Abstract
Background Thirty-day unplanned readmission following endoscopic transsphenoidal pituitary surgery (ETPS) occurs in up to 14% of patients. Delayed hyponatremia is one of the most common causes, accounting for 30% of readmissions and often occurs within 1 week of surgery. The authors' prior retrospective review identified endocrinology follow-up as protective factor.
Objectives Implementation of a multidisciplinary postoperative care (POC) pathway: (1) to reduce 30-day hospital readmissions following ETPS and (2) improve inpatient and outpatient coordination of care with endocrinologist.
Methods This study is a single institution temporal cohort study of patients prior to (control cohort) and after implementation of the POC pathway (intervention cohort). The POC pathway utilized postdischarge 1 to 1.5 L/d fluid restriction, postoperative days 5 to 7 serum sodium, and endocrinology follow-up within 1 week of discharge to stratify patients into tiered hyponatremia regimens.
Results A total of 542 patients were included in the study, 409 (75%) in the control cohort and 133 (25%) in the intervention cohort. All-cause readmission was significantly reduced following implementation of the POC pathway (14 vs. 6%, p = 0.015). Coordination with endocrinologist significantly increased in the inpatient (96 vs. 83%, p < 0.001) and outpatient (77 vs. 68%, p = 0.042) settings. Patients who were not in the POC pathway had the highest risk of readmission (odds ratio: 2.5; 95% confidence interval: 1.1–5.5).
Conclusion A multidisciplinary POC pathway incorporating endocrinologist in conjunction with postdischarge weight-based fluid restriction and postoperative serum sodium levels can safely be used to reduce 30-day readmissions following ETPS.
Keywords: quality improvement, postoperative care pathway, readmissions, transsphenoidal pituitary surgery, pituitary surgery, risk factors, endocrinology, coordination of care
Introduction
Endoscopic transsphenoidal pituitary surgery (ETPS) has become a very common procedure to address pathology involving the pituitary gland. 1 2 3 4 5 While morbidity and mortality following ETPS are low, postoperative complications are unique due to both surgical complications and metabolic derangements. 3 6 7 Perioperative management incorporating a multidisciplinary team of otolaryngologist, neurosurgeons, and endocrinologist is essential in providing high-quality patient care. 8 9
Thirty-day unplanned readmission rates for ETPS are reported to range from 4.6 to 13.9%. 6 10 11 12 13 14 15 16 17 18 Delayed hyponatremia is common following ETPS which can occur in up to 25% of patients, with more severe or symptomatic cases requiring readmission in up to 7.6% of patients. 6 11 17 19 20 21 The pathophysiology is consistent with the triphasic pituitary response characterized by early hypernatremia (diabetes insipidus [DI]), followed by delayed vasopressin release with hyponatremia, and finally, delayed hypernatremia from depleted vasopressin stores. 22 Readmission from delayed hyponatremia usually occurs on postoperative days (PODs) 5 to 7 which coincides with a nadir in serum sodium. 18 22
Although prior studies have linked white race, female sex, tumor size, preoperative hypopituitarism, and postoperative DI to delayed hyponatremia, there is no consensus agreement on reliable risk factors. Identification of patients at risk for delayed hyponatremia remains elusive. 10 18 20 23 24 25 Prior studies have utilized various postdischarge fluid restriction protocols and serum sodium laboratories within 1 week of surgery to identify patients at risk for delayed hyponatremia and reduce 30-day readmissions following ETPS. 26 27 28 29 30 31
Our team previously published a retrospective review of 409 patients who underwent ETPS with a 30-day readmission rate of 14%. Hyponatremia was the most common for readmission and accounted for 30% of all readmissions. Eighty-eight percent of those readmitted with delayed hyponatremia presented within 7 days of surgery. We identified postdischarge coordination with endocrinologist the only statistically significant protective factor for all-cause readmissions. 18
The findings of the prior study were used to develop a targeted multidisciplinary care pathway in conjunction with endocrinologist: (1) to reduce 30-day readmission following ETPS and (2) improve coordination of care with endocrinologist in the inpatient and outpatient settings.
Methods
Study Design
This is a single institution temporal cohort study compromised of patients prior to and after implementation of a postoperative care (POC) pathway. The preintervention cohort (control) underwent ETPS from December 2010 to 2018, while the postintervention cohort (intervention) underwent ETPS from July 2020 to May 2021 and included in the POC pathway. All patients who underwent ETPS for a pituitary adenoma were included. Patients were excluded from the study if they were <18 years of age at the time of surgery, or if they did not have a pituitary adenoma (i.e., craniopharyngioma, Rathke's cleft cyst, meningioma, malignancy, etc.). Patients were excluded from the intervention cohort if they were discharged with active DI requiring desmopressin treatment.
Postoperative Care Pathway
The POC pathway had two phases of care: (1) at the time of discharge and (2) postdischarge .
-
At discharge , patients:
Were placed on a weight-based fluid restriction protocol of 1 L/d if they weighed less than 100 kg or 1.5 L/d if they weighed more than 100 kg.
Received medication reconciliation with inpatient endocrinology.
Received an education pamphlet detailing POC including instructions to monitor for signs of sodium abnormalities.
-
During the postdischarge period , patients:
Continued fluid restriction until POD 10.
Had their serum sodium checked 5 to 7 days from surgery to coincide with the nadir in serum sodium.
Visited with our endocrinology team within 1 week of discharge when possible, or by telehealth when not possible.
