d-dimer in plasma from COVID-19 survivors and 26 healthy donors were quantified using standard commercially available enzyme-linked immunosorbent assay kits according to the manufacturers' instructions (R&D Systems, Minneapolis, Minn).
Statistical Analysis
The images were processed using MATLAB R2015a (MathWorks, Natick, Mass), and statistical analysis were conducted using GraphPad Prism version 8.1 (GraphPad Software Inc, San Diego, Calif) and MATLAB R2015a. Parametric data are presented as means ± SDs, and nonparametric data are presented as medians with interquartile ranges (IQRs; first quartile–third quartile). All image-derived variables were compared between groups using the nonparametric rank-sum test. The paired signed rank test was used for pairwise comparison of longitudinal data, and the effect of the time after discharge on normalized SUVs was assessed by linear regression. P < 0.05 was regarded as significant.
RESULTS
Clinical Characteristics of COVID-19 Pneumonia Survivors
In total, we enrolled 53 survivors of moderate to severe COVID-19 pneumonia (Fig. 1). Most survivors (33/53 [62.3%]) were male, and the mean age was 50 ± 14 years. The median length of hospitalization with COVID-19 was 9 days (range, 1–44 days; IQR, 5–15.3 days); 30 patients (56.6%) had moderate and 23 survivors (43.4%) had severe disease. Eleven patients (20.8%) required invasive mechanical ventilation. The median time from COVID-19 symptom onset to follow-up assessment was 78 days (range, 31–194 days; IQR, 59–120 days). Table 1 shows the demographic and clinical characteristics of the COVID-19 pneumonia survivors.
FIGURE 1.
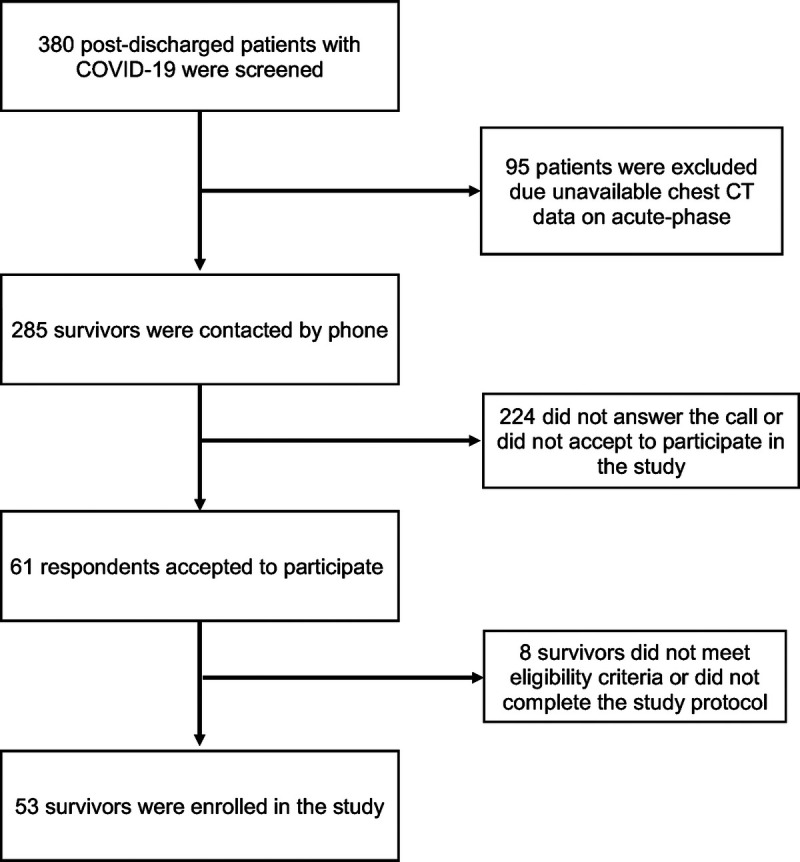
Patient inclusion flowchart.
TABLE 1.
