Abstract
Heart failure and preserved ejection fraction (EF) is a common disease with a poor prognosis and increasing prevalence in the community. The current treatment paradigm includes symptomatic therapy, such as diuretics, risk factor control, and treatment of comorbidities. According to the most recent European guidelines, there is no effective therapy in patients with heart failure and left ventricular EF ≥50%, while the pharmacological compounds normally used in heart failure with reduced EF could also be implemented in patients with EF slightly reduced (between 40 and 50%), with a recommendation class IIB. The recently published Empagliflozin Outcome Trial in Patients with Chronic Heart Failure with Preserved Ejection Fraction (EMPEROR-Preserved) study challenged current guidelines, showing for the first time in patients with heart failure and EF >40% better outcomes with the sodium-glucose cotransporter 2 (SGLT2) inhibitor empagliflozin than with placebo. This result was consistent in patients with and without diabetes, as well as in those with EF below and above 50%. The purpose of the review is to describe the rationale for this important finding and the main results of the EMPEROR-Preserved study and to provide some suggestions for the daily clinical management of SGLT2 inhibitors.
Keywords: SGLT2-inhibitors, HFpEF, Heart failure, Dapagliflozin, Empagliflozin
Introduction
Until 2021, the medical treatment of patients with heart failure and preserved ejection fraction (HFpEF) was mainly limited to diuretics to improve symptoms of heart failure, while no therapy had ever shown a benefit in terms of mortality or morbidity in these patients.1 The main causes of this failure include different and heterogeneous pathophysiological mechanisms underlying the diagnosis of HFpEF. According to the 2021 European guidelines, beta-blockers, mineralocorticoid receptor antagonists (MRAs), renin-angiotensin-aldosterone system inhibitors (RAASi) and neprilysin and angiotensin receptor inhibitor they could be implemented in patients with mildly reduced EF (i.e. between 41% and 49%, HFmrEF), but with a class of recommendation IIb.1 Indeed, the PARAGON-HF study recently showed a trend towards better clinical outcome in chronic renal failure patients treated with sacubitril/valsartan than valsartan itself, especially in the lower end of the EF spectrum, in women, in patients recently hospitalized for heart failure and in those with high sensitivity troponin values at baseline. However, while the Food and Drug Administration (FDA) has approved the use of sacubitril/valsartan in patients with ‘subnormal’ EF, the European Medicines Agency (EMA) has not made a decision. To satisfy the greatest unmet need in cardiology, in recent years the study ‘Empagliflozin Outcome Trial in Patients with Chronic Heart Failure with Preserved Ejection Fraction’ (EMPEROR-Preserved) was conducted to test the hypothesis of a beneficial effect of an inhibitor of the sodium-glucose cotransporter 2 (SGLT2i) in patients with HFpEF2. This study was recently published and has shown that, today, we have an effective therapy in HFpEF, namely SGLT2i empagliflozin. This drug has been shown to reduce the combined hospitalization endpoint for heart failure (HF) and cardiovascular death (CVD) in patients with HFpEF, compared to placebo. Importantly, the conclusions of this study apply to both HFpEF and HFmrEF, as enrolled patients could have both EF greater than 50% and between 40% and 50%, and in both patients with and without diabetes mellitus.2
The purpose of the review is to provide insights into the rationale and practical use of SGLT2i as a successful therapy in patients with HFpEF, considering as HFpEF all patients with EF > 40%, according to the enrollment criteria of contemporary HFpEF studies.
