Abstract
The greater male variability (GMV) hypothesis proposes that traits are more variable among males than females, and is supported by numerous empirical studies. Interestingly, GMV is also observed for human brain size and internal brain structure, a pattern which may have implications for sex-biased neurological and psychiatric conditions. A better understanding of neuroanatomical variability in non-human primates may illuminate whether certain species are appropriate models for these conditions. Here, we tested for sex differences in the variability of endocranial volume (ECV, a proxy for brain size) in a sample of 542 rhesus macaques (Macaca mulatta) from a large pedigreed free-ranging population. We also examined the components of phenotypic variance (additive genetic and residual variance) to tease apart the potential drivers of sex differences in variability. Our results suggest that males exhibit more variable ECVs, and that this pattern reflects either balancing/disruptive selection on male behaviour (associated with alternative male mating strategies) or sex chromosome effects (associated with mosaic patterns of X chromosome gene expression in females), rather than extended neurodevelopment among males. This represents evidence of GMV for brain size in a non-human primate species and highlights the potential of rhesus macaques as a model for sex-biased brain-based disorders.
Keywords: endocranial volume, rhesus macaques, sexual selection, variability
1. Introduction
The ‘greater male variability (GMV) hypothesis' posits that males tend to exhibit more physical and behavioural variability than females. This pattern has been observed in numerous mammalian species across many morphological traits, and appears to be particularly apparent in sexually selected traits [1–7]. The phenomenon is likely to reflect some combination of evolutionary and developmental mechanisms that produce and maintain greater inter-individual variability among males, including: (i) balancing or disruptive selection, (ii) sex differences in developmental schedules and (iii) sex chromosome effects.
While disruptive selection favours divergent (i.e. extreme) phenotypes, processes of balancing selection maintain phenotypic diversity within populations through various mechanisms. For instance, under negative frequency-dependent selection (one form of balancing selection), the fitness of a phenotype decreases as it becomes more common, which can lead to cyclical shifts in the frequency of different phenotypes over time, thereby preventing one phenotype from reaching fixation. When these mechanisms act on a trait within one sex only, this can lead to the exhibition of more variable phenotypes within that sex. Placental mammals tend to exhibit sex differences in reproductive potential, since female reproduction is most directly linked to longevity while male reproduction is primarily driven by fecundity [8–10]. Consequently, males often show greater reproductive variance between individuals [8] and are more likely to exhibit alternative reproductive strategies that are subject to balancing/disruptive selection [11]. Conversely, females may be more likely to undergo stabilizing selection in traits directly or indirectly related to reproduction, thereby reducing trait variability in females relative to males [11]. Furthermore, additional mechanisms of sexual selection (specifically, direct male–male contest competition) often drive the evolution of larger adult body sizes among male mammals. In these cases, males experience longer developmental periods that may leave them more susceptible to environmental effects on trait expression, which may produce larger trait variability in males. Finally, male mammals may be expected to exhibit more variability for traits influenced by genes on the X chromosome, since males can only express their single set of X-linked alleles, while females may express either of their two alternative sets (due to mosaic X chromosome inactivation (XCI) across cells) or both sets (for genes that escape XCI within cells). This may facilitate mosaic levels of trait expression and produce lower phenotypic variability in females [5,12].
The GMV hypothesis has important implications for our understanding of human variation, since numerous studies have demonstrated that self-identified males are more variable than self-identified females across several physical (including neuroanatomical), behavioural, cognitive and personality-related traits [13–26]. Studies demonstrating GMV in brain size and structure are almost exclusively focused on humans [20–26]. Attempts to identify this pattern among non-human primate brains are particularly rare, and are thus far limited to examinations of chimpanzee sulcal morphology [27] and strepsirrhine skull length [28], with the latter analysis limited by very low sample sizes. It therefore remains uncertain whether GMV in human neuroanatomy represents an unusual characteristic for the primate order, or whether it may represent a more widespread pattern among primates.
Rhesus macaques are of particular interest in this regard, as they are popular models for studying neurological and psychiatric conditions that may be linked to sex differences in human neuroanatomical variability, including male-biased conditions like autism spectrum disorder (ASD) [29] and schizophrenia [30]. Specifically, individuals with ASD or schizophrenia exhibit higher brain structure variability than control cases [31,32], suggesting a possible link between GMV and vulnerability for these conditions. Additionally, ASD aetiology may be more heterogeneous in males compared with females [31] and male-specific increases in brain gene expression variability throughout development have been linked to genetic risk for schizophrenia [33]. Whether rhesus macaques also exhibit greater male neuroanatomical variability is unknown.
To investigate potential sex differences in brain size variability (Aim 1), we analysed endocranial volumes (ECV) in a large post-mortem sample of free-ranging rhesus macaques. Previous work has demonstrated that ECV is a reliable estimate of brain size across species (e.g. primates [34], birds [35,36]) and within species (e.g. budgerigars [35]) throughout development (e.g. domestic chickens [37], American allegators [37]); however, the proportion of non-neural tissue within the adult cranium may vary across individuals and increase with age [38] (including in macaque males [39] and females [40]). Nevertheless, unless this proportion varies with age and ECV in a sex-specific manner, a phenomenon not reported in previous literature, then our analysis of ECV variability is likely to reflect brain size variability.
