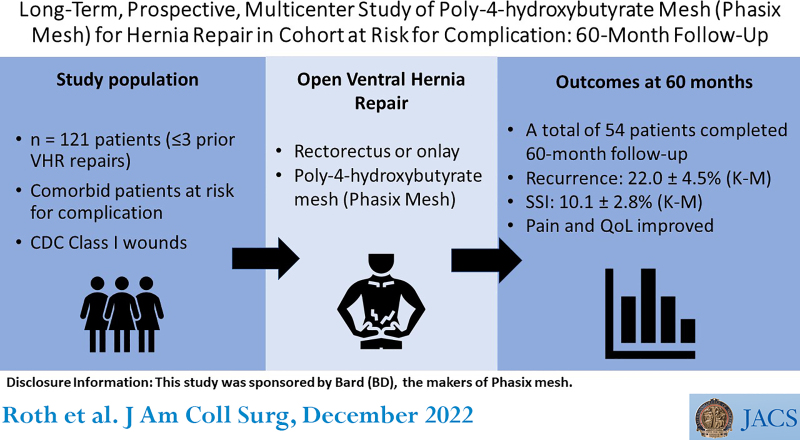
BACKGROUND:
Long-term resorbable mesh represents a promising technology for ventral and incisional hernia repair (VIHR). This study evaluates poly-4-hydroxybutyrate mesh (P4HB; Phasix Mesh) among comorbid patients with CDC class I wounds.
STUDY DESIGN:
This prospective, multi-institutional study evaluated P4HB VIHR in comorbid patients with CDC class I wounds. Primary outcomes included hernia recurrence and surgical site infection. Secondary outcomes included pain, device-related adverse events, quality of life, reoperation, procedure time, and length of stay. Evaluations were scheduled at 1, 3, 6, 12, 18, 24, 30, 36, and 60 months. A time-to-event analysis (Kaplan-Meier) was performed for primary outcomes; secondary outcomes were reported as descriptive statistics.
RESULTS:
A total of 121 patients (46 male, 75 female) 54.7 ± 12.0 years old with a BMI of 32.2 ± 4.5 kg/m2 underwent VIHR with P4HB Mesh (mean ± SD). Fifty-four patients (44.6%) completed the 60-month follow-up. Primary outcomes (Kaplan-Meier estimates at 60 months) included recurrence (22.0 ± 4.5%; 95% CI 11.7% to 29.4%) and surgical site infection (10.1 ± 2.8%; 95% CI 3.3 to 14.0). Secondary outcomes included seroma requiring intervention (n = 9), procedure time (167.9 ± 82.5 minutes), length of stay (5.3 ± 5.3 days), reoperation (18 of 121, 14.9%), visual analogue scale–pain (change from baseline –3.16 ± 3.35 cm at 60 months; n = 52), and Carolinas Comfort Total Score (change from baseline –24.3 ± 21.4 at 60 months; n = 52).
CONCLUSIONS:
Five-year outcomes after VIHR with P4HB mesh were associated with infrequent complications and durable hernia repair outcomes. This study provides a framework for anticipated long-term hernia repair outcomes when using P4HB mesh.
This prospective, multi-institutional study evaluated poly-4-hydroxybutyrate mesh for hernia repair in comorbid patients with CDC class I wounds. Outcomes included hernia recurrence, surgical site infection, pain, device-related adverse events, reoperation, quality of life, procedure time, and length of stay. Long-term (60-month) results showed infrequent complication and durable hernia repair.
Resorbable biomaterials have been used for abdominal wall reconstruction for decades, beginning with rapidly resorbing materials such as polyglactin 910 (a copolymer of glycolide and lactide) and polyglycolic acid.1,2 These materials resorb rapidly and lose mechanical strength and have been used primarily for temporary abdominal closure or other staged repairs rather than long-term hernia repair.1,2 More recently, hernia meshes comprised of resorbable polymers such as polyglycolide, polylactide, trimethylene carbonate, ultra-pure fibroin derived from silk, and poly-4-hydroxybutyrate (P4HB) have been used in abdominal wall reconstruction.1 These materials have longer resorption periods ranging from 4 to 36 months. Phasix Mesh (comprised of P4HB) offers one of the longest resorption periods and is essentially resorbed in 12 to 18 months.1,3,4 In hernia repair, a long-term resorption profile represents a unique advantage, allowing the mesh to provide mechanical support to the repair site during the critical period of healing and tissue remodeling, preventing early recurrence, and gradually transferring the load back to the abdominal wall.
Several clinical studies have reported outcomes associated with P4HB mesh with a variety of hernia repair techniques and patient populations.5-14 The majority of these studies have reported medium-term outcomes in the range of 18 to 36 months.5-13 The current study is uniquely designed to evaluate clinical outcomes of P4HB mesh along a continuum of early, intermediate, and long-term timeframes. Early and intermediate data have been published previously.5,6 The current study provides critical insight into long-term clinical outcomes such as hernia recurrence at 60 months after implementation. The strength of the repair at 60 months after implementation is solely dependent on the strength of the patient’s native abdominal wall tissue, as well as any tissue regenerated at the repair site over time. The objective of the current study is to evaluate long-term clinical outcomes of ventral and incisional hernia repair with P4HB mesh among patients at risk for postoperative complications.
