Abstract
Introduction
Abstinence symptoms present challenges to successful cessation of cigarette smoking. Chronic exposure to nicotine and long-term nicotine abstinence are associated with alterations in cortical and subcortical gray matter volumes (GMVs).
Aims and Methods
We aimed at examining changes in regional GMVs following overnight abstinence and how these regional functions relate to abstinence symptoms. Here, in a sample of 31 regular smokers scanned both in a satiety state and after overnight abstinence, we employed voxel-wise morphometry and resting-state functional connectivity (rsFC) to investigate these issues. We processed imaging data with published routines and evaluated the results with a corrected threshold.
Results
Smokers showed smaller GMVs of the left ventral hippocampus and right secondary somatosensory cortex (SII) after overnight abstinence as compared to satiety. The GMV alterations in right SII were positively correlated with changes in withdrawal symptom severity between states. Furthermore, right SII rsFC with the precentral gyrus was stronger in abstinence as compared to satiety. The inter-regional rsFC was positively correlated with motor impulsivity and withdrawal symptom severity during abstinence and negatively with craving to smoke during satiety.
Conclusions
These findings highlight for the first time the effects of overnight abstinence on cerebral volumetrics and changes in functional connectivity of a higher-order sensory cortex. These changes may dispose smokers to impulsive behaviors and aggravate the urge to smoke at the earliest stage of withdrawal from nicotine.
Implications
Overnight abstinence leads to changes in gray matter volumes and functional connectivity of the second somatosensory cortex in cigarette smokers. Higher somatosensory and motor cortical connectivity in abstinence is significantly correlated with trait motor impulsivity and withdrawal symptom severity. The findings add to the literature of neural markers of nicotine addiction.
Introduction
Nicotine use is a leading cause of morbidity and mortality.1 Despite the success of public health campaign to reduce prevalence of smoking, symptoms of nicotine abstinence, including inability to focus, restlessness, negative mood, and craving, continue to impede successful cessation.2,3 These symptoms typically set in a few hours following abstinence and may lead to impulsive and uncontrollable urge to smoke. Impulsivity conduces to smoking4 and relapse to smoking.5 For instance, greater trait impulsivity, as indexed by the total score of Barratt Impulsiveness Scale (BIS-11), predicted higher levels of craving and anxiety during a 48-h abstinence period6 and faster relapse to smoking following the 48 h of abstinence.7 Studies also showed that the attenuated response to monetary reward anticipation in the right caudate after an initial period of 24-h abstinence predicted greater likelihood of lapse, suggesting early abstinence as a critical period of vulnerability in cigarette smokers.8 Understanding abstinence-elicited shorter-term structural and functional changes of the brain may help in identifying neural markers to target for cessation treatments. Previous research demonstrated cerebral volumetric changes in chronic tobacco smokers and how these changes related to behavioral impulsivity,9,10 and examined how brain volumes predicted relapse in cessation treatments.11 However, no studies, to the best of our knowledge, have investigated these changes immediately after cessation of cigarette smoking.
Regional brain volumes are determined by cell sizes and density.12 Brain-derived neurotrophic factors (BDNFs) play vital roles in modulating activity-dependent synaptic plasticity among mature neurons, particularly in the hippocampus and neocortex.13,14 Studies have also shown that these neuromodulating effects are disrupted during acute withdrawal of nicotine in chronically exposed animals.15 Although no work has investigated the influences of acute nicotine withdrawal on GMVs, a recent study demonstrated reduction in anterodorsal hippocampal volumes following 12-h abstinence of alcohol in rats.16 Thus, GMVs seem amenable to the influences of abstinence from drug use on a short time scale, as with regional brain activation and connectivity.17,18 In human studies, participants showed significantly reduced regional cerebral blood flow (CBF) in the bilateral hippocampus extending into the midbrain, posterior cingulate cortex, ventral striatum, and occipital cortex after 4-h nicotine abstinence as compared to satiety.19 Another PET study showed that at the end of the overnight abstinence, global gray matter CBF and metabolic rate of oxygen (CMRO2) were both reduced by 17% relative to nonsmokers. At 15 min after renewed smoking, global CMRO2 increased by 11% in the left putamen and thalamus and right posterior cortical regions. At 60 and 105 min after renewed smoking, CBF increased by 8% and CMRO2 by 11%–12% in most brain regions.20 These findings indicate substantial and global disruption of CBF/CMRO2 just after overnight abstinence that can be restored by resumption of smoking. While these physiological changes may mediate the cognitive and emotional effects of nicotine withdrawal, the impacts on cerebral volumetrics are not clear.
Notably, a recent study reported increases in gray matter density (GMD) in the right frontal pole, and superior and middle frontal gyri as well as decreases in GMVs and cortical thickness in the right temporal pole after 24 h of acute sleep deprivation as compared to after normal sleep.21 The GMD increase may result from a net elevation in synaptic strength in many neuronal circuits in the state of being awake. Interestingly, total sleep loss is associated with impaired hippocampal BDNFs expression, along with memory and cognitive deficits,22 in accord with its impact on GMVs. Another study showed greater GMVs and white matter volumes (WMVs) of subcortical regions including the basal ganglia, hippocampus, and amygdala after 12 h of task practice in combination with sleep deprivation and the increases became even more significant after 24 h.23 Furthermore, the changes in GMVs and WMVs reverted after 8 h of recovery sleep, suggesting that structural brain alterations after acute sleep deprivation may be temporary. Thus, although scanty, evidence is available to support the prospective effects of acute changes in physiological states, potentially including very short-term abstinence from smoking, on brain volumetrics.
In the current study, we compared the GMVs within subjects between the states of overnight abstinence and satiety in cigarette smokers. We used voxel-based morphometry (VBM) and quantified the relationships between changes in GMVs, nicotine use metrics, and impulsivity of the participants. On the basis of the literature, we speculated that overnight abstinence negatively impacts the GMVs of brain regions critical to the manifestation of withdrawal-related symptoms and that the functional features of these regions may be related to nicotine use metrics and behavioral impulsivity.
