Abstract
Early life pain (ELP) experience alters adult pain behavior and increases injury-induced pain hypersensitivity, but the effect of ELP on adult functional brain connectivity is not known. We have performed continuous local field potential (LFP) recording in the awake adult male rats to test the effect of ELP on functional cortical connectivity related to pain behavior. Primary somatosensory cortex (S1) and medial prefrontal cortex (mPFC) LFPs evoked by mechanical hindpaw stimulation were recorded simultaneously with pain reflex behavior for 10 d after adult incision injury. We show that, after adult injury, sensory evoked S1 LFP δ and γ energy and S1 LFP δ/γ frequency coupling are significantly increased in ELP rats compared with controls. Adult injury also induces increases in S1-mPFC functional connectivity, but this is significantly prolonged in ELP rats, lasting 4 d compared with 1 d in controls. Importantly, the increases in LFP energy and connectivity in ELP rats were directly correlated with increased behavioral pain hypersensitivity. Thus, ELP alters adult brain functional connectivity, both within and between cortical areas involved in sensory and affective dimensions of pain. The results reveal altered brain connectivity as a mechanism underlying the effects of ELP on adult pain perception.
SIGNIFICANCE STATEMENT Pain and stress in early life has a lasting impact on pain behavior and may increase vulnerability to chronic pain in adults. Here, we record pain-related cortical activity and simultaneous pain behavior in awake adult male rats previously exposed to pain in early life. We show that functional connectivity within and between the somatosensory cortex and the medial prefrontal cortex (mPFC) is increased in these rats and that these increases are correlated with their behavioral pain hypersensitivity. The results reveal that early life pain (ELP) alters adult brain connectivity, which may explain the impact of childhood pain on adult chronic pain vulnerability.
Keywords: brain, cortex, early life, γ oscillation, neonatal, pain
Introduction
Exposure to pain and injury in early life pain (ELP) is associated with altered pain behavior in adults. Evidence from both human and animal studies shows that repeated painful procedures or surgical incision during a critical period of early postnatal development has significant long-term effects on pain processing (Walker et al., 2009a,b; Beggs et al., 2012b; Schwaller and Fitzgerald, 2014; van den Hoogen et al., 2018). The mechanisms underlying the effects of ELP involve changes in peripheral cutaneous innervation (Reynolds and Fitzgerald, 1995; De Lima et al., 1999; Beggs et al., 2012a; Boada et al., 2012), peripheral afferent sensitization (Walker et al., 2016; Liu et al., 2017; Dourson et al., 2021), spinal cord nociceptive circuitry (Torsney and Fitzgerald, 2003; J. Li and Baccei, 2019), early life spinal microglial activation (Moriarty et al., 2019), and altered descending brain stem pain control (Walker et al., 2015). There is also evidence from human studies of structural changes in the thalamus and cortex (Duerden et al., 2018) and functional changes in descending pain control from supraspinal sites (Walker et al., 2018). The importance of this extends into a wider area of the long-term consequences of early life stress and pain which, by inducing long-term alterations in brain function and behavior may lead to higher susceptibility to chronic pain (G.T. Jones et al., 2009; Denk et al., 2014; Ririe et al., 2021; Melchior et al., 2022). However, as yet, there is no evidence that ELP has any effect on adult cortical pain networks or on functional connectivity between the key cortical regions involved in the sensory and emotional dimensions of pain.
A wide network of brain areas is involved in acute pain processing, including primary (S1) and secondary (S2) somatosensory cortices, the medial prefrontal cortex (mPFC), insula, thalamus, and prefrontal areas (Apkarian et al., 2005; Duerden and Albanese, 2013; Tan and Kuner, 2021). To address whether ELP impacts on cortical function from the sensory-discriminative and emotional/cognitive perspectives, the S1 and mPFC are attractive targets (Tan and Kuner, 2021). S1 is a functionally defined part of the somatosensory and nociceptive system and processes sensory nociceptive information about pain from an early age in both rodents and humans (Chang et al., 2016, 2020b; L. Jones et al., 2022). S1 encodes nociceptive intensity and perceived pain intensity (Mancini et al., 2012) and γ-band oscillations in this area correlate with subjective pain perception (Ong et al., 2019). While mPFC is critically involved in numerous cognitive functions (Euston et al., 2012; Chang et al., 2020a) and emotion behavior (Cao et al., 2018; Huang et al., 2020), this area also plays an important role in the emotional and affective aspects of pain, and could modulate pain sensation by controlling the flow of afferent sensory stimuli into the dorsal horn through descending control pathways (Zhang et al., 2015; Huang et al., 2020). Here, we hypothesize that ELP alters pain-related connectivity in the adult S1 and mPFC and that this is associated with increases in adult pain-related behavior.
In this study, we used a well-established model of injury and postoperative pain: hind-paw plantar incision of skin and underlying muscle (Brennan et al., 1996; Beggs et al., 2012b) to examine the impact of ELP on pain behavior and associated neural activity in S1 and mPFC. We recorded local field potentials (LFPs) in S1 and mPFC in awake, freely moving adult rats and analyzed the oscillatory energy within those sensory evoked LFPs and the functional connectivity within and between these areas. Acute pain is associated with defined changes in cortical oscillations (Tan et al., 2021). In humans, γ-band oscillations in S1 correlate with subjective pain perception (Heid et al., 2020; Yue et al., 2020) and are strengthened in rodent S1 cortex during nociception and inflammatory pain in association with behavioral nociceptive hypersensitivity (Tan et al., 2019). We also analyze phase-amplitude coupling (PAC) and coherence of neuronal oscillations as putative mechanisms of regional and interareal communication (Buzsaki, 2004; Peng and Tang, 2016). Together, our results provide new insights into how ELP alters adult cortical function underlying sensory and emotional dimensions of pain behavior.
Materials and Methods
Experimental animals
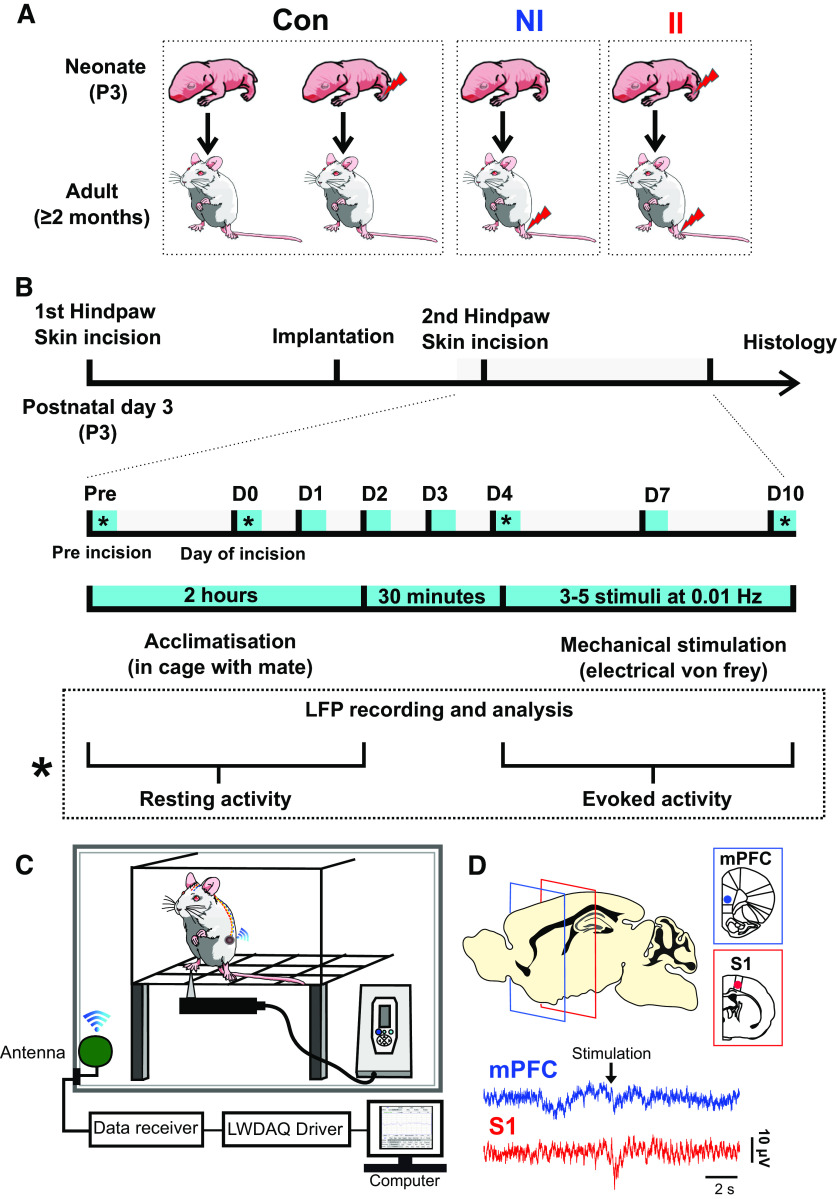
All experiments were performed in accordance with the United Kingdom Animal (Scientific Procedures) Act 1986. Reporting is based on the ARRIVE Guidelines for Reporting Animal Research developed by the National Center for Replacement, Refinement and Reduction of Animals in Research, London, United Kingdom. Male Sprague Dawley rat pups were obtained from the Biological Services Unit, University College London. Rats were housed in cages of five age-matched animals Postnatal day (P)21 or with the dam and littermates (P3–P21) under controlled environmental conditions (24–25°C; 50–60% humidity; 12/12 h light/dark cycle) with free access to food and water. In the case of rat pups, handling and maternal separation were kept to a minimum. All animals were exposed to the same standard caging, handling, and diet throughout development. The different experimental groups are represented in Figure 1A and protocol for probing the impact of nociceptive inputs in the early life on central pain processing and adult pain sensitivity is summarized in Figure 1B.
Figure 1.
Experiment design. A, Schematic of experimental groups. II: neonatal incision on postnatal day 3 and repeat incision two months later in adulthood (ELP model). NI: littermate control with equivalent anesthesia, handling and maternal separation on postnatal day 3 and first incision in adulthood. Con: pooled data from animals having neonatal incision only and from age-matched nonincised litter mates from the same colony. B, Experimental protocol for probing the impact of ELP on adult cortical pain processing and pain behavior. Upper scale, Timeline for recording cortical LFPs and pain behavior, where * marks days of simultaneous eVF hair stimulation and LFP recording. Lower box, Detail of testing protocol for recording resting LFPs and eVH evoked LFP recording on days marked *. C, Schematic of the experimental set-up for simultaneous recording of neural LFP activity in mPFC and S1 in awake adult rats using wireless telemetry while applying eVF hairs to the plantar hindpaw. D, Sample traces of simultaneous S1 and mPFC EPs evoked by mechanical eVF stimulation of the plantar hindpaw.
