Abstract
Background
Traumatic brachial plexus injuries (BPIs) in the nerve roots of C5 to T1 lead to the devastating loss of motor and sensory function in the upper extremity. Free functional gracilis muscle transfer (FFMT) is used to reconstruct elbow and shoulder function in adults with traumatic complete BPIs. The question is whether the gains in ROM and functionality for the patient outweigh the risks of such a large intervention to justify this surgery in these patients.
Questions/purposes
(1) After FFMT for adult traumatic complete BPI, what is the functional recovery in terms of elbow flexion, shoulder abduction, and wrist extension (ROM and muscle grade)? (2) Does the choice of distal insertion affect the functional recovery of the elbow, shoulder, and wrist? (3) Does the choice of nerve source affect elbow flexion and shoulder abduction recovery? (4) What factors are associated with less residual disability? (5) What proportion of flaps have necrosis and do not reinnervate?
Methods
We performed a retrospective observational study at Dr. Soetomo General Hospital in Surabaya, Indonesia. A total of 180 patients with traumatic BPIs were treated with FFMT between 2010 and 2020, performed by a senior orthopaedic hand surgeon with 14 years of experience in FFMT. We included patients with traumatic complete C5 to T1 BPIs who underwent a gracilis FFMT procedure. Indications were total avulsion injuries and delayed presentation (>6 months after trauma) or after failed primary nerve transfers (>12 months). Patients with less than 12 months of follow-up were excluded, leaving 130 patients eligible for this study. The median postoperative follow-up period was 47 months (interquartile range [IQR] 33 to 66 months). Most were men (86%; 112 of 130) who had motorcycle collisions (96%; 125 patients) and a median age of 23 years (IQR 19 to 34 years). Orthopaedic surgeons and residents measured joint function at the elbow (flexion), shoulder (abduction), and wrist (extension) in terms of British Medical Research Council (MRC) muscle strength scores and active ROM. A univariate analysis of variance test was used to evaluate these outcomes in terms of differences in distal attachment to the extensor carpi radialis brevis (ECRB), extensor digitorum communis and extensor pollicis longus (EDC/EPL), the flexor digitorum profundus and flexor pollicis longus (FDP/FPL), and the choice of a phrenic, accessory, or intercostal nerve source. We measured postoperative function with the DASH score and pain at rest with the VAS score. A multivariate linear regression analysis was performed to investigate what patient and injury factors were associated with less disability. Complications such as flap necrosis, innervation problems, infections, and reoperations were evaluated.
Results
The median elbow flexion muscle strength was 3 (IQR 3 to 4) and active ROM was 88° ± 46°. The median shoulder abduction grade was 3 (IQR 2 to 4) and active ROM was 62° ± 42°. However, the choice of distal insertion was not associated with differences in the median wrist extension strength (ECRB: 2 [IQR 0 to 3], EDC/EPL: 2 [IQR 0 to 3], FDP/FPL: 1 [IQR 0 to 2]; p = 0.44) or in ROM (ECRB: 21° ± 19°, EDC/EPL: 21° ± 14°, FDP/FPL: 13° ± 15°; p = 0.69). Furthermore, the choice of nerve source did not affect the mean ROM for elbow flexion (phrenic nerve: 87° ± 46°; accessory nerve: 106° ± 49°; intercostal nerves: 103° ± 50°; p = 0.55). No associations were found with less disability (lower DASH scores): young age (coefficient = 0.28; 95% CI -0.22 to 0.79; p = 0.27), being a woman (coefficient = -9.4; 95% CI -24 to 5.3; p = 0.20), and more postoperative months (coefficient = 0.02; 95% CI -0.01 to 0.05]; p = 0.13). The mean postoperative VAS score for pain at rest was 3 ± 2. Flap necrosis occurred in 5% (seven of 130) of all patients, and failed innervation of the gracilis muscle occurred in 4% (five patients).
Conclusion
FFMT achieves ROM with fair-to-good muscle power of elbow flexion, shoulder abduction, and overall function for the patient, but does not achieve good wrist function. Meticulous microsurgical skills and extensive rehabilitation training are needed to maximize the result of FFMT. Further technical developments in distal attachment and additional nerve procedures will pave the way for reconstructing a functional limb in patients with a flail upper extremity.
Level of Evidence
Level III, therapeutic study.
Introduction
Traumatic complete brachial plexus injuries (BPIs) are devastating injuries of the nerve roots of C5 to T1 and lead to loss of motor and sensory function in the upper extremity. When primary nerve reconstruction is not possible or sufficient, secondary surgery is indicated, including tendon transfer and free functional muscle transfer (FFMT). FFMT was first described in 1990 [2] to reconstruct upper extremity function, restore elbow flexion, and improve shoulder abduction and wrist stabilization [12, 13, 19, 20, 26, 27]. So far, the functional outcomes of FFMT have only been described in small case series [33]. Currently, reconstructive decision-making is mostly based on the experience of the surgeon and practical feasibility.
FFMT is an enormous operation that requires high microsurgical expertise. A free gracilis muscle flap is preferred because of its proximally based neurovascular pedicle, allowing for rapid reinnervation, and its long tendon length, which can be secured to the distal muscles to stabilize the wrist [14, 20]. The question is whether differences in the distal attachment of the gracilis muscle matter for elbow, shoulder, and wrist functional outcomes. Although most studies focused on mechanically improving joint function, more importantly, surgeons should focus on improving the patient’s function in activities of daily living [12, 13, 26]. Furthermore, because FFMT is infrequently performed in patients with BPIs, little is known about what patient or injury factors are associated with worse functional outcomes. Moreover, the risks of complications such as flap necrosis and reinnervation problems have not been consistently reported [33]. Do the benefits of FFMT outweigh the risks of such a large intervention to justify this procedure as a standard treatment for traumatic, complete BPI?
Therefore, we asked: (1) After FFMT for adult traumatic complete BPI, what is the functional recovery in terms of elbow flexion, shoulder abduction, and wrist extension (ROM and muscle grade)? (2) Does the choice of distal insertion affect the functional recovery of the elbow, shoulder, and wrist? (3) Does the choice of nerve source affect elbow flexion and shoulder abduction recovery? (4) What factors are associated with less residual disability? (5) What proportion of flaps have necrosis and do not reinnervate?
Patients and Methods
Study Design and Setting
We performed a retrospective, observational study of 130 patients treated with FFMT for traumatic BPIs at Dr. Soetomo General Hospital, Surabaya, Indonesia, between 2010 and 2020. All procedures were performed by one senior orthopaedic hand surgeon (HSO) with 19 years of experience in surgery for BPI. FFMT has been performed since 2008; since then, approximately 16 FFMTs have been performed per year [29]. All patients were perioperatively treated by a multidisciplinary team of one orthopaedic hand surgeon, assisting orthopaedic surgeons, residents, physiotherapists, and rehabilitation physicians.