Patients were stratified into a tiered treatment regimen based on their serum sodium values and severity of hyponatremia ( Fig. 1 ). Both cohorts were followed up for 30 days from discharge. The electronic health record (EHR) was retrospectively reviewed to evaluate for incidence and causes of 30-day readmissions. Incidence of unplanned 30-day readmission was compared between the intervention cohort and the control cohort to assess the effectiveness of the POC pathway.
Fig. 1.
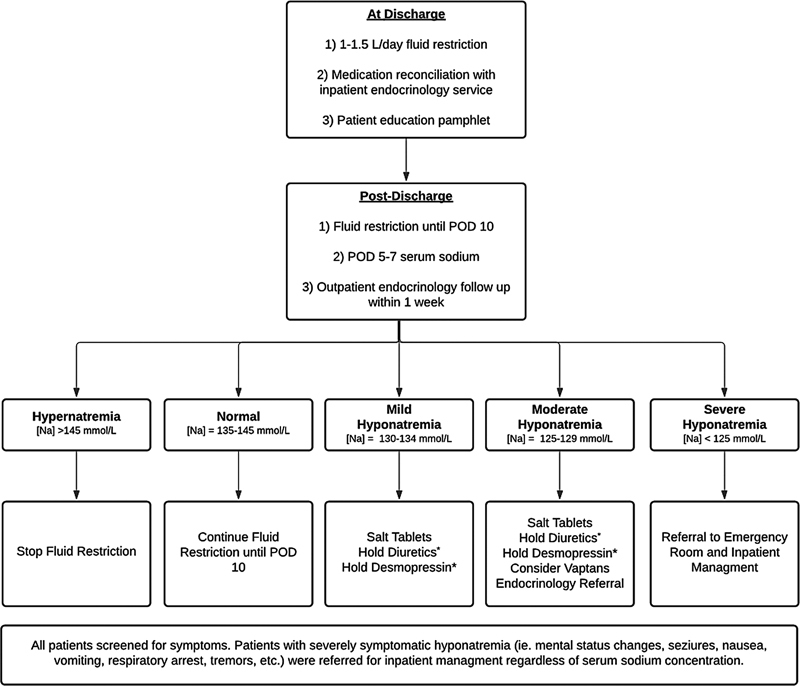
Postoperative care pathway. Na, serum sodium; POD, postoperative day; *, if applicable.
Variables and Definitions
Data were abstracted from the EHR system developed by Epic Systems (Epic Systems Corporation, Madison, Wisconsin, United States). Variables collected included patient demographics (age, sex, race, ethnicity, and body mass index [BMI]), clinical variables (relevant dates, laboratory values, complications, causes of readmission, and comorbidities), and relevant operative and radiologic variables (tumor size, skull base repair, intraoperative findings, and pathology).
Readmission refers to 30-day unplanned hospital readmission from the day of discharge. Adenomas were classified as either nonfunctioning or functioning depending on secretory characteristics based on preoperative laboratory analyses or permanent pathology. Radiologic data were used to calculate tumor volume. Intraoperative cerebrospinal fluid (CSF) leaks were classified as either (1) “ no leak” (absence of CSF leak confirmed by Valsalva maneuver), (2) “low-flow leak” (confirmed by Valsalva with small diaphragmatic defect), or (3) “high-flow leak” (moderate or large CSF leak usually with large diaphragmatic defect or opening of the arachnoid). 32 33 Per our ETPS standard postoperative protocol, patients are admitted to the neurosurgical intensive care unit for monitoring. Patients were retrospectively considered to have developed DI if they required treatment with desmopressin. Criteria for treatment with desmopressin includes urine output of > 250 mL/h for 2 consecutive hours, urine specific gravity of <1.005, and either a rising serum sodium or a serum sodium of ≥145 mmol/L. Postoperative steroid replacement was with hydrocortisone 10 mg/m 2 (∼10–25 mg) daily in two divided doses. Steroids were continued for 2 weeks until a serum cortisol was checked. The EHR was reviewed to determine utilization of inpatient endocrinology consultations and the pattern of postoperative follow-up with the endocrinologist (categorial variable: institutional, private/community, or none).
Data Analysis
The primary outcome of interest was unplanned 30-day hospital readmission from all causes. All statistical analyses were completed on Stata 14.0 software (StataCorp, College Station, Texas, United States). Two-tailed t -test was used to compare continuous variables between the groups. The Pearson's chi-square and Fisher's exact tests were used to compare categorical variables between the groups. Univariable and multivariable logistic regression were performed to identify predictors of readmission as well as risk and odds ratios (ORs). Incidence of readmissions between the control and intervention cohorts were used to calculate absolute risk reduction (ARR) and relative risk reduction (RRR). All statistics were two tailed and considered statistically significant if p < 0.05.
Institutional Review Board Approval
This study was approved by the University of Miami Miller School of Medicine Institutional Review Board (IRB #20181136).
Results
Patient Demographics and Readmission Rates
A total of 542 patients were included in the study; 409 patients were in the control cohort and 133 in the intervention cohort. The majority of the cohort was female (54%) and of white race (64%) and non-Hispanic (59%). Mean age of the cohort was 54.0 ± 16.4 years. Thirty-one percent of adenomas were functioning. There were no statistical differences between the cohorts with respect to age, sex, race, BMI, secretory adenomas, tumor volume, intraoperative CSF leaks, nasal septal flap repair, and length of stay ( Table 1 ). Additional demographic and population characteristics for patients readmitted and not readmitted in the intervention cohort are provided in Table 2 .