Rationale behind the use of SGLT2i in HFpEF
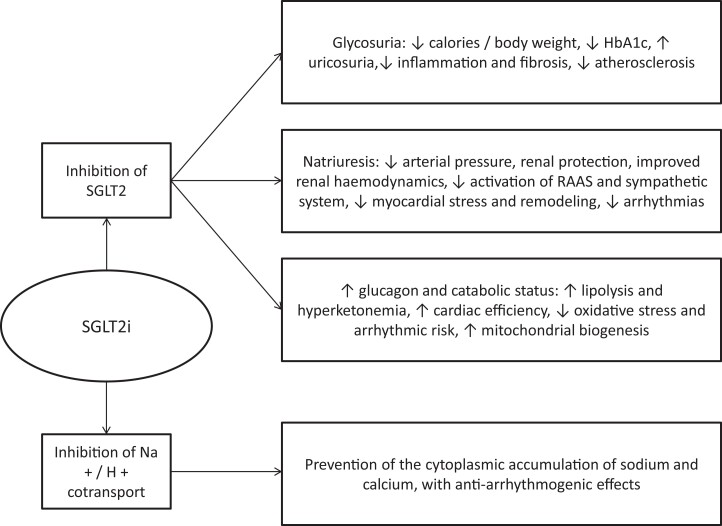
Mechanism of action of the SGLT2i (Figure 1)
Figure 1.
Mechanisms of action of SGLT2i and beneficial effects on the cardiovascular system.
SGLT2 proteins are mainly expressed in the proximal convoluted tubule of the kidneys and are responsible for the reabsorption of approximately 90% of the filtered glucose, along with sodium, making it the ideal target for lowering blood glucose by exploiting glucosuria in diabetes. Therefore, a new class of drugs has been developed to inhibit SGLT2, including dapagliflozin, empagliflozin, canagliflozin, while another compound, sotagliflozin, inhibits both renal SGLT2 and intestinal SGLT1. Inhibition of SGLT2 results in a lowering of the threshold for glycosuria from the usual value of 180–40 mg/dL of blood glucose levels. Importantly, people with genetically non-functional SGLT2 and severe glucosuria are generally healthy, with a low risk of hypotension and hypoglycemia, supporting the safety of SGLT2 inhibition even in non-diabetic patients. In addition to reducing glycated hemoglobin levels in patients with type 2 diabetes, SGLT2i has a significant effect on natriuresis and osmotic diuresis. However, unlike diuretics, they do not reduce intravascular volume, but instead reduce interstitial volume. Therefore, SGLT2i are associated with reductions in blood pressure around 3–5 mmHg, without increasing heart rate. Furthermore, other mechanisms, such as the reduction of arterial stiffness, may also be involved to explain this finding. Importantly, the urinary excretion of glucose caused by SGLT2i leads to a loss of calories and most studies have consistently shown a consequent weight loss of 2–3 kg. Therefore, SGLT2i have favorable effects on diabetes, hypertension and overweight/obesity, which in turn have a significant impact on left ventricular diastolic function. It has also been suggested that SGLT2i may improve cardiac metabolism and bioenergetics, shifting them towards ketone body oxidation, which has been shown to be associated with myocardial benefits. Furthermore, SGLT2i appear to play a role in ion exchange, as inhibition of the downstream Na + /H + myocardial cotransporter has been shown to lead to lower levels of sodium and therefore to lower levels of calcium in cardiomyocytes, which improves contractility and their mitochondrial function.
Data on SGLT2i and diastolic dysfunction
Dapagliflozin improved left ventricular diastolic dysfunction in patients with diabetes.3 In a small study of patients with type 2 diabetes and established cardiovascular disease, Verma et al. reported a significant reduction in left ventricular mass and an improvement in diastolic function 3 months after starting SGLT2i therapy.4
Data on cardiovascular outcome trials in diabetic patients
All of the above mechanisms of action provided the rationale for testing the cardiovascular benefits of SGLT2i, primarily in diabetic patients. In fact, the effect of this class of drugs has been successfully verified in several primary prevention studies in diabetics (EMPA-REG OUTCOME, CANVAS, CREDENCE and DECLARE-TIMI 58),5–8 showing a reduced incidence of HF, cardiac events and the preservation of renal function in patients who received SGLT2i.9,10 Importantly, despite these data that SGLT2i reduce the risk of HF in patients with type 2 diabetes, in these previous studies, most patients did not have HF at enrollment. Post-hoc characterization of the HF phenotype at the time of randomization or the onset of a post-randomization event suggested that not only patients with depressed EF but also patients with HFpEF may have benefited from treatment,11,12 but these analyzes were based on a small number of events and substantial data were missing. Therefore, more data was needed in patients with prevalent HF.
