Abstract
The reduced availability of human donor hearts compared with the needs of patients with advanced heart failure refractory to medical therapy has promoted the search for therapeutic alternatives to cardiac allografts. Porcine heart xenotransplantation represents one of the most promising frontiers in this field today. From the first researches in the 1960s to today, the numerous advances achieved in the field of surgical techniques, genetic engineering and immunosuppression have made it possible at the beginning of 2022 to carry out the first swine-to-human heart transplant, attaining a survival of 2 months after surgery. The main intellectual and experimental stages that have marked the history of xenotransplantation, the latest acquisitions in terms of genetic editing, as well as the improvement of immunosuppressive therapy are discussed analytically in this article in order to illustrate the underlying complexity of this therapeutic model.
Keywords: Heart failure refractory to medical therapy, Heart xenotransplantation, Genetic engineering
Introduction
Heart transplantation (HTx) is the treatment of choice for refractory advanced heart failure (HF), ensuring a median survival of 10–15 years.1 Advanced age and the coexistence of multiple comorbidities exclude most patients with advanced HF from this therapeutic option. Considering the limited number of hearts available from donors and the growing number of patients needing HTx, a rigorous selection of patients on the basis of eligibility and exclusion criteria, in accordance with international guidelines, appears essential.2 Mechanical circulatory supports such as left ventricular assist devices (LVADs) have progressively established themselves as a valid therapeutic alternative to HTx in those patients who are not candidates for HTx (destination therapy) or as a temporary measure while waiting for the appropriate organ (bridge to transplant), or to become HTx candidate (bridge to candidacy). These mechanical devices have been shown to offer survival at 1, 2, and 5 years comparable with HTx, albeit with a different quality of life and complications which must be considered.3 A significant discrepancy persists between the needs of patients with advanced HF and the therapies actually available.
To meet the demand for HTx, new approaches have been proposed to expand the donor pool, including the use of hearts from marginal donors, donation after circulatory death, ex vivo cardiac perfusion and xenotransplantation.
Heart transplantation in Italy
In 2021, 251 hearts were transplanted in Italy, a slightly higher number compared to the three-year period 2018–2020. According to the latest available report (year 2020), 50% of the patients who received an HTx were aged between 41 and 60 years, while about 28% were over 60 years old (compared to the previous 25%, average calculated on the 2000–2018), demonstrating a tendency to include older patients on the HTx list than in the past. Twenty five percent of heart transplants were carried out in national emergency, a sharp decline compared to previous data (44% in 2019). However, it should be considered that from 9 March 2020 the new protocol on the procedures for assigning the heart became operational, which divides the urgencies into two levels: the grade 1 urgency which provides for the access to the national list of donors, and the grade 2 urgency that refers to donors in the relevant macro-area. The new allocation criteria recognize the LVAD as a ‘strategy of clinical stabilization and prolongation of survival’ and they emphasize the importance of evaluating each patient for LVAD before placing it on the HTx urgency list. This standpoint was to fill the gap that separates donors from potential recipients. Actually, in the year 2020 the total number of enrolments in the HTx list was 992 (including 2019 untreated patients and new entries in 2020), compared to a total number of transplanted patients of 238, corresponding to a list fulfilling index of 24%. The mortality rate while on the list was ∼4%, with an average waiting time of ∼3 years and 7 months for patients on the Standard list and 8 months for patients on the Emergency list.4
Xenograft: the story
Among the possible strategies to reduce the gap between the number of patients on the transplant list and the organs available, xenotransplantation appears promising.