Methods
Volunteers, Informed Consents, and Assessments
Thirty-nine regular smokers (age 24–55 years) participated in the study. Participants completed a brief prescreening survey over phone and met eligibility criteria, including smoking at least 5 cigarettes daily for at least 2 years and having a body mass index of 19–38. Exclusion criteria included a history of or current major medical condition, history of head injury or brain concussion that resulted in loss of consciousness, history of or current Axis I psychiatric diagnosis including substance (except nicotine) use disorder according to DSM-IV, and current use of any psychotropic medications. The study was approved by the Institutional Review Board of Yale University. All research was performed in accordance with relevant guidelines and regulations, and written informed consent was obtained from each individual prior to participation.
A total of 8 participants were excluded from analyses because of incomplete scan (n = 5), smoking before the abstinence session (n = 2), or testing positive for cannabis on the day of scan (n = 1). The final sample size for VBM was 31 (15 women) with 11 Caucasian Americans, 9 African Americans, 2 Asians, and 9 Others in race. Two participants had excessive head motion during resting-state fMRI, and one did not have fMRI data; thus, the final sample size for resting-state fMRI analyses was 28 (13 women).
Participants completed two MRI scans, 1–2 weeks apart, each when they were sated with smoking and when they were overnight abstinent from smoking, with the order counter-balanced across subjects. Exhaled breath carbon monoxide (CO) level was verified using a Bedfont Micro+ breath CO monitor with abstinence defined by a CO level of <10 ppm.24 For the satiety session, participants were not required to remain abstinent prior to the scan. Participants also received urine toxicology tests prior to each MRI session to detect illicit substances and measure nicotine metabolite cotinine. The NicAlert test strip has 7 numbered zones (0–6); level 0 (0–10 ng/mL), 1 (10–30), 2 (30–100), 3 (100–200), 4 (200–500), 5 (500–1000), and 6 (>1000). The result was identified by locating the lowest level where a red band appeared. A reading of 3 or greater was considered positive for smoking, with a cotinine concentration of >100 ng/mL.25
Participants were assessed for nicotine use metrics and other clinical characteristics before the first MR scan, including daily consumption of cigarettes, years of smoking, and nicotine addiction severity with the Fagerström Test for Nicotine Dependence (FTND; range 0–10) with a higher score indicating more severe dependence.26 Participants were also assessed for impulsivity with BIS-11,27 with a higher total score and inattentional, motor, and nonplanning subscores indicating higher impulsivity. Pack years of each participant were calculated by multiplying the number of packs of cigarettes smoked per day by the number of years of smoking to reflect lifetime exposure to tobacco.
On the days of MRI, abstinence duration (ie, time since last cigarette, in hours) was recorded. Before each scan, 22 participants (14 women) were assessed for withdrawal symptoms with reference to the past 24 h using Hughes-Hatsukami Withdrawal Questionnaire,28 craving to smoke with a brief, 10-item version of the Questionnaire of Smoking Urges,29 and state anxiety with State-Trait Anxiety Inventory.30
We performed two-sample t tests to examine sex differences in the demographic and clinical measures and two (abstinence vs. satiety) by two (men vs. women) mixed-model ANOVAs on CO level, withdrawal duration, withdrawal symptom severity, craving to smoke, and state anxiety. Given that the urine cotinine level was an ordinal variable, we used a nonparametric interaction test of mixed-model ANOVA instead. Furthermore, we computed the correlation coefficients of Pearson’s regression to examine the relationships between clinical measures in men and women combined and separately and used slope tests to examine the sex differences in the correlations.31–33 For the clinical measures recorded for both scans, we also examined the differences in correlations between abstinence and satiety with slope tests.
MRI Data Acquisition and Preprocessing
A customized 3T Siemens Trio scanner with a standard 32-channel Siemens receiver head coil and a body transmission coil was used in the MRI scanning. T1-weighted high-resolution structural images were acquired using a 3D MPRAGE sequence in the axial plane parallel to the AC–PC line (FOV = 250 × 250 mm, matrix = 256 × 256, 176 sagittal slices with slice thickness = 1 mm and no gap, TR = 1900 ms, TE = 2.52 ms, TI = 900 ms, FA = 9°). Functional, BOLD signals were then acquired with a single-shot gradient echo planar imaging (EPI) sequence. Fifty-one axial slices parallel to the AC–PC line covering the whole brain were acquired (FOV = 210 × 210 mm, matrix = 84 × 84, 51 slices with slice thickness = 2.5 mm and no gap, TR = 1000 ms, TE = 30 ms, bandwidth = 2290 Hz/pixel, FA = 62°). One run of 10-min resting state fMRI scan (600 frames) was obtained for each visit of each volunteer with eye closed but awake. Physiological data (ie, cardiac and respiratory signals) associated with each functional MR scan were also acquired, using a standard Biopac pulse oximeter placed on a digit and a respiratory belt placed on the abdomen. These physiological signals were sampled at 1000 Hz.
We followed published routines and performed VBM to estimate the GMVs of brain regions with the CAT12 (Version 12.7) toolbox, following the suggested defaults34 (http://dbm.neuro.uni-jena.de/vbm). The details of VBM analysis have been described in our previous studies35,36 (Supplementary Methods). Weighted average image quality rating (IQR) was reported, and total intracranial volume (TIV) was estimated for the participants.
Likewise, we preprocessed the fMRI data as described earlier for resting-state functional connectivity (rsFC) analysis37 (Supplementary Methods).
Group Statistics of GMVs and rsFC
We performed paired-sample t tests for whole-brain GMVs and evaluated the results at voxel p < .001, uncorrected, in combination with a cluster p < .05, corrected for family-wise error (FWE) of multiple comparisons, on the basis of Gaussian random field theory as implemented in SPM, following current reporting standards.38 In addition to reporting the peak voxel Z values, we computed the effect sizes by approximating Cohen’s d from the t-statistics using the expression 39
For the clusters revealed in voxel-wise analysis (regions of interest or ROIs), we computed the regional GMVs for individual participants and performed linear correlations with clinical measures—pack years, FTND score, and BIS-11 total score and subscores—for each scan separately. Slope tests were used to examine differences in the correlations between abstinence and satiety.
