Abstract
Purpose After developing a protocol for evaluating, diagnosing, and treating postoperative endocrinopathy both during the hospitalization and during the immediate discharge period following resection of pituitary adenomas, we sought to assess the impact of this protocol on quality outcomes.
Methods An IRB-exempt, quality improvement initiated, Health Insurance Portability and Accountability Act (HIPAA)-compliant retrospective comparison of a pre-and-post-protocol cohort of all patients undergoing endoscopic endonasal resection of pituitary adenomas at NYU Langone Medical Center from January 2013 to December 2018. Demographic characteristics of the patients and their tumors with their postoperative outcomes were recorded. Quality outcomes regarding number of laboratory studies sent, rate of diabetes insipidus, length of stay, and readmission rate were also recorded. Statistical analysis was performed between the pre- and post-protocol groups.
Results There was a significant reduction in laboratory studies sent per patient (55.66 vs. 18.82, p <0.001). This corresponded with an overall cost reduction in laboratory studies of $255.95 per patient. There was a decrease in the overall number of patients treated with DDAVP (21.4% in the pre-protocol group vs. 8.9% in the post-protocol group, p = 0.04). All post-protocol patients requiring DDAVP at discharge were identified by 48 hours. There was no significant change in length of stay or need for hydrocortisone supplementation postoperatively between the two groups. Length of stay was driven mostly by need for reoperation during initial hospitalization. There was no significant change in the rate of 30-day readmission.
Conclusion Implementation of a postoperative management protocol results in a more efficient diagnosis and management of endocrinopathy after pituitary adenoma surgery which translates to decreased cost.
Keywords: pituitary adenoma, endoscopic endonasal surgery, quality improvement, safety, cost effectiveness
Introduction
Surgical resection of pituitary adenomas can lead to transient or permanent endocrinologic imbalances with variable clinical manifestations. While most patients tend to have mild symptoms, if hormonal imbalances are not recognized and appropriately treated, the consequences can be severe and even life-threatening. In patients undergoing surgical resection of pituitary adenomas, there is a 10 to 30% rate of temporary diabetes insipidus (DI), with approximately 50% resolving within 1 week and 80% resolving at 3 months. 1 Adrenal insufficiency occurs in approximately 5% of patients and can result in an Addisonian crisis if left undiagnosed. 1 2 These are two conditions that are easily managed with desmopressin (DDAVP) and hydrocortisone when identified accurately and promptly.
In most hospital systems, there has been a push to maximize cost effectiveness without compromising patient safety, and thus improve quality of care. With this push, there has been a shift away from indiscriminately sending frequent laboratories toward a more selective approach when choosing which laboratories to send as well as the frequency of laboratory draws. The adoption of a protocol allows for a systematic method of evaluating patients which leads to early diagnosis and treatment of postoperative endocrine abnormalities. It also allows for coordination of care amongst different providers within the treatment team, including neurosurgery, otolaryngology, endocrinology, and neurocritical care. A combination of these factors leads to shorter intensive care unit courses and shorter overall length of hospitalizations.
A secondary goal in the successful management of patients undergoing surgery for pituitary tumors is to prevent readmission to the hospital. Many studies have evaluated readmission rates after pituitary surgery. A review of over 1,200 cases demonstrated a readmission rate of 8.5% with the most common cause being hyponatremia (29.5%). 3 To reduce the rate of readmission for hyponatremia, some groups have demonstrated the effective use of outpatient fluid restriction during the first week postoperatively. 4 Coordination of care between neurosurgeons and endocrinologists is vital in the management of pituitary tumor patients, both perioperatively as well as in the long term. In particular, in the near postoperative period, all patients should have close follow up-with laboratory studies. These outpatient laboratory studies should be closely monitored by either a neurosurgeon, an endocrinologist, or ideally both.
Materials and Methods
This is an Institutional Review Board (IRB)-exempt, quality improvement initiated, Health Insurance Portability and Accountability Act (HIPAA)-compliant retrospective comparison of a pre- and post-protocol cohort of all patients undergoing endoscopic endonasal transsphenoidal resection of pituitary adenomas at NYU Langone Medical Center from January 2013 until December 2018. During these timeframes, the surgeons and approach surgeons remained the same. From January 2013 until February 2018 (during the “pre-protocol” period), there were no consistent guidelines regarding the postoperative management of patients undergoing endoscopic resection of pituitary adenomas. Decisions regarding the frequency of surveillance laboratories for DI or hypoadrenalism, which laboratories to send for evaluating DI, and the timing of DDAVP and hydrocortisone administration in the postoperative period were up to the discretion of the surgeon and the covering inpatient endocrinologist. Additionally, there was no standard for the outpatient management regarding recommendations for fluid intake or follow-up laboratory studies.