METHODS
The methods for this trial have been described in previous publications and are repeated here for clarity.5,6
Study design
This study represents a prospective, multicenter, open-label study to assess the safety, performance, and outcomes of poly-4-hydroxybutyrate mesh (Phasix Mesh, CR Bard, Inc) for primary ventral or incisional or multiply recurrent hernia repair in a cohort at risk for complications. This study has been registered with ClinicalTrials.gov (ClinicalTrials.gov/NCT01961687).
Patients were considered at risk for complications with 1 or more of the following comorbidities: BMI between 30 and 40 kg/m2 (inclusive), active smokers, COPD, diabetes mellitus, immunosuppression, coronary artery disease, chronic corticosteroid use (more than 6 months of systemic use), hypo-albuminemia (preoperative serum albumin less than 3.4 g/dL), advanced age (75 years or more), or renal insufficiency (serum creatinine concentration 2.5 mg/dL or more). Patients, investigators, and surgeons were not blinded to study treatment. The study was designed to treat 120 patients at 16 US sites. The protocol was approved by the IRB at each institution, and all patients provided informed consent before enrollment. Recruitment occurred through the surgical offices of the investigators during the period from October 2013 to January 2015. Investigators were selected based on experience with hernia repair techniques. No specific training was required for participation due to the similarity in technique required for P4HB mesh relative to other meshes.
Inclusion/exclusion criteria
Patients 18 years or older, with primary ventral, primary incisional, or recurrent incisional hernia (not to exceed 3 recurrences), were evaluated for eligibility, including 1 or more comorbidities listed here, class I surgical wound (defined by the CDC),15 and 10 to 350 cm2 hernia defect suitable for repair via retrorectus or onlay mesh (with or without myofascial release [MR]). Exclusion criteria included 4 or more previous hernia repairs (of the index repair), peritonitis, on or anticipated to be placed on chemotherapy during study period, BMI greater than 40 kg/m2, cirrhosis of the liver and/or ascites, American Society of Anesthesiology class IV or V, diagnosed HIV infection, life expectancy of less than 2 years at time of enrollment, planned intra-abdominal mesh placement or bridged repair, surgical wound designated class II (clean-contaminated), class III (contaminated), or class IV (dirty-contaminated) defined by the CDC15 (no device is currently indicated for use in contaminated or infected fields), active or latent systemic infection, pregnant or plans to become pregnant during study period, currently breastfeeding, enrolled in another clinical study within the last 30 days, part of site personnel directly involved with study, known allergy to the test device or component materials, or any condition that, in the opinion of the investigator, would preclude the use of the study device, or preclude the patient from completing the follow-up requirements.
Surgical technique
All patients were administered antibiotics according to hospital protocol and underwent open ventral hernia repair. Intraoperative inclusion and exclusion criteria were assessed and documented. Patients meeting intraoperative eligibility criteria received P4HB mesh, overlapping the defect by at least 5 cm with 6 to 12 resorbable sutures placed at approximately 5- to 6-cm intervals around the periphery. It is important to note that “5-cm overlap” encompasses overlap both cranial and caudal to the defect for a total of 5 cm longitudinally, rather than 5 cm cranially and 5 cm caudally, which would lead to 10 cm overlap overall. The same definition of overlap applies to the width dimension. The hernia defect was closed by approximating the fascial edges, including myofascial release, if required. The fascial and subcutaneous layers were closed with sutures, and the skin was closed with staples and/or sutures. Operative details including hernia defect size, mesh size, mesh position, repair technique, use of myofascial release, suture type, number of sutures to secure mesh, and procedural time were collected. Postoperative care was performed consistent with surgeon practice at each site.
Data collection
Postoperative patient visits were scheduled at 1, 3, 6, 12, 18, 24, 36, and 60 months, and a telephone interview was conducted at 30 months. Medical history, demographic information, and all current prescription and over-the-counter pain medications were recorded. The Pain Visual Analogue Scale (VAS) and quality-of-life assessments—Carolinas Comfort Scale and 12-Item Short Form Health Survey, version 2 (SF-12v2)—were completed preoperatively and at scheduled intervals.
Study endpoints
Primary endpoints included hernia recurrence and surgical site infections (SSI). Hernia recurrence was assessed by physical examination at each study visit. A recurrent hernia was defined as any hernia identified or confirmed by the investigator, during any study follow-up visit, within 7 cm of the repair. Hernia recurrence identified via incidental MRI or CT scan were evaluated by the operating surgeon for clinical significance and confirmation.
SSI was assessed by physical examination with confirmation by gram stain and culture. Superficial and deep SSI were classified according to CDC guidelines.16 Device-related complications and reoperations were also recorded.
Analysis population
Data are presented for all patients implanted with P4HB mesh. GraphPad Prism 6.01 statistical software was used to generate frequency counts and percentages (categorical variables) and mean ± SD (continuous variables). A time-to-event analysis (Kaplan-Meier) was performed for primary outcomes. Patients who did not complete the study were censored at the last available follow-up time. Fisher’s exact 2-tailed test was used to evaluate differences between various subgroups in analyses (p < 0.05 was considered statistically significant; no adjustment was made for multiple comparisons). All subgroup analyses were intended to be exploratory in nature and hypothesis-generating to inform the design of future trials. A paired, nonparametric Wilcoxon signed-rank test was performed to compare quality-of-life data at 60 months with baseline values (p < 0.05 was considered statistically significant). This work complies with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) criteria.17
RESULTS
Patient demographics
As shown in Figure 1 and Table 1, a total of 139 patients met the initial screening criteria for the study. Eighteen patients were ultimately excluded, and 121 patients were implanted with P4HB mesh.