We used the masks of the ROIs as seed regions to compute the whole-brain rsFC. The BOLD time courses of each voxel were averaged for the seed, and the correlation coefficient was computed between the average time course of each seed and the time courses of all other voxels for individual participants. The correlation matrix was Fisher’s z-transformed into z score maps. We used a whole-brain paired-sample t test to compare seed-based rsFC between abstinence and satiety, and evaluated the results at voxel p < .001, uncorrected, in combination with a cluster p < .05, corrected for FWE. Likewise, we computed the effect sizes with Cohen’s
We also computed the β estimates of the ROIs that were revealed in the whole-brain rsFC analyses and performed linear correlations with the same set of clinical measures for each scan separately. Again, slope tests were used to examine the difference in correlations between abstinence and satiety.
Results
Demographics, Clinical, and Global Volumetric Measures
The mean and SD values of demographic, clinical, and global volumetric measures are presented in Table 1. Two-sample t tests showed no significant sex differences in any of the demographic or clinical metrics (t’s ≤ 1.51, p’s ≥ .141). A two (abstinence vs. satiety) by two (men vs. women) mixed-model ANOVA showed a significant interaction effect on the CO level (F = 8.99, p = .006). Simple-effect tests showed that the CO level was significantly lower in abstinence versus satiety in women (p < .001) but not in men (p = .095) and there were no sex differences in the CO level in either abstinence (p = .430) or satiety (p = .259). The nonparametric interaction test of mixed-model ANOVA showed no state-by-sex interaction effect on urine cotinine level (F ≤ 0.10, p = .750).
Table 1.
Demographic, Clinical, and Global Volumetric Measures in Men and Women
| Mean ± SD | Two-sample t or ANOVA | |||||
|---|---|---|---|---|---|---|
| Men (n = 16) | Women (n = 15) | T | p | |||
| Age (years) | 41.7 ± 10.2 | 36.9 ± 8.6 | 1.40 | .17 | ||
| Scan interval (d) | 9.4 ± 4.8 | 9.2 ± 3.6 | 0.15 | .88 | ||
| Pack years | 17.1 ± 21.2 | 8.4 ± 7.0 | 1.51 | .14 | ||
| FTND score | 4.5 ± 2.8 | 4.6 ± 2.3 | −0.11 | .91 | ||
| BIS-11 total | 63.6 ± 11.1 | 63.5 ± 14.7 | 0.02 | .98 | ||
| BIS-11 inatt. | 16.1 ± 4.9 | 15.8 ± 6.0 | 0.18 | .86 | ||
| BIS-11 motor | 23.3 ± 5.9 | 22.2 ± 4.7 | 0.57 | .57 | ||
| BIS-11 nonpl. | 24.1 ± 4.4 | 25.5 ± 5.8 | −0.75 | .46 | ||
| Abstinence | Satiety | Abstinence | Satiety | F * | p * | |
|---|---|---|---|---|---|---|
| CO (ppm) | 9.6 ± 9.8 | 13.2 ± 11.7 | 7.0 ± 6.5 | 19.0 ± 13.7 | 9.00 | .01 |
| U. cotinine level | 5.3 ± 0.8 | 5.4 ± 0.8 | 5.4 ± 0.8 | 5.5 ± 0.9 | 0.10 | .75 |
| Withdr. dur. (h) | 11.1 ± 2.3 | 1.7 ± 1.7 | 11.6 ± 4.4 | 1.7 ± 3.3 | 0.25 | .62 |
| Withdr. sym. | 14.2 ± 7.7 | 8.6 ± 5.7 | 11.6 ± 6.4 | 7.8 ± 4.8 | 0.56 | .46 |
| Craving | 45.5 ± 16.6 | 32.9 ± 13.4 | 49.4 ± 15.4 | 31.7 ± 17.2 | 0.40 | .54 |
| State anxiety | 40.4 ± 16.6 | 35.1 ± 10.8 | 31.0 ± 10.6 | 29.0 ± 8.1 | 0.82 | .38 |
| TIV (ccm) | 1531 ± 93 | 1530 ± 88 | 1378 ± 114 | 1379 ± 112 | 0.33 | .57 |
| IQR (%) | 85.1 ± 1.0 | 85.1 ± 1.0 | 84.8 ± 1.3 | 84.8 ± 1.8 | 0.06 | .82 |
d, days; FTND, Fagerström Test for Nicotine Dependence; BIS-11, Barratt Impulsiveness Scale 11; inatt., inattentional; nonpl., nonplanning; CO, carbon monoxide; U, urine; Withdr, Withdrawal; dur. (h), duration (h); sym., symptom severity; TIV, total intracranial volume; ccm, cubic centimeter; IQR, image quality rating.
F and p values reflect the interaction effects of mixed-model ANOVA.
The main effects of state were significant for withdrawal duration (F = 385.76, p < .001), withdrawal symptom severity (F = 16.19, p = .001), craving to smoke (F = 14.16, p = .001), but not for state anxiety (F = 3.84, p = .064), TIV (F = 0.13, p = .717), or IQR (F < 0.01, p = .994). The main effects of sex were not significant for any of the clinical or global volumetric measures (F’s ≤ 2.76, p’s ≥ .112), except that men showed significantly higher TIV than women (F = 17.26, p < .001). There were no significant interaction effects for any of these measures (F’s ≤ 0.82, p’s ≥ 0.375).
Pack years were positively correlated with FTND score in men and women combined (r = 0.584, p = .001) as well as in men (r = 0.721, p = .002) but not in women (r = 0.395, p = .145) alone. The slope test confirmed the sex difference in the correlations (t = 3.231, p = .003). Pack years and FTND scores were not significantly correlated with BIS-11 total score or subscores across all subjects (p’s ≥ .116) or in men (p’s ≥ .194) or women (p’s ≥ .257) separately. Slope tests showed no significant sex differences in these correlations (p’s ≥ .168). In addition, BIS-11 total score and subscores were all significantly inter-correlated (r’s ≥ 0.555, p’s ≤ 0.002) in all subjects and in men (r’s ≥ 0.510, p’s ≤ .044, except for motor versus nonplanning with r = −0.138, p = .611) and in women (r’s ≥ 0.711, p’s ≤ .006, except for inattentional versus motor with r = 0.544, p = .055) separately. Slope tests showed significant sex differences in the correlations of BIS-11 motor and nonplanning subscores (r = −0.138 and 0.830 for men and women, respectively; slope test t = 3.821, p < .001) but not in any other correlations (p’s ≥ .073).
