Abstract
The immunoglobulin receptor GPVI (glycoprotein VI) is selectively expressed on megakaryocytes and platelets and is currently recognized as a receptor for not only collagen but also a variety of plasma and vascular proteins, including fibrin, fibrinogen, laminin, fibronectin, and galectin-3. Deficiency of GPVI is protective in mouse models of experimental thrombosis, pulmonary thromboembolism as well as in thromboinflammation, suggesting a role of GPVI in arterial and venous thrombus formation. In humans, platelet GPVI deficiency is associated with a mild bleeding phenotype, whereas a common variant rs1613662 in the GP6 gene is considered a risk factor for venous thromboembolism. However, preclinical studies on the inhibition of GPVI-ligand interactions are focused on arterial thrombotic complications. In this review we discuss the emerging evidence for GPVI in venous thrombus formation and leukocyte-dependent thromboinflammation, extending to venous thromboembolism, pulmonary thromboembolism, and cancer metastasis. We also recapitulate indications for circulating soluble GPVI as a biomarker of thrombosis-related complications. Collectively, we conclude that the current evidence suggests that platelet GPVI is also a suitable cotarget in the prevention of venous thrombosis due to its role in thrombus consolidation and platelet-leukocyte complex formation.
Keywords: embolism, glycoprotein, inflammation, thrombosis, venous thromboembolism
Highlights.
Platelets actively contribute to venous thrombosis with a suggested role of GPVI (glycoprotein VI).
Platelet GPVI contributes to thrombosis in inflammation and cancers.
Genetic variation in GP6 is a modest risk factor for venous thrombosis.
The immunoglobulin receptor GPVI (glycoprotein VI) has been widely studied as a platelet-activating receptor for collagen and is currently considered as a therapeutic target for arterial thrombotic complications. Here, we overview and discuss the indications for a role of GPVI also in venous thrombotic complications, including thromboembolism, thromboinflammation, and venous cancer thrombosis.
Interfaces of Platelet and Coagulation Activation
It is established that the processes of platelet activation and coagulation are highly interconnected in hemostasis and (arterial) thrombosis.1,2 The reported connection mechanisms are multiple, and involve in particular: (1) collagen- and thrombin-induced exposure on platelets of the procoagulant phospholipid phosphatidylserine, (2) subsequent enhanced generation of factor Xa and thrombin on these procoagulant platelets, (3) enforcement of platelet activation by the coagulation products thrombin and fibrin, and (4) thrombin-mediated fibrinogen proteolysis to form fibrin and stabilize the platelet thrombus.1,3,4 This concept of interactive thrombus-and-clot-formation is supported by static and flow studies, and it depicts platelets as thrombin- and fibrin-responsive cells, capable to aggregate and to generate massive amounts of thrombin and fibrin. Both the extrinsic (triggered by tissue factor) and intrinsic (activated by factor XIIa) coagulation pathways are considered to contribute to the thrombin generation process.5 Modeling studies furthermore support the concept of tissue factor and factor XII playing critical roles in the thrombus formation at both arterial and venous flow conditions.6,7
In the human body, the process is considered to start with the GPVI-dependent platelet adhesion to collagen present in the subendothelial matrix or the lesioned-atherosclerotic plaque.8 In the context of thromboinflammation, that is when inflammation leads to thrombosis, also other cells come into the play. On the inflamed endothelium, circulating platelets interact with leukocytes (neutrophils, monocytes) again resulting in thrombin and fibrin generation.9 In this article, we critically review the evidence for a role of GPVI in venous thrombosis alongside its contribution to arterial thrombosis.
GPVI and Thrombus Formation
GPVI is expressed exclusively on platelets and megakaryocytes. In the platelet membrane, GPVI is associated with the FcR γ (Fc receptor γ)-chain, which is responsible for the signaling via its immunoreceptor-tyrosine-based-activation-motif.10 Upon GPVI-ligand interaction and dimerization, the 2 tyrosine residues in the immunoreceptor-tyrosine-based-activation-motif become phosphorylated by Src-family kinases, which results in the binding and phosphorylation of the tyrosine kinase Syk (spleen tyrosine kinase) through its tandem SH2 (Src homology 2) domains. The ensuing phosphorylation cascade in the LAT signalosome leads to activation of phospholipase Cγ2 and phosphoinositide 3-kinases, culminating in a prolonged intracellular Ca2+ increase and other platelet responses.11
As a main signaling receptor for collagen, GPVI has widely been studied in the context of arterial thrombosis, whereas patient examinations suggest a limited but non-negligible role in hemostasis.12–14 An explanation is that in the absence of GPVI, hemostasis is preserved by parallel platelet pathways, for example, by platelet adhesion to collagen by the integrin α2β1 receptors and indirectly by GPIb-V-IX (glycoprotein Ib-V-IX) interacting with collagen-bound VWF (von Willebrand factor). Both GPVI and the FcR γ-chain have been found to be essential in murine arterial thrombosis models triggered by vascular injury and collagen exposure, regardless of the vascular bed, with a limited contribution to tail bleeding.15
The human GP6 gene contains 8 exons, of which the last encodes for a short intracellular and transmembrane domain (Figure [A] and [B]). Nine GP6 variants have been identified so far, the majority of which associates with a loss of receptor expression or function (Figure [B] through [D]). Individuals with platelet GPVI deficiency, carrying a common homozygous insertion in GP6 that prevents protein expression, were identified in 11 unrelated families in Chile.22 They all have normal platelet counts and no more than mild bleeding diathesis.12 The platelets were shown to be dysfunctional in flow-dependent thrombus formation, but not in adhesion to collagen surfaces.22 Gene mapping of a cohort of 1212 blood donors set the mutation incidence at 2.9% in the Chilean population, suggesting the existence of a large number of mutation carriers without clear symptoms.22 Whether these individuals are protected from thrombosis is unknown but of great interest. Furthermore, few patients have been identified with an acquired immune deficiency in platelet GPVI and associated thrombocytopenia.13
Figure.
GPVI (glycoprotein VI) structure and natural variants. A, Schematic presentation of the human GP6 gene structure. The first seven exons encode for the protein extracellular domain, with exons 3-4 encoding for domains D1 and D2, respectively. Exon 8 encodes for the transmembrane and short intracellular region. B, Cartoon showing the human GPVI protein domains with amino acid positions of the nine GPVI natural variants and mutations indicated. C, Representation of crystal structure of the GPVI binding site interacting with CRP (collagen-related peptide). D, Table of reported effects of GPVI variants on protein expression and platelet functions.16–21 FcR γ indicates Fc receptor γ; and VTE, venous thromboembolism.
In recent years, it has become clear that additional adhesive proteins in vessel wall or blood can function as ligands and agonists for GPVI, usually in conjunction with an integrin. These include vascular laminins (with integrin α6β1 as coreceptor),23,24 fibrillar fibronectin,25 and the basement membrane protein nidogen-1,26 leading to the suggestion that it functions as a pattern recognition receptor.27 In addition, it appeared that fibrinogen and fibrillar fibrin can bind and activate platelet GPVI.28–30 However, in comparison to collagens or synthetic collagen-like peptides, the fibrin- or fibrinogen-mediated GPVI activation was found to have limited Syk-mediated signaling capacity and to rely on integrin αIIbβ3.31
Additive Roles of GPVI and Thrombin
As a general scheme, in vitro flow perfusion studies point to a synergy of GPVI- and collagen-dependent platelet activation, aggregation, and procoagulant activity, together with a tissue factor-mediated generation of thrombin and fibrin.2,5,6 Similarly, mouse models of in vivo thrombus and fibrin formation have elucidated an additive contribution of GPVI-induced platelet activation and tissue factor–induced coagulation, in the mesenteric arterioles and venules.32,33 Complementarity of GPVI and thrombin activities also appeared from the structure of in vivo thrombi raised by collagen exposure, with an inner core of highly activated platelets, a transition zone, and an outer shell of loosely packed platelets, with fibrin fibers stabilizing the inner core.34 Similarly, GPVI was found to regulate the stability and hence thrombus structure.35
Mouse studies furthermore revealed a role of GPVI in the thrombin-sensitive ischemic stroke models, for example, via transient middle cerebral artery occlusion, in which GPVI depletion suppressed arterial platelet adhesion and drastically reduced infarct size in the brain, independently of platelet aggregation.36–38 Together, these findings draw attention to roles of platelet GPVI beyond the classical collagen-induced aggregation.
