Abstract
The therapy of transthyretin (TTR)-related cardiac amyloidosis consists, on the one hand, of the prevention and management of complications (supportive therapy) and on the other of treatments aimed at interrupting or slowing down the production and deposition of fibrils (disease-modifying therapy). This definition includes drugs that act on different phases of amyloidogenesis: (i) silencing of the gene encoding TTR (small interfering RNA: patisiran, vutrisiran; antisense oligonucleotides: inotersen, eplontersen; new CRISPR Cas-9 drug technology for editing in vivo DNA); (ii) stabilization of circulating TTR to inhibit its dissociation and subsequent assembly of the resulting monomers in amyloidotic fibrils (tafamidis, acoramidis, and tolcapone); (iii) destruction and re-absorption of already formed amyloid tissue deposits. Drugs related to the latter strategy (antibodies) are still the subject of Phase 1 or 2 studies.
Keywords: Cardiac amyloidosis, Disease-modifying therapy, transthyretin
Introduction
Amyloidosis consists of a heterogeneous group of conditions characterized by the accumulation in the extracellular area of insoluble fibrils composed of proteins mistakenly assembled through the amyloidogenesis process. These cause tissue damage and dysfunction of various organs, including the heart.1
Currently, 36 types of amyloidosis are known in relation to the precursor protein; the most frequent forms include transthyretin (TTR)-related amyloidosis, of which there are hereditary forms (ATTRv), a wild-type form (ATTRw), a group secondary to the accumulation of immunoglobulin light chains (AL) and the amyloidosis secondary to chronic inflammatory diseases (AA).2
Transthyretin is a tetramer consisting of identical monomers, each of 127 amino acids with beta-sheet morphology. It is mainly synthesized in the liver and released into the plasma, where it binds and transports thyroxine and the protein that binds retinol. The amyloidogenesis process first requires dissociation of the tetramer into monomers, followed by reorganization to form insoluble fibrils. The instability that leads to the dissociation of native TTR can be secondary to pathogenic mutations in the TTR gene (130 known mutations) or dependent on senescence (Figure 1). Biopsy studies have also suggested further pathogenic mechanisms underlying the formation of fibrils, starting from the evidence of different types of deposits (Type A fibrils consisting of TTR and truncated fragments and Type B fibrils formed by TTR) identifiable in ATTRv amyloidosis associated with different mutations. This molecular variability has been hypothesized to be linked to distinct disease phenotypes as well as a different response to treatment.3
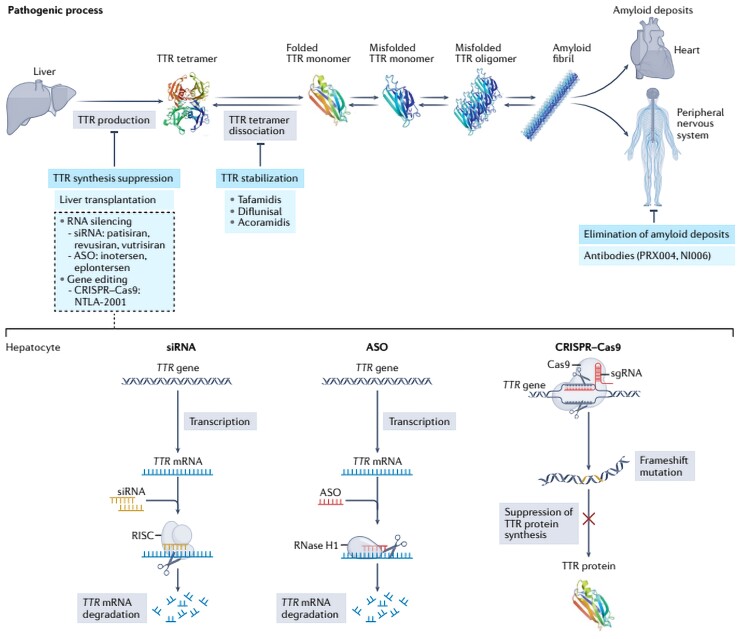
Figure 1.