Plantar hind-paw incision
Male rat pups on postnatal day 3 were anaesthetized and plantar hindpaw incision performed. Under general anesthesia with 2% isoflurane in 100% oxygen (flow rate, 1–1.5 l/min), a midline longitudinal incision was made through the skin and fascia extending from the midpoint of the heel to the proximal border of the first footpad and the underlying plantar muscle elevated and incised. The same relative length of incision was performed in adult animals as previously described (Brennan et al., 1996; Walker et al., 2009b). Skin edges were closed with 5–0 nylon suture (Ethicon). The whole procedure took 3–5 min. After plantar hindpaw incision, rats were placed in a recovery chamber and allowed to recover from the general anesthesia before returning to their home cage.
Four experimental groups were used. II: neonatal incision on postnatal day 3 and repeat incision two months later in adulthood. NI: littermate control with equivalent anesthesia, handling and maternal separation on postnatal day 3 and having incision in adulthood. Animals having neonatal incision and follow-up in adulthood (IN) and age-matched nonincised litter mates from the same colony (NN) were pooled data and used as control group (Con), because there was no significant difference between the two groups (Fig. 1A).
Pain hypersensitivity testing
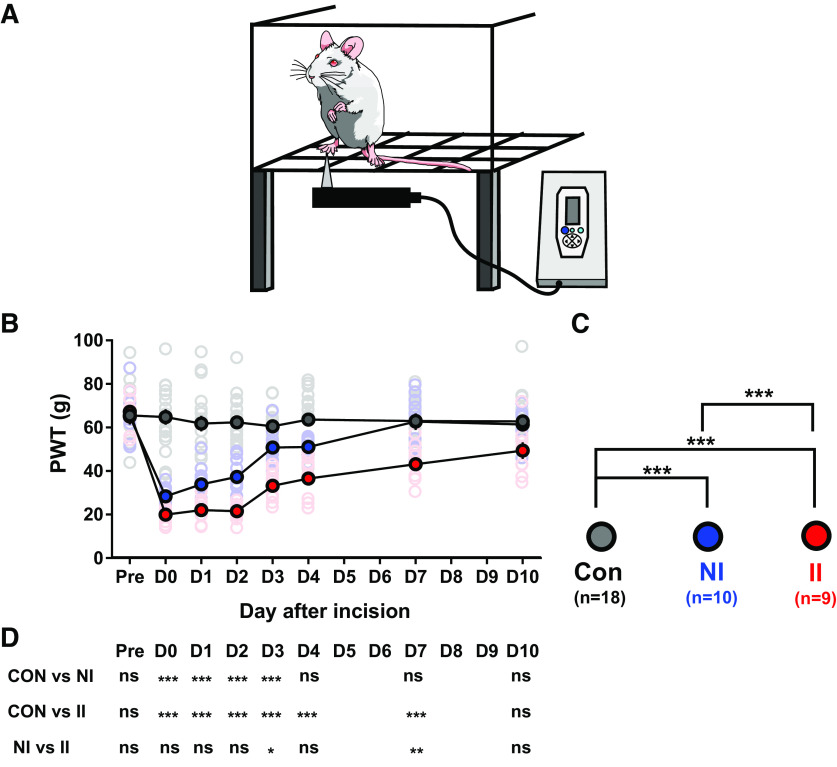
To test behavioral pain hypersensitivity following hind-paw incision, an electronic von Frey (eVF) unit (EVF4, Bioseb) was used to measure hindpaw mechanical flexion withdrawal thresholds (Ferrier et al., 2015, 2016). Following habituation for 30 min on an elevated mesh platform, a mechanical stimulus was applied to the plantar surface of the hindpaw adjacent to the distal half of the incision (Fig. 2). The electronic von Frey (eVF) apparatus, which has a measurement range of 0–500 g with 0.1-g resolution, consists of a plastic tip fitted in a hand-held force transducer, which was applied to the rat hindpaw from below with force (g) gradually increased until paw withdrawal. The force that induced paw withdrawal was digitized and recorded automatically by the unit and used as the threshold for mechanical nociception. For each recording session, the eVF was applied three to five times at ∼50-s intervals. Simultaneous recording from both S1 and mPFC accompanied testing of eVF withdrawal thresholds (Fig. 1C,D).
Figure 2.
ELP increases hyperalgesia following incision injury in adult rats. A, eVF hair testing of the plantar hindpaw adjacent to the wound (B) Plot of contralateral mechanical PWT, before (Pre) and up to 10 d after hindpaw incision in adult rats. Mean ± SEM with individual data superimposed. C, Statistical differences between groups using GLMs. D, Summary the post hoc pairwise comparisons with Bonferroni correction; *p < 0.05, **p < 0.01, ***p < 0.001. Nonincised controls (Con, n = 18), incision in adults without neonatal incision (NI, n = 10), and incision in adults with neonatal incision (II, n = 9).
Surgical preparation and transmitter implantation for long-term recording
Rats were anaesthetized with 2.5–3% isoflurane (Abbot, AbbVie Ltd.) in 100% oxygen (flow rate of 1–1.5 l/min) via gas anesthesia mask (Model 906, David Kopf Instruments) from a recently calibrated vaporizer (Harvard Apparatus). Body temperature was maintained with a heat blanket during surgery. A transmitter (A3028D-DDA, Open Source Instruments, Brandeis; Chang et al., 2011) was implanted subcutaneously with the depth recording electrodes (J-electrode (wire 125-μm dia 316SS 10-kΩ impedance), a Teflon-insulated stainless steel electrode, Open Source Instruments, Brandeis) positioned in mPFC (3.2 mm anterior, 0.5 mm lateral, 4 mm ventral) and primary somatosensory hindpaw cortex (1 mm posterior, 2.5 mm lateral, 2 mm ventral; Paxinos et al., 2013; Chang et al., 2016). The reference electrode was implanted over the cerebellum posterior to λ. The whole assembly was held in place with dental cement (Simplex Rapid, Acrylic Denture Polymer). A subcutaneous injection of bupivacaine and metacam was provided for postsurgical pain management. At the end of surgery, enrofloxacin (5 mg/kg, Baytril, Bayer Healthcare) and prewarm saline (0.5–1 ml) were administered subcutaneously. The animals were placed in a temperature controlled (25°C) recovery chamber until ambulatory and closely monitored at least 1–2 h before returning to their home cage to allow recovery for at least 14 d after surgery.
The transmitter, which has no adverse effects (Chang et al., 2016), was implanted for data recordings. During all recording sessions, continuous LFP recordings were recorded (bandpass filter: 0.2–160 Hz, 512-Hz sampling rate with 16-bit resolution) using LWDAQ Software (Open Source Instruments, Brandeis). Animals were carefully monitored daily and were euthanized at the end of experiment with carbon dioxide (CO2). The brain was removed and immediately immersed in 4% paraformaldehyde for >24 h before being transferred to 30% sucrose postfixation solution. Brain sections (40-μm-thick thickness) were cut using a microtome [Leica SM2000R, Leica Microsystems (UK) Ltd.] and stained with cresyl violet to allow histologic location of the electrode track. This procedure allowed us to verify recording electrode locations, and LFP data were only included in the study if electrode tips were located in mPFC and S1 (Fig. 1D).
Analysis of electrophysiology data
Data analysis was performed with Brainstorm (Tadel et al., 2011), which is free and open source for electrophysiology data visualization and processing through a simple and intuitive graphical user interface (GUI; http://neuroimage.usc.edu/brainstorm) and custom MATLAB scripts (The MathWorks Inc.).
Evoked LFP data processing: LFP preprocessing
For our initial analyses, continuous LFP recordings from each region were segmented into 10s epochs that lasted from 5 s before to 5 s after the peak of evoked LFP. Each epoch was visually inspected for artefacts before further analysis. Any epochs that, on visual inspection, exhibited electrode artifacts (i.e., abrupt vertical transients that do not modify the background activity) were excluded from subsequent analysis.
Time-frequency analysis
Activity changes in LFP in different frequency bands were calculated using the Hilbert transform (Le Van Quyen et al., 2001; Bruns, 2004; Tadel et al., 2011). Each epoch was filtered in various frequency bands with bandpass filters for δ (2–4 Hz), θ (4–8 Hz), α (8–12 Hz), β (12–30 Hz), and γ (30–90 Hz) band. The magnitude [µV/sqrt(Hz)] of the Hilbert transform of a narrow-band signal is a measure of the envelope of this signal, and therefore gives an indication of the activity in this frequency band. The energy magnitude data were then averaged across repetitions within each animal. Stimulus-induced changes in energy magnitude for each animal were then calculated by normalized to mean of baseline (−4 to –1 s).
Time-resolved PAC (tPAC) analysis
This approach measures cross-frequency coupling between bursts of high-frequency oscillations and the phase of lower frequency rhythms, over a time window, which slides along the electrophysiological data (Samiee and Baillet, 2017). mPFC and S1 time courses were examined for changes in phase of slow oscillation at δ band (2–4 Hz) coupled to the amplitude of a faster rhythm at γ (30–90 Hz) band. Phase and amplitude information were obtained via the Hilbert transform. The coupling between phase and amplitude was then quantified and Modulation Index values were calculated. To avoid edge artefacts, which can result in spurious Phase amplitude coupling (PAC) (Kramer et al., 2008), the first 2 s and last 2 s of each trial was used as buffer. These were then averaged across repetitions within each animal. Stimulus-induced PAC for each animal were then calculated by normalized to mean of baseline (−2.5 to −1 s).