Platelets and Venous Thrombosis
The effective treatment of venous thrombotic complications by a wide spectrum of anticoagulants implicates that especially thrombin (fibrin clotting) has an overall controlling role in venous thrombus formation, thus leaving a subordinate role for platelets. The spectrum of VTE includes deep venous thrombosis (DVT) and pulmonary thromboembolism. Worldwide VTE is the third common cause of cardiovascular mortality after coronary artery disease and stroke. Importantly, VTE is considered a long-term and phased disease since an initial (unprovoked) thromboembolism in the venous system can be followed by recurrent VTE, life-threatening emboli in the lungs, and a post-thrombotic syndrome.39 Phenotypically, the accepted model is that impaired blood flow together with hypercoagulability and endothelial dysfunction (Virchow triad) gradually lead to the formation of large-size venous red thrombi, composed of fibrin, platelets, and red cells; a process that is driven by thrombin and can start in the valve pockets of large veins.39,40
Activated platelets contribute to the venous thromboembolic events. Although less predominant than in arterial thrombi, platelet aggregates were found to comprise a relevant part of analyzed venous clots.41,42 This agrees with the outcome of in vitro microfluidic studies, which assign a role of GPVI and other platelet receptors in thrombus formation at both venous and arterial flow conditions.43 In adapted microfluidic chambers simulating venous flow disturbances around valves, it was found that platelets contribute here to the thrombus and clot growth by interacting with fibrin.7
Evidence for a consistent role of platelets in venous thrombosis furthermore comes from in vivo mouse studies. We note here that the present mouse venous thrombosis models have limitations in the way of triggering thrombus formation (usually flow restriction) and a short time of thrombus development (days to weeks). Nevertheless, it was shown that following veins flow restriction in such DVT models, platelets promote the recruitment of leukocytes (monocytes and neutrophils) to the activated endothelium. This resulted in a leukocyte-dependent coagulation process and the buildup of a venous thrombus or clot.44 It was proposed that leukocyte-expressed tissue factor was instrumental in the induction of thrombin generation.
In a similar mouse model of venous flow restriction, the amyloid precursor protein was identified as negative regulator of platelet-neutrophil interactions, fibrin-thrombus formation, and embolization; this led to the suggestion that the platelet-derived amyloid precursor protein may limit VTE.45 However, other mouse studies indicated that also platelet-expressed P-selectin and chemokines regulate leukocyte interactions.46 Furthermore, mouse platelet deficiency in the secretion-controlling protein SNAP23 (a condition leading to thrombocytopenia) resulted in an impaired thrombosis tendency in both arteries and veins.47 Collectively, these rodent studies suggest a partly leukocyte-dependent and partly fibrin-dependent role of platelets in the development of experimental VTE.
In humans, recent clinical studies to compare treatments of coronary artery disease advocate the combined use of antiplatelet and anticoagulant medication (dual pathway therapy). In the COMPASS trial (Cardiovascular Outcomes for People Using Anticoagulation Strategies), it was concluded that the combination of aspirin (weak platelet inhibitor) and rivaroxaban (factor Xa antagonist) resulted in a better cardiovascular outcome, when compared with single aspirin or rivaroxaban alone.48 Although the rivaroxaban treatment led to more bleeding events than aspirin intake, the dual pathway therapy resulted in less VTE events when compared with the monotherapies. This suggested a favorable effect of aspirin also for patients with only venous thrombosis. The INSPIRE initiative with 1200 patients concluded that aspirin plus anticoagulant treatment reduced the overall risk of recurrent VTE by more than one-third.49 However, later meta-analyses and post hoc studies did not confirm risk reduction of VTE by aspirin on top of anticoagulant.50–52 It is remarked here that aspirin in vitro is a weak platelet inhibitor, suppressing collagen-induced, but not thrombin-induced platelet responses. Altogether, this led us to speculate that stronger antiplatelet drugs are more effective in preventing venous thrombotic events, for instance in VTE or in atrial fibrillation.
Genetic Variation in GP6 and Venous Thrombosis
In 2007, the incidence of DVT was estimated at 1 per 1000 individuals per year, with a 10-year recurrent risk of about 30%.53 Several risk factors for DVT have been recognized, including age, hospitalization, cancer, pregnancy, anticonception, and surgery.53 Family studies have estimated that approximately half of the DVT cases are heritable.54 Yet, the identified genetic factors, although of large effect sizes, account for only a minority of all DVTs. Genetic factors link to deficits in the anticoagulant proteins, antithrombin protein C and protein S, as well as to a gain-of-function in the procoagulant proteins factor V (factor V Leiden or Padua) and prothrombin (G20210A mutation).54,55 Next to the genes of these (anti)coagulant factors, efforts have been made to search for additional (common) variants that associate with DVT, for a better risk prediction and understanding of the disease.
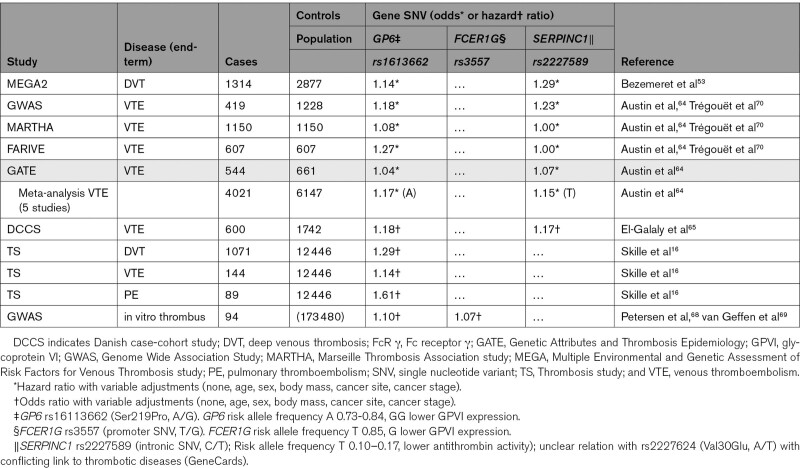
In a first population-based case-control study, the Leiden group examined 20 000 single nucleotide variants, which after a 4-stage refinement protocol resulted in 3 single nucleotide variants that significantly associated with DVT.53 A minor allele of the antithrombin gene SERPINC1 (T, rs2227589) had a modest prothrombotic tendency (Table 1). Interestingly, the same held for a major allele of GP6 (G, rs1613662), which is linked to a higher GPVI expression on platelets. A meta-analysis of 5 studies encompassing 4000 VTE cases and 6100 controls indicated that the 2 single nucleotide variants of SERPINC1 and GP6 counted as 15% higher risk factors for venous thrombosis in White patients (Table 1). Although was not seen for Black patients, the sample size was underpowered here for a definitive conclusion.64 In a prospective Danish case-cohort study, it was later confirmed that the heterozygous presence of this GP6 allele (G, rs1613662) moderately, but significantly, increased the hazard ratio for VTE.65 Since 2015, the research has identified 17 VTE-associated genes, predominantly of (anti)coagulation factors, including GP6, ABO, and secretion-regulating genes appearing as network disconnected entities.54 In a recent study of the association between this GP6 SNP and VTE risk in cancer, it was found that cancer-free AA-allele carriers had a 12-29% higher risk of VTE or DVT (nonsignificant) and a 53% to 61% higher risk of pulmonary thromboembolism.16 The different risk between genotypes, however, disappeared for the (lower numbers of) patients with active cancer, which suggested that the cancer state overruled mild effects of a variable GPVI expression.16
Table 1.
Epidemiological Support for a Role of Variation in Genes of GPVI, FcR γ-Chain, and Antithrombin in the Venous Thrombosis Risk
The risk GP6 variant rs1613662 consists of an A/G conversion, which introduces a serine to proline substitution in amino acid 219 and is considered to affect the expression of GPVI on platelets (Figure [B] and [D]).16 Platelets carrying the minor G-allele thus express less GPVI receptors and can be impaired in collagen-dependent adhesion and activation properties.66,67 Shear-dependent thrombus formation was found to be reduced in these individuals to a similar extent as in subjects carrying a variant (rs3557) of FCER1G encoding for the FcRγ-chain, which also associates with lower GPVI expression (Table 1).68,69 In agreement with the linkage between GPVI and FcRγ-chain, it appears that human platelet deficiency in GPVI is accompanied by a lower expression of the FcRγ-chain.22
In addition to the common variant P219S of GPVI, several other rare mutations in the GP6 gene are described that associate with an altered collagen-receptor binding or a reduced GPVI expression level on platelets (Figure [D]). Markedly, heterozygous deficiency in human GP6 seems to be accompanied by a no more than subtle effect on platelet function.12,22 Similarly, in heterozygous GP6+/− mice, no obvious platelet phenotype was found.71
Mouse GPVI and Venous Thrombosis
In mice, it is well established that antibody-mediated or genetic deficiency of either GPVI or FcRγ-chain results in abrogation of thrombus formation in the arterial circulation, with limited effects on tail bleeding.15,72 A few available reports also point to a role of mouse GPVI in venous thrombosis, although knowledge is still scarce here. In the murine microcirculation, it was observed that injury-induced thrombus formation in the venules required collagen-dependent platelet activation and tissue factor–induced thrombin generation, with a procoagulant role herein of phosphatidylserine-exposing platelets.32 Upon injury of the mouse mesenteric tissue, thrombus formation was reduced to a similar extent in the venules and arterioles, after the depletion of platelet GPVI with JAQ1 monoclonal antibody against GPVI or after genetic deletion of the FcRγ-chain.73
Acknowledging the limitations of current large-vein thrombosis models in mouse (eg, artificial flow restriction), these do point to a key role of platelets in general and of GPVI in particular. In transgenic mice with low levels of anticoagulant factors (antithrombin or protein C), it was established that a (fatal) thrombotic occlusion of large vessels was dependent on platelet activity next to tissue factor.74 In this context, considering that galectin-3–binding proteins can also act as GPVI ligands,75 an interesting finding is that the injection of galectin-3 enhanced the venous thrombosis induced by stasis.76 It should be stated, however, that next to GPVI, also platelet GPIb-V-IX and its ligand VWF can act as functional players in mouse venous thrombosis models.77,78 Moreover, in mouse embolization models, it was reported that GPVI depletion causes a transient protection against tissue factor–induced pulmonary thromboembolism.79 Collectively, these mouse studies lead to the attractive, but still unproven, supposition that GPVI acts as a central platelet receptor in the context of venous thrombus formation and embolization and by implication that anti-GPVI cotreatment might provide antithrombotic protection by affecting thrombus formation in a both collagen- and thrombin-dependent way.