Overview of the treatments available for transthyretin amyloidosis and their mechanism of action. Therapies that aim to inhibit transthyretin production include liver transplantation, small interfering RNA (transthyretin) gene silencers, and DNA editing therapies (CRISPR Cas9). The lower part of the figure summarizes their type of interaction with nucleic acids. The main stabilizing drugs of transthyretin are represented by tafamidis, diflunisal and acoarmidis. Some antibodies that bind to transthyretin have the potential to trigger immune-mediated amyloidolysis. Taken from Aimo et al.5
The therapy of TTR-related cardiac amyloidosis consists of the prevention and management of complications (supportive therapy) and the treatments aimed at interrupting or slowing down the organ damage secondary to the deposition of the fibrils (disease-modifying therapy). Supportive treatment is aimed at the management of heart failure, the prevention of arrhythmias and conduction disorders and the correction of valve defects. Vice versa, disease-modifying therapy acts on different phases of amyloidogenesis with the aim of changing the natural history of the disease. It can act upstream of the cascade by inhibiting the synthesis of TTR by hepatocytes (from orthotopic liver transplantation to the most recent gene silencing therapy), or it can stabilize the TTR tetramer to inhibit dissociation and subsequent assembly that form pathogenic fibrils.2,4 There is also a third line of therapeutic intervention aimed at the destruction and re-absorption of the already formed tissue deposits of amyloid; for the moment, however, none of the drugs referable to this strategy is already available in clinical practice.5
This review is dedicated to the disease-modifying therapy of ATTR and takes into consideration, chronologically, the different strategies that have occurred and overlapped, as well as possible future developments.
Stabilizers of the transthyretin
The destabilization of the TTR molecule, whose final stage is amyloidogenesis, begins with the dissociation of the TTR tetramers into monomers, which then lose their original structure according to a process called ‘misfolding’ and begin to aggregate, forming first oligomers and subsequently fibrils of amyloid substance. This critical process can occur because of aging in the wild-type form or be secondary to a pathogenetic mutation of the TTR gene. About 130 mutations are currently known. In any case, the crucial step in the amyloidogenesis process (rate-limiting of the entire cascade) is the stabilization by dissociation of the tetrameric structure of TTR into single monomers.6 Understanding this process, led to the synthesis of small molecules (typically tafamidis) capable of binding to critical points of the molecule and strengthening the bond between monomers.
It should be noted that the development of these drugs, typically tafamidis, occurred by mimicking a mechanism that is physiologically present in nature. In fact, alongside pathogenic mutations, there is a mutation (T119M) capable of stabilizing the molecule to the point of cancelling the pathogenic effects of other mutations possibly coexisting in the same gene.6 Tafamidis is the progenitor of this class of drugs of selective stabilizers of TTR. It is currently approved by regulatory agencies for the treatment of forms of polyneuropathy, as well as for the treatment of ATTR cardiomyopathy (the only approved drug for this indication).
Selective stabilizers
Tafamidis
Tafamidis is a derivative of benzoxazole, which acts by binding to the TTR tetramer in the portion of the protein responsible for binding thyroxine with high affinity and selectivity, thus inhibiting its dissociation into monomers. Tafamidis has been shown to slow disease progression and reduce neurological symptoms in patients with the Val30Met mutation.7 After initial Phase I and Phase II studies, a randomized, controlled, and double-blind trial involving 441 patients with wild-type or mutated cardiac amyloidosis, the ATTR-ACT trial, was published in 2018.8
Therapy with tafamidis reduced all-cause mortality and cardiovascular-related hospitalization compared with placebo. Furthermore, at the 30th month of follow-up, patients on therapy had a minor decline in 6MWT and KCCQ-OS score.9 The benefit was greatest for patients in NYHA Class I and II, while patients in NYHA Class III showed an increase in hospitalizations probably because the survival of this subgroup of patients with a more advanced stage of disease was increased. A recent sub-analysis of the ATTR-ACT investigated differences in the therapeutic response between the mutated and wild-type forms. Subjects with the mutant form had a worse long-term prognosis than the wild-type form in the placebo and the randomized tafamidis group. This was most likely due to the increased clinical severity of the patients with the mutant form at the time of randomization. Despite this, the reduction in the primary outcome (all-cause mortality and hospitalizations for heart failure) and the functional decline was similar in both mutated and wild-type group. Even more reassuring data on the drug’s safety profile and efficacy can be obtained from long-term follow-up studies. The ATTR-ACT study was not designed to compare the different therapeutic doses of the drug (80 vs. 20 mg): tafamidis 80 mg reduced the relative risk of mortality by 30% compared with the 20 mg dose with a concomitant reduction in NT-proBNP and without any change on the safety outcome.10
Follow-up data up to 58 months were recently published, not only tafamidis therapy continued to provide a survival benefit over placebo, but this was also evident in patients with more advanced disease. First, the curves of NYHA III patients who initially did not have a benefit in the ATTR-ACT also began to diverge in a statistically significant manner with a longer follow-up. Second, patients who started therapy late when switching from the placebo arm to the tafamidis arm also had an improvement in survival compared with an arm of patients who continued placebo therapy extrapolated from the original study.11
Other selective stabilizers under study
AG10
AG10 (acoramidis) is a potent selective stabilizer of TTR which has been designed, similarly as for tafamidis, to mimic the protective influence that the T119M mutation has on the TTR tetramers, however, having the peculiarity of forming a hydrogen bond with the same serine residues in position 117 that stabilize the T119M variant. In some in vitro studies, its stabilizing power appears to be greater than that of tafamidis.