Table 1. Demographics and population characteristics of the entire cohort.
| Demographic factor | Entire cohort | Control | Intervention | p -Value a | |
|---|---|---|---|---|---|
| n (%) | 542 | 409 (75.5%) | 133 (24.5%) | ||
| Readmission, all cause | 65 (12.0%) | 57 (13.9%) | 8 (6.0%) | 0.015 | |
| Readmission, hyponatremia | 20 (3.7%) | 17 (4.2%) | 3 (2.3%) | 0.31 | |
| Admission age, y (mean ± SD) | 54.0 ± 16.4 | 53.9 ± 16.5 | 54.6 ± 16.2 | 0.65 | |
| Female sex | 294 (54.2%) | 219 (53.5%) | 75 (56.4%) | 0.57 | |
| Race | White | 348 (64.2%) | 262 (64.1%) | 86 (64.7%) | 0.99 |
| African American | 108 (19.9%) | 82 (20.0%) | 26 (19.5%) | ||
| Other | 86 (15.9%) | 65 (15.9%) | 21 (15.8%) | ||
| Hispanic | 224 (41.2%) | 163 (39.8) | 61 (45.9%) | 0.221 | |
| BMI (mean ± SD) | 30.0 ± 6.7 | 29.8 ± 6.7 | 30.4 ± 6.6 | 0.39 | |
| Functioning adenoma | 158 (31.0%) | 119 (39.2%) | 39 (33.6%) | 0.484 | |
| Tumor volume, cm 3 (mean ± SD) | 12.8 ± 17.1 | 12.8 ± 16.9 | 12.7 ± 17.9 | 0.94 | |
| Intraoperative CSF leak | Any leak | 296 (54.6%) | 218 (53.3%) | 78 (58.6%) | 0.28 |
| Low-flow leak | 199 (67.2%) | 153 (70.2% | 46 (59.0%) | 0.10 | |
| High-flow leak | 97 (32.8%) | 65 (29.8%) | 32 (41.0%) | ||
| Nasal septal flap | 69 (12.9%) | 50 (12.2%) | 19 (15.2%) | 0.39 | |
| Length of stay, d (mean ± SD) | 2.7 ± 1.9 | 2.8 ± 2.1 | 2.5 ± 1.4 | 0.17 | |
| Endocrine, inpatient | 467 (86.5%) | 340 (83.3%) | 127 (96.2%) | <0.001 | |
| Endocrine, outpatient | 380 (70.2%) | 278 (68.0%) | 102 (77.3%) | 0.042 | |
| Type of outpatient endocrine | Institution | 229 (59.8%) | 146 (52.1%) | 83 (80.6%) | <0.001 |
| Private | 154 (40.2%) | 134 (47.9%) | 20 (19.4%) | ||
| Postoperative diabetes insipidus | 236 (43.9%) | 183 (45.1%) | 53 (40.2%) | 0.32 | |
Abbreviations: BMI, body mass index; CSF, cerebrospinal fluid; SD, standard deviation.
Note: Values presented as number (%) unless otherwise indicated. Bold indicates statistically significant p -Values.
Reflects two-tailed t -test for continuous variables or a Pearson's chi-square/Fisher's exact test to compare categorical data between the groups.
Table 2. Demographics and population characteristics of the intervention cohort stratified by readmission.
| Demographic factor | Intervention cohort | Not readmitted | Readmitted | p -Value a | |
|---|---|---|---|---|---|
| n (%) | 133 (100%) | 125 (94.0%) | 8 (6.0%) | ||
| Readmission, hyponatremia | 3 (2.3%) | 3 (37.5%) | |||
| Admission age, y (mean ± SD) | 54.6 ± 16.2 | 55.2 ± 14.7 | 44.7 ± 31.7 | 0.075 | |
| Sex (female) | 75 (56.4%) | 70 (56.0%) | 5 (62.5%) | 0.72 | |
| Race | White | 86 (64.7%) | 80 (64.0%) | 6 (75.0%) | 0.81 |
| Hispanic | 61 (45.9) | 58 (46.4%) | 3 (37.5%) | 0.624 | |
| BMI (mean ± SD) | 30.4 ± 6.6 | 30.5 ± 6.6 | 28.7 ± 6.1 | 0.44 | |
| Length of stay, d (mean ± SD) | 2.5 ± 1.4 | 2.5 ± 1.4 | 2.6 ± 0.7 | 0.79 | |
| Functioning adenoma | 39 (33.0%) | 36 (33.0%) | 3 (42.9%) | 0.594 | |
| Histology | Lactotroph | 17 (12.9%) | 16 (12.9%) | 1 (12.5%) | 0.86 |
| Somatotroph | 6 (4.5%) | 6 (4.8%) | 0 (0.0%) | ||
| Corticotroph | 19 (14.4%) | 18 (14.5%) | 1 (12.5%) | ||
| Gonadotroph | 59 (44.7%) | 56 (45.2%) | 3 (37.5%) | ||
| Multiple | 31 (23.5%) | 28 (22.6%) | 3 (37.5%) | ||
| Intraoperative CSF leak | Any leak | 78 (58.6%) | 73 (58.4%) | 5 (62.5%) | 0.82 |
| Low-flow leak | 46 (34.6%) | 45 (61.6%) | 1 (20%) | 0.067 | |
| High-flow leak | 32 (24.1%) | 28 (38.4%) | 4 (75%) | ||
| Nasal septal flap | 19 (15.2%) | 17 (14.5%) | 2 (25.0%) | 0.42 | |
| Tumor volume, mm 3 (mean ± SD) | 12,656.2 ± 17,869.0 | 12,569.2 ± 17,821.2 | 14,048.7 ± 20,041.6 | 0.83 | |
| Endocrine, inpatient | 127 (96.2%) | 120 (96.8%) | 7 (87.5%) | 0.18 | |
| Endocrine, outpatient | 102 (77.3%) | 96 (77.4%) | 6 (75.0%) | 0.87 | |
| Type of outpatient endocrine | University of Miami | 83 (80.6%) | 78 (80%) | 5 (83%) | 0.86 |
| Private | 20 (19.4%) | 19 (20%) | 1 (17%) | ||
| Serum sodium checked | 81 (62.3%) | 75 (60.0% | 6 (75%) | 0.217 | |
| Serum sodium, mmol/L (mean ± SD) | 139.0 ± 4.1 | 139.4 ± 3.6 | 135.0 ± 6.7 | 0.004 | |
| Hyponatremia | Any | 11 (14%) | 10 (13.3%) | 1 (16.7%) | 0.040 |
| Mild | 9 (11.1%) | 9 (12%) | 0 (0%) | ||
| Moderate | 2 (2.5%) | 1 (1.3%) | 1 (16.7%) | <0.001 | |
| Postoperative diabetes insipidus | 53 (40.2%) | 47 (37.9%) | 6 (75.0%) | 0.038 | |
Abbreviations: BMI, body mass index; CSF, cerebrospinal fluid; SD, standard deviation.