Figure 1.
Flow of patients throughout the study period. P4HB, poly-4-hydroxybutyrate.
Table 1.
Preoperative Data: Patient Demographics and Hernia Data
| Demographic and hernia characteristic | Data |
|---|---|
| Patient treated with P4HB mesh, n | 121 |
| Patients with 5-year follow-up, n (%) | 54 (44.6) |
| Sex, n (%) | |
| Male | 46 (38) |
| Female | 75 (62) |
| Ethnicity, n (%) | |
| Not Hispanic or Latino | 113 (93.4) |
| Hispanic or Latino | 8 (6.6) |
| Race, n (%) | |
| Black | 5 (4.1) |
| White | 116 (95.9) |
| Age, y, mean ± SD | 54.7 ± 12.0 |
| BMI, kg/m2, mean ± SD | 32.2 ± 4.5 |
| Patient with previous abdominal incision, n (%) | 117 (96.7) |
| No. of previous incisions, mean ± SD | 3.0 ± 2.2 |
| Previous incision at current hernia site | 62 (51.2) |
| No. of comorbidities, n (%) | |
| 1 | 42 (34.7) |
| 2 | 45 (37.2) |
| 3 | 25 (20.7) |
| ≥4 | 9 (7.4) |
| Comorbidity, n (%) | |
| Obesity | 95 (78.5) |
| COPD | 34 (28.1) |
| Coronary artery disease | 26 (21.5) |
| Diabetes | 40 (33.1) |
| Active smoker | 28 (23.1) |
| Immunosuppressed | 10 (8.3) |
| Chronic corticosteroid use | 6 (5.0) |
| Hypoalbuminemia | 3 (2.5) |
| Advanced age | 6 (5.0) |
| Renal insufficiency | 1 (0.8) |
| Hernia diagnosis, n (%) | |
| Primary ventral | 17 (14.0) |
| Primary incisional | 54 (44.6) |
| Recurrent ventral | 15 (12.4) |
| Recurrent incisional | 35 (28.9) |
| Hernia location, n (%) | |
| Midline | 102 (84.3) |
| Suprapubic | 5 (4.1) |
| Subxiphoid | 3 (2.5) |
| Other | 11 (9.1) |
P4HB, poly-4-hydroxybutyrate.
The majority of patients were non-Hispanic/Latino, White, females with a mean ± SD age of 54.7 ± 12.0 years and BMI of 32.2 ± 4.5 kg/m2. Nearly all of the patients (n = 117, 96.7%) had a history of abdominal incisions(s). Previous incisions were located at the current hernia site in approximately half of the patients (n = 62, 51.2%). Patient-reported comorbidities included obesity, diabetes, COPD, active smoker, coronary artery disease, immunosuppression, advanced age, chronic corticosteroid use, hypoalbuminemia, and renal insufficiency, which are reported in Table 1. Approximately two-thirds of the patients reported 2 or more comorbidities (n = 79, 65.3%).
Preoperative data
Hernias were divided between primary hernias (n = 71, 58.7%; Table 1) and recurrent hernias (n = 50, 41.3%). The majority of the hernias were located in the midline (n = 102, 84.3%), with a small number of suprapubic, subxiphoid, or “other” locations (ie superior to umbilicus, midline to suprapubic, lower abdomen, subcostal, flank, paramedian, and midline to subxiphoid).
Operative characteristics
Detailed operative data are shown in Table 2. The majority of patients underwent ventral hernia repair with P4HB mesh via retrorectus repair (n = 88, 72.7%), with a mean defect length of 14.7 ± 5.6 cm, width of 8.6 ± 3.4 cm, and area of 108.2 ± 68.2 cm2. Approximately one-third of the defects were classified as “Swiss cheese” defects (n = 39, 32.2%). In patients undergoing retrorectus repair, 45 used MR, with the majority undergoing posterior (n = 26) or Ramirez/open repair (n = 15) and only a few open/endoscopic (n = 2) or endoscopic/minimally invasive (n = 2) repairs. In those patients undergoing retrorectus repair without MR, the majority underwent Rives-Stoppa repair (n = 41 of 43). In patients undergoing onlay repairs, the majority did not require MR (n = 24), and a few (n = 8) used MR in a Ramirez/open fashion. Additionally, a single patient (n = 1, 0.8%) underwent open Rives-Stoppa repair of a right flank hernia and was not classified as retrorectus or onlay, but rather as “other.”
Table 2.
Perioperative Data: Mesh/Defect Size and Operative Technique
| Mesh/defect size and operative technique | Data |
|---|---|
| Defect, cm, mean ± SD | |
| Length | 14.7 ± 5.6 |
| Width | 8.6 ± 3.4 |
| Mesh, cm, mean ± SD | |
| Length | 26.4 ± 5.1 |
| Width | 21.3 ± 4.9 |
| Fixation, n (%) | |
| Suture | 116 (95.9) |
| Mechanical | 4 (3.3) |
| Unknown | 1 (0.8) |
| Fascia reapproximated, n (%) | 113 (93.4) |
| Operative technique, n (%) | |
| Retrorectus | 43 (35.5) |
| Retrorectus with MR | 45 (37.2) |
| Onlay | 24 (19.8) |
| Onlay with MR | 8 (6.6) |
| Other | 1 (0.8) |
| Negative pressure wound therapy, n (%) | 13 (10.7) |
| No. of drains, n (%) | 107 (88.4) |
| 0 | 14 (11.6) |
| 1 | 30 (24.8) |
| 2 | 60 (49.6) |
| ≥3 | 17 (14.0) |
MR, myofascial release.