Soluble GPVI as a Biomarker in Thrombotic Diseases
Both in humans and mice, GPVI is stably expressed on resting platelets, but it can be extracellularly cleaved in the presence of antibodies or agonists, resulting in shedding of the GPVI extracellular ectodomain. The proteolysis results in a platelet population that is essentially devoid of functionally active GPVI.80,81 Shedding of GPVI is primarily mediated by ADAM proteases (a disintegrin and metalloprotease), which become enzymatically active upon platelet activation. From experiments with mice deficient in ADAM10 or ADAM17, it was postulated that yet another enzyme can contribute to antibody-induced GPVI shedding.82 In humans, ADAM10 is primarily responsible for the GPVI cleavage secondary to conditions including receptor activation, high shear stress, or exposure to Ca2+-ionophore or factor Xa.81,83–85 In contrast to the GPVI shedding, ADAM17-induced cleavage of GPIbα appears to be a constitutive process upon platelet aging, which occurs independently of GPVI ligands.86
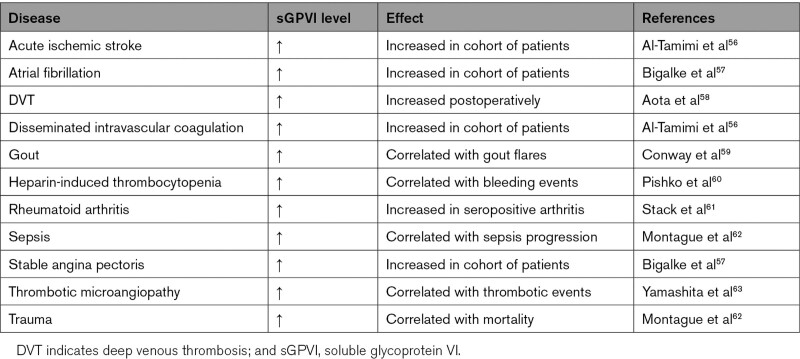
Because GPVI is selectively expressed on platelets and megakaryocytes, the presence in the circulation of sGPVI (soluble GPVI) has been postulated as a biomarker reflecting platelet activation in vivo. Elevated plasma levels of sGPVI are reported in circumstances with a prothrombotic propensity, such as acute ischemic stroke, thrombotic microangiopathy, rheumatoid arthritis, disseminated intravascular coagulation, atrial fibrillation, and DVT (Table 2).56–58,61 Along the same line, elevated sGPVI accompanied the major bleeding events in patients with heparin-induced thrombocytopenia, that is, a condition relying on prior platelet activation through the low-affinity Fc receptor, FcγRIIA.60 Higher levels of circulating sGPVI are also seen during sepsis progression, in worse-outcome trauma patients, or at gout flares.59,62
Table 2.
Changes in Circulating sGPVI Observed in Thrombotic and Related Diseases
Although the majority of studies on cleaved GPVI relate to arterial thrombotic conditions, elevated sGPVI was also observed in patients developing DVT postoperatively, suggesting that the cleaved fragment can act as a biomarker in venous thrombosis.58 In support of this, GPVI shedding can be induced by fibrin clots in sepsis or trauma.62 Taken together, these data suggest that the presence of soluble GPVI reflects in situ platelet activation in cases of multiple longer-term cardiovascular-related pathologies. Other diagnostic markers of platelet activation, such as β-thromboglobulin and platelet factor 4,87 or of coagulant activity, such as thrombin-antithrombin complexes and D-dimers,88 may rather detect more acute thrombotic events. A future well-structured study to compare these various platelet activation-dependent biomarkers may help to better understand the pathologies of distinct thrombotic diseases.
Platelet GPVI in the Wider Context of Thromboinflammatory Conditions
The term thromboinflammation is used to describe a condition where thrombotic and inflammatory events are pathological and lead to organ damage. It also implies a role of platelets in the vascular-related inflammation, often occurring in the microcirculation. Thromboinflammation was first described as a pathological process in sepsis and in ischemia-reperfusion injury.9 The thromboinflammatory process is supposed to be driven by coagulation activation, with anticoagulant therapies being effective although with bleeding side effects.89 Mechanistically, is not well understood how platelets contribute to a vascular inflammatory potential, but they likely support this by the release of chemokines and cytokines.90 An attractive model here is that the activated endothelial cells of the inflamed vessel wall express P-selectin and VWF, which triggers leukocyte and platelet adhesion, after which leukocyte-expressed tissue factor induces the formation of a venous clot.44 Support for a platelet secretory role in the clotting process comes from the use of mice with platelet secretion defects.47 In mouse models of nonsterile thromboinflammation, platelets were found to stimulate neutrophil granular release in a GPVI-dependent manner.91 In pneumonia-induced sepsis, GPVI appeared regulate the formation of platelet-leukocyte complexes.92 However, based on this limited knowledge, it appears that at the inflamed vascular beds GPVI can contribute to the inflammation process, besides its involvement in thrombus formation.
Platelets furthermore help to maintain vascular integrity and prevent blood loss from leaky vessels in inflammatory conditions. The few published mouse studies on inflammatory hemostasis indicate that platelet GPVI plays a role in the regulation of vascular integrity albeit in an organ- and stimulus-dependent manner, alongside the receptors CLEC2 (C-type lectin 2) and GPIb-IX-V.93–96 However, there are no reports on inflammatory hemostasis or bleeding in GPVI-deficient individuals.
Inflammation-propagating effects of platelets have also been examined in the context of coronavirus disease 2019 (COVID-19). An infection by severe acute respiratory syndrome coronavirus-2 can lead to a wide range of clinical manifestations, varying from absence of symptoms to severe pneumonia which can progress into an acute respiratory distress syndrome and sepsis. In subjects at risk (elderly, patients with comorbidities), massive vascular inflammation is frequently observed, culminating in disseminated intravascular coagulopathy, arterial or venous thrombosis, and pulmonary thromboembolism.97,98 The underlying condition is characterized as (pulmonary) endothelialitis and thromboinflammation.9 An assumption is that platelets, likely by interacting with leukocytes, promote the thromboinflammatory activity in SARS-CoV-2 infections.99 Reports are, however, inconsistent regarding alterations in platelet responses in severely diseased patients with COVID-19, ranging from negative priming100,101 to positive priming.102,103 Because low platelet counts are uncommon,97,104 it is unlikely that platelet activation is a disease trigger. A clinical study on the effect of GPVI antagonist glenzocimab in severe acute respiratory syndrome coronavirus-2 syndrome is in current progress (https://www.clinicaltrials.gov; Unique identifier: NCT04659109).
Role of GPVI in Cancer-Induced Thrombosis
The relation between cancers and (venous) thrombosis was already discovered in the nineteenth century.105 Nowadays, it appears that (venous) thrombotic events are the second leading cause of death in cohorts of cancer patients, whereas conversely, a history of idiopathic venous thrombosis increases the susceptibility for developing cancer.106 A high risk score for VTE is a predictive variable for earlier mortality in treated cancer patients.107
In spite of the fact that cancers are different in origin, development, and fate, there is increasing evidence that platelet interaction with a tumor exposed to the circulation promotes cancer dissemination and metastasis.108 Platelets can influence tumor cells by several mechanisms, including (1) the release of granular growth factors, matrix proteins, and inflammatory mediators, (2) the expression of P-selectin and other cell-adhesive receptors, and (3) the fibrin-mediated interaction of immune cells with a tumor.108–110
The few available mouse studies point to a role of platelet GPVI especially in tumor metastasis. Platelet GPVI deficiency resulted in less metastatic foci after the implantation of tumor cells into mice.75,111 A recently suggested mechanism is that GPVI mediates platelet interaction with cancer cell-derived galectin.75 We speculate that GPVI can also promote metastasis via the binding to fibrin clots, which are formed around tumor cells expressing tissue factor.
Clinical Possibilities for GPVI Antagonism and Agonists
Selected inhibitors of platelet GPVI are close to enter the clinic for treatment of thrombosis. A clinical study is under way with a GPVI-blocking Fab (ACT017, glenzocimab), aiming to treat acute ischemic stroke.112 In transgenic mice carrying the human GP6 gene, glenzocimab was found to be effective in thrombus suppression, without impacting GPVI-dependent inflammatory hemostasis.113 However, multiple inhibitors of protein tyrosine kinases downstream of GPVI, that is, Syk and Btk (Bruton tyrosine kinase), have extensively been evaluated in clinical trials and are currently prescribed for the treatment of B cell malignancies.114–117 This type of chemotherapy is mostly well tolerated in diseased patients while causing only limited side effects such as bleeding, nausea, vomiting, or diarrhea.118,119 GPVI-dependent antiplatelet effects can be expected from the use of these drugs as reported for the Btk inhibitor ibrutinib, prescribed to target B cells in chronic lymphocytic leukemia, along with effects on GPIb-mediated platelet responses.120 With the introduction into the clinic of more tyrosine kinase inhibitors, it will be interesting to see if the antithrombotic effects eventually reported will link to a suppression of GPVI activation in platelets, such as already shown for ibrutinib in the setting of deep vein.119,121
GPVI-Fibrin-Thrombin Loop as a Novel Target
Given the recent evidence for a role of GPVI in fibrin(ogen)-dependent thrombus formation and stability,28,35 we speculate that in the venous thrombosis setting especially fibrin may act as a relevant GPVI agonist. Although the signaling strength of fibrin to GPVI is only low,31 we note that venous thrombus formation is usually a slow process, in comparison to arteries. This raises the possibility that a weak but prolonged GPVI signal might be pathophysiologically relevant. Because in the venous setting, GPVI will act on platelets in conjunction with thrombin, anti-GPVI treatment is likely to enforce the antithrombotic effect of anticoagulant drugs in venous thrombotic diseases. However, trials still need to be performed to support this idea.