Note: Values presented as number (%) unless otherwise indicated. Bold indicates statistically significant p -Values.
Reflects two-tailed t -test for continuous variables or a Pearson's chi-square/Fisher's exact test to compare categorical data between the groups.
There was significant reduction in all-cause 30-day readmissions from 14 to 6% ( p = 0.015) following implementation of the POC pathway. Hyponatremia was the most common cause of readmission in both cohorts; however, there was a nonsignificant trend toward decreased incidence of readmission from hyponatremia from 4 to 2% following the POC pathway ( p = 0.31). There was a significant ARR for all-cause readmission of 8% (95% confidence interval [CI]: 3–13%) and significant RRR of 57% (95% CI: 12–79%). ARR for readmission from hyponatremia was 2% (95% CI: −1 to 5%), and RRR 46% (95% CI: −82 to 84%) ( Table 3 ).
Table 3. Causes of readmission and risk reduction.
| Cause for readmission | Control | Intervention | ARR (95% CI) | RRR (95% CI) | p -Value |
|---|---|---|---|---|---|
| n (%) | 409 (75.5%) | 133 (24.5%) | |||
| All cause | 57 (13.9%) | 8 (6.0%) | 7.9 (2.7–13.2) | 56.8 (11.9–78.9) | 0.0146 |
| Hyponatremia | 17 (4.2%) | 3 (2.3%) | 1.9 (−1.3–5.1) | 45.7 (−82.3–83.8) | 0.3124 |
| Hypernatremia | 5 (1.2%) | 1 (0.8%) | 0.5 (−1.3–2.3) | 38.5 (−422–92.8) | 0.6523 |
| Adrenal insufficiency | 5 (1.2%) | 1 (0.8%) | 0.5 (−1.3–2.3) | 38.5 (−422–92.8) | 0.6523 |
| Pain/headache | 16 (3.9%) | 4 (3.0%) | 0.9 (−2.6–4.4) | 23.1 (−125–80.1) | 0.6308 |
| CSF leak | 14 (3.4%) | 3 (2.3%) | 1.2 (−1.9–4.2) | 34.1 (−125–80.8) | 0.5023 |
| Epistaxis | 11 (2.7%) | 2 (1.5%) | 1.8 (−1.4–3.8) | 44.1 (−149–87.4) | 0.4375 |
Note: Bold indicates statistically significant p -Values.
Abbreviations: ARR, absolute risk reduction; CI, confidence interval; CSF, cerebrospinal fluid; RRR, relative risk reduction.
Coordination with Endocrinologist and Incidence of Delayed Hyponatremia
There was a significant increase in patients seen by endocrinology as an inpatient (83 vs. 96%; p < 0.001) and outpatient (68 vs. 77%; p = 0.0.42) following implementation of the POC pathway. Follow-up with institutional endocrinologist increased significantly from 52 to 81% ( p < 0.001) ( Table 1 ). Serum sodium was collected during PODs 5 to 7 in 62% of patients in the intervention cohort. Mean serum sodium was 139 ± 4.1 mmol/L. Serum sodium in those who were readmitted following the POC pathway was significantly lower than those who were not readmitted in the POC pathway (135.0 ± 4.1 vs. 139.4 0 ± 3.6 mmol/L; p = 0.004) ( Table 2 ).
In the intervention cohort, hyponatremia was identified in 11 (14%) patients and hypernatremia was identified in 1 (1.2%) patient who had their serum sodium checked. Of the 11 patients with delayed hyponatremia, 9 patients (81%) had mild hyponatremia and 2 patients (18%) had moderate hyponatremia. One of the 11 patients (9%) with delayed hyponatremia required readmission for treatment (see later). The remaining 10 patients (91%) were managed uneventfully as an outpatient with 1 L fluid restriction and salt tablets. The patient with hypernatremia had a serum sodium of 147 mmol/L, which required no additional management other than discontinuing fluid restriction.
Causes of Readmission
In the intervention cohort, a total of eight patients (6%) were readmitted, of whom three patients (38%) were readmitted with hyponatremia ( Table 2 ).
Of the three patients who were readmitted with hyponatremia, one had moderate hyponatremia identified on their postoperative serum sodium requiring readmission on POD 7. The two other patients were readmitted with moderate hyponatremia within 3 days of discharge, prior to being captured by the postdischarge laboratory check. Each patient readmitted with hyponatremia underwent fluid restriction to 1 L and received 2 g of salt tablets. One of the patients readmitted with hyponatremia had a concurrent CSF leak that required surgical repair. None of the hyponatremia readmissions required hypertonic saline.