In men and women combined, state anxiety was significantly correlated with withdrawal symptom severity in both abstinent (r = 0.59, p = .004) and sated (r = 0.60, p = .003) states; craving to smoke was not significantly correlated with withdrawal symptom severity or with state anxiety in either state (p’s ≥ .054). Slope test did not show significant state differences in any of the correlations (p’s ≥ .207). Furthermore, the differences (abstinence–satiety) in these three measures were not significantly inter-correlated (p’s ≥ .054).
Regional GMVs: Whole Brain Analyses and Clinical Correlations
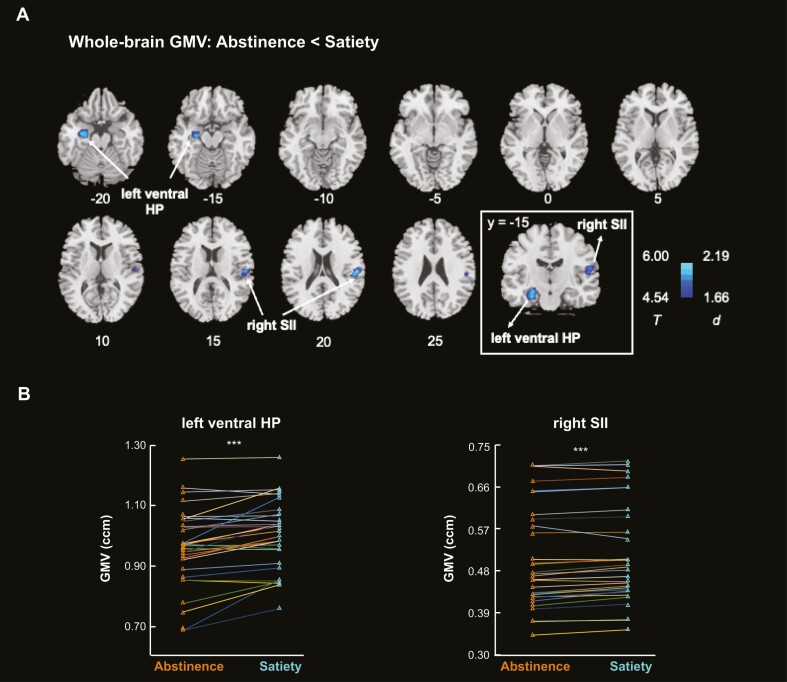
The whole-brain paired-sample t test identified lower GMVs in two clusters in abstinence versus satiety, at voxel level p < .001 uncorrected and cluster level p < .05 corrected for FWE, each in the left ventral hippocampus (cluster size k = 414, MNI coordinates x = −32, y = −9, z = −21, peak Z = 4.83) and the right second somatosensory cortex or SII (k = 209, x = 53, y = −23, z = 20, peak Z = 4.54) (Figure 1A). We extracted the GMVs for these two clusters and visualized the differences across abstinence and satiety across subjects in Figure 1B. Specifically, 25 and 27 of the 31 subjects showed lower GMVs in abstinence versus satiety for the left ventral hippocampus and right SII, respectively. Nonparametric tests showed no significant differences in any demographic or clinical measures between those who showed higher versus lower GMVs across satiety and abstinence for either left ventral hippocampus or right SII (p’s ≥ .057).
Figure 1.
(A) Whole-brain analysis identified the left ventral HP and right SII showing smaller GMVs in abstinence versus satiety. The inset shows the coronal section of the two clusters at y = −15. Color bar shows T value and Cohen’s d for the clusters. (B) Connected-line plots show the GMVs of left ventral HP and right SII of each participant in abstinence versus satiety: 25 and 27 of the 31 subjects showed lower GMVs in abstinence versus satiety for the left ventral HP and right SII, respectively. GMV, gray matter volume; HP, hippocampus; SII, secondary somatosensory cortex. ***p < .001.
Post hoc paired-sample t test showed that, left ventral hippocampus GMV was smaller in abstinence versus satiety (0.962 ± 0.130 vs. 1.003 ± 0.115, t = −4.86, p < .001; Figure 1B—left panel); right SII GMV was also smaller in abstinence versus satiety (0.506 ± 0.108 vs. 0.513 ± 0.106, t = −4.20, p < .001; Figure 1B—right panel). No clusters showed greater GMVs in the state of abstinence relative to satiety. The TIV did not show significant differences in abstinence versus satiety (1456.88 ± 128.38 vs. 1457.38 ± 125.53 ccm; t = −0.35, p = .728).
Regardless of abstinence or satiety, the left ventral hippocampus or right SII GMVs were not significantly correlated with pack years, FTND score, BIS-11 total score or subscores, withdrawal symptom severity, craving to smoke, or state anxiety (p’s ≥ .114). Slope tests showed no significant differences in these correlations between the two states (p’s ≥ .618). The statistics are summarized in Supplementary Table S1. With age, sex, race, TIV, and IQR as covariates, the findings remain nonsignificant (Supplementary Table S2).
However, the differences (satiety–abstinence) in right SII GMV were significantly correlated with the differences (satiety–abstinence) in withdrawal symptom severity (r = 0.482, p = .023). The state differences in the GMV of left ventral hippocampus or right SII were not significantly correlated with pack years, FTND scores, BIS-11 total or subscores, or with the differences between states in craving to smoke or state anxiety (p’s ≥ .149).