The first xenotransplant attempt in humans was performed in 1964 by James Hardy, who implanted a chimpanzee heart in the chest of the 64-year-old Body Rush, who died two hours after the operation. Subsequently, other attempts were made at xenograft, both orthotopic and heterotopic, using hearts of different animal species (including chimpanzees, sheep, baboons, and pigs). However, all these attempts failed within hours or days mainly due to hyperacute rejection, microvascular thrombosis, or excessive discrepancy between the size of the donor and recipient heart.5
Since then, in order to understand the reasons for xenotransplant failure and find possible solutions, numerous preclinical studies have been performed, mostly exploiting HTx from pigs to non-human primates.5 In fact, although there is a greater similarity between non-human and human primates, a transplant between non-human and human primates carries an excessive infectious risk linked to the easy passage of animal viruses from the primate to the human species, with the risk of generating pandemics. For this reason, the Food and Drug Administration has banned xenotransplantation between non-human primates and humans.6 On the other hand, the hearts of pigs have numerous advantages: in addition to having a lower infectious risk, they are easily available, they have an adequate size to replace the human heart, and they are susceptible to genetic modifications. Nonetheless, non-human primates continue to be employed as receptor animals in preclinical studies due to their high similarity to humans. Table 1 summarizes the various types of pig to non-human primate transplants that have been performed and their rationale.7
Table 1.
Pig to non-human primate xenograft models
| Xenograft model | Description | Features | Accepted for clinical trials | Scientific rationale |
|---|---|---|---|---|
| Abdominal heterotopic | System with two anastomoses: the infrarenal aorta supports the coronary arteries of the donor's heart while the recipient's inferior vena cava drains blood from the donor's pulmonary artery | The pig's heart is contractile but cannot support the recipient's circulation | No | To study the mechanisms of rejection and the possible patterns of immunosuppression. |
| Orthotopic | The recipient's heart is replaced with that of the pig. | It can support circulation but with difficulty. | Yes | To study the mechanisms of rejection and the possible patterns of immunosuppression. Evaluate the hemodynamic efficacy of the transplant. |
| Intrathoracic heterotopic | The pig's heart is placed on the right side of the recipient's chest and supports circulation. | It combines the safety of heterotopic transplantation with the fact of having a heart capable of supporting circulation. | Yes | To study the mechanisms of rejection and the possible patterns of immunosuppression. Evaluate the hemodynamic efficacy of the transplant. |
The main barriers to the success of xenograft in the above-mentioned preclinical studies were immunological rejection and coagulation problems.5 The immunological barrier represents the greatest limitation to xenotransplantation, and it can lead to hyperacute, acute or chronic rejection. This immunological incompatibility was partially resolved thanks to the use of genetically modified pigs and the recipient's immunosuppression.5,8
Gene editing
Thanks to genetic engineering techniques it is possible to add, remove, or modify genetic material at certain sites of the deoxyribonucleic acid (DNA). With the recent creation of the CRISPR/Cas9 technique by E. Charpentier and J.A. Doudna, the genome editing process has become widely accessible. In the context of HTx, and in particular of xenotransplantation, these techniques have made it possible to optimize the survival of the graft and therefore to make xenotransplantation a real alternative to heart allograft.
