Abstract
The US Preventive Services Task Force recommends lung cancer screening (LCS) to promote early lung cancer detection, and tobacco cessation services are strongly recommended in adjunct. Screen ASSIST (NCT03611881) is a randomized factorial trial to ascertain the best tobacco treatment intervention for smokers undergoing LCS; trial outreach is conducted during 3 recruitment points (RPs): when LCS is ordered (RP1), at screening (RP2), and following results (RP3). Among 177 enrollees enrolled from April 2019 to March 2020, 31.6% enrolled at RP1, 13.0% at RP2, and 55.4% at RP3. The average number of enrollees (per 1000 recruitment days) was 2.26 in RP1, 3.37 in RP2, and 1.04 in RP3. LCS provides an opportunity to offer tobacco treatment at multiple clinical timepoints. Repeated and proactive outreach throughout the LCS experience was beneficial to enrolling patients in tobacco cessation services.
In 2013 the US Preventive Services Task Force recommended lung cancer screening (LCS) to promote early lung cancer detection among high-risk individuals; smoking accounts for nearly 87% of lung cancer deaths (1). Tobacco cessation services are strongly recommended in adjunct to LCS to help reduce cancer rates and increase access to tobacco treatment (2). Failure to advise patients about tobacco treatment at the time of LCS may lead to erroneous conclusions that LCS is adequate to protect lung health, irrespective of ongoing tobacco use (3).
Screen ASSIST (NCT03611881) is a randomized factorial trial approved by the Massachusetts General Brigham Institutional Review Board to ascertain the best combination of behavioral, pharmacological, and community-based services to assist smokers who are undergoing LCS to quit smoking. Our previous research demonstrated that patients undergoing screening need time and repeated opportunities to engage in tobacco treatment (4). The LCS process includes several points of contact, creating a structure for repeated outreach during a clinical experience. We developed a systems-based intervention to integrate and offer tobacco treatment to smokers who have an LCS test ordered within a large health-care system. Our outreach includes recruitment approaches at 3 clinical timepoints: 1) when LCS is ordered, 2) at screening, and 3) following receipt of LCS results. At each recruitment point (RP), a multimodal outreach effort is used, including a video (5), mailed letters, and phone calls, which are targeted to an individual’s trial enrollment status and test results. Our recruitment model is “a cascade of exposure” in which we assess how varying and repeated efforts engage patients. We describe recruitment yield at each clinical timepoint, including proportion of participants enrolled at each timepoint, days until enrollment at each RP, and, to assess expediency, average of enrollees per days in recruitment.
Participants who are scheduled to undergo LCS are eligible if they have any cigarette use in the past 30 days, speak English or Spanish, fulfill the 2015 Medicare coverage criteria (aged 55-80 years and 30+ pack/years), have telephone access, and are willing to discuss tobacco use (6). Patients undergoing an LCS as part of a diagnostic evaluation, or who are unable to give informed consent, are excluded. A live feed of electronic health record data is integrated into the study database, tracking individuals who enroll, refuse, or remain undecided. At RP1, a research assistant conducts patient outreach via phone; patients can enroll, refuse, or be undecided. At RP2, a clinic staff member hands out a tablet computer, which identifies the patient’s study status and shows a targeted recruitment video to reinforce participation; individuals can enroll, refuse, or be undecided. At RP3, individuals receive a phone call and an outreach video tailored to their test results and enrollment status; individuals can enroll, refuse, or remain undecided until 90 days past the screening date.