Seed-Based rsFCs and Clinical Correlations
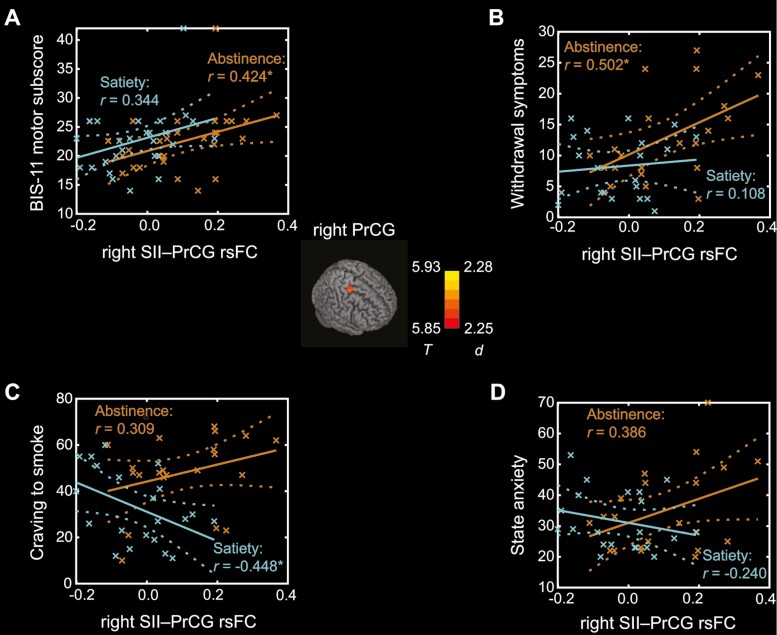
Whole-brain paired-sample t test did not identify any clusters showing significant differences in rsFC with the left ventral hippocampus. With the right SII as a seed, the rsFC with right precentral gyrus (cluster size k = 71, MNI coordinates x = 24, y = −14, z = 54, peak Z = 4.70) was stronger in abstinence versus satiety (Figure 2; center).
Figure 2.
Right precentral gyrus (PrCG) shows stronger rsFC with the right secondary somatosensory cortex (SII) in abstinence versus satiety (center). Color bar shows T value and Cohen’s d. Correlations between right SII-PrCG rsFC and (A) BIS-11 motor subscore, (B) withdrawal symptoms, (C) craving to smoke, and (D) state anxiety in the state of abstinence and satiety. Crosses represent individual data points and dashed lines represent 95% confidence intervals of the mean regressions (solid lines). *p < .05.
Stronger rsFC between right SII and precentral gyrus (PrCG) was significantly correlated with higher BIS-11 motor impulsivity (r = 0.424, p = .031) and more withdrawal symptoms (r = 0.502, p = .021) in abstinence but not in satiety (r = 0.344, p = .085 and r = 0.108, p = .642, respectively) (Figure 2A and 2B). As shown in Figure 2C, stronger right SII-PrCG rsFC was significantly associated with less craving to smoke in satiety (r = −0.448, p = .042) but not significantly in abstinence (r = 0.309, p = .173). The correlation between right SII-PrCG rsFC was not significantly correlated with state anxiety either in abstinence (r = 0.386, p = .084) or in satiety (r = −0.240, p = .294) (Figure 2D). Slope tests showed significant differences between two states only in the correlations of right SII-PrCG rsFC with craving to smoke (t = 2.55, p = .015). The statistics are summarized in Supplementary Table S3. With age, sex, and race as covariates, the findings remained similar (Supplementary Table S4).
The difference in the right SII–PrCG rsFC between the two states (abstinence – satiety) was not significantly correlated with pack years, FTND score, BIS-11 total or subscores, or with state differences in withdrawal symptoms, craving to smoke, or anxiety (p’s ≥ .154).
Discussion
We presented, to the best of our knowledge, the first evidence of regional GMV changes following overnight nicotine abstinence in cigarette smokers. Smokers showed significant reductions in the GMVs of left ventral hippocampus and right SII as well as stronger SII-precentral cortical rsFC in abstinence versus satiety. Greater GMV alterations in the right SII were associated with greater differences in the severity of withdrawal symptoms between the two states. The right SII-precentral cortical rsFC was positively correlated with motor impulsivity and withdrawal symptom severity in abstinence, and negatively with craving to smoke in satiety. We discussed the main findings below.
Hippocampal and SII GMVs in Abstinence Versus Satiety
Left ventral hippocampus GMV was lower in smokers in abstinence as compared to satiety, in accord with previous evidence showing decreases in hippocampus GMV after 4-week abstinence from smoking in humans.11 The current findings suggest that ventral hippocampus GMV may start to diminish at the very early stage of abstinence. Decrements in hippocampal volumes were also noted earlier in patients with alcohol use disorder following 14 days of abstinence and in chronically exposed rats following 12 h of abstinence.16 Furthermore, the levels of glutamate and glutamine were associated negatively with hippocampal volumes, suggesting neuronal loss because of excessive glutamatergic activity during alcohol withdrawal.16 Thus, the changes in ventral hippocampus GMV may relate to hyperglutamatergic neurotoxicity and potentially the decrease in BDNFs, as discussed earlier, during the onset of nicotine withdrawal.
The hippocampus is critical to learning and memory and associating environmental cues with craving and drug use.40 The dorsal hippocampus has been implicated in relapse to drug-seeking during long-term abstinence,41 whereas the ventral hippocampus is involved in the regulation of anxiety-driven behaviors and stress responses,42,43 both of which are central to clinical manifestations of nicotine withdrawal. We found significant increases in withdrawal symptom severity, craving to smoke, and trend-level increases in state anxiety in abstinence versus satiety. An earlier study showed that anxiety escalated within the first 3.5 h and continued to increase at 18-h.44 The average withdrawal duration was 11 h in our study, well within this range. However, we did not observe significant correlations between left ventral hippocampus GMV and these clinical measures in our small sample.
We also observed lower GMV of right-hemispheric SII in smokers during overnight abstinence versus satiety, with greater GMV alterations associated with greater differences in the severity of withdrawal symptoms between the two states. This finding suggests neuroplasticity of the associative somatosensory cortex in response to acute nicotine abstinence. A network involving the insula, the primary somatosensory cortex (SI) and SII, in the right hemisphere especially, is critical for the representation of affective states and visceral awareness, in addition to general somatosensory sensations.45 Both SI and SII along with the right posterior insula showed parametric elevation in activation when individuals attended to aversive emotions.46 Previous studies have documented GMV or GMD reduction in SII in chronic pain disorders.47 Another study demonstrated lower somatosensory cortical GMVs in correlation with higher levels of somatic complaints in otherwise healthy subjects.48 Thus, it is tempting to speculate that the decreases in SII GMV may relate to physical discomforts arising from acute nicotine abstinence.