Participants
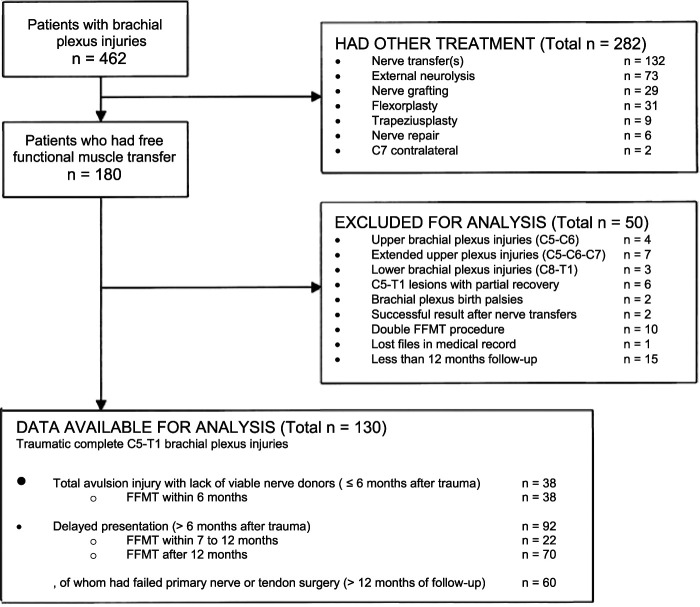
Between 2010 and 2020, we treated 462 patients for traumatic BPI. Because of the extent of the injury, 180 patients were eligible for FFMT. We generally recommended FFMT in patients with complete BPI after total avulsion injuries who have a lack of viable nerve donors (≤ 6 months after trauma), after delayed presentation (> 6 months after trauma), and after failed primary nerve reconstructive surgery or tendon surgery (> 12 months after intervention) that resulted in a nonfunctional elbow or shoulder in terms of muscle strength (< M3). All patients were examined with a preoperative muscle chart as well as an electromyography and nerve conduction velocity test to confirm the diagnosis [27]. We included patients with traumatic complete C5 to T1 BPIs who had a follow-up duration of at least 12 months after FFMT. The maximum expected effect of FFMT is mostly achieved after 1 year because the short pedicle of the FFMT allows rapid reinnervation (1 mm per day) of end-to-end neurorrhaphy [14, 20]. Patients with bilateral FFMT to achieve hand function were excluded (5.6%; 10 of 180 patients). A further 12% (22) with brachial plexus birth palsies; upper (C5 to C6), upper extended (C5, C6, and C7), and lower plexus injuries (C8 to T1); and partial recovery after total BPI (C5 to T1) were excluded. Furthermore, 1.1% (two patients) were excluded because they had successful primary nerve procedures that would influence the outcomes of elbow or shoulder function. Lastly, 9% (16) had incomplete datasets or were lost before the minimum study follow-up of 12 months, leaving 130 patients (28%) for analysis (Fig. 1).
Fig. 1.
This flowchart shows all patients who had surgical treatment for brachial plexus injury between 2010 and 2020 at Dr. Soetomo General Hospital; FFMT = free functional muscle transfer.
Patients’ Baseline Data
One hundred thirty patients were included, with a median postoperative follow-up duration of 47 months (interquartile range [IQR] 33 to 66 months). At the time of surgery, the median age was 23 years (IQR 19 to 34 years), and 86% (112 of 130) were men. Most brachial plexus injuries were motorcycle collisions (97%; 125 of 129 patients) (Table 1). Preoperatively the mean Medical Research Council (MRC) muscle strength score for elbow flexion was 0 ± 0.4 for all patients, 97% of whom had a grade of M0 at baseline. Two patients had a grade of M1 and three patients had a grade of M2. Furthermore, the preoperative mean active ROM for elbow flexion was 1° ± 11°, and 98% of patients had ROM of 0°. Moreover, the mean preoperative MRC score for shoulder abduction was 0 ± 0.5 and the mean active ROM was 3° ± 12°. Lastly, for wrist extension, the mean preoperative MRC score was 0 ± 0.2 and ROM was 0° ± 0.9°.
Table 1.
Patient characteristics (n = 130 patients)
| Patient characteristic | Value |
| Age in years at the time of FFMT, median (IQR) | 23 (19-34) |
| Men, % (n) | 86 (112) |
| BMI in kg/m2, mean ± SDa | 23.8 ± 4.53 |
| Affected dominant side, % (n)a | 62 (51) |
| Interval between trauma and surgery in months, mean ± SD (n)a | 14 (6.0-29) |
| ≤ 6 months | 3.8 ± 0.31 (38) |
| 7 to 12 months | 8.9 ± 0.36 (22) |
| > 12 months | 45 ± 6.2 (69) |
| Postoperative follow-up time in months, median (IQR) | 47 (33-66) |
| Type of injury, % (n)a | |
| Complete C5 to T1, preganglionic | 47 (55) |
| C5, C6, C7 postganglionic; C8 to T1 preganglionic | 19 (22) |
| Complete C5 to T1, postganglionic | 34 (39) |
| Mechanism of injury, % (n)a | |
| Low-velocity motorcycle collision | 31 (40) |
| High-velocity motorcycle collision | 66 (85) |
| Industrial crush injury | 1.6 (2) |
| Fall from height | 1.6 (2) |
Missing data: BMI: 36% (47 of 130) of data are missing; affected dominant side: 37% (48 of 130) of data are missing; interval between trauma and surgery: 0.7% (1 of 130) of data are missing; type of injury: 11% (14 of 130) of data are missing; mechanism of injury: 0.7% (1 of 130) of data are missing; FFMT = free functional muscle transfer.
Surgical Technique
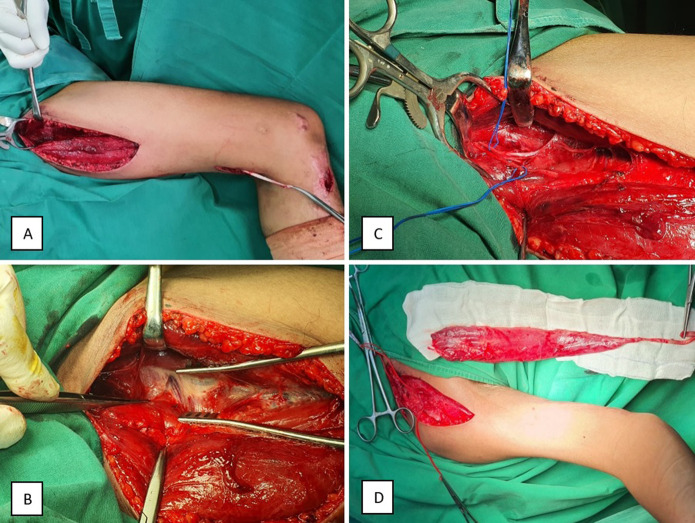
First, the entire brachial plexus trajectory was surgically exposed with a supraclavicular and infraclavicular approach. To establish a definitive surgical plan, intraoperatively, we used electrical nerve stimulation and ultrasound. Viable resources were isolated and the proximal recipient site of the free muscle donor was prepared. Second, the patient was positioned with the contralateral leg abducted and externally rotated, with flexion of the knee and hip [12]. The gracilis muscle flap, anterior branch of the obturator nerve, medial circumflex artery, and two concomitant veins were harvested with three small incisions, including a 3-cm longitudinal incision at the pes anserine to cut the insertion, a 5-cm transverse incision in the medial aspect of the distal thigh at the musculotendinous junction, and a 15-cm longitudinal incision in the medial thigh to dissect the origin proximally at the suprapubic area (Fig. 2A-C). In a few thin patients, an additional skin paddle (15 cm) was harvested. The distal recipient site for the gracilis flap was prepared by creating a subcutaneous tunnel on the anteromedial aspect of the arm along the medial distal humerus and incisions distally over the anterior cubital fossa and anterior or posterior aspect of the forearm (Fig. 3) [18]. The surgeon used a 4.5× magnifying glass to perform microsurgery, creating an end-to-end vascular anastomosis, followed by an end-to-end neurorrhaphy. The gracilis flap with the tendon was placed underneath the lacertus fibrosus and mobile wad compartment. To maximize the length of the tendon, the full length of the gracilis muscle (44 cm) and tendon (6 cm) was used (Fig. 2D) [23], which was transferred to the upper arm and secured to the distal tendons to stabilize the wrist and fingers (Fig. 3) [14]. After distal attachment, the patient’s arm was positioned in elbow flexion and wrist extension to correct the length of the muscle and adjust the tension of the flap to maintain the resting muscle length. Furthermore, the patency of the artery, nerve, and vein was evaluated.
Fig. 2.