Time-resolved phase locking analysis
To evaluate the functional connectivity between mPFC and S1, we estimated phase-locking value (PLV) between the LFPs simultaneously recorded at the two areas in different frequency bands (Lachaux et al., 1999). To do this we (1) bandpass filtered the LFPs at S1 and mPFC in the δ (2–4 Hz), θ (4–8 Hz), α (8–12 Hz), β (12–30 Hz), and γ (30–90 Hz) frequency bands; (2) applied Hilbert transform to the band-passed signals; (3) calculate the instantaneous PLV between mPFC and S1. PLVs were then averaged across repetitions within each animal. Stimulus-induced magnitude changes in LFP energy for each animal were then calculated by normalized to mean of baseline (−4 to –1 s)
Quantification and statistical analysis
Statistical analysis was performed using GraphPad Prism 6 (GraphPad Software), SPSS (Statistical Product and Service Solutions, IBM). All data are presented as mean ± SEM. Comparisons of means were performed using one way ANOVA with Tukey's post hoc test if the data were normally distributed; Kruskal–Wallis test with post hoc Dunn's multiple comparisons test if the data were not normally distributed (with the Shapiro–Wilk test used to assess normality of the data distributions). Generalized linear model (GLM) Type III tests followed by Bonferroni post hoc tests were used for analysis of repeated-measures behavior data. Differences were considered statistically significant at p < 0.05. Estimation statistics (open source estimation program available on https://www.estimationstats.com; Ho et al., 2019) were used to compute the change of electrophysiological data in mPFC/S1 in response to eVF stimulation with days following injury (D0, D4, and D10), compared with preinjury activity. Mean differences are shown using Cumming estimation plots, with each graphed as a bootstrap sampling distribution (5000 bootstrap samples). The p value(s) reported are the likelihood(s) of observing the effect size(s), if the null hypothesis of zero difference is true. For each permutation p value, 5000 reshuffles of the control and test labels were performed; p < 0.05 is considered a significant difference. Pearson correlation was applied to calculate the correlation between pain sensitivity and electrophysiological data. The significance threshold for all correlation tests was set at p < 0.05.
Results
ELP increases injury-induced hyperalgesia and pain in adult life
Behavioral pain threshold testing confirmed the impact of ELP on adult pain behavior, as described previously (Beggs et al., 2012b; Moriarty et al., 2019). We measured the amplitude and duration of hindlimb withdrawal reflexes in response to eVF hair stimulation following incision injury in adult male rats. Figure 2 shows von Frey hair pain thresholds in adult ELP male rats before and 10 d after an adult hindpaw incision (II). This is compared with age- matched animals with no ELP, experiencing their first hindpaw incision in adulthood (NI) and control rats that have ELP only or no incisions at all (Con; Fig. 1).
Hindpaw incision injury caused von Frey hair thresholds to fall in both groups of adult rats (NI and II) compared with the control (Con) group, indicating a significant postinjury pain and hyperalgesia. Consistent with previous reports (Beggs et al., 2012b; Moriarty et al., 2019), animals that experienced ELP (II, n = 9) developed significantly lower paw withdraw thresholds (PWTs), compared with injured animals with no ELP (NI, n = 10). In addition, as in earlier studies (Walker et al., 2009b), ELP resulted in more prolonged as well as enhanced hyperalgesia, lasting up to 10 d after hindpaw incision, compared with 3–4 d in non ELP rats (Fig. 2).
ELP increases postinjury evoked δ and γ activity in adult S1
To test the impact of ELP experience on pain-related neural activity in S1 and mPFC, we next investigated evoked potentials (EPs) and oscillatory neural activity in S1 and mPFC evoked by mechanical stimulation (von Frey hair, eVF) following incision injury. EP amplitudes in S1 and mPFC did not differ between groups and so to gain further insight into the pattern and time course of evoked cortical activity following incision injury, the EP energy was analyzed in the δ (2–4 Hz), θ (4–8 Hz), α (8–12 Hz), β (12–30 Hz), and γ (30–90 Hz) frequency bands. Figures 3 and 4 show a significant increase in the eVF evoked δ energy (Fig. 3C,D) and γ energy (Fig. 4C,D) in S1 following incision in ELP rats, which is not observed in the other adult rat groups, NI and Con. The sensory evoked data in Figures 3 and 4 has been normalized to baseline (a period before stimulation), removing any effect of increased γ power in the S1 and PFC caused by the surgical pain alone, and revealing only eVF stimulus evoked energy changes. These stimulus evoked increases in δ and γ energy in ELP rats were recorded in II groups only, in the 2–3 h postinjury (D0) and had recovered by 4 d postinjury. They were not observed in mPFC.
Figure 3.
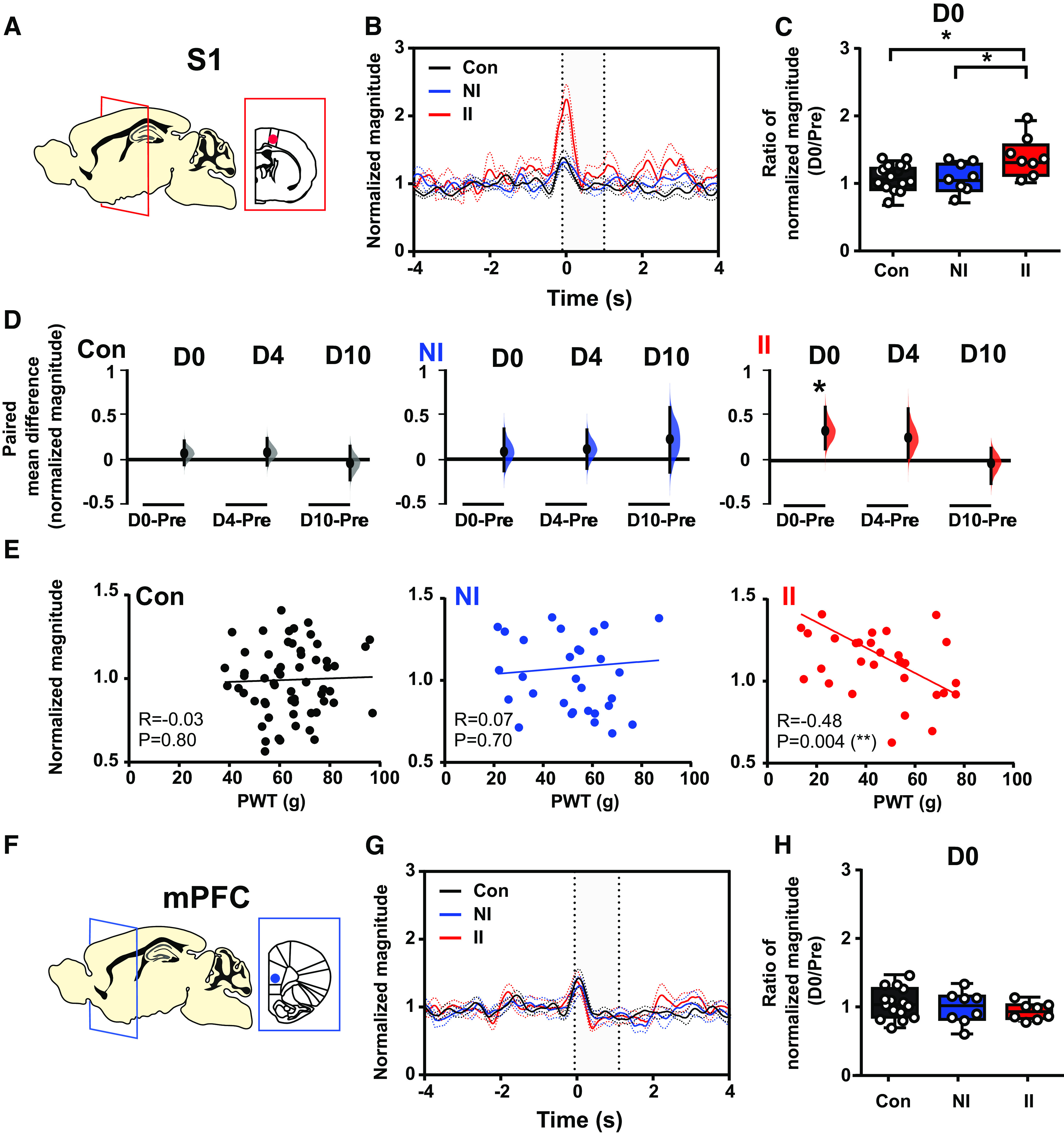
Stimulus-evoked δ energy in SI increases after adult incision injury only in animals who experienced ELP. Electrophysiological responses in the (A) somatosensory cortex (S1) and (F) mPFC to mechanical (eVF) stimulation of the hindpaw following adult injury in ELP rats (II, red) and non-ELP rats (NI, blue) and controls (Con, black). Peristimulus normalized δ frequency (2–4 Hz) oscillations (mean ± SEM) in S1 (B) and mPFC (G) on the day of adult incision injury (D0). Comparison of the injury-induced changes in stimulus-evoked δ energy in S1 (C) and mPFC (H), expressed as a ratio of normalized magnitude (D0/Pre), between groups. D, The enhancement of injury-induced changes in sensory evoked S1 δ energy returned to preinjury level by 10 d (D10) following injury. The paired mean difference for comparisons is shown as Cumming estimation. Each paired mean difference is plotted as a bootstrap sampling distribution; 95% confidence intervals are indicated by the ends of the vertical error bars. Statistical analysis was performed using a permutation t test (randomization: 5000). E, Correlations between PWT and stimulus-evoked S1 δ activity (normalized magnitude). The scatter plots represent the correlations between PWT and normalized energy (Pre to D10) with continuous lines showing the linear regression. Pearson correlation coefficient (R) with significance (p value) is presented in the figures. Nonincised adult controls (Con, n = 15), incision in adults without neonatal incision (NI, n = 8), and incision in adults with neonatal incision (II, n = 8).
Figure 4.