We predicted that male rhesus macaques would exhibit greater variability for absolute ECV (Prediction 1a) and relative ECV (Prediction 1b), in accordance with the GMV hypothesis, and with previous reports of neuroanatomical GMV in humans [20–26] and chimpanzees [27]. Male rhesus macaques are subject to sex-specific selective pressures, including direct male–male contest competition, as demonstrated by intermediate body and canine size dimorphism [41,42]. This moderate level of sexual size dimorphism leads to extended development, including neurodevelopment [43], among males, which is similar to patterns observed in humans (years to peak cerebral volume: rhesus macaque F = 4, M = 6; human F = 10.5, M = 14.5; i.e. males develop about 50% slower) [43,44]. Given that brain and body size are correlated within and across species [45] (including rhesus macaques [46]), any observations of GMV in absolute ECV may simply reflect GMV in body size; however, since relative ECV (measured with ECV and body size proxies for each individual specimen) controls for inter-individual variation in body size, GMV in relative ECV may implicate mechanisms acting on this trait (or on the processes linking ECV and body size) specifically. In addition, although higher ranking males tend to have greater reproductive success than low ranking males, this correlation is relatively low [47,48], suggesting there may be multiple behavioural routes to reproductive success in males that could potentially be under balancing selection. Finally, genes expressed in the brain tend to be located on the X chromosome in rodents and primates, including macaques [49], and X chromosome genes affect the development of brain and region size in humans [50,51] and mice [52,53]. These characteristics of the rhesus macaque sociosexual system, in addition to the fact that rhesus macaques exhibit a typical mammalian XY sex chromosome structure, suggests that any of the aforementioned mechanisms may produce GMV in this species.
To investigate the potential drivers of sex differences in brain size variability (Aim 2), we examined sex differences in the components of phenotypic variance, namely additive genetic and residual (environmental) variances. Previous work has demonstrated that brain size and structure are heritable in humans [54] and non-human primates, including rhesus macaques [55], baboons [56] and chimpanzees [27,57]. If GMV is a result of sex differences in developmental schedules, males should exhibit higher environmental variance than females (Prediction 2a). If GMV is a result of balancing or disruptive selection in males, or sex chromosome effects, males should exhibit higher additive genetic variance than females (Prediction 2b).
2. Material and methods
(a) . Subjects
The data used in this study were presented in a previous study [55]. Briefly, A.C. collected morphological measurements for 542 free-ranging rhesus macaques (300 F/242 M) from the Caribbean Primate Research Center (CPRC) skeletal collections at the University of Puerto Rico (UPR) with permission granted through J.H.'s long-term memorandum of understanding (MoU) with CPRC/UPR. Individuals ranged in age at death from 6 to 31 years (mean = 12.8, s.d. = 5.2; females: mean = 13.0, s.d. = 5.0; males: mean = 12.7, s.d. = 5.4). We obtained age at death from the demographic database. Previous work on this population suggested that adult ECV is reached at approximately 4 years in females and approximately 6 years in males and does not change during the adult lifespan [43]. In line with this, linear models suggested that age (scaled) was not correlated with ECV (scaled) in either sex (males: slope = 0.059, p = 0.246; females: slope = 0.053, p = 0.221) (electronic supplementary material, figure S1). However, age (scaled) did predict body size (geometric mean, cubed and scaled) in females (males: slope = 0.066, p = 0.095; females: slope = 0.130, p < 0.001) (electronic supplementary material, figure S1). Given that body size (geometric mean, cubed and scaled) predicted ECV (scaled) in both sexes (males: slope = 0.269, p < 0.001; females: slope = 0.357, p < 0.001) (electronic supplementary material, figure S1) (consistent with previous work relating brain and body weight in this species [46]), we included age at death as a covariate to account for possible age-related changes in body size and relative ECV (see below for model details).
(b) . Morphological measurements
A.C. measured ECV by pouring 2 mm glass beads into the cranium of each specimen via the foramen magnum. Subsequently, A.C. poured beads into a graduated cylinder and recorded the volume [58]. To estimate body size, we used two body size proxies [59] (femoral length (FL) and femoral mediolateral breath (FMLB) [60]), measured by A.C., to calculate a geometric mean (). We also performed an intraclass correlation analysis to assess intra-rater reliability for all morphological measurements (ICC > 0.95).
(c) . Statistical analysis
We performed all statistical analyses in R 4.0.2 [61].
To examine potential sex differences in ECV phenotypic variance (Aim 1; Predictions 1a,b) and its components (Aim 2; Predictions 2a,b), we used generalized linear mixed models (GLMMs) which incorporate relatedness information in the form of a pedigree (i.e. a quantitative genetic ‘animal' model [62]). We obtained access to the pedigree via J.H.'s long-term MoU with CPRC/UPR. The CPRC maintains a pedigree database, which contains information on behavioural dams (identified via field observation and available for all specimens in the study) and genetic dams and sires (identified via microsatellite panel and available for individuals born after 1985) [63].
Owing to the inherently high correlations between the sex chromosome relatedness structure and autosomal chromosomes relatedness structure in natural populations, it can be difficult to separately estimate autosomal chromosomal inheritance and sex chromosomal inheritance of a single trait in quantitative genetic models [64]. Simulation modelling has shown that estimation of the additive autosomal genetic variance is a good approximation of both forms of additive genetic variance (i.e. the cumulative variance explained by autosomal and sex chromosomes) [64]. Here, we use quantitative genetic modelling estimating autosomal chromosome genetic variance which is likely to be an accurate estimator of both autosomal and sex chromosome genetic variance.
Across all models described below, we followed other studies [27,57] and used the default prior for the mean and variance of fixed effects for Gaussian-family models in MCMCglmm, and an inverse-Wishart prior for the random effects and residual variances (V = 1, nu = 1). All continuous variables were scaled and centred prior to further analysis (mean = 0; s.d. = 1). Models were run for 1 000 000 iterations, sampling every 100 iterations with a burn-in of 500 000. We ensured proper mixing occurred by visually inspecting trace and density plots. Autocorrelation was below 0.1 and effective sample sizes were greater than 1000 for all variables. We implemented Heidelberger and Welch's convergence diagnostic to test that the sampled values came from a stationary distribution, and all variables passed this stationary test. We also conducted half-width tests, which remove up to half the chain to test that the means are estimated from a chain that has converged and found that all variables passed this test. Finally, we ran each chain twice and confirmed convergence using the Gelman–Rubin statistic [65].