The fascia was reapproximated in 113 (93.4%) patients, and P4HB mesh with a mean length (26.4 ± 5.1 cm), width (21.3 ± 4.9 cm), and area (461.7 ± 173.5 cm2) was used to reinforce the repair in all treated patients (n = 121, 100%). P4HB mesh was fixated to the abdominal wall using a mean of 12.5 ± 13.5 fixation points. Nearly all fixation was accomplished with sutures (n = 116, 95.9%), but a small number of patients received mechanical or other types of fixation. Almost all of the patients (n = 107, 88.4%) required at least 1 drain, and the longest overall drain duration was 11.9 ± 8.9 days. The average surgical procedure time was 167.9 ± 82.5 min (mean ± SD, Table 3). Only a small percentage of patients required negative pressure wound therapy (n = 13, 10.7%), and the majority of those wounds were open (n = 10 of 13). A single patient (n = 1) had both an open and a closed wound that required negative pressure wound therapy. The average length of stay was 5.3 ± 5.3 days (mean ± SD, Table 3).
Table 3.
Study Endpoints: Primary and Secondary
| Study endpoint | Data |
|---|---|
| Primary, Kaplan-Meier at 60 mo, %, mean ± SD (95% CI) | |
| Recurrence, n = 20 | 22.0 ± 4.5 (11.7–29.4) |
| Surgical site infection, n = 12 | 10.1 ± 2.8 (3.3–14.0) |
| Secondary | |
| Surgical procedure time, min, mean ± SD | 167.9 ± 82.5 |
| Length of stay, d, mean ± SD | 5.3 ± 5.3 |
| Recurrence by hernia type, n (%) | |
| Primary ventral | 1 (5.9) |
| Primary incisional | 10 (18.5) |
| Recurrent ventral | 3 (20.0) |
| Recurrent incisional | 6 (17.1) |
| Reoperation rate, n (%) | 18 (14.9) |
| Time to reoperation, d, mean ± SD | 700.4 ± 553.2 |
Postoperative outcomes
A total of 54 (44.6%) patients completed the study at the 60-month follow-up visit (Fig. 1 and Table 1). The majority of the patients who did not complete the study were lost to follow-up after 3 attempts to contact them (n = 19). Another group of patients withdrew consent or chose to end their participation when the study period was extended (n = 12). Similarly, several study sites elected not to extend the study beyond the originally planned 24-month final time point, which removed an additional 6 patients from later follow-up.
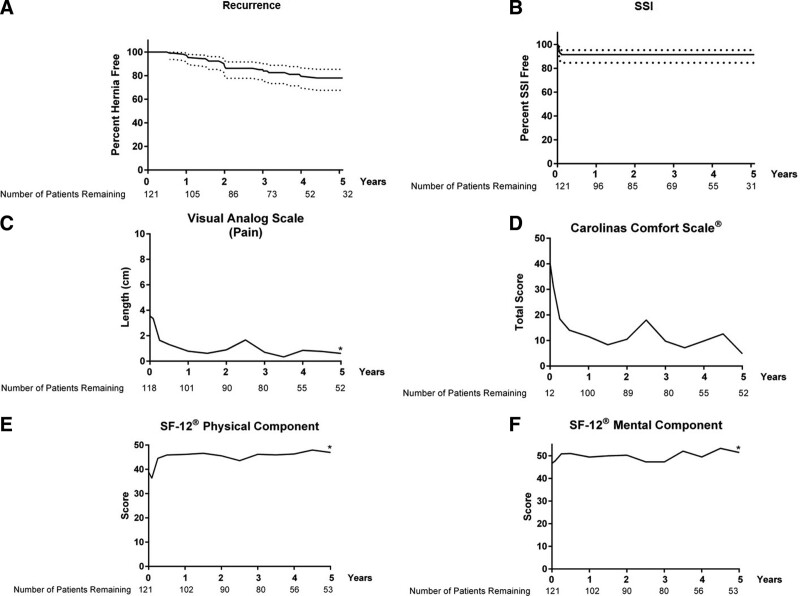
The primary outcomes of recurrence and SSI are summarized in Figure 2 and Table 3. Using Kaplan-Meier methods to account for censoring, the 60-month recurrence rates were estimated to be 22.0% ± 4.5% (mean ± SD; 95% CI 11.7% to 29.4%) for all patients treated with P4HB mesh. The number of patients remaining in the trial at each time interval is noted below the x-axis. On average, the time to recurrence was 771.6 ± 397.1 days (mean ± SD). A small number of patients experienced SSI. Again, using Kaplan-Meier methods to account for censoring, the 60-month SSI rates were estimated to be 10.1% ± 2.8% (mean ± SD; 95% CI 3.3% to 14.0%) for all patients treated with P4HB mesh. The average time to SSI was 15.2 ± 9.3 days (mean ± SD), and no SSIs were reported 37 days after implementation.
Figure 2.
Primary and secondary study endpoints: (A and B) Kaplan-Meier curves for hernia recurrence and surgical site infection (SSI) in all patients treated with poly-4-hydroxybutyrate mesh (dotted lines = 95% CI; there were no additional SSI after 37 days). (C) Visual Analogue Scale for Pain (mean). (D) Carolinas Comfort Scale–Total Score (mean). (E) SF-12 Physical Component Score (mean). (F) SF-12 Mental Component Score (mean). *p < 0.05 (baseline vs 60 months).