Functional Connectivity Between Right SII and Precentral Gyrus
Nicotine withdrawal is associated with deficits in neurocognitive functions including attention, working memory, and response inhibition.49 For instance, a behavioral study showed that smokers undergoing 24-h nicotine abstinence preferred immediate cigarettes over delayed money, but did not alter preference between immediate small versus delayed large sum of money, suggesting that acute nicotine deprivation may lead to impulsive decision making on nicotine-related rewards.50 Indeed, we found stronger rsFC between right SII and precentral gyrus in abstinence versus satiety, in correlation with higher motor impulsivity and more severe withdrawal symptoms in abstinence and, in contrast, with lower levels of craving in satiety. Motor impulsivity reflects “acting without thinking”.27 The precentral gyrus is implicated in sensorimotor integration51 and motor impulsivity.52 For instance, the somatomotor cortex showed higher activation in correlation with BIS-11 motor impulsiveness during proactive control in a Go/No-go task.53 Furthermore, the precentral gyrus showed higher responses not only to cue-54 but also withdrawal-induced18 craving in smokers. Thus, higher SII rsFC with precentral gyrus may serve to integrate somatosensation and interoception to impact craving and urge to smoke as smokers experience acute nicotine deprivation.
The findings relating rsFC strength to clinical manifestations are correlational. Glutamatergic signaling contributes to the motivation to smoke and may be associated with nicotine withdrawal symptoms.55 Previous studies have suggested corticolimbic rsFCs as intermediate phenotypes to explain the relationships between altered glutamatergic signaling and addiction symptomatology such as craving.56,57 Studies combining MR functional and spectroscopic imaging may investigate how cortical rsFCs mediate glutamatergic signaling and withdrawal-induced craving and other symptoms at early as well as later stages of nicotine deprivation, elucidating the mechanistic processes of nicotine withdrawal.
Limitations of the Study, Other Considerations, and Conclusions
A few limitations should be considered. First, our findings need to be considered as preliminary and verified in a larger sample as well as investigated further for sex differences. In particular, men relative to women showed significantly less difference in CO level between abstinence and satiety, suggesting that some male participants may not be truly abstinent overnight. On the other hand, we wish to emphasize that, despite the small sample size, the imaging findings were evaluated at a corrected threshold and would help future work in power analyses and formulation of specific hypotheses on the volumetric correlates of nicotine withdrawal. Second, we only assessed impulsivity as a personality trait along with the first MR scan. Although impulsivity is typically considered a stable trait, researchers may investigate how GMV changes in abstinence would associate with impulsivity via laboratory behavioral paradigms. Third, hippocampal volume measures are influenced by tissue water levels.58 We cannot rule out the possibility that the volumetric changes during acute nicotine abstinence may reflect dehydration-induced changes in water signals. Previous studies demonstrated significant GMV reduction in association with dehydration in the caudate nucleus and cerebellum.59 Thus, ensuring adequate fluid intake within 24 h before scanning and controlling the use of alcohol or medications that may affect brain morphometry should be noted for future work.60,61 In addition, we need to acknowledge the broader possibility that the structural alterations could be related to transient changes in blood flow or other physiological signals.61 Finally, volumetric brain changes owing to sleep deprivation reverted after recovery sleep.23 It would be of great interest to explore the recovery effects on GMVs in smokers.
To conclude, the current study reports the effects of overnight abstinence on regional volumetrics and changes in functional connectivity of the SII that may dispose smokers to impulsive behaviors and aggravate the urge to smoke. A better understanding of the underlying mechanisms may help in elucidating the etiological processes of nicotine addiction and in formulating new treatments for smoking cessation.
Supplementary Material
A Contributorship Form detailing each author’s specific involvement with this content, as well as any supplementary data, are available online at https://academic.oup.com/ntr.
Acknowledgments
This work was supported by a National Institute of Health (NIH) grant DA045189 to C-S.R.L. The NIH is otherwise not involved in conceptualization of the study, data analysis and interpretation, or the decision to publish the current results.
Contributor Information
Yu Chen, Department of Psychiatry, Yale University School of Medicine, New Haven, CT 06520, USA.
Isha Dhingra, Department of Psychiatry, Yale University School of Medicine, New Haven, CT 06520, USA.
Shefali Chaudhary, Department of Psychiatry, Yale University School of Medicine, New Haven, CT 06520, USA.
Lisa Fucito, Department of Psychiatry, Yale University School of Medicine, New Haven, CT 06520, USA.
Chiang-Shan R Li, Department of Psychiatry, Yale University School of Medicine, New Haven, CT 06520, USA; Department of Neuroscience, Yale University School of Medicine, New Haven, CT 06520, USA; Inter-department Neuroscience Program, Yale University, New Haven, CT 06520, USA; Wu Tsai Institute, Yale University, New Haven, CT 06520, USA.
Competing Interests
The authors declare no competing interests in the current study.
Data Availability
The data shown this study will be shared on request to the corresponding author. MATLAB codes will be shared upon request.