One patient was readmitted with hypernatremia of 147 mmol/L and acute kidney injury 26 days from discharge. However, this patient's PODs 5 to 7 serum sodium was 141 mmol/L. This patient was treated with desmopressin and told to drink to thirst. There were no significant differences in the rate of readmission from hypernatremia between our control and intervention cohort following implementation of the POC pathway. The other causes of readmission are outlined in Table 3 .
Unwarranted Emergency Room Visits
When looking at unplanned emergency room (ER) visits that did not lead to an admission, 10% of patients in the control cohort and 6% of those in the intervention cohort were seen in the ER ( p = 0.1685) within 30 days of discharge. However, the incidence of ER visits due to hyponatremia significantly decreased from 3.7 to 0% following implementation of the POC pathway ( p = 0.0279). While not significant, we did not have any unplanned ER visits from hypernatremia following implementation of the POC pathway. Visits from adrenal insufficiency, pain, CSF leaks, and epistaxis were similar between both cohorts ( Table 4 ).
Table 4. Unplanned emergency room visits without subsequent admission.
| Reason for ER visit | Control | Intervention | ARR (95% CI) | p -Value |
|---|---|---|---|---|
| n (%) | 409 (75.5%) | 133 (24.5%) | ||
| All cause | 42 (10.3%) | 8 (6.0%) | 4.3 (−0.7–9.3) | 0.1685 |
| Hyponatremia | 15 (3.7% | 0 (0%) | 3.7 (1.6–5.8) | 0.0279 |
| Hypernatremia | 2 (0.5% | 0 (0%) | 0.5 (−0.8–1.8) | 1.0 |
| Adrenal insufficiency | 5 (1.2%) | 2 (1.5%) | 0.3 (−2.0–2.6) | 0.6822 |
| Pain/headache | 10 (2.4%) | 3 (2.3%) | 0.2 (−2.7–3.1) | 1.0 |
| CSF leak | 5 (1.2%) | 1 (0.8%) | 0.5 (−1.3–2.3) | 1.0 |
| Epistaxis | 5 (1.2%) | 2 (1.5%) | 0.3 (−2.1–2.6) | 0.6822 |
Abbreviations: ARR, absolute risk reduction; CI, confidence interval; CSF, cerebrospinal fluid; ER, emergency room; RRR, relative risk reduction.
Multivariable Analysis and Predictors of Readmission
When looking at only the patients readmitted with hyponatremia in the entire cohorts or the intervention cohort, none of the variables in this study was predictive of 30-day readmission from hyponatremia on either univariable or multivariable analysis. However, when analyzing risk factors for all-cause readmission across the entire cohort, multivariable analysis found patients in the control cohort at the greatest risk of readmission compared with those in the intervention cohort (OR: 2.5; 95% CI: 1.1–5.5). Other significant risks for readmission included patients of white race and those without outpatient endocrinology follow-up ( Table 5 ).
Table 5. Univariable and multivariable odds ratio for all-cause 30-day readmissions.
| Risk factor | Univariable analysis | Multivariable analysis | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p -Value | OR | 95% CI | p -Value | |
| White | 2.0 | 1.1–3.7 | 0.025 | 2.2 | 1.2–4.1 | 0.014 |
| No endocrine follow-up | 1.8 | 1.1–3.1 | 0.028 | 1.9 | 1.1–3.2 | 0.02 |
| Control cohort | 2.5 | 1.2–5.5 | 0.018 | 2.5 | 1.1–5.5 | 0.023 |
| High-flow CSF leak | 2.2 | 1.1–4.1 | 0.021 | 1.7 | 0.8–3.7 | 0.196 |
| NSF | 2.1 | 1.1–4.0 | 0.03 | 1.4 | 0.6–3.3 | 0.382 |
Note: Bold indicates statistically significant p -Values.
Abbreviations: CI, confidence interval; CSF, cerebrospinal fluid; NSF, nasoseptal flap; OR, odds ratio.
Discussion
Delayed hyponatremia is the most common cause of readmission following ETPS and most commonly occurs 5 to 7 days from surgery. Prior studies have utilized various strategies including postoperative serum sodium and fluid restriction protocols to decrease 30-day readmissions.
Bohl et al published their POC pathway which utilized a PODs 5 to 7 screening serum sodium to identify patients with hyponatremia. However, fluid restriction was only utilized following identification of hyponatremia, and thus, they were not able to reduce readmissions from hyponatremia. 31
Other studies have shown success in managing hyponatremia using fluid restriction preemptively. Prior studies using a 2.5 and 1.6 to 1.8 L/d fluid restriction protocols demonstrated significant reduction in the incidence of postoperative delayed hyponatremia. 28 29
Deaver et al used a 1.5 L/d fluid restriction protocol and a 7-day serum sodium from discharge to significantly reduce readmissions from hyponatremia from 8 to 2% ( p = 0.04). 27 Similarly, Burke et al used a 1 L/d fluid restriction protocol and a 1-week postoperative serum sodium to significantly reduce readmission from hyponatremia from 3 to 0% ( p = 0.003) and the incidence of delayed hyponatremia (38 vs. 14%, p < 0.001). 30 Neither study found an increased risk of readmission from hypernatremia in fluid restricted cohorts. Finally, Snyder et al used a 1 L/d 7 postoperative fluid restriction and a PODs 7 to 10 serum sodium, and while they found significantly lower rates of postoperative syndrome of inappropriate antidiuretic hormone secretion (5 vs. 15%, p = 0.01), they were not able to demonstrate a reduction in hospital readmissions. 34 Other investigators have reported on modifications of fluid restriction protocols starting on PODs 4 to 8 in 57 patients and found no incidence or readmissions from hyponatremia. 26
Findings from our previously published quality improvement review was used to develop a targeted, multidisciplinary care pathway utilizing 1 to 1.5 L/d of fluid restriction, postdischarge serum sodium, and a tiered treatment regimen in coordination with endocrinologist. Following implementation of our POC pathway, we were able to significantly reduce all-cause 30-day readmissions from 14 to 6%. While not statistically significant, we were able to reduce readmissions from delayed hyponatremia from 4 to 2%, an RRR of 47%, which implies a clinically significant improvement in quality of patient care. Additionally, we were able to significantly reduce unplanned ER visits from hyponatremia following implementation of the POC pathway from 3.7 to 0% without increased incidence of unplanned ER visits from hypernatremia. Furthermore, multivariate analysis showed that those in the control cohort were at higher risk of readmission.
