Abstract
The myocardial bridge (MB) is a common anomaly of the coronary tree, very often clinically silent. The artery typically involved is the left anterior descending in its proximal and/or middle portion. MB can cause ischaemia with various mechanisms, directly proportional to the degree of compression of the intra-myocardial tract, which impairs the coronary flow. It is a dynamic phenomenon that is affected by the adrenergic tone and is therefore often brought by physical exercise. MB, when symptomatic, often begins with angina from exertion; some patients have more severe conditions such as unstable angina or myocardial infarction. Coronary vasospasm related to MB-induced endothelial dysfunction can explain a number of cases that come to observation even with catastrophic pictures such as ventricular fibrillation caused by ischaemia. The diagnostic workup includes the non-invasive study using computed tomography angiography and the invasive study of the haemodynamic impact using pressure and Doppler guides. In symptomatic cases, drug therapy with a beta-blocker is enough to manage angina. When it fails, there is the option of coronary angioplasty or surgical treatment techniques.
Keywords: Myocardial bridge, Angina, Ischaemia, Myocardial infarction, Sudden death, MINOCA, Vasospasm, Pressure drive, Disease-free coronary artery
The myocardial bridge (MB) is a common anomaly, generally benign, characterized by the presence of myocardial fibres (the actual bridge) that run above an epicardial coronary artery, whose tract under the muscle fibres is called intra-myocardial or tunnelled. In general, MB is found occasionally on coronary angiography or coronary angiography and is asymptomatic. However, it can cause angina or be the substrate for acute coronary events. Since the prevalence of these abnormalities is very high and the clinical spectrum is broad, this condition still represents a challenge for the cardiologist to date.
Epidemiology
MB is a very common anomaly, which can be found in more than 30% of the population, based on autoptic studies.1 Coronary computed tomography (CT) angiography (cCTA) has good sensitivity, as shown by the prevalence of about 20% reported in the literature with this method, not far from post-mortem data.2 Coronary angiography has a much lower sensitivity, which we will explain later (2–6% of patients studied). MB mainly concerns the left anterior descending coronary artery (LAD) in its proximal or middle portion; the circumflex and right coronaries are more rarely involved.3
Pathophysiology
The amount of myocardial fibres that runs above the artery makes it possible to distinguish between superficial (1–2 mm of myocardial) and deep (>2 mm of myocardium) bridge; this distinction is relevant because the depth is proportional to the compression exerted in systole: the superficial bridges are typically asymptomatic.4 MB has historically been considered a benign entity because coronary perfusion occurs in diastole, while the compression of myocardial fibres with alteration of flow in the tunnelled tract occurs in systole. During exercise, the increase in cardiac inotropism and heart rate, on the one hand, rises the compression exerted on the intra-myocardial tract, slowing the rapid proto-diastolic coronary flow phase, and, on the other hand, reduces the relative time of diastole itself, increasing the possibility of inducing ischaemia. There is also a ‘steal’ mechanism to the detriment of the collateral branches that originate from the tunnelled tract: the increased flow velocities that are generated when the blood crosses the segment compressed in tele-systole/proto-diastole lead to a drop in pressure in the collateral branches due to the Venturi effect; hence, the longer the intra-myocardial tract is, the more it is possible to have ischaemia of the collaterals emerging from the affected segment. This phenomenon, which is typically observed in the septal branches, explains how it is possible to observe ischaemia of the septum even in the presence of normal perfusion of the distal territories of the left anterior descendant (cardiac apex).
Spontaneous coronary dissection should also be considered, a rare event that can occur due to stresses on the intima of the tunnelled tract.
The presence of MB has been related to the development of atherosclerosis, with a mechanism that probably acts on shear stress, the stress generated on the vascular walls by the flow of blood: the reduction of shear stress can favour endothelial dysfunction and atherosclerosis;5 the systolic interruption of the flow alters its laminarity by reducing the shear stress upstream of the tunnelled section; on the contrary, shear stress is greater on the intramyocardial segment due to the increased flow velocities. Therefore, the presence of MB could favour atherosclerosis in the tract immediately upstream of the intra-myocardial segment, while the latter would be spared.6 The relative distance between the epicardial adipose tissue and the tunnelled tract could also reduce the arrival of pro-inflammatory signals and cells involved in atherosclerosis;7 in the same way, the compression exerted by the MB, favouring lymphatic drainage, could increase the local clearance of leukocytes. The onset of intra-myocardial tract vasospasm may explain why previously asymptomatic patients develop symptoms.8 Similarly, the appearance of obstructive atherosclerosis in the segment upstream of the MB accentuates the pressure drop downstream of the same; the development of left ventricular hypertrophy makes the supply–demand imbalance more likely and reduces the reserve of the microcirculation, compressing it.