The SOLOIST-WHF trial
The SOLOIST-WHF study was conducted in diabetic patients with acute HF. A total of 1222 patients were randomized to sotagliflozin vs. placebo and followed for a median of 9.0 months. The primary endpoint was the total number of CVDs and HFs and urgent HF visits (considering both first and subsequent events). There were 600 events, including 245 in the sotagliflozin group and 355 in the placebo group. Therefore, the event rate per 100 patient-years was lower in the sotagliflozin group than in the placebo group (51.0 vs. 76.3; hazard ratio 0.67; 95% confidence interval, 0.52–0.85; P < 0.001). Among the side effects, diarrhea was more common with sotagliflozin than placebo (6.1% vs. 3.4%), as was severe hypoglycemia (1.5% vs. 0.3%). Sotagliflozin was associated with a significant reduction in visits for cardiovascular reasons, HF and urgent visits for HF also in a subset of patients with HFpEF.13 However, the number of events was too small to allow a reliable estimate of the treatment effect.
The EMPEROR-Preserved trial
EMPEROR-Preserved was a double-blind study that compared SGLT2i empagliflozin vs. placebo in patients with HFpEF, which was defined as EF > 40%. 5988 patients with NYHA class II-IV HF were randomly assigned to receive empagliflozin (10 mg once daily) or placebo, in addition to their usual therapy.2 The study was designed and developed to address three endpoints: CVD or HF; total HF (first episode and any recurring ones); and preservation of renal function, measured as changes in the slope of the estimated glomerular filtration rate (eGFR). In a median of 26.2 months, a primary outcome event occurred in 415 of 2997 patients (13.8%) in the empagliflozin group and in 511 of 2991 patients (17.1%) in the placebo group (hazard ratio 0.79; 95% confidence interval, 0.69–0.90; P < 0.001). This effect was shown to be mainly related to a lower risk of HF in the empagliflozin group, with a relative risk reduction of 28%. The effects of empagliflozin appeared consistent in patients with or without diabetes mellitus.
The eGFR slope was significantly preserved, with a mean change of 1.36 mL/min/1.73 m2 per year.
Overall, the study achieved all three primary goals. However, although there was a 9% advantage in CVD reduction, it did not reach statistical significance. Regarding the subgroup analyzes, for the primary endpoint, there was no heterogeneity based on gender or considering EF greater than or less than 50%. Dividing the enrolled patients into three groups according to whether the EF was 40% −50%, 50% −60% and >60%, the P-value of the interaction was 0.21 and the hazard ratio <1 for the three groups. Finally, empagliflozin treatment was generally safe. Simple genital and urinary tract infections and hypotension have been reported more frequently with empagliflozin.
Subsequent sub-analysis of the trial shows that empagliflozin exerts a similar clinical effect and has a similar safety profile regardless of the age of the patients14 and the presence of diabetes mellitus.15 Furthermore, the use of MRA does not affect the efficacy of empagliflozin, which has also been shown to be able to reduce hyperkalaemia, regardless of the intake of MRA.16 Empagliflozin improved quality of life and its clinical effects were independent of baseline status.17 Very important is the finding that statistical significance for the separation between the empagliflozin arm and the placebo arm occurred by day 18 for the primary endpoint (HR at 18 days 0.41, 95% CI 0.17–0, 99) and the statistical significance of this benefit has been maintained thereafter.18 The same can be said for the improvement of the quality of life.17 Finally, the analysis of numerous endpoints of in-hospital HF (need for intensive therapy or vasopressors/inotropes) and out-of-hospital (need to intensify diuretic therapy, changes in the NYHA class) showed a positive and early effect of empagliflozin vs. placebo.19
EMPEROR-pooled
The EMPEROR-pooled20 represents a combined analysis of the two randomized trials EMPEROR-Reduced and EMPEROR-Preserved.2 A total of 9718 patients were included in this analysis. Evaluation showed that empagliflozin reduced the risk of HF to a similar extent (approximately 30%) in EMPEROR-Preserved and EMPEROR-Reduced. The magnitude of the effect on HF was similar over a wide range of EF less than 65%, with attenuation of the drug effect for higher EF values (65% or greater). The analysis also found that empagliflozin reduced the risk of major renal outcomes in EMPEROR-Reduced, but not in EMPEROR-Preserved. However, in EMPEROR-Preserved, by redefining renal outcomes with more stringent criteria, pre-treatment EF influenced the effect of empagliflozin on renal outcomes in a similar way to the drug’s effect on HF.
