Abstract
Parental care is critical for successful reproduction in mammals. Recent work has implicated the hormone prolactin in regulating male parental behavior, similar to its established role in females. Male laboratory mice show a mating-induced suppression of infanticide (normally observed in virgins) and onset of paternal behavior 2 weeks after mating. Using this model, we sought to investigate how prolactin acts in the forebrain to regulate paternal behavior. First, using c-fos immunoreactivity in prolactin receptor (Prlr) Prlr-IRES-Cre-tdtomato reporter mouse sires, we show that the circuitry activated during paternal interactions contains prolactin-responsive neurons in multiple sites, including the medial preoptic nucleus, bed nucleus of the stria terminalis, and medial amygdala. Next, we deleted Prlr from three prominent cell types found in these regions: glutamatergic, GABAergic, and CaMKIIα. Prlr deletion from CaMKIIα, but not glutamatergic or GABAergic cells, had a profound effect on paternal behavior as none of these KO males completed the pup-retrieval task. Prolactin was increased during mating, but not in response to pups, suggesting that the mating-induced secretion of prolactin is important for establishing the switch from infanticidal to paternal behavior. Pharmacological blockade of prolactin secretion at mating, however, had no effect on paternal behavior. In contrast, suppressing prolactin secretion at the time of pup exposure resulted in failure to retrieve pups, with exogenous prolactin administration rescuing this behavior. Together, our data show that paternal behavior in sires is dependent on basal levels of circulating prolactin acting at the time of interaction with pups, mediated through Prlr on CaMKIIα-expressing neurons.
SIGNIFICANCE STATEMENT Parental care is critical for offspring survival. Compared with maternal care, however, the neurobiology of paternal care is less well understood. Here we show that the hormone prolactin, which is most well known for its female-specific role in lactation, has a role in the male brain to promote paternal behavior. In the absence of prolactin signaling specifically during interactions with pups, father mice fail to show normal retrieval behavior of pups. These data demonstrate that prolactin has a similar action in both males and females to promote parental care.
Keywords: parental care, paternal behavior, paternal care, prolactin, prolactin receptor
Introduction
Parental care is critical for offspring survival and is an important component of successful reproduction in many species. While maternal care is the most common form of parental provisioning in mammals, a number of species also show paternal care, with males making significant contributions to the raising of the young. The transition to a parental state involves many significant physiological and behavioral changes. In females, the hormones associated with pregnancy and lactation, such as estradiol, prolactin, and oxytocin, have long been known to facilitate maternal care by acting on complex neural circuits required to display maternal behaviors (for review, see Smiley et al., 2019). The role and timing of hormonal signaling in the expression of paternal care, however, are less well understood.
The anterior pituitary hormone prolactin has a well-defined role in lactation and the establishment of maternal behaviors (for review, see Bridges, 2020). In males, circulating prolactin levels are positively related to paternal care in a number of mammalian species, including humans (for review, see Saltzman and Ziegler, 2014; Bales and Saltzman, 2016), but there has been little causal evidence supporting a role for prolactin in paternal behavior. It has been recently shown that species differences in prolactin secretion dynamics can lead to differences in the expression of paternal care. Tight suppression of prolactin secretion by the tuberoinfundibular dopamine neurons in rats is associated with the absence of paternal care, whereas the less rigid control of prolactin secretion in mice allows for higher basal levels of prolactin, which are associated with high levels of paternal care (Stagkourakis et al., 2020). Furthermore, it was shown that prolactin mediates paternal behavior in male mice via the prolactin receptor (Prlr) in the medial preoptic nucleus (MPN) of the hypothalamus (Stagkourakis et al., 2020), an important regulatory node in the parental brain network (Kohl, 2017). Prlrs are expressed throughout the male mouse brain (Kokay et al., 2018), however, in multiple regions known to be important for parental behavior in both sexes, such as the bed nucleus of the stria terminalis (BNST), periventricular nucleus (PVN), and medial amygdala (MeA) (Kohl et al., 2017). Therefore, the aim of the present study was to better understand how prolactin regulates paternal behavior by determining whether prolactin-responsive neurons throughout the parental regulatory circuits are activated during the expression of paternal behavior, and identifying the broad neuronal subtypes involved in mediating prolactin action on these circuits. Specifically, we aimed to determine whether prolactin's effects are primarily driven by excitatory (i.e., glutamatergic) or inhibitory (i.e., GABAergic) actions from Prlr-expressing neurons.
The second aim of the study was to investigate the temporal dynamics of circulating prolactin to identify critical periods of prolactin exposure for paternal behavior. Virgin male mice are infanticidal and display aggressive behaviors toward pups, but undergo a dramatic transition to pup-directed caregiving behaviors following mating (vom Saal, 1985). The mechanisms behind this remarkable behavioral transition are still unknown. Mating is known to cause a significant rise in circulating prolactin in both humans and rodents (Krüger et al., 2002; Valente et al., 2021), which was previously thought to be involved in the refractory period between ejaculations in males (Brody and Krüger, 2006). However, a recent study has shown that this is not the case in mice (Valente et al., 2021); therefore, the function of the mating-induced rise in prolactin remains unclear. We hypothesized that mating-induced prolactin may serve as a signal to initiate the transition away from infanticidal behavior to paternal care. Alternatively (or additionally), prolactin may be elevated in sires during interaction with pups. In females, prolactin (or its placental homolog, placental lactogen) is high during pregnancy, and is further stimulated by the suckling stimulus during interactions with pups (for review, see Phillipps et al., 2020), but whether pup-induced increases in prolactin occur in sires is unknown. Therefore, we hypothesized that pup interactions would drive increases in circulating prolactin in fathers and that blocking this increase would disrupt paternal behaviors.
Materials and Methods
All procedures were approved by University of Otago Animal Ethics Committee in compliance with the New Zealand Animal Welfare Act.
Animals
Adult C57BL/6J mice were sourced from the Biomedical Research Facility (University of Otago, Dunedin, New Zealand) from stock regularly refreshed from The Jackson Laboratory (IMSR catalog #JAX:000664, RRID:IMSR_JAX:000664). Mice were housed in individually ventilated cages with shredded-paper nesting material and kept in temperature-controlled rooms (22 ± 1°C) on 12:12 h reverse light/dark cycles (lights on at 20:00 h) with ad libitum chow and water. All experiments were conducted during the dark cycle under sodium lighting (which is not detected by rodents) (McLennan and Taylor-Jeffs, 2016). Mice were 8-12 weeks of age when used.
To identify neurons that express the Prlr, we used a transgenic mouse line in which Cre recombinase is expressed under the control of the Prlr promoter. Generation and characterization of these Prlr-IRES-Cre mice have been previously described and result in specific labeling of cells expressing the long form of the Prlr (Kokay et al., 2018; Aoki et al., 2019). Heterozygous Prlr-IRES-Cre mice were crossed with Ai9 Cre-dependent tdtomato reporter mice (B6.Cg-Gt(ROSA)26Sortm9(CAG-tdtomato)Hze/J; IMSR catalog #JAX:007909, RRID:IMSR_JAX:007909, The Jackson Laboratory) (Madisen et al., 2010) to generate Prlr-IRES-Cre/tdtomato reporter mice (Kokay et al., 2018). As current immunohistochemistry methods lack the necessary sensitivity to reliably detect the low abundance Prlr in neurons, this reporter line is an invaluable tool for identifying Prlr-expressing cells in the brain.
To knock out the Prlr in specific subpopulations of neurons (GABA, glutamate, and CaMKIIα), we crossed Prlr lox/lox mice (Brown et al., 2016a) with mice in which Cre recombinase expression is driven by the vesicular GABA transporter promoter (Prlrlox/lox/VGat-Cre mice), the vesicular glutamate transporter 2 promoter (Prlrlox/lox/VGlut-Cre mice), or the CaMKIIα promoter (Prlrlox/lox/CamK-Cre mice) (Vong et al., 2011; Brown et al., 2016a). Prlr deletion in CaMKIIα-expressing neurons is predominantly restricted to the mouse forebrain, with little or no detection observed in the hindbrain (Solà et al., 1999) or outside the brain (Casanova et al., 2001). The pattern of recombination driven by this Cre line has been extensively characterized previously (Casanova et al., 2001), and we have found that Prlr deletion using this line includes subpopulations of both GABAergic and glutamatergic neurons (Brown et al., 2016a; Gustafson et al., 2020). Respective littermate Cre-negative Prlrlox/lox mice for each genotype were used as control animals. As previously described (Brown et al., 2016a), the Prlrlox/lox construct was designed such that the WT exon 5 and an inverted eGFP (functional enhanced green fluorescent protein) reporter are flanked by lox66 and lox71 sites. A Cre-mediated inversion transposes the eGFP into the correct orientation upstream of exon 5 in the Prlr, with its translation stop signal effectively preventing translation of large parts of the Prlr gene (exons 5-10). eGFP can thus be used as marker both for successful recombination and for the normal sites of expression of Prlr, with control (cre-negative Prlrlox/lox) mice showing no eGFP expression, indicative of intact Prlr.
Pup exposure assays
The pup exposure assay used in sires (Experiments 1, 2, 3.2, and 3.3, described below) was based on that used by Tsuneoka et al. (2015). Briefly, virgin males were paired with a novel WT female and cohoused together until day 3 postpartum (pp; the first day pups were observed was counted as day 1 pp). On day 3 pp, males were removed and individually housed in a new cage for 24 h. On the following day (day 4 pp), males were brought into a quiet testing room and allowed to acclimate for 15 min. Cage lids were replaced with clear Plexiglas, and a video camera was placed directly above the cage to record behaviors. Following the acclimation period, 4 pups (age 4 d) from the male's original home cage were placed in his new cage, opposite his nest. Paternal behaviors were recorded for 30 min.
For pup tests using virgin males (Experiments 1 and 3.4), males were separated into individual cages 24 h before testing. On the day of testing, 2 foster pups (3-6 d old) were placed in the cage, opposite of the nest, and behavior was observed for 10 min. All virgin pup tests were observed live; and if males were aggressive toward a pup (potential infanticidal responses), the pups were immediately removed and killed, and testing ceased. If males ignored or showed parental responses toward pups, then pups were removed after completion of the 10 min test and returned to their home cage.
All pup retrieval testing was conducted between 09:00 h and 12:00 h. Control males underwent all the same procedures as pup-exposed males, except that only a hand was briefly placed in the cage, but no pups were added. For all pup exposure tests, the behaviors measured included sniffing pups (nose in contact with part of a pup's body), retrieving pups (picking up pups with their mouth and carrying into the nest), nesting alone (in nest with no pups), or huddling (hovering over at least one pup in the nest). Behaviors were scored from videos using the program BORIS (Friard and Gamba, 2016) using a scan-sampling method every 15 s (Lonstein and Fleming, 2002; Tsuneoka et al., 2015). This method yields similar proportions of time spent engaged in each behavior as when full durations of each behavior are recorded (unpublished data). The number of instances for each behavior was divided by the total number of observations per video., 120 observations for a 30 min video) to give the proportion of time an animal engaged in each behavior. The researcher scoring videos was blind to conditions.
Mating behavior assay
Males were individually housed and tested in their home cage. For mating tests, one sexually receptive female was placed in the male's home cage and mating behavior was both observed live and video recorded. Once a male had ejaculated, testing ceased, and the female was returned to her original home cage. Female stimuli used for all mating tests were reproductively experienced, ovariectomized WT animals, which were brought into receptivity by using a standard protocol of injecting estradiol (0.01 mg injection s.c., dissolved in sesame oil, vol = 0.1 ml, Sigma 815) 48 h prior and progesterone (0.05 mg injection s.c., dissolved in sesame oil, vol = 0.1 ml, Sigma P0130) 4 h before testing (Liu et al., 2020). All females were injected at 09:00 h, and mating behavior tests took place between 13:00 h and 17:00 h. Mating behaviors were scored from videos using the program BORIS (Friard and Gamba, 2016), including latency to first mount and to ejaculate, the number of mounts and intromissions, and duration of each mounting bout. The researcher scoring videos was blind to condition.
Blood sampling and prolactin assay
Whole blood samples were collected from the tail vein of mice following previously described methods (Steyn et al., 2011; Guillou et al., 2015). Mice were habituated to blood sampling procedures by being handled daily in a gentle restraint device (cardboard tube) for 3 weeks before blood collection. At the beginning of the sampling period, the tail tip (<1 mm from the end of the tail) was cut with a sharp scalpel blade, and then the tail was gently squeezed to encourage a drop of blood to form at the site of the cut; 12 µl of whole blood was collected with a pipette at each sampling point and immediately diluted 1:10 in 0.01 m PBS containing 0.05% Tween and 0.2% bovine serum (PBST-BSA) and snap-frozen on dry ice. Samples were stored at −80°C until analysis. Blood prolactin concentrations were measured with an ELISA, which has been described previously (Kirk et al., 2017). Values that were not detectable by the ELISA were assigned a value of 0.1 ng/ml (the limit of detection; range 0.1-20 ng/ml). The interplate coefficient of variation was 2.6% and the intraplate coefficient of variations were 0.23%-2.22%.
Immunofluorescence detection of c-fos
Mice were deeply anesthetized with sodium pentobarbital (100 mg/kg−1; i.p. injection) before transcardial perfusion with 4% PFA in 0.1 mol L−1 PB, pH 7.4. Brains were postfixed in the same fixative overnight before being cryoprotected in 30% sucrose solution for 2 d and stored at −80°C. Brains were cut into three series of 30-µm-thick sections on a freezing microtome and kept in cryoprotectant at −20°C until processing. For immunofluorescence detection of c-fos, the protocol used was adapted from Brown et al. (2019). Briefly, sections were incubated in rabbit anti-cfos primary antibody (rabbit polyclonal Anti-c-fos, 1:5000, Abcam catalog #ab190289, RRID:AB_2737414) for 48 h at 4°C, followed by a 60 min incubation in biotinylated goat anti-rabbit IgG (1:200; Vector Laboratories catalog #BA-1000, RRID:AB_2313606). Sections were then incubated in Vector Elite avidin-biotin-HRP complex (1:100) for 45 min, before a 20 min incubation in Biotin-XX Tyramide (0.3%; Invitrogen). Finally, sections were incubated in a Streptavidin 647 IgG (1:400; AlexaFluor; Invitrogen) for 2 h at 37°C. Images were captured using a Nikon A1 inverted confocal using a 20× objective. Z stacks were collected with images taken 1.4 µm apart. For all runs (including below), control sections that had the primary antibody omitted were included in each batch. No specific staining was observed in any of these sections.
The number of tdtomato-labeled cells (Prlr), c-fos labeled cells, and double-labeled cells (with both tdtomato and c-fos) were manually counted in each ROI using NIS-Elements AR Analysis software, Nikon (NIS-Elements, RRID:SCR_014329) by a researcher blind to conditions. Cell count numbers were divided by the area analyzed (mm2) to calculate the density of cells per region for each animal. Area outlines were drawn based on the stereotaxic mouse brain atlas (Franklin and Paxinos, 2013). One hemisphere per section for each brain region was counted for each animal. Images of compressed z stacks were pseudo-colored magenta and cyan to be color-blind friendly and prepared in FIJI distribution of ImageJ (National Institutes of Health; RRID:SCR_002285).
eGFP immunohistochemistry
To quantify eGFP immunoreactivity across our three Prlrlox/lox models (Experiment 2; see Fig. 4), separate groups of male Prlrlox/lox/CamK-Cre+, Prlrlox/lox/VGlut-Cre+, Prlrlox/lox/VGat-Cre+, and respective littermate Prlrlox/loxCre– controls (n = 6 per group) mice were perfused, and brains were collected (as described above) at 8-12 weeks of age. The protocol performed was as described previously (R. S. Brown et al., 2016a; Kokay et al., 2018) using rabbit polyclonal anti-GFP antibody (1:30,000, Invitrogen catalog #A-6455, RRID:AB_221570, Thermo Fisher Scientific) and biotinylated goat anti-rabbit IgG secondary antibody at 1:200 (Vector Laboratories catalog #BA-1000, RRID:AB_2313606). Images were taken on an Olympus AX70 brightfield microscope using 4× and 10× objectives. One hemisphere per section for each brain area was counted for each animal. Brain ROIs were drawn based on the stereotaxic mouse brain atlas (Franklin and Paxinos, 2013). For all cell counts, the optical densities in each section were thresholded, automatically quantified in ImageJ software (National Institutes of Health; RRID:SCR_003070), and expressed as cell density (number of counted cells per mm2 of the area measured). Image analysis was performed by a researcher blind to conditions.
Figure 4.