Conclusive Model and Perspective
Collectively the studies in this review support the concept of VTE as a disease process, where platelet GPVI may play a role at different stages, although multiple questions remain (Table 3). A mechanistical question is whether fibrin is indeed the principal GPVI agonist under conditions of venous thrombosis and embolism. Assuming that the answer is yes, we propose that the formation of a venous thrombus is steered by a slow but continuous GPVI-thrombin-fibrin feedforward loop, in which fibrin-bound platelets expose low levels of phosphatidylserine in a GPVI-dependent manner, after which the activity of locally generated thrombin is dampened by its binding to fibrin. Under flow conditions in vitro, we have observed that GPVI consolidates rather than propagates the formation of a thrombus.31 However, once stasis is reached, this loop can still allow the gradual growing of a clot over time, balanced in vivo by endothelial activity. Future elucidation of the crystal structure resolving the binding sites of collagen and fibrin(ogen) to GPVI (Figure [C]), and development of selective inhibitors based on the fibrin binding site, will help to resolve the importance of this feedforward loop. So far, it is also known that binding to fibrinogen relies on the avidity of GPVI-GPVI interactions.122
Table 3.
A Summary of What Is Currently Known and Unknown Regarding Platelet GPVI, Murine VT, and VTE
At the inflamed and then less antithrombotic endothelium, we propose that GPVI can influence thrombus formation in multiple ways. The endothelial activation leads to VWF release and P-selectin expression, which results in capturing and activation of both platelets and neutrophils in part via GPVI.44 In this setting, GPVI can increase the thromboinflammatory status by promoting neutrophil granular release91 and support the thrombus formation through fibrin binding.28,31 The GPVI-activated and procoagulant platelets will furthermore contribute to the generation of thrombin and fibrin.
At present, it is unclear how the interindividual variation in platelet GPVI levels fits in this scenario. However, in analogy to the other genetic risk factors of venous thrombosis (defects in the anticoagulants protein C, protein S, antithrombin), one can assume that GPVI expression level determines the activity of these interactions as in the proposed GPVI-thrombin-fibrin loop. All these intriguing aspects together make GPVI an attractive receptor for further studies on VTE and for a deeper understanding of the sets of mouse and human data (Table 3).
Article Information
Acknowledgments
We thank Sam Montague for textual suggestions and Alex Slater (both University of Birmingham) for contributing to the figure. All authors cowrote, revised, and reviewed the article.
Sources of Funding
G. Perrella is supported by a Birmingham-Maastricht PhD studentship. S.P. Watson acknowledges the support of the British Heart Foundation (BHF; CH03/003).
Disclosures
None.
Nonstandard Abbreviations and Acronyms
- ADAM
- a disintegrin and metalloprotease
- Btk
- Bruton tyrosine kinase
- COVID-19
- coronavirus disease 2019
- DVT
- deep venous thrombosis
- FcR γ
- Fc receptor γ
- GPVI
- glycoprotein VI
- sGPVI
- soluble GPVI
- Syk
- spleen tyrosine kinase
- VTE
- venous thromboembolism
- VWF
- von Willebrand factor
For Sources of Funding and Disclosures, see page 2689.
Contributor Information
Magdolna Nagy, Email: m.nagy@maastrichtuniversity.nl.
Steve P. Watson, Email: s.p.watson@bham.ac.uk.
References
- 1.Heemskerk JW, Cosemans JM, van der Meijden PE. Gresele P, Kleiman NS, Lopez JA, Page CP, eds. Platelets and coagulation. In: Platelets in Thrombotic and Non-Thrombotic Disorders. 2017. Springer Verlag, Berlin: 447–462 [Google Scholar]
- 2.van der Meijden PEJ, Heemskerk JWM. Platelet biology and functions: new concepts and clinical perspectives. Nat Rev Cardiol. 2019;16:166–179. doi: 10.1038/s41569-018-0110-0 [DOI] [PubMed] [Google Scholar]
- 3.Wu J, Heemskerk JWM, Baaten CCFMJ. Platelet membrane receptor proteolysis: implications for platelet function. Front Cardiovasc Med. 2020;7:608391. doi: 10.3389/fcvm.2020.608391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sang Y, Roest M, de Laat B, de Groot PG, Huskens D. Interplay between platelets and coagulation. Blood Rev. 2021;46:100733. doi: 10.1016/j.blre.2020.100733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brouns SLN, van Geffen JP, Campello E, Swieringa F, Spiezia L, van Oerle R, Provenzale I, Verdoold R, Farndale RW, Clemetson KJ, et al. Platelet-primed interactions of coagulation and anticoagulation pathways in flow-dependent thrombus formation. Sci Rep. 2020;10:11910. doi: 10.1038/s41598-020-68438-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diamond SL. Systems analysis of thrombus formation. Circ Res. 2016;118:1348–1362. doi: 10.1161/CIRCRESAHA.115.306824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lehmann M, Schoeman RM, Krohl PJ, Wallbank AM, Samaniuk JR, Jandrot-Perrus M, Neeves KB. Platelets drive thrombus propagation in a hematocrit and glycoprotein VI-dependent manner in an in vitro venous thrombosis model. Arterioscler Thromb Vasc Biol. 2018;38:1052–1062. doi: 10.1161/ATVBAHA.118.310731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Versteeg HH, Heemskerk JW, Levi M, Reitsma PH. New fundamentals in hemostasis. Physiol Rev. 2013;93:327–358. doi: 10.1152/physrev.00016.2011 [DOI] [PubMed] [Google Scholar]
- 9.Jackson SP, Darbousset R, Schoenwaelder SM. Thromboinflammation: challenges of therapeutically targeting coagulation and other host defense mechanisms. Blood. 2019;133:906–918. doi: 10.1182/blood-2018-11-882993 [DOI] [PubMed] [Google Scholar]
- 10.Nieswandt B, Watson SP. Platelet-collagen interaction: is GPVI the central receptor? Blood. 2003;102:449–461. doi: 10.1182/blood-2002-12-3882 [DOI] [PubMed] [Google Scholar]
- 11.Fernandez DI, Kuijpers MJ, Heemskerk JW. Platelet calcium signalling by G-protein coupled and ITAM-linked receptors regulating anoctamin-6 and procoagulant activity [published online December 26, 2020]. Platelets. doi: 10.1080/09537104.2020.185910 [DOI] [PubMed] [Google Scholar]
- 12.Matus V, Valenzuela G, Sáez CG, Hidalgo P, Lagos M, Aranda E, Panes O, Pereira J, Pillois X, Nurden AT, et al. An adenine insertion in exon 6 of human GP6 generates a truncated protein associated with a bleeding disorder in four Chilean families. J Thromb Haemost. 2013;11:1751–1759. doi: 10.1111/jth.12334 [DOI] [PubMed] [Google Scholar]
- 13.Loyau Inserm S, Faille D, Gautier P, Nurden P, Jandrot-Perrus M, Ajzenberg N. Absence of bleeding upon dual antiplatelet therapy in a patient with a immune GPVI deficiency. Platelets. 2021;32:705–709. doi: 10.1080/09537104.2020.1787974 [DOI] [PubMed] [Google Scholar]
- 14.Nagy M, van Geffen JP, Stegner D, Adams DJ, Braun A, de Witt SM, Elvers M, Geer MJ, Kuijpers MJE, Kunzelmann K, et al. Comparative analysis of microfluidics thrombus formation in multiple genetically modified mice: link to thrombosis and hemostasis. Front Cardiovasc Med. 2019;6:99. doi: 10.3389/fcvm.2019.00099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baaten CCFMJ, Meacham S, de Witt SM, Feijge MAH, Adams DJ, Akkerman JN, Cosemans JMEM, Grassi L, Jupe S, Kostadima M, et al. A synthesis approach of mouse studies to identify genes and proteins in arterial thrombosis and bleeding. Blood. 2018;132:e35–e46. doi: 10.1182/blood-2018-02-831982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Skille H, Paulsen B, Hveem K, Gabrielsen ME, Brumpton B, Hindberg K, Gran OV, Rosendaal FR, Brækkan SK, Hansen JB. Genetic variation of platelet glycoprotein VI and the risk of venous thromboembolism. Haematologica. 2020;105:e358–e360. doi: 10.3324/haematol.2019.231225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dumont B, Lasne D, Rothschild C, Bouabdelli M, Ollivier V, Oudin C, Ajzenberg N, Grandchamp B, Jandrot-Perrus M. Absence of collagen-induced platelet activation caused by compound heterozygous GPVI mutations. Blood. 2009;114:1900–1903. doi: 10.1182/blood-2009-03-213504 [DOI] [PubMed] [Google Scholar]