A Phase II study conducted on 49 patients with amyloid heart disease demonstrated the tolerability of AG10 and the ability to reaching adequate plasma concentration levels and almost completely stabilizing TTR.12
A Phase III study (ATTRIBUTE-CM) is currently underway. This multicentre, randomized, double-blind study enrolled 632 participants and consists of two survey phases: Phase A, with a 12-month follow-up aims to assess the variation in 6MWT, Phase B, with a follow-up of 30 months, will focus on reducing all-cause mortality and hospitalization for cardiovascular causes. At the present time, only Phase A has come to an end. In this phase acoramidis (dose of 800 mg twice a day) has not reached the primary endpoint, represented by an improvement in 6MWT. However, the treatment showed an improvement in quality of life and a reduction in plasma concentrations of NT-proBNP.13
Tolcapone
Tolcapone is a molecule already used in the therapy of Parkinson’s disease and is able to bind to the binding sites of T4 with TTR, stabilizing it in a similar way to what is done by tafamidis and acoramidis. A particularly interesting feature of the drug is its ability to overcome the blood-brain barrier. A subgroup of variant forms of TTR is characterized specifically by a leptomeningeal and retinal localization of the disease, supported by a local production of TTR; unfortunately, tafamidis is not able to control these manifestations, not passing the blood-brain barrier and therefore not acting on the TTR produced by the choroid plexuses and the retina. The tolcapone study is currently underway.14,15
Non-selective stabilizers
Diflunisal
Diflunisal falls into the category of non-steroidal anti-inflammatory drugs and manages to occupy the portions of the TTR responsible for binding with thyroxine, thus stabilizing it.
Initially, doubts arose about its use in patients with heart failure, as a chronic inhibition of cyclo-oxygenases can aggravate a pre-existing condition of kidney dysfunction leading to fluid overload and arterial hypertension.
At present, in fact, the only randomized placebo trial on diflunisal has been conducted in patients with mutant TTR variants suffering from polyneuropathy. In this context, diflunisal was able to halt the progression of the disease by improving mental status and quality of patient life at 24 months of follow-up.16
Clinical data in patients with amyloid heart disease can be drawn from individual case reports centres and a single single-arm open-label study.17 In selected patients, where it has been excluding renal failure and advanced heart failure, diflunisal appears to be effective and safe when combined with proton pump inhibitors. In any case, in the absence of more solid data, its use is limited for now.
Transthyretin gene silencers
The second category of drugs for treating TTR-related amyloidosis is represented by molecules which, through the silencing of the TTR gene within hepatocytes, reduce the production of circulating TTR, influencing the natural history of the disease. This category includes:
Small interfering RNAs (siRNA) (patisiran, revusiran, and vutrisiran).
Antisense oligonucleotides (ASO) (inotersen and eplontersen).
The new DNA editing technology.
The liver transplant performed for the first time in the ATTRv in the early 90s already represents the first surgical-gene therapy, definitively silencing the production of mutated forms of TTR. The transplant proved to be particularly effective in the forms with exclusive neurological involvement (Val30Met in Portugal) in the presence of other mutations with mixed phenotype, the distant results are somewhat disappointing due to the resumption of the formation of amyloid deposits (in this case wild-type) favoured by the existence of small amyloid deposits before the transplant itself (nest effect).
Antisense oligonucleotides
Antisense oligonucleotides are synthetic single-stranded oligonucleotides with a length of 16–20 base pairs which, by binding to target mRNA, promote their degradation through various mechanisms, including the endogenous ribonuclease (RNAase H1). These molecules are widely used in treating various conditions such as cytomegalovirus retinitis, Duchenne muscular dystrophy and homozygous familial hypercholesterolaemia.18
Inotersen
It is a subcutaneous injectable formulation rapidly absorbed and bound by plasma proteins. Within 24 h of administration, a reduction of more than 90% in Cmax is observed, indicative of a rapid molecule transfer into the tissues. The half-life is one month and is eliminated in the urine5.