Clinical presentation
As mentioned earlier, MB is an anatomic variant often devoid of any clinical impact. In an attempt to classify it, Schwarz analyzed a cohort of 157 patients with MB on the LAD, with pain, and without obstructive coronary artery disease and categorized them into three groups: Group A composed of patients who had no documentation of inducible ischaemia and who did not need treatment; Group B composed of the patients with documentation of ischaemia on non-invasive tests, and Group C had alterations documented with Doppler or intra-coronary pressure guides. In these last two groups, due to the refractoriness of anginal symptoms despite beta-blocker therapy, some patients (24) required stent implantation during follow-up.9 On analyzing a series of patients undergoing coronary angiography, it was found that patients with MB tend to be younger, smokers, and with a lower prevalence of the classic risk factors for atherosclerotic disease.10 Patients with MB may experience angina or equivalent angina or manifest ischaemic changes on the exercise ECG in the absence of symptoms. Rarely, MB is associated with the acute coronary syndrome (ACS) with a mechanism linked to vasospasm or atherosclerosis upstream of the tunnelled tract. However, the majority of people with MB-related ACS experience unstable angina (troponin negative) rather than myocardial infarction. This could be linked to vasospasm, thus explaining the high prevalence of smokers in MB patients who eventually need coronary angiography. More rarely, MB can be responsible for myocardial infarction and normal coronary artery (MINOCA). In a recent work, the presence of MB was in fact a risk factor for MINOCA with adjusted odds ratio (OR) equal to 3.28, 95% confidence interval (CI; 2.34; 4.61) P < 0.001.11 Similarly, in 310 patients with myocardial ischaemia in the absence of coronary artery disease (both MINOCA—acute and NOCAD—stable ), including 53 (17.1%) with MB, an acetylcholine (ACh) test was performed: the presence of MB was an independent predictor of positivity to ACh for epicardial vasospasm (documented in 97% of cases at the MB level; hazard ratio, 2.57; 95% CI, 1.24–5.33; P = 0.011); it is noteworthy that patients with positive ACh and MB tests showed a significantly higher re-hospitalization rate for unstable angina than patients with one or none of these two characteristics [major cardiovascular events (MACEs) 28.6 vs. 5.7—0%].12 It should be emphasized that, in this cohort of patients, those who had documentation of MB but were negative for the ACh test did not show any event during follow-up.12 This highlights how the pathophysiology of MB is complex: indeed, it is due to the interaction among anatomical variant, patient characteristics, and trigger factors.
Diagnostics
The most classic examination with which the MB can be highlighted is coronary angiography; however, the diagnosis can be missed easily given the dynamic nature of this anomaly and its variability with the autonomic and haemodynamic settings. The classic criterion for defining the presence of a significant MB is a reduction of the vascular lumen on coronary angiography of at least 70% in systole with persistence of compression >35% in meso-tele diastole. These parameters make coronary angiography an insensitive examination for the diagnosis of MB: the use of intra-coronary nitrates increases the degree of systolic compression, thus increasing the sensitivity of the examination. In the literature, a more sensitive criterion for angiographic diagnosis is sometimes used, which requires only the systolic reduction >50% of the coronary diameter. The diagnosis can be corroborated by invasive imaging such as intravascular ultrasound (IVUS) or optical coherence tomography (OCT). The first of the two methods provides a precise estimate of the degree of compression exerted by the MB, allowing the measurement of the minimal luminal area (MLA) and provides the pathognomonic image of a ‘crescent,’ a hyperechoic arc that partially surrounds the coronary artery and which seems to be due to myocardial fibres overlying the lumen itself; OCT, due to the high speed of withdrawal, does not allow to appreciate the cyclical nature of systolic–diastolic compression, thus potentially being less informative than IVUS. What should be emphasized is that neither coronary angiography nor IVUS nor OCT provide functional information on MB that can correlate with ischaemia. Hence, other invasive investigations are needed such as the study of the coronary reserve by pressure guidance with hyperaemic [fractional flow reserve (FFR)] or non-hyperaemic [instantaneous wave-free ratio (iFR)] method. This last approach appears more suitable, in theory, because the analysis is performed in the diastolic phase of the cardiac cycle, thus excluding perturbations that occur in systole by the MB, which can make the FFR measurement unreliable.13 Not surprisingly, iFR is more sensitive in documenting obstructive MB than FFR. It should be noted that the use of intracoronary nitrates, adenosine, and dobutamine can facilitate the diagnosis by reproducing a haemodynamic condition that is similar to physiological stress, unmasking significant diastolic gradients, detectable with pressure guides.