(i) . Aim 1: investigating potential sex differences in brain size variability
We first constructed the following three models to investigate sex differences in the variability of absolute ECV (Model 1; Prediction 1a), relative ECV (Model 2; Prediction 1b) and body size (Model 3):
-
(1)
ECV ~ age at death + sex (random = VA * sex; rcov = VR * sex)
-
(2)
ECV ~ body size (GM3) + age at death + sex (random = VA * sex; rcov = VR * sex)
-
(3)
body size (GM3) ~ age at death + sex (random = VA * sex; rcov = VR * sex)
In Models 2 and 3, the linear body size proxy (GM) was raised to the third power to ensure the same dimensionality among ECV and body size measures. In all models, the additive genetic variance (estimated by the inverse relatedness matrix estimated by the pedigree) was included as a random effect. Within each model, both the additive genetic and residual terms were partitioned into their effects for males and females separately. For each model, we estimated sex-specific phenotypic variances as the sums of the sex-specific genetic and residual variances for each sample of the posterior distribution (VP= VA + VR; VP = phenotypic variance; VA = additive genetic variance; VR = residual (environmental) variance), extracted mean estimates and 68% highest posterior density (HPD) intervals (i.e. the interval of values that contains 68% of the posterior probability) from the sex-specific phenotypic variance distributions, and compared them to test if mean phenotypic variance was higher in males (Predictions 1a,b).
As an additional test of sex differences in variability, we also extracted residuals from Models 1–3 and tested for significant sex differences in the variance of these residuals using permutation tests, following previous work [21,23,27]. We calculated the log male-to-female variance across the residuals (positive values = greater male variability; negative values = greater female variability), randomly permuted the sex variable among the residuals 10 000 times and calculated the proportion of permuted log male-to-female variance ratios (absolute value) greater than the observed ratio (absolute value). This proportion is referred to here as ‘pPERM' and represents a two-sided test of sex differences in variability. A positive log male-to-female variance ratio and pPERM < 0.05 would indicate greater male variability (Predictions 1a,b).
In addition, within each sex, we calculated the average range size for ECV across 1000 random samples for every possible sample size (from n = 2 to the actual subset sample size). The average range sizes were plotted against sample size to demonstrate that each distribution reached a horizontal asymptote.
(ii) . Aim 2: investigating the potential drivers of sex differences in brain size variability
To test whether residual (Prediction 2a) and/or additive genetic variance (Prediction 2b) contributed to sex differences in phenotypic variance, we compared Models 1–3 to reduced models which either: (1) did not partition additive genetic variance by sex, (2) did not partition residual variance by sex or (3) did not partition either additive genetic or residual variance by sex. We compared model fits using the Deviance Information Criterion (DIC). We also extracted mean estimates and 68% HPD intervals from the sex-specific variance distributions for all models (where applicable) and compared them.
3. Results
(a) . Aim 1: investigating potential sex differences in brain size variability
Consistent with Prediction 1a, males exhibited more variable absolute ECVs, as demonstrated by their higher mean phenotypic variances with non-overlapping 68% HPD intervals between the sexes (phenotypic variance: males: mean = 0.670 [0.598, 0.717], females: mean = 0.533 [0.481, 0.568]), and significantly more variable residual values (log M-to-F variance ratio = 0.396; pPERM = 0.001) (electronic supplementary material, figures S2, S3 and table S1). Since males also exhibited more variable body sizes (phenotypic variance: males: mean = 0.501 [0.454, 0.544], females: mean = 0.296 [0.269, 0.318]; M-to-F variance ratio = 0.352; pPERM = 0.007) (electronic supplementary material, figures S2, S3 and table S1), GMV in absolute ECV may reflect correlated development between ECV and body size. However, consistent with Prediction 1b, males also exhibited more variable relative ECVs (phenotypic variance: males: mean = 0.646 [0.581, 0.696], females: mean = 0.505 [0.459, 0.542]; M-to-F variance ratio = 0.288; pPERM = 0.016) (figure 1; electronic supplementary material, figures S2, S3 and table S1). Across all models, males were significantly larger (pMCMC < 0.05) (electronic supplementary material, table S1). In all models of relative ECV, body size was a significant, positive predictor (pMCMC < 0.05) (electronic supplementary material, table S1), and in all models of absolute ECV and of body size, age at death was a significant, positive predictor (pMCMC < 0.05) (electronic supplementary material, table S1). ECV increasing with age is not consistent with our exploratory linear model results (see Material and methods) or with previous work on ECV variation in this population [43], which may reflect differences in statistical modelling approaches, sample sizes and age distributions.
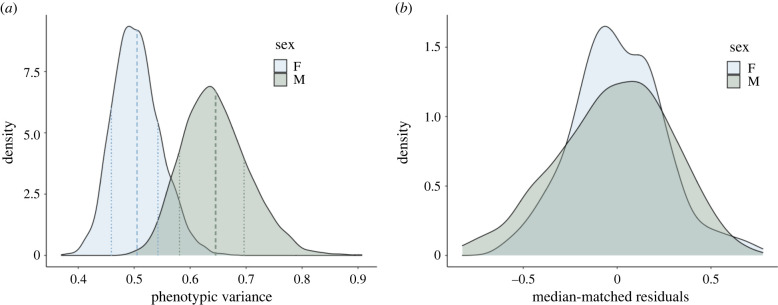
Figure 1.