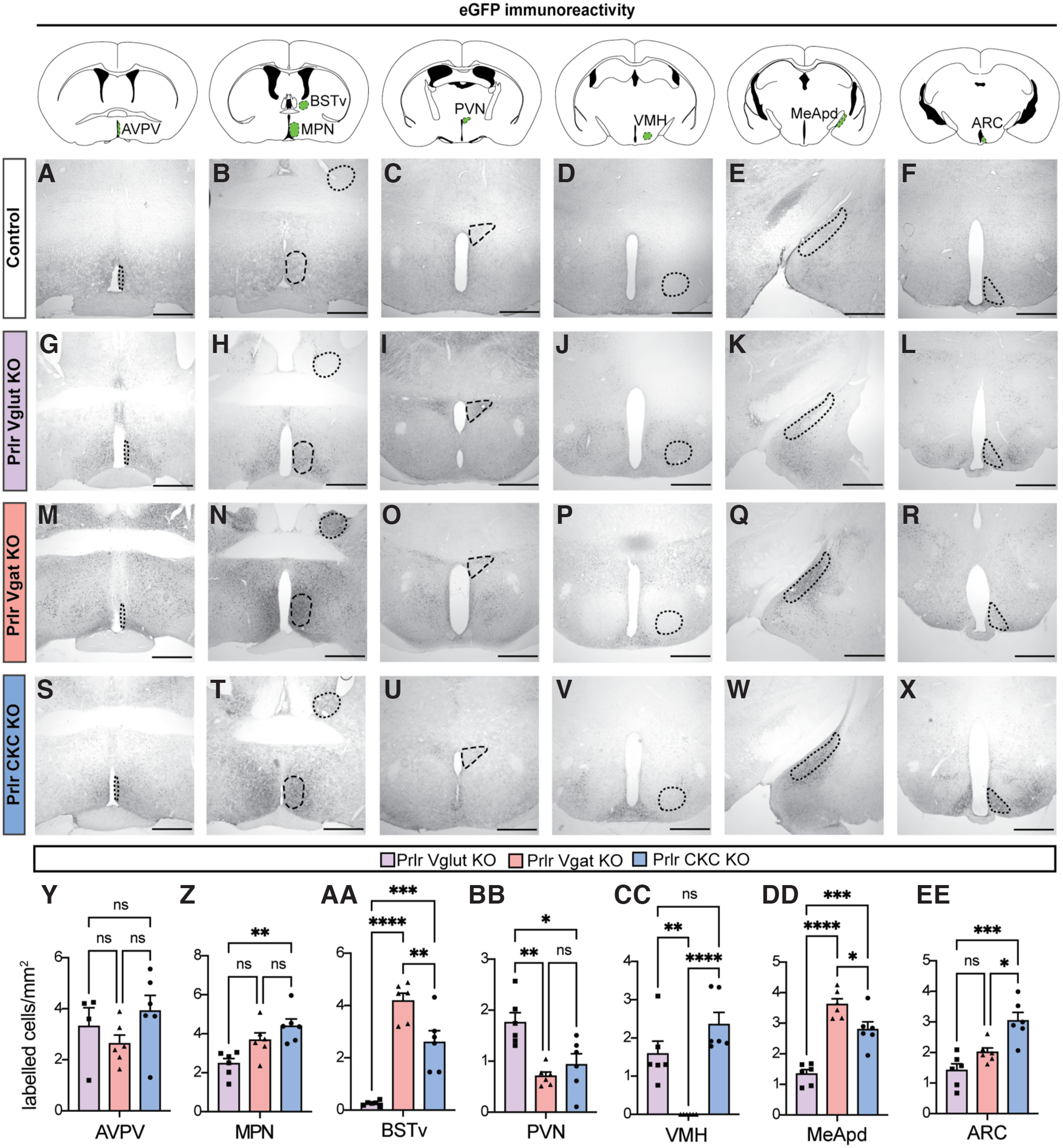
Varying degrees of Prlr deletion are observed in Prlr Vglut, Vgat, and CKC KO males. The Prlrlox/lox mouse model used in this paper was designed such that Cre-mediated inversion deletes the Prlr gene and knocks eGFP into place, meaning that eGFP can be used as marker for successful recombination (e.g., Prlr deletion). We compared eGFP immunoreactivity across the forebrain of male Prlrlox/lox/VGlut-Cre+ (Prlr Vglut KO, purple bars), Prlrlox/lox/VGat-Cre+ (Prlr Vgat KO, orange bars), and Prlrlox/lox/CamK-Cre+ (Prlr CKC KO, blue bars) (n = 6 per group). Colored areas on atlas drawings represent the brain region that was quantified. None of control brains from any group showed eGFP expression in any part of the brain (indicative of no recombination/Prlr deletion; e.g., intact Prlr), so only one set of control brains (Prlr CKC Cre-negative) is shown for comparison (A–F), and was not included in the analysis. Representative images from Prlr Vglut KO (G–L), Prlr Vgat KO (M–R), and Prlr CKC KO (S–X) brains. Y–EE, In all models, there was some Prlr deletion in every brain region examined, but the degree of this was markedly different in each mouse line, reflecting the composition of neuronal subtypes expressed in each region. Bar graphs represent mean ± SEM. ns, Nonsignificant (p > 0.05). *p < 0.05. **p < 0.01. ***p < 0.001. ****p < 0.0001. Scale bars, 50 µm.
pSTAT5 immunohistochemistry
To measure pSTAT5 activity in Prlrlox/lox/CamK-Cre mice (Experiment 2; see Fig. 6), a separate cohort of Prlrlox/lox/CamK-Cre+ and Prlrlox/loxCre– control male (n = 6 per group) were injected with ovine prolactin (5 mg/kg injection i.p., dissolved in PBS/130 mm NaCl, pH 8, National Institutes of Health, National Hormone & Peptide Program) 45 min before perfusion, as in Brown et al. (2010). Immunohistochemical labeling for pSTAT5 was undertaken as previously described (Brown et al., 2010) using Phospho-Stat5 (Tyr694) primary antibody (pSTAT5 Tyr 694, 1:1000; Cell Signaling Technology catalog # 9351, RRID:AB_2315225) and biotinylated goat anti-rabbit IgG secondary antibody at 1:200 (Vector Laboratories catalog #BA-1000, RRID:AB_2313606). Images were collected and analyzed as described for eGFP, above.
Figure 6.
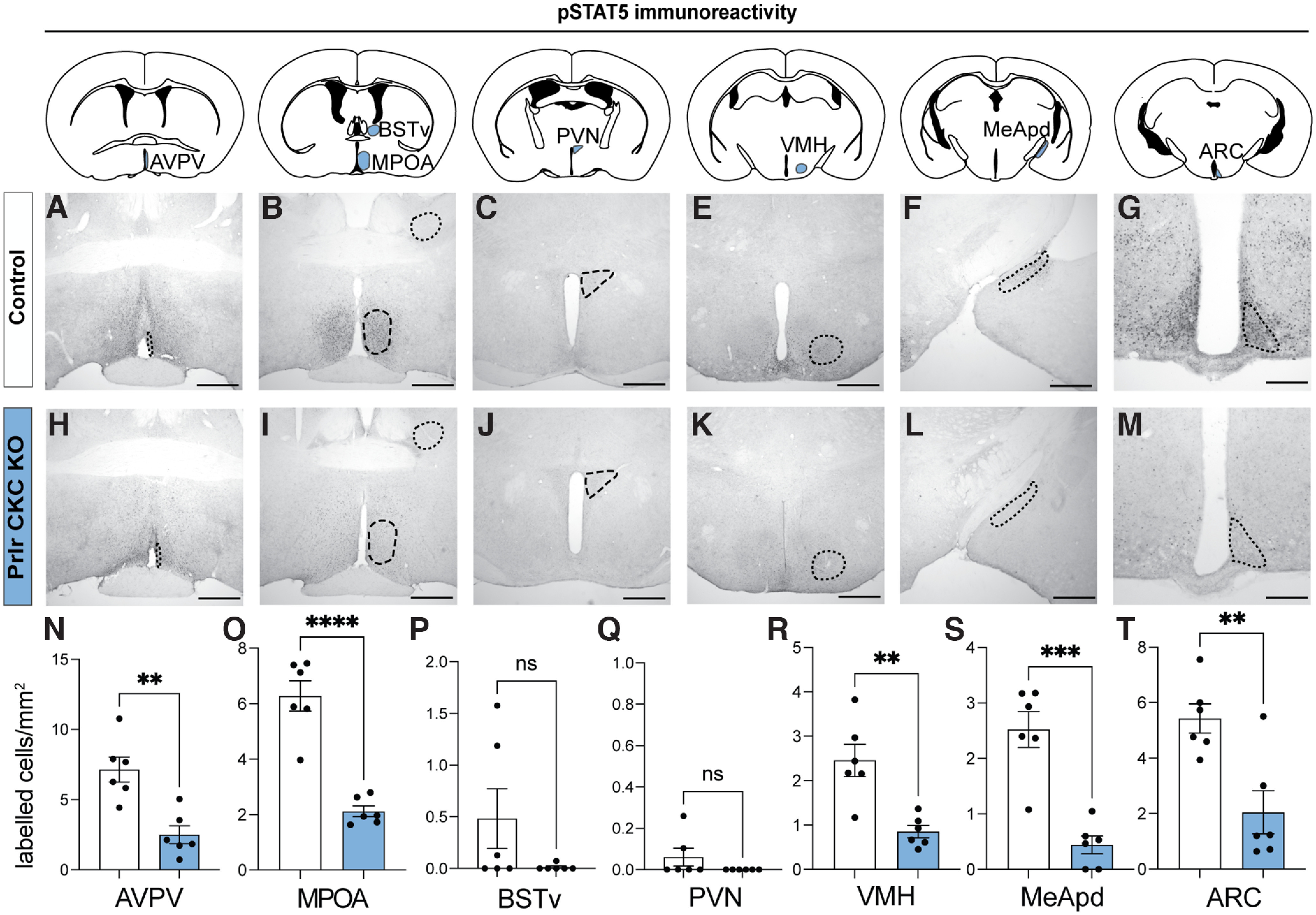
pSTAT5 activity is significantly reduced in Prlr CKC KO males. A–M, Prlr CKC KO males (n = 6) and cre-negative control males (n = 6) were injected with ovine prolactin 45 min before perfusion to assess pSTAT5 (a reliable marker of Prlr activation) immunoreactivity across the forebrain. Colored areas on atlas drawings represent the brain regions that were quantified. Control males (with fully intact Prlr) show a robust response to prolactin (high levels of pSTAT5 immunoreactivity) (A–G), whereas Prlr CKC KO males show markedly reduced levels of pSTAT5 activity in response to prolactin (H–M), confirming significant Prlr deletion in the AVPA, MPN, VMH, MeApd, and ARC (N–T). For all bar graphs, data points represent individual subjects and are presented as mean ± SEM. ns, Nonsignificant (p > 0.05). **p < 0.01. ***p < 0.001. ****p < 0.0001. Scale bars, 50 µm.
Experimental design
Experiment 1: characterizing c-fos expression in Prlr-responsive neurons following pup interactions
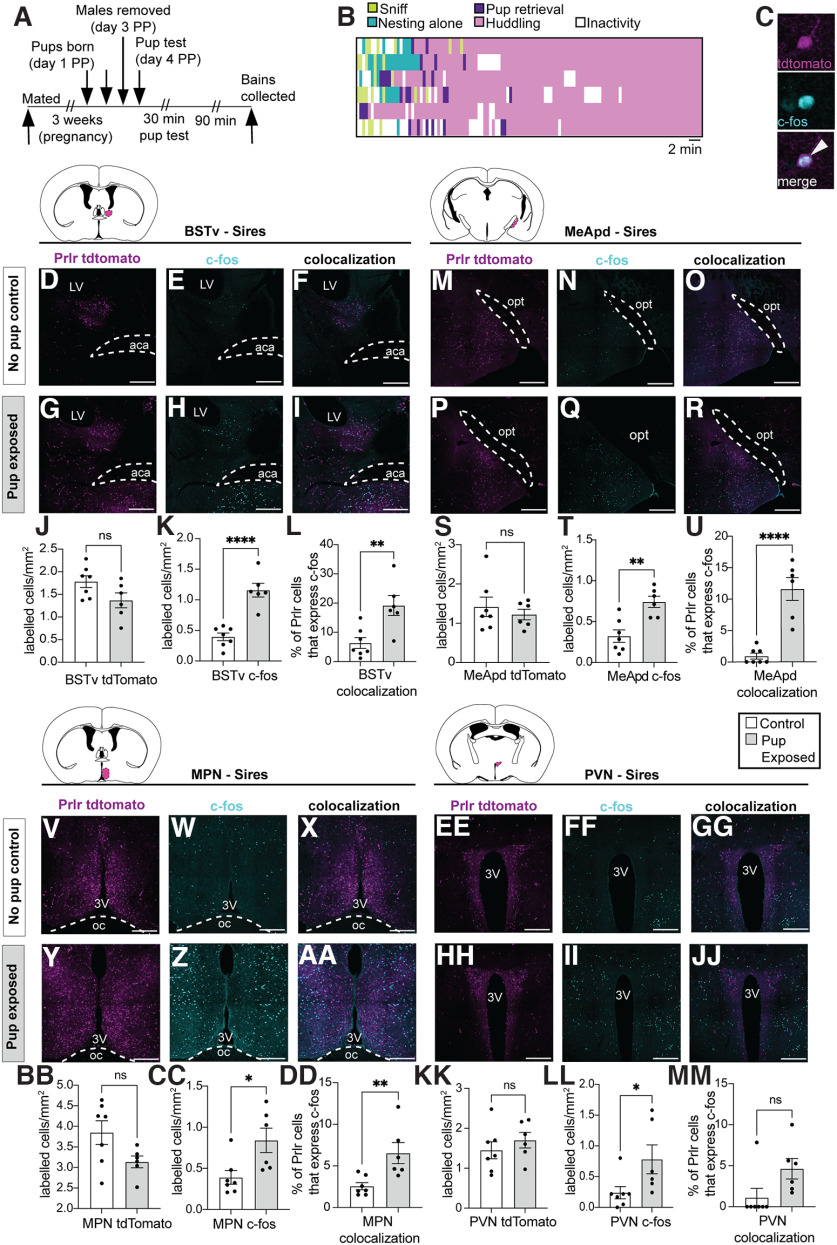
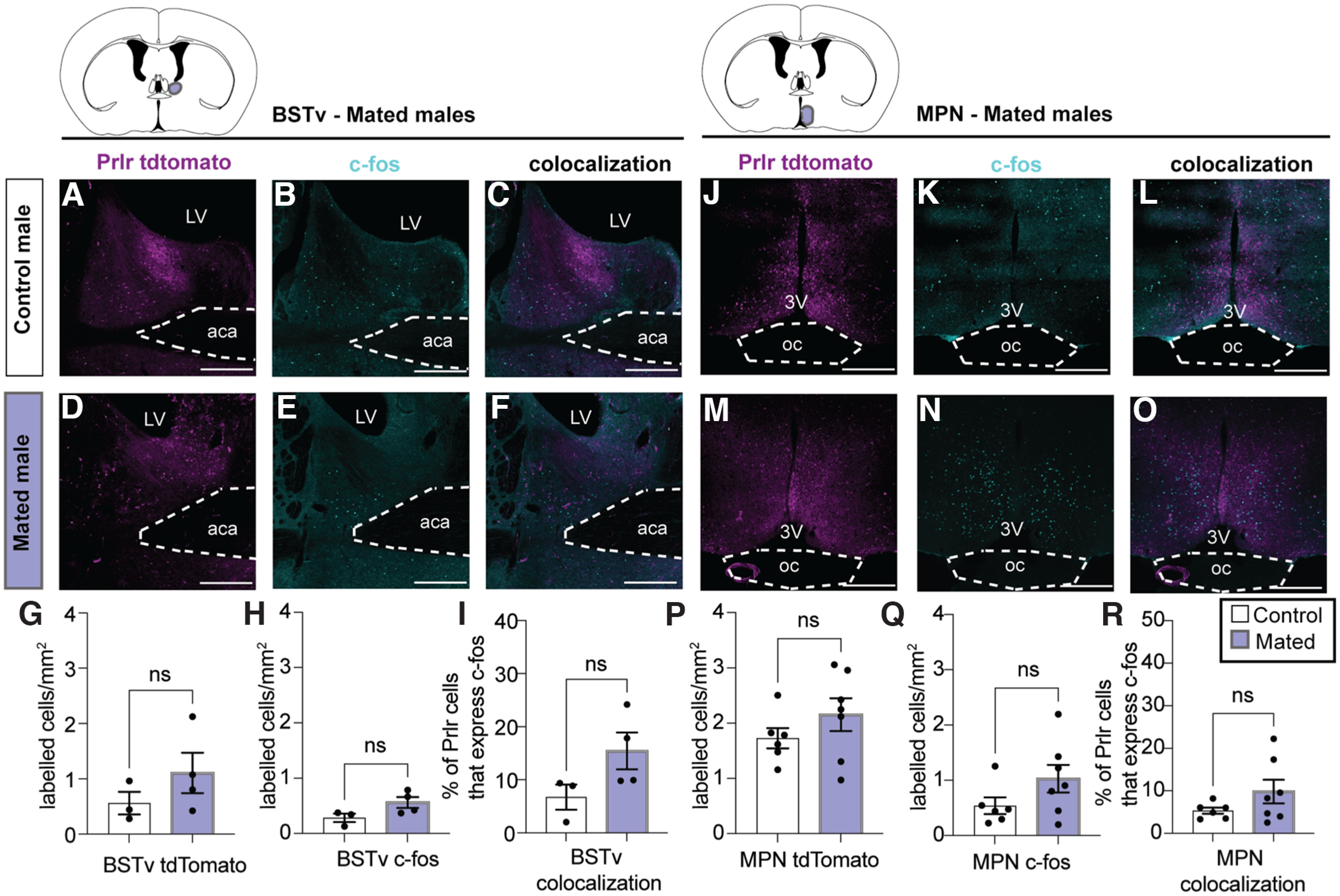
Our first aim was to determine which populations of Prlr-responsive neurons were activated in response to pup interactions in sires. To address this, c-fos immunoreactivity (a marker of recent cellular activation) was measured in Prlr-IRES-Cre-tdtomato reporter mouse sires that were either exposed to pups or received no pup exposure (controls). Paternal behavior was recorded for the first 30 min after pups were placed in the cage (as described above). Males were left undisturbed in cages with pups for an additional 90 min following the test before brains were collected to enable maximal detection of pup-induced c-fos immunoreactivity (Tsuneoka et al., 2015). Six of 7 sires retrieved pups to the nest and huddled over the pups for the majority of the test (see Fig. 1B). One pup-exposed male did not retrieve any pups and therefore was not included in the data analysis. For comparison, virgin Prlr-IRES-Cre/tdtomato reporter males were also exposed to pups to evaluate reproduction-driven changes in neuronal activation in response to pups. Following the 10 min pup assay, pups were removed and males were left in the cage for an additional 90 min before brain collection. For these experiments, c-fos, tdtomato (indicative of Prlr expression), and c-fos+tdtomato colabeled immunoreactivity was quantified in the ventral BNST (BNSTv), posteroventral division of the MeA (MeApd), MPN, and PVN. These brain regions were chosen as they are known to be involved in paternal behavior (Tsuneoka et al., 2015; Bales and Saltzman, 2016) and to express the Prlr (Kokay et al., 2018). Finally, to confirm whether c-fos expression in Prlr-expressing neurons was unique to pup interactions, c-fos was assessed in a separate cohort of male mice following their first mating experience (other social interaction control). Once a male had ejaculated, the female was removed and brains were collected 90 min later. Additional control male brains (that did not mate) were collected at equivalent time points to mated males. c-fos, tdtomato, and c-fos+tdtomato colabeled immunoreactivity was quantified in the MPN and BNSTv.