These photographs show harvest of the gracillis muscle flap. (A) The gracilis was approached through three small incisions: a 3-cm longitudinal incision at the pes anserine, a 5-cm transverse incision in the medial aspect of the distal thigh, and a 15-cm longitudinal incision in the medial thigh. (B) The adductor longus was retracted anteriorly to cut the gracilis origin at the inferior ramus of the pubis. (C) We identified the anterior branch of the obturator nerve, medial circumflex artery, and two concomitant veins. (D) The gracilis was dissected from the thigh, with a full-length gracilis muscle (44 cm) and tendon (6 cm).
Fig. 3.
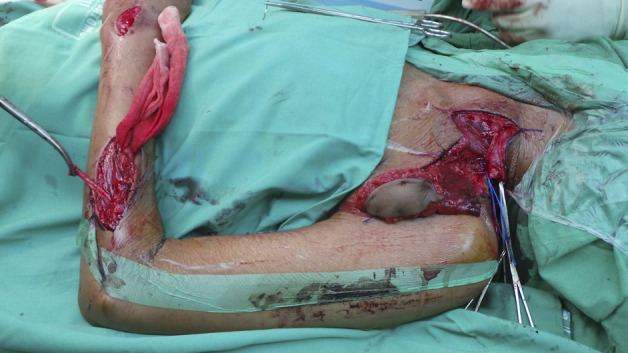
This figure shows distal attachment of the gracilis tendon to the extensor carpi radialis brevis for wrist stabilization, performed in 84% (110 of 130) of patients. The gracilis flap was proximally attached to the distal one-third of the clavicle with a subcutaneous tunnel on the anteromedial aspect of the arm along the medial distal humerus.
The primary goal of all FFMT procedures was to achieve elbow flexion, followed by shoulder abduction and stabilization of the wrist. The gracilis tendon was distally attached to the extensor carpi radialis brevis (ECRB) for wrist extension in 85% (110 of 130) of patients. In patients who asked the surgeon to focus on the recovery of hand function, the gracilis tendon was attached to the extensor digitorum communis (EDC) and extensor pollicis longus (EPL) for finger extension in 12% (16 patients), and to the flexor digitorum profundus (FDP) and flexor pollicis longus (FPL) for finger flexion in 3% (four patients) of patients. Proximal attachment was performed in the distal third of the clavicle in most patients and in the proximal humeral bone in one patient. From May 2011 to May 2015, the transverse cervical artery and vein were mostly used as resources in 35% (46 of 130) of patients. From May 2015 to March 2020, an improved technique with a smaller thoracoacromial artery and cephalic vein was used in 63% (82) of patients because the level of the artery source is closer to the medial pedicle of the gracilis muscle [12]. In two patients, both arteries were not available, so the thoracodorsal artery was used. Moreover, the phrenic nerve (12 cm) was mostly used as the nerve donor in 84% of patients (109 of 130), leaving the spinal accessory nerve free for additional nerve transfer to the suprascapular nerve for shoulder abduction and rotation in 19% (25) of patients. If the phrenic nerve was not viable, the spinal accessory nerve was used as a donor in 10% (13 patients). The intercostal nerves were alternatively used in 5% (seven patients), and the thoracodorsal nerve was used in one patient. Extended brachial plexus reconstruction was performed in 46% (60 of 130) of patients at least 1 year before FFMT. External neurolysis was performed to remove scar tissue along the nerve in 10% (13 patients), accessory to the suprascapular or axillary nerve transfer in 28% (36), or shoulder arthrodesis in 5% (seven) for shoulder abduction. Intraplexal or extraplexal nerve transfer was performed in the radial nerve for wrist and finger extension in one patient, in the median nerve for hand pinch function in 8% (10 of 130), in the ulnar nerve for hand grasp function in 2% (three patients), and in the musculocutaneous nerve for elbow flexion in 11% (14) of all patients. All patients had failed primary nerve procedures, and these procedures did not influence measurements after FFMT.
The postoperative rehabilitation program started with edema control for 3 weeks, as well as 1 month of shoulder immobilization with mostly a foreslap splint from the insertion of the deltoid to the midshaft of the metacarpal. The elbow was held in 90° of flexion, forearm in supination, and wrist in slight flexion to put the gracilis muscle in the resting position and protect the distal attachment of the gracilis in the ECRB. Passive movement exercises of the wrist and fingers were initiated at 1 week postoperatively, followed by shoulder abduction and elbow flexion after 3 weeks, as permitted by the surgeon. After hospital discharge, the patients attended follow-up visits at our tertiary hospital with a specialized rehabilitation team for BPI. One month postoperatively, neuromuscular re-education was started with electrical stimulation and biofeedback. After 2 months, patients began active and assistive movement exercises, including the use of gravity-minimalized positions to improve active ROM, followed by strengthening exercises starting at 10 weeks postoperatively. However, patients who were unable to return to the clinic because of long travel distances continued their rehabilitation program at the nearest general hospital in their region.
Data Sources and Measurement
Information on patient characteristics, BPI lesions, preoperative evaluations, operative techniques, and postoperative outcomes were obtained from the hospital’s database, surgical reports, and medical records. Existing videos, photographs, and handwritten notes that were taken during the patient’s regular care were reviewed, which were documented by the treating team of one senior orthopaedic hand surgeon, orthopaedic surgeons, and residents. All preoperative and postoperative videos with outcomes of muscle strength and ROM were reassessed by the same senior surgeon, together with one researcher who was not involved in patient care. Participants were invited to attend additional follow-up visits from November 2017 to January 2018 and annual organized patient gatherings from 2017 to 2020 as part of regular patient care. Information on a patient’s ability to perform certain daily activities, work, sports, or performing arts [26] was prospectively collected during follow-up visits and by questionnaires sent through email. Patients who lived too far away to visit the hospital were asked to send a homemade video according to the rules of an instruction video.
Primary and Secondary Study Outcomes
Our primary study goal was to evaluate the functional recovery of the elbow (flexion), shoulder (abduction), and wrist (extension). To achieve this, we defined different grades of muscle strength by the British MRC score, with scores of 0 to 2 (of 5) considered to indicate a poor outcome, 3 as fair, 4 as good, and 5 as excellent [1]. Furthermore, active ROM was measured with a goniometer. Evaluation of MRC grades based on the video was adjusted to visual assessment only, and grades were defined as M0: no visible muscle contraction or movement; M1: visible muscle contraction, but no movement in the supine position against gravity; M2: active movement in the supine position, but no movement against gravity while standing; M3: active movement possible against gravity, but no strength to keep the position against gravity higher than 90°; M4: active ROM of more than 90° against gravity, with the patient able to hold a heavy bag; and M5: full active ROM while the patient is holding heavy weights. Our secondary goal was to explore whether the choice of distal attachment to either the ECRB, EDC/EPL, or FDP/FPL affected the functional recovery of the elbow, shoulder, and wrist. Therefore, a univariate analysis of variance test was performed. Third, the effect of the choice of the source of the phrenic nerve, accessory spinal nerve, or intercostal nerves was also evaluated in terms of motor outcomes, using a univariate analysis of variance test, but only in patients with distal attachment to the ECRB to account for confounding. Similarly, the outcomes for shoulder abduction with and without an additional accessory to the suprascapular nerve were compared. Our fourth goal was to investigate what patient and injury factors might be associated with less residual disability, such as age; gender; affected dominant side; the time between injury and surgery; type of injury; mechanism of injury; postoperative months; surgical procedure, including distal and proximal attachment and the source of the artery and nerve; and additional procedures before or during FFMT, using multivariate linear regression analysis. To evaluate the patient’s postoperative disabilities, the validated DASH score was used [19, 30], with a version translated into Indonesian, using 5-point ordinal scale questions; a score of 0 to 20 (of 100) was considered no difficulty, 21 to 40 mild difficulty, 41 to 60 moderate difficulty, 61 to 80 severe difficulty, and 81 to 100 an inability to perform any task. For overall pain assessment at rest, the validated VAS score was used [1], with a scale of 1 to 10 (no pain to the worst pain possible).