Baseline Demographics
| Characteristics | N = 53 |
|---|---|
| Age, mean (SD), y | 50 (14) |
| Sex (male), n (%) | 33 (62.3%) |
| BMI, mean (SD), kg/m2 | 29.9 (4.8) |
| Underweight | 0 (0%) |
| Normal weight | 6 (11.3%) |
| Overweight | 26 (49.1%) |
| Class I obesity | 13 (24.5%) |
| Class II obesity | 6 (11.3%) |
| Class III obesity | 2 (3.8%) |
| Comorbidities, n (%) | |
| 0 | 12 (22.6%) |
| 1 | 21 (39.6%) |
| 2 | 9 (17.0%) |
| ≥3 | 11 (20.8%) |
| Length of hospital stay, median (IQR), d | 9 (5–15.3) |
| Severity clinical classification (WHO), n (%) | 53 (100%) Hospitalized WHO ordinal scale 4–9) |
| Moderate (scale 4–5) | 30 (56.6%) |
| Severe (scale 6–9) | 23 (43.4%) |
| Mechanical ventilation, n (%) | 11 (20.8%) |
| Time from onset of COVID-19 symptoms and follow-up assessment, median (IQR), d | 78 (59–120) |
Data are expressed as mean (SD), median (IQR), or no. (%), as indicated.
WHO, World Health Organization.
All survivors reported at least 1 persistent symptom at the follow-up assessment, and 41.5% reported more than 6 symptoms. The most frequently reported symptoms were fatigue (38/53 [71.7%]), breathlessness/dyspnea (34/53 [64.2%]), difficulty remembering or concentrating (33/53 [62.3%]), anxiety or depression [30/53 (56.6%]), pain or discomfort (24/53 [45.3%]), and problems with the performance of usual activities (24/53 [45.3%]). Other respiratory symptoms were persistent cough (17/53 [32.1%]) and pain on breathing (11/53 [20.8%]). One patient (1.9%) reported deep vein thrombosis in the follow-up. No other thrombotic event was reported after discharge.
Morphological and Quantitative CT Findings
Visual analysis of the follow-up CT images showed that 19 (35.8%) of the 53 COVID-19 survivors had normal lung parenchyma. Persistent abnormalities were observed in 64.2% (34/53) of the survivors; they consisted of GGOs (25/34 [73.5%]), reticular abnormalities without fibrosis (22/34 [64.7%]), and fibrotic-like changes (10/34 [29.4%]). No patient presented consolidation areas. Semiquantitative visual scores indicated that the extent of lung involvement was less than 5% in 29.4% (10/34) of patients, 6% to 25% in 38.2% (13/34), 26% to 50% in 23.5% (8/34), and 51% to 75% in 8.8% (3/34) of patients. No participant had more than 75% lung involvement.
Quantitative densitometric analysis revealed larger areas of normal lung parenchyma, fewer high-attenuation (dense) areas, and increased TLVs on follow-up CT images than on acute-phase images, indicating the tendency for recovery (Table 2). Compared with control images, follow-up CT images from COVID-19 survivors showed denser parenchyma and similar lung volumes. Regions of normal attenuation had similar mean densities in both groups, but larger volumes in control subjects than in COVID-19 survivors (994 [850, 1314] vs 787 [537, 1152] mL; P = 0.016). Conversely, COVID-19 survivors had larger volumes of high-attenuation areas (895 [665, 1125] vs 619 [437, 749] mL; P < 0.001). These findings indicate the recovery of the lung volume but persistence of areas of pulmonary infiltration.
TABLE 2.
Comparison of the Extension of Pulmonary Changes in Baseline (Acute-Phase) and Follow-up CT (Survivors)
| Acute-Phase CT | Follow-up CT | P | |
|---|---|---|---|
| TLV, mL | 3174 (2488, 4034) | 4094 (3452, 5053) | <0.001 |
| Normal attenuation, ≤−700 HU (% TLV) | 58.2 (51.1, 75.3) | 85.5 (78.5, 88.4) | <0.001 |
| High attenuation, >700 HU (% TLV) | 39.1 (22.5, 46.9) | 14.3 (10.6, 20.9) | <0.001 |
The values inside the parentheses represent the first and third quartiles.