Males exhibit more variable relative ECVs than females. (a) Density plots of the posterior distributions of phenotypic variance for males (green) and females (blue) from a GLMM of relative ECV (in which both the additive genetic and residual terms were partitioned into their effects for males and females separately). Dashed lines indicate sex-specific means and dotted lines indicate 68% HPD intervals for these means. (b) Median-matched (0 = median for each sex) density plots of male (green) and female (blue) residual distributions from a GLMM of relative ECV (in which both the additive genetic and residual terms were partitioned into their effects for males and females separately).
Given that measurements are available for more females than males, we expect that our estimates of GMV are conservative. Our resampling procedure confirmed that the sex-specific distributions approached a horizontal asymptote, suggesting our sample sizes were sufficient to capture population-level variation (electronic supplementary material, figure S4).
(b) . Aim 2: investigating the potential drivers of sex differences in brain size variability
We did not find support for Prediction 2a (i.e. greater residual (environmental) variance among males), but we did find some support for Prediction 2b (i.e. greater additive genetic variance among males). Specifically, contrary to Prediction 2a, models that estimated sex-specific residual variance performed worse (i.e. had higher DIC values) than similar models with unpartitioned residual variance (electronic supplementary material, table S1). However, for all measures, the best-fit models (i.e. with the lowest DIC values) included sex-specific additive genetic variance (electronic supplementary material, table S1) and males exhibited higher mean additive genetic variances in each of these models, with non-overlapping 68% HPD intervals for relative ECV and body size (relative ECV: males: mean = 0.483 [0.410, 0.561], females: mean = 0.347 [0.278, 0.406]; absolute ECV: males: mean = 0.514 [0.432, 0.580], females: mean = 0.382 [0.319, 0.445]; body size: males: mean = 0.367 [0.316, 0.420], females: mean = 0.168 [0.133, 0.206]).
4. Discussion
This work demonstrates GMV in ECV, a proxy for brain size, in a non-human primate model species, the rhesus macaque. Specifically, we found that males exhibit more variable absolute ECVs than females (in support of Prediction 1a), which may reflect that males also exhibit more variable body sizes. However, males also exhibit more variable relative ECVs (in support of Prediction 1b), suggesting there is GMV in ECV above and beyond that observed for body size. We did not find evidence that males exhibit greater environmental (residual) variance, suggesting that the patterns of GMV observed here may not reflect sex differences in developmental schedules (contra Prediction 2a). Rather, greater additive genetic variance among males suggests that GMV in rhesus macaque ECV is likely to reflect some combination of sexual selection mechanisms and sex chromosome effects (in support of Prediction 2b). Although ECV is a reliable estimate of brain size and shape across species [34–36] and within species throughout development [35,37], our analyses cannot account for the effects of inter-individual and age-related variation in the proportion of non-neural tissue. This proportion is likely to increase with age in rhesus macaques, since previous work suggests that brain volume decreases with age in this species [39,40]. Nevertheless, unless this measure varies across this population in a manner that would produce more similar brain sizes among males compared with females (despite males having more variable ECVs), then our observation of GMV in ECV is likely to reflect GMV in brain size.
As mentioned above, development is relatively longer in male (versus female) rhesus macaques [43]. Given that longer cranial ontogeny could potentially expose males to more environmental factors that influence cranial size development, we predicted that these factors may lead male rhesus macaques to display greater ECV variability (Prediction 2a). However, we did not find support for this, as models that estimated sex-specific residual variances performed worse than similar models with unpartitioned residual variance. This suggests that sex differences in exposure to physical and/or social environments during development cannot account for observed sex differences in ECV variability. Rather, our results suggest that sexual selection mechanisms and/or sex chromosome effects are likely to explain our observation of GMV in rhesus macaque ECV (Prediction 2b). Specifically, we found that the best-fit models for all measures included sex-specific additive genetic variance, and males exhibited higher mean additive genetic variances in these models (although the HPD intervals did marginally overlap for absolute ECV).
Given that both absolute and relative ECV are heritable [55,66], greater variability in ECV among males may, in theory, reflect selection on cognition and behaviour—specifically, disruptive or balancing selection on male reproductive strategies. Male rhesus macaques are subject to an interesting mix of sexual selection pressures, including direct male–male competition, reflected by intermediate body and canine size dimorphism [67], indirect male–male competition, reflected by large relative testis volume [68] and mechanisms of indirect female mate choice, such as female preference for males with darker red faces [69]. Accordingly, although dominance rank predicts male rhesus macaque reproductive success, male reproductive skew is relatively low and may reflect that males tend to queue for dominance rank instead of fighting directly [48]. Together, these mechanisms are expected to create several routes to reproductive success in males, which would produce and maintain variation in male behavioural phenotypes instead of generating one male phenotype that is under directional selection. Previous comparative work suggests that either of these alternative routes (e.g. greater investment in direct or indirect male–male competition) may be facilitated by increased brain size [70,71] and/or introduce constraints on brain size (in the form of, e.g. tissue/energetic trade-offs) [72,73]. Accordingly, if alternative male reproductive tactics are differentially coupled to brain size in this species, this may lead to more variable ECVs among males. Alternatively, the female fitness optimum for ECV may be narrower than the fitness optimum in males, which would suggest that our results reflect stronger stabilizing selection in females. Colby et al. [55] used linear and quadratic selection gradients to investigate selection in this sample and did not find evidence of selection on absolute or relative ECV in either sex, which suggests that stabilizing selection on females may not underlie the sex differences in ECV variability observed in this study. However, a lack of evidence for selection may reflect that: (1) this population is not currently under selection for ECV or (2) selection is occurring in this population, but larger sample sizes would be required to detect it. Furthermore, if balancing or disruptive selection are occurring in this population, they would not be detectable using linear or quadratic models. Accordingly, we cannot rule out selection as a potential explanation for greater ECV variability among male rhesus macaques.