- 18.Watkins NA, O’Connor MN, Rankin A, Jennings N, Wilson E, Harmer IJ, Davies L, Smethurst PA, Dudbridge F, Farndale RW, et al. Definition of novel GP6 polymorphisms and major difference in haplotype frequencies between populations by a combination of in-depth exon resequencing and genotyping with tag single nucleotide polymorphisms. J Thromb Haemost. 2006;4:1197–1205. doi: 10.1111/j.1538-7836.2006.01937.x [DOI] [PubMed] [Google Scholar]
- 19.Ezumi Y, Uchiyama T, Takayama H. Molecular cloning, genomic structure, chromosomal localization, and alternative splice forms of the platelet collagen receptor glycoprotein VI. Biochem Biophys Res Commun. 2000;277:27–36. doi: 10.1006/bbrc.2000.3624 [DOI] [PubMed] [Google Scholar]
- 20.Sokol J, Skerenova M, Biringer K, Simurda T, Kubisz P, Stasko J. Glycoprotein VI gene variants affect pregnancy loss in patients with platelet hyperaggregability. Clin Appl Thromb Hemost. 2018;24(9_suppl):202S–208S. doi: 10.1177/1076029618802358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rudzik R, Dziedziejko V, Rać ME, Sawczuk M, Maciejewska-Skrendo A, Safranow K, Pawlik A. Polymorphisms in GP6, PEAR1A, MRVI1, PIK3CG, JMJD1C, and SHH genes in patients with unstable angina. Int J Environ Res Public Health. 2020;17:E7506. doi: 10.3390/ijerph17207506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagy M, Perrella G, Dalby A, Becerra MF, Garcia Quintanilla L, Pike JA, Morgan NV, Gardiner EE, Heemskerk JWM, Azócar L, et al. Flow studies on human GPVI-deficient blood under coagulating and noncoagulating conditions. Blood Adv. 2020;4:2953–2961. doi: 10.1182/bloodadvances.2020001761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inoue O, Suzuki-Inoue K, McCarty OJ, Moroi M, Ruggeri ZM, Kunicki TJ, Ozaki Y, Watson SP. Laminin stimulates spreading of platelets through integrin alpha6beta1-dependent activation of GPVI. Blood. 2006;107:1405–1412. doi: 10.1182/blood-2005-06-2406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schaff M, Tang C, Maurer E, Bourdon C, Receveur N, Eckly A, Hechler B, Arnold C, de Arcangelis A, Nieswandt B, et al. Integrin α6β1 is the main receptor for vascular laminins and plays a role in platelet adhesion, activation, and arterial thrombosis. Circulation. 2013;128:541–552. doi: 10.1161/CIRCULATIONAHA.112.000799 [DOI] [PubMed] [Google Scholar]
- 25.Maurer E, Schaff M, Receveur N, Bourdon C, Mercier L, Nieswandt B, Dubois C, Jandrot-Perrus M, Goetz JG, Lanza F, et al. Fibrillar cellular fibronectin supports efficient platelet aggregation and procoagulant activity. Thromb Haemost. 2015;114:1175–1188. doi: 10.1160/TH14-11-0958 [DOI] [PubMed] [Google Scholar]
- 26.Lakshmanan HHS, Melrose AR, Sepp AI, Mitrugno A, Ngo ATP, Khader A, Thompson R, Sallee D, Pang J, Mangin PH, et al. The basement membrane protein nidogen-1 supports platelet adhesion and activation. Platelets. 2021;32:424–428. doi: 10.1080/09537104.2020.1745170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Montague SJ, Patel P, Martin EM, Slater A, Garcia Quintanilla L, Perrella G, Kardeby C, Nagy M, Mezzano D, Mendes PM, Watson SP. Platelet activation by charged ligands and nanoparticles: platelet glycoprotein receptors as pattern recognition receptors. Platelets. 2021. In press. doi: 10.1080/09537104.2021.1945571 [DOI] [PubMed] [Google Scholar]
- 28.Mammadova-Bach E, Ollivier V, Loyau S, Schaff M, Dumont B, Favier R, Freyburger G, Latger-Cannard V, Nieswandt B, Gachet C, et al. Platelet glycoprotein VI binds to polymerized fibrin and promotes thrombin generation. Blood. 2015;126:683–691. doi: 10.1182/blood-2015-02-629717 [DOI] [PubMed] [Google Scholar]
- 29.Onselaer MB, Hardy AT, Wilson C, Sanchez X, Babar AK, Miller JLC, Watson CN, Watson SK, Bonna A, Philippou H, et al. Fibrin and D-dimer bind to monomeric GPVI. Blood Adv. 2017;1:1495–1504. doi: 10.1182/bloodadvances.2017007732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alshehri OM, Hughes CE, Montague S, Watson SK, Frampton J, Bender M, Watson SP. Fibrin activates GPVI in human and mouse platelets. Blood. 2015;126:1601–1608. doi: 10.1182/blood-2015-04-641654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perrella G, Huang J, Provenzale I, Swieringa F, Heubel-Moenen FCJI, Farndale RW, Roest M, van der Meijden PEJ, Thomas M, Ariëns RAS, et al. Nonredundant roles of platelet glycoprotein VI and integrin αIIbβ3 in fibrin-mediated microthrombus formation. Arterioscler Thromb Vasc Biol. 2021;41:e97–e111. doi: 10.1161/ATVBAHA.120.314641 [DOI] [PubMed] [Google Scholar]
- 32.Kuijpers MJ, Munnix IC, Cosemans JM, Vlijmen BV, Reutelingsperger CP, Egbrink MO, Heemskerk JW. Key role of platelet procoagulant activity in tissue factor-and collagen-dependent thrombus formation in arterioles and venules in vivo differential sensitivity to thrombin inhibition. Microcirculation. 2008;15:269–282. doi: 10.1080/10739680701653517 [DOI] [PubMed] [Google Scholar]
- 33.Berny MA, Munnix IC, Auger JM, Schols SE, Cosemans JM, Panizzi P, Bock PE, Watson SP, McCarty OJ, Heemskerk JW. Spatial distribution of factor Xa, thrombin, and fibrin(ogen) on thrombi at venous shear. PLoS One. 2010;5:e10415. doi: 10.1371/journal.pone.0010415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Welsh JD, Stalker TJ, Voronov R, Muthard RW, Tomaiuolo M, Diamond SL, Brass LF. A systems approach to hemostasis: 1. The interdependence of thrombus architecture and agonist movements in the gaps between platelets. Blood. 2014;124:1808–1815. doi: 10.1182/blood-2014-01-550335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ahmed MU, Kaneva V, Loyau S, Nechipurenko D, Receveur N, Le Bris M, Janus-Bell E, Didelot M, Rauch A, Susen S, et al. Pharmacological blockade of glycoprotein VI promotes thrombus disaggregation in the absence of thrombin. Arterioscler Thromb Vasc Biol. 2020;40:2127–2142. doi: 10.1161/ATVBAHA.120.314301 [DOI] [PubMed] [Google Scholar]
- 36.Kleinschnitz C, Pozgajova M, Pham M, Bendszus M, Nieswandt B, Stoll G. Targeting platelets in acute experimental stroke: impact of glycoprotein Ib, VI, and IIb/IIIa blockade on infarct size, functional outcome, and intracranial bleeding. Circulation. 2007;115:2323–2330. doi: 10.1161/CIRCULATIONAHA.107.691279 [DOI] [PubMed] [Google Scholar]
- 37.Stoll G, Kleinschnitz C, Nieswandt B. Molecular mechanisms of thrombus formation in ischemic stroke: novel insights and targets for treatment. Blood. 2008;112:3555–3562. doi: 10.1182/blood-2008-04-144758 [DOI] [PubMed] [Google Scholar]
- 38.Schuhmann MK, Kraft P, Bieber M, Kollikowski AM, Schulze H, Nieswandt B, Pham M, Stegner D, Stoll G. Targeting platelet GPVI plus rt-PA administration but not α2β1-mediated collagen binding protects against ischemic brain damage in mice. Int J Mol Sci. 2019;20:2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wolberg AS, Rosendaal FR, Weitz JI, Jaffer IH, Agnelli G, Baglin T, Mackman N. Venous thrombosis. Nat Rev Dis Primers. 2015;1:15006. doi: 10.1038/nrdp.2015.6 [DOI] [PubMed] [Google Scholar]
- 40.Montoro-García S, Schindewolf M, Stanford S, Larsen OH, Thiele T. The role of platelets in venous thromboembolism. Semin Thromb Hemost. 2016;42:242–251. doi: 10.1055/s-0035-1570079 [DOI] [PubMed] [Google Scholar]
- 41.Lippi G, Favaloro EJ. Venous and arterial thromboses: two sides of the same coin? Semin Thromb Hemost. 2018;44:239–248. doi: 10.1055/s-0037-1607202 [DOI] [PubMed] [Google Scholar]