These findings suggest that fluid restriction protocol and utilization of endocrinologist may contribute to lower incidence of postoperative hyponatremia. While we were not able to show significant reduction in readmissions from hyponatremia, the data show trends toward lower incidence of readmission from hyponatremia with hyponatremia having the most absolute reduction in incidence of those readmitted. The data on unplanned ER visits among those who were not readmitted further demonstrate a significant reduction in hyponatremia. This likely represents the ability of the POC pathway to identify patients at risk of hyponatremia and exercise interventions in coordination with our endocrinologist to limit worsening sodium levels. The authors believe that with a higher powered study, statistical difference in readmissions from hyponatremia will be identified.
The incidence of hyponatremia in our intervention cohort was 14% based on the postoperative serum sodium laboratories. We are not able to determine the incidence of hyponatremia in our control cohort since postoperative serum sodium laboratories were not routinely part of our postdischarge care pathway. Therefore, we cannot determine the change in the incidence of delayed hyponatremia between our control and intervention cohorts following implementation of our POC pathway. However, the incidence of delayed hyponatremia in our intervention cohort is similar to those reported in other fluid restricted cohorts. 28 29 30 Finally, we did not have any significant changes in the rate of readmissions from delayed hypernatremia between our cohorts following implementation of our POC pathway.
One of the strengths of our POC pathway was integrating endocrinologist routinely in the inpatient and outpatient care of patients undergoing ETPS. This enabled creation of multidisciplinary care team for patients with health care providers who can manage and educate patients on surgical and metabolic issues that may occur following surgery. Endocrinologist can provide patients additional education on metabolic changes such as adrenal insufficiency and electrolyte abnormalities that may occur postoperatively. Furthermore, inclusion of endocrinologist in the inpatient setting can facilitate access to outpatient care. Other studies also implemented follow-up with either a nurse practitioner, neurosurgeon, or endocrinologist in their POC pathways demonstrating success in reducing the incidence of delayed hyponatremia 26 as well as readmissions 27 from delayed hyponatremia.
Our findings in conjunction with the findings from the aforementioned studies suggest that 1 L to 1.5 L/d fluid restriction can safely be used to lower the incidence of postoperative hyponatremia and hospital readmissions. Higher powered studies may ultimately demonstrate statistical significance similar to Deaver et al and Burke et al.
Limitations
This study has several limitations. Due to the overall low rate of readmissions following ETPS, lack of power may have limited identification of statistically significant predictor variables. While we were not able to demonstrate a statistically significant decrease in readmissions from hyponatremia, it would be reasonable to attribute the decrease in all-cause readmission to increase hospital awareness of readmissions as well as advancements in endoscopic surgery and reconstructive techniques. However, the additional findings of significantly reduced ER visits related to hyponatremia following implementation of the POC pathway provide additional support for the effectiveness of the pathway. Our POC pathway is ongoing; with inclusion of more patients in our POC pathway, we may be able to identify additional statistically significant variables in future analysis. Future studies with larger cohorts may be powered enough to detect these small changes. Additionally, a meta-analysis of the current literature can help identify significant variables that may predict or reduce readmissions. Second, as a tertiary center with a large referral area, patients may have been readmitted to an outside facility potentially leading to underestimation of readmission rates. This issue was minimized by our protocol to see all ETPS patients 6 to 12 weeks after surgery, capturing information on 30-day readmission at another facility. It is also difficult to completely characterize the extent of patient adherence to fluid restriction. However, medication reconciliation and discharge pamphlets attempted to educate patients to the best of our ability to facilitate adherence to the protocol. We also translated our educational pamphlet into Spanish to help overcome potential barriers in communication in our Spanish-speaking patients. Finally, while we were not able to show statistical differences in readmissions from delayed hyponatremia, we were able to show statistical differences in all-cause readmission. While these findings may also reflect institutional changes in overall management during the study period, the reduction in other causes of readmission such as adrenal insufficiency, pain, CSF leaks, and epistaxis was not as strong. We also showed a significant decrease in unplanned ER visits from hyponatremia without significant differences in other causes of unplanned ER visits which also supports the utilization of the POC pathway to reduce the incidence of delayed hyponatremia.
Conclusion
Delayed hyponatremia is common following ETPS and one of the most common causes of hospital readmissions. Patients are usually readmitted on PODs 5 to 7 coinciding with a nadir in serum sodium following the triphasic pituitary response. A targeted, multidisciplinary care pathway with emphasis on fluid restriction, postoperative sodium level testing, and coordination of endocrinology follow-up significantly impacted 30-day readmission in our cohort. Utilization of 1 to 1.5 L/d of fluid restriction at the time of discharge and a serum sodium within 1 week of surgery can safely be suggested to reduce readmissions following ETPS and improve quality of patient care.
