Abstract
Atherosclerotic cardiovascular disease is a systemic condition involving several vascular districts. The most involved vascular bed, beyond the coronary district, is represented by the peripheral arteries, whose involvement can give rise to cerebrovascular or peripheral events. PCSK9 inhibitors (PCSK9i) have established themselves as safe and effective drugs in reducing cholesterol linked to low-density lipoprotein (LDL-C), a causative factor of disease, with a consequent reduction in cardiovascular events. The two main studies on anti-PCSK9 antibodies, the FOURIER study for evolocumab and the ODYSSEY OUTCOMES study for alirocumab, highlighted the effectiveness in reducing LDL-C levels and its translation in a lower event rate of around 15%. Sub-analysis of these two trials showed how PCSK9i prevent cerebrovascular and/or peripheral events and how patients with already known cerebrovascular or peripheral disease benefit more from the action of these drugs than patients who do not have a widespread disease. Current evidence suggests that the preventive action of cerebrovascular and peripheral events is mainly expressed through reducing LDL-C levels. Although there are data regarding the association of PCSK9 levels and inflammatory status, propensity for thrombosis and platelet aggregation, these are currently less robust and do not justify a cardiovascular event reduction action that is independent of the action on LDL-C.
Keywords: PCSK9, Atherosclerotic cardiovascular disease, Secondary prevention
Introduction
PCSK9 inhibitors (PCSK9i) monoclonal antibodies represent a therapeutic novelty for the treatment of hypercholesterolaemia, as they use a new therapeutic target and a completely different mechanism of action than the other drugs used so far.1 These have led to a substantial change in the clinical management of hypercholesterolaemia and atherosclerotic cardiovascular disease (ASCVD) due to their high efficacy in lowering cholesterol linked to low-density lipoprotein (LDL-C) and their effects on reducing cardiovascular events.2,3 PCSK9 inhibitors have been studied in large clinical trial programs involving a variety of patient settings. Patients with a history of cardiovascular disease, heterozygous and homozygous familial hypercholesterolaemia, mixed dyslipidaemia, and statin-intolerant patients were enrolled.4,5 In all these cases, PCSK9i demonstrated a marked LDL-C cholesterol-lowering effect, even higher than 60%, allowing most patients to reach therapeutic targets. Furthermore, this cholesterol-lowering effect was observed regardless of the intensity of the underlying lipid-lowering treatment. The results of the Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk (FOURIER) study with evolocumab and the Evaluation of Cardiovascular Outcomes After an Acute Coronary Syndrome During Treatment With Alirocumab (ODYSSEY OUTCOMES) study with alirocumab provided strong scientific evidence of the role of PCSK9i in cardiovascular prevention.2,3 Both FOURIER and ODYSSEY OUTCOMES are studies conducted on patients in secondary prevention, therefore at very high cardiovascular risk, in the stable phase with evolocumab and in the more acute phase with alirocumab, in both cases, patients treated with high potency statins and with LDL-C >70 mg/dL. The current extensive use of evolocumab and alirocumab has provided robust real-life evidence on their efficacy and safety. These data, together with the sub-analyzes of the two outcome trials on evolocumab and alirocumab, also provided evidence with respect to the reduction in cerebrovascular events and peripheral arterial events.
PCSK9 inhibitors: FOURIER and ODYSSEY OUTCOMES clinical trials
The FOURIER trial involved 27 564 patients with ASCVD and LDL-C >70 mg/dL on statin therapy and demonstrated that inhibition of PCSK9 with evolocumab (140 mg every 2 weeks or 420 mg every month) produces a mean reduction in levels of LDL-C of 59% with an average value of LDL-C reached of 30 mg/dL.2 In the group of patients treated with evolocumab, 42% achieved LDL-C levels <25 mg/dL. Compared with the placebo group, evolocumab reduced the risk of the primary endpoint by 15%. This occurred in 9.8% of evolocumab-treated patients and 11.3% of placebo-treated patients [hazard ratio (HR): 0.85, 95% CI: 0.79–0.92, P < 0.001].2 This preventive effect was observed regardless of statin intensity at baseline and LDL-C at baseline. It was also noted that the lower the LDL-C levels achieved with treatment, the lower the incidence of CV events.