The NEURO-trial (Phase III, double-blind and placebo-controlled trial) investigated the efficacy and safety of inotersen 300 mg once weekly for 15 months in a population of 172 patients with Stages I- and II-related TTRv polyneuropathy. In terms of primary efficacy endpoint, inotersen demonstrated a significant improvement in the neurological picture at 66 weeks [−19.7 points in the modified Neuropathy Impairment Score + 7 (mNIS + 7) and −11.7 points in the Norfolk Quality of Life score Diabetic Neuropathy (Norfolk QOl-DN)]. There was also a significant and stable reduction in circulating TTR concentration at 13 weeks (−79%), without, however observing a significant correlation between protein reduction and clinical improvement. However, significant differences were observed between the two groups in terms of echocardiographic variables [wall thickness, left ventricular (LV) mass, LV ejection fraction, E/e′ wave] in the 15-month follow-up. Regarding the safety, five deaths were reported in the treatment group, including four related to the progression of the underlying disease and one due to intracranial haemorrhage secondary to severe thrombocytopenia (<10 000 platelets/m3). In fact, in 54% of treated patients, there was a reduction in platelet count below 140 000/m3, presumably secondary to immune-mediated mechanisms (given the improvement during glucocorticoid therapy and the presence of circulating anti-platelet antibodies). Three cases of glomerulonephritis have also been reported, two of which were associated with a reduced glomerular filtrate. Among the minor side effects, nausea, fever, vomiting, and anaemia have been reported in part also related to the underlying disease.5,19
Inotersen has been approved by the EMA and the FDA for the treatment of patients with Grade I and II TTRv-related polyneuropathy at a dose of 284 mg/week; contraindicated in the presence of thrombocytopenia (<100 000/m3), GFR <45 mL/min or urinary protein/creatinine ratio >1 g/g).5
Eplontersen
It is an ASO having the same sequence as inotersen but conjugated to 3-year-old N-acetyl galactosamine, which facilitates its uptake by hepatocytes to increase its ability to reduce the expression of the TTR gene. Like inotersen, eplontersen is also administered subcutaneously and is characterized by rapid systemic distribution and a half-life of 2–6 weeks 20; the Phase I trial conducted on healthy volunteers showed no adverse effects other than headache and transient elevation of ALT and CPK.5
A Phase II clinical trial is currently underway that enrols subjects with ATTR-CM who have completed 24 months of treatment with inotersen and are proposed to switch to eplontersen; the study has as its primary endpoint the trend of echocardiographic, laboratory and functional parameters and the results are expected in 2025. A Phase III trial is also underway on subjects affected by Stage I and II TTR polyneuropathy which will end in 2024. Finally, a trial will begin in 2024 that will enrol 750 subjects affected by ATTR-CM with the primary efficacy endpoint of reducing adverse cardiovascular events at 120 weeks.5
Small interfering RNA
Small interfering RNA are a class of drugs capable of binding complementary mRNA molecules and causing their degradation, blocking the expression of both mutated and wild-type TTR.
Patisiran
Patisiran, a molecule encapsulated in lipid nanoparticles, administered by intravenous infusion, and internalized specifically by hepatocytes, belongs to this class of drugs. Already in the first phase 2 studies, it was shown that two doses of 0.3 mg/kg of patisiran every 3 weeks reduce serum TTR levels by about 80%, with a maximum reduction of 96%.20 This inhibition was observed for both ATTRwt amyloidosis and ATTRv (V30M), as well as in patients already being treated with TTR tetramer stabilizing drugs. There was also an improvement in the scores for neuropathy compared with patients treated with placebo. Subsequently, in the Phase III APOLLO study, a randomized, double-blind study enrolling 225 patients with ATTRv amyloidosis and polyneuropathy, patients treated with patisiran (0.3 mg/kg once every 3 weeks for 18 months) showed significant improvement in neuropathy, with an improvement in the mNIS + 7 scale in 56% in patients treated with patisiran (compared with 4% in patients treated with placebo). The incidence of serious adverse effects found in the different studies is similar in patients treated with patisiran or placebo (28% vs. 36%); the most commonly reported adverse effects with patisiran include peripheral oedema, infusion-related reactions and visual disturbances. These results led to the approval of patisiran for treating adults with ATTRv amyloid polyneuropathy in both the USA and the European Union. However, in Europe, patisiran has only been approved for the treatment of mild to moderate polyneuropathy (Stages 1 and 2).
Interestingly, in a sub-analysis of the APOLLO study, which studied patients with echocardiographic evidence of cardiac involvement (patients with LV hypertrophy with no history of aortic stenosis or arterial hypertension), patients treated with patisiran showed, compared with the placebo group, a reduction in the degree of LV dysfunction, an improvement in the longitudinal ventricular strain, an increase in cardiac output, a slight decrease in thickness, and a reduction in the rate of hospitalizations and all-cause mortality.21 These results show that patisiran not only slows the progression of LV functional damage but also promotes reverse remodelling (i.e., recovery of LV structure and function); this effect has not so far been demonstrated for inotersen and is currently being evaluated for tafamidis. The APOLLO-B study is now underway, aimed at investigating the impact of patisiran, specifically in patients with amyloid heart disease who have never taken other disease-modifying therapies.22
Revusiran
Another siRNA, revusiran, was investigated in a Phase 1 study (ENDEAVOUR), aimed at assessing functional capacity and serum TTR levels in 200 patients with ATTRv amyloid-induced cardiomyopathy.23 Patients were randomized 2:1 to the revusiran or placebo arms, with revusiran administered subcutaneously, 500 mg daily for 5 months and then once weekly for 18 months. The study was stopped due to the excessive cardiac mortality found in patients treated with revusiran. Although the causes were not clarified, the pharmacological evaluation of revusiran was stopped.