Finally, our results may reflect sex chromosome effects. While males can only express a single set of X-linked alleles throughout all of their somatic cells, females may exhibit variable X chromosome gene expression across cells (due to mosaic XCI) or within cells (due to XCI escapees), leading to more intermediate levels of trait expression and lower population variability in females [5,12]. These effects may be particularly strong on neurodevelopment since brain-expressed genes and genes associated with brain size tend to be located on the X chromosome [49–53]. This may reflect evolutionary dynamics unique to the X chromosome, including the ‘faster-X effect' (i.e. more rapid evolution of X chromosome genes due to a relatively lower effective population size than autosomes) and the accumulation of sex-biased genes on the X chromosome (potentially reflecting resolved sexual antagonism) [74,75]. Owing to limited data availability, the current dataset did not provide the power to separately estimate autosomal versus sex chromosomal additive genetic effects (see Material and methods) [64]. Nevertheless, previous work has also provided indirect evidence for sex chromosome effects on sex differences in trait variability, including greater size correlations between brain areas in human and chimpanzee males (versus females) [21,23,27] and higher phenotypic variability among females in species with homogametic (e.g. ZZ) males [5].
While sex differences in phenotypic variability have been demonstrated in many taxa and across numerous traits, almost all studies demonstrating sex differences in neuroanatomical or behavioural variability have focused on humans. Here, we show that greater neuroanatomical variability among males is not only present in humans [26], but also another primate species, the rhesus macaque. Accordingly, this work supports the use of rhesus macaques as an animal model for sex-biased human neurological and psychiatric conditions. To tease apart which factors predominantly account for observed sex differences in neuroanatomical variability, future studies should focus on taxa that exhibit a diverse array of mating systems, sex-specific developmental schedules and sex chromosome compositions.
Acknowledgements
We thank the Caribbean Primate Research Center, notably Terry Kensler, for providing access to skeletal specimens, pedigree and demographic data used in the analyses. We thank two anonymous reviewers and Dr Locke Rowe for valuable feedback on this manuscript. We also thank Scott Williams for advice on morphometric measurements and data analysis. The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of the University of Puerto Rico or the Office of Research Infrastructure Programs of The National Institutes of Health.
Contributor Information
Abigail E. Colby, Email: aec619@nyu.edu.
Alex R. DeCasien, Email: alex.decasien@gmail.com.
Data accessibility
All data used in the analyses presented in this study are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.ffbg79cz0 [76].
Other supplementary material can be found at [77].
Authors' contributions
A.C.: conceptualization, data curation, formal analysis, methodology, writing—original draft; A.D.: conceptualization, formal analysis, methodology, writing—original draft; E.C.: conceptualization, formal analysis, writing—review and editing; J.H.: conceptualization, supervision, writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
We declare we have no competing interests.
Funding
The Cayo Santiago field station is supported by the University of Puerto Rico and the Office of Research Infrastructure Programs of The National Institutes of Health (grant no. 2 P40 OD012217).
References
- 1.Ellis H. 1894. Man and woman: a study of human secondary sexual characters. London, UK: Walter Scott. [Google Scholar]
- 2.Pomiankowski A, Møller AP. 1995. A resolution of the lek paradox. Proc. R. Soc. B 260, 21-29. ( 10.1098/rspb.1995.0054) [DOI] [Google Scholar]
- 3.Zajitschek SR, et al. 2020. Sexual dimorphism in trait variability and its eco-evolutionary and statistical implications. eLife 9, e63170. ( 10.7554/eLife.63170) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wyman MJ, Rowe L. 2014. Male bias in distributions of additive genetic, residual, and phenotypic variances of shared traits. Am. Nat. 184, 326-337. ( 10.1086/677310) [DOI] [PubMed] [Google Scholar]
- 5.Reinhold K, Engqvist L. 2013. The variability is in the sex chromosomes. Evolution 67, 3662-3668. ( 10.1111/evo.12224) [DOI] [PubMed] [Google Scholar]
- 6.Nakagawa S, Poulin R, Mengersen K, Reinhold K, Engqvist L, Lagisz M, Senior AM. 2015. Meta-analysis of variation: ecological and evolutionary applications and beyond. Methods Ecol. Evol. 6, 143-152. ( 10.1111/2041-210X.12309) [DOI] [Google Scholar]
- 7.Beery AK. 2018. Inclusion of females does not increase variability in rodent research studies. Curr. Opin. Behav. Sci. 23, 143-149. ( 10.1016/j.cobeha.2018.06.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bateman AJ. 1948. Intra-sexual selection in Drosophila. Heredity 2, 349-368. ( 10.1038/hdy.1948.21) [DOI] [PubMed] [Google Scholar]
- 9.Trivers RL. 1972. Parental investment and sexual selection. In Sexual selection and the descent of man (ed. Campbell B), pp. 136-179. Chicago, IL: Aldine Publishing Company. [Google Scholar]