18F-FDG PET-CT Findings
The quantitative analysis of PET images revealed a significantly higher total (whole-lung) metabolic volume for COVID-19 survivors than for control subjects (0.290 [0.255, 0.320) vs 0.237 [0.201, 0.285] liver-corrected mean SUV; P = 0.001). This difference in metabolic activity was driven primarily by larger areas of high attenuation in COVID-19 survivors (0.330 [0.306, 0.353] vs 0.296 [0.266, 0.321]; P = 0.001). However, measurements performed in normally aerated regions also reflected more metabolic activity in COVID-19 survivors than in control subjects (0.235 [0.210, 0.540] vs 0.206 [0.180, 0.243]; P = 0.035; Figs. 2 and 3). The interval from symptom onset to PET/CT examination had a minor effect on metabolic activity, explaining less than 8% of the variability among survivors (linear regression of SUV vs days, slope = 0.00045 [95% confidence interval, −0.00028 to 0.00037], r2 = 0.002, P = 0.780).
FIGURE 2.
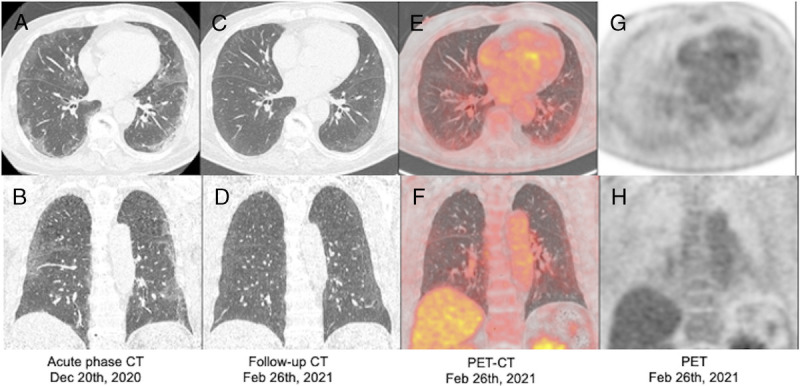
Chest CT images of a patient with COVID-19 pneumonia (acute-phase) and follow-up CT and chest-dedicated 18F-FDG PET/CT images of the same patient. Acute-phase CT images (A and B) show bilateral peripheral GGOs and linear opacities. Those obtained 70 days after symptom onset (C and D) depict partial absorption of the opacifications. 18F-FDG PET/CT images (E–H) show greater uptake in the lung periphery, overlapping the mild areas of GGOs.
FIGURE 3.
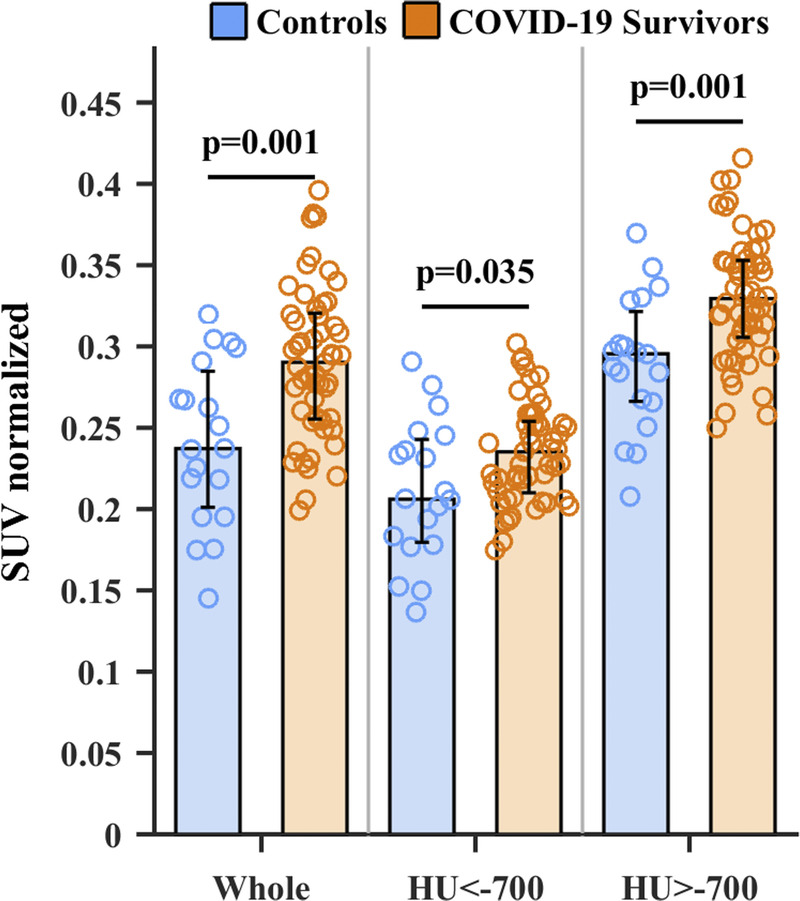
Liver-corrected mean SUV from the whole lung, areas <−700 HU, and areas >−700 HU. Columns are median; error bars are first and third quartiles, and circles are individual data. Metabolic activity was higher in survivors than in control subjects in the whole-lung parenchyma and normal- and high-attenuation areas.