Lastly, we aimed to identify complications such as flap necrosis, lack of flap reinnervation, bowstringing (with a wrong gracilis pathway leading to elbow extension instead of elbow flexion), infection of the surgical site or anywhere else in the body, and indications for reoperation with a second FFMT. All patients had a postoperative follow-up of at least 1 year, with a median duration time of 47 months (IQR of 33 to 66 months).
Ethical Approval
Institutional review board approval and written informed consent were obtained.
Statistical Analysis
For the statistical analysis, SPSS Statistics, version 25, was used (IBM Corp). Graphics were designed using Tableau 2020.4.0. We considered p values < 0.05 significant, but the effect size was considered more relevant. Furthermore, patients were stratified by early (≤ 6 months), delayed (7 to 12 months), or late intervention (> 12 months) to consider confounding by indication.
A multivariate linear regression model was used, programmed with R Studio version 1.3.1093. The factors of age (ρ coefficient = 0.21; 95% confidence interval [CI] -0.04 to 0.44; p = 0.10), gender (ρ coefficient = -0.20; 95% CI -0.43 to 0.06; p = 0.13), and postoperative months (ρ coefficient = 0.20; 95% CI -0.28 to 0.22; p = 0.12) were included in the regression analysis, because the Spearman rho correlation had a p value < 0.2 (mild association). Because of the retrospective nature of our study, a formal power analysis was not performed.
Results
Elbow, Shoulder, and Wrist Muscle Function and ROM
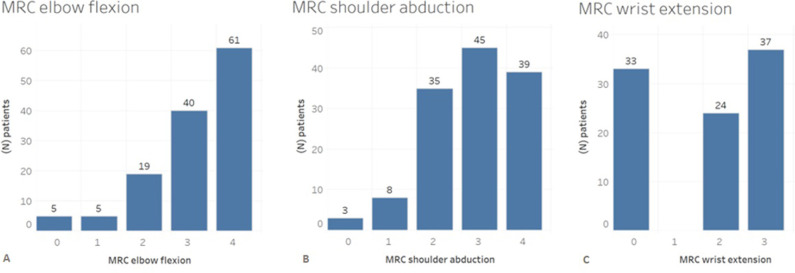
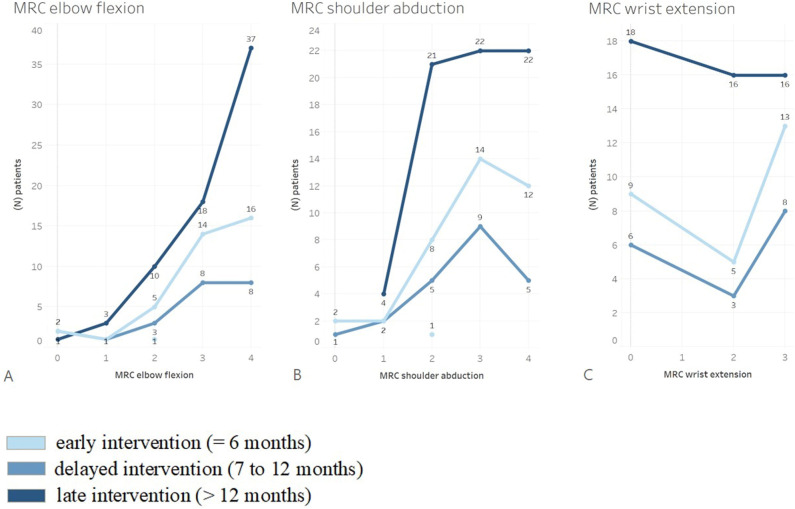
The median elbow flexion muscle strength was 3 (IQR 3 to 4) (Table 2) and active ROM was 88° ± 46° (Fig. 4). The median shoulder abduction grade was 3 (IQR 2 to 4) (Table 2) and active ROM was 62° ± 42° (Fig. 4). Forty-seven percent of the patients (61 of 130 patients) had a grade of M4 and 31% (40 patients) had a grade of M3 for elbow flexion (Fig. 5A). However, there was no difference in the median elbow flexion strength, with differences in time between injury and surgery (within 6 months: 3 [IQR 3 to 4], between 7 and 12 months: 3 [IQR 2 to 4], and later than 12 months: 3 [IQR 3 to 4]; p = 0.32) (Fig. 6A) or ROM (within 6 months: 92° ± 48°, between 7 and 12 months: 68° ± 45°, later than 12 months: 68° ± 49°; p = 0.07).
Table 2.
Effects of distal gracilis attachment on muscle strength and ROM
| Parameter | All (n = 130) | ECRB (n = 110) | EDC/EPL (n = 16) | FDP/FPL (n = 4) | p value |
| Elbow flexion MRC, median (IQR) | 3 (3-4) | 3 (3-4) | 3 (2-4) | 4 (2-4) | 0.44 |
| Elbow flexion ROM in degrees, mean ± SD | 88 ± 46 | 89 ± 46 | 74 ± 47 | 113 ± 19 | 0.27 |
| Shoulder abduction MRC, median (IQR) | 3 (2-4) | 3 (2-4) | 3 (2-3) | 3 (2-3) | 0.41 |
| Shoulder abduction ROM in degrees, mean ± SD | 62 ± 42 | 63 ± 44 | 56 ± 34 | 46 ± 17 | 0.60 |
| Wrist extension MRC, median (IQR)a | 2 (0-3) | 2 (0-3) | 2 (0-3) | 1 (0-2) | 0.43 |
| Wrist extension ROM in degrees, mean ± SDa | 20 ± 18 | 21 ± 19 | 21 ± 14 | 13 ± 15 | 0.69 |
No association was found between the distal attachment of the gracilis free muscle flap and postoperative muscle strength grades and ROM of elbow flexion, shoulder abduction, and wrist extension (p > 0.05).
Sample size for wrist extension: all n = 94, ECRB n = 80, EDC/EPL n = 10, FDP/FPL n = 4. MRC = Medical Research Council; ECRB = extensor carpi radialis brevis; EDC/EPL = extensor digitorum communis and extensor pollicis longus; FDP/FPL = flexor digitorum profundus and flexor pollicis longus.
Fig. 4.
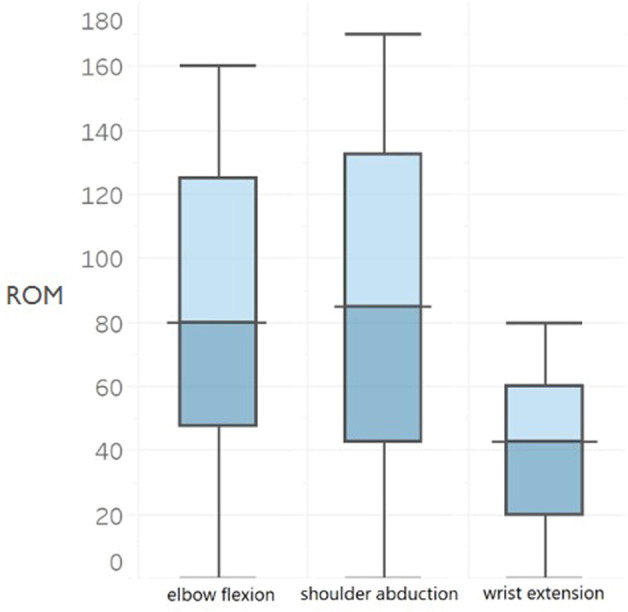
This figure shows three boxplots with postoperative ROM for elbow flexion (n = 130 patients), shoulder abduction (n = 130), and wrist extension (n = 94) after FFMT in patients with total BPIs. The middle line shows the median, the borders of the boxplots show the interquartile range, and the outer lines show the minimum and maximum ROM values; FFMT = free functional muscle transfer; BPI = brachial plexus injury.