The results of all the mentioned studies are summarized in Table 1.
Table 1.
Summary of the results of all the mentioned studies
| Trial | Population and drug | History of diabetes mellitus | History of heart failure | Median of follow-up | Mortality from all causes | Cardiovascular mortality | Hospitalization for heart failure | Cardiovascular mortality + hospitalization for heart failure | Kidney benefits | Other effects | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Phase II trials | IDDIA3 | Dapagliflozin (n = 30) vs. placebo (n = 30) | 100% | 0% | 24 weeks | NR | NR | NR | NR | NR | Diastolic stress test: improvement in left ventricular diastolic reserve, and ‘from effort and E/and’ from effort |
| Verma et al. 20164 | Empagliflozin (n = 10) | 100% | NR | 3 months | NR | NR | NR | NR | NR | Reduction in left ventricular mass and improvement in diastolic function (e′) | |
| Phase III trials cardiovascular outcome trials | EMPAREG-OUTCOME5,9 | Empagliflozin (n = 4687) vs. placebo (n = 2333) | 100% | 10% | 3.1 years | −32% | −38% | −35% | −34% | Substantial Loss of kidney function, end-stage kidney disease or death from kidney disease: −46% Acute kidney damage: −24% |
Cardiovascular mortality + hospitalization for heart failure in patients with a history of heart failure: NS |
| CANVAS Program6,7,9,10,12 | Canagliflozin (n = 5795) vs. placebo (n = 4347) | 100% | 14% | 2.4 years | NS | NS | −33% | −22% | Substantial Loss of kidney function, end-stage kidney disease or death from kidney disease: −47% 40% reduction in eGFR, renal replacement therapy or renal death: −40% |
Cardiovascular mortality + hospitalization for heart failure in patients with a history of heart failure: −39% Hospitalization for heart failure in patients with a history of heart failure: −49% Fatal or hospitalized heart failure episodes: −30% All-cause mortality in patients with a history of heart failure: −30% |
|
| DECLARE-TIMI 588–11 | Dapagliflozin (n = 8582) vs. placebo (n = 8578) | 100% | 10% | 4.2 years | NS | NS | −27% | −17% | Substantial Loss of kidney function, end-stage kidney disease or death from kidney disease: −47% End-stage renal disease: −69% Dialysis, transplant or death from kidney disease: −58% Acute kidney damage: −31% |
Cardiovascular mortality + hospitalization for heart failure in patients with a history of heart failure: −21% (mainly HFrEF: −38%) Hospitalization for heart failure in patients with HFrEF: −36% Cardiovascular mortality in patients with HFrEF: −45% All-cause mortality in patients with HFrEF: −41% |
|
| Phase III trials heart failure | DAPA-HF | Dapagliflozin (n = 2373) vs. placebo (n = 2371) | 42% | 100% | 18.2 months | −17% | −18% | −30% | −25% | Worsening of renal function: NS | Cardiovascular mortality + total hospitalizations for heart failure: −25% |
| EMPEROR-Reduced | Empagliflozin (n = 1863) vs. placebo (n = 1867) | 50% | 100% | 16 months | NS | NS | −31% | −25% | Improvement in the eGFR slope (+1.73 mL/min/1.73 m2 per year) Chronic dialysis or renal transplantation or sustained reduction of at least 40% in eGFR or eGFR stably <15 mL/min/1.73 m2 (if baseline eGFR ≥ 30 mL/min/1.73 m2) or <10 mL/min/1.73 m2 ( if baseline eGFR <30 mL/min/1.73 m2): −50% |
Total hospitalizations for heart failure: −30% | |
| SOLOIST-WHF13 | Sotagliflozin (n = 608) vs. placebo (n = 614) | 100% | 100% | 9 months | NS | NS | −36% (including urgent visits for heart failure) | −29% | NS | Cardiovascular mortality + hospitalization for heart failure + urgent visits for heart failure: −33% | |