Thromboinflammation and Endothelial Cell Activation Analysis
Endotheliopathy and thromboinflammation are essential components of acute-phase COVID-19 pathology. To gain insights on mechanisms involved in the persistence of metabolic activity in the lung, we evaluated the persistence of biomarkers of thromboinflammation and endothelial activation in survivors when compared with healthy volunteers. COVID-19 survivors exhibited significant biomarker elevation compared with control subjects, such as Ang-2 (192 [IQR, 1.7–3.2] vs 1.243 [1.053–1.562] ng/mL), soluble VCAM (1.38 [1.05–1.72] vs 0.7 [0.58–0.82] ng/mL), soluble ICAM (18.6 [IQR, 15.5–27.4] vs 10.4 [IQR, 8.65–11.9] ng/mL), d-dimer (521 [IQR, 325.8–800.8] vs 326 [IQR, 190–392.1] ng/mL), and IL-6 (7 [IQR, 3–17] vs 3 [IQR, 0–4] pg/mL). Although the elevation of endothelial activation and thromboinflammation biomarkers persists in this cohort of COVID-19 survivors, the levels of the biomarkers are mildly elevated compared with those reported in acute hospitalized COVID-19 cases.16–18 Of note, Ang-1 levels are also elevated (89.2 [IQR, 46.71–129.5] vs 5.61 [IQR, 3.56–8.24] ng/mL), suggesting that mechanisms associated with the restoration of endothelial homeostasis are also taking place in these subjects (Fig. 4).
FIGURE 4.
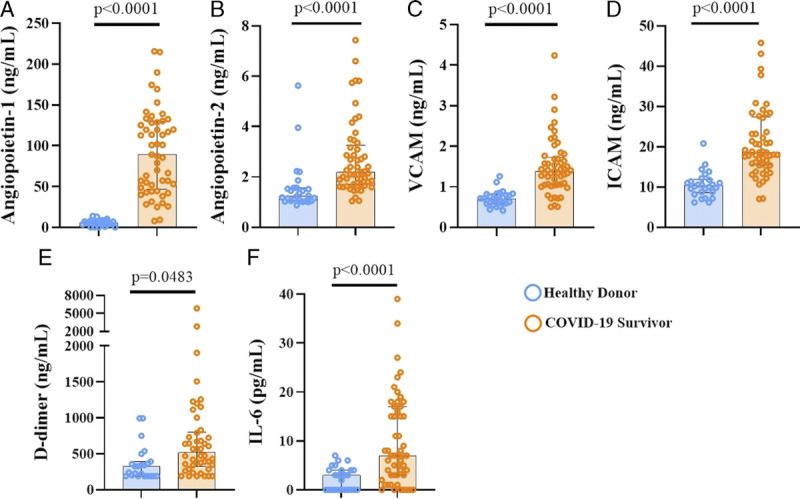
Persistence of elevated biomarkers of endothelial cell activation, coagulation, and inflammation. Plasmatic concentrations of endothelial cell activation markers, Ang-1 (A) and Ang-2 (B), VCAM-1 (C) and human ICAM-1 (D), coagulation activation marker d-dimer (E), and the proinflammatory cytokine IL-6 (F), are shown. Median and IQR are represented.