Figure 1.
Prolactin-responsive neurons in the ventral part of the BNST (BSTv), MeApd, and MPN are activated by pup exposure in sires. Male Prlr-IRES-Cre/tdtomato reporter mice were used to identify pup-induced cell activation (c-fos) in Prlr-containing neurons. A, Schematic of pup retrieval test used to induce c-fos in response to pup interactions. PP, Postpartum. B, Ethogram of paternal behavior during the 30 min pup test. Each pup-exposed male (n = 6) retrieved pups to the nest and spent the majority of the time huddling over pups. C, Representative high-powered images showing a tdtomato (Prlr) labeled cell (magenta), c-fos immunoreactivity (cyan), and the merged image to show an example of a colocalized cell (white arrow). D–R, V–JJ, Representative images of tdtomato labeling (indicative of Prlr; magenta), c-fos labeling (cyan), and colocalization of tdtomato+c-fos (white) in control (top rows) and pup-exposed sires (bottom rows). J–U, BB–MM, corresponding cell counts and comparisons between control and pup exposed males for above images. Colored areas on atlas drawings represent the brain regions examined: BSTv, MeApd, MPN, and PVN. L, U, DD, Pup-exposed males (gray bars) had significantly higher c-fos expression in Prlr-expressing cells in the BSTv, MeApd, and MPN compared with control males (white bars). Bar graphs represent individual data points (black circles) and mean ± SEM. ns, Nonsignificant (p > 0.05). *p < 0.05. **p < 0.01. ****p < 0.0001. 3V, Third ventricle; aca, anterior commissure; oc, optic chiasm. Scale bars, 50 µm.
Experiment 2: effects of neuronal Prlr deletion on paternal behavior in sires
We next wanted to confirm that paternal behavior in mouse sires was (1) dependent on neuronal expression of the Prlr and (2) test whether this effect was mediated by excitatory (i.e., glutamatergic) or inhibitory (i.e., GABAergic) neurons. Three conditional Prlr KO lines were generated (described above) in which Prlr was genetically deleted from glutamatergic (Prlr Vglut KO; Vong et al., 2011; Brown et al., 2017), GABAergic (Prlr Vgat KO; Vong et al., 2011; Brown et al., 2017), or a combined inhibitory/excitatory population of CaMKIIα expressing-forebrain neurons (Prlr CKC KO; Casanova et al., 2001; Brown et al., 2016a). Adult male KOs and littermate Cre-negative Prlrlox/lox control mice were tested as sires for paternal behavior using the pup retrieval test.
Experiment 3: identifying critical periods of prolactin exposure for paternal behavior
Finally, we aimed to identify critical periods of prolactin exposure that may be important for both the mating-induced transition away from infanticidal behavior and the expression of paternal care. These experiments also enabled us to establish whether the effects observed in the above Prlr-neuron KO studies were driven by circulating prolactin (as opposed to any potential source of brain-derived prolactin).
Experiment 3.1: characterizing circulating prolactin levels in males
Using C57/B6J mice, circulating prolactin levels in males were characterized before, during, and after mating, and throughout the pup rearing period. Males were housed in a normal 12:12 light cycle room (lights on at 08:00 h) and blood samples collected at 09:00 h (light phase) and 21:00 h (dark phase). Males were then paired with a WT female. Mating and pregnancies were monitored by daily morning checks for vaginal plugs and female weight gain. Each male was blood sampled between 09:00 h and 10:00 h on days 12, 20, 35, 50, and 60 after mating (day of plug was determined as day 1). Males remained with females and pups during the duration of blood sampling period.
Experiment 3.2: is mating-induced prolactin required for the transition to paternal care?
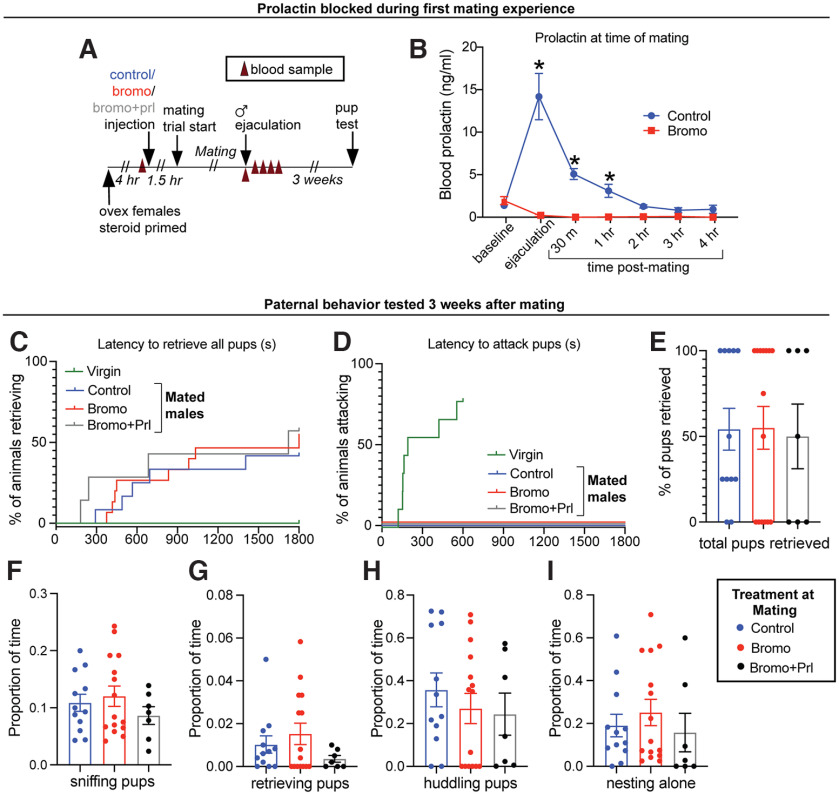
C57BL/6J males were randomly assigned to one of three treatments groups: (1) bromocriptine injection (a D2 agonist, which prevents prolactin secretion from the pituitary, 200 μg injections s.c., vol = 0.3 ml, Sigma B2134; n = 9) (Brown et al., 2010; Valente et al., 2021); 1.5 h before mating; (2) vehicle injection (10% ethanol dissolved in sterile saline; n = 9) 1.5 h before mating; or (3) bromocriptine 1.5 h and ovine prolactin (injections described above) administered 45 min before the mating test (n = 7). Males had a blood sample taken before the bromocriptine/vehicle injection (baseline) and at 30 min and 1, 2, 3, and 4 h after ejaculation to confirm treatment effects. Males remained singly housed after mating until they were tested 20-24 d later (the normal delay between mating and birth of pups) with foster pups (aged 3-5 d). Although males were not cohoused with females, which differs from pup retrieval task paradigm described above, C57BL/6J mice will still undergo that mating-induced suppression of infanticide and onset of paternal care with mating alone (vom Saal, 1985; Brown, 1993). Importantly, unlike some other rodents, paternal care is not reliant on the prior cohousing with a pregnant female and/or pups in mice, although there can be potential additive effects of cohousing (Brown, 1993). In addition, mated male laboratory mice do not differentiate between their own and foster pups and as sires, will readily retrieve either to the nest (Alsina-Llanes and Olazábal, 2018). Blood samples were collected 1 h before (pre-pup sample) and immediately following the pup exposure test (post-pup sample). For this pup retrieval test, males were presented with 2 foster pups. Although the pup retrieval tests generally uses 4 pups, only 2 pups were used based on ethical concerns, to avoid risk to additional pups (Lonstein et al., 2002) if infanticidal behavior was not suppressed following treatment. To confirm that bromocriptine had no effects on mating behavior, a separate group of C57BL/6J males were injected with bromocriptine (n = 9) or vehicle (n = 8) 1.5 h before the mating assay and sexual behaviors were quantified as described above.
Experiment 3.3: is circulating prolactin required to show paternal behavior in sires?
C57BL/6J males were mated and cohoused with the female until day 3 pp, when they were individually caged. On the morning of testing (day 4 pp), males had a blood sample taken (pre-pup sample) before receiving either a bromocriptine (n = 8) or vehicle (n = 6) injection 1.5 h before the pup test, or bromocriptine 1.5 h and ovine prolactin 45 min before the pup test (n = 6) (injection details outlined above). 4 pups (age 4 d) from the male's original home cage were used as pup stimuli for the pup exposure assay. A second blood sample was taken immediately following the 30 min pup exposure test (post-pup sample).
Experiment 3.4: does prolactin affect pup-directed behavior in virgin males?
C57BL/6J virgin males were injected with prolactin or vehicle either 45 min before a pup exposure assay (acute effects of prolactin; n = 7 per group) or 3 weeks before the pup test (delayed effects of prolactin, n = 10 per group). To verify that prolactin is not required for infanticidal behavior, virgin C57BL/6J males were either injected with bromocriptine (as described above, n = 9) or vehicle (n = 9) for 1.5 h before the virgin pup test.
Statistical analysis
No statistical methods were used to predetermine sample sizes, but our sample sizes are similar to those reported in previous publications (e.g., Wu et al., 2014; Brown et al., 2017). Data were analyzed using PRISM 8 and 9 (GraphPad Prism, RRID:SCR_002798). In all cases, data were checked for normality, all tests were two-tailed, and significance was accepted if p values were <0.05. All statistical analyses, including group means/medians, SEM, 95% CIs, and R2 squared (η2) values for effect size estimates are provided in Tables 1–11, with the corresponding figure panels listed.
Table 1.
Statistical test, parameters, and outcomes for each data panel in Figure 1
| Corresponding panel | Test | Brain region | Parameter | t | df | p | Control group (mean) | Pup-exposed group (mean) | SEM | 95% CI | R2 (η2) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| J | t test | BSTv | tdTomato (no. of labeled cells/mm2) | 1.99 | 11.00 | 0.072 | 1.78 | 1.37 | 0.21 | −0.872 to 0.044 | 0.26 |
| K | t test | BSTv | c-fos (no. of labeled cells/mm2) | 6.23 | 11.00 | <0.001 | 0.40 | 1.16 | 0.12 | 0.493-1.031 | 0.78 |
| L | t test | BSTv | % of Prlr cells that express c-fos | 3.43 | 11.00 | 0.006 | 6.37 | 19.19 | 3.74 | 4.583-21.060 | 0.52 |
| S | t test | MeApd | tdTomato (no. of labeled cells/mm2) | 0.66 | 11.00 | 0.524 | 1.42 | 1.22 | 0.29 | −0.842 to 0.454 | 0.04 |
| T | t test | MeApd | c-fos (no. of labeled cells/mm2) | 4.04 | 11.00 | 0.002 | 0.32 | 0.74 | 0.10 | 0.191-0.648 | 0.60 |
| U | t test | MeApd | % of Prlr cells that express c-fos | 6.11 | 11.00 | <0.001 | 0.93 | 11.59 | 1.75 | 6.813-14.49 | 0.77 |
| BB | t test | MPN | tdTomato (no. of labeled cells/mm2) | 2.11 | 11.00 | 0.059 | 3.85 | 3.14 | 0.34 | −1.456 to 0.031 | 0.29 |
| CC | t test | MPN | c-fos (no. of labeled cells/mm2) | 2.75 | 11.00 | 0.019 | 0.39 | 0.84 | 0.16 | 0.090-0.811 | 0.41 |
| DD | t test | MPN | % of Prlr cells that express c-fos | 3.16 | 11.00 | 0.009 | 2.56 | 6.54 | 1.26 | 1.209-6.742 | 0.48 |
| KK | t test | PVN | tdTomato (no. of labeled cells/mm2) | 0.87 | 11.00 | 0.403 | 1.45 | 1.70 | 0.29 | −0.388 to 0.895 | 0.06 |
| LL | t test | PVN | c-fos (no. of labeled cells/mm2) | 2.40 | 11.00 | 0.046 | 0.24 | 0.78 | 0.24 | 0.012-1.073 | 0.32 |
| MM | t test | PVN | % of Prlr cells that express c-fos | 2.10 | 11.00 | 0.060 | 1.12 | 4.64 | 1.68 | −0.171 to 7.208 | 0.29 |
Table 11.
Statistical test, parameters, and outcomes for each data panel in Figure 11
| Corresponding panel | Test | Parameter | z score | p | Control group (proportion) | Prolactin (acute) group (proportion) | |||
|---|---|---|---|---|---|---|---|---|---|
| A | Two proportion z test | Proportion of males with attack response | −1.08 | 0.28 | 0.29 | 0.57 | — | — | — |
| Two proportion z test | Proportion of males with ignore response | 0.00 | 1.00 | 0.43 | 0.43 | — | — | — | |
| Two proportion z test | Proportion of males with paternal response | 1.53 | 0.13 | 0.29 | 0.00 | — | — | — |
| Test | Parameter | χ2 | df | p | Control group (median latency) | Median latency - Prolactin (acute) group | Hazard ratio (Mantel–Haenszel) | 95% CI of ratio | |
|---|---|---|---|---|---|---|---|---|---|
| B | Survival analysis/Mantel–Cox Log-rank test | Latency to attack pups: acute effects of prolactin | 1.11 | 1 | 0.29 | undefined | 222.00 | 0.42 | 0.081-2.133 |
| C | Survival analysis/Mantel–Cox Log-rank test | Latency to retrieve pups: acute effects of prolactin | 2.16 | 1 | 0.14 | undefined | undefined | 8.03 | 0.500-128.900 |
| Test | Parameter | z score | p | Control group (proportion) | Prolactin (delayed) group (proportion) | ||||
|---|---|---|---|---|---|---|---|---|---|
| D | Two proportion z test | Proportion of males with attack response | −1.79 | 0.07 | 0.30 | 0.70 | — | — | — |
| Two proportion z test | Proportion of males with ignore response | 0.91 | 0.36 | 0.50 | 0.30 | — | — | — | |
| Two proportion z test | Proportion of males with paternal response | 1.49 | 0.14 | 0.20 | 0.00 | — | — | — |
| Test | Parameter | χ2 | df | p | Control group (median latency) | Prolactin (delayed) group (median latency) | Hazard ratio (Mantel–Haenszel) | 95% CI of ratio | |
|---|---|---|---|---|---|---|---|---|---|
| E | Survival analysis/Mantel–Cox Log-rank test | Latency to attack pups: delayed effects of prolactin | 3.71 | 1 | 0.05 | undefined | 150.00 | 0.27 | 0.071-1.023 |
| F | Survival analysis/Mantel–Cox Log-rank test | Latency to retrieve pups: delayed effects of prolactin | 2.11 | 1 | 0.15 | undefined | undefined | 7.81 | 0.487-125.100 |
| Test | Parameter | z score | p | Control group (proportion) | Treated group (proportion) | ||||
|---|---|---|---|---|---|---|---|---|---|
| G | Two proportion z test | Proportion of males with attack response | 0.53 | 0.60 | 0.78 | 0.67 | — | — | — |
| Two proportion z test | Proportion of males with ignore response | −0.53 | 0.60 | 0.22 | 0.33 | — | — | — |
| Test | Parameter | χ2 | df | p | Control group (median latency) | Bromo group (median latency) | Hazard ratio (Mantel–Haenszel) | 95% CI of ratio | |
|---|---|---|---|---|---|---|---|---|---|
| H | Survival analysis/Mantel–Cox Log-rank test | Latency to attack pups: effects of bromocriptine | 0.36 | 1 | 0.55 | 191.00 | 378.00 | 1.42 | 0.455-4.426 |
For Experiments 1-3, all analyses between two groups (control and treated/KO groups) were conducted with t tests if data were normally distributed or Mann–Whitney U tests if data were not normally distributed (see Tables 1–11). In Experiments 2 and 3, group differences in the latency to retrieve pups, latency to attack pups, latency to first mount a female, and latency to first ejaculate were analyzed using survival analysis and curve comparison with the Mantel–Cox Log-rank test (Swart et al., 2021). Latency to attack pups in Experiment 3.2 (see Fig. 8D) was not analyzed as no mated males performed this behavior. Hazard ratios (Mantel–Haenszel tests) and 95% CIs are reported as an estimate of the effect size for the comparison of latency data.