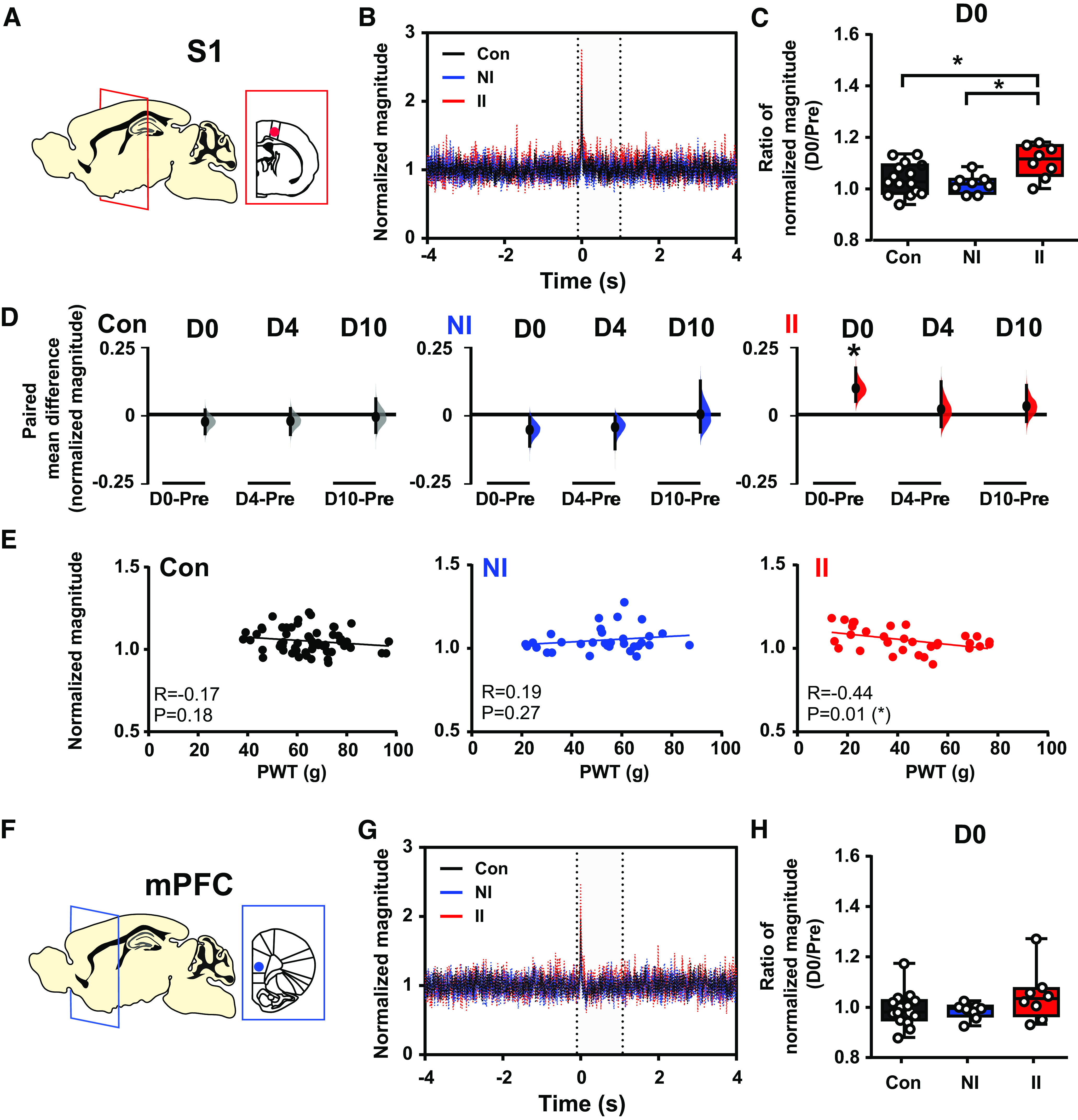
Stimulus-evoked γ energy in SI increases after adult incision injury only in animals who experienced ELP. Electrophysiological responses in the (A) somatosensory cortex (S1) and (F) mPFC to mechanical (eVF) stimulation of the hindpaw following injury in ELP adult rats (II, red), non-ELP rats (NI, blue), and controls (Con, black). Peristimulus normalized γ frequency (30–90 Hz) oscillations (mean ± SEM) in S1 (B) and mPFC (G). Comparison of changes in stimulus-evoked γ energy in S1 (C) and mPFC (H), expressed as a ratio of normalized magnitude (D0/Pre), between groups. D, The enhancement of injury-induced changes in sensory evoked S1 γ energy returned to preinjury level by 4 d (D4) following injury. The paired mean difference for comparison is shown as Cumming estimation. Each paired mean difference is plotted as a bootstrap sampling distribution; 95% confidence intervals are indicated by the ends of the vertical error bars. Statistical analysis was performed using a permutation t test (randomization: 5000). E, Correlations between PWT and stimulus evoked S1 γ activity (normalized magnitude). The scatter plots represent the correlations between PWT and normalized energy (Pre to D10) with continuous lines showing the linear regression. Pearson correlation coefficient (R) with significance (p value) is presented in the figures. Nonincised adult controls (Con, n = 15), incision in adults without neonatal incision (NI, n = 8), and incision in adults with neonatal incision (II, n = 8).
Importantly, the magnitude of S1 evoked δ and γ activity was significantly correlated to pain sensitivity, or fall in behavioral von Frey hair PWT, as indicated by the inverse correlation of S1 δ power (Fig. 3E) and S1 γ power (Fig. 4E) with PWT in II male adult rats.
ELP increases postinjury evoked δ-γ coupling in adult S1
Since δ and γ energy evoked by mechanical stimulation (eVF) postinjury is increased in S1 in ELP rats, we next asked whether ELP altered cross-frequency coupling [δ (2–4 Hz) vs γ (30–90 Hz)] associated with the observed differences in pain sensitivity following hindpaw incision. Cross-frequency interaction (Florin and Baillet, 2015). Here, to evaluate event related changes in PAC, we used tPAC. Figure 5 shows a significant enhancement of evoked δ-γ coupling in S1 immediately postincision (D0) in II rats (Fig. 5A–C). This increase in δ-γ coupling was not seen in mPFC (Fig. 5D). The enhanced evoked δ-γ coupling in S1 coupling potentially provides a mechanism for investigating local-to-wide networks synchronization and was observed on the day of injury and return to preinjury levels by 4 d (D4) postincision in II rats. There was no significant alteration in S1 evoked δ-γ coupling in NI and Con rats (Fig. 5E). To determine whether this increase in evoked S1 δ-γ coupling is associated with the enhanced pain sensitivity, we subsequently examined the correlation between the two measures. A significant inverse correlation was found between δ-γ coupling and PWT in II rats, but not in NI and Con rats (Fig. 5F). Thus, pain-related stimulus evoked δ-γ coupling in the somatosensory cortex, and its association with pain behaviors is selectively increased in adult ELP rats.
Figure 5.
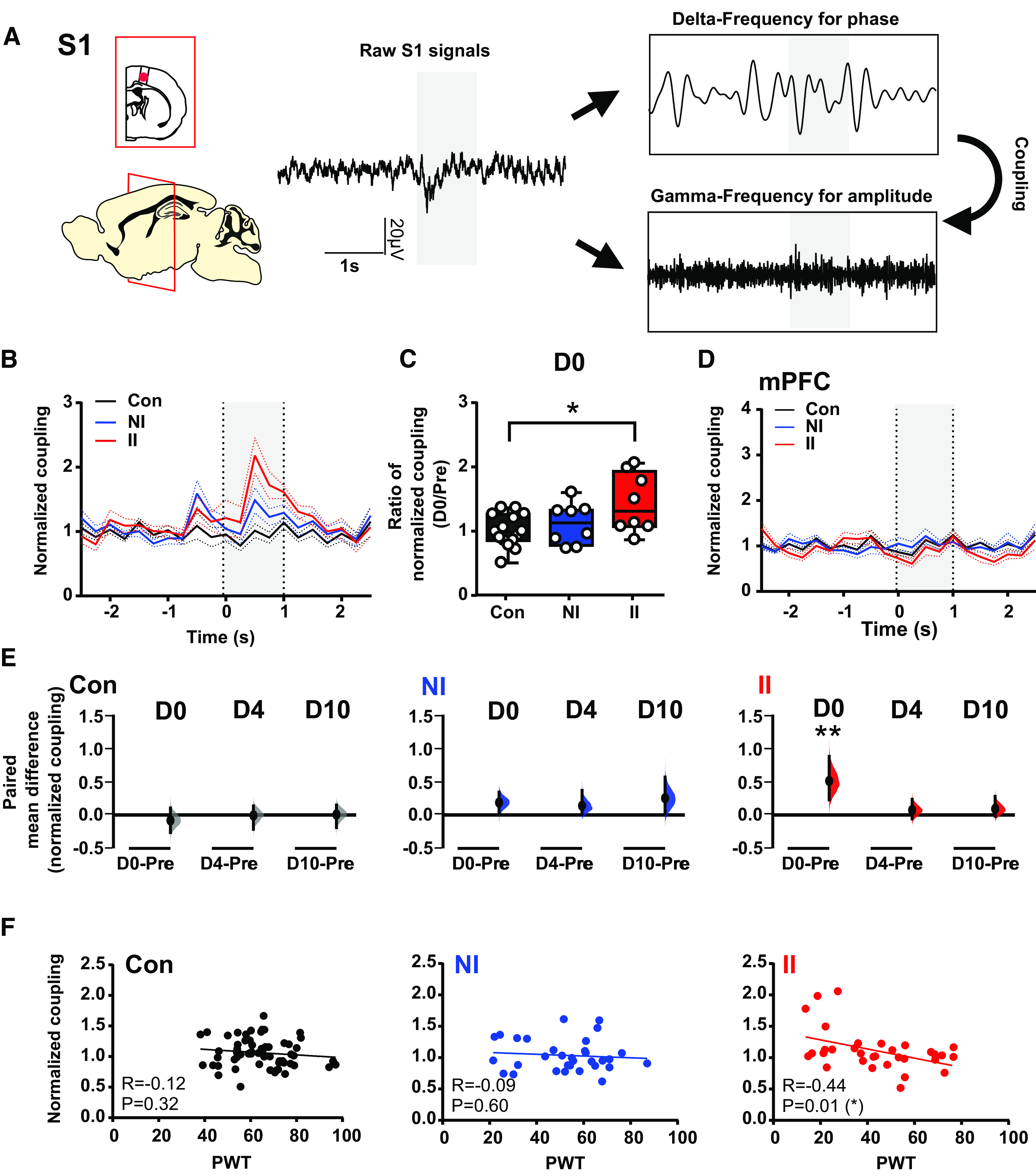
Stimulus-evoked δ-γ cross-frequency coupling in SI increases after adult injury only in animals who experienced ELP. A, Sample trace of LFP recorded in S1 during hindpaw mechanical stimulation (eVF) and a diagram illustrating the principle of cross-frequency coupling. Peristimulus normalized time-resolved δ-γ coupling in S1 (B) and mPFC (D) on the day of adult injury (D0), data are presented as mean ± SEM. C, Comparison of the injury-induced changes in stimulus-evoked δ-γ coupling in S1, expressed as a ratio of normalized magnitude (D0/Pre), between groups. E, The enhancement of pain-induced changes in stimulus-evoked δ-γ coupling in S1 returned to preinjury level by 4 d (D4) following injury. The paired mean difference for comparisons is shown as Cumming estimation. Each paired mean is plotted as a bootstrap sampling distribution; 95% confidence intervals are indicated by the ends of the vertical error bars. Statistical analysis was performed using permutation t test (randomization: 5000). F, Correlations between PWT and δ-γ modulation in S1 expressed as normalized modulation index. The scatter plots represent correlations between PWT and normalized δ-γ coupling with continuous line as linear regression. Pearson correlation coefficient (R) with significance (p value); *p < 0.05, **p < 0.01. Nonincised adult controls (Con, n = 15), incision in adults without neonatal incision (NI, n = 8), and incision in adults with neonatal incision (II, n = 8).