DISCUSSION
This prospective study revealed increased lung parenchyma density and metabolic activity in survivors of moderate to severe COVID-19 pneumonia at 31 to 194 days after hospitalization. The increase in metabolic activity occurred in high-attenuation areas (infiltrates) and even in normal-attenuation areas. Survivors also presented increased systemic thromboinflammation and endothelial cell activation biomarkers relative to control subjects. These data suggest that systemic immune activation and lung parenchymal inflammation persist months after SARS-CoV-2 infection, with multiple implications.
COVID-19 can clearly cause a long-term multisystem syndrome, frequently referred to as long COVID syndrome or post–acute sequelae of SARS-CoV-2 infection, in a significant proportion of survivors. The precise definition of this syndrome remains controversial, given its underlying immune mechanisms. Prolonged and persistent symptoms after COVID-19 infection are frequent, reported in 30% of outpatients19 and up to 93% of patients following hospitalization.10 All survivors in our study reported at least 1 persistent symptom. This higher prevalence of persistent symptoms may be related to the severity of acute disease or a shorter interval between the acute phase and follow-up than in the study by Sigfrid et al.10 In agreement with previous reports,3 fatigue, dyspnea, and neurocognitive manifestations were the most common symptoms reported by patients in the present study.
The persistence of respiratory symptoms, especially dyspnea and cough, for more than 2 months after symptom onset seems to be common among COVID-19 survivors. The reported prevalence of such persistence for 1 to 12 months after hospitalization with COVID-19 ranges from 5% to 81%.20 Wu et al21 recently reported the improvement of respiratory symptoms after 1 year in patients who had moderate disease. In contrast, Huang et al22 reported a slight deterioration of dyspnea scores between 6 and 12 months after COVID-19 and absence of improvement in exercise capacity and diffusing capacity for carbon monoxide, but gradual recovery of the total lung capacity and lung imaging abnormalities. Patterns of recovery for respiratory symptoms, functional respiratory capacity, and radiological abnormalities remain to be determined.
The prevalence of lung parenchymal changes on chest radiographs and CT images of COVID-19 pneumonia survivors varies, depending on the study population, interval after infection, and severity of initial illness.23 The most common persistent CT abnormalities are GGOs, reticular abnormalities, and fibrotic changes.6,23,24 In our study, persistent lung abnormalities were observed on follow-up CT images in 64.2% of survivors, consistent with the reported prevalence of 56% to 88%.5,21,25,26 Ground-glass opacities were the most frequent finding, occurring in 73.5% of survivors with CT abnormalities, and fibrotic-like changes occurred in 29.4% of these patients. The prevalence of fibrotic-like changes in COVID-19 pneumonia survivors with lung abnormalities at 4 and 6 months were 19.3% and 56.3%, respectively.6,26 In the present study, densitometric analysis showed a tendency for gradual resolution over time, but the lung parenchyma of COVID-19 survivors was significantly denser than that of control subjects, with similar TLVs.
PET/CT is the most sensitive imaging modality for the assessment of cell metabolism and the magnitude of inflammatory/metabolic activity.27 This inflammatory/metabolic phenotype results in high 18F-FDG uptake. We observed more metabolic activity in survivors' lungs than in those of control subjects, mainly overlapping regions of greater density. PET/CT has been used to study COVID-19 survivors or those with long COVID syndrome in a few studies, consisting of case reports and small case series,28–32 and in only 1 study, patients have been prospectively examined at a later stage (>30 days between symptom onset and PET/CT examination).29 In that study, Sollini et al32 examined 13 adult patients with at least 1 symptom persisting and highlighted the multisystemic aspect of long COVID syndrome, with increased FDG uptake in several organs, including the lungs in 4 patients.