Figure 8.
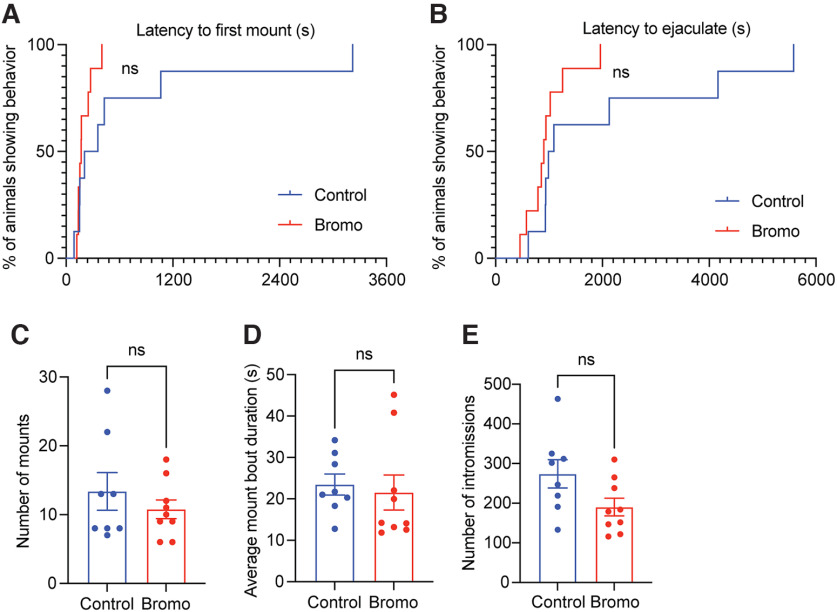
Mating-induced prolactin surge is not required to show subsequent paternal behaviors in mated males. A, B, C57BL/6J male mice were treated with either bromocriptine (bromo) to prevent prolactin secretion (B; n = 15) or vehicle-control (n = 12) 1.5 h prior, or bromocriptine 1.5 h and ovine prolactin (prl) 45 min before their first mating experience (n = 7). This treatment did not affect mating behavior (see Fig. 9; Table 9). C–I, Paternal behaviors were assessed 3 weeks later (the normal length between mating and birth of pups) using a pup retrieval assay. C, D, Neither bromocriptine nor bromocriptine+prolactin treatment at mating caused an effect on latency to retrieve pups when tested 3 weeks later, nor did it cause males to retain infanticidal behavior after mating. Virgin data are from Figure 11G,H and were included in C, D for visual comparison but were not included in the data analysis. As expected, mated males from all three treatment groups performed pup retrieval behavior, in contrast to virgin males that do not show retrieval behavior (C). Similarly, no mated males in any treated group showed any infanticidal behavior, whereas most virgin males rapidly show infanticidal behavior (D). Bromocriptine or bromocriptine+prolactin had no effect on any other paternal behaviors measured (E–I). ovex, Ovariectomized.
In Experiment 2, group differences in eGFP-labeled cell densities between the three Prlr KO models (Prlr CKC, Vglut, and Vgat KO males) were analyzed using a one-way ANOVA. As Cre-negative Prlrlox/lox mice (with intact Prlr) show no eGFP immunoreactivity in the brain, eGFP was only quantified and compared in Cre+ males. Pairwise comparisons between models were conducted using Tukey's multiple comparisons test. Differences in eGFP-labeled cell densities between the three KO models were analyzed for each brain region separately (Table 4).
Table 4.
Statistical test, parameters, and outcomes for each data panel in Figure 4
| Corresponding panel | Test | Brain region | F (DFn, DFd) | p | R2 (η2) | Tukey's multiple comparisons test: GFP (no. of labeled cells/mm2) | Mean difference | 95% CI | Adjusted p |
|---|---|---|---|---|---|---|---|---|---|
| Y | One-way ANOVA | AVPV | F(2,13) = 1.580 | 0.243 | 0.20 | Prlr CKC KO vs Prlr Vglut KO | 0.61 | −1.533 to 2.743 | 0.740 |
| AVPV | Prlr CKC KO vs Prlr Vgat KO | 1.29 | −0.625 to 3.199 | 0.216 | |||||
| AVPV | Prlr Vglut KO vs Prlr Vgat KO | 0.68 | −1.456 to 2.820 | 0.685 | |||||
| Z | One-way ANOVA | MPN | F(2,15) = 8.719 | 0.003 | 0.54 | Prlr CKC KO vs Prlr Vglut KO | 1.93 | 0.717-3.146 | 0.002 |
| MPN | Prlr CKC KO vs Prlr Vgat KO | 0.72 | −0.492 to 1.936 | 0.299 | |||||
| MPN | Prlr Vglut KO vs Prlr Vgat KO | −1.21 | −2.424 to 0.005 | 0.051 | |||||
| AA | One-way ANOVA | BSTv | F(2,15) = 41.06 | <0.001 | 0.85 | Prlr CKC KO vs Prlr Vglut KO | 2.34 | 1.213-3.473 | <0.001 |
| BSTv | Prlr CKC KO vs Prlr Vgat KO | −1.58 | −2.705 to −0.445 | 0.007 | |||||
| BSTv | Prlr Vglut KO vs Prlr Vgat KO | −3.92 | −5.048 to −2.788 | <0.001 | |||||
| BB | One-way ANOVA | PVN | F(2,15) = 10.45 | 0.001 | 0.58 | Prlr CKC KO vs Prlr Vglut KO | −0.82 | −1.450 to −0.194 | 0.010 |
| PVN | Prlr CKC KO vs Prlr Vgat KO | 0.23 | −0.399 to 0.857 | 0.620 | |||||
| PVN | Prlr Vglut KO vs Prlr Vgat KO | 1.05 | 0.423-1.679 | 0.002 | |||||
| CC | One-way ANOVA | VMH | F(2,15) = 21.26 | <0.001 | 0.74 | Prlr CKC KO vs Prlr Vglut KO | 0.77 | −0.186 to 1.730 | 0.125 |
| VMH | Prlr CKC KO vs Prlr Vgat KO | 2.36 | 1.400-3.316 | <0.001 | |||||
| VMH | Prlr Vglut KO vs Prlr Vgat KO | 1.59 | 0.628-2.544 | 0.002 | |||||
| DD | One-way ANOVA | MeApd | F(2,15) = 34.44 | <0.001 | 0.82 | Prlr CKC KO vs Prlr Vglut KO | 1.46 | 0.735-2.174 | <0.001 |
| MeApd | Prlr CKC KO vs Prlr Vgat KO | −0.81 | −1.535 to −0.095 | 0.026 | |||||
| MeApd | Prlr Vglut KO vs Prlr Vgat KO | −2.27 | −2.989 to −1.550 | <0.001 | |||||
| EE | One-way ANOVA | ARC | F(2,15) = 14.71 | <0.001 | 0.66 | Prlr CKC KO vs Prlr Vglut KO | 1.62 | 0.837-2.412 | <0.001 |
| ARC | Prlr CKC KO vs Prlr Vgat KO | 1.04 | 0.248-1.824 | 0.010 | |||||
| ARC | Prlr Vglut KO vs Prlr Vgat KO | −0.59 | −1.376 to 0.199 | 0.162 |
For Experiment 3.1, differences in blood prolactin concentration within the same animals (e.g., between the light and dark phase in virgin males and before and after pup test) were analyzed using a paired t test. Blood prolactin concentrations measured across time after mating were analyzed using a repeated-measures ANOVA. Post hoc comparisons between groups (time of sample after mating) were compared with the baseline sample group (before mating) using Dunnett's test to correct for multiple comparisons. The number of missing blood samples (because of inability to collect sufficient quantities of blood) were as follows: day 12 (n = 3), day 20 (n = 1), day 50 (n = 1), and day 60 (n = 1). Blood prolactin concentrations across the days after mating were analyzed using a repeated-measures ANOVA. Post hoc comparisons between groups (day of sample after mating) were analyzed using Tukey's test to correct for multiple comparisons.
For Experiment 3.2, differences in blood prolactin concentrations following ejaculation between bromocriptine and vehicle control groups following mating were analyzed using a two-way repeated-measures ANOVA, with post hoc comparisons made using Bonferroni's test to correct for multiple comparisons. Blood prolactin concentrations before and after pup exposure test (that occurred 3 weeks after mating) were analyzed using a paired t test. Sufficient blood samples could not be collected at both time points for 1 control male and 3 bromocriptine-treated males. Differences in paternal behavior between control, bromocriptine, and bromocriptine+prolactin-treated groups were analyzed using a one-way ANOVA for each behavior separately. Post hoc comparisons between the treated groups were compared with the vehicle control group using Dunnett's multiple comparisons test.
For Experiment 3.3, differences in paternal behavior between control, bromocriptine, and bromocriptine+prolactin groups were analyzed using the Kruskal–Wallis test followed by Dunn's multiple comparisons post hoc test. Blood prolactin concentrations before and after pup exposure test were analyzed using a paired t test. A sufficient amount of blood could not be collected at both time points from 1 control male and 1 bromocriptine-treated male. Correlational analyses between circulating prolactin concentrations and paternal behavior were conducted for each behavior using Pearson's R test.
For Experiment 3.4, the proportion of virgin males showing attacking, ignore, or paternal responses was compared between treated males (acute prolactin, delayed prolactin, or bromocriptine) and their respective vehicle-treated controls using two proportion z tests.
Results
Experiment 1: pup-induced activation of prolactin-responsive neurons in sires differs from pup-exposed virgin males and recently mated males
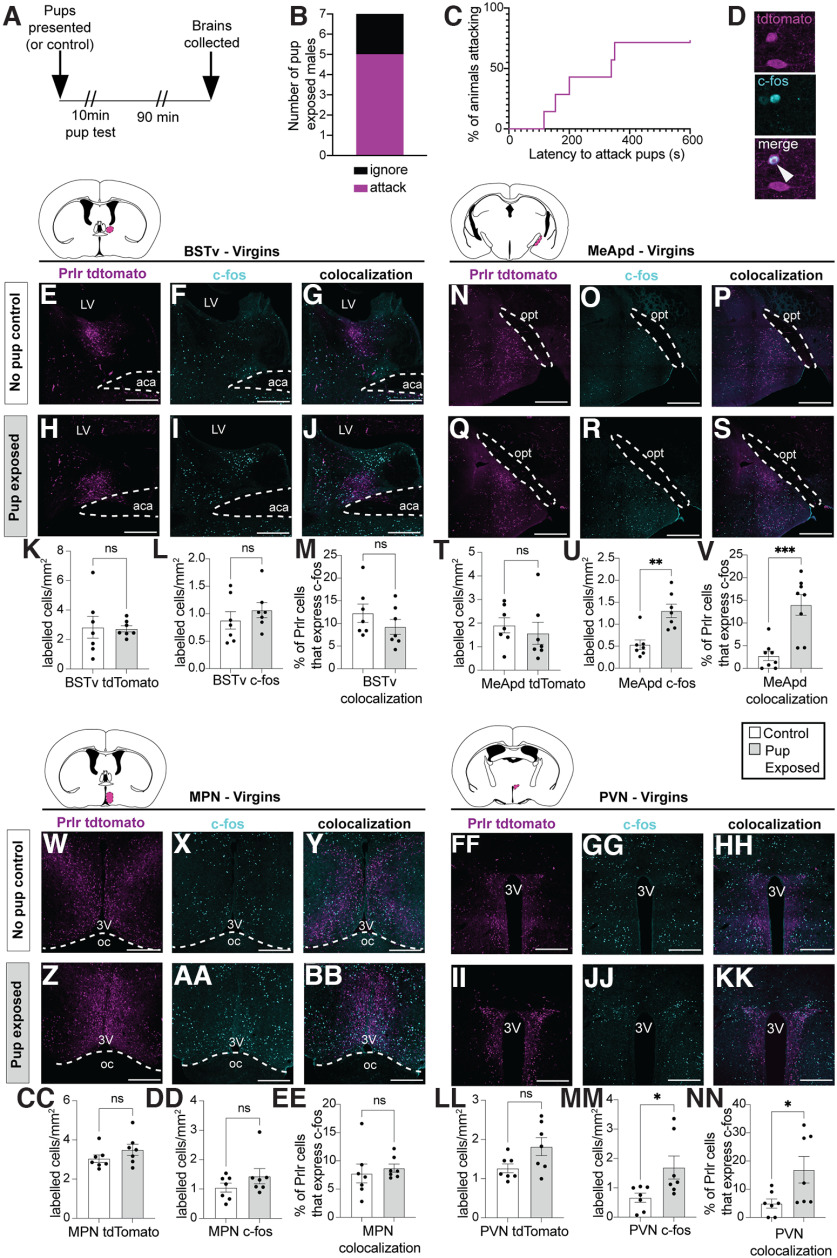
Our first aim was to identify sites within the neural circuitry that are activated during paternal interactions that contained prolactin-responsive neurons using Prlr-IRES-Cre-tdtomato reporter mouse sires. Consistent with previous reports, pup-induced increases in c-fos immunoreactivity were observed in the BNSTv, MeApd, MPN, and PVN relative to nonexposed control animals (Fig. 1K,T,CC,LL; Table 1). A significant portion of prolactin-responsive cells were activated in response to paternal interactions, with pup-exposed fathers showing a 2- to 4-fold increase in c-fos expression in Prlr-expressing cells in the BNSTv (Fig. 1L) and MPN (Fig. 1DD), with the MeApd (Fig. 1U) showing a 12-fold increase over the very low levels of c-fos seen in controls (Table 1). In comparison, pup-exposed Prlr-IRES-Cre-tdtomato virgin males, which are generally aggressive toward pups, showed significant pup exposure-induced c-fos immunoreactivity in Prlr-containing cells in the MeApd and PVN (Fig. 2V,NN; Table 2). However, unlike sires, there was no significant pup-induced c-fos in Prlr-containing cells in the MPN or BNSTv in virgin males (Fig. 2M,EE; Table 2) or in recently mated males (Fig. 3I,R; Table 3). Together, these data show that, although Prlr-expressing neurons in the MeApd respond to pups in both virgins and sires, activation of Prlr-expressing neurons in the MPN and BNSTv only occurs after pup interactions in sires.
Figure 2.
Prlr containing neurons in the PVN and MeApd are activated by pup exposure in virgin mice. A, Schematic of pup exposure assay used with virgin Prlr-IRES-Cre-tdtomato males (n = 7 per group). B, Five of the 7 pup-exposed males tested attacked pups, while 2 males ignored pups. C, Latency to attack pups across all males. Males were only exposed to pups for 10 min or until attacking occurred, in which case, pups were immediately removed and testing ceased. D, Representative high-powered image showing a tdtomato/Prlr-labeled cell (magenta), c-fos immunoreactivity (cyan), and the merge of the two images to show an example of a colocalized cell (white arrow). E–S, W–KK, Representative images of tdtomato labeling (indicative of Prlr; magenta), c-fos labeling (cyan), and colocalization of tdtomato+c-fos are shown for each brain region examined in control virgins (top rows) and pup-exposed virgins (bottom rows). K–V, CC–NN, corresponding cell counts and comparisons between control and pup exposed males for above images. Colored areas on atlas drawings represent the brain regions examined: BSTv, MeApd, MPN, and PVN. Pup-exposed virgin males (gray bars) had significantly more c-fos expression in Prlr-expressing cells compared with control males (white bars) in the MeApd and PVN, but not the BSTv or MPN. Bar graphs represent individual data points (black circles) and mean ± SEM. ns, Nonsignificant (p > 0.05). *p < 0.05. **p < 0.01. ***p < 0.001. 3V, Third ventricle; aca, anterior commissure; oc, optic chiasm. Scale bars, 50 µm.