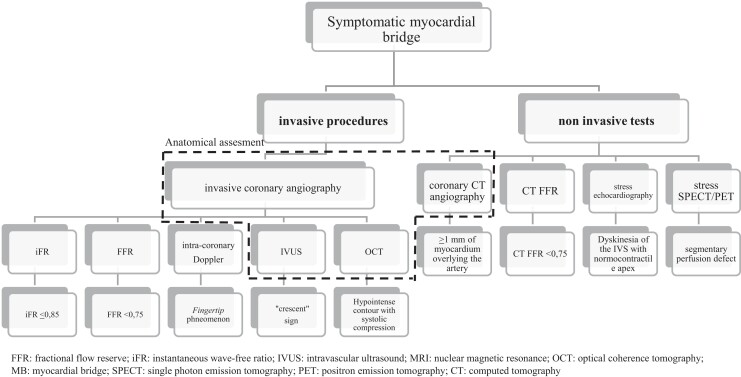
As an alternative or complementary to the invasive methods described earlier, MB can be studied with non-invasive imaging (see Figure 1). The pivotal approach is cCTA, with which the finding of MBs reaches high prevalences (in some reports, it reaches almost 50% of patients).14 In accordance with the pathophysiological premises set out earlier, only the deepest (>2 mm) and longest (>25 mm) bridges documented with this method seem to have the potential to create ischaemia, while the majority of those documented on cCTA are not accompanied by ischaemia and/or symptoms. The classic search methods of ischaemia such as single-photon emission computed tomography (SPECT) and stress echocardiogram are usable and provide valuable information. A review of the diagnostic methods is presented in Table 1.
Figure 1.
Overview of diagnostic tests that can be used for the study of MB.
Table 1.
Characteristics of the diagnostic methods that can be used for the myocardial bridge
| Type of test | Diagnostic criteria | Pro | Cons | Functional information |
|---|---|---|---|---|
| Invasive methods | ||||
| Coronary angiogram | Squeezing the vessel (milking effect) |
|
|
No |
| IVUS | ‘Crescent’ sign |
|
|
No |
| OCT | Fusiform contour attenuated with systolic compression |
|
|
No |
| Intracoronary Doppler | Fingertip phenomenon: (i) quick acceleration of the proto-diastolic flow; (ii) rapid meso-diastolic deceleration; and (iii) diastolic meso-tele plateau |
|
|
Yes |
| FFR | FFR ≤0.75 |
|
|
Yes |
| iFR | iFR ≤0.85 |
|
|
Yes |
| ||||
| Coronary CT angiography | >1 mm of myocardium that runs above the coronary: superficial MB; >2 mm MB deep; >5 mm MB very deep |
|
|
No |
| CT-FFR | FFR ≤0.75 |
|
|
Yes |
| SPECT | Defects of segmental perfusions during stress |
|
|
Yes |
| PET | Defects of segmental perfusions during stress |
|
|
Yes |
| Stress echocardiogram | Segmental hypokinesia during stress |
|
|
Yes |
| Cardiac MRI | Sub-endocary segmental hypokinesia during stress |
|
|
Yes |
FFR, fractional flow reserve; iFR, instantaneous wave-free ratio; IVUS, intravascular ultrasound; MRI, nuclear magnetic resonance imaging; OCT, optical coherence tomography; MB, myocardial bridge; SPECT, single-photon emission tomography; PET, positron emission tomography; CT, computed tomography.