Secondary endpoints included surgical procedure time, length of stay, reoperation rate, seroma requiring intervention, device-related adverse events, and quality-of-life assessments and are depicted in Figure 2 and Tables 3 and 4. Out of a total of 20 hernia recurrences, slightly more than half required reoperation (n = 12), and 8 required no further action. An additional 6 patients required reoperation due to SSI, intestinal perforation, hematoma, nontherapeutic laparotomy, or fat necrosis. P4HB mesh was explanted at the time of reoperation in 4 patients. A single patient (1) underwent 2 reoperation procedures. The first was debridement of skin and subcutaneous tissue necrosis at 28 days after implementation, and the second reoperation occurred at 1,023 days after implementation for a hernia recurrence. A total of 9 patients experienced a seroma that required intervention, occurring an average of 175.5 ± 382.3 days after implementation (mean ± SD). Device-related adverse events were ascribed by the individual investigators according to their judgment. Some investigators qualified recurrence as device-related, and others did not. Because recurrence is an expected possible outcome of hernia repair, recurrences were excluded from device-related adverse events in Table 3. There were a total of 8 device-related adverse events, with a small number of patients affected by seroma, deep incisional wound infection, diastasis, pyrexia, small bowel obstruction, or abdominal pain. Seroma and SSI that were apparent at the level of the mesh were ascribed by the investigator as “device-related” in contrast with those observed in a different tissue plane. The earliest reported adverse events averaged 634.2 ± 491.8 days after implementation (mean ± SD).
Table 4.
Quality-of-Life Metrics
| Quality-of-life metric | Baseline | 60-mo score | Change from baseline | |||
|---|---|---|---|---|---|---|
| n | Score | n | Score | p Value | ||
| Visual Analogue Scale: Pain, cm, mean ± SD | 118 | 3.5 ± 3.2 | 0.6 ± 1.7 | 52 | –3.16 ± 3.35 | <0.0001 |
| Carolinas Comfort Scale: Total Score, mean ± SD | 12 | 40.1 ± 28.6 | 4.9 ± 14.2 | 52 | –24.3 ± 21.4 | 0.50 |
| SF-12 Physical Component Score, mean ± SD | 121 | 38.9 ± 9.8 | 47.0 ± 9.6 | 53 | 7.4 ± 10.0 | <0.0001 |
| SF-12 Mental Component Score, mean ± SD | 121 | 46.7 ± 10.8 | 51.4 ± 9.7 | 53 | 3.2 ± 10.8 | 0.03 |
VAS for pain decreased significantly from 3.5 ± 3.2 before surgery (baseline) to 0.6 ± 1.7 at 60 months (p < 0.0001). Carolinas Comfort Scale–Total Score trended down, but did not reach statistical significance with a score of 40.1 ± 28.6 before surgery (baseline) and a score of 4.9 ± 14.2 at 60 months (p = 0.50). For the SF-12v2 scores, the Physical Component Score increased significantly from 38.9 ± 9.8 before surgery (baseline) to 47.0 ± 9.6 at 60 months (p < 0.0001). Similarly, the SF-12v2 Mental Component Score increased significantly from baseline (46.7 ± 10.8) to 60 months (51.4 ± 9.7, p = 0.03). Quality-of-life assessment values for all intermediate time points are depicted graphically in Figure 2.
In addition to the overall rates of recurrence and SSI, several subgroups were investigated using Fisher’s exact 2-tailed test (Table 5). Surgical technique and mesh-to-tissue overlap were the most remarkable results. Patients who underwent onlay repair experienced significantly greater risk of recurrence compared with those who underwent a retrorectus repair (p = 0.02). Additionally, patients with less than 5 cm or less than 10 cm mesh-to-tissue overlap in both length and width dimensions experienced greater risk of recurrence compared with patients who received larger pieces of P4HB mesh. Hernia-related complications were graded according to the Clavien-Dindo classification system (Supplemental Digital Content 1, http://links.lww.com/JACS/A134).18 There were no grade IVa, IVb, or V complications and very few grade II, IIIa, or IIIb complications. There were several grade I complications, with the majority attributed to pain/tenderness, abdominal wound dehiscence, hematoma, and seroma.
Table 5.
Raw Percentages and Fisher’s Exact Test p Values for Subanalyses of Primary Outcomes
| Characteristic | Recurrence rate | SSI | ||||
|---|---|---|---|---|---|---|
| n/N | Calculated raw percentage | Fisher’s exact test 2-tailed p Value | n/N | Calculated raw percentage | Fisher’s exact test 2-tailed p Value | |
| All patients | 20/121 | 16.5 | n/a | 12/121 | 9.9 | n/a |
| Sex | 0.61 | 0.03 * | ||||
| Male | 9/46 | 19.6 | 1/46 | 2.2 | ||
| Female | 11/75 | 14.7 | 11/75 | 14.7 | ||
| No. of comorbidities | >0.99 | 0.06 | ||||
| 1 | 7/42 | 16.7 | 1/42 | 2.4 | ||
| ≥2 | 13/79 | 16.5 | 11/79 | 13.9 | ||
| Operative technique | >0.99 | 0.36 | ||||
| With MR† | 9/53 | 17.0 | 7/53 | 13.2 | ||
| Without MR† | 11/67 | 16.4 | 5/67 | 7.5 | ||
| Retrorectus vs onlay | 0.02* | >0.99 | ||||
| Retrorectus† | 10/88 | 11.4 | 9/88 | 10.2 | ||
| Onlay† | 10/32 | 31.3 | 3/32 | 9.4 | ||
| Total overlap in length and width | 0.04* | 0.13 | ||||
| <10 cm | 13/53 | 24.5 | 8/53 | 15.1 | ||
| ≥10 cm | 7/68 | 10.3 | 4/68 | 5.9 | ||
| Total overlap in length and width | 0.005* | 0.69 | ||||
| <5 cm | 8/20 | 40.0 | 1/20 | 5.0 | ||
| ≥5 cm | 12/101 | 11.9 | 11/101 | 10.9 | ||
| SSI | >0.99 | n/a | ||||
| Patients with SSI | 2/12 | 16.7 | 12/12 | 100.0 | ||
| Patients without SSI | 18/109 | 16.5 | 0/109 | 0.0 | ||
p < 0.05.