Table 2.
Statistical test, parameters, and outcomes for each data panel in Figure 2
| Corresponding panel | Test | Brain region | Parameter | t | df | p | Control group (mean) | Pup-exposed group (mean) | SEM | 95% CI | R2 (η2) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| K | t test | BSTv | tdTomato (no. of labeled cells/mm2) | 0.12 | 12.00 | 0.904 | 2.83 | 2.74 | 0.77 | −1.776 to 1.586 | 0.00 |
| L | t test | BSTv | c-fos (no. of labeled cells/mm2) | 0.89 | 12.00 | 0.393 | 0.88 | 1.06 | 0.21 | −0.271 to 0.641 | 0.06 |
| M | t test | BSTv | % of Prlr cells that express c-fos | 1.16 | 12.00 | 0.269 | 12.25 | 9.24 | 2.60 | −8.672 to 2.653 | 0.10 |
| T | t test | MeApd | tdTomato (no. of labeled cells/mm2) | 0.60 | 12.00 | 0.561 | 1.91 | 1.57 | 0.57 | −1.577 to 0.898 | 0.03 |
| U | t test | MeApd | c-fos (no. of labeled cells/mm2) | 4.10 | 12.00 | 0.002 | 0.53 | 1.30 | 0.19 | 0.362-1.183 | 0.58 |
| V | t test | MeApd | % of Prlr cells that express c-fos | 5.26 | 12.00 | <0.001 | 2.65 | 15.35 | 2.42 | 7.444-17.970 | 0.70 |
| CC | t test | MPN | tdTomato (no. of labeled cells/mm2) | 1.31 | 12.00 | 0.214 | 3.05 | 3.50 | 0.34 | −0.298 to 1.201 | 0.13 |
| DD | t test | MPN | c-fos (no. of labeled cells/mm2) | 1.28 | 12.00 | 0.225 | 1.05 | 1.44 | 0.30 | −0.272 to 1.044 | 0.12 |
| EE | t test | MPN | % of Prlr cells that express c-fos | 0.52 | 12.00 | 0.610 | 7.76 | 8.72 | 1.82 | −3.018 to 4.927 | 0.02 |
| LL | t test | PVN | tdTomato (no. of labeled cells/mm2) | 2.23 | 12.00 | 0.046 | 0.06 | 0.34 | 0.12 | 0.006-0.5423 | 0.29 |
| MM | t test | PVN | c-fos (no. of labeled cells/mm2) | 2.43 | 12.00 | 0.032 | 0.66 | 1.69 | 0.42 | 0.107-1.950 | 0.33 |
| NN | t test | PVN | % of Prlr cells that express c-fos | 2.41 | 12.00 | 0.033 | 4.95 | 16.89 | 4.96 | 1.145-22.750 | 0.33 |
Figure 3.
Prlr-containing neurons are not activated during mating in the BSTv or MPN. c-fos was assessed in the BSTv and MPN of Prlr-IRES-Cre-tdtomato reporter males following their first mating experience compared with control males who were not exposed to a female. A–O, Representative images from control males (top row) and mated males (bottom row) of tdtomato-labeled cells (magenta, indicative of a Prlr-expressing cell), c-fos (cyan), and the number of colocalized (tdtomato + c-fos, white). There were no differences in the density of tdtomato-labeled cells (G,P), c-fos-labeled cells (H,Q), or the percentage of Prlr cells expressing c-fos in the BSTv or MPN (I,R) between controls (white bars) and mated males (purple bars). Bar graphs represent individual data points (black circles) and mean ± SEM. ns, Nonsignificant (p > 0.05). LV, Lateral ventricle; 3V, third ventricle; aca, anterior commissure; oc, optic chiasm. Scale bars, 50 µm.
Table 3.
Statistical test, parameters, and outcomes for each data panel in Figure 3
| Corresponding panel | Test | Brain region | Parameter | t | df | p | Control group (mean) | Mated group (mean) | SEM | 95% CI | R2 (η2) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| G | t test | BSTv | tdTomato (no. of labeled cells/mm2) | 1.18 | 5.00 | 0.293 | 0.56 | 1.11 | 0.46 | −0.649 to 1.740 | 0.22 |
| H | t test | BSTv | c-fos (no. of labeled cells/mm2) | 2.08 | 5.00 | 0.092 | 0.28 | 0.56 | 0.13 | −0.065 to 0.623 | 0.46 |
| I | t test | BSTv | % of Prlr cells that express c-fos | 1.91 | 5.00 | 0.115 | 6.76 | 15.45 | 4.55 | −3.020 to 20.390 | 0.42 |
| P | t test | MPN | tdTomato (no. of labeled cells/mm2) | 1.18 | 11.00 | 0.264 | 1.73 | 2.16 | 0.36 | −0.372 to 1.226 | 0.11 |
| Q | t test | MPN | c-fos (no. of labeled cells/mm2) | 1.60 | 11.00 | 0.138 | 0.54 | 1.03 | 0.31 | −0.185 to 1.162 | 0.19 |
| R | t test | MPN | % of Prlr cells that express c-fos | 1.43 | 11.00 | 0.180 | 5.33 | 9.79 | 3.11 | −2.391 to 11.310 | 0.16 |
Experiment 2: Prlr KO in forebrain CaMKIIα-expressing neurons, but not Vgat+ or Vglut2+ expressing neurons, impairs paternal behavior
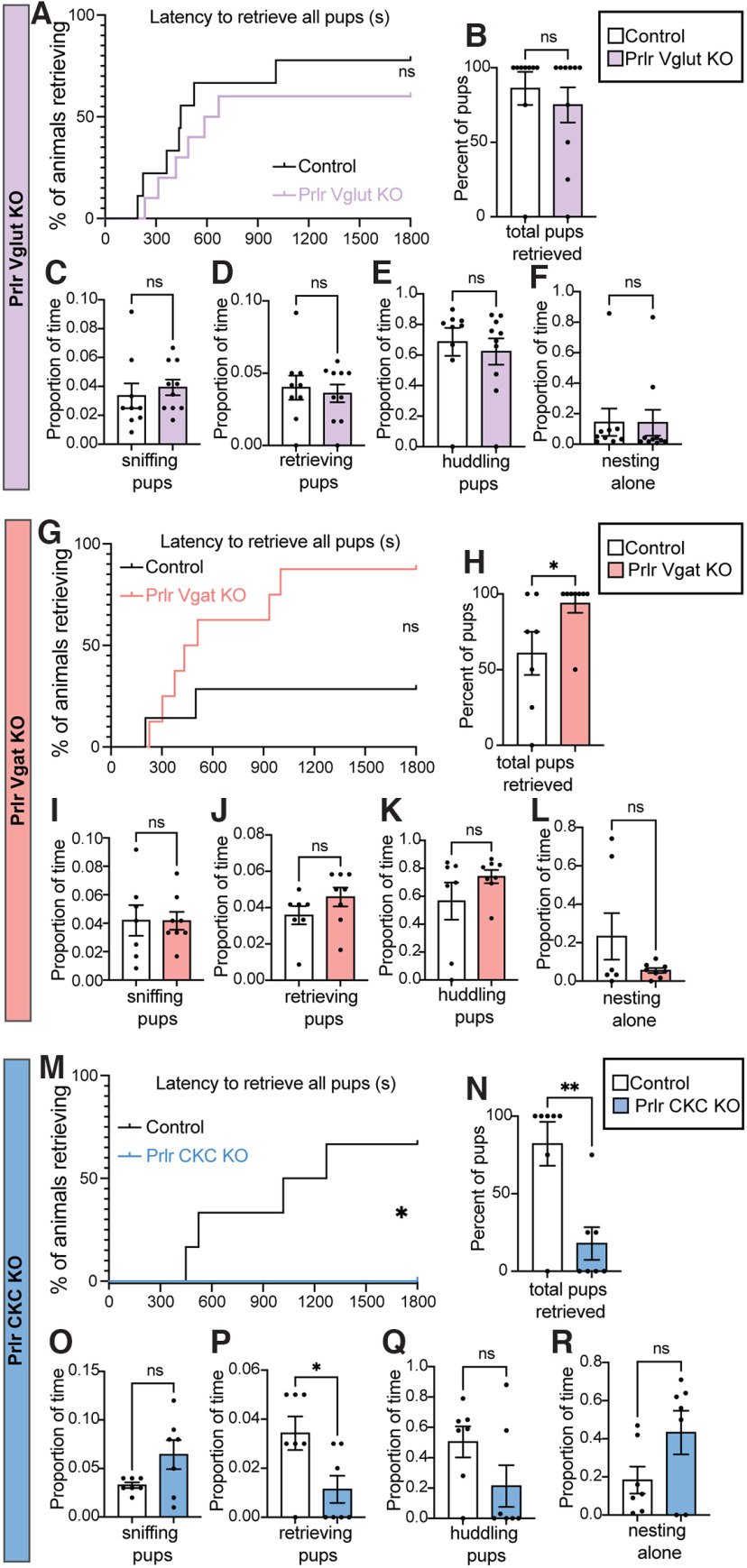
Following identification that populations of Prlr-expressing cells exhibited pup-induced c-fos immunoreactivity, we aimed to confirm that Prlr in the brain was necessary for paternal behavior in mouse sires and, if so, to begin to determine which population(s) of Prlr-expressing cells were involved. In Prlr Vglut KO, Prlr Vgat KO, and Prlr CKC KO male mice, there was some Prlr deletion in every brain region examined, but the degree of this deletion was markedly different in each mouse line (Fig. 4), reflecting the composition of neuronal subtypes expressed in each region. As expected, none of the respective littermate Cre-negative Prlrlox/lox control mice showed any eGFP expression, indicative of intact Prlr. Neither Prlr Vglut KO nor Prlr Vgat KO males showed any detectable deficits in paternal behaviors compared with Cre-negative control males (Fig. 5A–L; Table 5). Although the Prlr Vgat KO males appeared to have retrieved more than controls (Fig. 5H), this may be because control males in this group were more variable and performed less pup retrieval relative to similar Prlrlox/lox Cre- controls (Fig. 5B,N). In contrast, we observed significant deficits in Prlr CKC KO males, as none of these males retrieved all pups to the nest (Fig. 5M,N; Table 5; Movie 1). Although Prlr CKC KO mice are hyperprolactinemic because of the Prlr deletion in the arcuate nucleus (ARC), which controls negative feedback of prolactin secretion (i.e., the removal of negative feedback results in chronically high prolactin levels) (Brown et al., 2016b), Prlr CKC KO males showed a marked reduction in functional Prlr as assessed by pSTAT5 (a robust marker for Prlr activation) (Brown et al., 2010) (Fig. 6; Table 6) in a number of forebrain regions that correspond with Prlr deletion (Fig. 4S–X), including the anteroventral periventricular nucleus of the hypothalamus (AVPV), PVN MPN, ventromedial hypothalamus (VMH), MeApd, and the ARC. These results indicate that Prlr expression in CaMKIIα-expressing neurons is necessary for paternal care behavior and that Prlr deletion in either GABA or glutamate populations alone is not sufficient to disrupt paternal behavior. Furthermore, as supported by our c-fos data (Fig. 1), multiple regions may be involved in a prolactin-sensitive network that controls pup retrieval behavior.
Figure 5.
Prlr KO in CaMKIIα-expressing forebrain neurons disrupts paternal behavior. Prlr KO in glutamergic (Vglut, A–F) or GABAergic (Vgat, G–L) neurons did not result in any deficits in paternal behavior, compared with control males. In contrast, Prlr KO in CaMKIIa neurons (CKC, M–R) males showed significant deficits in pup retrieval behaviors, with none of the Prlr CKC KO males retrieving the full set of 4 pups to the nest (M,N). Although not statistically different from controls, most Prlr CKC KO males spent the majority of their time nesting alone and not huddling pups (Q,R). For all bar graphs, data points represent individual subjects and are presented as mean ± SEM. ns, Nonsignificant (p > 0.05). *p < 0.05. **p < 0.01.
Table 5.
Statistical test, parameters, and outcomes for each data panel in Figure 5
| Corresponding panel | Test | Genotype | Parameter | χ2 | df | p | Control group (median latency) | KO group (median latency) | Hazard ratio (Mantel–Haenszel) | 95% CI of ratio | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| A | Survival analysis/Mantel–Cox Log-rank test | Prlr Vglut KO | Latency to retrieve pups | 0.77 | 1 | 0.379 | 445.0 | 627.0 | 1.64 | 0.5435-4.961 | — |
| G | Survival analysis/Mantel–Cox Log-rank test | Prlr Vgat KO | Latency to retrieve pups | 3.88 | 1 | 0.049 | Undefined | 471.5 | 0.26 | 0.069-0.994 | — |
| M | Survival analysis/Mantel–Cox Log-rank test | Prlr CKC KO | Latency to retrieve pups | 5.57 | 1 | 0.018 | 1143.0 | Undefined | 11.11 | 1.504-82.130 | — |
| Test | Genotype | Parameter | U | p | Sum of ranks | Control group (median) | KO group (median) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| B | Mann–Whitney U | Prlr Vglut KO | % of pups retrieved | 37.00 | 0.52 | 98, 92 | 100.00 | 100.00 | — | — | — |
| E | Mann–Whitney U | Prlr Vglut KO | Proportion of time huddling pups | 35.50 | 0.46 | 99.5, 90.5 | 0.79 | 0.71 | — | — | — |
| F | Mann–Whitney U | Prlr Vglut KO | Proportion of time nesting alone | 29.50 | 0.22 | 105.5, 84.5 | 0.05 | 0.03 | — | — | — |
| H | Mann–Whitney U | Prlr Vgat KO | % of pups retrieved | 11.50 | 0.03 | 39.5, 80.5 | 75.00 | 100.00 | — | — | — |
| K | Mann–Whitney U | Prlr Vgat KO | Proportion of time huddling pups | 19.50 | 0.35 | 47.5, 72.5 | 0.07 | 0.04 | — | — | — |
| L | Mann–Whitney U | Prlr Vgat KO | Proportion of time nesting alone | 24.00 | 0.67 | 60, 60 | 0.06 | 0.05 | — | — | — |
| N | Mann–Whitney U | Prlr CKC KO | % of pups retrieved | 5.50 | 0.02 | 71.5, 33.5 | 100.00 | 0.00 | — | — | — |
| P | Mann–Whitney U | Prlr CKC KO | Proportion of time retrieving pups | 8.00 | 0.04 | 69, 36 | 0.03 | 0.00 | — | — | — |
| Q | Mann–Whitney U | Prlr CKC KO | Proportion of time huddling pups | 12.50 | 0.13 | 64.5, 40.5 | 0.59 | 0.00 | — | — | — |
| Test | Genotype | Parameter | t | df | p | Control group (mean) | KO (mean) | SEM | 95% CI | R2 (η2) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| C | t test | Prlr Vglut KO | Proportion of time sniffing | 0.39 | 17 | 0.701 | 0.04 | 0.04 | 0.01 | −0.026 to 0.018 | 0.01 |
| D | t test | Prlr Vglut KO | Proportion of time retrieving pups | 0.50 | 17 | 0.622 | 0.69 | 0.62 | 0.13 | −0.328 to 0.202 | 0.01 |
| I | t test | Prlr Vgat KO | Proportion of time sniffing | 0.02 | 13 | 0.984 | 0.04 | 0.04 | 0.01 | −0.026 to 0.026 | <0.001 |
| J | t test | Prlr Vgat KO | Proportion of time retrieving pups | 1.38 | 13 | 0.191 | 0.04 | 0.05 | 0.01 | −0.006 to 0.026 | 0.13 |
| O | t test | Prlr CKC KO | Proportion of time sniffing | 2.07 | 12 | 0.061 | 0.03 | 0.06 | 0.02 | −0.002 to 0.065 | 0.26 |
| R | t test | Prlr CKC KO | Proportion of time nesting alone | 1.86 | 12 | 0.088 | 0.18 | 0.43 | 0.13 | −0.043 to 0.543 | 0.22 |
Table 6.