Vutrisiran
Vutrisiran, also known as ALN-TTRsc02, is a siRNA which is administered percutaneously, and which allows administration at greater intervals than patisiran, approximately every 3 months. The phase III HELIOS-A study is currently underway, where already at 9 months, it was shown that in patients in the vutrisiran group, there was an improvement in neurological symptoms assessed by the mNIS + 7 and Norfolk OOL-DN scales.24 Another study whose results are awaited is the phase III HELIOS-B study, in which patients with TTR-related amyloid heart disease, both wild-type and mutated forms, are evaluated to evaluate the possible effects of the drug also in terms of cardiac involvement.25
DNA editing
CRISPR Cas9 editing
This is a genomic editing strategy based on using a nuclease (Cas9) linked to a single-stranded RNA capable of presumably irreversibly silencing the target gene. In fact, after a single administration, a 97% reduction in serum TTR for up to 12 months has been shown in transgenic mouse models. Considering this, Gillmore et al.26 performed a Phase I trial on subjects suffering from TTRv polyneuropathy not previously treated with gene silencers, in which there was a significant reduction in serum TTR following a single administration. This strategy would present an advantage over ASO siRNAs which require serial infusions.
Drugs that promote the degradation and re-absorption of amyloid deposits at tissue level
Despite the undoubted ability to stabilize TTR or to silence the genes that produce it, none of the drugs previously described can significantly affect the amyloid deposits already formed. In the ATTR-ACT study, after 30 months of treatment, there was no significant reduction in LV wall thickness compared with placebo.8 In a sub-analysis of the APOLLO study relating to patients with mixed phenotype, therefore with cardiac involvement, a reduction in wall thickness compared with placebo was found, which was not confirmed in the general study population, including also subjects not meeting the criteria for amyloidotic cardiomyopathy.21
Two lines of intervention have been tested in recent years:
chemical degradation of deposits and
immune-mediated amyloidolysis.
Epigallocatechin-3-gallate
Epigallocatechin-3-gallate is the catechin most present in green tea. In the face of anecdotal reports, no controlled clinical trials have ever been developed. This therapy, therefore, remains devoid of scientific evidence. It binds the soluble TTR tetramers reducing the likelihood of dissociation, inhibits the aggregation of oligomers and promotes the breakdown of TTR fibrils. A placebo-controlled study in subjects with TTR-related amyloidotic cardiomyopathy (both wild-type and hereditary) showed neither increased survival nor improved echocardiography and NT-proBNP.27
Doxycycline and tauroursodeoxycholic acid
Combination therapy with doxycycline and tauroursodeoxycholic acid (TUDCA) has been shown to be effective in promoting the removal of amyloid fibrils in transgenic mice carrying the Val30Met mutation.28 The combination of these molecules, in fact performs a synergistic action on different steps of amyloidogenesis and tissue damage: doxycycline destructures the fibrils, facilitating their re-absorption, while TUDCA reduces the toxicity of TTR fibrils. In a small trial involving 28 subjects, 1 with TTR-wild type amyloidosis and the others with TTRv forms, there was a dropout of 86% of subjects secondary to side effects and ineffectiveness of the drug (with an increase in NT-proBNP >30%).29 A Phase II study on 20 subjects (85% represented by forms of ATTRv) demonstrated non-progression of cardiomyopathy and stability of the neurological picture with 12-month treatment.29
Immune-mediated amyloidolysis
New therapeutic strategies are now becoming increasingly important based on the use of monoclonal antibodies, able to bind to specific points of the fibrils already formed and therefore to opsonize the deposits, exposing them to the activity of native immunity and ultimately to the destructive action of macrophages.
It should be noted that the binding points with antibodies can be located both directly on the fibrils and on other proteins that constantly accompany amyloid deposits such as serum amyloid protein (SAP).
The first to be studied were antibodies binding to SAP, a plasma glycoprotein produced by hepatocytes that represents one of the scaffold proteins of amyloid deposits in all tissues.30 In a Phase I study conducted in patients with systemic amyloidosis, the use of anti-SAP antibodies had shown a reduction in the accumulation of fibrils in the various organs; however, a Phase II study in patients with cardiac amyloidosis was terminated prematurely due to an apparent change in the benefit/risk profile in this type of patient.