†n = 120.
MR, myofascial release; n/a, not applicable; SSI, surgical site infection.
DISCUSSION
The long-term evaluation of hernia repair outcomes in the US creates many challenges due to the healthcare delivery system and migratory population. This study represents unique long-term insight into the outcomes of ventral hernia repair 5 years after repair. Very few studies report on outcomes with a 5-year duration of follow-up, yet even fewer studies are prospective. This study highlights the challenges with hernia follow-up in that approximately 50% of the patients were evaluated at 5 years. Nevertheless, this study provides important insight into outcomes among those undergoing repair with an absorbable P4HB mesh at a timeframe when the mesh is no longer providing any contribution to the strength of the repair. Approximately 4 of 5 patients were free of hernia at the conclusion of the study. Among the 20 known recurrences, 12 patients underwent reoperation for hernia recurrence. These results are consistent with long-term recurrence19 and reoperation20 rates reported with synthetic mesh. This study compares well with another study reporting on long-term outcomes with P4HB mesh.21 In a retrospective series of 31 patients with a median follow-up of 98 months, recurrences after P4HB repairs occurred in 12.9% with a 10% reoperation rate. Additionally, the current study provides insight into long-term complications associated with mesh. No mesh-related complications were identified beyond the early postoperative period, and none of the patients developed mesh infection or mesh-related complications throughout the entirety of the study period. Due to the relatively low number of patients completing the 60-month follow-up, the ability to draw definitive conclusions about long-term mesh-related complications is limited. However, the observations of the lack of long-term mesh-related complications are an important finding in an era in which the drawbacks of mesh have called into question the benefits of mesh use.
The current study provides insight into the risk for both mesh complications and recurrence through the use of fascial approximation with reinforcement with a P4HB absorbable mesh. Further work is required to delineate the best populations for each type of repair and mesh, but an absorbable mesh provides a high likelihood of long-term success in selected patients. Future hernia research should focus on patient factors beyond comorbidities that may help delineate the need for permanent mesh as opposed to absorbable mesh during ventral hernia repair.
The concept of an absorbable mesh is not without precedent. In fact, a study involving another absorbable mesh product composed of polyglycolide-trimethylene carbonate demonstrated 17% recurrence and 18% SSI 24 months after retrorectus or intraperitoneal implantation in clean-contaminated or contaminated hernias.22 However, the current study provides even greater insight into long-term outcomes with 60-month follow-up. Future prospective randomized trials will be necessary to delineate the risks and benefits of absorbable vs synthetic mesh.
Independent of mesh type, this study highlights variability in outcomes related to technique. Similar to other studies,23 patients undergoing preperitoneal or retrorectus repairs experienced fewer recurrences than those undergoing an onlay repair. Although this should not imply that onlay repairs should be abandoned, use of a retrorectus technique is likely to reduce the risk for hernia recurrence in absorbable mesh hernia repairs.
Additional factors beyond mesh placement such as degree of overlap and mesh-to-defect ratio similarly impact hernia outcomes. The placement of large meshes with significant overlap beyond the defects of a hernia is accepted as surgical dogma. This study has demonstrated the impact of limited overlap on hernia recurrence rates. Patients with less than 10 cm of total mesh overlap relative to defect in the longitudinal access (5 cm on either side) had a 3-fold increase in hernia recurrence rates, which was even greater for patients with less than 5 cm of total longitudinal mesh overlap (2.5 cm on either side). Interestingly, lateral mesh overlap less than 10 cm overall did not impact hernia recurrence rates, likely related to the offloading of midline tension associated with the hernia dissection. This suggests that the placement of mesh that extends both superior and inferior to the incision is an important aspect of hernia repair and may be a significant driver for hernia recurrences. Interestingly, among the onlay repairs in the study, many patients did not have mesh overlap extending above and below the defect. This might explain the increased recurrence rate reported in the onlay group.
Although the study was not powered to evaluate the impact of SSI on recurrence, those with postoperative SSI did not experience increased long-term hernia recurrence rates, contrary to published outcomes with synthetic mesh.24 An unexpected finding was the reduced incidence of SSI rates in male patients compared with female patients. In the subanalyses, we were unable to delineate a rationale for this difference based on technique or patient characteristics. Despite this finding, sex did not impact hernia recurrence rates. Female sex has been previously reported to be associated with increased risk for poor outcomes in ventral hernia repair independent of comorbid conditions.25 Whether this difference is a result of chance, an unstudied variable, or sex alone will require future investigation.