- 42.Staessens S, Denorme F, Francois O, Desender L, Dewaele T, Vanacker P, Deckmyn H, Vanhoorelbeke K, Andersson T, De Meyer SF. Structural analysis of ischemic stroke thrombi: histological indications for therapy resistance. Haematologica. 2020;105:498–507. doi: 10.3324/haematol.2019.219881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.de Witt SM, Swieringa F, Cavill R, Lamers MM, van Kruchten R, Mastenbroek T, Baaten C, Coort S, Pugh N, Schulz A, et al. Identification of platelet function defects by multi-parameter assessment of thrombus formation. Nat Commun. 2014;5:4257. doi: 10.1038/ncomms5257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.von Brühl ML, Stark K, Steinhart A, Chandraratne S, Konrad I, Lorenz M, Khandoga A, Tirniceriu A, Coletti R, Köllnberger M, et al. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J Exp Med. 2012;209:819–835. doi: 10.1084/jem.20112322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Canobbio I, Visconte C, Momi S, Guidetti GF, Zarà M, Canino J, Falcinelli E, Gresele P, Torti M. Platelet amyloid precursor protein is a modulator of venous thromboembolism in mice. Blood. 2017;130:527–536. doi: 10.1182/blood-2017-01-764910 [DOI] [PubMed] [Google Scholar]
- 46.Postea O, Vasina EM, Cauwenberghs S, Projahn D, Liehn EA, Lievens D, Theelen W, Kramp BK, Butoi ED, Soehnlein O, et al. Contribution of platelet CX(3)CR1 to platelet-monocyte complex formation and vascular recruitment during hyperlipidemia. Arterioscler Thromb Vasc Biol. 2012;32:1186–1193. doi: 10.1161/ATVBAHA.111.243485 [DOI] [PubMed] [Google Scholar]
- 47.Williams CM, Li Y, Brown E, Poole AW. Platelet-specific deletion of SNAP23 ablates granule secretion, substantially inhibiting arterial and venous thrombosis in mice. Blood Adv. 2018;2:3627–3636. doi: 10.1182/bloodadvances.2018023291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eikelboom JW, Connolly SJ, Bosch J, Dagenais GR, Hart RG, Shestakovska O, Diaz R, Alings M, Lonn EM, Anand SS, et al. ; COMPASS Investigators. Rivaroxaban with or without Aspirin in Stable Cardiovascular Disease. N Engl J Med. 2017;377:1319–1330. doi: 10.1056/NEJMoa1709118 [DOI] [PubMed] [Google Scholar]
- 49.Simes J, Becattini C, Agnelli G, Eikelboom JW, Kirby AC, Mister R, Prandoni P, Brighton TA; INSPIRE Study Investigators (International Collaboration of Aspirin Trials for Recurrent Venous Thromboembolism). Aspirin for the prevention of recurrent venous thromboembolism: the INSPIRE collaboration. Circulation. 2014;130:1062–1071. doi: 10.1161/CIRCULATIONAHA.114.008828 [DOI] [PubMed] [Google Scholar]
- 50.Valeriani E, Porreca E, Weitz JI, Schulman S, Candeloro M, Di Nisio M. Impact of concomitant antiplatelet therapy on the efficacy and safety of direct oral anticoagulants for acute venous thromboembolism: systematic review and meta-analysis. J Thromb Haemost. 2020;18:1661–1671. doi: 10.1111/jth.14807 [DOI] [PubMed] [Google Scholar]
- 51.Prandoni P, Lensing AWA, Prins MH, Gebel M, Pap AF, Homering M, Bauersachs R, Beyer-Westendorf J, Bounameaux H, Cohen AT, et al. Benefits and risks of extended treatment of venous thromboembolism with rivaroxaban or with aspirin. Thromb Res. 2018;168:121–129. doi: 10.1016/j.thromres.2018.06.009 [DOI] [PubMed] [Google Scholar]
- 52.Van Galen J, Pava L, Wright C, Elbadawi A, Hamer A, Chaturvedi A, Cameron SJ. Effect of platelet inhibitors on thrombus burden in patients with acute pulmonary embolism. Platelets. 2021;32:138–140. doi: 10.1080/09537104.2020.1732329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bezemer ID, Bare LA, Doggen CJ, Arellano AR, Tong C, Rowland CM, Catanese J, Young BA, Reitsma PH, Devlin JJ, et al. Gene variants associated with deep vein thrombosis. JAMA. 2008;299:1306–1314. doi: 10.1001/jama.299.11.1306 [DOI] [PubMed] [Google Scholar]
- 54.Morange PE, Suchon P, Trégouët DA. Genetics of Venous Thrombosis: update in 2015. Thromb Haemost. 2015;114:910–919. doi: 10.1160/TH15-05-0410 [DOI] [PubMed] [Google Scholar]
- 55.Simioni P, Tormene D, Spiezia L, Tognin G, Rossetto V, Radu C, Prandoni P. Inherited thrombophilia and venous thromboembolism. Semin Thromb Hemost. 2006;32:700–708. doi: 10.1055/s-2006-951298 [DOI] [PubMed] [Google Scholar]
- 56.Al-Tamimi M, Grigoriadis G, Tran H, Paul E, Servadei P, Berndt MC, Gardiner EE, Andrews RK. Coagulation-induced shedding of platelet glycoprotein VI mediated by factor Xa. Blood. 2011;117:3912–3920. doi: 10.1182/blood-2010-08-301523 [DOI] [PubMed] [Google Scholar]
- 57.Bigalke B, Stellos K, Weig HJ, Geisler T, Seizer P, Kremmer E, Pötz O, Joos T, May AE, Lindemann S, et al. Regulation of platelet glycoprotein VI (GPVI) surface expression and of soluble GPVI in patients with atrial fibrillation (AF) and acute coronary syndrome (ACS). Basic Res Cardiol. 2009;104:352–357. doi: 10.1007/s00395-009-0779-7 [DOI] [PubMed] [Google Scholar]
- 58.Aota T, Naitoh K, Wada H, Yamashita Y, Miyamoto N, Hasegawa M, Wakabayashi H, Yoshida K, Asanuma K, Matsumoto T, et al. Elevated soluble platelet glycoprotein VI is a useful marker for DVT in postoperative patients treated with edoxaban. Int J Hematol. 2014;100:450–456. doi: 10.1007/s12185-014-1676-x [DOI] [PubMed] [Google Scholar]
- 59.Conway R, Murphy CL, Madigan A, Kavanagh P, Geraghty L, Redmond N, Helbert L, Carey JJ, Dunne E, Kenny D, et al. Increased platelet reactivity as measured by plasma glycoprotein VI in gout. Platelets. 2018;29:821–826. doi: 10.1080/09537104.2017.1366974 [DOI] [PubMed] [Google Scholar]
- 60.Pishko AM, Andrews RK, Gardiner EE, Lefler DS, Cuker A. Soluble glycoprotein VI is a predictor of major bleeding in patients with suspected heparin-induced thrombocytopenia. Blood Adv. 2020;4:4327–4332. doi: 10.1182/bloodadvances.2020002861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stack JR, Madigan A, Helbert L, Dunne E, Gardiner EE, Andrews RK, Finan R, Smyth E, Kenny D, McCarthy GM. Soluble glycoprotein VI, a specific marker of platelet activation is increased in the plasma of subjects with seropositive rheumatoid arthritis. PLoS One. 2017;12:e0188027. doi: 10.1371/journal.pone.0188027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Montague SJ, Delierneux C, Lecut C, Layios N, Dinsdale RJ, Lee CS, Poulter NS, Andrews RK, Hampson P, Wearn CM, et al. Soluble GPVI is elevated in injured patients: shedding is mediated by fibrin activation of GPVI. Blood Adv. 2018;2:240–251. doi: 10.1182/bloodadvances.2017011171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yamashita Y, Naitoh K, Wada H, Ikejiri M, Mastumoto T, Ohishi K, Hosaka Y, Nishikawa M, Katayama N. Elevated plasma levels of soluble platelet glycoprotein VI (GPVI) in patients with thrombotic microangiopathy. Thromb Res. 2014;133:440–444. doi: 10.1016/j.thromres.2013.11.023 [DOI] [PubMed] [Google Scholar]
- 64.Austin H, De Staercke C, Lally C, Bezemer ID, Rosendaal FR, Hooper WC. New gene variants associated with venous thrombosis: a replication study in White and Black Americans. J Thromb Haemost. 2011;9:489–495. doi: 10.1111/j.1538-7836.2011.04185.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.El-Galaly TC, Severinsen MT, Overvad K, Steffensen R, Vistisen AK, Tjønneland A, Kristensen SR. Single nucleotide polymorphisms and the risk of venous thrombosis: results from a Danish case-cohort study. Br J Haematol. 2013;160:838–841. doi: 10.1111/bjh.12132 [DOI] [PubMed] [Google Scholar]
- 66.Yee DL, Bray PF. Clinical and functional consequences of platelet membrane glycoprotein polymorphisms. Semin Thromb Hemost. 2004;30:591–600. doi: 10.1055/s-2004-835679 [DOI] [PubMed] [Google Scholar]
- 67.Joutsi-Korhonen L, Smethurst PA, Rankin A, Gray E, IJsseldijk M, Onley CM, Watkins NA, Williamson LM, Goodall AH, de Groot PG, et al. The low-frequency allele of the platelet collagen signaling receptor glycoprotein VI is associated with reduced functional responses and expression. Blood. 2003;101:4372–4379. doi: 10.1182/blood-2002-08-2591 [DOI] [PubMed] [Google Scholar]