The ODYSSEY OUTCOMES study enrolled patients with acute coronary syndrome within 12 months prior to inclusion, and with an LDL-C >70 mg/dL (or a non-HDL-C value >100 mg/dL, or an apolipoprotein B >80 mg/dL) despite treatment with the maximum tolerated dose of statins, whether or not with ezetimibe.3 Patients were randomized to alirocumab or placebo and followed for a mean of 2.8 years, titrating alirocumab doses (75 or 150 mg/dL every 2 weeks) to achieve a therapeutic target of LDL-C between 25 and 50 mg/dL. The composite primary endpoint (death from CVD, MI, stroke, or hospitalization for angina) occurred in 9.5% of alirocumab-treated patients and 11.1% of placebo-treated patients with a 15% reduction (HR: 0.85, 95% CI: 0.78–0.93, P < 0.001).3
Alirocumab and evolocumab have also been shown to have effects on the reduction of coronary atheroma and effects on the stabilization of the fibrous cap of coronary plaques.6–8
PCSK9 inhibitors and impact on cerebrovascular events
A pre-specified sub-analysis of the FOURIER study sought to investigate the benefit of evolocumab in preventing stroke and stroke subtypes in the overall population and in specific patient subgroups (including those with prior ischaemic stroke).9 Among the enrolled patients, 503 strokes occurred, of which 421 (84%) were of the ischaemic type. Evolocumab significantly reduced all strokes (1.5 vs. 1.9%; HR: 0.79, 95% CI: 0.66–0.95, P = 0.01) and ischaemic stroke (1.2 vs. 1.6%; HR: 0.75, 95% CI: 0.62–0.92, P = 0.005), with no difference in haemorrhagic stroke (0.21 vs. 0.18%; HR: 1.16, 95% CI: 0.68–1.98, P = 0.59).9 These results were also consistent across subgroups, including patients with prior ischaemic stroke, in which HR was (95% CI) 0.90 (0.68–1.19) for all strokes and 0.92 (0.68–1.25) for ischaemic stroke.
An interesting meta-analysis by Cordero et al.10 collected all available studies on PCSK9i, including 81 700 patients, of which 41 979 were treated with PCSK9i (17 244 with evolocumab; 13 720 with bococizumab and 11 015 with alirocumab): the analysis shows that treatment with PCSK9i reduces the incidence of ACS by 19% (RR: 0.81, 95% CI: 0.76–0.87) and of stroke by 25% (RR: 0.75, 95% CI: 0.65–0.85). Similar data emerge from another meta-analysis by Jin Qin et al.,11 in which seven randomized clinical trials were analyzed with 57 440 participants, of which 29 850 treated with PCSK9i: the results confirm the ability of PCSK9i to significantly reduce the risk of total stroke events and ischaemic stroke without increasing the risk of haemorrhagic stroke or neurocognitive pathologies. In detail, PCSK9i were associated with a 23% reduction in the risk of total stroke (RR: 0.77, 95% CI: 0.67–0.88, P < 0.001) and a 24% reduction in ischaemic stroke (RR: 0.76, 95% CI: 0.66–0.89, P < 0.001) compared with the control group.11 In light of these results, the reduction in cerebrovascular events appears to be related to the ability of PCSK9i to reduce LDL-C levels.12
PCSK9 inhibitors and impact on peripheral atherosclerotic disease
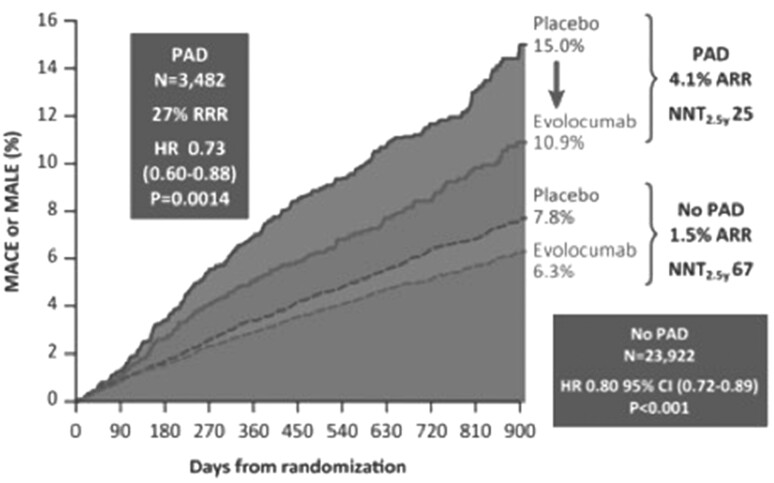