Hyperacute rejection of the transplanted pig heart (minutes–hours) is due to the presence of preformed antibodies directed against carbohydrates present on pig epithelial cells. These carbohydrates, expressed on the membrane of intestinal bacteria and in the tissues of mammals, including pigs, are absent in humans and primates,9 which are therefore provided with an antibody response against them. The first of these antigens to be discovered was α-1,3-Galactose (α-Gal). Two other important antigens involved in hyperacute rejection are Neu5GC (N-glycolylneuraminic acid) and Sda (Sid antigen, named after the discoverer Sidney Smith). Through genomic editing techniques, it is possible to generate knock-out pigs for the genes of the mentioned antigens (respectively GGTA1, CMAH, and β4GalNT2) in order to obtain hearts that do not express α-Gal, Neu5GC, and Sda, thus minimizing the probability of hyperacute rejection of the organ.5,9
In addition to silencing certain genes, it is possible to induce the expression of proteins/enzymes useful for modulating different molecular processes in the recipient animal.5,8 In fact, there is an incompatibility between species that does not allow a correct interaction between the complement molecules and the coagulation factors of the recipient with the regulatory proteins of the donor and which contributes to acute organ rejection (days–weeks).9 The transgenic expression of human proteins regulating the coagulation cascade and complement in the donor pig [such as thrombomodulin, endothelial receptor for protein c, tissue factor inhibitor, and cluster of differentiation (CD) 39] seems effective in modulating the prothrombotic phenomena that are triggered after xenograft and increase the resistance of the transplanted organ to complement mediated damage.5,8
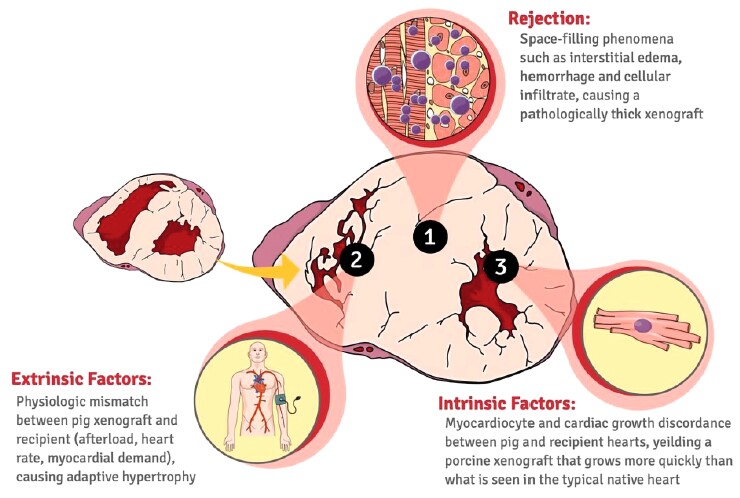
Gene editing has also enabled to address a further problem in xenotransplantation, that of the overgrowth of the donor organ in the recipient organism, resulting in dysfunction of the xenograft within one month of surgery. This phenomenon is supposed to be partly due to rejection phenomena, partly to hemodynamic differences between the different species and, above all, to the fact that the donor pig, for the same weight, is an organism still growing compared with the recipient baboon which has almost reached its target weight (Figure 1). A possible solution to this problem is represented by the knock-out of the growth hormone receptor in the donor's heart, which has been shown to reduce the growth of the xenograft in the recipient without the need for additional drugs.10
Figure 1.
Potential pathophysiological mechanisms underlying the excessive growth of the pig heart transplanted into the baboon (orthotopic transplant). Possible causes include an immunological response against xenograft, a discrepancy in physiological parameters, and a discordant growth potential between donor and recipient species.10
Finally, the inactivation by genetic engineering techniques of endogenous porcine retroviruses, which are integrated into the pig genome, has reduced the risk of transmission of potentially pathogenic viruses to humans.5
Currently, the main challenge lies in identifying the best combination of genetic modifications to reduce the risk of xenotransplantation failure.