Statistical test, parameters, and outcomes for each data panel in Figure 6
| Corresponding panel | Test | Brain region | Parameter | t | df | p | Control group (mean) | Prlr CKC KO group (mean) | SEM | 95% CI | R2 (η2) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | t test | AVPV | pSTAT5: no. of labeled cells/mm2 | 4.26 | 10 | 0.002 | 7.15 | 2.50 | 1.09 | −7.072 to −2.212 | 0.64 |
| O | t test | MPN | pSTAT5: no. of labeled cells/mm2 | 7.13 | 10 | <0.001 | 6.28 | 2.12 | 0.58 | −5.460 to −2.860 | 0.84 |
| R | t test | VMH | pSTAT5: no. of labeled cells/mm2 | 4.12 | 10 | 0.002 | 2.46 | 0.85 | 0.39 | −2.471 to −0.737 | 0.63 |
| S | t test | MeApd | pSTAT5: no. of labeled cells/mm2 | 5.74 | 10 | <0.001 | 2.52 | 0.44 | 0.36 | −2.887 to −1.273 | 0.77 |
| T | t test | ARC | pSTAT5: no. of labeled cells/mm2 | 3.61 | 10 | 0.005 | 5.43 | 2.05 | 0.94 | −5.474 to −1.298 | 0.57 |
| Test | Genotype | Parameter | U | p | Sum of ranks | Control group (median) | Prlr CKC KO group (median) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| P | BSTv | pSTAT5: no. of labeled cells/mm2 | 10.50 | 0.18 | 46.5, 31.5 | 0.06 | 0.00 | — | — | — | |
| Q | PVN | pSTAT5: no. of labeled cells/mm2 | 12.00 | 0.45 | 45, 33 | 0.00 | 0.00 | — | — | — |
Prlr KO in CaMKIIα-expressing neurons disrupts pup retrieval behavior (separate file). Video shows a representative example of a control sire (cre-negative Prlrlox/lox) and a CaMKIIα-neuron-specific Prlr KO (Prlr CKC KO) sire behavior during the pup retrieval test. Each video shows the first 2 min of the test and is sped up to 4× speed. The control male quickly retrieves pups to the nest. In contrast, while the Prlr CKC KO male investigates/sniffs the pups, he does not retrieve the pups to the nest.
Experiment 3: circulating prolactin is required for paternal behavior in sires, but not for the mating-induced transition to paternal care
With Prlr expression in the brain clearly necessary for paternal behavior, we subsequently aimed to investigate the temporal dynamics of circulating prolactin to identify critical periods of prolactin exposure for paternal behavior. Virgin males had relatively low prolactin levels, which did not differ between the light and dark cycles (Fig. 7A; Table 7). Consistent with previous reports (Valente et al., 2021), males showed a transient mating-induced surge of prolactin that lasted 30 min after ejaculation (Fig. 7B; Table 7). In contrast, we saw no increase from circulating prolactin from virgin levels in sires during the pup-rearing period (Fig. 7C; Table 7).
Figure 7.
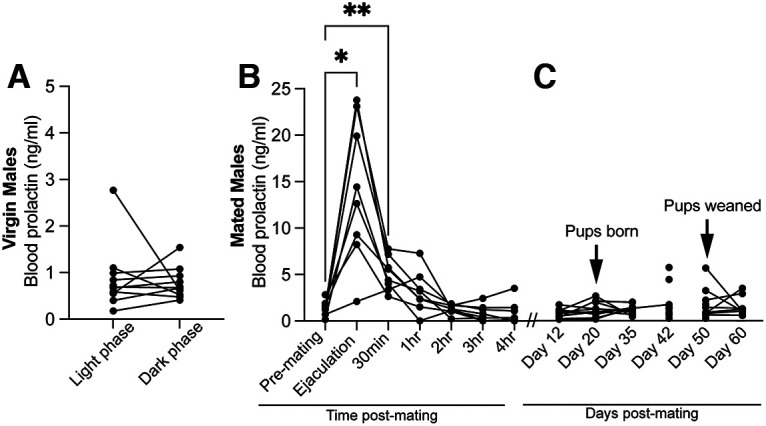
Circulating prolactin profiles in virgin and mated males. Prolactin concentrations measured in blood samples taken from C57BL/6J male mice. Virgin male mice have relatively low prolactin levels, which do not differ between the light and dark cycles (A), but show a transient mating-induced surge of prolactin that lasts up to 30 min after ejaculation (B). Asterisks indicate when prolactin is significantly higher than premating baseline concentrations. *p < 0.05. **p < 0.01. In contrast, we did not detect any significant increases in prolactin in sires during the pup rearing period (C).
Table 7.
Statistical test, parameters, and outcomes for each data panel in Figure 7
| Corresponding panel | Test | Parameter | t | df | p | Mean of differences | SD of differences | SEM of differences | 95% CI | R2 (η2) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | paired t test | Virgin male blood prolactin (light vs dark phase) | 0.41 | 9.00 | 0.69 | −0.11 | 0.82 | 0.26 | −0.690 to 0.478 | 0.02 | — | — |
| Test | Parameter | F (DFn, DFd) | p | Dunnett's multiple comparisons test | Mean difference | 95% CI of difference | Adjusted p | Baseline (mean) | Treatment group (mean) | Mean difference | SE of difference | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | Repeated-measures ANOVA | Mated male blood prolactin (time post-mating) | F(1.249, 8.536) = 19.39 | 0.001 | Baseline (pre-mating) vs ejaculation | −12.80 | −21.99 to −3.606 | 0.01 | 1.39 | 14.19 | −12.80 | 2.76 |
| Baseline (pre-mating) vs 30 min | −3.69 | −6.172 to −1.209 | 0.01 | 1.39 | 5.08 | −3.69 | 0.74 | |||||
| Baseline (pre-mating) vs 1 h | −1.72 | −4.496 to 1.057 | 0.27 | 1.39 | 3.11 | −1.72 | 0.83 | |||||
| Baseline (pre-mating) vs 2 h | 0.12 | −1.162 to 1.403 | 1.00 | 1.39 | 1.27 | 0.12 | 0.38 | |||||
| Baseline (pre-mating) vs 3 h | 0.57 | −1.088 to 2.223 | 0.73 | 1.39 | 0.82 | 0.57 | 0.50 | |||||
| Baseline (pre-mating) vs 4 h | 0.45 | −1.831 to 2.738 | 0.95 | 1.39 | 0.94 | 0.45 | 0.65 |
| Test | Parameter | F (DFn, DFd) | p | Tukey's multiple comparisons test | Mean difference | 95% CI of difference | Adjusted p | Mean 1 | Mean 2 | Mean difference | SE of difference | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | Repeated-measures ANOVA | Mated male blood prolactin (days post-mating) | F(1.859, 22.31) = 1.541 | 0.24 | day 12 vs day 20 | −0.55 | −1.496 to 0.4003 | 0.37 | 0.62 | 1.17 | −0.55 | 0.26 |
| day 12 vs day 35 | −0.81 | −2.838 to 1.216 | 0.69 | 0.62 | 1.44 | −0.81 | 0.55 | |||||
| day 12 vs day 42 | −5.00 | −18.17 to 8.169 | 0.73 | 0.62 | 5.62 | −5.00 | 3.60 | |||||
| day 12 vs day 50 | −2.05 | −7.509 to 3.412 | 0.72 | 0.62 | 2.67 | −2.05 | 1.44 | |||||
| day 12 vs day 60 | −3.19 | −7.013 to 0.6258 | 0.11 | 0.62 | 3.82 | −3.19 | 1.01 | |||||
| day 20 vs day 35 | −0.26 | −2.055 to 1.529 | 0.99 | 1.17 | 1.44 | −0.26 | 0.52 | |||||
| day 20 vs day 42 | −4.45 | −15.35 to 6.450 | 0.72 | 1.17 | 5.62 | −4.45 | 3.14 | |||||
| day 20 vs day 50 | −1.50 | −6.021 to 3.020 | 0.84 | 1.17 | 2.67 | −1.50 | 1.27 | |||||
| day 20 vs day 60 | −2.65 | −7.436 to 2.144 | 0.43 | 1.17 | 3.82 | −2.65 | 1.35 | |||||
| day 35 vs day 42 | −4.19 | −14.10 to 5.721 | 0.70 | 1.44 | 5.62 | −4.19 | 2.91 | |||||
| day 35 vs day 50 | −1.24 | −5.760 to 3.285 | 0.92 | 1.44 | 2.67 | −1.24 | 1.30 | |||||
| day 35 vs day 60 | −2.38 | −8.397 to 3.632 | 0.74 | 1.44 | 3.82 | −2.38 | 1.73 | |||||
| day 42 vs day 50 | 2.95 | −7.829 to 13.73 | 0.92 | 5.62 | 2.67 | 2.95 | 3.10 | |||||
| day 42 vs day 60 | 1.81 | −11.19 to 14.80 | 1.00 | 5.62 | 3.82 | 1.81 | 3.74 | |||||
| day 50 vs day 60 | −1.15 | −9.155 to 6.865 | 1.00 | 2.67 | 3.82 | −1.15 | 2.31 |
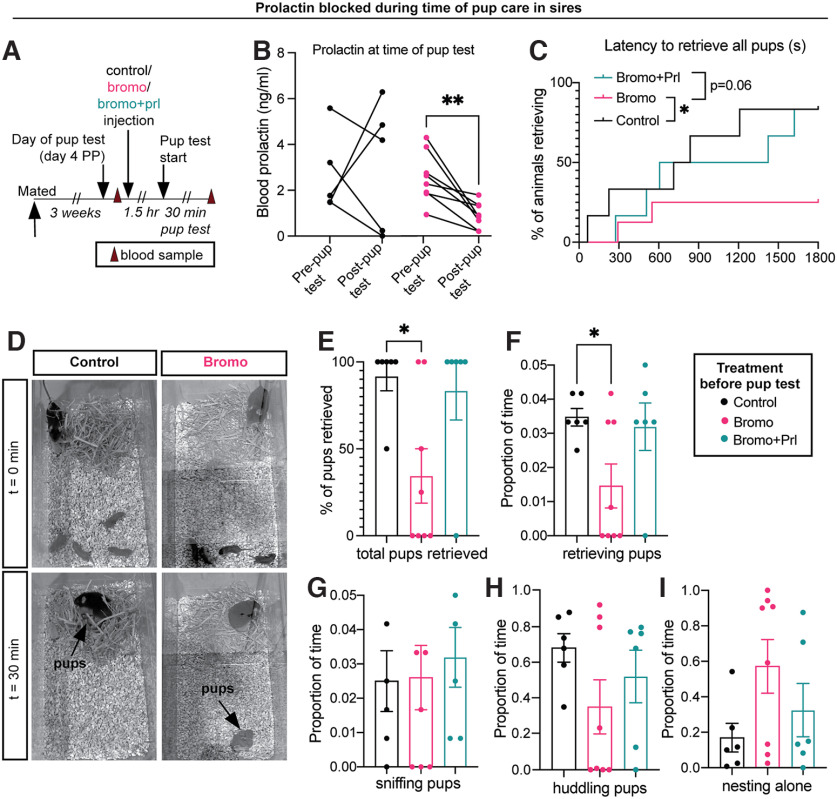
As paternal care in laboratory mice is dependent on ejaculation during mating (vom Saal, 1985), we hypothesized that mating-induced prolactin may be required to signal the transition into paternal care. Importantly, blocking prolactin with bromocriptine (Fig. 8B) before mating did not affect mating behavior (Fig. 9; Table 9). Contrary to our prediction, however, preventing the mating-induced prolactin surge had no significant effect on subsequent paternal behavior or circulating prolactin levels when tested 3 weeks later (the normal time between mating and birth of offspring; Fig. 8C–I; Table 8). Likewise, administering both bromocriptine and prolactin (rescue control) at mating did not significantly affect subsequent paternal behavior. Notably, bromocriptine-treated animals did not show any infanticidal responses (Fig. 8D), indicating that mating-induced prolactin release is not required for the transition to paternal care, nor the suppression of infanticidal behaviors.
Figure 9.
Suppressing prolactin at mating does not affect mating behavior. Mating behavior was assessed in C57/BL6J male mice that were treated with bromocriptine (n = 9, red) or vehicle (n = 7, blue) 1.5 h before mating. Bromocriptine did not affect the latency to first mount (A), latency to ejaculation (B), the number of mounts (C), mount duration (D), or number of intromission (E) compared with control males.
Table 9.
Statistical test, parameters, and outcomes for each data panel in Figure 9
| Corresponding panel | Test | Parameter | χ2 | df | p | Control group (median latency) | Bromo group (median latency) | Hazard ratio (Mantel–Haenszel) | 95% CI of ratio | |
|---|---|---|---|---|---|---|---|---|---|---|
| A | Survival analysis/Mantel–Cox Log-rank test | Latency to first mount | 2.78 | 1 | 0.12 | 281 | 168 | 0.42 | 0.143-1.244 | — |
| B | Survival analysis/Mantel–Cox Log-rank test | Latency to ejaculate | 2.94 | 1 | 0.09 | 1039 | 903 | 0.39 | 0.139-1.144 | — |
| Test | Parameter | t | df | p | Control group (mean) | Bromo group (mean) | SEM | 95% CI | R2 (η2) | |
|---|---|---|---|---|---|---|---|---|---|---|
| C | t test | No. of mounts | 0.88 | 15 | 0.39 | 13.38 | 10.78 | 2.94 | −8.865 to 3.671 | 0.05 |
| D | t test | Average mount bout duration (s) | 0.38 | 15 | 0.71 | 23.48 | 21.54 | 5.09 | −12.77 to 8.904 | 0.01 |
| E | t test | No. of intromissions | 2.04 | 15 | 0.06 | 274.00 | 190.30 | 41.03 | −171.1 to 3.788 | 0.22 |
Table 8.
Statistical test, parameters, and outcomes for each data panel in Figure 8
| Corresponding panel | Test | Parameter | Fixed effects | p | F (DFn, DFd) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | Two-way repeated-measures ANOVA | Blood prolactin (ng/ml) | Time | <0.01 | F(1.281,15.16) = 13.95 | — | — | — | — | — | — | — |
| Treatment | <0.0001 | F(1,12) = 34.27 | — | — | — | — | — | — | — | |||
| Time × Treatment | <0.0001 | F(6,71) = 15.14 | — | — | — | — | — | — | — |
| Bonferroni's multiple comparisons test | Parameter | Mean difference | 95% CI of difference | Adjusted p | Control group (mean) | Bromo group (mean) | Difference (mean) | SE of difference | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | −0.56 | −2.474 to 1.359 | >0.99 | 1.39 | 1.95 | −0.56 | 0.54 | — | — | — | |
| After ejaculation | 13.96 | 3.781-24.140 | 0.01 | 14.19 | 0.23 | 13.96 | 2.72 | — | — | — | |
| 30 m | 5.07 | 2.673-7.469 | 0.00 | 5.08 | 0.01 | 5.07 | 0.64 | — | — | — | |
| 1 h | 3.07 | 0.170-5.975 | 0.04 | 3.11 | 0.04 | 3.07 | 0.77 | — | — | — | |
| 2 h | 1.21 | 0.539-1.889 | 0.00 | 1.27 | 0.06 | 1.21 | 0.19 | — | — | — | |
| 3 h | 0.74 | −0.362 to 1.836 | 0.29 | 0.82 | 0.09 | 0.74 | 0.30 | — | — | — | |
| 4 h | 0.93 | −0.985 to 2.838 | 0.71 | 0.94 | 0.01 | 0.93 | 0.48 | — | — | — |
| Test | Parameter | χ2 | df | p | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | Survival analysis/Mantel–Cox Log-rank test | Latency to retrieve pups | 0.59 | 2 | 0.75 | — | — | — | — | — | — | — |
| Test | Parameter | F (DFn, DFd) | p | Dunnett's multiple comparisons test | Mean difference | 95% CI of difference | Adjusted p | Mean 1 | Mean 2 | Mean difference | SE of difference | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E | One-way ANOVA | % of pups retrieved | F(2,23) = 0.69 | 0.51 | control vs bromo | 26.11 | −26.57 to 78.79 | 0.42 | 65.00 | 38.89 | 26.11 | 22.29 |
| control vs bromo+prl | 15.00 | −41.51 to 71.51 | 0.76 | 65.00 | 50.00 | 15.00 | 23.91 | |||||
| F | Proportion of time sniffing | F(2,22) = 0.96 | 0.40 | control vs bromo | −0.03 | −0.09 to 0.03 | 0.44 | 0.09 | 0.12 | −0.03 | 0.02 | |
| control vs bromo+prl | 0.005 | −0.06 to 0.07 | 0.98 | 0.09 | 0.09 | 0.00 | 0.03 | |||||
| G | Proportion of time retrieving pups | F(2,22) = 1.48 | 0.25 | control vs bromo | 0.004 | −0.001 to 0.009 | 0.20 | 0.006 | 0.003 | 0.004 | 0.002 | |
| control vs bromo+prl | 0.003 | −0.003 to 0.009 | 0.39 | 0.006 | 0.004 | 0.003 | 0.002 | |||||
| H | Proportion of time huddling pups | F(2,22) = 2.50 | 0.11 | control vs bromo | 0.25 | −0.03 to 0.52 | 0.08 | 0.34 | 0.09 | 0.25 | 0.12 | |
| control vs bromo+prl | 0.05 | −0.24 to 0.35 | 0.88 | 0.34 | 0.29 | 0.05 | 0.12 | |||||
| I | Proportion of time nesting alone | F(2,22) = 0.14 | 0.89 | control vs bromo | −0.04 | −0.28 to 0.20 | 0.89 | 0.19 | 0.23 | −0.04 | 0.10 | |
| control vs bromo+prl | −0.05 | −0.3060 to 0.1995 | 0.84 | 0.19 | 0.24 | −0.05 | 0.11 |
We next aimed to determine whether paternal behavior in sires is dependent on circulating prolactin at the time of pup care. In support of our hypothesis, suppression of circulating prolactin (Fig. 10B; Table 10) caused significant deficits in pup retrieval behavior, similar to that seen in the Prlr CKC KO males (Fig. 5M,N), with most males failing to retrieve all of the pups during the task (Fig. 10C–E; Table 10). Notably, administering prolactin following bromocriptine treatment rescued pup retrieval behavior, with nearly all bromocriptine+prolactin males retrieving pups, similar to controls (Fig. 10F). Surprisingly, pup interactions did not cause an acute increase in prolactin in control fathers (Fig. 10B; Table 10), indicating that basal levels of circulating prolactin are sufficient to stimulate pup retrieval behavior. There were no significant correlations between prolactin and any paternal behavior (Table 8). No males showed any infanticidal responses. This effect of prolactin is unique to sires, as prolactin exposure did not affect virgin male behavior (Fig. 11; Table 11). Together, these data demonstrate that prolactin action is specifically required at the time of pup exposure in father mice for the display of paternal responses.