Conflict of Interest None declared.
Note
Interim results of this study were presented at the 31st Annual North American Skull Base Society Meeting in Phoenix, Arizona, United States.
References
- 1.Chibbaro S, Ganau M, Gubian A. The role of endoscopic endonasal approach in the multimodal management of giant pituitary adenoma: case report and literature review. Asian J Neurosurg. 2018;13(03):888–892. doi: 10.4103/ajns.AJNS_97_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gittleman H, Ostrom Q T, Farah P D. Descriptive epidemiology of pituitary tumors in the United States, 2004-2009. J Neurosurg. 2014;121(03):527–535. doi: 10.3171/2014.5.JNS131819. [DOI] [PubMed] [Google Scholar]
- 3.Gondim J A, Almeida J P, Albuquerque L A. Endoscopic endonasal approach for pituitary adenoma: surgical complications in 301 patients. Pituitary. 2011;14(02):174–183. doi: 10.1007/s11102-010-0280-1. [DOI] [PubMed] [Google Scholar]
- 4.Messerer M, Cossu G, George M, Daniel R T. Endoscopic endonasal trans-sphenoidal approach: minimally invasive surgery for pituitary adenomas. J Vis Exp. 2018;(131):55896. doi: 10.3791/55896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Villwock J A, Villwock M, Deshaies E, Goyal P. Significant increases of pituitary tumors and resections from 1993 to 2011. Int Forum Allergy Rhinol. 2014;4(09):767–770. doi: 10.1002/alr.21356. [DOI] [PubMed] [Google Scholar]
- 6.Alzhrani G, Sivakumar W, Park M S, Taussky P, Couldwell W T. Delayed complications after transsphenoidal surgery for pituitary adenomas. World Neurosurg. 2018;109:233–241. doi: 10.1016/j.wneu.2017.09.192. [DOI] [PubMed] [Google Scholar]
- 7.Chowdhury T, Prabhakar H, Bithal P K, Schaller B, Dash H H. Immediate postoperative complications in transsphenoidal pituitary surgery: a prospective study. Saudi J Anaesth. 2014;8(03):335–341. doi: 10.4103/1658-354X.136424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fan Y P, Lv M H, Feng S Y. Full endoscopic transsphenoidal surgery for pituitary adenoma-emphasized on surgical skill of otolaryngologist. Indian J Otolaryngol Head Neck Surg. 2014;66 01:334–340. doi: 10.1007/s12070-011-0317-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carminucci A S, Ausiello J C, Page-Wilson G. Outcome of implementation of a multidisciplinary team approach to the care of patients after transsphenoidal surgery. Endocr Pract. 2016;22(01):36–44. doi: 10.4158/EP15894.OR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bur A M, Brant J A, Newman J G. Incidence and risk factors for prolonged hospitalization and readmission after transsphenoidal pituitary surgery. Otolaryngol Head Neck Surg. 2016;155(04):688–694. doi: 10.1177/0194599816652379. [DOI] [PubMed] [Google Scholar]
- 11.Hendricks B L, Shikary T A, Zimmer L A. Causes for 30-day readmission following transsphenoidal surgery. Otolaryngol Head Neck Surg. 2016;154(02):359–365. doi: 10.1177/0194599815617130. [DOI] [PubMed] [Google Scholar]
- 12.Cote D J, Dasenbrock H H, Muskens I S. Readmission and other adverse events after transsphenoidal surgery: prevalence, timing, and predictive factors. J Am Coll Surg. 2017;224(05):971–979. doi: 10.1016/j.jamcollsurg.2017.02.015. [DOI] [PubMed] [Google Scholar]
- 13.Barber S M, Liebelt B D, Baskin D S. Incidence, etiology and outcomes of hyponatremia after transsphenoidal surgery: experience with 344 consecutive patients at a single tertiary center. J Clin Med. 2014;3(04):1199–1219. doi: 10.3390/jcm3041199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graboyes E M, Kallogjeri D, Saeed M J, Olsen M A, Nussenbaum B. Postoperative care fragmentation and thirty-day unplanned readmissions after head and neck cancer surgery. Laryngoscope. 2017;127(04):868–874. doi: 10.1002/lary.26301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rizvi Z H, Ferrandino R, Luu Q, Suh J D, Wang M B. Nationwide analysis of unplanned 30-day readmissions after transsphenoidal pituitary surgery. Int Forum Allergy Rhinol. 2019;9(03):322–329. doi: 10.1002/alr.22241. [DOI] [PubMed] [Google Scholar]
- 16.Shaftel K A, Cole T S, Little A S. National trends in hospital readmission following transsphenoidal surgery for pituitary lesions. Pituitary. 2020;23(02):79–91. doi: 10.1007/s11102-019-01007-0. [DOI] [PubMed] [Google Scholar]
- 17.Younus I, Gerges M M, Dobri G A, Ramakrishna R, Schwartz T H. Readmission after endoscopic transsphenoidal pituitary surgery: analysis of 584 consecutive cases. J Neurosurg. 2019:1–6. doi: 10.3171/2019.7.JNS191558. [DOI] [PubMed] [Google Scholar]