Fig. 5.
This bar chart shows the postoperative MRC strength for (A) elbow flexion, (B) shoulder abduction, and (C) wrist extension after FFMT in 130 patients with total BPIs. The number of patients with the same MRC outcome is also shown per bar; MRC = Medical Research Council; FFMT = free functional muscle transfer; BPI = brachial plexus injury.
Fig. 6.
These graphs show the postoperative MRC strength for (A) elbow flexion, (B) shoulder abduction, and (C) wrist extension after FFMT in 130 patients with total BPIs, stratified for the difference in time between injury and surgery: patients with early intervention (≤ 6 months) (light blue), patients with delayed intervention (7 to 12 months) (medium blue), and patients with late intervention (> 12 months) (dark blue). The number of patients with the same MRC outcome is also shown; MRC = Medical Research Council; FFMT = free functional muscle transfer; BPI = brachial plexus injury.
For shoulder abduction, 30% (39 of 130) of all patients had a grade of M4, and 35% (45 patients) had a grade of M3 (Fig. 5B). In 94 patients, the median postoperative MRC score for wrist extension was 2 (IQR 0 to 3) (Table 2) and active ROM was 20° ± 18° (Fig. 4). Of these patients, 39% (37 of 94) had a grade of M3 and 26% (24) had a grade of M2 for wrist extension (Fig. 5C). Furthermore, differences in time between injury and surgery did not result in differences in the median MRC strength score and mean ROM of shoulder abduction (Fig. 6B) and wrist extension (Fig. 6C) (p > 0.05).
Effects of Distal Attachment Selection
The choice of distal insertion was not associated with differences in the median elbow flexion strength (ECRB: 3[IQR 3 to 4], EDC/EPL: 3 [IQR 2 to 4], FDP/FPL: 4 [IQR 2 to 4]; p = 0.44) or ROM (ECRB: 89° ± 46°, EDC/EPL: 74° ± 47°, FDP/FPL: 113° ± 19°; p = 0.27). Furthermore, no effect was found regarding the choice of distal attachment with median shoulder abduction strength (ECRB: 3 [IQR 2 to 4]; EDC/EPL: 3 [IQR 2 to 3], FDP/FPL: 3 [IQR 2 to 3]; p = 0.41) or ROM (ECRB: 63° ± 44°, EDC/EPL: 56° ± 34°, FDP/FPL: 46° ± 17°; p = 0.60). Lastly, the outcomes of wrist extension remained poor, and no association was found for median wrist extension strength (ECRB: 2 [IQR 0 to 3], EDC/EPL: 2 [IQR 0 to 3], FDP/FPL: 1 [IQR 0 to 2]; p = 0.43) and ROM (ECRB: 21° ± 19°, EDC/EPL: 21° ± 14°, FDP/FPL: 13° ± 15°; p = 0.69), regardless of the location of distal attachment (Table 2).
Nerve Source
We found no difference among nerve source in terms of median elbow flexion strength (phrenic nerve: 3 [IQR 3 to 4] and mean ROM 87° ± 46°, 94 patients; accessory nerve: 4 [IQR 3 to 4] and mean ROM 106° ± 49°, 11 patients; intercostal nerves: 4 [IQR 2 to 4]; p = 0.57; mean ROM: 103° ± 50°, four patients; p = 0.55) or for median shoulder abduction strength (phrenic nerve: 3 [IQR 2 to 4] and mean ROM 63° ± 44°; accessory nerve: 3 [IQR 2 to 4]; mean ROM 61° ± 42°; intercostal nerves: 4 [IQR 3 to 4]; p = 0.46; mean ROM 88° ± 47°; p = 0.61) in patients with FFMT to the ECRB. Furthermore, there were no differences in the median shoulder abduction muscle strength between patients who had an additional nerve transfer of the accessory to the suprascapular or axillary nerve (3 [IQR 3 to 4]; mean ROM 63° ± 43°) and those who did not (3 [IQR 2 to 4]; p = 0.95 and mean ROM 62° ± 42°; p = 0.89) (Table 3).
Table 3.
Effects of different nerve sources on muscle strength and ROM
| Parameter | Phrenic nerve (n = 94) | Accessory nerve (n = 11) | Intercostal nerves (n = 4) | p value |
| Elbow flexion MRC, median (IQR) | 3 (3-4) | 4 (3-4) | 4 (2-4) | 0.57 |
| Elbow flexion ROM in degrees, mean ± SD | 87 ± 46 | 106 ± 49 | 103 ± 50 | 0.55 |
| Shoulder abduction MRC, median (IQR) | 3 (2-4) | 3 (2-4) | 4 (3-4) | 0.46 |
| Shoulder abduction ROM in degrees, mean ± SD | 63 ± 44 | 61 ± 42 | 88 ± 47 | 0.61 |
| Wrist extension MRC, median (IQR)a | 2 (0-3) | 3 (2-3) | 2 (2-2) | 0.24 |
| Wrist extension ROM in degrees, mean ± SDa | 19 ± 18 | 31 ± 26 | 30 ± 10 | 0.25 |
In patients with free functional gracilis muscle transfer with distal attachment to the extensor carpi radialis brevis, no association was found between the use of a nerve source and postoperative muscle strength grades and ROM of elbow flexion, shoulder abduction, and wrist extension (p > 0.05).
Sample size for wrist extension: all n = 76, phrenic nerve n = 69, accessory nerve n = 4, intercostal nerves n = 3; MRC = Medical Research Council.
Factors Associated With Lower Extremity Disability
The median postoperative DASH score was 33 (IQR 18 to 52, obtained in 62 patients who attended a follow-up visit at a mean of 36 ± 20 months after FFMT). No associations were found with less disability (lower DASH scores), including young age (coefficient = 0.28; 95% CI -0.22 to 0.79; p = 0.27), being a woman (coefficient = -9.4; 95% CI -24 to 5.3; p = 0.20), and more postoperative months (coefficient = 0.02; 95% CI -0.01 to 0.05; p = 0.13) (Table 4). Furthermore, the extent of recovery of elbow flexion strength or ROM was not associated with age, gender, BMI, affected dominant side, the interval between injury and surgery, type of injury, and additional procedures (p > 0.2). The median postoperative DASH score was not associated with differences in time between the injury and surgery (within 6 months: 33 [IQR 20 to 73], between 7 and 12 months: 30 [IQR 13 to 51], after 12 months: 29 [IQR 18 to 48]; p = 0.49). The optional DASH for work was obtained from 52 patients (median 47 [IQR 31 to 81]), and the DASH for sports and performing arts was obtained from 35 patients (median 63 [IQR 42 to 94]). The mean VAS score for postoperative pain at rest was 3 ± 2 in 121 patients (Table 5).
Table 4.