Prognosis and treatment
The management of patients with symptomatic MB is poorly codified: each case must be evaluated on its own, based on the anatomical characteristics of the anomaly, the clinical picture, and the comorbidities. Key information is the presence or absence of anginal symptoms or equivalent and of ischaemia: in the absence of these two elements, the prognosis of MB is reasonably good: it could not be otherwise, given the enormous prevalence of this anatomical variant; for this same reason, the strict Italian regulations of the Organizational Committee for Cardiology for Sport Fitness (COCIS), in the 2017 revision, suggest careful for patients with cCTA finding of the MB longer than >10 mm and deep >3 mm: in the absence of symptoms and if SPECT does not document inducible ischaemia (or FFR negative on coronary angiography) there are no contraindications to even strenuous agonistic activity. However, annual reassessment is recommended as there is, as illustrated above, the possibility that symptoms and/or inducible ischaemia appear over the years. To support the prognosis of MB there are data on a cohort of patients with hypertrophic cardiomyopathy in which as many as 11.4% had documentation of the anomaly on coronary angiography: MB does not seem to have had an impact on prognosis at a median follow-up of 4.2 years.15 Similarly, no fatal events were observed at the 5-year follow-up in Schwartz’s series.9 However, it is important to distinguish patients who present with ACS, in whom it is important to evaluate the role of the MB and to exclude local complications such as dissections and vasospasm; if not recognized and treated, the risk of fatal events in these patients with unstable presentation exists and could explain the presence in the literature of patients with MB who suffered sudden death.16 It should be borne in mind that the relationship between mortality and MB lacks clear confirmation: the most recent meta-analysis on the subject showed that in patients with MB vs. non-MB, an OR for MACEs) is equal to 1.53 (1.01–2.30, P < 0.001). The result, with a CI that borders on identity, is undermined by the heterogeneity, small size, and paucity of information of the 21 studies used in the analysis.17 Furthermore, for the cardiovascular death, no significant correlation was found.17
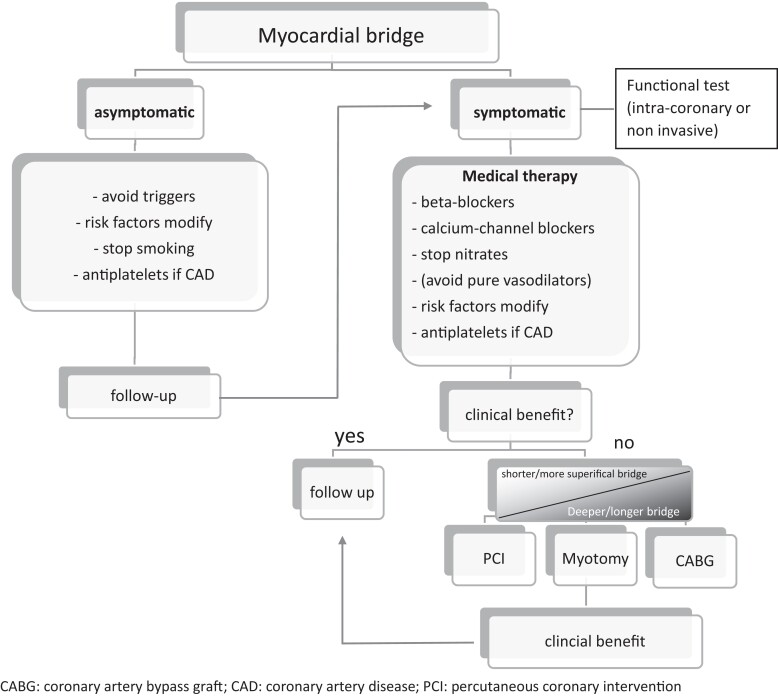
A therapeutic option (see Figure 2), in case of symptoms, is drug therapy as the first mandatory step. Beta-blockers are the historically preferred class due to their ability to reduce inotropism, thus decreasing compression on the tunnelled tract, and to increase diastolic flow by reducing the heart rate. The reduction of sympathetic tone is also a class effect that is particularly useful in this clinical setting. The use of non-thienopyridine calcium antagonists is common, due to both a contraindication to the beta-blocker and evidence of vasospasm. Nitrates are to be used with caution because they can exacerbate perfusion problems due to the pressure drop of the coronary vessels adjacent to the MB with an increase in retrograde flow; they can also trigger reflex tachycardias. Finally, the correction of risk factors for atherosclerosis, with particular attention to smoking, must be strictly applied, even in asymptomatic patients, due to the increased risk of atherosclerosis and vasospasm that could be connected to the presence of MB.18
Figure 2.