In the context of AL amyloidosis, the subject of study is the monoclonal antibody CAEL-101; in a Phase IA/B study it was shown that CAEL-101 mAb infusions were well tolerated, and most of the patients treated showed an important haematological response, with improvement in organ function (especially in the case of heart involvement), with a median response time of 3 weeks.31
The NEOD-001 monoclonal antibody was evaluated in the Phase III study VITAL, where it was shown that there were no statistically significant differences between patients treated with the drug compared with patients in the placebo group32; however, a post hoc analysis suggests the potential survival benefit in patients at high risk of mortality (Mayo Stage IV).
In the context of TTR-related amyloidosis, a monoclonal antibody PRX004 was developed; In a Phase, I study (terminated prematurely due to the COVID-19 pandemic), in seven patients with ATTRv-CA not only was the safety of the drug demonstrated but there was also an improvement in neurological symptoms and heart function after only 9 months of treatment. An open-label Phase I study is currently underway in which 21 patients with ATTRv have been enrolled to determine the safety and tolerability of the molecule.33 Others studied are Ab-A and NI006, which showed affinity in binding both ATTRv and ATTRwt, with the ability to eliminate amyloid deposits both ex vivo on autopsy findings and in vivo on mice with human TTR-graft at the cardiac level.34,35 The Phase I study for NI006 is still ongoing, in which secondary clinical endpoints such as 6MWT, NT-proBNP and TnT will be explored.
Huge milestones have been achieved over the last few years. However, there is still a need to translate these results from the bench to clinical practice so that patients can benefit directly from them.
The prescription of disease-modifying drugs for the ATTR
At present, the only treatments that are safe and effective and that can be prescribed for the treatment of TTR-related amyloidosis are tafamidis and patisiran. Based on the results of the ATTR-ACT study, tafamidis 61 mg received approval from the US regulatory authority in 2019 and from the European regulatory authority in 2020 for the treatment of TTR cardiac amyloidosis, both in the genetic variant and in the wild-type form.8
Patisiran, considering the results of the APOLLO study, is currently only indicated to treat patients with variant TTR-related amyloidosis and neurological or mixed phenotype, with Stage 1 and Stage 2 polyneuropathy. Its approval by the European and US regulatory body took place in 2018.
Although tafamidis is the first disease-modifying therapy specifically approved for the treatment of amyloid cardiomyopathy, it is important to consider that it is a long-term, expensive therapy that shows clinically tangible efficacy after a medium to long-term treatment. Hence the enormous importance of defining reliable criteria to predict and monitor the effectiveness of the treatment.
Indications and reimbursement of treatment with tafamidis
In Italy, tafamidis 61 mg can be prescribed in patients with clinical–instrumental evidence of NYHA Class II or I amyloid cardiomyopathy (if there are or have been signs of heart failure).
At what stages of the disease is it most appropriate to initiate disease-modifying therapy? In an ideal world, as soon as possible, but you must deal with economic problems and also have to realize that treatment can be futile both if started too early and if started too late (e.g. at a very old age or in an advanced stage of heart disease). Therefore, which is the ideal candidate?
While it seems logical to exclude patients in NYHA Class IV, which was not studied in the ATTR-ACT trial, the decision to exclude patients in NYHA Class III was partly driven by a less clear benefit of treatment in these patients (who had a lengthening of life expectancy but at the expense of a greater number of hospitalizations). On the one hand, starting treatment in the advanced stages of the disease is not considered convenient as well as clinically useful. On the other hand, the question arises of when to start treatment in the earliest stages of the disease. In fact, a recent study carried out at the NAC of London showed that patients considered in NAC Stage Ia (category of patients effectively excluded from the ATTR-ACT study), i.e. those who receive an equivalent dose of furosemide <0.75 mg/kg and who have an NT-proBNP ≤500 or ≤1000 ng/L in the presence of AF, showed a life expectancy substantially overlapping the general population with similar sex and age.36 Therefore, given the economic weight of the treatment, at present, these data discourage the routine initiation of early treatment with tafamidis in such patients.