ELP increases postinjury evoked S1-mPFC connectivity in adult rats
The increased pain-related signal processing in ELP found in adult S1, was not observed in mPFC. Since alterations in pain processing in mPFC may depend on connections with other areas of the cerebral cortex, we next examined the functional connectivity between the S1 and mPFC in ELP rats. To explore this, we used PLV, a statistical method used to investigate task-induced changes in long range synchronization of neural activity (Lachaux et al., 1999), which provides an index of phase synchrony between two signals over a specific time period.
On the day of injury (D0), 2–3 h after the incision, a significant increase in S1-mPFC PLV in response to eVF stimulation occurred in both ELP and non ELP rats following hindpaw incision (NI and II). There was no significant difference between the two injured groups (Fig. 6A–C). This increase in phase locking was restricted to the θ band and was not observed in other frequency bands (δ: F(2,28) = 0.16, p = 0.85; α: F(2,28) = 1.75, p = 0.19; β: F(2,28) = 0.96, p = 0.39; γ: F(2,28) = 2.41, p = 0.10). Importantly, a clear difference emerges on inspection of the time course of this effect postinjury, which reveals that the increased θ phase locking is maintained until 4 d postinjury in ELP (II) rats, compared with non-ELP (NI) groups (Fig. 6D,E). We further examined correlation coefficients with pain behavior to determine whether the increased S1-mPFC PLV in the θ band is associated with pain hypersensitivity. A significant inverse correlation between S1-mPFC PLV and PWT is seen in both NI and II rats, but not in uninjured Con rats (Fig. 6F).
Figure 6.
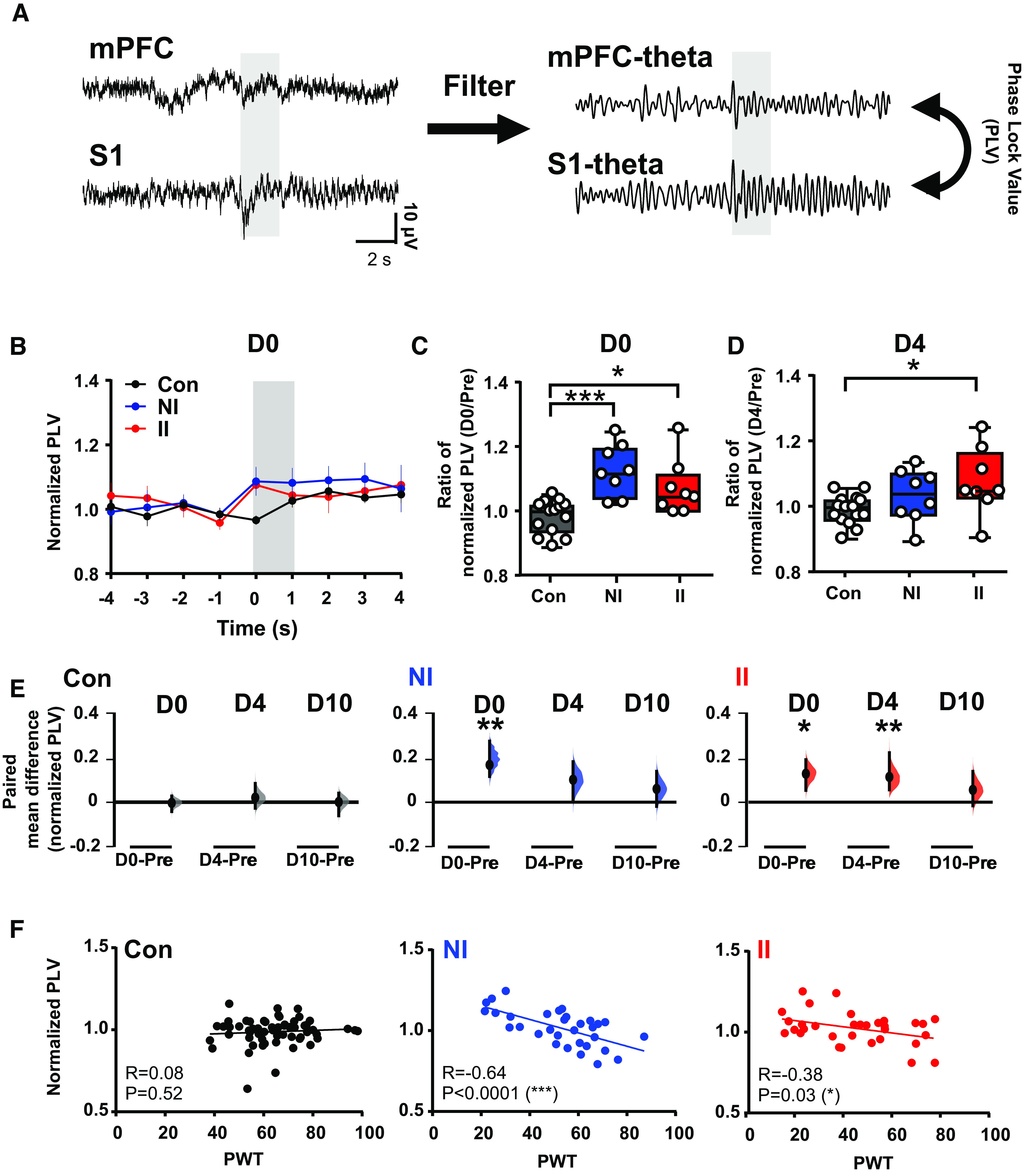
Stimulus-evoked S1-mPFC β phase coupling is enhanced after adult injury and is prolonged in animals who experienced ELP. A, An example of simultaneous recording of stimulus evoked LFPs in S1 and mPFC, before (left) and after (right) filtering for phase coupling measurement at θ frequency. B, Peristimulus normalized S1-mPFC PLV at θ frequency following injury, presented as mean ± SEM. C, Comparison of changes in S1-mPFC PLV at θ on the day of injury (D0) and (D) 4 d following injury (D4), expressed as a ratio of normalized PLV (D0/Pre), between groups. E, The enhancement of injury-induced changes in sensory evoked S1-mPFC PLV at θ returned to preinjury level by 4 d (D4) in the NI group, whereas a longer lasting increase in S1-mPFC PLV at θ was found in II. As a bootstrap sampling distribution, 95% confidence intervals are indicated by the ends of the vertical error bars. Statistical analysis was performed using a permutation t test (randomization: 5000). F, Correlations between PWT and stimulus evoked S1-mPFC phase lock θ oscillations. The scatter plots represent correlations between PWT and normalized δ-γ coupling with continuous line as linear regression. Pearson correlation coefficient (R) with significance (p value); *p < 0.05, **p < 0.01. Nonincised adult controls (Con, n = 15), incision in adults without neonatal incision (NI, n = 8) and incision in adults with neonatal incision (II, n = 8).
Discussion
The results presented here provide novel insights into the effects of ELP on adult cortical pain networks. Using telemetric recording of LFPs in the S1 and mPFC in awake adult mice we show that ELP results in significant changes in neural connectivity in the adult S1 and mPFC related to postinjury pain hypersensitivity.
We used a well-established model of ELP, incision on the plantar hindpaw, which when applied at a critical stage of development, is known to cause lasting changes in pain behavior and increased postinjury pain hypersensitivity in adult life (Walker et al., 2009b; Beggs et al., 2012b; Schwaller and Fitzgerald, 2014). The effect is likely to be driven by altered peripheral nociceptor sensitization (Jankowski et al., 2014; Walker et al., 2016; Dourson et al., 2021) and microglial activation in the dorsal horn of the spinal cord (Beggs et al., 2012b; Moriarty et al., 2019) resulting in altered synaptic connectivity and reduced dynorphin inhibition in the dorsal horn of the spinal cord (J. Li and Baccei, 2016, 2019; Brewer et al., 2020). Brainstem descending pain control is also altered in adults following early life incision (Walker et al., 2015) but the current data are the first to show changes in functional cortical pain networks following ELP. By recording simultaneous behavioral and cortical LFP responses to the same mechanical stimulus, we show that following ELP δ and γ energy and δ/γ modulation are increased in S1, together with increased phase-locking connectivity with mPFC, all directly correlated with behavioral pain hypersensitivity.
The data provide new insight into the central mechanisms whereby exposure to painful sensory experience in early life alters adult pain experience. The mPFC and S1 have key roles in cortical pain processing (Tan and Kuner, 2021); mPFC receives ascending nociceptive input, but also exerts important top-down regulation of sensory and affective processes of pain (Kummer et al., 2020), whereas S1 is the first level of pain perception and encodes nociceptive intensity and perceived pain intensity (Fields, 2012; Mancini et al., 2012). Pain is a complex phenomenon that depends on communication between different brain areas, which is served by neural oscillations and connectivity involving short-range and long-range communication processes (Baliki et al., 2011; Baliki and Apkarian, 2015; Kucyi and Davis, 2015; Ploner et al., 2017; Tan et al., 2021) and it is these oscillations that we have focused on here.
The results reveal a significantly greater noxious-evoked γ in S1 in injured rats with ELP compared with controls. In humans, γ-band oscillations in the S1 correlate with subjective pain perception (Zhang et al., 2012; Heid et al., 2020) and in mice they are specifically strengthened, independently of any motor component, in the S1 cortex during nociception and are elevated during pain hypersensitivity (Tan et al., 2019). Nociceptive C fiber stimulation drives γ activity in adult rat S1 (Chang et al., 2020b) and γ oscillations generated by optogenetic activation of parvalbumin-expressing inhibitory interneurons in the S1 cortex enhance nociceptive sensitivity and induce aversive avoidance behavior, while activating a network of prefrontal cortical and subcortical centers, including descending serotonergic facilitatory pathways (Tan et al., 2021). Recent evidence suggests that γ oscillations reflect strong coupling of neural activity with fast spiking interneurons in the superficial layers of the S1 contralateral to the stimulated side (Yue et al., 2020). The increased energy of γ oscillations, considered one of the most promising biomarkers of pain in the brain, is important evident for increased postinjury pain perception in ELP animals.
Evoked activity in the δ frequency was also observed in the S1 of injured ELP rats. Event-related δ oscillations serve active sensory and cognitive functional roles across different sensory domains (Arnal and Giraud, 2012; Knyazev, 2012; Fardo et al., 2017) and play an crucial role in S1 sensory perception (Schroeder and Lakatos, 2009). δ oscillations association with pain has been demonstrated elsewhere and may reflect coupling in thalamocortical loops (Sarnthein et al., 2006; Walton et al., 2010; Peng and Tang, 2016). The lack of δ frequency changes in mPFC supports the proposal that thalamo-S1 pathways are altered in ELP rats. Indeed, in human infants, ELP is associated with volume loss in the somatosensory thalamus accompanied by disruptions in thalamic metabolic growth and thalamocortical pathway maturation (Brummelte et al., 2012; Duerden et al., 2018).