We also found significantly more 18F-FDG uptake in the normal lung parenchyma of survivors than in that of control subjects, consistent with the finding of Sollini et al.32 These findings suggest that sustained inflammatory changes occur in the normal lung parenchyma of patients with long COVID syndrome. 18F-FDG uptake in normal lung parenchyma has been reported for other inflammatory pulmonary diseases, including acute lung injury,8,33,34 idiopathic pulmonary fibrosis,35 chronic obstructive pulmonary disease,36 and other infections.27,37 In these settings, the increased metabolic activity is associated with increased numbers of cells, accumulated through migration or local proliferation and cellular activation.33
The underlying pathophysiological mechanisms involved in the persistent clinical manifestations of COVID-19 survivors and in the lung PET/CT alterations here described are poorly understood at present. Accumulating evidence places thromboinflammation and endotheliopathy as central components in pathophysiology and as determinants of mortality in acute COVID-19 patients.38–40 Accordingly, biomarkers of inflammation, coagulation, and endothelial activation, including d-dimers, Ang-1/Ang-2, and IL-6, are early predictors of respiratory distress and mortality during acute COVID-19.16–18 Our findings demonstrate that biomarkers of thromboinflammation and endothelial cell activation characteristic of severe acute COVID-19 are sustained long after hospital discharge, even in moderate COVID-19 patients, and raise the intriguing possibility that this may contribute to long COVID pathogenesis. In our study, only 1 patient reported a deep vein thrombosis episode at follow-up. However, subclinical cases may have occurred, as systematic investigations for these events were outside the scope of this study.
This study has some limitations. First, to ensure that the control subjects had not had SARS-CoV-2 infection, we used CT and PET-CT images from a study conducted before the COVID-19 pandemic. Thus, we could not completely match survivors and control subjects. In addition, we lacked information on previous symptom histories for survivors before COVID-19 pneumonia. On the other side, the study has several strengths. It was prospective, and clinical, imaging, and immunometabolic data were acquired on the same day. Imaging quantification was performed with the use of advanced processing techniques to ensure the accuracy of the results. Biomarkers of inflammation, coagulation, and endothelial were examined. Finally, to our knowledge, our cohort is the largest group of patients to undergo PET/CT after recovery from COVID-19 pneumonia.
How long the post–COVID-19 inflammatory response can persist in the lungs remains unclear. We found increased inflammatory metabolic activity in lung areas of high and normal lung attenuation several months after recovery from COVID-19 pneumonia in this study. This activity and the persistence of plasma markers of thromboinflammation and endothelial activation can have implications for our understanding of the in vivo pathogenesis and long-lasting effects of COVID-19 pneumonia, eventually impacting treatment. Studies with longer follow-up periods are needed to clarify the impacts of persistent lung inflammation in COVID-19 survivors.
Footnotes
Conflicts of interest and sources of funding: The authors declare that they have no conflicts of interest. This work was supported by grants from the D'Or Institute for Research and Education, Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (numbers E-26/202.751/2018, E-26/202.785/2017, E-26/203.001/2018, E-26/203.279/2017, and E-26/211.867/2016), and Conselho Nacional de Desenvolvimento Científico e Tecnológico (numbers 02839/2017-8, 302702/2017-2, and 312410/2017-4).
Author Contributions: R.S.R., P.H.R.d.C., G.M.R., F.A.B., and M.M.B. wrote the manuscript; J.d.T.-M. and P.A.G.M. contributed to patient recruitment and management; R.M.-G. and E.D.H. performed sample processing, flow cytometry, and enzyme-linked immunosorbent assays; G.M.R., A.R.C, and R.B. performed the computational imaging and statistical analyses; M.M.B. performed the visual CT analysis; S.A.d.A. contributed to PET/CT acquisition and analysis; R.S.R., P.H.R.d.C., F.A.B., W.A.Z., and P.T.B. are responsible for the experimental design, manuscript review, and study supervision. All authors reviewed the final manuscript.
Contributor Information
Gabriel Motta Ribeiro, Email: gabrielcasulari@poli.ufrj.br.
Miriam Menna Barreto, Email: miriam.menna@gmail.com.