Peripheral atherosclerotic disease (PAD) is a common manifestation of systemic atherosclerosis and affects hundreds of millions of people worldwide.13 This is characterized by a risk of cardiovascular death from 2 to 4 times greater than in unaffected patients and is still often undiagnosed and consequently not treated.13 Patients with lower limb PAD have an increased risk of major adverse cardiovascular events (MACEs) and major adverse limb events (MALEs). The use of lipid-lowering drugs and the reduction of LDL-C levels have been shown to reduce the risk of cardiovascular events, the risk of lower limb events, and the need for amputation. A sub-analysis by Bonaca et al.14 of the FOURIER study demonstrated the benefit on cardiovascular events in PAD patients treated with evolocumab and the reduction of the risk of MALE.14 The results showed that evolocumab treatment reduced the risk of major limb events by 42% (HR: 0.58, 95% CI: 0.38–0.88, P = 0.0093) (Figure 1). Furthermore, as the presence of PAD increases the risk of MACE—including cardiovascular death, myocardial infarction, stroke, hospitalization for unstable angina, and revascularization—the absolute risk reduction for these endpoints was greater in the PAD subgroup than in the overall FOURIER cohort of 27 564 patients: 35 vs. 1.6%. Symptomatic patients with PAD treated with evolocumab achieved a median LDL of 31 mg/dL (IQR: 19–49), compared with a median of 94 mg/dL (IQR: 81–112) at baseline. The event reduction effect also seems in this case mainly linked to the reduction of LDL-C levels.
Figure 1.
Impact of evolocumab on MACE and MALE in patients with and without PAD.
In a more recent sub-analysis, the burden of acute arterial events in the various vascular districts and the effect of the reduction of LDL-C values with evolocumab on these events were studied.15 The endpoints considered were acute arterial events, defined as a composite of coronary events (death from CHD, MI, or urgent coronary revascularization), cerebrovascular (ischaemic stroke, transient ischaemic attack, or urgent cerebral revascularization) or peripheral vascular (acute limb ischaemia, amputation major or urgent peripheral revascularization). Results showed that patients with polyvascular disease had the highest rate of acute arterial events (18.7%), followed by those with symptomatic PAD (13.2%), previous stroke (10.6%), and previous MI (10.3%). Evolocumab reduced the risk of a first acute arterial event by 19% compared with placebo (HR: 0.81, 95% CI: 0.74–0.88, P < 0.001), and with reductions in acute coronary events (HR: 0.83, 95% CI: 0.75–0.91, P < 0.001), acute cerebrovascular (HR: 0.77, 95% CI: 0.65–0.92, P = 0.004), and acute peripheral vascular events (HR: 0.58, 95% CI: 0.38–0.88, P = 0.01). The magnitude of the reduction in the risk of first acute arterial events with evolocumab increased over time; a 16% reduction (HR: 0.84, 95% CI: 0.75–0.95, P = 0.004) in the first year followed by a 24% reduction (HR: 0.76, 95% CI: 0.67–0.85, P < 0.001) after 12 months.15
The involvement of peripheral vascular districts was also analyzed in patients treated with alirocumab. A pre-specified analysis of the ODYSSEY OUTCOMES trial evaluated the association between polyvascular disease and the risk of MACE and death and the impact of alirocumab on these events.