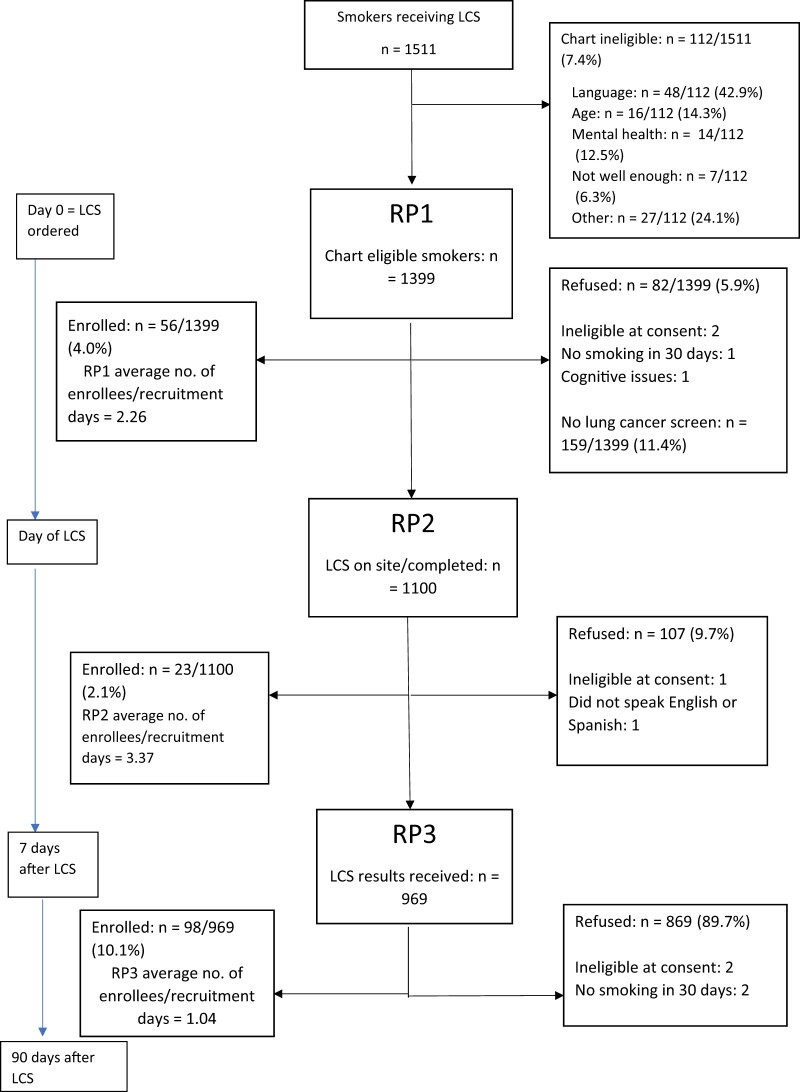
We tracked recruitment efforts and yield among smokers who were screened from April 2019 to March 2020 (Figure 1), at which point we ceased clinic-based tablet computer procedures. In total, 1511 charts of smokers undergoing LCS were screened; 92.6% were chart eligible. Of the 1399 chart eligible, 177 enrolled and 159 did not attend LCS (3% enrolled). Of enrollees, 54.2% were female and 47.8% were male. Of female enrollees, the median age was 64.3 years; 8% of enrollees identified as Black, 4% as Hispanic, and 88% as White. Of male enrollees, the median age was 64 years; 9% identified as Black, 6% identified as Hispanic, and 80% as White. There were no statistically significant sociodemographic differences between enrollees and nonenrollees.
Figure 1.
Recruitment flow and outreach efforts at each clinical recruitment timepoint including enrollment yield among smokers. Values within brackets are values at the 25th and 75th percentiles. LCS = lung cancer screening; RP1 = recruitment point before LCS; RP2 = recruitment point day of LCS to 7 days after; RP3 = recruitment point after LCS results received.