- 68.Petersen R, Lambourne JJ, Javierre BM, Grassi L, Kreuzhuber R, Ruklisa D, Rosa IM, Tomé AR, Elding H, van Geffen JP, et al. Platelet function is modified by common sequence variation in megakaryocyte super enhancers. Nat Commun. 2017;8:16058. doi: 10.1038/ncomms16058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.van Geffen JP, Brouns SLN, Batista J, McKinney H, Kempster C, Nagy M, Sivapalaratnam S, Baaten CCFMJ, Bourry N, Frontini M, et al. High-throughput elucidation of thrombus formation reveals sources of platelet function variability. Haematologica. 2019;104:1256–1267. doi: 10.3324/haematol.2018.198853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Trégouët DA, Heath S, Saut N, Biron-Andreani C, Schved JF, Pernod G, Galan P, Drouet L, Zelenika D, Juhan-Vague I, et al. Common susceptibility alleles are unlikely to contribute as strongly as the FV and ABO loci to VTE risk: results from a GWAS approach. Blood. 2009;113:5298–5303. doi: 10.1182/blood-2008-11-190389 [DOI] [PubMed] [Google Scholar]
- 71.Lockyer S, Okuyama K, Begum S, Le S, Sun B, Watanabe T, Matsumoto Y, Yoshitake M, Kambayashi J, Tandon NN. GPVI-deficient mice lack collagen responses and are protected against experimentally induced pulmonary thromboembolism. Thromb Res. 2006;118:371–380. doi: 10.1016/j.thromres.2005.08.001 [DOI] [PubMed] [Google Scholar]
- 72.Nieswandt B, Schulte V, Bergmeier W, Mokhtari-Nejad R, Rackebrandt K, Cazenave JP, Ohlmann P, Gachet C, Zirngibl H. Long-term antithrombotic protection by in vivo depletion of platelet glycoprotein VI in mice. J Exp Med. 2001;193:459–469. doi: 10.1084/jem.193.4.459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Munnix IC, Strehl A, Kuijpers MJ, Auger JM, van der Meijden PE, van Zandvoort MA, oude Egbrink MG, Nieswandt B, Heemskerk JW. The glycoprotein VI-phospholipase Cgamma2 signaling pathway controls thrombus formation induced by collagen and tissue factor in vitro and in vivo. Arterioscler Thromb Vasc Biol. 2005;25:2673–2678. doi: 10.1161/01.ATV.0000193568.71980.4a [DOI] [PubMed] [Google Scholar]
- 74.Heestermans M, Salloum-Asfar S, Streef T, Laghmani EH, Salvatori D, Luken BM, Zeerleder SS, Spronk HMH, Korporaal SJ, Kirchhofer D, et al. Mouse venous thrombosis upon silencing of anticoagulants depends on tissue factor and platelets, not FXII or neutrophils. Blood. 2019;133:2090–2099. doi: 10.1182/blood-2018-06-853762 [DOI] [PubMed] [Google Scholar]
- 75.Mammadova-Bach E, Gil-Pulido J, Sarukhanyan E, Burkard P, Shityakov S, Schonhart C, Stegner D, Remer K, Nurden P, Nurden AT, et al. Platelet glycoprotein VI promotes metastasis through interaction with cancer cell-derived galectin-3. Blood. 2020;135:1146–1160. doi: 10.1182/blood.2019002649 [DOI] [PubMed] [Google Scholar]
- 76.DeRoo EP, Wrobleski SK, Shea EM, Al-Khalil RK, Hawley AE, Henke PK, Myers DD, Jr, Wakefield TW, Diaz JA. The role of galectin-3 and galectin-3-binding protein in venous thrombosis. Blood. 2015;125:1813–1821. doi: 10.1182/blood-2014-04-569939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bergmeier W, Chauhan AK, Wagner DD. Glycoprotein Ibalpha and von Willebrand factor in primary platelet adhesion and thrombus formation: lessons from mutant mice. Thromb Haemost. 2008;99:264–270. doi: 10.1160/TH07-10-0638 [DOI] [PubMed] [Google Scholar]
- 78.Brill A, Fuchs TA, Chauhan AK, Yang JJ, De Meyer SF, Köllnberger M, Wakefield TW, Lämmle B, Massberg S, Wagner DD. von Willebrand factor-mediated platelet adhesion is critical for deep vein thrombosis in mouse models. Blood. 2011;117:1400–1407. doi: 10.1182/blood-2010-05-287623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schulte V, Reusch HP, Pozgajová M, Varga-Szabó D, Gachet C, Nieswandt B. Two-phase antithrombotic protection after anti-glycoprotein VI treatment in mice. Arterioscler Thromb Vasc Biol. 2006;26:1640–1647. doi: 10.1161/01.ATV.0000225697.98093.ed [DOI] [PubMed] [Google Scholar]
- 80.Bergmeier W, Rabie T, Strehl A, Piffath CL, Prostredna M, Wagner DD, Nieswandt B. GPVI down-regulation in murine platelets through metalloproteinase-dependent shedding. Thromb Haemost. 2004;91:951–958. doi: 10.1160/TH03-12-0795 [DOI] [PubMed] [Google Scholar]
- 81.Baaten CCFMJ, Swieringa F, Misztal T, Mastenbroek TG, Feijge MAH, Bock PE, Donners MMPC, Collins PW, Li R, van der Meijden PEJ, et al. Platelet heterogeneity in activation-induced glycoprotein shedding: functional effects. Blood Adv. 2018;2:2320–2331. doi: 10.1182/bloodadvances.2017011544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bender M, Hofmann S, Stegner D, Chalaris A, Bösl M, Braun A, Scheller J, Rose-John S, Nieswandt B. Differentially regulated GPVI ectodomain shedding by multiple platelet-expressed proteinases. Blood. 2010;116:3347–3355. doi: 10.1182/blood-2010-06-289108 [DOI] [PubMed] [Google Scholar]
- 83.Stephens G, Yan Y, Jandrot-Perrus M, Villeval JL, Clemetson KJ, Phillips DR. Platelet activation induces metalloproteinase-dependent GP VI cleavage to down-regulate platelet reactivity to collagen. Blood. 2005;105:186–191. doi: 10.1182/blood-2004-07-2842 [DOI] [PubMed] [Google Scholar]
- 84.Gardiner EE, Karunakaran D, Shen Y, Arthur JF, Andrews RK, Berndt MC. Controlled shedding of platelet glycoprotein (GP)VI and GPIb-IX-V by ADAM family metalloproteinases. J Thromb Haemost. 2007;5:1530–1537. doi: 10.1111/j.1538-7836.2007.02590.x [DOI] [PubMed] [Google Scholar]
- 85.Rabie T, Varga-Szabo D, Bender M, Pozgaj R, Lanza F, Saito T, Watson SP, Nieswandt B. Diverging signaling events control the pathway of GPVI down-regulation in vivo. Blood. 2007;110:529–535. doi: 10.1182/blood-2006-11-058107 [DOI] [PubMed] [Google Scholar]
- 86.Gardiner EE, Andrews RK. Platelet receptor expression and shedding: glycoprotein Ib-IX-V and glycoprotein VI. Transfus Med Rev. 2014;28:56–60. doi: 10.1016/j.tmrv.2014.03.001 [DOI] [PubMed] [Google Scholar]
- 87.Blanke H, Praetorius G, Leschke M, Seitz R, Egbring R, Strauer BE. [Significance of the thrombin-antithrombin III complex in the diagnosis of pulmonary embolism and deep venous thrombosis–comparison with fibrinopeptide A, platelet factor 4 and beta-thromboglobulin]. Klin Wochenschr. 1987;65:757–763. doi: 10.1007/BF01743250 [DOI] [PubMed] [Google Scholar]
- 88.van der Putten RF, Glatz JF, Hermens WT. Plasma markers of activated hemostasis in the early diagnosis of acute coronary syndromes. Clin Chim Acta. 2006;371:37–54. doi: 10.1016/j.cca.2006.03.005 [DOI] [PubMed] [Google Scholar]
- 89.Fan Y, Jiang M, Gong D, Zou C. Efficacy and safety of low-molecular-weight heparin in patients with sepsis: a meta-analysis of randomized controlled trials. Sci Rep. 2016;6:25984. doi: 10.1038/srep25984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Thomas MR, Storey RF. The role of platelets in inflammation. Thromb Haemost. 2015;114:449–458. doi: 10.1160/TH14-12-1067 [DOI] [PubMed] [Google Scholar]
- 91.Gros A, Syvannarath V, Lamrani L, Ollivier V, Loyau S, Goerge T, Nieswandt B, Jandrot-Perrus M, Ho-Tin-Noé B. Single platelets seal neutrophil-induced vascular breaches via GPVI during immune-complex-mediated inflammation in mice. Blood. 2015;126:1017–1026. doi: 10.1182/blood-2014-12-617159 [DOI] [PubMed] [Google Scholar]
- 92.Claushuis TAM, de Vos AF, Nieswandt B, Boon L, Roelofs JJTH, de Boer OJ, van ‘t Veer C, van der Poll T. Platelet glycoprotein VI aids in local immunity during pneumonia-derived sepsis caused by gram-negative bacteria. Blood. 2018;131:864–876. doi: 10.1182/blood-2017-06-788067 [DOI] [PubMed] [Google Scholar]