- 18.Ghiam M K, Chyou D E, Dable C L. 30-day readmissions and coordination of care following endoscopic transsphenoidal pituitary surgery: experience with 409 patients. J Neurol Surg B Skull Base. 2021;83 02:e410–e418. doi: 10.1055/s-0041-1729980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krogh J, Kistorp C N, Jafar-Mohammadi B, Pal A, Cudlip S, Grossman A. Transsphenoidal surgery for pituitary tumours: frequency and predictors of delayed hyponatraemia and their relationship to early readmission. Eur J Endocrinol. 2018;178(03):247–253. doi: 10.1530/EJE-17-0879. [DOI] [PubMed] [Google Scholar]
- 20.Bohl M A, Ahmad S, Jahnke H. Delayed hyponatremia is the most common cause of 30-day unplanned readmission after transsphenoidal surgery for pituitary tumors. Neurosurgery. 2016;78(01):84–90. doi: 10.1227/NEU.0000000000001003. [DOI] [PubMed] [Google Scholar]
- 21.AACE Neuroendocrine and Pituitary Scientific Committee . Woodmansee W W, Carmichael J, Kelly D, Katznelson L, Neuroendocrine A. Pituitary scientific C. American Association of Clinical Endocrinologists and American College of Endocrinology Disease State Clinical Review: postoperative management following pituitary surgery. Endocr Pract. 2015;21(07):832–838. doi: 10.4158/EP14541.DSCR. [DOI] [PubMed] [Google Scholar]
- 22.Olson B R, Gumowski J, Rubino D, Oldfield E H. Pathophysiology of hyponatremia after transsphenoidal pituitary surgery. J Neurosurg. 1997;87(04):499–507. doi: 10.3171/jns.1997.87.4.0499. [DOI] [PubMed] [Google Scholar]
- 23.Hensen J, Henig A, Fahlbusch R, Meyer M, Boehnert M, Buchfelder M. Prevalence, predictors and patterns of postoperative polyuria and hyponatraemia in the immediate course after transsphenoidal surgery for pituitary adenomas. Clin Endocrinol (Oxf) 1999;50(04):431–439. doi: 10.1046/j.1365-2265.1999.00666.x. [DOI] [PubMed] [Google Scholar]
- 24.Jahangiri A, Wagner J, Tran M T. Factors predicting postoperative hyponatremia and efficacy of hyponatremia management strategies after more than 1000 pituitary operations. J Neurosurg. 2013;119(06):1478–1483. doi: 10.3171/2013.7.JNS13273. [DOI] [PubMed] [Google Scholar]
- 25.Taylor S L, Tyrrell J B, Wilson C B.Delayed onset of hyponatremia after transsphenoidal surgery for pituitary adenomas Neurosurgery 19953704649–653., discussion 653–654 [DOI] [PubMed] [Google Scholar]
- 26.Winograd D, Staggers K A, Sebastian S, Takashima M, Yoshor D, Samson S L. An effective and practical fluid restriction protocol to decrease the risk of hyponatremia and readmissions after transsphenoidal surgery. Neurosurgery. 2020;87(04):761–769. doi: 10.1093/neuros/nyz555. [DOI] [PubMed] [Google Scholar]
- 27.Deaver K E, Catel C P, Lillehei K O, Wierman M E, Kerr J M. Strategies to reduce readmissions for hyponatremia after transsphenoidal surgery for pituitary adenomas. Endocrine. 2018;62(02):333–339. doi: 10.1007/s12020-018-1656-7. [DOI] [PubMed] [Google Scholar]
- 28.Takeuchi K, Nagatani T, Okumura E, Wakabayashi T.A novel method for managing water and electrolyte balance after transsphenoidal surgery: preliminary study of moderate water intake restriction Nagoya J Med Sci 201476(1-2):73–82. [PMC free article] [PubMed] [Google Scholar]
- 29.Matsuyama J, Ikeda H, Sato S, Yamamoto K, Ohashi G, Watanabe K. Early water intake restriction to prevent inappropriate antidiuretic hormone secretion following transsphenoidal surgery: low BMI predicts postoperative SIADH. Eur J Endocrinol. 2014;171(06):711–716. doi: 10.1530/EJE-14-0530. [DOI] [PubMed] [Google Scholar]
- 30.Burke W T, Cote D J, Iuliano S I, Zaidi H A, Laws E R. A practical method for prevention of readmission for symptomatic hyponatremia following transsphenoidal surgery. Pituitary. 2018;21(01):25–31. doi: 10.1007/s11102-017-0843-5. [DOI] [PubMed] [Google Scholar]
- 31.Bohl M A, Ahmad S, White W L, Little A S. Implementation of a postoperative outpatient care pathway for delayed hyponatremia following transsphenoidal surgery. Neurosurgery. 2018;82(01):110–117. doi: 10.1093/neuros/nyx151. [DOI] [PubMed] [Google Scholar]
- 32.Conger A, Zhao F, Wang X. Evolution of the graded repair of CSF leaks and skull base defects in endonasal endoscopic tumor surgery: trends in repair failure and meningitis rates in 509 patients. J Neurosurg. 2018;130(03):861–875. doi: 10.3171/2017.11.JNS172141. [DOI] [PubMed] [Google Scholar]
- 33.Umamaheswaran P, Krishnaswamy V, Krishnamurthy G, Mohanty S. Outcomes of surgical repair of skull base defects following endonasal pituitary surgery: a retrospective observational study. Indian J Otolaryngol Head Neck Surg. 2019;71(01):66–70. doi: 10.1007/s12070-018-1511-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Snyder M H, Asuzu D T, Shaver D E, Vance M L, Jane J A. Routine postoperative fluid restriction to prevent syndrome of inappropriate antidiuretic hormone secretion after transsphenoidal resection of pituitary adenoma. J Neurosurg. 2021;136(02):405–412. doi: 10.3171/2021.1.JNS203579. [DOI] [PubMed] [Google Scholar]