Immunosuppression
The development over the years of an effective immunosuppression regimen has further improved the survival of animal xenograft models. As in cardiac allograft, immunosuppression involves two stages: induction and maintenance. For the induction, antithymocyte globulins and the anti-CD20 monoclonal antibody are used to suppress the response of T and B lymphocytes, respectively. Maintenance, on the other hand, involves the use of lymphocyte suppressants such as mycophenolate mofetil and, in the first two to three months, the use of corticosteroids and monoclonal antibodies that antagonize interleukin-6 and tumour necrosis factor-alpha.10
An innovative molecule introduced in this field is a monoclonal antibody that antagonizes CD40. CD40 is a costimulatory protein present on antigen-presenting cells and its interaction with CD154 in T lymphocytes is necessary to develop an effective immune response. Initially, a monoclonal antibody against CD154 was developed but its use resulted in an increased incidence of thromboembolic complications. Subsequently, the use of a monoclonal antibody against CD40, both during induction and maintenance, proved to increase the survival of the transplanted organ.10
It should also be considered that immunosuppressive therapy has been shown to be more effective than anticoagulant/antiplatelet drugs in reducing post-HTx procoagulative and prothrombotic phenomena, suggesting that the immune response is the main mechanism of coagulation alterations after transplantation.5,8
The necessary lifetime administration of immunosuppressive therapy carries an increased risk of infectious and neoplastic complications and direct toxicity induced by these drugs. A strategy to solve this problem could be represented by the induction of an immunological tolerance, whereby the recipient's immune system is modified to recognize the donor's antigens as ‘self’.5,8 A further strategy could be carrying out a knock in or a knock-out of porcine leukocyte antigens or immune system regulatory molecules in the donor animal. This would lead to a reduction in the adaptive immune response, but it could induce an immunodeficiency in the pig and make it more susceptible to infectious complications.11
The results of the xenograft experiments
The survival of hearts transplanted from pig to non-human primate is significantly increased thanks to genetic modifications of the donor heart and the improvement of immunosuppressive therapy in the recipient, reaching 945 days in the heterotopic transplant and 264 days in the orthotopic model.10 Limited to the available follow-up, late xenograft dysfunction due to vasculopathy has been rarely reported,5 unlike what happens in human allografts.
These advances in animal models were preparatory to the first pig-to-human compassionate use xenograft on 7 January 2022. The recipient was the 57-year-old David Bennett, who was placed in extracorporeal circulation for cardiogenic shock in the course of incessant ventricular tachycardia, but then he was judged unsuitable either for HTx or long-term mechanical support. The transplanted organ was obtained by modifying 10 genes, including the deletion of three porcine surface antigens to which humans have performed antibodies, the deletion of the gene coding for growth hormone and the introduction of six human genes involved in the immune response12 (of which, specifically, two genes with regulatory action on complement, two on coagulation, and two on inflammation). Immunosuppression induction and maintenance regimens were based on rituximab, anti-CD40, antithymocyte globulin, C1 esterase inhibitor, mycophenolate, and corticosteroids. The post-operative course was characterized by multiple complications, including suppurative peritonitis and generalized sepsis and inflammation requiring general surgery and intravenous immunoglobulin administration. The subsequent development of dilation and pseudohypertrophy of the graft with diastolic dysfunction and irreversible heart damage led to the suspension of vital supports with death two months after surgery. The xenograft, on post-mortem examination, was markedly oedematous with infarct areas and endothelial damage,13 albeit in the absence of thrombosis which is usually present in allograft rejection. However, a new form of rejection with cellular and biohumoral mechanisms still to be studied cannot be excluded. Furthermore, death could have been favoured by the administration of intravenous immunoglobulins, as well as by the presence, probably in latent form, of porcine cytomegalovirus in the transplanted organ, although all donor pigs are routinely screened to identify and remove this virus.14
Future prospects and innovations
Several obstacles still limit the affirmation and diffusion of xenotransplantation as an alternative to allograft. At the same time, other therapeutic options are being explored to provide useful solutions to address the growing needs of HTx, including total artificial heart, myocardial regeneration and gene therapy. ‘Artificial heart’ devices have been already designed and tested on humans. The best results were obtained with SynCardia, a device consisting of artificial ventricles and mechanical valves, which guaranteed survival beyond one year up to transplantation in 72% of the 42 patients supported with SynCardia with only two deaths attributable to the device, however at price of a high rate of ischaemic, haemorrhagic, and infectious complications.15 In the field of myocardial regeneration, the bioartificial heart is the most ambitious project, aimed at building the entire organ in vivo starting from a decellularized porcine heart scaffold transplanted in a heterotopic site with the aim of being repopulated by human cells which, under adequate mechanical and electrical stimulation, can mature and organize themselves in parenchyma and vessels.16 Complete removal of porcine cells, the main source of tissue immunogenicity, could minimize or eliminate the need for immunosuppressive therapy. Another frontier in the treatment of advanced HF is gene therapy; the possibility of inducing angiogenesis in the ischaemic heart or modulating cardiac function in HF through small ribonucleic acids (RNAs) causing targeted modifications of myocardial cells’ gene expression could obviate the need to resort to HTx.17
Conclusions
Heart xenograft poses complex issues of advanced surgery, molecular biology, gene therapy, and immunosuppression. Advances in genetic engineering have made this approach a promising alternative to cardiac allograft. Although at the moment there are major limitations to its structured use, among the main ones the infectious risk (linked to the transmission of porcine viruses) and late rejection (not yet explored in humans), heart xenotransplantation heralds the possibility of contributing to the needs of those patients with advanced HF who still lack treatment options today.