| EMPEROR-Preserved2 | Empagliflozin (n = 2997) vs. placebo (n = 2991) | 49% | 100% | 26.2 months | NS | NS | −29% | −21% | Improvement in the eGFR slope (+1.36 mL/min/1.73 m2 per year) Composite renal endpoint: NS |
Total hospitalizations for heart failure: −27% |
Practical tips for using SGLT2i
SGLT2i are safe drugs, well tolerated, do not cause hypotension or electrolyte imbalance, and have a diuretic and natriuretic effect, targeting the proximal convoluted tubule and working in synergy with loop diuretics. After an initial drop in eGFR, they are protective over kidney function. They do not cause hypoglycemia, even in non-diabetic patients, and do not require a progressive dose increase. These important characteristics explain why they have been named the ‘smartest diuretics’.
However, some caveats in their use are mandatory:
Glomerular filtrate:
Immediately after initiation of SGLT2i therapy, a transient minimal drop in eGFR of approximately 5 mL/min/1.73 m2 occurred in the EMPEROR-Reduced study population. Interestingly, a meta-analysis from the EMPA-REG OUTCOME, CANVAS, DECLARE TIMI 58, and CREDENCE studies compared SGLT2i and DPP4i and showed no eGFR decline in the higher eGFR population strata, also confirming the immediate eGFR reduction after SGLT2i initiation only in lower eGFR strata (<45 mL/min/1.73 m2).9 However, this event is not accompanied by severe acute kidney injury, renal adverse events or hospitalization. Conversely, the risk of hospitalization for severe acute kidney injury was even reduced. Finally, the medium-term results (at 30 and 90 days) and the long-term results were clearly favorable to therapy with SGLT2. The EMPEROR-Reduced and EMPEROR-Preserved studies enrolled patients with eGFR as low as 20 mL/min/1.73 m2, with no serious adverse events reported in the lower end of the eGFR spectrum. In addition, in the Dapagliflozin and Prevention of Adverse Outcomes in Chronic Kidney Disease (DAPA-CKD) study, patients with chronic kidney disease, with and without type 2 diabetes (eGFR ≥25 ml/min/1.73 m2) were enrolled. Of note, dapagliflozin reduced the risk of renal failure and cardiovascular death/hospitalization for HF and prolonged survival in patients with chronic renal failure with or without type 2 diabetes, regardless of history of HF. Considering all these data, it seems reasonable to allow patients with eGFR as low as 20–25 ml/min/1.73 m2 to safely receive these drugs.
Euglycemic ketoacidosis:
The increase in glucosuria after taking SGLT2i reduces blood sugar and insulin production, with a reduced insulin/glucagon ratio and mild ketogenesis. In the case of severely reduced insulin production or prolonged fasting, this mechanism may be sufficient to cause diabetic ketoacidosis. Euglycemic diabetic ketoacidosis is characterized by metabolic acidosis and anion gap ketonuria but without the characteristic sign of hyperglycemia, which is artificially kept low by the glucosuria in progress. The treatment of diabetic euglycemic ketoacidosis is represented by IV infusion of insulin and maintenance of normal blood sugar with glucose infusion, up to the normalization of blood gas analysis and ketonuria. This rare and potentially life-threatening adverse event is an absolute contraindication to the reintroduction of SGLT2i.