Out of 18 924 enrolled patients, 17 370 had involvement of a single vascular district (coronary artery disease), 1405 had polyvascular disease involving two vascular districts (coronary artery disease and peripheral arterial or cerebrovascular disease) and 149 had polyvascular disease involving three vascular beds (coronary, peripheral arterial, and cerebrovascular disease). There was an increase in the incidence of MACE with the increase in the vascular districts involved (10, 22.2, and 39.7%, respectively). In the alirocumab group of patients, there was an absolute risk reduction of 1.4, 1.9, and 13%. Therefore, intensive reduction of LDL-C levels with alirocumab is critical in patients with polyvascular disease.
Final considerations
Dyslipidaemia plays a fundamental role in the development of atherosclerotic disease and consequently the reduction of LDL-C levels is the cornerstone of stroke and PAD prevention strategies. However, when we talk about the benefits of lipid-lowering therapy, we must also consider the pleiotropic mechanisms that mediate, at least in part, the beneficial effect of lipid-lowering therapies in the prevention of cerebrovascular and cardiovascular outcomes. These include the improvement of endothelial function, the reduction of oxidative stress and vascular inflammation, the stabilization of atherosclerotic plaques and the reduction of platelet aggregation. Evidence suggesting that the effect of PCSK9i on cardiovascular events is influenced, albeit minimally, by mechanisms that transcend the cholesterol-lowering effect is less robust. The ATHERO-AF study showed a direct correlation between PCSK9 levels and platelet activation.16 Similarly, in the PCSK9-REACT study,17 a modest correlation was observed between increased serum levels of PCSK9 and platelet reactivity in patients undergoing percutaneous angioplasty.17 There are also data on the association between PCSK9 levels and inflammation. In the SPUM-ACS study, PCSK9 levels were associated with higher levels of the inflammatory marker highly sensitive C-reactive protein.18 PCSK9 levels are also associated with several traditional vascular risk factors, such as age, obesity, hypertension, diabetes mellitus, and smoking.19 Although some data may suggest the effects of PCSK9i on pathogenetic mechanisms of cerebral ischaemia and acute peripheral ischaemia, including inflammation, thrombosis, and apoptosis, at present, further studies are needed to conclude that PCSK9i protect against stroke or peripheral vascular events beyond their effect on the lipid profile.
Contributor Information
Arturo Cesaro, Department of Translational Medical Sciences, University of Campania ‘Luigi Vanvitelli’, Naples; Division of Cardiology, A.O.R.N. ‘Sant’Anna and San Sebastiano’, Caserta.
Vincenzo Acerbo, Department of Translational Medical Sciences, University of Campania ‘Luigi Vanvitelli’, Naples; Division of Cardiology, A.O.R.N. ‘Sant’Anna and San Sebastiano’, Caserta.
Giuseppe Raucci, Department of Translational Medical Sciences, University of Campania ‘Luigi Vanvitelli’, Naples; Division of Cardiology, A.O.R.N. ‘Sant’Anna and San Sebastiano’, Caserta.
Paolo Calabrò, Department of Translational Medical Sciences, University of Campania ‘Luigi Vanvitelli’, Naples; Division of Cardiology, A.O.R.N. ‘Sant’Anna and San Sebastiano’, Caserta.