Association of patient and injury factors with the DASH in patients with traumatic complete BPI treated with FFMT
| Univariate analysis | Multivariate linear regression analysis | |||||
| DASH (n = 62) | ρa | (95% CI) | p value | βb | (95% CI) | p value |
| Gender | -0.20 | (-0.43 to 0.06) | 0.13 | -9.4 | (-24 to 5.3) | 0.20 |
| Age at time of surgery | 0.21 | (-0.04 to 0.44) | 0.10 | 0.28 | (-0.22 to 0.79) | 0.27 |
| BMI | -0.01 | (-0.26 to 0.24) | 0.93 | |||
| Mechanism of injury | 0.02 | (-0.23 to 0.27) | 0.90 | |||
| Affected dominant side | -0.12 | (-0.36 to 0.14) | 0.39 | |||
| Diagnosis | -0.05 | (-0.33 to 0.16) | 0.73 | |||
| Time between injury and surgery | -0.09 | (-0.06 to 0.43) | 0.49 | |||
| Postoperative months | 0.20 | (-0.28 to 0.22) | 0.12 | 0.02 | (-0.01 to 0.05) | 0.13 |
| Distal attachment | -0.03 | (-0.09 to 0.40) | 0.80 | |||
| Source of nerve | -0.03 | (-0.20 to 0.30) | 0.83 | |||
| Source of artery | 0.16 | (-0.43 to 0.06) | 0.21 | |||
| Additional procedure | 0.06 | (-0.04 to 0.44) | 0.67 | |||
The factors of age, gender, and postoperative months were included in the regression analysis because Spearman rho correlation “ρ” had a p value < 0.2; mild association, with univariate analysis for nonparametric data. No associations were found with less disability (lower DASH scores): young age, being a woman, and more postoperative months, because the sample size (n = 62) is small.
Spearman rho (ρ) coefficient represents a magnitude of correlation, in the range of no association (ρ = 0) to monotonic correlation (ρ = -1 or 1). Weak association: p > 0.2; mild association: p < 0.2; strong association: p < 0.05.
Beta (β) coefficient represents the degree of change in the outcome variable of DASH for every unit of change in the predictor variable; FFMT = free functional muscle transfer; BPI = brachial plexus injuries.
Table 5.
Effect of the timing of FFMT and outcome after FFMT in terms of DASH score and VAS pain score
| Score | Within 6 months | 7-12 months | > 12 months | p value |
| DASH, median (IQR) | 33 (20-73) | 30 (13-51) | 29 (18-48) | 0.49 |
| DASH work, median (IQR) | 43 (27-63) | 41 (25-78) | 69 (33-81) | 0.22 |
| DASH sports/performing arts, median (IQR) | 53 (36-75) | 44 (33-67) | 69 (47-94) | 0.24 |
| VAS pain, mean ± SD | 3 ± 2 | 3 ± 2 | 3 ± 2 | 0.70 |
Sample size of patients with DASH scores, with a scale of 0 (no disability) to 100 (not able to perform activities of daily living) after FFMT: all n = 62, FFMT within 6 months after trauma n = 17, between 7 and 12 months after trauma n = 12, more than 12 months after trauma n = 33. Sample size of patients with VAS scores for pain at rest, with a scale of 0 (no pain) to 10 (unbearable pain), after FFMT: all n = 121, FFMT within 6 months after trauma n = 35, between 7 and 12 months after trauma n = 21, more than 12 months after trauma n = 64.
Complications
Complications were observed in 15% (20 of 130) of patients during or after surgery (Table 6). Flap necrosis occurred in 5% (seven of 130) of all patients; the cervical transverse artery was involved in 3% (four of 130) and the thoracoacromial artery was involved in 2% (three of 130). Failed innervation of the gracilis muscle occurred in 4% (five patients). In 4% (five patients), an infection occurred; one patient had postoperative elbow flexion muscle strength of 0. Furthermore, in 2% (two patients) of patients, bowstringing occurred. In another patient, the gracilis tendon did not reach the distal attachment to the ECRB; therefore, a palmaris longus tendon graft was used. However, the outcome of elbow flexion muscle strength was 0 in this patient. The median postoperative elbow flexion strength in these patients was 2 (IQR 2 to 2) and ROM was 30° (IQR 20° to 40°).
Table 6.
Complications (20 of 130 patients)
| Complication | Value, % (n) |
| Flap necrosis | 5 (7) |
| Did not reinnervate | 4 (5) |
| Bowstringing | 2 (2) |
| Shortage of gracilis length | 1 (1) |
| Infection | 4 (5) |
| Reoperation with second FFMT | 11 (14) |
| Rehabilitation insufficiency | 18 (23) |
Of all patients with complications, in 11% (14 of 130 patients) a reoperation with a second FFMT of the other gracilis muscle was performed. Among these patients, seven had a distal attachment to the FDP/FPL, six had an attachment to the ECRB, and one patient had an attachment to the EDC/EPL. Proximal attachment to the clavicle was performed in 79% (11 of 14) of patients and to the humerus in three. The intercostal nerves were used as a donor in 71% (10) of patients, the accessory nerve was used in 14% (two), the phrenic nerve was used in 14% (two), and the thoracodorsal nerve was used in one patient. After the second FFMT in these 14 patients, the median elbow flexion strength was 4 (IQR 3 to 4), with a mean ROM of 97° ± 49°. The median shoulder abduction muscle strength was 3 (IQR 2 to 4), with a mean ROM of 75° ± 41°. However, the median wrist extension muscle strength was 0 (IQR 0 to 0), with a mean ROM of 22° ± 22°.
Discussion
Patients with a flail arm after traumatic complete BPI require active ROM and fair muscle strength of the elbow to gain functionality. FFMT can reconstruct elbow flexion and shoulder abduction in patients with traumatic, complete BPIs. In our study, patients with early, delayed, and late reconstruction were evaluated with different techniques for attaching the distal tendon and donor nerves. We aimed to evaluate the functional outcomes of FFMT in terms of muscle strength and ROM and to discuss the choice of distal attachment, nerve and artery source, associated patient and injury factors, and the risk of complications.
Limitations
First, for this study, it was not possible to prospectively collect data at the same follow-up intervals. Unfortunately, we did not evaluate preoperative DASH and VAS scores in these patients. Therefore, we cannot make statements regarding any improvement in daily functioning and experience of pain. Furthermore, the disadvantage of longitudinal research is the increased risk of selective dropout. Patients might have been more willing to come for a postoperative evaluation if they hoped for improvement or subsequent surgery. Likewise, patients with no improvement after surgery might have been less motivated to visit the hospital. Postoperative outcomes were slightly lower in patients who had follow-up shorter than 2 years than in those who had longer follow-up; additionally, 17 patients were lost before 1 year of follow-up, indicating transfer bias. The limitation of missing patient data results from the daily practice and registration system used in Dr. Soetomo General Hospital, which used paper documents before an electronic system. Therefore, performing retrospective research in countries such as Indonesia remains challenging. Although missing patients are usually doing worse, the findings might represent a best-case scenario in terms of the results of this intervention. However, almost one-fifth of these patients live on other islands and had no access to specialized rehabilitation training at their home base. Suboptimal rehabilitation resulted in worse postoperative outcomes than intended. Another 10 patients were excluded from the analysis because they underwent double FFMT to reconstruct the hand’s function. In this study, we included 20 patients who also had a request for hand prehension, in whom a single FFMT to the distal FDP/FPL (four of 20 patients) or EDC/EPL (16 patients) was performed, instead of to the ECRB, as is usually done. However, these patients had the same preoperative muscle strength and ROM as patients who had FFMT with distal attachment to the ECRB. We compared outcomes regarding the choice of distal insertion, with the remark that these small numbers of subsets have insufficient power to allow us to conclude about potentially important differences.
Another disadvantage in this retrospective study was observer bias because the postoperative outcomes of muscle strength and ROM were reported by different orthopaedic surgeons and residents. The British MRC grading is recommended as the best method for assessing functional motor recovery [20, 25], although it is subjective. The intrarater agreement of the MRC score is high (kappa > 0.8) [25], which minimizes interobserver bias. Furthermore, patient outcomes were reassessed by a senior orthopaedic surgeon and a researcher who was not involved in patient care. Because some patients from other islands of Indonesia lived too far away to visit our hospital for a follow-up examination, some observations of MRC for strength and ROM were based on homemade videos, which gave less reliable results regarding motor strength. This resulted in assessment bias. The video follow-up visits also limited our ability to classify motor strength as M0 or M1. Patients who were able to hold a heavy bag against resistance had a score of M4; however, differences in the weight of the bag might have led to an overestimation of the results in some patients.