Figure 10.
Circulating prolactin is required to show paternal behaviors in sires. A, C57BL/6J sires were treated with either bromocriptine (bromo), which suppresses prolactin (B; n = 8) or vehicle (n = 6) 1.5 h before or bromocriptine 1.5 h and ovine prolactin (prl) 45 min before the pup retrieval test. Pup interactions did not increase prolactin in vehicle-control males (B). C, Bromocriptine-treated sires were slower to retrieve pups, relative to controls, but this was rescued by prolactin (bromo+prl group). D, Representative photographs of the beginning (t = 0 min) and end (t = 30 min) of the pup retrieval test between control (vehicle) and bromo-treated males. Most control males readily received pups to the nest, whereas bromo-treated males did not retrieve pups to the nest (E, F). Other behaviors, including sniffing (G), huddling (H), and nesting alone (I), were not statistically different between treatment groups. Bromocriptine+prolactin-treated males were not significantly different from controls in any test. Bar graphs represent individual data points (circles) and mean ± SEM. ns, Nonsignificant (p > 0.05). *p < 0.05. **p < 0.01.
Table 10.
Statistical test, parameters, and outcomes for each data panel in Figure 10
| Corresponding panel | Test | Parameter | t | df | p | Mean of differences | SD of differences | SEM of differences | 95% CI | R2 (η2) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | paired t test | Blood prolactin (pre- vs post-pup test): control males | 0.27 | 4 | 0.80 | 0.41 | 3.34 | 1.50 | −3.742 to 4.562 | 0.02 | — | — |
| paired t test | Blood prolactin (pre- vs post-pup test): Bromo males | 4.08 | 7 | 0.00 | −1.66 | 1.15 | 0.41 | −2.616 to −0.696 | 0.70 | — | — |
| Test | Parameter | Groups | χ2 | df | p | Control group (median latency) | Bromo group (median latency) | Hazard ratio (Mantel–Haenszel) | 95% CI of ratio | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | Survival analysis/Mantel–Cox Log-rank test | Latency to retrieve pups | Control vs Bromo | 3.96 | 1 | 0.04 | 774.00 | undefined | 4.86 | 0.96-21.22 | — | — |
| Survival analysis/Mantel–Cox Log-rank test | Latency to retrieve pups | Bromo vs Bromo+prl | 3.66 | 1 | 0.06 | undefined | 1015.00 | 0.22 | 0.05-1.04 | — | — | |
| Survival analysis/Mantel–Cox Log-rank test | Latency to retrieve pups | Control vs Bromo+prl | 0.16 | 1 | 0.68 | 774.00 | 1015.00 | 1.31 | 0.37-4.68 | — | — |
| Test | Parameter | H | p | Dunn's multiple comparisons test | Mean rank difference | Adjusted p | Mean rank 1 | Mean rank 2 | Mean rank difference | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E | Kruskal–Wallis | % of pups retrieved | 6.80 | 0.03 | Control vs Bromo | 6.52 | 0.04 | 13.33 | 6.81 | 6.52 | — | — |
| Control vs Bromo+Prl | 0.75 | >0.99 | 13.33 | 12.58 | 0.75 | — | — | |||||
| F | Kruskal–Wallis | Proportion of time retrieving pups | 4.24 | 0.13 | Control vs Bromo | 5.44 | 0.15 | 12.75 | 7.31 | 5.44 | — | — |
| Control vs Bromo+Prl | 0.25 | >0.99 | 12.75 | 12.50 | 0.25 | — | — | |||||
| H | Kruskal–Wallis | Proportion of time huddling pups | 1.30 | 0.54 | Control vs Bromo | 3.60 | 0.51 | 12.67 | 9.06 | 3.60 | — | — |
| Control vs Bromo+Prl | 2.42 | 0.95 | 12.67 | 10.25 | 2.42 | — | — |
| Test | Parameter | F (DFn, DFd) | p | Dunnett's multiple comparisons test | Mean difference | 95% CI of difference | Adjusted p | Mean 1 | Mean 2 | Mean difference | SE of difference | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| G | One-way ANOVA | Proportion of time sniffing | F(2,17) = 0.15 | 0.86 | Control vs Bromo | −0.001 | −0.03 to 0.03 | 1.00 | 0.03 | 0.03 | 0.00 | 0.01 |
| Control vs Bromo+Prl | −0.01 | −0.04 to 0.03 | 0.83 | 0.03 | 0.03 | −0.01 | 0.01 | |||||
| I | One-way ANOVA | Proportion of time nesting alone | F(2,17) = 2.28 | 0.13 | Control vs Bromo | −0.40 | −0.87 to 0.06 | 0.09 | 0.17 | 0.57 | −0.40 | 0.19 |
| Control vs Bromo+Prl | −0.16 | −0.65 to 0.34 | 0.67 | 0.17 | 0.33 | −0.16 | 0.21 |
| (data not shown in figure) | Pearson r | 95% CI | R 2 | p (two-tailed) | Pearson r | 95% CI | R 2 | p (two-tailed) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pre-pup prl vs no. of pups retrieved | 0.1113 | −0.4684 to 0.6240 | 0.01239 | 0.7173 | — | post-pup prl vs no. of pups retrieved | 0.1898 | −0.4034 to 0.6707 | 0.03603 | 0.5345 | — | |
| pre-pup prl vs latency retrieve all 4 pups | −0.1753 | −0.6623 to 0.4158 | 0.03074 | 0.5667 | — | post-pup prl vs latency retrieve all 4 pups | 0.07064 | −0.4998 to 0.5983 | 0.004991 | 0.8186 | — | |
| pre-pup prl vs time spent sniffing | −0.2691 | −0.7142 to 0.3310 | 0.07239 | 0.3741 | — | post-pup prl vs time spent sniffing | 0.4037 | −0.1895 to 0.7810 | 0.1629 | 0.1714 | — | |
| pre-pup prl vs time spent in nest alone | −0.06304 | −0.5934 to 0.5055 | 0.003974 | 0.8379 | — | post-pup prl vs time spent in nest alone | −0.2464 | −0.7021 to 0.3524 | 0.06071 | 0.4171 | — | |
| pre-pup prl vs time spent huddling | 0.07182 | −0.4989 to 0.5991 | 0.005159 | 0.8156 | — | post-pup prl vs time spent huddling | 0.1625 | −0.4267 to 0.6549 | 0.02641 | 0.5958 | — | |
| pre-pup prl vs time spent retrieving | 0.06744 | −0.5022 to 0.5963 | 0.004548 | 0.8267 | — | post-pup prl vs time spent retrieving | 0.1262 | −0.4565 to 0.6332 | 0.01593 | 0.6812 | — |
Figure 11.
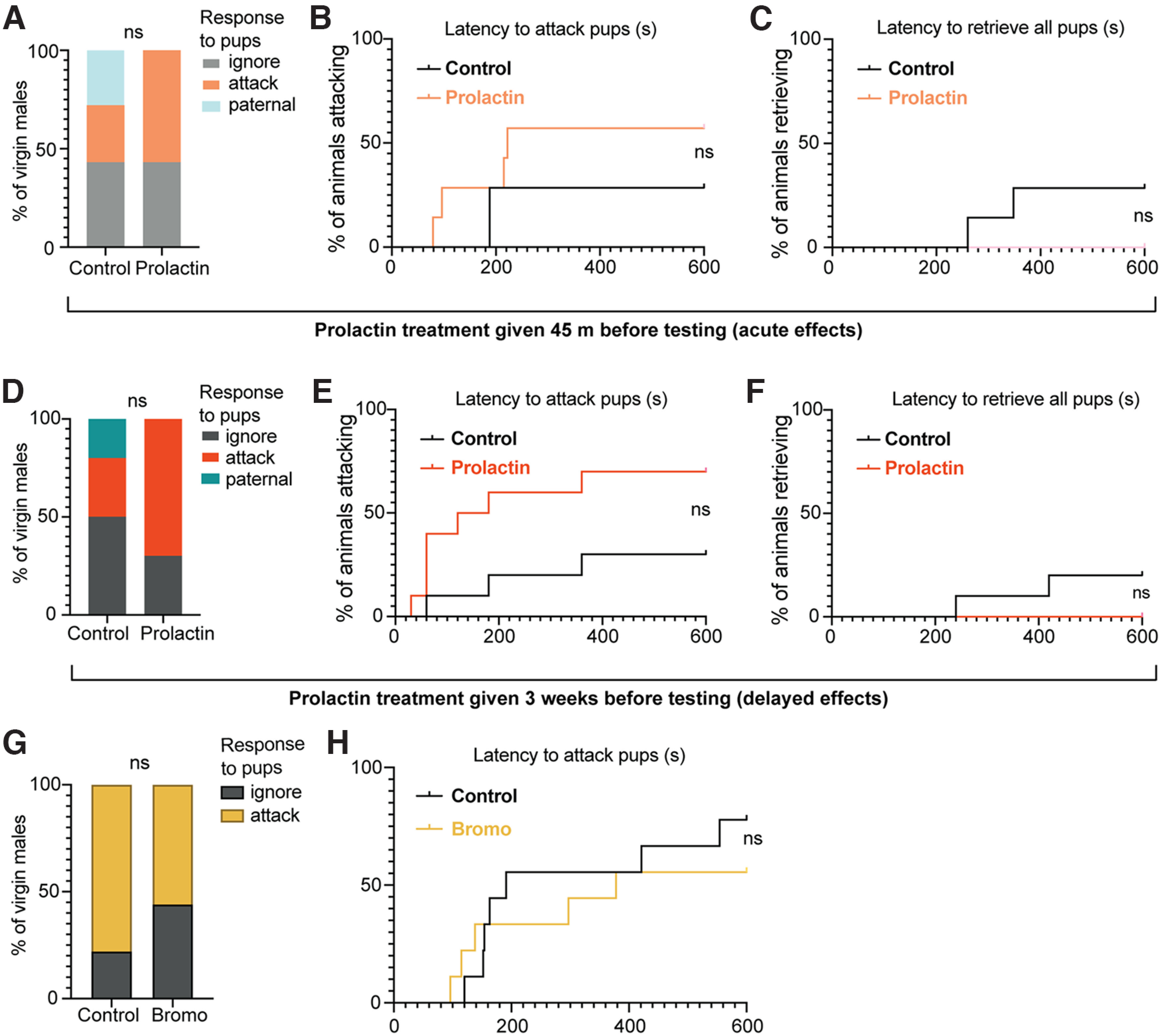
Prolactin exposure does not affect attacking or paternal behavior in virgin male mice. Neither acute prolactin treatment (45 min before the pup retrieval test) nor delayed prolactin exposure (administered 3 weeks before the pup test) in virgin C57/B6J mice affected the proportion of males which showed attacking, ignoring, or paternal responses toward pups (A,D), the latency to attack (B,E), or latency to retrieve pups (C,F) compared with vehicle-control males. Bromocriptine (bromo) treatment (1.5 h before the pup retrieval test) also had no effect on the proportion of males which showed attacking or ignoring responses toward pups (G), or the latency to attack pups (H). None of the virgin males showed paternal responses in this experiment. ns, Nonsignificant (p > 0.05).
Discussion
To date, prolactin has been most well recognized for its role in lactation and the accompanying maternal care in female mammals. The present data build on our previous findings to provide strong causal evidence that basal circulating prolactin acting through the neuronal Prlr is required for the normal paternal responses in mouse sires. In the present study, we found that (1) paternal interactions with pups induce c-fos immunoreactivity in prolactin-responsive neurons in multiple regions, including the BNSTv, MPN, and MeApd in sires; (2) Prlr deletion restricted to Vgat+ or Vglut2+ neurons is not sufficient to disrupt paternal care, while a Prlr KO on CaMKIIα-expressing neurons causes significant deficits in pup retrieval behavior; and (3) this behavioral effect requires basal circulating prolactin present in sires at the time of interactions with pups, although there is no acute activation of prolactin secretion in response to pups.
The first aim of the present study was to determine whether prolactin-responsive neurons throughout the parental regulatory circuits are activated during the expression of paternal behavior. Using c-fos as an indicator of neural responses to pup interactions, we found that Prlr-expressing cells were activated in the MPN and BNSTv of sires and in the MeApd of both sires and virgins. The MPN and BNST are highly interconnected, mainly through GABAergic connections (Tachikawa et al., 2013), and have long been known to be critical nodes in the parenting behavior circuits (Numan and Insel, 2003). Prlr-containing neurons in the MPN are required for both maternal (Brown et al., 2017) and paternal (Stagkourakis et al., 2020) behaviors; however, the role of prolactin/Prlr in the BNST is less studied. These areas were not significantly activated in virgin or mated males, indicating that pup-induced activation of Prlr-expressing cells in the MPN and BNSTv may depend on prior mating experience and/or other physiological changes that co-occur in sires. The MeA is involved in downstream processing of sensory cues controlling social behavior and is highly connected with the MPN and BNST (Kohl et al., 2017). In virgin female rats, the MeA receives signals from olfactory bulb and vomeronasal organ which inhibit maternal care, with MeA lesions or exposure to ovarian hormones releasing this inhibition, causing the display of maternal behavior (Numan et al., 1993). As the population of MeApd neurons is mostly GABAergic (Keshavarzi et al., 2014), our data showing that a Vgat-specific deletion of Prlr did not impair pup retrieval suggests that prolactin action on these cells may not be involved in this response in males. However, the role of prolactin signaling in the MeA during maternal and paternal behavior has not been explored and warrants further investigation.
Our next aim was to begin to identify the cell types involved in prolactin-mediated effects on paternal behaviors and to evaluate whether these were primarily driven by excitatory or inhibitory actions. Given the widespread nature of Prlr activation in response to pups in sires, we focused on three broad subpopulations of neurons found throughout these regions: glutamate, GABA, and CaMKIIα. While the role of glutamatergic transmission has not been as well studied in paternal behavior, GABAergic signaling has been implicated in regulating both the infanticidal and paternal response in male mice in the MPN and MeA (Chen et al., 2019; Dimén et al., 2021). Despite this, we found that males with Prlr deleted from either glutamatergic or GABAergic cells did not show any deficits in paternal behavior. Similarly, in female mice, a Prlr Vglut KO, Prlr Vgat KO, or combined Prlr Vglut+Vgat KO did not result in any significant deficits in maternal behavior, whereas complete deletion of Prlr from all neurons in the MPN using an AAV-Cre profoundly disrupted maternal behavior (Brown et al., 2017). This may also be the case for males as it appears that Prlr action is not mediated solely by excitatory (i.e., glutamatergic) or inhibitory (i.e., GABAergic) neurons. Paternal care expression therefore may require prolactin action on both populations (and perhaps in a site-specific way) or is driven by prolactin action on another unknown population (e.g., peptidergic) that was not targeted by either the Vgat or Vglut Cre lines. In support of the first possibility, we found that deleting the Prlr from CaMKIIα-expressing neurons, a population that includes both glutamatergic and GABAergic neurons, resulted in a profound effect on paternal behavior, with none of the Prlr CKC KO males retrieving the full set of pups to the nest. These results corroborate our previous findings that a complete Prlr deletion from the MPN using an AAV-Cre approach also resulted in similar deficits in pup retrieval behavior (Stagkourakis et al., 2020). Although more work is needed to further characterize the specific Prlr-expressing cells, we have identified that Prlr-expressing CaMKIIα forebrain cells are critical for the display of paternal behavior in fathers. While the MPN population may be essential, it is unlikely that prolactin action on this circuit is strictly limited to the MPN, based on our c-fos data.