Uncomplicated genital infections:
SGLT2i increases the risk of genital infection (mainly Candida fungal infection), which is already high in diabetic patients, compared to placebo. Infection is preventable with better hygiene and responds well to regular antifungal therapy. Discontinuing the drug after an uncomplicated fungal genital infection does not lead to a better prognosis.
Complicated genital infections (Fournier’s gangrene):
An increased incidence of this rare and life-threatening perineal bacterial fasciitis was demonstrated in 55 patients receiving SGLT2i from 2013 to 2019. Risk factors are poorly controlled diabetes, obesity, male gender, immunosuppression, poor hygiene, substance abuse. Case reports suggest an increased risk with SGLT2i, but they are too few to generate a specific indication or contraindication. A high index of suspicion is recommended if a patient receiving SGLT2i develops genital pain or edema (rare in uncomplicated fungal infections) or unexplained fever.
Acute ischemia of the lower limbs:
A twice as high risk of below-knee amputation with canagliflozin was observed in the CANVAS study and in a meta-analysis including patients primarily receiving canagliflozin.6 These results were not observed in trials with other SGLT2i, despite sufficient events, so the effect of canagliflozin is not generalizable to other SGLT2i.
Conclusions
Since the recognition of HFpEF, its therapy has been called the greatest unmet need in cardiology. From this perspective, the recent FDA approval of the use of sacubitril/valsartan in a subset of HFpEF patients, those with ‘below normal’ EF, could be seen as a great achievement, a first recognition that a subgroup of patients with HFpEF could have effective therapy. However, the real revolution in this field is now represented by the results of the EMPEROR-Preserved study. It would seem that HFpEF finally has a successful therapy, namely SGLT2i empagliflozin. Importantly, according to a comparison of the effect pattern of sacubitril/valsartan and empagliflozin in the PARAGON-HF and EMPEROR-Preserved studies, the magnitude of risk reduction of HF endpoints appears to be greater with SGLT2i than with sacubitril/valsartan for most patients with HFpEF. Furthermore, the cardiovascular beneficial effect of empagliflozin, represented mainly by the reduction of HF, its safety profile, together with the ease of use of the drug, will probably facilitate its implementation in clinical practice. The publication of the results of a second phase 3 study in HFpEF and HFmrEF, the DELIVER study (NCT03619213) with dapagliflozin, a natural extension of DAPA-HF, is awaited with great excitement; a recent press release from the Company announced that dapagliflozin significantly reduced the combined CVD + HF endpoint in a population very similar to that of EMPEROR-Preserved (https://www.tctmd.com/news/deliver-trial-scores-win-dapagliflozin-hfpef).
Contributor Information
Edoardo Sciatti, Cardiology Unit, Cardiovascular Department, ASST Papa Giovanni XXIII.
Mauro Gori, Cardiology Unit, Cardiovascular Department, ASST Papa Giovanni XXIII.
Emilia D’elia, Cardiology Unit, Cardiovascular Department, ASST Papa Giovanni XXIII.
Attilio Iacovoni, Cardiology Unit, Cardiovascular Department, ASST Papa Giovanni XXIII.
Michele Senni, Cardiology Unit, Cardiovascular Department, ASST Papa Giovanni XXIII; Bicocca University of Milan.