Walter Araujo Zin, Email: walter_zin@hotmail.com.
Júlia de Toledo-Mendes, Email: juliatoledo.mendes@gmail.com.
Philippe Alcantara G. Martins, Email: philippe.martins19@gmail.com.
Sergio Altino de Almeida, Email: altino.sergio@gmail.com.
Rodrigo Basílio, Email: riddlexter@gmail.com.
Remy Martins-Gonçalves, Email: philippe.martins19@gmail.com.
Eugênio Damaceno Hottz, Email: eugeniohottz@gmail.com.
Patricia T. Bozza, Email: pbozza@gmail.com.
Fernando A. Bozza, Email: bozza.fernando@gmail.com.
Alysson Roncally Silva Carvalho, Email: roncally.carvalho@gmail.com.
Paulo Henrique Rosado-de-Castro, Email: paulo.rosado@idor.org.
REFERENCES
- 1.COVID Live. Coronavirus statistics. Worldometer [Internet]. Available at: https://www.worldometers.info/coronavirus/. Accessed March 29, 2022.
- 2.Carfì A Bernabei R Landi F, et al. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324:603–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nalbandian A Sehgal K Gupta A, et al. Post–acute COVID-19 syndrome. Nat Med. 2021;27:601–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siddiqui S, Brightling CE. Pathological disease in the lung periphery after acute COVID-19. Lancet Respir Med. 2021;9:1089–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vijayakumar B Tonkin J Devaraj A, et al. CT lung abnormalities after COVID-19 at 3 months and 1 year after hospital discharge. Radiology. 2022;303:444–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Han X Fan Y Alwalid O, et al. Six-month follow-up chest CT findings after severe COVID-19 pneumonia. Radiology. 2021;299:E177–E186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balbi M Conti C Imeri G, et al. Post-discharge chest CT findings and pulmonary function tests in severe COVID-19 patients. Eur J Radiol. 2021;138:109676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodrigues RS Bozza FA Hanrahan CJ, et al. 18F-fluoro-2-deoxyglucose PET informs neutrophil accumulation and activation in lipopolysaccharide-induced acute lung injury. Nucl Med Biol. 2017;48:52–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.WHO Working Group on the Clinical Characterisation and Management of COVID-19 Infection . A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis. 2020;20:e192–e197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sigfrid L Drake TM Pauley E, et al. Long COVID in adults discharged from UK hospitals after COVID-19: a prospective, multicentre cohort study using the ISARIC WHO clinical characterisation protocol. Lancet Reg Health Eur. 2021;8:100186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hansell DM Bankier AA MacMahon H, et al. Fleischner Society: glossary of terms for thoracic imaging. Radiology. 2008;246:697–722. [DOI] [PubMed] [Google Scholar]
- 12.Pan F Zheng C Ye T, et al. Different computed tomography patterns of coronavirus disease 2019 (COVID-19) between survivors and non-survivors. Sci Rep. 2020;10:11336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hofmanninger J Prayer F Pan J, et al. Automatic lung segmentation in routine imaging is primarily a data diversity problem, not a methodology problem. Eur Radiol Exp. 2020;4:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Newell JD, Sieren J, Hoffman EA. Development of quantitative computed tomography lung protocols. J Thorac Imaging. 2013;28:266–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hinoshita T Ribeiro GM Winkler T, et al. Inflammatory activity in atelectatic and normally aerated regions during early acute lung injury. Acad Radiol. 2020;27:1679–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lucas C Wong P Klein J, et al. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature. 2020;584:463–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang L Yan X Fan Q, et al. d-Dimer levels on admission to predict in-hospital mortality in patients with COVID-19. J Thromb Haemost. 2020;18:1324–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smadja DM Guerin CL Chocron R, et al. Angiopoietin-2 as a marker of endothelial activation is a good predictor factor for intensive care unit admission of COVID-19 patients. Angiogenesis. 