- 10.Clutton-Brock TH. 1988. Reproductive success: studies of individual variation in contrasting breeding systems. Chicago, IL: University of Chicago Press. [Google Scholar]
- 11.Taborsky M, Oliveira RF, Brockmann HJ. 2008. The evolution of alternative reproductive tactics: concepts and questions. In Alternative reproductive tactics (eds Oliveira RF, Taborsky M, Brockmann HJ), pp. 1-22. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 12.James J. 1973. Covariances between relatives due to sex-linked genes. Biometrics 29, 584-588. ( 10.2307/2529178) [DOI] [PubMed] [Google Scholar]
- 13.Lehre A, Lehre KP, Laake P, Danbolt NC. 2009. Greater intrasex phenotype variability in males than in females is a fundamental aspect of the gender differences in humans. Dev. Psychobiol. 51, 198-206. ( 10.1002/dev.20358) [DOI] [PubMed] [Google Scholar]
- 14.Johnson MB, et al. 2009. Functional and evolutionary insights into human brain development through global transcriptome analysis. Neuron 62, 494-509. ( 10.1016/j.neuron.2009.03.027) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feingold A. 1992. Sex differences in variability in intellectual abilities: a new look at an old controversy. Rev. Educ. Res. 62, 61-84. ( 10.3102/00346543062001061) [DOI] [Google Scholar]
- 16.Karwowski M, Jankowska DM, Gralewski J, Gajda A, Wiśniewska E, Lebuda I. 2016. Greater male variability in creativity: a latent variables approach. Think. Skills Creat. 22, 159-166. ( 10.1016/j.tsc.2016.10.005) [DOI] [Google Scholar]
- 17.Else-Quest NM, Hyde JS, Goldsmith HH, Van Hulle CA. 2006. Gender differences in temperament: a meta-analysis. Psychol. Bull. 132, 33. ( 10.1037/0033-2909.132.1.33) [DOI] [PubMed] [Google Scholar]
- 18.Borkenau P, McCrae RR, Terracciano A. 2013. Do men vary more than women in personality? A study in 51 cultures. J. Res. Personal. 47, 135-144. ( 10.1016/j.jrp.2012.12.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Del Giudice M, Gangestad S. 2022. No evidence against the greater male variability hypothesis: a commentary on Harrison et al.’s meta-analysis of animal personality. PsyArXiv. ( 10.31234/osf.io/6ua8r) [DOI] [Google Scholar]
- 20.Lange N, Giedd JN, Castellanos FX, Vaituzis AC, Rapoport JL. 1997. Variability of human brain structure size: ages 4–20 years. Psychiatry Res. Neuroimaging 74, 1-12. ( 10.1016/S0925-4927(96)03054-5) [DOI] [PubMed] [Google Scholar]
- 21.Wierenga LM, Sexton JA, Laake P, Giedd JN, Tamnes CK. 2018. A key characteristic of sex differences in the developing brain: greater variability in brain structure of boys than girls. Cereb. Cortex 28, 2741-2751. ( 10.1093/cercor/bhx154) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wierenga LM, Bos MG, van Rossenberg F, Crone EA. 2019. Sex effects on development of brain structure and executive functions: greater variance than mean effects. J. Cogn. Neurosci. 31, 730-753. ( 10.1162/jocn_a_01375) [DOI] [PubMed] [Google Scholar]
- 23.Wierenga LM, et al. 2020. Greater male than female variability in regional brain structure across the lifespan. Hum. Brain Mapp. 43, 470-499. ( 10.1002/hbm.25204) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Forde NJ, Jeyachandra J, Joseph M, Jacobs GR, Dickie E, Satterthwaite TD, Shinohara RT, Ameis SH, Voineskos AN. 2020. Sex differences in variability of brain structure across the lifespan. Cereb. Cortex 30, 5420-5430. ( 10.1093/cercor/bhaa123) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ritchie SJ, et al. 2018. Sex differences in the adult human brain: evidence from 5216 UK biobank participants. Cereb. Cortex 28, 2959-2975. ( 10.1093/cercor/bhy109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williams CM, Peyre H, Toro R, Ramus F. 2021. Neuroanatomical norms in the UK Biobank: the impact of allometric scaling, sex, and age. Hum. Brain Mapp. 42, 4623-4642. ( 10.1002/hbm.25572) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DeCasien AR, Sherwood CC, Schapiro SJ, Higham JP. 2020. Greater variability in chimpanzee (Pan troglodytes) brain structure among males. Proc. R. Soc. B 287, 20192858. ( 10.1098/rspb.2019.2858) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jenkins PD, Albrecht GH. 1991. Sexual dimorphism and sex ratios in Madagascan prosimians. Am. J. Primatol. 24, 1-14. ( 10.1002/ajp.1350240102) [DOI] [PubMed] [Google Scholar]
- 29.Bauman MD, Iosif AM, Ashwood P, Braunschweig D, Lee A, Schumann CM, Van de Water J, Amaral DG. 2013. Maternal antibodies from mothers of children with autism alter brain growth and social behavior development in the rhesus monkey. Transl. Psychiatry 3, e278-e278. ( 10.1038/tp.2013.47) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gil-da-Costa R, Stoner GR, Fung R, Albright TD. 2013. Nonhuman primate model of schizophrenia using a noninvasive EEG method. Proc. Natl Acad. Sci. USA 110, 15 425-15 430. ( 10.1073/pnas.1312264110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zabihi M, et al. 2019. Dissecting the heterogeneous cortical anatomy of autism spectrum disorder using normative models. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 4, 567-578. ( 10.1016/j.bpsc.2018.11.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alnæs D, et al. 2019. Brain heterogeneity in schizophrenia and its association with polygenic risk. JAMA Psychiatry 76, 739. ( 10.1001/jamapsychiatry.2019.0257) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen J, Cao H, Meyer-Lindenberg A, Schwarz E. 2018. Male increase in brain gene expression variability is linked to genetic risk for schizophrenia. Transl. Psychiatry 8, 140. ( 10.1038/s41398-018-0200-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Isler K, Kirk EC, Miller JM, Albrecht GA, Gelvin BR, Martin RD. 2008. Endocranial volumes of primate species: scaling analyses using a comprehensive and reliable data set. J. Hum. Evol. 55, 967-978. ( 10.1016/j.jhevol.2008.08.004) [DOI] [PubMed] [Google Scholar]