Contributor Information
Gianfranco Sinagra, Cardiothoracovascular Department, Cardiology, Giuliano Isontina University Health Authority (ASUGI), University of Trieste. European Reference Network for rare, low-prevalence, or complex diseases of the Heart (ERN GUARD-Heart).
Linda Pagura, Cardiothoracovascular Department, Cardiology, Giuliano Isontina University Health Authority (ASUGI), University of Trieste. European Reference Network for rare, low-prevalence, or complex diseases of the Heart (ERN GUARD-Heart); Cardiothoracovascular Department, Cardiac Surgery, Giuliano Isontina University Health Authority (ASUGI).
Cinzia Radesich, Cardiothoracovascular Department, Cardiology, Giuliano Isontina University Health Authority (ASUGI), University of Trieste. European Reference Network for rare, low-prevalence, or complex diseases of the Heart (ERN GUARD-Heart).
Giulia Gagno, Cardiothoracovascular Department, Cardiology, Giuliano Isontina University Health Authority (ASUGI), University of Trieste. European Reference Network for rare, low-prevalence, or complex diseases of the Heart (ERN GUARD-Heart).
Antonio Cannata’, Cardiothoracovascular Department, Cardiology, Giuliano Isontina University Health Authority (ASUGI), University of Trieste. European Reference Network for rare, low-prevalence, or complex diseases of the Heart (ERN GUARD-Heart).
Davide Barbisan, Cardiothoracovascular Department, Cardiology, Giuliano Isontina University Health Authority (ASUGI), University of Trieste. European Reference Network for rare, low-prevalence, or complex diseases of the Heart (ERN GUARD-Heart).
Marco Cittar, Cardiothoracovascular Department, Cardiology, Giuliano Isontina University Health Authority (ASUGI), University of Trieste. European Reference Network for rare, low-prevalence, or complex diseases of the Heart (ERN GUARD-Heart).
Alessia Paldino, Cardiothoracovascular Department, Cardiology, Giuliano Isontina University Health Authority (ASUGI), University of Trieste. European Reference Network for rare, low-prevalence, or complex diseases of the Heart (ERN GUARD-Heart).
Maria Perotto, Cardiothoracovascular Department, Cardiology, Giuliano Isontina University Health Authority (ASUGI), University of Trieste. European Reference Network for rare, low-prevalence, or complex diseases of the Heart (ERN GUARD-Heart).
Marco Mase’, Cardiothoracovascular Department, Cardiology, Giuliano Isontina University Health Authority (ASUGI), University of Trieste. European Reference Network for rare, low-prevalence, or complex diseases of the Heart (ERN GUARD-Heart).
Matteo Dal Ferro, Cardiothoracovascular Department, Cardiology, Giuliano Isontina University Health Authority (ASUGI), University of Trieste. European Reference Network for rare, low-prevalence, or complex diseases of the Heart (ERN GUARD-Heart).
Enzo Mazzaro, Cardiothoracovascular Department, Cardiac Surgery, Giuliano Isontina University Health Authority (ASUGI).
Marco Merlo, Cardiothoracovascular Department, Cardiology, Giuliano Isontina University Health Authority (ASUGI), University of Trieste. European Reference Network for rare, low-prevalence, or complex diseases of the Heart (ERN GUARD-Heart).