Limitations of this study include the fact that this is not a randomized trial. However, it is a prospective trial with 5-year follow-up. Long-term follow-up is always a challenge with studies like this, especially in the US with multiple insurers and a lack of standardized health records. We also acknowledge the limitations of our statistical analyses, namely that the current study was not powered to detect differences between subgroups of interest. Nevertheless, we found these initial subgroup analyses interesting and provocative, and future investigation is warranted. Additionally, this study did not use the minimal clinically important difference (MCID) score to contextualize the results and differentiate between “statistically significant” and “clinically relevant” outcomes, particularly as they relate to patient-centered outcomes such as quality-of-life metrics. Although the published literature reports MCID for VAS and SF-12 v.2 in spinal procedures, caution should be exercised in using MCID generated for a different disease state. Several published studies also report MCID for hernia-related quality-of-life metrics (Hernia-related Quality-of-Life survey and modified Activities Assesment Scale ), but none report on VAS, Carolinas Comfort Scale, and SF-12 in a hernia population. Future studies are certainly warranted to generate MCID for more outcomes associated specifically with abdominal wall reconstruction.
CONCLUSIONS
Five-year outcomes after ventral and incisional hernia repair with P4HB mesh are associated with infrequent complications and durable hernia repair outcomes. Although limited by the extended study duration and associated loss to follow-up, this study provides a framework for anticipated long-term hernia repair outcomes when using P4HB mesh.
Author Contributions
Study conception and design: All authors
Acquisition of data: All authors
Analysis and interpretation of data: Deeken, Badhwar, Roth
Drafting of manuscript: Deeken, Roth
Critical revision: Badhwar, Deeken, Roth
Supplementary Material
Abbreviations and Acronyms
- MCID =
- minimal clinically important change
- MR =
- myofascial release
- P4HB =
- poly-4-hydroxybutyrate
- SF-12v2 =
- 12-Item Short Form Health Survey, version 2
- SSI =
- surgical site infection
- VAS =
- Visual Analogue Scale
Disclosure Information: This study was sponsored by Becton Dickinson (BD). Authors were reimbursed for expenses related to the conduct of the study.
Disclosures outside the scope of this work: Dr Roth’s institute received grant funding from BD during the study period and receives grant funding from Advanced Medical Solutions. Dr Roth is a paid consultant to Johnson & Johnson and BD, holds stock in Miromatrix, and receives speaker fees from Allergan. Dr Selzer’s institute received grant funding from Becton Dickinson. Dr Selzer is a paid consultant to Cook Biotechnology and Becton Dickinson, and was a paid consultant to PolyNovo, Inc. Dr Poulose receives research funding from Advanced Medical Solutions and BD, is a paid consultant to Ethicon, and is the majority owner of EndoEvolve, LLC. Dr Goldblatt is a paid consultant to WL Gore, Medtronic, and Allergan. Dr Goldblatt’s institute receives research funding from WL Gore and Medtronic. Dr Deeken is a paid consultant to Johnson & Johnson, Medtronic, SurgiMatrix, Tissium, Surgical Innovation Associates, Americas Hernia Society Quality Collaborative, Colorado Therapeutics, TelaBio, and Aran Biomedical; is the owner of Covalent Bio, LLC; and holds the following issued patents: 2009293001, 2334257, 2,334,257UK, 602009046407.8, 2,334,257FR, 16/043,849, and 2,737,542. Dr Anthone receives consulting fees and speaker honoraria from Becton Dickinson. Dr Pierce receives research support from Intuitive Surgical Solutions, and his spouse is a salaried employee of CareFusion, which falls under the overall umbrella of the parent company, Becton Dickinson. Dr Bittner receives consulting/speaker fees and travel support from Cook Biotech, Inc, Intuitive Surgical, Inc, Becton Dickinson, and CMR Surgical Ltd. Dr Linn receives consultant/speaker fees from WL Gore. Dr Hope receives consultant fees, honoraria, and research support from Intuitive, WL Gore, and Medtronic, and honoraria payments from Becton Dickinson. Dr Martindale is a paid consultant to Allergan, CR Bard, Inc/Davol/Becton Dickinson, and Nestle. Dr Badhwar is an employee of Becton Dickinson. Dr Parra-Davila receives consultant/speaker fees from Bard, Inc/Davol/Becton Dickinson, Intuitive, Johnson & Johnson, and Medtronic; research funds from CR Bard, Inc/Davol/Becton Dickinson; and proctoring fees from Intuitive. Dr Earle is a paid consultant to Becton Dickinson and Medtronic and is a paid advisory board member at Via Surgical. Dr Mancini is a paid consultant to Stryker Endoscopy and receives speaker fees from Gore Medical and Medtronic. Dr Greenberg receives grant funding from Becton Dickinson and Medtronic, and course registration, travel, and lodging from Intuitive. Dr Romamelli is a paid consultant to New View Surgical. Dr Salluzzo’s institute receives grant funding from Davol, Inc. Dr Dunn receives grant funding from Becton Dickinson. Other authors have nothing to disclose.
Support: Drs Selzer and Poulose receive salary support from the Abdominal Core Health Quality Collaborative.
Presented as a poster at the Society of American Gastrointestinal and Endoscopic Surgeons 2019 Annual Meeting, Baltimore, MD, April 2019, and at the Americas Hernia Society 2021 Annual Meeting, Austin, TX, September 2021.
Supplemental digital content is available for this article.