- 35.Iwaniuk AN, Nelson JE. 2002. Can endocranial volume be used as an estimate of brain size in birds? Can. J. Zool. 80, 16-23. ( 10.1139/z01-204) [DOI] [Google Scholar]
- 36.Ocampo D, Sánchez C, Barrantes G. 2020. Do different methods yield equivalent estimations of brain size in birds? Brain Behav. Evol. 95, 113-122. ( 10.1159/000509383) [DOI] [PubMed] [Google Scholar]
- 37.Watanabe A, Gignac PM, Balanoff AM, Green TL, Kley NJ, Norell MA. 2019. Are endocasts good proxies for brain size and shape in archosaurs throughout ontogeny? J. Anat. 234, 291-305. ( 10.1111/joa.12918) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tobias P. 1994. The craniocerebral interface in early hominids. In Integr. Paths Past Paleoanthropological Adv. Honor F Clark Howell, vol. 2 (eds Corruccini RS, Ciochon RL), pp. 185-203. Englewood, NJ: Prentice Hall. [Google Scholar]
- 39.Wisco JJ, Killiany RJ, Guttmann CR, Warfield SK, Moss MB, Rosene DL. 2008. An MRI study of age-related white and gray matter volume changes in the rhesus monkey. Neurobiol. Aging 29, 1563-1575. ( 10.1016/j.neurobiolaging.2007.03.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen X, et al. 2013. Brain aging in humans, chimpanzees (Pan troglodytes), and rhesus macaques (Macaca mulatta): magnetic resonance imaging studies of macro- and microstructural changes. Neurobiol. Aging 34, 2248-2260. ( 10.1016/j.neurobiolaging.2013.03.028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Plavcan JM, Van Schaik CP. 1997. Intrasexual competition and body weight dimorphism in anthropoid primates. Am. J. Phys. Anthropol. 103, 37-68. () [DOI] [PubMed] [Google Scholar]
- 42.Plavcan JM, van Schaik CP. 1992. Intrasexual competition and canine dimorphism in anthropoid primates. Am. J. Phys. Anthropol. 87, 461-477. ( 10.1002/ajpa.1330870407) [DOI] [PubMed] [Google Scholar]
- 43.Konigsberg L, Falk D, Hildebolt C, Vannier M, Cheverud J, Helmkamp RC. 1990. External brain morphology in rhesus macaques (Macaca mulatta). J. Hum. Evol. 19, 269-284. ( 10.1016/0047-2484(90)90069-N) [DOI] [Google Scholar]
- 44.Lenroot RK, et al. 2007. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. NeuroImage 36, 1065-1073. ( 10.1016/j.neuroimage.2007.03.053) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jerison HJ. 1973. Evolution of the brain and intelligence. New York, NY: Academic Press. [Google Scholar]
- 46.Herndon JG, Tigges J, Klumpp SA, Anderson DC. 1998. Brain weight does not decrease with age in adult rhesus monkeys. Neurobiol. Aging 19, 267-272. ( 10.1016/S0197-4580(98)00054-2) [DOI] [PubMed] [Google Scholar]
- 47.Berard JD, Nürnberg P, Epplen JT, Schmidtke J. 1993. Male rank, reproductive behavior, and reproductive success in free-ranging rhesus macaques. Primates 34, 481-489. ( 10.1007/BF02382659) [DOI] [Google Scholar]
- 48.Dubuc C, Muniz L, Heistermann M, Engelhardt A, Widdig A. 2011. Testing the priority-of-access model in a seasonally breeding primate species. Behav. Ecol. Sociobiol. 65, 1615-1627. ( 10.1007/s00265-011-1172-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nguyen DK, Disteche CM. 2006. Dosage compensation of the active X chromosome in mammals. Nat. Genet. 38, 47-53. ( 10.1038/ng1705) [DOI] [PubMed] [Google Scholar]
- 50.Raznahan A, Lee NR, Greenstein D, Wallace GL, Blumenthal JD, Clasen LS, Giedd JN. 2016. Globally divergent but locally convergent X- and Y-chromosome influences on cortical development. Cereb. Cortex 26, 70-79. ( 10.1093/cercor/bhu174) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Raznahan A, Disteche CM. 2021. X-chromosome regulation and sex differences in brain anatomy. Neurosci. Biobehav. Rev. 120, 28-47. ( 10.1016/j.neubiorev.2020.10.024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Raznahan A, Lue Y, Probst F, Greenstein D, Giedd J, Wang C, Lerch J, Swerdloff R. 2015. Triangulating the sexually dimorphic brain through high-resolution neuroimaging of murine sex chromosome aneuploidies. Brain Struct. Funct. 220, 3581-3593. ( 10.1007/s00429-014-0875-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vousden DA, Corre C, Spring S, Qiu LR, Metcalf A, Cox E, Lerch JP, Palmert MR. 2018. Impact of X/Y genes and sex hormones on mouse neuroanatomy. NeuroImage 173, 551-563. ( 10.1016/j.neuroimage.2018.02.051) [DOI] [PubMed] [Google Scholar]