Neural oscillations play an important role in the integration and segregation of brain regions that are important for pain processing. Low-frequency oscillations (e.g., δ, θ) mediate long-range communication at slow timescales across distant brain regions and are crucial for functional integration in large-scale brain networks. In contrast, high-frequency brain oscillations (e.g., γ) are more transient and focal and thus important for local neuronal synchrony in cortical areas (Canolty and Knight, 2010). Understanding these spatiotemporal and oscillatory aspects in the context of pain-related neural responses will therefore inform the neural mechanisms underlying pain-sensation. Studies of neural oscillations related to pain have identified several functional bands, especially θ, δ, and γ bands, implicated in nociceptive processing (Kim and Davis, 2021; Luo et al., 2021). δ oscillations are changes in the thalamus and S1, as well as the coupling between the thalamus and S1, in laser-induced pain (X. Li et al., 2017) and in neuropathic disease (Walton et al., 2010). Furthermore, a recent study suggested that activity δ combined with other oscillations is responsible for the coding of pain perception, indicating that perception as an overall reflection of the pain state may contain complex information and involve additional brain areas (Luo, 2021). On the other hand, γ oscillations in S1 predict the pain intensity induced by laser stimulation in both humans and rodents (Hu and Iannetti, 2019; Yue et al., 2020) and the pain level in chronic pain patients (Parker et al., 2020), indicating γ oscillations may contain more specific information about pain. Therefore, the combination of neural oscillations is essential for encoding perceptive and sensory measures of pain. Our findings highlight that pain-related sensory evoked neuronal activity in S1, which is associated with both low-frequency and high-frequency oscillatory rhythms mediating functional integration at both local and large-scale brain networks, are altered by ELP experiences.
Overall, these results indicate that the changes in δ and γ activity in S1 are functionally linked to the behavioral hypersensitivity in injured rats with ELP. However, given the distinct intrinsic spatiotemporal properties of low-frequency and high-frequency oscillations, we further examined the transient modulation of high-frequency amplitude (γ) by low-frequency phase (δ) in relation to pain sensitivity and found enhanced evoked S1 δ-γ modulation in injured rats with ELP. Because the high-frequency activity reflects local cortical processing, while low-frequency brain rhythms are dynamically entrained across distributed brain regions by both external sensory input and internal cognitive events, cross frequency modulation between low and high frequency is thought to contribute to information flow from large-scale brain networks to the fast, local cortical processing (Cardin et al., 2009; Canolty and Knight, 2010). Phase-amplitude cross-frequency coupling strength changes quickly in response to sensory, motor, and cognitive events (Schroeder and Lakatos, 2009) and abnormalities of cross frequency modulation may contribute to abnormal routing of information flow in chronic pain (Ploner et al., 2017). Our results suggest that such abnormal routing of information may occur in adults following ELP.
While the S1 reflects sensory discriminative aspects of pain, the PFC is associated with the affective aspect of pain, providing top-down modulation of sensory and affective processes, including inhibition of both sensory and affective pain signals by descending projections to the various brain and spinal cord regions (Ji and Neugebauer, 2014; Bräscher et al., 2016; Kummer et al., 2020). Enhanced functional connectivity during procedural pain has been observed in several areas involved in pain perception: somatosensory cortices, anterior insula, anterior cingulate cortex and thalamus and mPFC (Bräscher et al., 2016; Galambos et al., 2019). Here, we tested whether communication between S1 and mPFC was affected by ELP using synchronization in the θ range as a measure of connectivity. θ synchronization is proposed to be involved in large scale integration between long range multiple brain regions (von Stein and Sarnthein, 2000), especially in mPFC (Colgin, 2011; O'Neill et al., 2013; Esmaeili and Diamond, 2019), consistent with human data showing that prefrontal-sensorimotor connectivity is increased in tonic pain (Nickel et al., 2020). Our results show that adult incision injury does indeed produce a marked increase in evoked θ S1-mPFC connectivity, highly correlated to behavioral pain sensitivity in both ELP and control groups, but this increase is prolonged in ELP, lasting for 4 d compared with only 1 d in controls. Our data suggests that the connection between sensory and affective pain processing is enhanced in ELP rats which may underpin the wider social, emotional and cognitive life-long impact of ELP beyond increased pain perception (Ranger et al., 2018; de Kort et al., 2021; Ririe et al., 2021).
Our demonstration that ELP affects the cortical dynamics and connectivity underlying adult pain perception has important translational implications. Hospitalized infants exposed to ELP as a result of necessary clinical care, despite efforts to control that exposure (Laudiano-Dray et al., 2020; Eccleston et al., 2021), display long-term structural and functional brain changes (Ranger and Grunau, 2014; Walker, 2019). Early life adversity, including stress and pain, has been reported to increase the risk of persistent pain in adults (Victoria and Murphy, 2016; Nelson et al., 2017) and it is possible that the changes reported here underlie an increased vulnerability to chronic pain in adults exposed to ELP. Pain is the perceptual consequence of the complex interactions of many cortical areas, including the somatosensory, prefrontal cortices, and limbic areas (e.g., thalamus) and both animal (Eto et al., 2011) and human (Geha et al., 2008; Ichesco et al., 2012) studies reveal functional and structural changes in these specific areas of the cerebral cortex in chronic pain conditions. Furthermore, S1 and mPFC closely interact in chronic pain (Kong et al., 2013; A.F. Jones and Sheets, 2020; Kummer et al., 2020). This reorganization of local cortical circuits provides a mechanism for abnormal activity underlying chronic pain and early life adversity, including stress and pain, may not only have long-term effects on nociceptive processing, but also increase the risk of persistent pain in the adult by altering normal brain development and function (Brummelte et al., 2012; Schneider et al., 2018; Chau et al., 2019).
In conclusion, we have demonstrated that painful sensory experiences in early life have a significant effect on the function of adult pain-related cortical circuits. This change is likely driven by altered peripheral nociceptor and spinal cord circuit function following early life injury. Changes in regional and interregional neural oscillations in S1 and mPFC caused by painful experience in early life play a key role in altered nociceptive processing and may predispose to an adaptive mechanism underlying chronification of pain. Understanding the effects of ELP on developing cortical pain networks will increase our understanding of individual susceptibility to pain in adult life (Denk et al., 2014).
Footnotes
This work was supported by the Biotechnology and Biological Sciences Research Council Grant BB/R00823X/1 (to M.F. and P.C.) and the Medical Research Council Grant MR/L019248/1 (to L.F. and M.F.).
The authors declare no competing financial interests.
References
- Apkarian AV, Bushnell MC, Treede RD, Zubieta JK (2005) Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain 9:463–484. 10.1016/j.ejpain.2004.11.001 [DOI] [PubMed] [Google Scholar]
- Arnal LH, Giraud AL (2012) Cortical oscillations and sensory predictions. Trends Cogn Sci 16:390–398. 10.1016/j.tics.2012.05.003 [DOI] [PubMed] [Google Scholar]
- Baliki MN, Apkarian AV (2015) Nociception, pain, negative moods, and behavior selection. Neuron 87:474–491. 10.1016/j.neuron.2015.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliki MN, Baria AT, Apkarian AV (2011) The cortical rhythms of chronic back pain. J Neurosci 31:13981–13990. 10.1523/JNEUROSCI.1984-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beggs S, Alvares D, Moss A, Currie G, Middleton J, Salter MW, Fitzgerald M (2012a) A role for NT-3 in the hyperinnervation of neonatally wounded skin. Pain 153:2133–2139. 10.1016/j.pain.2012.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beggs S, Currie G, Salter MW, Fitzgerald M, Walker SM (2012b) Priming of adult pain responses by neonatal pain experience: maintenance by central neuroimmune activity. Brain 135:404–417. 10.1093/brain/awr288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boada MD, Gutierrez S, Giffear K, Eisenach JC, Ririe DG (2012) Skin incision-induced receptive field responses of mechanosensitive peripheral neurons are developmentally regulated in the rat. J Neurophysiol 108:1122–1129. 10.1152/jn.00399.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bräscher A-K, Becker S, Hoeppli M-E, Schweinhardt P (2016) Different brain circuitries mediating controllable and uncontrollable pain. J Neurosci 36:5013–5025. 10.1523/JNEUROSCI.1954-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan TJ, Vandermeulen EP, Gebhart GF (1996) Characterization of a rat model of incisional pain. Pain 64:493–502. 10.1016/0304-3959(95)01441-1 [DOI] [PubMed] [Google Scholar]
- Brewer CL, Li J, O'Conor K, Serafin EK, Baccei ML (2020) Neonatal injury evokes persistent deficits in dynorphin inhibitory circuits within the adult mouse superficial dorsal horn. J Neurosci 40:3882–3895. 10.1523/JNEUROSCI.0029-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummelte S, Grunau RE, Chau V, Poskitt KJ, Brant R, Vinall J, Gover A, Synnes AR, Miller SP (2012) Procedural pain and brain development in premature newborns. Ann Neurol 71:385–396. 10.1002/ana.22267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns A (2004) Fourier-, Hilbert- and wavelet-based signal analysis: are they really different approaches? J Neurosci Methods 137:321–332. 10.1016/j.jneumeth.2004.03.002 [DOI] [PubMed] [Google Scholar]
- Buzsaki G (2004) Neuronal oscillations in cortical networks. Science 304:1926–1929. [DOI] [PubMed] [Google Scholar]