Early identification of responders and treatment monitoring
Long-term follow-up data on patients with variant amyloidosis and neurological phenotype treated with tafamidis showed that ∼34% of patients show the complete arrest of disease progression, 36% partial arrest, and 30% progression, i.e. they are considered non-responders. It is interesting to note how a low severity of the disease, female sex, and high native circulating TTR values at the start of treatment predict a positive response to tafamidis.37
The importance of identifying the patient responder is inherent in the fact that patients who do not respond optimally to treatment should be treated with a different drug or with several drugs at the same time. But how can we identify non-responders? Unlike AL amyloidosis, where circulating antibodies easily allow to evaluate the response to treatment of the underlying haematological disease, with the help of other biomarkers (such as natriuretic peptides and troponin), in the case of TTR amyloidosis the question becomes more complex. Certainly, regarding treatment with tafamidis, the degree of stabilization of TTR may represent a marker of response. In the ATTR-ACT study, the fixed dose of 80 mg of tafamidis ensured a degree of stabilization that did not require monitoring of circulating TTR and its degree of stabilization. This may be true in most patients, but stabilization is not necessarily maximal in some individuals with rare mutations (not present in the ATTR-ACT study). The stabilization of TTR can be measured directly with various methods, not currently applicable in the routine clinical practice. Alternatively, the dosage of circulating TTR can be considered an indirect measure of the degree of stabilization of its tetramers. Furthermore, circulating TTR values were found to be a prognostic index in patients treated with TTR stabilizers.38
Currently what are the optimal biomarkers to evaluate the response to therapy in amyloid heart disease remains in doubt. Future studies will evaluate which non-invasive markers can predict response to treatment and allow choosing the best therapy. Currently, serial measurement of troponin, NT-proBNP, ECG and measurement of echocardiographic parameters (e.g. myocardial thickness, longitudinal ventricular strain, ejection fraction, myocardial contraction fraction, and stroke volume) and magnetic resonance imaging (e.g. extracellular volume), appears to be the best way to assess disease progression, both for their cost, their safety profile and reproducibility.
Contributor Information
Anna Cantone, Cardiovascular Centre, University of Ferrara, Italy.
Federico Sanguettoli, Cardiovascular Centre, University of Ferrara, Italy.
Beatrice Dal Passo, Cardiovascular Centre, University of Ferrara, Italy.
Matteo Serenelli, Cardiovascular Centre, University of Ferrara, Italy.
Claudio Rapezzi, Cardiovascular Centre, University of Ferrara, Italy; Maria Cecilia Hospital, GVM Care & Research, Cotignola, Ravenna, Italy.
References
- 1. Rapezzi C, et al. Cardiac amyloidosis: the great pretender. Heart Fail Rev 2015;20:117–124. [DOI] [PubMed] [Google Scholar]
- 2. Garcia-Pavia P, et al. Diagnosis and treatment of cardiac amyloidosis. A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur J Heart Fail 2021;23:512–526. [DOI] [PubMed] [Google Scholar]
- 3. Suhr OB, Lundgren E, Westermark P. One mutation, two distinct disease variants: unravelling the impact of transthyretin amyloid fibril composition. J Intern Med 2017;281:337–347. [DOI] [PubMed] [Google Scholar]
- 4. Emdin M, et al. Treatment of cardiac transthyretin amyloidosis: an update. Eur Heart J 2019;40:3699–3706. [DOI] [PubMed] [Google Scholar]
- 5. Aimo A, et al. RNA-targeting and gene editing therapies for transthyretin amyloidosis. Nat Rev Cardiol; doi: 10.1038/S41569-022-00683-Z. Published online ahead of print. [DOI] [PubMed] [Google Scholar]
- 6. Hammarström P, Schneider F, Kelly JW. Trans-suppression of misfolding in an amyloid disease. Science 2001;293:2459–2462. [DOI] [PubMed] [Google Scholar]
- 7. Coelho T, et al. Tafamidis for transthyretin familial amyloid polyneuropathy: a randomized, controlled trial. Neurology 2012;79:785–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Maurer MS, et al. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med 2018;379:1007–1016. [DOI] [PubMed] [Google Scholar]
- 9. Rapezzi C, et al. Efficacy of tafamidis in patients with hereditary and wild-type transthyretin amyloid cardiomyopathy: further analyses from ATTR-ACT. JACC Heart Fail 2021;9:115–123. [DOI] [PubMed] [Google Scholar]
- 10. Damy T, et al. Efficacy and safety of tafamidis doses in the tafamidis in transthyretin cardiomyopathy clinical trial (ATTR-ACT) and long-term extension study. Eur J Heart Fail 2021;23:277–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Elliott P, et al. Long-term survival with tafamidis in patients with transthyretin amyloid cardiomyopathy. Circ Heart Fail 2022;15:E008193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Judge DP, et al. Transthyretin stabilization by AG10 in symptomatic transthyretin amyloid cardiomyopathy. J Am Coll Cardiol 2019;74:285–295. [DOI] [PubMed] [Google Scholar]
- 13. Topline results from Phase 3 ATTRibute-CM study | BridgeBio. https://bridgebio.com/news/bridgebio-pharma-reports-month-12-topline-results-from-phase-3-attribute-cm-study/.