Algorithm for the treatment of MB.
In the case of failure of drugs in achieving good control of symptoms and/or ischaemia, the next step is coronary angioplasty [percutaneous coronary intervention (PCI)] or surgery. Before proceeding invasively, a careful anatomical evaluation using cCTA is, however, essential.
The implantation of drug-eluting stent within the tunnelled segment has the rationale of preventing systolic compression on the intramyocardial tract itself. Obviously, there are no trial data that have examined the impact of PCI vs. a more conservative approach. The previous studies report complications related to the stent, in particular, due to incomplete coverage of the tunnelled tract (a problem that can be solved by stimulation with dobutamine to observe the real extension of the bridge before implantation), the stent fracture, or the coronary perforation. It should be considered that classic complications related to the stent, such as thrombosis and restenosis, could be favoured by the compression forces that are exerted on the stent itself and by the risk of malaposition connected to the coronary artery without atherosclerotic pathology and/or with marked vasoreactivity that can lead to underestimate its real calibre; the failure and reoperation rate therefore appears to be higher than the rate of PCI performed on atherosclerotic plaque,9,19 and so treatment has to be intended only for drug-refractory cases. Surgical revascularization is a valid alternative to PCI; however, in the literature, it is known how the use of arterial graft, typically the left internal mammary artery (LIMA), for the bypass of bridges of the LAD, is burdened by a very high rate of long-term failures. The hypothesized mechanism is that of the competitive flow coming from the native artery, which interferes with the patency of the arterial graft; the proof of this is the fact that patients in whom atherosclerotic stenosis coexists upstream of the bridge have a much higher rate of LIMA patency than patients without concomitant obstructive coronary artery disease.20 A further possibility offered by surgery is the myectomy of the bridge itself, an operation that aims to restore normal anatomy. It is not recommended for particularly deep (>5 mm) and long (>25 mm) bridges.
Conclusions
The MB is a disease with a very broad clinical spectrum, generally asymptomatic for the entire life of the patient. The high prevalence, especially with increasingly used diagnostic methods [cCTA and cardiac magnetic resonance (CMR)], makes the MB an entity with which the cardiologist must likely deal. Symptomatic cases must be managed carefully, especially if with unstable presentation, using an invasive approach, which can confirm or exclude the haemodynamic impact of the MB. The treatment of cases refractory to medical therapy appears feasible with PCI, possibly followed by surgery in the case of failure of the percutaneous approach. The absence of randomized trials and shared diagnostic protocols constitute a knowledge gap, to be filled in the years to come to be able to give clear answers to a rapidly increasing number of patients.
Contributor Information
Andrea Santucci, Division of Cardiology, Perugia Hospital.
Francesca Jacoangeli, Division of Cardiology, Perugia Hospital.
Sara Cavallini, Division of Cardiology, Perugia Hospital.
Matteo d’Ammando, Division of Cardiology, Perugia Hospital.
Francesca de Angelis, Division of Cardiology, Perugia Hospital.
Claudio Cavallini, Division of Cardiology, Perugia Hospital.