REFERENCES
- 1.Deeken CR, Matthews BD. Characterization of the mechanical strength, resorption properties, and histologic characteristics of a fully absorbable material (poly-4-hydroxybutyrate-PHASIX Mesh) in a porcine model of hernia repair. ISRN Surg. 2013;2013:238067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dayton MT, Buchele BA, Shirazi SS, et al. Use of an absorbable mesh to repair contaminated abdominal-wall defects. Arch Surg. 1986;121:954–960. [DOI] [PubMed] [Google Scholar]
- 3.Martin DP, Badhwar A, Shah DV, et al. Characterization of poly-4-hydroxybutyrate mesh for hernia repair applications. J Surg Res. 2013;184:766–773. [DOI] [PubMed] [Google Scholar]
- 4.Instructions for Use - Phasix Mesh, C. R. Bard, Inc. (Warwick, RI). Available at: https://www.bd.com/en-us/products-and-solutions/products/product-families/phasix-mesh. Accessed June 5, 2022.
- 5.Roth JS, Anthone GJ, Selzer DJ, et al. Prospective evaluation of poly-4-hydroxybutyrate mesh in CDC class I/high-risk ventral and incisional hernia repair: 18-month follow-up. Surg Endosc. 2018;32:1929–1936. [DOI] [PubMed] [Google Scholar]
- 6.Roth JS, Anthone GJ, Selzer DJ, et al. Prospective, multicenter study of P4HB (Phasix™) mesh for hernia repair in cohort at risk for complications: 3-year follow-up. Ann Med Surg (Lond). 2021;61:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Plymale MA, Davenport DL, Dugan A, et al. Ventral hernia repair with poly-4-hydroxybutyrate mesh. Surg Endosc. 2018;32:1689–1694. [DOI] [PubMed] [Google Scholar]
- 8.Messa CA, 4th, Kozak G, Broach RB, et al. When the mesh goes away: an analysis of poly-4-hydroxybutyrate mesh for complex hernia repair. Plast Reconstr Surg Glob Open. 2019;7:e2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Christopher AN, Patel V, Othman S, et al. Onlay poly-4-hydroxybutyrate (P4HB) mesh for complex hernia: early clinical and patient reported outcomes. J Surg Res. 2021;264:199–207. [DOI] [PubMed] [Google Scholar]
- 10.Levy AS, Bernstein JL, Premaratne ID, et al. Poly-4-hydroxybutyrate (Phasix) mesh onlay in complex abdominal wall repair. Surg Endosc. 2021;35:2014–2058. [DOI] [PubMed] [Google Scholar]
- 11.Buell JF, Sigmon D, Ducoin C, et al. Initial experience with biologic polymer scaffold (poly-4-hydroxybuturate) in complex abdominal wall reconstruction. Ann Surg. 2017;266:185–188. [DOI] [PubMed] [Google Scholar]
- 12.Rognoni C, Cuccurullo D, Borsoi L, et al. Clinical outcomes and quality of life associated with the use of a biosynthetic mesh for complex ventral hernia repair: analysis of the “Italian Hernia Club” registry. Sci Rep. 2020;10:10706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pakula A, Skinner R. Outcomes of open complex ventral hernia repairs with retromuscular placement of poly-4-hydroxybutyrate bioabsorbable mesh. Surg Innov. 2020;27:32–37. [DOI] [PubMed] [Google Scholar]
- 14.Mellia JA, Othman S, Naga HI, et al. Outcomes of poly-4-hydroxybutyrate mesh in ventral hernia repair: a systematic review and pooled analysis. Plast Reconstr Surg Glob Open. 2020;8:e3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention ( 2017. Surgical site infection protocol. Available at: https://wwwcdcgov/nhsn/pdfs/pscmanual/9pscssicurrentpdf. Accessed February 15, 2017.
- 16.Mangram A, Horan T, Pearson M, et al. Guideline for prevention of surgical site infection, 1999. Am J Infect Control. 2014;20:250–280. [DOI] [PubMed] [Google Scholar]
- 17.von Elm E, Altman DG, Egger M, et al.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. [DOI] [PubMed] [Google Scholar]
- 18.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burger JW, Luijendijk RW, Hop WC, et al. Long-term follow-up of a randomized controlled trial of suture versus mesh repair of incisional hernia. Ann Surg. 2004;240:578–583; discussion 583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kokotovic D, Bisgaard T, Helgstrand F. Long-term recurrence and complications associated with elective incisional hernia repair. JAMA. 2016;316:1575–1582. [DOI] [PubMed] [Google Scholar]
- 21.Buell J, Flaris A, Raju S, Hauch A, et al. Long-term outcomes in complex abdominal wall reconstruction repaired with absorbable biologic polymer scaffold (poly-4-hydroxybutyrate). Ann Surg. 2021;1:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosen MJ, Bauer JJ, Harmaty M, et al. Multicenter, prospective, longitudinal study of the recurrence, surgical site infection, and quality of life after contaminated ventral hernia repair using biosynthetic absorbable mesh: the COBRA study. Ann Surg. 2017;265:205–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holihan JL, Nguyen DH, Nguyen MT, et al. Mesh location in open ventral hernia repair: a systematic review and network meta-analysis. World J Surg. 2016;40:89–99. [DOI] [PubMed] [Google Scholar]
- 24.Otero J, Huber AT, Heniford BT. Laparoscopic hernia repair. Adv Surg. 2019;53:1–19. [DOI] [PubMed] [Google Scholar]
- 25.Schlosser KA, Maloney SR, Thielan O, et al. Outcomes specific to patient sex after open ventral hernia repair. Surgery. 2020;167:614–619. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.