References
- 1. Mach F, Baigent C, Catapano ALet al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J 2020;41:111–188. [DOI] [PubMed] [Google Scholar]
- 2. Sabatine MS, Giugliano RP, Keech ACet al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med 2017;376:1713–1722. [DOI] [PubMed] [Google Scholar]
- 3. Schwartz GG, Steg PG, Szarek Met al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379:2097–2107. [DOI] [PubMed] [Google Scholar]
- 4. Cesaro A, Fimiani F, Gragnano Fet al. New frontiers in the treatment of homozygous familial hypercholesterolemia. Heart Fail Clin 2022:18:177–188. [DOI] [PubMed] [Google Scholar]
- 5. Iannuzzo G, Gentile M, Bresciani Aet al. Inhibitors of protein convertase subtilisin/kexin 9 (PCSK9) and acute coronary syndrome (ACS): the state-of-the-art. J Clin Med 2021;10:1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nicholls SJ, Puri R, Anderson Tet al. Effect of evolocumab on progression of coronary disease in statin-treated patients. JAMA 2016;316:2373–2384. [DOI] [PubMed] [Google Scholar]
- 7. Nicholls SJ, Kataoka Y, Nissen SEet al. Effect of evolocumab on coronary plaque phenotype and burden in statin-treated patients following myocardial infarction. JACC Cardiovasc Imaging 2022;15:1308–1321. [DOI] [PubMed] [Google Scholar]
- 8. Räber L, Ueki Y, Otsuka Tet al. Effect of alirocumab added to high-intensity statin therapy on coronary atherosclerosis in patients with acute myocardial infarction: the PACMAN-AMI randomized clinical trial. JAMA 2022;327:1771–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Giugliano RP, Pedersen TR, Saver JLet al. Stroke prevention with the PCSK9 (proprotein convertase subtilisin-kexin type 9) inhibitor evolocumab added to statin in high-risk patients with stable atherosclerosis. Stroke 2020;51:1546–1554. [DOI] [PubMed] [Google Scholar]
- 10. Cordero A, Rodríguez-Mañero M, Fácila Let al. Prevention of myocardial infarction and stroke with PCSK9 inhibitors treatment: a metanalysis of recent randomized clinical trials. J Diabetes Metab Disord. 2020;19:759–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Qin J, Liu L, Su XDet al. The effect of PCSK9 inhibitors on brain stroke prevention: a systematic review and meta-analysis. Nutr Metab Cardiovasc Dis 2021;31:2234–2243. [DOI] [PubMed] [Google Scholar]
- 12. Moustafa B, Testai FD. Efficacy and safety of PCSK9 inhibitors in stroke prevention. J Stroke Cerebrovasc Dis 2021;30:106057. [DOI] [PubMed] [Google Scholar]
- 13. Aboyans V, Ricco J-B, Bartelink M-LELet al. 2017 ESC guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European Society for Vascular Surgery (ESVS). Eur Heart J 2018;39:763–816. [DOI] [PubMed] [Google Scholar]
- 14. Bonaca MP, Nault P, Giugliano RPet al. Low-density lipoprotein cholesterol lowering with evolocumab and outcomes in patients with peripheral artery disease. Circulation 2018;137:338–350. [DOI] [PubMed] [Google Scholar]
- 15. Oyama K, Giugliano RP, Tang Met al. Effect of evolocumab on acute arterial events across all vascular territories: results from the FOURIER trial. Eur Heart J 2021;42:4821–4829. [DOI] [PubMed] [Google Scholar]
- 16. Pastori D, Ettorre E, Carnevale Ret al. Interaction between serum endotoxemia and proprotein convertase subtilisin/kexin 9 (PCSK9) in patients with atrial fibrillation: a post-hoc analysis from the ATHERO-AF cohort. Atherosclerosis 2019;289:195–200. [DOI] [PubMed] [Google Scholar]
- 17. Navarese EP, Kolodziejczak M, Winter MPet al. Association of PCSK9 with platelet reactivity in patients with acute coronary syndrome treated with prasugrel or ticagrelor: the PCSK9-REACT study. Int J Cardiol 2017;227:644–649. [DOI] [PubMed] [Google Scholar]
- 18. Gencer B, Montecucco F, Nanchen Det al. Prognostic value of PCSK9 levels in patients with acute coronary syndromes. Eur Heart J 2016;37:546–553. [DOI] [PubMed] [Google Scholar]
- 19. Cesaro A, Bianconi V, Gragnano Fet al. Beyond cholesterol metabolism: the pleiotropic effects of proprotein convertase subtilisin/kexin type 9 (PCSK9). genetics, mutations, expression, and perspective for long-term inhibition. BioFactors 2020;46:367–380. [DOI] [PubMed] [Google Scholar]