References
- 1. Hostiuc S, Negoi I, Rusu MC, Hostiuc M. Myocardial bridging: a meta-analysis of prevalence. J Forensic Sci 2018;63:1176–1185. [DOI] [PubMed] [Google Scholar]
- 2. Roberts W, Charles SM, Ang Cet al. . Myocardial bridges: a meta-analysis. Clin Anat 2021;34:685–709. [DOI] [PubMed] [Google Scholar]
- 3. Rajendran R, Hegde M. The prevalence of myocardial bridging on multidetector computed tomography and its relation to coronary plaques. Pol J Radiol 2019;84:e478–e483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Uusitalo V, Saraste A, Pietila M, Kajander S, Bax JJ, Knuuti J. The functional effects of intramural course of coronary arteries and its relation to coronary atherosclerosis. JACC Cardiovasc Imaging 2015;8:697–704. [DOI] [PubMed] [Google Scholar]
- 5. Hung OY, Brown AJ, Ahn SG, Veneziani A, Giddens DP, Samady H. Association of wall shear stress with coronary plaque progression and transformation. Interv Cardiol Clin 2015;4:491–502. [DOI] [PubMed] [Google Scholar]
- 6. Corban MT, Hung OY, Eshtehardi Pet al. . Myocardial bridging: contemporary understanding of pathophysiology with implications for diagnostic and therapeutic strategies. J Am Coll Cardiol 2014;63:2346–2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Verhagen SN, Rutten A, Meijs MFet al. . Relationship between myocardial bridges and reduced coronary atherosclerosis in patients with angina pectoris. Int J Cardiol 2013;167:883–888. [DOI] [PubMed] [Google Scholar]
- 8. Sara JDS, Corban MT, Prasad Met al. . Prevalence of myocardial bridging associated with coronary endothelial dysfunction in patients with chest pain and non-obstructive coronary artery disease. EuroIntervention 2020;15:1262–1268. [DOI] [PubMed] [Google Scholar]
- 9. Schwarz ER, Gupta R, Haager PKet al. . Myocardial bridging in absence of coronary artery disease: proposal of a new classification based on clinical-angiographic data and long-term follow-up. Cardiology 2009;112:13–21. [DOI] [PubMed] [Google Scholar]
- 10. Podolec J, Wiewiorka L, Siudak Zet al. . Prevalence and clinical presentation of myocardial bridge on the basis of the National Polish Percutaneous Interventions Registry and the Classification of Rare Cardiovascular Diseases. Kardiol Pol 2018;77:465–470. [DOI] [PubMed] [Google Scholar]
- 11. Mohlenkamp S, Hort W, Ge J, Erbel R. Update on myocardial bridging. Circulation 2002;106:2616–2622. [DOI] [PubMed] [Google Scholar]
- 12. Montone RA, Gurgoglione FL, Del Buono MGet al. . Interplay between myocardial bridging and coronary spasm in patients with myocardial ischemia and non-obstructive coronary arteries: pathogenic and prognostic implications. J Am Heart Assoc 2021;10:e020535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tarantini G, Barioli A, Nai Fovino Let al. . Unmasking myocardial bridge-related ischemia by intracoronary functional evaluation. Circ Cardiovasc Interv 2018;11:e006247. [DOI] [PubMed] [Google Scholar]
- 14. Nakanishi R, Rajani R, Ishikawa Y, Ishii T, Berman DS. Myocardial bridging on coronary CTA: an innocent bystander or a culprit in myocardial infarction? J Cardiovasc Comput Tomogr 2012;6:3–13. [DOI] [PubMed] [Google Scholar]
- 15. Tian T, Wang YL, Wang JZet al. . Myocardial bridging as a common phenotype of hypertrophic cardiomyopathy has no effect on prognosis. Am J Med Sci 2014;347:429–433. [DOI] [PubMed] [Google Scholar]
- 16. Ki YJ. Myocardial bridging presenting as myocardial ischaemia induced cardiac arrest: a case report. BMC Cardiovasc Disord 2021;21:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hostiuc S, Rusu MC, Hostiuc M, Negoi RI, Negoi I. Cardiovascular consequences of myocardial bridging: a meta-analysis and meta-regression. Sci Rep 2017;7:14644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sternheim D, Power DA, Samtani R, Kini A, Fuster V, Sharma S. Myocardial bridging: diagnosis, functional assessment, and management: JACC state-of-the-art review. J Am Coll Cardiol 2021;78:2196–2212. [DOI] [PubMed] [Google Scholar]
- 19. Ernst A, Bulum J, Separovic Hanzevacki J, Lovric Bencic M, Strozzi M. Five-year angiographic and clinical follow-up of patients with drug-eluting stent implantation for symptomatic myocardial bridging in absence of coronary atherosclerotic disease. J Invasive Cardiol 2013;25:586–592. [PubMed] [Google Scholar]
- 20. Ji Q, Shen J, Xia L, Ding W, Wang C. Surgical treatment of symptomatic left anterior descending myocardial bridges: myotomy vs. bypass surgery. Surg Today 2020;50:685–692. [DOI] [PubMed] [Google Scholar]