2020;23:611–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Logue JK Franko NM McCulloch DJ, et al. Sequelae in adults at 6 months after COVID-19 infection. JAMA Netw Open. 2021;4:e210830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Montani D Savale L Noel N, et al. Post–acute COVID-19 syndrome. Eur Respir Rev. 2022;31:210185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu X Liu X Zhou Y, et al. 3-Month, 6-month, 9-month, and 12-month respiratory outcomes in patients following COVID-19–related hospitalisation: a prospective study. Lancet Respir Med. 2021;9:747–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang C Huang L Wang Y, et al. 6-Month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Solomon JJ Heyman B Ko JP, et al. CT of post-acute lung complications of COVID-19. Radiology. 2021;301:E383–E395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kwee TC, Kwee RM. Chest CT in COVID-19: what the radiologist needs to know. Radiographics. 2020;40:1848–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shah AS Wong AW Hague CJ, et al. A prospective study of 12-week respiratory outcomes in COVID-19–related hospitalisations. Thorax. 2021;76:402–404. [DOI] [PubMed] [Google Scholar]
- 26.Morin L Savale L Pham T, et al. Writing Committee for the COMEBAC Study Group . Four-month clinical status of a cohort of patients after hospitalization for COVID-19. JAMA. 2021;325:1525–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eibschutz LS Rabiee B Asadollahi S, et al. FDG-PET/CT of COVID-19 and other lung infections. Semin Nucl Med. 2022;52:61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fu C Zhang W Li H, et al. FDG PET/CT evaluation of a patient recovering from COVID-19. Eur J Nucl Med Mol Imaging. 2020;47:2703–2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ajuria-Illarramendi O, Martinez-Lorca A, Orduña-Diez MDP. [18F]FDG-PET/CT in different COVID-19 phases. IDCases. 2020;21:e00869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bai Y Xu J Chen L, et al. Inflammatory response in lungs and extrapulmonary sites detected by [18F] fluorodeoxyglucose PET/CT in convalescing COVID-19 patients tested negative for coronavirus. Eur J Nucl Med Mol Imaging. 2021;48:2531–2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thornton A Fraioli F Wan S, et al. Evolution of 18F-FDG PET/CT findings in patients after COVID-19: an initial investigation. J Nucl Med. 2022;63:270–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sollini M Morbelli S Ciccarelli M, et al. Long COVID hallmarks on [18F]FDG-PET/CT: a case-control study. Eur J Nucl Med Mol Imaging. 2021;48:3187–3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rodrigues RS Miller PR Bozza FA, et al. FDG-PET in patients at risk for acute respiratory distress syndrome: a preliminary report. Intensive Care Med. 2008;34:2273–2278. [DOI] [PubMed] [Google Scholar]
- 34.de Prost N, Tucci MR, Melo MF. Assessment of lung inflammation with 18F-FDG PET during acute lung injury. AJR Am J Roentgenol. 2010;195:292–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Win T Thomas BA Lambrou T, et al. Areas of normal pulmonary parenchyma on HRCT exhibit increased FDG PET signal in IPF patients. Eur J Nucl Med Mol Imaging. 2014;41:337–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Subramanian DR Jenkins L Edgar R, et al. Assessment of pulmonary neutrophilic inflammation in emphysema by quantitative positron emission tomography. Am J Respir Crit Care Med. 2012;186:1125–1132. [DOI] [PubMed] [Google Scholar]
- 37.Intriago B Danùs M Calvo N, et al. Influenza-like infection can result in diffuse fluordeoxyglucose uptake in the lungs. Clin Nucl Med. 2009;34:737–738. [DOI] [PubMed] [Google Scholar]
- 38.Nicolai L Leunig A Brambs S, et al. Immunothrombotic dysregulation in COVID-19 pneumonia is associated with respiratory failure and coagulopathy. Circulation. 2020;142:1176–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ackermann M Verleden SE Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in COVID-19. N Engl J Med. 2020;383:120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goshua G Pine AB Meizlish ML, et al. Endotheliopathy in COVID-19–associated coagulopathy: evidence from a single-centre, cross-sectional study. Lancet Haematol. 2020;7:e575–e582. [DOI] [PMC free article] [PubMed] [Google Scholar]