Among the 177 enrollees, 56 (31.6%) enrolled immediately after an LCS test was ordered (RP1), 23 (13.0%) enrolled at screening (RP2), and 98 (55.4%) enrolled upon receipt of their test results (RP3). Of the RP3 enrollees, 87% were Lung-RADS assessment categories for LCS scores 1 or 2 (negative or benign appearance or behavior), which is consistent with the population prevalence (7). Figure 1 also shows the median and interquartile range (IQR) for days to enrollment from chart screen: 12 days (IQR = 7-17 days) in RP1, 3 days (IQR = 1-7 days) in RP2, and 1-week postscreen 86.5 (IQR = 29-135 days) days in RP3. The average number of enrollees per 1000 recruitment days was, respectively, 2.26 (56 enrolled per 24 759 recruitment days) in RP1, 3.37 (23 enrolled per 6828 recruitment days) in RP2, and 1.04 (98 enrolled per 94 012 recruitment days) in RP3. To standardize the enrollment rates that occur between the differing timepoints, we present a rate—the number of people enrolled over the number of days in which people were able to be recruited at each timepoint—and then standardized this to per 1000 days of recruitment.
Our aim was to explore the yield for tobacco treatment enrollment into a clinical research trial across 3 LCS clinical timepoints. More than one-half of participants enrolled after receipt of the LCS results; more than 30% enrolled before their LCS. Enrollment at the screening site yielded the highest average of enrollees per recruitment day; the prescreen period yielded the second-highest average of enrollees per recruitment day. Although the greatest proportion of participants enrolled after LCS, participants recruited before or at the time of LCS could begin tobacco counseling earlier. It is unknown if RP3 yielded the highest proportion of enrollees because of 1) the teachable moment (removal of uncertainty and lowering of emotional distress), 2) having more time to decide whether to enroll or accept tobacco treatment support, or 3) the cumulative effect of repeated outreach efforts.
Study limitations include the inability to deconstruct each mode of outreach, including if patients watched the entirety of videos. Although the current sample is relatively homogeneous, the recently expanded US Preventive Services Task Force eligibility guidelines, decreasing patient age, and smoking history duration may enhance the diversity of patients undergoing LCS. After March 2020, we suspended in-clinic recruitment; post-pandemic, we will need to reflect on how to best leverage the LCS appointment.
LCS provides an opportunity to offer tobacco treatment resources at multiple clinical timepoints. Repeated and proactive outreach at several clinical timepoints in the LCS experience appeared to be beneficial, in different ways, to engaging patients in tobacco treatment. Integration of repeated outreach strategies by leveraging clinical timepoints should be explored; the entirety of the screening process is an opportunity for patients to make an informed decision about their health.
Funding
The Screen ASSIST study is funded by the National Cancer Institute through an R01 grant (5R01CA218123-02). This grant was awarded to Elyse R. Park, Ph.D., MPH, Jennifer Haas, M.D., and Nancy Rigotti, M.D.
Notes
Role of the funder: The funder did not play a role in the design of the study; the collection, analysis and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication.
Disclosures: NR receives royalties from UpToDate and consults for and received research funding from Achieve Life Sciences. Authors EP, JN, EN, SM, IG, CM, AW, DL, YC, and JH declare no conflict of interests or disclosures.
Author contributions: EP: Conceptualization, Methodology, Writing—Original Draft, Writing—Reviewing and Editing, Supervision, Project administration. JN: Conceptualization, Writing—original draft, Writing—Reviewing and Editing, Supervision, Project administration. EN: Writing—Original Draft, Writing, Reviewing and Editing, Project administration. SM: Data curation, Formal analysis. IG: Project administration. CM: Writing- review and editing, Project administration. AW: Supervision, Project administration, Writing—Review and Editing. DL: Supervision, Writing—Review and Editing. YC: Validation, Data curation. NR: Supervision, Conceptualization. JH: Supervision, Conceptualization.
Prior presentations: Some of the data from this study was previously presented in March 2020 at the Society on Nicotine and Tobacco Research (SRNT) in New Orleans, LA.
Contributor Information
Elyse R Park, Health Promotion and Resiliency and Intervention Research Program, Department of Medicine, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA; Mongan Institue, Department of Medicine, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA; Tobacco Research and Treatment Center, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA; Department of Psychiatry, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA.