References
- 1. Lund LH, Edwards LB, Kucheryavaya AYet al. . The registry of the international society for heart and lung transplantation: thirtieth official adult heart transplant report–2013; focus theme: age. J Heart Lung Transplant 2013;32:951–164 . [DOI] [PubMed] [Google Scholar]
- 2. McDonagh TA, Metra M, Adamo Met al. . ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the task force for the diagnosis and treatment of acute and chronic heart failure of the European society of cardiology (ESC) with the special contribution of the heart failure association (HFA) of the ESC. Eur Heart J 2021;42:4901. [DOI] [PubMed] [Google Scholar]
- 3. DeFilippis EM, Clerkin K, Truby LKet al. . ECMO As a bridge to left ventricular assist device or heart transplantation. JACC Heart Fail 2021;9:281–289. [DOI] [PubMed] [Google Scholar]
- 4. Centro Nazionale Trapianti . Donazione & trapianto di organi, tessuti e cellule staminali emopoietiche; Valutazione di qualità dell'attività del trapianto di cuore 2000–2018; Attività annuale Rete Nazionale Trapianti. Report 2020; Regolamento delle urgenze di cuore in ambito nazionale e di macroarea. Available online at: https://www.trapianti.salute.gov.it.
- 5. Shu S, Ren J, Song J. Cardiac xenotransplantation: a promising way to treat advanced heart failure. Heart Fail Rev 2022;27:71–91. [DOI] [PubMed] [Google Scholar]
- 6. Schoenrath F, Falk V, Emmert MY. Xenotransplantation in the era of a zoonotic pandemic. Eur Heart J 2021;42:1283–1285. [DOI] [PubMed] [Google Scholar]
- 7. Postrach J, Bauer A, Schmoeckel M, Reichart B, Brenner P. Heart xenotransplantation in primate models. Methods Mol Biol 2012;885:155–168. [DOI] [PubMed] [Google Scholar]
- 8. Cooper DKC, Ekser B, Tector AJ. Immunobiological barriers to xenotransplantation. Int J Surg 2015;23:211–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pierson RN, Fishman JA, Lewis GDet al. . Progress toward cardiac Xenotransplantation. Circulation 2020;142:1389–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mohiuddin MM, Goerlich CE, Singh AKet al. . Progressive genetic modifications of porcine cardiac xenografts extend survival to 9 months. Xenotransplantation 2022;29:e12744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cooper DKC, Hara H. You cannot stay in the laboratory forever”: taking pig kidney xenotransplantation from the laboratory to the clinic. EBioMedicine 2021;71:103562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rabin RC. In a First, Man Receives a Heart From a Genetically Altered Pig. The New York Times. Gen 10, 2022. Available online at: https://www.nytimes.com/2022/01/10/health/heart-transplant-pig-bennett.html.
- 13. Braunwald E. Cardiac xenotransplantation: a new path for the treatment of advanced heart failure? Eur Heart J 2022;43:3014–3015. [DOI] [PubMed] [Google Scholar]
- 14. Regalado A. The gene-edited pig heart given to a dying patient was infected with a pig virus. MIT Technology Review. May 4, 2022. Available online at: https://www.technologyreview.com/2022/05/04/1051725/xenotransplant-patient-died-received-heart-infected-with-pig-virus.
- 15. Torregrossa G, Morshuis M, Varghese Ret al. . Results with SynCardia total artificial heart beyond 1 year. ASAIO J 2014;60:626–634. [DOI] [PubMed] [Google Scholar]
- 16. Taylor DA, Frazier OH, Elgalad A, Hochman-Mendez C, Sampaio LC. Building a total bioartificial heart: harnessing nature to overcome the current hurdles. Artif Organs 2018;42:970–982. [DOI] [PubMed] [Google Scholar]
- 17. Cannata A, Ali H, Sinagra G, Giacca M. Gene therapy for the heart lessons learned and future perspectives. Circ Res 2020;126:1394–1414. [DOI] [PubMed] [Google Scholar]