References
- 1. McDonagh TA, Metra M, Adamo Met al. ESC Scientific Document Group . 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021;42:3599–3726. [DOI] [PubMed] [Google Scholar]
- 2. Anker SD, Butler J, Fiippatos Get al. EMPEROR-Preserved Trial Investigators . Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385:1451–1461. [DOI] [PubMed] [Google Scholar]
- 3. Shim CY, Seo J, Cho Iet al. Randomized, controlled trial to evaluate the effect of dapagliflozin on left ventricular diastolic function in patients with type 2 diabetes mellitus: the IDDIA trial. Circulation 2021;143:510–512. [DOI] [PubMed] [Google Scholar]
- 4. Verma S, Garg A, Yan ATet al. Effect of empagliflozin on left ventricular mass and diastolic function in individuals with diabetes: an important clue to the EMPA-REG OUTCOME trial? Diabetes Care 2016;39:e212–e213. [DOI] [PubMed] [Google Scholar]
- 5. Zinman B, Wanner C, Lachin JMet al. EMPA-REG OUTCOME Investigators . Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117–2128. [DOI] [PubMed] [Google Scholar]
- 6. Neal B, Perkovic V, Mahaffey KWet al. CANVAS Program Collaborative Group . Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017;377:644–657. [DOI] [PubMed] [Google Scholar]
- 7. Rådholm K, Figtree G, Perkovic Vet al. Canagliflozin and heart failure in type 2 diabetes mellitus: results from the CANVAS program. Circulation 2018;138:458–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wiviott SD, Raz I, Bonaca MPet al. DECLARE–TIMI 58 Investigators . Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019;380:347–357. [DOI] [PubMed] [Google Scholar]
- 9. Neuen BL, Young T, Heerspink HJLet al. SGLT2 inhibitors for the prevention of kidney failure in patients with type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol 2019;7:845–854. [DOI] [PubMed] [Google Scholar]
- 10. Zelniker TA, Wiviott SD, Raz Iet al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet 2019;393:31–39. [DOI] [PubMed] [Google Scholar]
- 11. Kato ET, Silverman MG, Mosenzon Oet al. Effect of dapagliflozin on heart failure and mortality in type 2 diabetes mellitus. Circulation 2019;139:2528–2536. [DOI] [PubMed] [Google Scholar]
- 12. Figtree GA, Rådholm K, Barrett TDet al. Effects of canagliflozin on heart failure outcomes associated with preserved and reduced ejection fraction in type 2 diabetes mellitus. Circulation 2019;139:2591–2593. [DOI] [PubMed] [Google Scholar]
- 13. Bhatt DL, Szarek M, Steg PGet al. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med 2021;384:117–128. [DOI] [PubMed] [Google Scholar]
- 14. Böhm M, Butler J, Filippatos Get al. EMPEROR-Preserved Trial Committees and Investigators . Empagliflozin improves outcomes in patients with heart failure and preserved ejection fraction irrespective of age. J Am Coll Cardiol 2022;80:1–18. [DOI] [PubMed] [Google Scholar]
- 15. Filippatos G, Butler J, Farmakis Det al. EMPEROR-Preserved Trial Committees and Investigators . Empagliflozin for heart failure with preserved left ventricular ejection fraction with and without diabetes. Circulation 2022;146:676–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ferreira JP, Butler J, Zannad Fet al. Mineralocorticoid receptor antagonists and empagliflozin in patients with heart failure and preserved ejection fraction. J Am Coll Cardiol 2022;79:1129–137. [DOI] [PubMed] [Google Scholar]
- 17. Butler J, Filippatos G, Jamal Siddiqi Tet al. Empagliflozin, health status, and quality of life in patients with heart failure and preserved ejection fraction: the EMPEROR-Preserved trial. Circulation 2022;145:184–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Butler J, Siddiqi TJ, Filippatos Get al. Early benefit with empagliflozin in heart failure with preserved ejection fraction: insights from the EMPEROR-preserved trial. Eur J Heart Fail 2022;24:245–248. [DOI] [PubMed] [Google Scholar]
- 19. Packer M, Butler J, Zannad Fet al. Effect of empagliflozin on worsening heart failure events in patients with heart failure and preserved ejection fraction: EMPEROR-preserved trial. Circulation 2021;144:1284–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Packer M, Butler J, Zannad Fet al. EMPEROR Study Group . Empagliflozin and major renal outcomes in heart failure. N Engl J Med 2021;385:1531–1533. [DOI] [PubMed] [Google Scholar]