References
- 1. Grant BF, Shmulewitz D, Compton WM.. Nicotine use and DSM-IV nicotine dependence in the United States, 2001-2002 and 2012-2013. Am J Psychiatry. 2020;177(11):1082–1090. [DOI] [PubMed] [Google Scholar]
- 2. Farris SG, Zvolensky MJ, Schmidt NB.. Smoking-specific experiential avoidance cognition: explanatory relevance to pre- and post-cessation nicotine withdrawal, craving, and negative affect. Addict Behav. 2015;44:58–64. doi: 10.1016/j.addbeh.2014.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kassel JD, Stroud LR, Paronis CA.. Smoking, stress, and negative affect: correlation, causation, and context across stages of smoking. Psychol Bull. 2003;129(2):270–304. [DOI] [PubMed] [Google Scholar]
- 4. Yakir A, Rigbi A, Kanyas K, et al. Why do young women smoke? III. Attention and impulsivity as neurocognitive predisposing factors. Eur Neuropsychopharmacol. 2007;17(5):339–351. [DOI] [PubMed] [Google Scholar]
- 5. Pattij T, De Vries TJ.. The role of impulsivity in relapse vulnerability. Curr Opin Neurobiol. 2013;23(4):700–705. [DOI] [PubMed] [Google Scholar]
- 6. VanderVeen JW, Cohen LM, Cukrowicz KC, Trotter DR.. The role of impulsivity on smoking maintenance. Nicotine Tob Res. 2008;10(8):1397–1404. [DOI] [PubMed] [Google Scholar]
- 7. Doran N, Spring B, McChargue D, Pergadia M, Richmond M.. Impulsivity and smoking relapse. Nicotine Tob Res. 2004;6(4):641–647. [DOI] [PubMed] [Google Scholar]
- 8. Sweitzer MM, Geier CF, Denlinger R, et al. Blunted striatal response to monetary reward anticipation during smoking abstinence predicts lapse during a contingency-managed quit attempt. Psychopharmacology (Berl). 2016;233(5):751–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yang Z, Zhang Y, Cheng J, Zheng R.. Meta-analysis of brain gray matter changes in chronic smokers. Eur J Radiol. 2020;132:109300. [DOI] [PubMed] [Google Scholar]
- 10. Zhong J, Shi H, Shen Y, et al. Voxelwise meta-analysis of gray matter anomalies in chronic cigarette smokers. Behav Brain Res. 2016;311:39–45. [DOI] [PubMed] [Google Scholar]
- 11. Froeliger B, Kozink RV, Rose JE, et al. Hippocampal and striatal gray matter volume are associated with a smoking cessation treatment outcome: results of an exploratory voxel-based morphometric analysis. Psychopharmacology (Berl). 2010;210(4):577–583. [DOI] [PubMed] [Google Scholar]
- 12. Anderson BJ. Plasticity of gray matter volume: the cellular and synaptic plasticity that underlies volumetric change. Dev Psychobiol. 2011;53(5):456–465. [DOI] [PubMed] [Google Scholar]
- 13. Gorski JA, Zeiler SR, Tamowski S, Jones KR.. Brain-derived neurotrophic factor is required for the maintenance of cortical dendrites. J Neurosci. 2003;23(17):6856–6865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lu B. BDNF and activity-dependent synaptic modulation. Learn Mem. 2003;10(2):86–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kim J, Yang JH, Ryu IS, et al. Interactions of glutamatergic neurotransmission and brain-derived neurotrophic factor in the regulation of behaviors after nicotine administration. Int J Mol Sci. 2019;20(12):2943. doi: 10.3390/ijms20122943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Frischknecht U, Hermann D, Tunc-Skarka N, et al. Negative association between MR-spectroscopic glutamate markers and gray matter volume after alcohol withdrawal in the hippocampus: a translational study in humans and rats. Alcohol Clin Exp Res. 2017;41(2):323–333. [DOI] [PubMed] [Google Scholar]
- 17. Connolly CG, Foxe JJ, Nierenberg J, Shpaner M, Garavan H.. The neurobiology of cognitive control in successful cocaine abstinence. Drug Alcohol Depend. 2012;121(1-2):45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Huang W, King JA, Ursprung WW, et al. The development and expression of physical nicotine dependence corresponds to structural and functional alterations in the anterior cingulate-precuneus pathway. Brain Behav. 2014;4(3):408–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Franklin TR, Jagannathan K, Hager N, et al. Brain substrates of early (4h) cigarette abstinence: identification of treatment targets. Drug Alcohol Depend. 2018;182:78–85. doi: 10.1016/j.drugalcdep.2017.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vafaee MS, Gjedde A, Imamirad N, et al. Smoking normalizes cerebral blood flow and oxygen consumption after 12-hour abstention. J Cereb Blood Flow Metab. 2015;35(4):699–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sun J, Zhao R, Yang X, et al. Alteration of brain gray matter density after 24 h of sleep deprivation in healthy adults. Front Neurosci. 2020;14:754. doi: 10.3389/fnins.2020.00754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rahmani M, Rahmani F, Rezaei N.. The brain-derived neurotrophic factor: missing link between sleep deprivation, insomnia, and depression. Neurochem Res. 2020;45(2):221–231. [DOI] [PubMed] [Google Scholar]
- 23. Bernardi G, Cecchetti L, Siclari F, et al. Sleep reverts changes in human gray and white matter caused by wake-dependent training. Neuroimage. 2016;129:367–377. doi: 10.1016/j.neuroimage.2016.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ernst M, Matochik JA, Heishman SJ, et al. Effect of nicotine on brain activation during performance of a working memory task. Proc Natl Acad Sci USA. 2001;98(8):4728–4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gruzdys V, Signorelli H, Johnson-Davis KL.. NicAlert™ test strip performance comparison with LC-MS/MS and immunoassay methods for nicotine and cotinine. Arch Clin Toxicol (Middlet). 2020;2(2):19–24. [Google Scholar]
- 26. Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO.. The Fagerström test for nicotine dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86(9):1119–1127. [DOI] [PubMed] [Google Scholar]
- 27. Stanford MS, Mathias CW, Dougherty DM, et al. Fifty years of the barratt impulsiveness scale: an update and review. Pers Individ Differ. 2009;47(5):385–395. [Google Scholar]
- 28. Hughes JR, Hatsukami D.. Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry. 1986;43(3):289–294. [DOI] [PubMed] [Google Scholar]
- 29. Cox LS, Tiffany ST, Christen AG.. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine Tob Res. 2001;3(1):7–16. [DOI] [PubMed] [Google Scholar]
- 30. Spielberger CD. State-trait Anxiety Inventory for Adults. Washington, DC: APA PsycTests; 1983. [Google Scholar]
- 31. Chen Y, Ide JS, Li CS, et al. Gray matter volumetric correlates of dimensional impulsivity traits in children: sex differences and heritability. Hum Brain Mapp. 2022;43(8):2634–2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chen Y, Li C-SR.. Striatal gray matter volumes, externalizing traits, and N-back task performance: An exploratory study of sex differences using the human connectome project data. J Exp Psychopathol. 2022;13(1):20438087221080057. [Google Scholar]