- 54.Peper JS, Brouwer RM, Boomsma DI, Kahn RS, Hulshoff Pol HE. 2007. Genetic influences on human brain structure: a review of brain imaging studies in twins. Hum. Brain Mapp. 28, 464-473. ( 10.1002/hbm.20398) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Colby AE, Kimock CM, Higham JP. 2021. Endocranial volume is variable and heritable, but not related to fitness, in a free-ranging primate. Sci. Rep. 11, 1-11. ( 10.1038/s41598-021-81265-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rogers J, Kochunov P, Lancaster J, Shelledy W, Glahn D, Blangero J, Fox P. 2007. Heritability of brain volume, surface area and shape: an MRI study in an extended pedigree of baboons. Hum. Brain Mapp. 28, 576-583. ( 10.1002/hbm.20407) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gómez-Robles A, Hopkins WD, Schapiro SJ, Sherwood CC. 2016. The heritability of chimpanzee and human brain asymmetry. Proc. R. Soc. B 283, 20161319. ( 10.1098/rspb.2016.1319) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Logan CJ, Clutton-Brock TH. 2013. Validating methods for estimating endocranial volume in individual red deer (Cervus elaphus). Behav. Processes 92, 143-146. ( 10.1016/j.beproc.2012.10.015) [DOI] [PubMed] [Google Scholar]
- 59.Delson E, Terranova CJ, Jungers WL, Sargis EJ, Jablonski NG. 2000. Body mass in Cercopithecidae (Primates, Mammalia): estimation and scaling in extinct and extant taxa. Anthropol. Pap. Am. Mus. Nat. Hist. 83, 1-159. [Google Scholar]
- 60.Jolly CJ. 1972. The classification and natural history of Theropithecus (Simopithecus) (Andrews, 1916) baboons of the African Plio-Pleistocene. Bull. Br. Mus. Nat. Hist. Geol. 22, 1-123. [Google Scholar]
- 61.R Core Team.2020. R: A language and environment for statistical computing (version 4.0.2). R Found. Stat. Comput. (www.r-project.org. )
- 62.Kruuk LE. 2004. Estimating genetic parameters in natural populations using the ‘animal model’. Phil. Trans. R. Soc. Lond. B 359, 873-890. ( 10.1098/rstb.2003.1437) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Widdig A, et al. 2016. Genetic studies on the Cayo Santiago rhesus macaques: a review of 40 years of research. Am. J. Primatol. 78, 44-62. ( 10.1002/ajp.22424) [DOI] [PubMed] [Google Scholar]
- 64.Larsen CT, Holand AM, Jensen H, Steinsland I, Roulin A. 2014. On estimation and identifiability issues of sex-linked inheritance with a case study of pigmentation in Swiss barn owl (Tyto alba). Ecol. Evol. 4, 1555-1566. ( 10.1002/ece3.1032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gelman A, Rubin DB. 1992. Inference from iterative simulation using multiple sequences. Stat. Sci. 7, 457-472. [Google Scholar]
- 66.Cheverud J, Falk D, Vannier M, Konigsberg L, Helmkamp RC, Hildebolt C. 1990. Heritability of brain size and surface features in rhesus macaques (Macaca mulatta). J. Hered. 81, 51-57. ( 10.1093/oxfordjournals.jhered.a110924) [DOI] [PubMed] [Google Scholar]
- 67.Plavcan JM. 2001. Sexual dimorphism in primate evolution. Am. J. Phys. Anthropol. 116, 25-53. ( 10.1002/ajpa.10011) [DOI] [PubMed] [Google Scholar]
- 68.Harcourt AH, Harvey PH, Larson SG, Short RV. 1981. Testis weight, body weight and breeding system in primates. Nature 293, 55-57. ( 10.1038/293055a0) [DOI] [PubMed] [Google Scholar]
- 69.Dubuc C, Allen WL, Maestripieri D, Higham JP. 2014. Is male rhesus macaque red color ornamentation attractive to females? Behav. Ecol. Sociobiol. 68, 1215-1224. ( 10.1007/s00265-014-1732-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pawłowskil B. 1998. Neocortex size, social skills and mating success in primates. Behaviour 135, 357-368. ( 10.1163/156853998793066285) [DOI] [Google Scholar]
- 71.Kelley TC, Higdon JW, Ferguson SH. 2014. Large testes and brain sizes in odontocetes (order Cetacea, suborder Odontoceti): the influence of mating system on encephalization. Can. J. Zool. 92, 721-726. ( 10.1139/cjz-2014-0044) [DOI] [Google Scholar]
- 72.Pitnick S, Jones KE, Wilkinson GS. 2006. Mating system and brain size in bats. Proc. R. Soc. B 273, 719-724. ( 10.1098/rspb.2005.3367) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Simmons LW, Emlen DJ. 2006. Evolutionary trade-off between weapons and testes. Proc. Natl Acad. Sci. USA 103, 16 346-16 351. ( 10.1073/pnas.0603474103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vicoso B, Charlesworth B. 2006. Evolution on the X chromosome: unusual patterns and processes. Nat. Rev. Genet. 7, 645-653. ( 10.1038/nrg1914) [DOI] [PubMed] [Google Scholar]
- 75.Vicoso B, Charlesworth B. 2009. Effective population size and the faster-X effect: an extended model. Evolution 63, 2413-2426. ( 10.1111/j.1558-5646.2009.00719.x) [DOI] [PubMed] [Google Scholar]
- 76.Colby AE, DeCasien AR, Cooper EB, Higham JP. 2022. Data from: Greater variability in rhesus macaque (Macaca mulatta) endocranial volume among males than females. Dryad Digital Repository. ( 10.5061/dryad.ffbg79cz0) [DOI] [PMC free article] [PubMed]
- 77.Colby AE, DeCasien AR, Cooper EB, Higham JP. 2022. Greater variability in rhesus macaque (Macaca mulatta) endocranial volume among males than females. Figshare. ( 10.6084/m9.figshare.c.6251490) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Colby AE, DeCasien AR, Cooper EB, Higham JP. 2022. Data from: Greater variability in rhesus macaque (Macaca mulatta) endocranial volume among males than females. Dryad Digital Repository. ( 10.5061/dryad.ffbg79cz0) [DOI] [PMC free article] [PubMed]
- Colby AE, DeCasien AR, Cooper EB, Higham JP. 2022. Greater variability in rhesus macaque (Macaca mulatta) endocranial volume among males than females. Figshare. ( 10.6084/m9.figshare.c.6251490) [DOI] [PMC free article] [PubMed]
Data Availability Statement
All data used in the analyses presented in this study are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.ffbg79cz0 [76].
Other supplementary material can be found at [77].