Motor and ROM Recovery After Muscle Transfer
After free gracilis muscle transfer, most patients gained motor strength to overcome gravity (≥ M3) and achieved functional ROM in terms of elbow flexion (in 78%) and shoulder abduction (in 75%). A systemic review [33] stated that 65% to 87% of patients achieved a useful power grade of M3 or more for elbow flexion after FFMT. With 14 years of experience performing FFMT and performing 16 procedures per year, we achieved a mean ROM of 88° ± 46° in these patients for elbow flexion and ROM of 62° ± 42° for shoulder abduction.
Distal Transfer Choice and Wrist Function
Differences in the placement of the distal attachment of the gracilis muscle did not lead to differences in shoulder, elbow, and wrist strength and ROM. The optimal placement of the distal tendon of the gracilis transfer is still being discussed. Maldonado et al. [21] concluded that distal attachment to the FDP/FPL achieves better elbow flexion strength and ROM than attachment to the biceps tendon. Although no studies, to our knowledge, have compared the effect of distal insertion on motor outcomes, other small case series have described some alternatives to distal attachment to the biceps, forearm extensor, or flexor tendons [3, 17]. So far, the best results were achieved by Doi et al. [9]. Despite these promising results, worldwide experience with FFMT is limited. More studies investigating this matter are needed to give evidence-based conclusions about the best distal insertion of the gracilis muscle.
Choice of Nerve Source
Differences in the choice of nerve source did not lead to differences in shoulder, elbow, and wrist strength and ROM. In this study, most of the patients (84%) had a phrenic nerve source. Other studies also reported successful outcomes with the intercostal nerves [7] or an accessory nerve donor [4, 32], but had worse outcomes when a nerve graft was needed [7, 11]. Oliver et al. [24] found better outcomes for elbow flexion in patients treated with an accessory nerve donor for FFMT than in those with a phrenic nerve source. Phrenic nerve transfer reduces respiratory function, whereas the use of three or four intercostal nerves has less effect on lung capacity [5, 31, 34]. However, with the use of a phrenic nerve donor, most pulmonary function parameters gradually recover to preoperative levels within 1 year [34]. In our study, postoperative lung capacity was not evaluated. Furthermore, an additional suprascapular or axillary nerve transfer did not lead to better shoulder abduction strength and ROM. Unfortunately, the postoperative outcomes for other rotator cuff functions were not consistently collected for this study.
Factors Associated With Disability
Older age, men, and fewer postoperative months were not associated with worse outcomes (higher DASH scores). However, Doi et al. [9, 10] stated that age older than 60 years is a contraindication for FFMT. Sixty-two patients had DASH scores representing mild disability after surgery, with a median DASH score of 33 points, indicating that they still have a fair-to-poor ability to perform bilateral activities for which force is needed or to position the arms above the head. Impairment in hand function has a large physical and psychological impact and greatly limits daily function. According to Kachooei et al. [16], the dominant hand’s condition, being a woman, the extent of trauma, and shoulder and hand involvement are associated with higher DASH scores. However, in the current study, these factors were not correlated with DASH scores. BMI is also considered important for postoperative functional recovery [21, 28], although no association was found in this study. The DASH questionnaire is a convenient method to evaluate disabilities in daily functioning [25] but not the quality of life. This shows the need to adopt alternative ways to evaluate the patient’s full condition; for example, the Ulm questionnaire, which assesses the patient’s perception of disability and treatment satisfaction [6, 26]; the World Health Organization’s Quality-of-Life questionnaire, which evaluates psychologic health and social relationships [16]; and the SF-36, which assesses physical and social health [1, 8].
Complications
Five percent of the patients had flap necrosis and 4% experienced reinnervation problems. Preoperative planning with an electromyography and nerve conduction velocity test, chest radiography, and intraoperative electrical nerve stimulation to evaluate the viable nerve source is greatly important to prevent end-to-end neurorrhaphy problems. To prevent vascular anastomosis, regular ultrasound control is necessary. It has been reported that flap necrosis occurs in 4% to 17% of patients [33]. Flap necrosis occurred more when the transverse cervical artery source was used than when the thoracoacromial artery was used. The thoracoacromial artery is smaller in diameter and closer to the medial pedicle of the gracilis muscle than the transverse cervical artery. When harvesting the vascular pedicle, an additional concomitant vein should also be harvested as a backup. Furthermore, 4% of all patients had an infection. Preoperative blood testing to assess the patient’s health status and pre-existing infections also helps to avoid perioperative infections. Moreover, postoperative rehabilitation also plays an important role in the recovery of upper extremity movement after surgery [15, 22]. Patients did not always return to the hospital for follow-up and rehabilitation. This could be related to low literacy, financial problems, visits to traditional or religious healers, and long travel distances to the hospital. To achieve the optimal result, multidisciplinary collaboration with rehabilitation specialists is essential, with the aggressive and sustained rehabilitation program recommended by Doi et al. [9].
Conclusion
Overall, free functional gracilis muscle transfer may improve ROM, with fair-to-good muscle power in elbow flexion, shoulder abduction, and overall function for the patient, whereas wrist function remained nonfunctional. FFMT of the gracilis muscle is a viable treatment option for patients with a flail upper limb after failure of primary nerve surgery, delayed intervention, or total root avulsions. Meticulous microsurgical skills and extensive rehabilitation training are needed for an optimal FFMT. Further research should focus on gains in ROM and function for the patient regarding different FFMT techniques, as well as distal attachment, nerve source, and pathways of the subcutaneous tunnel. With the heterogenicity of BPI, confounding by indication is inevitable and comparison studies remain complex.
Acknowledgment
We thank the brachial plexus team of Dr. Soetomo General Hospital and Universitas Airlangga, Surabaya, Indonesia.
Footnotes
Each author certifies that there are no funding or commercial associations (consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted article related to the author or any immediate family members.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Ethical approval for this study was obtained from Leiden University Medical Center, Leiden the Netherlands (number C17.092/SH/sh) and Dr. Soetomo General Academic Hospital, Surabaya, Indonesia (number 1399/KEPK/VIII/2019).
This work was performed at Dr. Soetomo General Hospital, Surabaya, Indonesia.
Contributor Information
Tawatha C. Steendam, Email: t.c.steendam@lumc.nl, tawathacamilla@gmail.com.
Rob G. H. H. Nelissen, Email: r.g.h.h.nelissen@lumc.nl.
Martijn J. A. Malessy, Email: Malessy@lumc.nl.
Mohammad H. Basuki, Email: basukimh@gmail.com.
Airlangga B. P. Sihotang, Email: anggasihotang@gmail.com.