- 14. Sant’Anna R, et al. Repositioning tolcapone as a potent inhibitor of transthyretin amyloidogenesis and associated cellular toxicity. Nat Commun 2016;71:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pinheiro F, et al. Tolcapone, a potent aggregation inhibitor for the treatment of familial leptomeningeal amyloidosis. FEBS J 2021;288:310–324. [DOI] [PubMed] [Google Scholar]
- 16. Berk JL, et al. The diflunisal trial: update on study drug tolerance and disease progression. Amyloid 2011;18:196–197. [DOI] [PubMed] [Google Scholar]
- 17. Castaño A, Helmke S, Alvarez J, Delisle S, Maurer MS. Diflunisal for ATTR cardiac amyloidosis. Congest Heart Fail 2012;18:315–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hayashi Y, Jono H. Recent advances in oligonucleotide-based therapy for transthyretin amyloidosis: clinical impact and future prospects. Biol Pharm Bull 2018;41:1737–1744. [DOI] [PubMed] [Google Scholar]
- 19. Benson MD, et al. Inotersen treatment for patients with hereditary transthyretin amyloidosis. N Engl J Med 2018;379:22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Viney NJ, et al. Ligand conjugated antisense oligonucleotide for the treatment of transthyretin amyloidosis: preclinical and phase 1 data. ESC Heart Fail 2021;8:652–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Solomon SD, et al. Effects of patisiran, an RNA interference therapeutic, on cardiac parameters in patients with hereditary transthyretin-mediated amyloidosis. Circulation 2019;139:431–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. APOLLO-B: a study to evaluate patisiran in participants with transthyretin amyloidosis with cardiomyopathy (ATTR amyloidosis with cardiomyopathy)—full text view—ClinicalTrials.gov. https://www.clinicaltrials.gov/ct2/show/NCT03997383.
- 23. Judge DP, et al. Phase 3 multicenter study of revusiran in patients with hereditary transthyretin-mediated (hATTR) amyloidosis with cardiomyopathy (ENDEAVOUR). Cardiovasc Drugs Ther 2020;34:357–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Adams D, et al. HELIOS-A: results from the phase 3 study of vutrisiran in patients with hereditary transthyretin-mediated amyloidosis with polyneuropathy (S8.003). Neurology 2022;98 (18 Supplement):2974. [Google Scholar]
- 25. HELIOS-B: a study to evaluate vutrisiran in patients with transthyretin amyloidosis with cardiomyopathy—full text view—ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT04153149.
- 26. Gillmore JD, et al. CRISPR-Cas9 in vivo gene editing for transthyretin amyloidosis. N Engl J Med 2021;385:493–502. [DOI] [PubMed] [Google Scholar]
- 27. Prove cliniche su light chain (AL) amyloidosis: epigallocatechin-3-gallate (EGCG), placebo—Registro degli studi clinici—ICH GCP. https://ichgcp.net/it/clinical-trials-registry/NCT02015312.
- 28. Cardoso I, Martins D, Ribeiro T, Merlini G, Saraiva MJ. Synergy of combined doxycycline/TUDCA treatment in lowering transthyretin deposition and associated biomarkers: studies in FAP mouse models. J Transl Med 2010;8:Article number: 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wixner J, Pilebro B, Lundgren HE, Olsson M, Anan I. Effect of doxycycline and ursodeoxycholic acid on transthyretin amyloidosis. Amyloid 2017;24:78–79. [DOI] [PubMed] [Google Scholar]
- 30. Richards DB, et al. Therapeutic clearance of amyloid by antibodies to serum amyloid P component. N Engl J Med 2015;373:1106–1114. [DOI] [PubMed] [Google Scholar]
- 31. Edwards CV, et al. Phase 1a/b study of monoclonal antibody CAEL-101 (11–1F4) in patients with AL amyloidosis. Blood 2021;138:2632–2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. The VITAL amyloidosis study, a global phase 3, efficacy and safety study of NEOD001 in patients with AL amyloidosis—full text view—ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT02312206.
- 33. A study of PRX004 in subjects with amyloid transthyretin (ATTR) amyloidosis—full text view—ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT03336580.
- 34. George J, et al. A novel monoclonal antibody targeting aggregated transthyretin facilitates its removal and functional recovery in an experimental model. Eur Heart J 2020;41:1260–1270. [DOI] [PubMed] [Google Scholar]
- 35. Michalon A, et al. A human antibody selective for transthyretin amyloid removes cardiac amyloid through phagocytic immune cells. Nat Commun 2021;12:3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Law S, et al. Characteristics and natural history of early-stage cardiac transthyretin amyloidosis. Eur Heart J 2022;43:2622–2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Monteiro C, et al. Predictive model of response to tafamidis in hereditary ATTR polyneuropathy. JCI Insight 2019;4:e126526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hanson JLS, et al. Use of serum transthyretin as a prognostic indicator and predictor of outcome in cardiac amyloid disease associated with wild-type transthyretin. Circ Heart Fail 2018;11:e004000. [DOI] [PMC free article] [PubMed] [Google Scholar]