- Canolty RT, Knight RT (2010) The functional role of cross-frequency coupling. Trends Cogn Sci 14:506–515. 10.1016/j.tics.2010.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao W, Lin S, Xia Q, Du Y, Yang Q, Zhang M, Lu Y, Xu J, Duan S, Xia J, Feng G, Xu J, Luo J (2018) Gamma oscillation dysfunction in mPFC leads to social deficits in neuroligin 3 R451C knockin mice. Neuron 97:1253–1260.e7. 10.1016/j.neuron.2018.02.001 [DOI] [PubMed] [Google Scholar]
- Cardin JA, Carlén M, Meletis K, Knoblich U, Zhang F, Deisseroth K, Tsai L-H, Moore CI (2009) Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature 459:663–667. 10.1038/nature08002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang P, Hashemi KS, Walker MC (2011) A novel telemetry system for recording EEG in small animals. J Neurosci Methods 201:106–115. 10.1016/j.jneumeth.2011.07.018 [DOI] [PubMed] [Google Scholar]
- Chang P, Fabrizi L, Olhede S, Fitzgerald M (2016) The development of nociceptive network activity in the somatosensory cortex of freely moving rat pups. Cereb Cortex 26:4513–4523. 10.1093/cercor/bhw330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang P, Bush D, Schorge S, Good M, Canonica T, Shing N, Noy S, Wiseman FK, Burgess N, Tybulewicz VLJ, Walker MC, Fisher EMC (2020a) Altered hippocampal-prefrontal neural dynamics in mouse models of down syndrome. Cell Rep 30:1152–1163.e4. 10.1016/j.celrep.2019.12.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang P, Fabrizi L, Fitzgerald M (2020b) Distinct age-dependent C fiber-driven oscillatory activity in the rat somatosensory cortex. eNeuro 7:ENEURO.0036-20.2020. 10.1523/ENEURO.0036-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chau CMY, Ranger M, Bichin M, Park MTM, Amaral RSC, Chakravarty M, Poskitt K, Synnes AR, Miller SP, Grunau RE (2019) Hippocampus, amygdala, and thalamus volumes in very preterm children at 8 years: neonatal pain and genetic variation. Front Behav Neurosci 13:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgin LL (2011) Oscillations and hippocampal–prefrontal synchrony. Curr Opin Neurobiol 21:467–474. 10.1016/j.conb.2011.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kort AR, Joosten EA, Patijn J, Tibboel D, van den Hoogen NJ (2021) Neonatal procedural pain affects state, but not trait anxiety behavior in adult rats. Dev Psychobiol 63:e22210. 10.1002/dev.22210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lima J, Alvares D, Hatch DJ, Fitzgerald M (1999) Sensory hyperinnervation after neonatal skin wounding: effect of bupivacaine sciatic nerve block. Br J Anaesth 83:662–664. 10.1093/bja/83.4.662 [DOI] [PubMed] [Google Scholar]
- Denk F, McMahon SB, Tracey I (2014) Pain vulnerability: a neurobiological perspective. Nat Neurosci 17:192–200. 10.1038/nn.3628 [DOI] [PubMed] [Google Scholar]
- Dourson AJ, Ford ZK, Green KJ, McCrossan CE, Hofmann MC, Hudgins RC, Jankowski MP (2021) Early life nociception is influenced by peripheral growth hormone signaling. J Neurosci 41:4410–4427. 10.1523/JNEUROSCI.3081-20.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duerden EG, Albanese MC (2013) Localization of pain-related brain activation: a meta-analysis of neuroimaging data. Hum Brain Mapp 34:109–149. 10.1002/hbm.21416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duerden EG, Grunau RE, Guo T, Foong J, Pearson A, Au-Young S, Lavoie R, Chakravarty MM, Chau V, Synnes A, Miller SP (2018) Early procedural pain is associated with regionally-specific alterations in thalamic development in preterm neonates. J Neurosci 38:878–886. 10.1523/JNEUROSCI.0867-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccleston C, Fisher E, Howard RF, Slater R, Forgeron P, Palermo TM, Birnie KA, Anderson BJ, Chambers CT, Crombez G, Ljungman G, Jordan I, Jordan Z, Roberts C, Schechter N, Sieberg CB, Tibboel D, Walker SM, Wilkinson D, Wood C (2021) Delivering transformative action in paediatric pain: a Lancet Child and Adolescent Health Commission. Lancet Child Adolesc Health 5:47–87. 10.1016/S2352-4642(20)30277-7 [DOI] [PubMed] [Google Scholar]
- Esmaeili V, Diamond ME (2019) Neuronal correlates of tactile working memory in prefrontal and vibrissal somatosensory cortex. Cell Rep 27:3167–3181.e5. 10.1016/j.celrep.2019.05.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eto K, Wake H, Watanabe M, Ishibashi H, Noda M, Yanagawa Y, Nabekura J (2011) Inter-regional contribution of enhanced activity of the primary somatosensory cortex to the anterior cingulate cortex accelerates chronic pain behavior. J Neurosci 31:7631–7636. 10.1523/JNEUROSCI.0946-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euston DR, Gruber AJ, McNaughton BL (2012) The role of medial prefrontal cortex in memory and decision making. Neuron 76:1057–1070. 10.1016/j.neuron.2012.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fardo F, Vinding MC, Allen M, Jensen TS, Finnerup NB (2017) Delta and gamma oscillations in operculo-insular cortex underlie innocuous cold thermosensation. J Neurophysiol 117:1959–1968. 10.1152/jn.00843.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrier J, Bayet-Robert M, Dalmann R, El Guerrab A, Aissouni Y, Graveron-Demilly D, Chalus M, Pinguet J, Eschalier A, Richard D, Daulhac L, Marchand F, Balayssac D (2015) Cholinergic neurotransmission in the posterior insular cortex is altered in preclinical models of neuropathic pain: key role of muscarinic M2 receptors in donepezil-induced antinociception. J Neurosci 35:16418–16430. 10.1523/JNEUROSCI.1537-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrier J, Marchand F, Balayssac D (2016) Assessment of mechanical allodynia in rats using the electronic von Frey test. Bio-protocol 6:e1933. 10.21769/BioProtoc.1933 [DOI] [Google Scholar]
- Fields HL (2012) Pain and the primary somatosensory cortex. Pain 153:742–743. 10.1016/j.pain.2012.01.034 [DOI] [PubMed] [Google Scholar]
- Florin E, Baillet S (2015) The brain's resting-state activity is shaped by synchronized cross-frequency coupling of neural oscillations. Neuroimage 111:26–35. 10.1016/j.neuroimage.2015.01.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galambos A, Szabó E, Nagy Z, Édes AE, Kocsel N, Juhász G, Kökönyei G (2019) A systematic review of structural and functional MRI studies on pain catastrophizing. J Pain Res 12:1155–1178. 10.2147/JPR.S192246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geha PY, Baliki MN, Harden RN, Bauer WR, Parrish TB, Apkarian AV (2008) The brain in chronic CRPS pain: abnormal gray-white matter interactions in emotional and autonomic regions. Neuron 60:570–581. 10.1016/j.neuron.2008.08.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heid C, Mouraux A, Treede R-D, Schuh-Hofer S, Rupp A, Baumgärtner U (2020) Early gamma-oscillations as correlate of localized nociceptive processing in primary sensorimotor cortex. J Neurophysiol 123:1711–1726. 10.1152/jn.00444.2019 [DOI] [PubMed] [Google Scholar]
- Ho J, Tumkaya T, Aryal S, Choi H, Claridge-Chang A (2019) Moving beyond P values: data analysis with estimation graphics. Nat Methods 16:565–566. 10.1038/s41592-019-0470-3 [DOI] [PubMed] [Google Scholar]
- Hu L, Iannetti GD (2019) Neural indicators of perceptual variability of pain across species. Proc Natl Acad Sci U S A 116:1782–1791. 10.1073/pnas.1812499116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang WC, Zucca A, Levy J, Page DT (2020) Social behavior is modulated by valence-encoding mPFC-amygdala sub-circuitry. Cell Rep 32:107899. 10.1016/j.celrep.2020.107899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichesco E, Quintero A, Clauw DJ, Peltier S, Sundgren PM, Gerstner GE, Schmidt-Wilcke T (2012) Altered functional connectivity between the insula and the cingulate cortex in patients with temporomandibular disorder: a pilot study. Headache 52:441–454. 10.1111/j.1526-4610.2011.01998.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowski MP, Ross JL, Weber JD, Lee FB, Shank AT, Hudgins RC (2014) Age-dependent sensitization of cutaneous nociceptors during developmental inflammation. Mol Pain 10:34. 10.1186/1744-8069-10-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji G, Neugebauer V (2014) CB1 augments mGluR5 function in medial prefrontal cortical neurons to inhibit amygdala hyperactivity in an arthritis pain model. Eur J Neurosci 39:455–466. 10.1111/ejn.12432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AF, Sheets PL (2020) Sex-specific disruption of distinct mPFC inhibitory neurons in spared-nerve injury model of neuropathic pain. Cell Rep 31:107729. 10.1016/j.celrep.2020.107729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones GT, Power C, Macfarlane GJ (2009) Adverse events in childhood and chronic widespread pain in adult life: results from the 1958 British Birth Cohort Study. Pain 143:92–96. 10.1016/j.pain.2009.02.003 [DOI] [PubMed] [Google Scholar]
- Jones L, Verriotis M, Cooper RJ, Laudiano-Dray MP, Rupawala M, Meek J, Fabrizi L, Fitzgerald M (2022) Widespread nociceptive maps in the human neonatal somatosensory cortex. Elife 11:e71655. 10.7554/eLife.71655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JA, Davis KD (2021) Neural oscillations: understanding a neural code of pain. Neuroscientist 27:544–570. 10.1177/1073858420958629 [DOI] [PubMed] [Google Scholar]
- Knyazev GG (2012) EEG delta oscillations as a correlate of basic homeostatic and motivational processes. Neurosci Biobehav Rev 36:677–695. 10.1016/j.neubiorev.2011.10.002 [DOI] [PubMed] [Google Scholar]
- Kong J, Spaeth B, Wey H-Y, Cheetham A, Cook AH, Jensen K, Tan Y, Liu H, Wang D, Loggia ML, Napadow V, Smoller JW, Wasan AD, Gollub RL (2013) S1 is associated with chronic low back pain: a functional and structural MRI study. Mol Pain 9:43. 10.1186/1744-8069-9-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer MA, Tort ABL, Kopell NJ (2008) Sharp edge artifacts and spurious coupling in EEG frequency comodulation measures. J Neurosci Methods 170:352–357. 10.1016/j.jneumeth.2008.01.020 [DOI] [PubMed] [Google Scholar]
- Kucyi A, Davis KD (2015) The dynamic pain connectome. Trends Neurosci 38:86–95. 10.1016/j.tins.2014.11.006 [DOI] [PubMed] [Google Scholar]
- Kummer KK, Mitrić M, Kalpachidou T, Kress M (2020) The medial prefrontal cortex as a central hub for mental comorbidities associated with chronic pain. IJMS 21:3440. 10.3390/ijms21103440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachaux JP, Rodriguez E, Martinerie J, Varela FJ (1999) Measuring phase synchrony in brain signals. Hum Brain Mapp 8:194–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laudiano-Dray MP, Pillai Riddell R, Jones L, Iyer R, Whitehead K, Fitzgerald M, Fabrizi L, Meek J (2020) Quantification of neonatal procedural pain severity: a platform for estimating total pain burden in individual infants. Pain 161:1270–1277. 10.1097/j.pain.0000000000001814 [DOI] [PubMed] [Google Scholar]