References
- 1.Ahmed-Labib M, Golan JD, Jacques L. Functional outcome of brachial plexus reconstruction after trauma. Neurosurgery. 2007;61:1016-1022. [DOI] [PubMed] [Google Scholar]
- 2.Akasara Y, Hara T, Takahashi M. Restoration of elbow flexion and wrist extension in brachial plexus paralyses by means of free muscle transplantation innervated by intercostal nerve. Ann Chir Main Memb Super. 1990;9:341-350. [DOI] [PubMed] [Google Scholar]
- 3.Armagil M, Ünsal S, Yıldırım T, et al. Outcome of free gracilis muscle transfer for the restoration of elbow flexion in traumatic brachial plexus palsy. Jt Dis Relat Surg. 2021;32:633-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barrie K, Steinmann S, Shin A, Spinner R, Bishop A. Gracilis free muscle transfer for restoration of function after complete brachial plexus avulsion. Neurosurg Focus. 2004;16:E8. [DOI] [PubMed] [Google Scholar]
- 5.Chalidapong P, Sananpanich K, Kraisarin J, Blumroongkit C. Pulmonary and biceps function after intercostal and phrenic nerve transfer for brachial plexus injuries. J Hand Surg Br. 2004;29:8-11. [DOI] [PubMed] [Google Scholar]
- 6.Choi PD, Novak CB, Mackinnon SE, Kline DG. Quality of life and functional outcome following brachial plexus injury. J Hand Surg Am. 1997;22:605-612. [DOI] [PubMed] [Google Scholar]
- 7.Chung D, Carver N, Wei F. Result of functioning free muscle transplantation for elbow flexion. J Hand Surg Am . 1996;21:1071-1077. [DOI] [PubMed] [Google Scholar]
- 8.Dodakundi C, Doi K, Hattori Y, et al. Outcome of surgical reconstruction after traumatic total brachial plexus palsy. J Bone Joint Surg Am. 2013;95:1505-1512. [DOI] [PubMed] [Google Scholar]
- 9.Doi K, Hattori Y, Sakamoto S, Dodakunda C, Satbhai B, Montales T. Current procedure of double free muscle transfer for traumatic total brachial plexus palsy. J Bone Joint Surg Am. 2013;95:1505-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doi K, Hattori Y, Yamazaki H, Wahegaonkar AL, Addosooki A, Watanabe M. Importance of early passive mobilization following double free gracilis muscle transfer. Plast Reconstr Surg. 2008;121:2037-2045. [DOI] [PubMed] [Google Scholar]
- 11.Elzinga K, Zuo K, Olson J, Morhart M, Babicki Chan K. Double free gracillis muscle transfer after complete brachial plexus injury: first Canadian experience. Plast Surg (Oakv) . 2014;22:26-29. [PMC free article] [PubMed] [Google Scholar]
- 12.Estrella EP, Montales TD. Functioning free muscle transfer for the restoration of elbow flexion in brachial plexus injury patients. Injury. 2016;47:2525-2533. [DOI] [PubMed] [Google Scholar]
- 13.Felici N, Zaami S, Ciancolini G, et al. Cost analysis of brachial plexus injuries: variability of compensation by insurance companies before and after surgery. Handchír Mikrochir Plast Chir. 2014;46:85-89. [DOI] [PubMed] [Google Scholar]
- 14.Giuffre JL, Bishop AT, Shin AY. Harvest of an entire gracilis muscle and tendon for use in functional muscle transfer: a novel technique. J Reconstr Microsurg. 2012;28:349-358. [DOI] [PubMed] [Google Scholar]
- 15.Havton LA, Caristedt T. Repair and rehabilitation of plexus and root avulsions in animal models and patients. Curr Opin Neurol. 2009;22:570-574. [DOI] [PubMed] [Google Scholar]
- 16.Kachooei A, Moradi A, Janssen S, Ring D. The influence of dominant limb involvement on DASH and QuickDASH. Hand (N Y). 2015;10:512-515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kay S, Pinder R, Wiper J, Hart A, Jones F, Yates A. Microvascular free functioning gracilis transfer with nerve transfer to establish elbow flexion. J Plast Reconstr Aesthet Surg. 2010;63:1142-1149. [DOI] [PubMed] [Google Scholar]
- 18.Limthongthang R, Bachoura A, Songcharoen P, Osterman AL. Adult brachial plexus injury: evaluation and management. Orthop Clin North Am. 2013;44:591-603. [DOI] [PubMed] [Google Scholar]
- 19.Madura T, Doi K, Hattori Y, Sakamoto S, Shimoe T. Free functioning gracilis transfer for reanimation of elbow and hand in total traumatic brachial plexopathy in children. J Hand Surg Eur Vol. 2018; 43:596-608. [DOI] [PubMed] [Google Scholar]
- 20.Maldonado AA, Kircher MF, Spinner RJ, Bishop AT, Shin AY. Free functioning gracilis muscle transfer versus intercostal nerve transfer to musculocutaneous nerve for restoration of elbow flexion after traumatic adult brachial pan-plexus injury. Plast Reconstr Surg. 2016;138:483e-488e. [DOI] [PubMed] [Google Scholar]
- 21.Maldonado AA, Romero-Brufau S, Kircher MF, Spinner RJ, Bishop AT, Shin AY. Free functioning gracilis muscle transfer for elbow flexion reconstruction after traumatic adult brachial pan-plexus injury: where is the optimal distal tendon attachment for elbow flexion? Plast Reconstr Surg. 2017;139:128-136. [DOI] [PubMed] [Google Scholar]
- 22.Martin E, Senders JT, DiRisio AC, Smith TR, Broekman MDL. Timing of surgery in traumatic brachial plexus injury: a systematic review. J Neurosurg. 2018;1:1-13. [DOI] [PubMed] [Google Scholar]
- 23.Morris SF, Yang D. Gracilis muscle: arterial and neural basis for subdivision. Ann Plast Surg. 1999;42:630-633. [DOI] [PubMed] [Google Scholar]
- 24.Oliver JD, Beal C, Graham EM, Santosa KB, Hu MS. Functioning free muscle transfer for brachial plexus injury: a systematic review and pooled analysis comparing functional outcomes of intercostal nerve and spinal accessory nerve grafts. J Reconstr Microsurg. 2020;36:567-571. [DOI] [PubMed] [Google Scholar]
- 25.Paternostro-Sluga T, Grim-Stieger M, Posch M, et al. Reliability and validity of the Medical Research Council (MRC) scale and a modified scale for testing muscle strength in patients with radial palsy. J Rehabil Med. 2008;40:665-671. [DOI] [PubMed] [Google Scholar]
- 26.Rasulić L, Savić A, Živković B, et al. Outcome after brachial plexus injury surgery and impact on quality of life. Acta Neurochir (Wien) . 2017;159:1257-1264. [DOI] [PubMed] [Google Scholar]
- 27.Sakellariou VI, Badilas NK, Stavropoulos NA, et al. Treatment options for brachial plexus injuries. ISRN Orthop. 2014:314137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Socolovsky M, Martins RS, Di Masi G, Bonilla G, Siqueira MG. Influence of body mass index on the outcome of brachial plexus surgery: are there any differences between elbow and shoulder results? Acta Neurochir (Wien). 2014;156:2337-2344. [DOI] [PubMed] [Google Scholar]
- 29.Suroto H, Antoni I, Siyo A, et al. Traumatic brachial plexus injury in Indonesia: an experience from a developing country. J Reconstr Microsurg. Published online September 1, 2021. DOI: 10.1055/s-0041-1735507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wali AR, Santiago-Dieppa DR, Brown JM, Mandeville R. Nerve transfer versus muscle transfer to restore elbow flexion after pan-brachial plexus injury: a cost-effectiveness analysis. Neurosurg Focus. 2017;43:E4. [DOI] [PubMed] [Google Scholar]
- 31.Xu WD, Gu YD, Lu JB, Yu C, Zhang CG, Xu JG. Pulmonary function after complete unilateral phrenic nerve transection. J Neurosurg. 2005;103:464-467. [DOI] [PubMed] [Google Scholar]
- 32.Yang Y, Yang J, Fu G, et al. Functioning free gracilis transfer to reconstruct elbow flexion and quality of life in global brachial plexus injured patients. Sci Rep. 2016;6:22479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yi Lee TM, Sechachalam S, Satkunanantham M. Systematic review on outcome of free functioning muscle transfers for elbow flexion in brachial plexus injuries. J Hand Surg Eur Vol. 2019;44:620-627. [DOI] [PubMed] [Google Scholar]
- 34.Zheng MX, Qiu YQ, Xu WD, Xu JG. Long-term observation of respiratory function after unilateral phrenic nerve and multiple intercostal nerve transfer for avulsed brachial plexus injury. Neurosurgery. 2012;70:796-801. [DOI] [PubMed] [Google Scholar]