Jordan M Neil, Health Promotion and Resiliency and Intervention Research Program, Department of Medicine, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA; Department of Family and Preventive Medicine, University of Oklahoma Health Sciences Center, Oklahoma City, OK, USA; TSET Health Promotion Research Center, Stephenson Cancer Center, Oklahoma City, OK, USA.
Elise Noonan, Health Promotion and Resiliency and Intervention Research Program, Department of Medicine, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA; Mongan Institue, Department of Medicine, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA.
Sydney E Howard, Division of General Internal Medicine, Department of Medicine, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA.
Irina Gonzalez, Health Promotion and Resiliency and Intervention Research Program, Department of Medicine, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA; Mongan Institue, Department of Medicine, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA.
Caylin Marotta, Division of General Internal Medicine, Department of Medicine, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA.
Amy J Wint, Division of General Internal Medicine, Department of Medicine, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA.
Douglas E Levy, Mongan Institue, Department of Medicine, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA; Tobacco Research and Treatment Center, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA.
Yuchiao Chang, Tobacco Research and Treatment Center, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA; Division of General Internal Medicine, Department of Medicine, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA.
Nancy A Rigotti, Mongan Institue, Department of Medicine, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA; Tobacco Research and Treatment Center, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA; Division of General Internal Medicine, Department of Medicine, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA.
Jennifer S Haas, Mongan Institue, Department of Medicine, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA; Division of General Internal Medicine, Department of Medicine, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA.
Data Availability
The investigators will ensure that all data sharing is conducted in a manner according to the Institutional Review Board (IRB) policy and the National Institute of Health (NIH) guidelines. Data from human subjects will be shared through mechanisms protecting participant identity and following data sharing policies. We will provide a study data file to the National Institutes of Health (NIH) dbGaP (database of Genotypes and Phenotypes) website upon clinical trial data collection completion.
References
- 1. US Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress. A Report of the Surgeon General. Atlanta: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. [Google Scholar]
- 2. Nguyen-Grozavu FT, Pierce JP, Sakuma KLK, et al. Widening disparities in cigarette smoking by race/ethnicity across education level in the United States. Prev Med. 2020;139:106220. doi: 10.1016/j.ypmed.2020.106220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zeliadt SB, Heffner JL, Sayre G, et al. Attitudes and perceptions about smoking cessation in the context of lung cancer screening. JAMA Intern Med. 2015;175(9):1530-1537. doi: 10.1001/jamainternmed.2015.3558 [DOI] [PubMed] [Google Scholar]
- 4. Lennes IT, Luberto CM, Carr AL, et al. Project reach: piloting a risk-tailored smoking cessation intervention for lung screening. J Health Psychol. 2020;25(10-11):1472-1482. doi: 10.1177/1359105318756500 [DOI] [PubMed] [Google Scholar]
- 5. Neil JM, Chang Y, Goshe B, et al. A web-based intervention to increase smokers’ intentions to participate in a cessation study offered at the point of lung screening: factorial randomized trial. JMIR Form Res. 2021;5(6):e28952. doi: 10.2196/28952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Neil JM, Marotta C, Gonzalez I, et al. Integrating tobacco treatment into lung cancer screening practices: study protocol for the Screen ASSIST randomized clinical trial. Contemp Clin Trials. 2021;111:106586. doi: 10.1016/j.cct.2021.106586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Deffebach ME, Humphrey L.. Screening for lung cancer. In: Givens J., ed. UpToDate, Inc; 2022. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The investigators will ensure that all data sharing is conducted in a manner according to the Institutional Review Board (IRB) policy and the National Institute of Health (NIH) guidelines. Data from human subjects will be shared through mechanisms protecting participant identity and following data sharing policies. We will provide a study data file to the National Institutes of Health (NIH) dbGaP (database of Genotypes and Phenotypes) website upon clinical trial data collection completion.