- 93.Rayes J, Jadoui S, Lax S, Gros A, Wichaiyo S, Ollivier V, Denis CV, Ware J, Nieswandt B, Jandrot-Perrus M, et al. The contribution of platelet glycoprotein receptors to inflammatory bleeding prevention is stimulus and organ dependent. Haematologica. 2018;103:e256–e258. doi: 10.3324/haematol.2017.182162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wichaiyo S, Lax S, Montague SJ, Li Z, Grygielska B, Pike JA, Haining EJ, Brill A, Watson SP, Rayes J. Platelet glycoprotein VI and C-type lectin-like receptor 2 deficiency accelerates wound healing by impairing vascular integrity in mice. Haematologica. 2019;104:1648–1660. doi: 10.3324/haematol.2018.208363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rayes J, Watson SP, Nieswandt B. Functional significance of the platelet immune receptors GPVI and CLEC-2. J Clin Invest. 2019;129:12–23. doi: 10.1172/JCI122955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Boulaftali Y, Hess PR, Getz TM, Cholka A, Stolla M, Mackman N, Owens AP, III, Ware J, Kahn ML, Bergmeier W. Platelet ITAM signaling is critical for vascular integrity in inflammation. J Clin Invest. 2013;123:908–916. doi: 10.1172/JCI65154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Connors JM, Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135:2033–2040. doi: 10.1182/blood.2020006000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bikdeli B, Madhavan MV, Jimenez D, Chuich T, Dreyfus I, Driggin E, Nigoghossian C, Ageno W, Madjid M, Guo Y, et al. ; Global COVID-19 Thrombosis Collaborative Group, Endorsed by the ISTH, NATF, ESVM, and the IUA, Supported by the ESC Working Group on Pulmonary Circulation and Right Ventricular Function. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC State-of-the-art review. J Am Coll Cardiol. 2020;75:2950–2973. doi: 10.1016/j.jacc.2020.04.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hottz ED, Azevedo-Quintanilha IG, Palhinha L, Teixeira L, Barreto EA, Pão CRR, Righy C, Franco S, Souza TML, Kurtz P, et al. Platelet activation and platelet-monocyte aggregate formation trigger tissue factor expression in patients with severe COVID-19. Blood. 2020;136:1330–1341. doi: 10.1182/blood.2020007252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Taus F, Salvagno G, Canè S, Fava C, Mazzaferri F, Carrara E, Petrova V, Barouni RM, Dima F, Dalbeni A, et al. Platelets promote thromboinflammation in SARS-CoV-2 pneumonia. Arterioscler Thromb Vasc Biol. 2020;40:2975–2989. doi: 10.1161/ATVBAHA.120.315175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Denorme F, Manne BK, Portier I, Petrey AC, Middleton EA, Kile BT, Rondina MT, Campbell RA. COVID-19 patients exhibit reduced procoagulant platelet responses. J Thromb Haemost. 2020;18:3067–3073. doi: 10.1111/jth.15107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zaid Y, Puhm F, Allaeys I, et al. Platelets can associate with SARS-Cov-2 RNA and are hyperactivated in COVID-19. Circ Res. 2020;127:1404–1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sriram K, Insel PA. Inflammation and thrombosis in COVID-19 pathophysiology: proteinase-activated and purinergic receptors as drivers and candidate therapeutic targets. Physiol Rev. 2021;101:545–567. doi: 10.1152/physrev.00035.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Thachil J, Tang N, Gando S, Falanga A, Cattaneo M, Levi M, Clark C, Iba T. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020;18:1023–1026. doi: 10.1111/jth.14810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Varki A. Trousseau’s syndrome: multiple definitions and multiple mechanisms. Blood. 2007;110:1723–1729. doi: 10.1182/blood-2006-10-053736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mahajan A, Brunson A, White R, Wun T. The epidemiology of cancer-associated venous thromboembolism: an update. Semin Thromb Hemost. 2019;45:321–325. doi: 10.1055/s-0039-1688494 [DOI] [PubMed] [Google Scholar]
- 107.Kuderer NM, Culakova E, Lyman GH, Francis C, Falanga A, Khorana AA. A validated risk score for venous thromboembolism is predictive of cancer progression and mortality. Oncologist. 2016;21:861–867. doi: 10.1634/theoncologist.2015-0361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Schlesinger M. Role of platelets and platelet receptors in cancer metastasis. J Hematol Oncol. 2018;11:125. doi: 10.1186/s13045-018-0669-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ho-Tin-Noé B, Goerge T, Cifuni SM, Duerschmied D, Wagner DD. Platelet granule secretion continuously prevents intratumor hemorrhage. Cancer Res. 2008;68:6851–6858. doi: 10.1158/0008-5472.CAN-08-0718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Palumbo JS, Talmage KE, Massari JV, La Jeunesse CM, Flick MJ, Kombrinck KW, Jirousková M, Degen JL. Platelets and fibrin(ogen) increase metastatic potential by impeding natural killer cell-mediated elimination of tumor cells. Blood. 2005;105:178–185. doi: 10.1182/blood-2004-06-2272 [DOI] [PubMed] [Google Scholar]
- 111.Jain S, Russell S, Ware J. Platelet glycoprotein VI facilitates experimental lung metastasis in syngenic mouse models. J Thromb Haemost. 2009;7:1713–1717. doi: 10.1111/j.1538-7836.2009.03559.x [DOI] [PubMed] [Google Scholar]
- 112.Voors-Pette C, Lebozec K, Dogterom P, Jullien L, Billiald P, Ferlan P, Renaud L, Favre-Bulle O, Avenard G, Machacek M, et al. Safety and tolerability, pharmacokinetics, and pharmacodynamics of ACT017, an antiplatelet GPVI (Glycoprotein VI) Fab. Arterioscler Thromb Vasc Biol. 2019;39:956–964. doi: 10.1161/ATVBAHA.118.312314 [DOI] [PubMed] [Google Scholar]
- 113.Jadoui S, Le Chapelain O, Ollivier V, Mostefa-Kara A, Di Meglio L, Dupont S, Gros A, Nomenjanahary MS, Desilles JP, Mazighi M, et al. Glenzocimab does not impact glycoprotein VI-dependent inflammatory haemostasis. Haematologica. 2021;106:2000–2003. doi: 10.3324/haematol.2020.270439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang ML, Rule S, Martin P, Goy A, Auer R, Kahl BS, Jurczak W, Advani RH, Romaguera JE, Williams ME, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2013;369:507–516. doi: 10.1056/NEJMoa1306220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Byrd JC, Harrington B, O’Brien S, Jones JA, Schuh A, Devereux S, Chaves J, Wierda WG, Awan FT, Brown JR, et al. Acalabrutinib (ACP-196) in relapsed chronic lymphocytic leukemia. N Engl J Med. 2016;374:323–332. doi: 10.1056/NEJMoa1509981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Burke JM, Shustov A, Essell J, Patel-Donnelly D, Yang J, Chen R, Ye W, Shi W, Assouline S, Sharman J. An open-label, phase II trial of entospletinib (GS-9973), a selective spleen tyrosine kinase inhibitor, in diffuse large B-cell lymphoma. Clin Lymphoma Myeloma Leuk. 2018;18:e327–e331. doi: 10.1016/j.clml.2018.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hamlin PA, Flinn IW, Wagner-Johnston N, Burger JA, Coffey GP, Conley PB, Michelson G, Leeds JM, Der K, Kim Y, et al. Efficacy and safety of the dual SYK/JAK inhibitor cerdulatinib in patients with relapsed or refractory B-cell malignancies: results of a phase I study. Am J Hematol. 2019;94:E90–E93. doi: 10.1002/ajh.25387 [DOI] [PubMed] [Google Scholar]
- 118.Friedberg JW, Sharman J, Sweetenham J, Johnston PB, Vose JM, Lacasce A, Schaefer-Cutillo J, De Vos S, Sinha R, Leonard JP, et al. Inhibition of Syk with fostamatinib disodium has significant clinical activity in non-Hodgkin lymphoma and chronic lymphocytic leukemia. Blood. 2010;115:2578–2585. doi: 10.1182/blood-2009-08-236471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tullemans BME, Nagy M, Sabrkhany S, Griffioen AW, Oude Egbrink MGA, Aarts M, Heemskerk JWM, Kuijpers MJE. Tyrosine kinase inhibitor pazopanib inhibits platelet procoagulant activity in renal cell carcinoma patients. Front Cardiovasc Med. 2018;5:142. doi: 10.3389/fcvm.2018.00142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Busygina K, Jamasbi J, Seiler T, Deckmyn H, Weber C, Brandl R, Lorenz R, Siess W. Oral Bruton tyrosine kinase inhibitors selectively block atherosclerotic plaque-triggered thrombus formation in humans. Blood. 2018;131:2605–2616. doi: 10.1182/blood-2017-09-808808 [DOI] [PubMed] [Google Scholar]
- 121.Nicolson PL, Welsh JD, Chauhan A, Thomas MR, Kahn ML, Watson SP. A rationale for blocking thromboinflammation in COVID-19 with Btk inhibitors. Platelets. 2020;31:685–690. doi: 10.1080/09537104.2020.1775189 [DOI] [PubMed] [Google Scholar]
- 122.Xu RG, Gauer JS, Baker SR, Slater A, Martin EM, McPherson HR, Duval C, Manfield IW, Bonna AM, Watson SP, et al. GPVI (Glycoprotein VI) interaction with fibrinogen is mediated by avidity and the fibrinogen αC-region. Arterioscler Thromb Vasc Biol. 2021;41:1092–1104. doi: 10.1161/ATVBAHA.120.315030 [DOI] [PMC free article] [PubMed] [Google Scholar]