- 33. Zar JH. Biostatistical Analysis. India: Pearson Education; 1999. [Google Scholar]
- 34. Gaser C, Dahnke R.. CAT-a computational anatomy toolbox for the analysis of structural MRI data. HBM. 2016;2016:336–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chen Y, Chaudhary S, Wang W, Li C-SR.. Gray matter volumes of the insula and anterior cingulate cortex and their dysfunctional roles in cigarette smoking. Addict Neurosci. 2021;(1):100003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ide JS, Li HT, Chen Y, et al. Gray matter volumetric correlates of behavioral activation and inhibition system traits in children: an exploratory voxel-based morphometry study of the ABCD project data. Neuroimage. 2020;220:117085. doi: 10.1016/j.neuroimage.2020.117085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chen Y, Li G, Ide JS, Luo X, Li C-SR.. Sex differences in attention deficit hyperactivity symptom severity and functional connectivity of the dorsal striatum in young adults. Neuroimage Rep. 2021;1(2):100025. [Google Scholar]
- 38. Poldrack RA, Fletcher PC, Henson RN, et al. Guidelines for reporting an fMRI study. Neuroimage. 2008;40(2):409–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kleber B, Veit R, Moll CV, et al. Voxel-based morphometry in opera singers: increased gray-matter volume in right somatosensory and auditory cortices. Neuroimage. 2016;133:477–483. [DOI] [PubMed] [Google Scholar]
- 40. Lehmann VE, Kenny PJ.. Hippocampal plasticity may drive cocaine relapse. Proc Natl Acad Sci USA. 2020;117(48):30003–30005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Werner CT, Mitra S, Auerbach BD, et al. Neuroadaptations in the dorsal hippocampus underlie cocaine seeking during prolonged abstinence. Proc Natl Acad Sci USA. 2020;117(42):26460–26469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Adhikari A, Topiwala MA, Gordon JA.. Synchronized activity between the ventral hippocampus and the medial prefrontal cortex during anxiety. Neuron. 2010;65(2):257–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Vachon-Presseau E, Roy M, Martel MO, et al. The stress model of chronic pain: evidence from basal cortisol and hippocampal structure and function in humans. Brain. 2013;136(Pt 3):815–827. [DOI] [PubMed] [Google Scholar]
- 44. Morrell HE, Cohen LM, al’Absi M.. Physiological and psychological symptoms and predictors in early nicotine withdrawal. Pharmacol Biochem Behav. 2008;89(3):272–278. [DOI] [PubMed] [Google Scholar]
- 45. Khalsa SS, Rudrauf D, Feinstein JS, Tranel D.. The pathways of interoceptive awareness. Nat Neurosci. 2009;12(12):1494–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Straube T, Miltner WH.. Attention to aversive emotion and specific activation of the right insula and right somatosensory cortex. Neuroimage. 2011;54(3):2534–2538. [DOI] [PubMed] [Google Scholar]
- 47. Mayer EA, Gupta A, Kilpatrick LA, Hong JY.. Imaging brain mechanisms in chronic visceral pain. Pain. 2015;156(Suppl 1):S50–S63. doi: 10.1097/j.pain.0000000000000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wei D, Du X, Li W, et al. Regional gray matter volume and anxiety-related traits interact to predict somatic complaints in a non-clinical sample. Soc Cogn Affect Neurosci. 2015;10(1):122–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ashare RL, Falcone M, Lerman C.. Cognitive function during nicotine withdrawal: implications for nicotine dependence treatment. Neuropharmacology. 2014;76(Pt B):581–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mitchell SH. Effects of short-term nicotine deprivation on decision-making: delay, uncertainty and effort discounting. Nicotine Tob Res. 2004;6(5):819–828. [DOI] [PubMed] [Google Scholar]
- 51. Cooke DF, Graziano MS.. Sensorimotor integration in the precentral gyrus: polysensory neurons and defensive movements. J Neurophysiol. 2004;91(4):1648–1660. [DOI] [PubMed] [Google Scholar]
- 52. Schilling C, Kuhn S, Romanowski A, et al. Cortical thickness correlates with impulsiveness in healthy adults. Neuroimage. 2012;59(1):824–830. [DOI] [PubMed] [Google Scholar]
- 53. Gavazzi G, Rossi A, Orsolini S, et al. Impulsivity trait and proactive cognitive control: an fMRI study. Eur J Neurosci. 2019;49(9):1171–1179. [DOI] [PubMed] [Google Scholar]
- 54. Culbertson CS, Bramen J, Cohen MS, et al. Effect of bupropion treatment on brain activation induced by cigarette-related cues in smokers. Arch Gen Psychiatry. 2011;68(5):505–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Li X, Semenova S, D’Souza MS, Stoker AK, Markou A.. Involvement of glutamatergic and GABAergic systems in nicotine dependence: implications for novel pharmacotherapies for smoking cessation. Neuropharmacology. 2014;76(Pt B):554–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Abulseoud OA, Ross TJ, Nam HW, et al. Short-term nicotine deprivation alters dorsal anterior cingulate glutamate concentration and concomitant cingulate-cortical functional connectivity. Neuropsychopharmacology. 2020;45(11):1920–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Moeller SJ, London ED, Northoff G.. Neuroimaging markers of glutamatergic and GABAergic systems in drug addiction: relationships to resting-state functional connectivity. Neurosci Biobehav Rev. 2016;61:35–52. doi: 10.1016/j.neubiorev.2015.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Regenold WT. Lithium and increased hippocampal volume-more tissue or more water? Neuropsychopharmacology. 2008;33(7):1773–4; author reply 1775. doi: 10.1038/sj.npp.1301524 [DOI] [PubMed] [Google Scholar]
- 59. Streitburger DP, Moller HE, Tittgemeyer M, et al. Investigating structural brain changes of dehydration using voxel-based morphometry. PLoS One. 2012;7(8):e44195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Franklin TR, Wetherill RR, Jagannathan K, et al. Limitations of the use of the MP-RAGE to identify neural changes in the brain: recent cigarette smoking alters gray matter indices in the striatum. Front Hum Neurosci. 2014;8:1052. doi: 10.3389/fnhum.2014.01052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Franklin TR, Wang Z, Shin J, et al. A VBM study demonstrating “apparent” effects of a single dose of medication on T1-weighted MRIs. Brain Struct Funct. 2013;218(1):97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data shown this study will be shared on request to the corresponding author. MATLAB codes will be shared upon request.