Our next step was to determine at what time point(s) prolactin exposure was critical for the onset of paternal care. Unlike females, which are exposed to high levels of prolactin in pregnancy and lactation, males typically do not exhibit major changes in circulating prolactin levels before offspring interactions with the exception of mating. Since mating with ejaculation is the necessary stimulus for paternal care in mice, we hypothesized that mating-induced prolactin may serve as a signal to initiate the transition to paternal care. Contrary to our hypothesis, however, when mating-induced prolactin was blocked, it did not have a significant effect on subsequent paternal behavior. These data suggest that the mating-induced release of prolactin is not required for the subsequent expression of paternal behavior. One caveat to this interpretation, however, was that, in this specific experiment, the control males appeared to perform less paternal behaviors relative to other control males (e.g., Fig. 5). One factor that could account for this difference is that mated males in this experiment were not cohoused with pregnant females or pups in between mating and the pup retrieval test (as they were mated with steroid-primed ovariectomized females), whereas the other males tested (e.g., Fig. 5) were cohoused with females and litters until day 3 postpartum (see Materials and Methods). Although paternal care in this mouse strain is not reliant on cohousing with a female, there can be potential additive effects of this, along with prior experience with the pups on paternal behavior (Brown, 1993). Notably, no bromocriptine-treated animals showed infanticidal responses, indicating that the mating-induced prolactin surge is clearly not required for the transition away from infanticide.
Aside from mating, basal circulating levels of prolactin were generally low, and remained unchanged throughout interactions with pups. This pattern is in contrast to some other paternal rodent species, such as Mongolian gerbils (Brown et al., 1995), Djungarian hamsters (Reburn and Wynne-Edwards, 1999), and California mice (Gubernick and Nelson, 1989), which show increased prolactin levels as fathers. Nonetheless, blocking pituitary prolactin secretion with bromocriptine resulted in similar pup retrieval deficits as observed in the Prlr CKC KO males. Importantly, exogenous prolactin administration prevented the effect of bromocriptine, and rescued pup retrieval. We suggest that there is a threshold, permissive, effect of prolactin, rather than a linear relationship, as individual variation in prolactin concertation did not directly relate to the amount of care provided. In male mice, prolactin is the only identified lactogen to act through the Prlr, with growth hormone failing to bind to the Prlr in mice (Bartke and Kopchick, 2015) and placental lactogen only relevant in female mice (Cohick et al., 1996). It has also been confirmed that circulating prolactin can be transported past the blood–brain barrier to access central Prlr (Brown et al., 2016b; Barad et al., 2020), and suppression of pituitary secretion of prolactin completely eliminates pSTAT5 expression in the brain (Brown et al., 2010), suggesting that there is no central source of prolactin under most conditions. Notably, the effect of prolactin-promoting paternal behaviors is dependent on mating experience and/or other internal factors present in fathers, as injecting prolactin alone into virgin males did not cause them to show paternal behavior (as shown in Fig. 11), when tested acutely or after a period of time following prolactin treatment to match the mating-induced onset of parental care.
In conclusion, our data show that, while the mating-induced transition away from infanticidal behaviors is not likely to be prolactin-dependent, following mating experience, paternal behavior is dependent on basal levels of circulating prolactin acting at the Prlr on CaMKIIα-expressing forebrain neurons during the interaction with pups. Our results provide further support for the concept that the suppression of infanticide and the onset of paternal caring behaviors occur via two different, parallel pathways and have separate neuroendocrine regulatory mechanisms (Kohl et al., 2017). These data provide a long-sought explanation for the role of prolactin in males, and show that prolactin acts equivalently in both sexes to promote offspring care. Given that humans are among the few mammalian species that exhibit paternal care, it is likely that this mechanism will be important in men as well as women.
Footnotes
This work was supported by the Health Research Council of New Zealand, the Marsden Fund–Royal Society of New Zealand, and British Society of Neuroendocrinology Project Support Grant. We thank Pene Knowles for genotyping the animals; and Genieve Yeo for assistance with behavior video coding.
The authors declare no competing financial interests.
References
- Alsina-Llanes M, Olazábal D (2018) Do sires and juvenile male mice (C57BL/6) contribute to the rearing of the offspring? Acta Ethol 21:185–193. [Google Scholar]
- Aoki M, Wartenberg P, Grünewald R, Phillipps HR, Wyatt A, Grattan DR, Boehm U (2019) Widespread cell-specific prolactin receptor expression in multiple murine organs. Endocrinology 160:2587–2599. 10.1210/en.2019-00234 [DOI] [PubMed] [Google Scholar]
- Bales KL, Saltzman W (2016) Fathering in rodents: neurobiological substrates and consequences for offspring. Horm Behav 77:249–259. 10.1016/j.yhbeh.2015.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barad Z, Khant Aung Z, Grattan DR, Ladaman SR, Brown RS (2020) Impaired prolactin transport into the brain and functional responses to prolactin in aged male mice. J Neuroendocrinol 32:e12889. 10.1111/jne.12889 [DOI] [PubMed] [Google Scholar]
- Bartke A, Kopchick JJ (2015) The forgotten lactogenic activity of growth hormone: important implications for rodent studies. Endocrinology 156:1620–1622. 10.1210/en.2015-1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges RS (2020) The behavioral neuroendocrinology of maternal behavior: past accomplishments and future directions. Horm Behav 120:104662. 10.1016/j.yhbeh.2019.104662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody S, Krüger TH (2006) The post-orgasmic prolactin increase following intercourse is greater than following masturbation and suggests greater satiety. Biol Psychology 71:312–315. 10.1016/j.biopsycho.2005.06.008 [DOI] [PubMed] [Google Scholar]
- Brown RE (1993) Hormonal and experiential factors influencing parental behaviour in male rodents: an integrative approach. Behav Processes 30:1–27. 10.1016/0376-6357(93)90009-G [DOI] [PubMed] [Google Scholar]
- Brown RE, Murdoch T, Murphy PR, Moger WH (1995) Hormonal responses of male gerbils to stimuli from their mate and pups. Horm Behav 29:474–491. 10.1006/hbeh.1995.1275 [DOI] [PubMed] [Google Scholar]
- Brown RS, Kokay IC, Herbison AE, Grattan DR (2010) Distribution of prolactin-responsive neurons in the mouse forebrain. J Comp Neurol 518:92–102. 10.1002/cne.22208 [DOI] [PubMed] [Google Scholar]
- Brown RS, Kokay IC, Phillipps HR, Yip SH, Gustafson P, Wyatt A, Larsen CM, Knowles P, Ladyman SR, LeTissier P, Grattan DR (2016a) Conditional deletion of the prolactin receptor reveals functional subpopulations of dopamine neurons in the arcuate nucleus of the hypothalamus. J Neurosci 36:9173–9185. 10.1523/JNEUROSCI.1471-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RS, Wyatt AK, Herbison RE, Knowles PJ, Ladyman SR, Binart N, Banks WA, Grattan DR (2016b) Prolactin transport into mouse brain is independent of prolactin receptor. FASEB J 30:1002–1010. 10.1096/fj.15-276519 [DOI] [PubMed] [Google Scholar]
- Brown RS, Aoki M, Ladyman SR, Phillipps HR, Wyatt A, Boehm U, Grattan DR (2017) Prolactin action in the medial preoptic area is necessary for postpartum maternal nursing behavior. Proc Natl Acad Sci USA 114:10779–10784. 10.1073/pnas.1708025114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RS, Khant Aung Z, Phillipps HR, Barad Z, Lein HJ, Boehm U, Szawka RE, Grattan DR (2019) Acute suppression of LH secretion by prolactin in female mice is mediated by kisspeptin neurons in the arcuate nucleus. Endocrinology 160:1323–1332. 10.1210/en.2019-00038 [DOI] [PubMed] [Google Scholar]
- Casanova E, Fehsenfeld S, Mantamadiotis T, Lemberger T, Greiner E, Stewart AF, Schütz G (2001) A CamKIIα iCre BAC allows brain-specific gene inactivation. Genesis 31:37–42. 10.1002/gene.1078 [DOI] [PubMed] [Google Scholar]
- Chen PB, Hu RK, Wu YE, Pan L, Huang S, Micevych PE, Hong W (2019) Sexually dimorphic control of parenting behavior by the medial amygdala. Cell 176:1206–1221.e18. 10.1016/j.cell.2019.01.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohick CB, Dai G, Xu L, Deb S, Kamei T, Levan G, Szpirer C, Szpirer J, Kwok SC, Soares MJ (1996) Placental lactogen-I variant utilizes the prolactin receptor signaling pathway. Mol Cell Endocrinol 116:49–58. 10.1016/0303-7207(95)03695-4 [DOI] [PubMed] [Google Scholar]
- Dimén D, Puska G, Szendi V, Sipos E, Zelena D, Dobolyi Á (2021) Sex-specific parenting and depression evoked by preoptic inhibitory neurons. iScience 24:103090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin KB, Paxinos G (2013) Paxinos and Franklin's The mouse brain in stereotaxic coordinates. Amsterdam: Elsevier. [Google Scholar]
- Friard O, Gamba M (2016) BORIS: a free, versatile open-source event-logging software for video/audio coding and live observations. Methods Ecol Evol 7:1325–1330. 10.1111/2041-210X.12584 [DOI] [Google Scholar]
- Gubernick DJ, Nelson RJ (1989) Prolactin and paternal behavior in the biparental California mouse, Peromyscus californicus. Horm Behav 23:203–210. 10.1016/0018-506x(89)90061-5 [DOI] [PubMed] [Google Scholar]
- Guillou A, Romanò N, Steyn F, Abitbol K, Le Tissier P, Bonnefont X, Chen C, Mollard P, Martin AO (2015) Assessment of lactotroph axis functionality in mice: longitudinal monitoring of PRL secretion by ultrasensitive-ELISA. Endocrinology 156:1924–1930. 10.1210/en.2014-1571 [DOI] [PubMed] [Google Scholar]
- Gustafson P, Ladyman SR, McFadden S, Larsen C, Aung ZK, Brown RS, Bunn SJ, Grattan DR (2020) Prolactin receptor-mediated activation of pSTAT5 in the pregnant mouse brain. J Neuroendocrinol 32:e12901. 10.1111/jne.12901 [DOI] [PubMed] [Google Scholar]
- Keshavarzi S, Sullivan RK, Ianno DJ, Sah P (2014) Functional properties and projections of neurons in the medial amygdala. J Neurosci 34:8699–8715. 10.1523/JNEUROSCI.1176-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk SE, Xie TY, Steyn FJ, Grattan DR, Bunn SJ (2017) Restraint stress increases prolactin-mediated phosphorylation of signal transducer and activator of transcription 5 in the hypothalamus and adrenal cortex in the male mouse. J Neuroendocrinol 29. 10.1111/jne.12477 [DOI] [PubMed] [Google Scholar]
- Kohl J, Autry AE, Dulac C (2017) The neurobiology of parenting: a neural circuit perspective. Bioessays 39:1–11. 10.1002/bies.201600159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokay IC, Wyatt A, Phillipps HR, Aoki M, Ectors F, Boehm U, Grattan DR (2018) Analysis of prolactin receptor expression in the murine brain using a novel prolactin receptor reporter mouse. J Neuroendocrinol 30:e12634. 10.1111/jne.12634 [DOI] [PubMed] [Google Scholar]
- Krüger TH, Haake P, Hartmann U, Schedlowski M, Exton MS (2002) Orgasm-induced prolactin secretion: feedback control of sexual drive? Neurosci Biobehav Rev 26:31–44. 10.1016/s0149-7634(01)00036-7 [DOI] [PubMed] [Google Scholar]
- Liu ZW, Jiang N, Tao X, Wang XP, Liu XM, Xiao SY (2020) Assessment of sexual behavior of male mice. J Vis Exp 5:157. 10.3791/60154 [DOI] [PubMed] [Google Scholar]
- Lonstein JS, Fleming AS (2002) Parental behaviors in rats and mice. Curr Protoc Neurosci. Chapter 8:Unit 8.15. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, Rood BD, De Vries GJ (2002) Parental responsiveness is feminized after neonatal castration in virgin male prairie voles, but is not masculinized by perinatal testosterone in virgin females. Horm Behav 41:80–87. 10.1006/hbeh.2001.1740 [DOI] [PubMed] [Google Scholar]
- Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, Lein ES, Zeng H (2010) A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci 13:133–140. 10.1038/nn.2467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLennan IS, Taylor-Jeffs J (2016) The use of sodium lamps to brightly illuminate mouse houses during their dark phases: laboratory animals. Lab Anim 38:384–392. [DOI] [PubMed] [Google Scholar]
- Numan M, Insel TR (2003) Neuroanatomy of maternal behavior. In: The neurobiology of parental behavior: hormones, brain, and behavior, pp 107–189. New York: Springer. [Google Scholar]
- Numan M, Numan MJ, English JB (1993) Excitotoxic amino acid injections into the medial amygdala facilitate maternal behavior in virgin female rats. Horm Behav 27:56–81. 10.1006/hbeh.1993.1005 [DOI] [PubMed] [Google Scholar]
- Phillipps HR, Yip SH, Grattan DR (2020) Patterns of prolactin secretion. Mol Cell Endocrinol 502:110679. 10.1016/j.mce.2019.110679 [DOI] [PubMed] [Google Scholar]
- Reburn CJ, Wynne-Edwards KE (1999) Hormonal changes in males of a naturally biparental and a uniparental mammal. Horm Behav 35:163–176. 10.1006/hbeh.1998.1509 [DOI] [PubMed] [Google Scholar]
- Saltzman W, Ziegler TE (2014) Functional significance of hormonal changes in mammalian fathers. J Neuroendocrinol 26:685–696. 10.1111/jne.12176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smiley KO, Ladyman SR, Gustafson P, Grattan DR, Brown RS (2019) Neuroendocrinology and adaptive physiology of maternal care. In: Neuroendocrine regulation of behavior (Coolen LM, Grattan DR, eds), pp 161–210. Current topics in behavioral neurosciences. Cham, Switzerland: Springer. [DOI] [PubMed] [Google Scholar]
- Solà C, Tusell JM, Serratosa J (1999) Comparative study of the distribution of calmodulin kinase II and calcineurin in the mouse brain. J Neurosci Res 57:651–662. [DOI] [PubMed] [Google Scholar]
- Stagkourakis S, Smiley KO, Williams P, Kakadellis S, Ziegler K, Bakker J, Brown RS, Harkany T, Grattan DR, Broberger C (2020) A neuro-hormonal circuit for paternal behavior controlled by a hypothalamic network oscillation. Cell 182:960–975.e15. 10.1016/j.cell.2020.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steyn FJ, Huang L, Ngo ST, Leong JW, Tan HY, Xie TY, Parlow AF, Veldhuis JD, Waters MJ, Chen C (2011) Development of a method for the determination of pulsatile growth hormone secretion in mice. Endocrinology 152:3165–3171. 10.1210/en.2011-0253 [DOI] [PubMed] [Google Scholar]
- Swart JM, Grattan DR, Ladyman SR, Brown RS (2021) Changes in maternal motivation across reproductive states in mice: a role for prolactin receptor activation on GABA neurons. Horm Behav 135:105041. 10.1016/j.yhbeh.2021.105041 [DOI] [PubMed] [Google Scholar]
- Tachikawa KS, Yoshihara Y, Kuroda KO (2013) Behavioral transition from attack to parenting in male mice: a crucial role of the vomeronasal system. J Neurosci 33:5120–5126. 10.1523/JNEUROSCI.2364-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuneoka Y, Tokita K, Yoshihara C, Amano T, Esposito G, Huang AJ, Yu LM, Odaka Y, Shinozuka K, McHugh TJ, Kuroda KO (2015) Distinct preoptic-BST nuclei dissociate paternal and infanticidal behavior in mice. EMBO J 34:2652–2670. 10.15252/embj.201591942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valente S, Marques T, Lima SQ (2021) No evidence for prolactin's involvement in the post-ejaculatory refractory period. Commun Biol 4:10. 10.1038/s42003-020-01570-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- vom Saal FS (1985) Time-contingent change in infanticide and parental behavior induced by ejaculation in male mice. Physiol Behav 34:7–15. 10.1016/0031-9384(85)90069-1 [DOI] [PubMed] [Google Scholar]
- Vong L, Ye C, Yang Z, Choi B, Chua S, Lowell BB (2011) Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neurons. Neuron 71:142–154. 10.1016/j.neuron.2011.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Autry AE, Bergan JF, Watabe-Uchida M, Dulac CG (2014) Galanin neurons in the medial preoptic area govern parental behaviour. Nature 509:325–330. 10.1038/nature13307 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Prlr KO in CaMKIIα-expressing neurons disrupts pup retrieval behavior (separate file). Video shows a representative example of a control sire (cre-negative Prlrlox/lox) and a CaMKIIα-neuron-specific Prlr KO (Prlr CKC KO) sire behavior during the pup retrieval test. Each video shows the first 2 min of the test and is sped up to 4× speed. The control male quickly retrieves pups to the nest. In contrast, while the Prlr CKC KO male investigates/sniffs the pups, he does not retrieve the pups to the nest.