- Le Van Quyen M, Foucher J, Lachaux J-P, Rodriguez E, Lutz A, Martinerie J, Varela FJ (2001) Comparison of Hilbert transform and wavelet methods for the analysis of neuronal synchrony. J Neurosci Methods 111:83–98. 10.1016/S0165-0270(01)00372-7 [DOI] [PubMed] [Google Scholar]
- Li J, Baccei ML (2016) Neonatal tissue damage promotes spike timing-dependent synaptic long-term potentiation in adult spinal projection neurons. J Neurosci 36:5405–5416. 10.1523/JNEUROSCI.3547-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Baccei ML (2019) Neonatal injury alters sensory input and synaptic plasticity in GABAergic interneurons of the adult mouse dorsal horn. J Neurosci 39:7815–7825. 10.1523/JNEUROSCI.0509-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Zhao Z, Ma J, Cui S, Yi M, Guo H, Wan Y (2017) Extracting neural oscillation signatures of laser-induced nociception in pain-related regions in rats. Front Neural Circuits 11:71. 10.3389/fncir.2017.00071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Green KJ, Ford ZK, Queme LF, Lu P, Ross JL, Lee FB, Shank AT, Hudgins RC, Jankowski MP (2017) Growth hormone regulates the sensitization of developing peripheral nociceptors during cutaneous inflammation. Pain 158:333–346. 10.1097/j.pain.0000000000000770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo H, Huang Y, Green AL, Aziz TZ, Xiao X, Wang S (2021) Neurophysiological characteristics in the periventricular/periaqueductal gray correlate with pain perception, sensation, and affect in neuropathic pain patients. Neuroimage Clin 32:102876. 10.1016/j.nicl.2021.102876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini F, Haggard P, Iannetti GD, Longo MR, Sereno MI (2012) Fine-grained nociceptive maps in primary somatosensory cortex. J Neurosci 32:17155–17162. 10.1523/JNEUROSCI.3059-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchior M, Kuhn P, Poisbeau P (2022) The burden of early life stress on the nociceptive system development and pain responses. Eur J Neurosci 55:2216–2241. 10.1111/ejn.15153 [DOI] [PubMed] [Google Scholar]
- Moriarty O, Tu Y, Sengar AS, Salter MW, Beggs S, Walker SM (2019) Priming of adult incision response by early life injury: neonatal microglial inhibition has persistent but sexually dimorphic effects in adult rats. J Neurosci 39:3081–3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson SM, Cunningham NR, Kashikar-Zuck S (2017) A conceptual framework for understanding the role of adverse childhood experiences in pediatric chronic pain. Clin J Pain 33:264–270. 10.1097/AJP.0000000000000397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickel MM, Ta Dinh S, May ES, Tiemann L, Hohn VD, Gross J, Ploner M (2020) Neural oscillations and connectivity characterizing the state of tonic experimental pain in humans. Hum Brain Mapp 41:17–29. 10.1002/hbm.24784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill PK, Gordon JA, Sigurdsson T (2013) Theta oscillations in the medial prefrontal cortex are modulated by spatial working memory and synchronize with the hippocampus through its ventral subregion. J Neurosci 33:14211–14224. 10.1523/JNEUROSCI.2378-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong WY, Stohler CS, Herr DR (2019) Role of the prefrontal cortex in pain processing. Mol Neurobiol 56:1137–1166. 10.1007/s12035-018-1130-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker T, Huang Y, Raghu ALB, FitzGerald JJ, Green AL, Aziz TZ (2020) Dorsal root ganglion stimulation modulates cortical gamma activity in the cognitive dimension of chronic pain. Brain Sciences 10:95. 10.3390/brainsci10020095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Ashwell KWS, Tork I (2013) Atlas of the developing rat nervous system. San Diego: Academic Press. [Google Scholar]
- Peng W, Tang D (2016) Pain related cortical oscillations: methodological advances and potential applications. Front Comput Neurosci 10:9. 10.3389/fncom.2016.00009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploner M, Sorg C, Gross J (2017) Brain rhythms of pain. Trends Cogn Sci 21:100–110. 10.1016/j.tics.2016.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranger M, Grunau RE (2014) Early repetitive pain in preterm infants in relation to the developing brain. Pain Manag 4:57–67. 10.2217/pmt.13.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranger M, Tremblay S, Chau CMY, Holsti L, Grunau RE, Goldowitz D (2018) Adverse behavioral changes in adult mice following neonatal repeated exposure to pain and sucrose. Front Psychol 9:2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds ML, Fitzgerald M (1995) Long-term sensory hyperinnervation following neonatal skin wounds. J Comp Neurol 358:487–498. 10.1002/cne.903580403 [DOI] [PubMed] [Google Scholar]
- Ririe DG, Eisenach JC, Martin TJ (2021) A painful beginning: early life surgery produces long-term behavioral disruption in the rat. Front Behav Neurosci 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samiee S, Baillet S (2017) Time-resolved phase-amplitude coupling in neural oscillations. Neuroimage 159:270–279. 10.1016/j.neuroimage.2017.07.051 [DOI] [PubMed] [Google Scholar]
- Sarnthein J, Stern J, Aufenberg C, Rousson V, Jeanmonod D (2006) Increased EEG power and slowed dominant frequency in patients with neurogenic pain. Brain 129:55–64. 10.1093/brain/awh631 [DOI] [PubMed] [Google Scholar]
- Schneider J, Duerden EG, Guo T, Ng K, Hagmann P, Bickle Graz M, Grunau RE, Chakravarty MM, Hüppi PS, Truttmann AC, Miller SP (2018) Procedural pain and oral glucose in preterm neonates: brain development and sex-specific effects. Pain 159:515–525. 10.1097/j.pain.0000000000001123 [DOI] [PubMed] [Google Scholar]
- Schroeder CE, Lakatos P (2009) Low-frequency neuronal oscillations as instruments of sensory selection. Trends Neurosci 32:9–18. 10.1016/j.tins.2008.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwaller F, Fitzgerald M (2014) The consequences of pain in early life: injury-induced plasticity in developing pain pathways. Eur J Neurosci 39:344–352. 10.1111/ejn.12414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadel F, Baillet S, Mosher JC, Pantazis D, Leahy RM (2011) Brainstorm: a user-friendly application for MEG/EEG analysis. Comput Intell Neurosci 2011:879716. 10.1155/2011/879716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan LL, Kuner R (2021) Neocortical circuits in pain and pain relief. Nat Rev Neurosci 22:458–471. 10.1038/s41583-021-00468-2 [DOI] [PubMed] [Google Scholar]
- Tan LL, Oswald MJ, Heinl C, Retana Romero OA, Kaushalya SK, Monyer H, Kuner R (2019) Gamma oscillations in somatosensory cortex recruit prefrontal and descending serotonergic pathways in aversion and nociception. Nat Commun 10:983. 10.1038/s41467-019-08873-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan LL, Oswald MJ, Kuner R (2021) Neurobiology of brain oscillations in acute and chronic pain. Trends Neurosci 44:629–642. 10.1016/j.tins.2021.05.003 [DOI] [PubMed] [Google Scholar]
- Torsney C, Fitzgerald M (2003) Spinal dorsal horn cell receptive field size is increased in adult rats following neonatal hindpaw skin injury. J Physiol 550:255–261. 10.1113/jphysiol.2003.043661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Hoogen NJ, Patijn J, Tibboel D, Joosten BA, Fitzgerald M, Kwok CHT (2018) Repeated touch and needle-prick stimulation in the neonatal period increases the baseline mechanical sensitivity and postinjury hypersensitivity of adult spinal sensory neurons. Pain 159:1166–1175. 10.1097/j.pain.0000000000001201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victoria NC, Murphy AZ (2016) Exposure to early life pain: long term consequences and contributing mechanisms. Curr Opin Behav Sci 7:61–68. 10.1016/j.cobeha.2015.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Stein A, Sarnthein J (2000) Different frequencies for different scales of cortical integration: from local gamma to long range alpha/theta synchronization. Int J Psychophysiol 38:301–313. 10.1016/S0167-8760(00)00172-0 [DOI] [PubMed] [Google Scholar]
- Walker SM (2019) Long-term effects of neonatal pain. Semin Fetal Neonatal Med 24:101005. 10.1016/j.siny.2019.04.005 [DOI] [PubMed] [Google Scholar]
- Walker SM, Franck LS, Fitzgerald M, Myles J, Stocks J, Marlow N (2009a) Long-term impact of neonatal intensive care and surgery on somatosensory perception in children born extremely preterm. Pain 141:79–87. 10.1016/j.pain.2008.10.012 [DOI] [PubMed] [Google Scholar]
- Walker SM, Tochiki KK, Fitzgerald M (2009b) Hindpaw incision in early life increases the hyperalgesic response to repeat surgical injury: critical period and dependence on initial afferent activity. Pain 147:99–106. 10.1016/j.pain.2009.08.017 [DOI] [PubMed] [Google Scholar]
- Walker SM, Fitzgerald M, Hathway GJ (2015) Surgical injury in the neonatal rat alters the adult pattern of descending modulation from the rostroventral medulla. Anesthesiology 122:1391–1400. 10.1097/ALN.0000000000000658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker SM, Beggs S, Baccei ML (2016) Persistent changes in peripheral and spinal nociceptive processing after early tissue injury. Exp Neurol 275:253–260. 10.1016/j.expneurol.2015.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker SM, O'Reilly H, Beckmann J, Marlow N; EPICure@19 Study Group (2018) Conditioned pain modulation identifies altered sensitivity in extremely preterm young adult males and females. Br J Anaesth 121:636–646. 10.1016/j.bja.2018.05.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton KD, Dubois M, Llinás RR (2010) Abnormal thalamocortical activity in patients with Complex Regional Pain Syndrome (CRPS) Type I. Pain 150:41–51. 10.1016/j.pain.2010.02.023 [DOI] [PubMed] [Google Scholar]
- Yue L, Iannetti GD, Hu L (2020) The neural origin of nociceptive-induced gamma-band oscillations. J Neurosci 40:3478–3490. 10.1523/JNEUROSCI.0255-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZG, Hu L, Hung YS, Mouraux A, Iannetti GD (2012) Gamma-band oscillations in the primary somatosensory cortex–a direct and obligatory correlate of subjective pain intensity. J Neurosci 32:7429–7438. 10.1523/JNEUROSCI.5877-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Gadotti VM, Chen L, Souza IA, Stemkowski PL, Zamponi GW (2015) Role of prelimbic GABAergic circuits in sensory and emotional aspects of neuropathic pain. Cell Rep 12:752–759. 10.1016/j.celrep.2015.07.001 [DOI] [PubMed] [Google Scholar]