In March 2018, a protocol approved by a multidisciplinary pituitary team and the Neurosurgery Quality Assurance Committee at our institution was adopted for both the inpatient and outpatient management of patients following endoscopic surgery for pituitary adenomas ( Supplementary Material , available in online version only). The protocol called for discontinuation of intravenous fluids once the patient is awake enough to tolerate adequate oral intake as well as removal of the foley catheter within 24 hours of surgery. It also specified which laboratories to be sent and at what frequency over the first 3 days postoperatively to evaluate for DI and hypoadrenalism. Triggers such as increased urine output (UOP >250 mL/h over 2 hours or >400 mL/h over 1 hour), elevated serum sodium (Na >145), and low urine specific gravity (SG <1.005) resulted in more frequent laboratory checks. Within the protocol, there was an algorithm for the treatment of DI based on the patient's level of wakefulness and the presence of an intact thirst mechanism. Finally, once patients were discharged, the protocol limited fluid intake in the outpatient setting to 1 L/d (four glasses of water per day) for 1 week. All patients had a basic metabolic panel 1 week postop. Labs relevant to the patient's particular pathology and clinical history were also sent at that time. Extent of resection was determined by a neuroradiologist with an MRI done 6 months after surgery. Readmissions included inpatient stays greater than 24 hours.
We retrospectively reviewed the data for the pre-protocol (January 2013 to February 2018) and post-protocol (March 2018 to December 2018) cohorts. Statistical analyses comparing the two groups were performed using IBM SPSS Statistics Version 25. Pearson Chi-square test was used to compare categorical variables. Students t -test was used to compare continuous variables. Multiple logistic regression was performed to determine independent factors associated with DI. Linear regression was used to evaluate factors associated with length of stay. p -Values less than or equal to 0.05 were considered statistically significant.
Results
A total of 171 patients were included in the study. There were 126 patients included in the pre-protocol group and 45 patients in the post-protocol group. There was no statistically significant difference in gender ( p = 0.133) or number of recurrent tumors treated ( p = 0.602) between the two groups. The average age of patients treated in the pre-protocol group was slightly more than those treated in the post-protocol group (53.6 vs. 47.8 years, p = 0.029). There was a lower percentage of microadenomas (6.3 vs. 15.6%, p = 0.024) and secreting tumors (18.3 vs. 35.6%, p = 0.018) in the pre-protocol group than in the post-protocol group ( Table 1 ).
Table 1. Table displaying patient demographics as well as tumor characteristics in the pre-protocol and post-protocol groups.
| Pre-protocol | Post-protocol | p -Value | |
|---|---|---|---|
| Gender | |||
| Male | 64 (50.8%) | 17 (37.8%) | 0.133 |
| Female | 62 (49.2%) | 28 (62.2%) | |
| Age | 53.6 | 47.8 | 0.029 |
| Tumor | |||
| New | 102 (81.1%) | 38 (84.4%) | 0.602 |
| Recurrent | 24 (19.0%) | 7 (15.6%) | |
| Macroadenoma | 118 (93.7%) | 37 (84.4%) | 0.024 |
| Microadenoma | 8 (6.3%) | 8 (15.6%) | |
| Secretion status | |||
| Non-secretor | 103 (81.7%) | 29 (64.4%) | 0.018 |
| Secretor | 23 (18.3%) | 16 (35.6%) | |
| Types of secretors | |||
| ACTH | 7 (5.6%) | 7 (15.6%) | 0.094 |
| GH | 11 (8.7%) | 5 (11.1%) | |
| Prolactin | 5 (4.0%) | 3 (6.7%) | |
| FSH | 0 (0%) | 1 (2.2%) |
Abbreviations: ACTH, adrenocorticotropic hormone; FSH, follicle-stimulating hormone; GH, growth hormone.
Gross total resection, determined by first postoperative imaging within 6 months of surgery, was achieved at similar rates between pre- and post-protocol groups (64.3 and 73.3%, p = 0.269). The rates of intraoperative cerebrospinal fluid leak (CSF) (49.2 vs. 55.6%, p = 0.465) and postoperative CSF leak (6.3 vs. 4.4%, p = 0.448) were not significantly different between the two groups ( Table 2 ). There was also no statistical difference in the rate of reoperation during the initial hospitalization (5.6 vs. 8.9%, p = 0.43).
Table 2. Table depicting surgical results, rates of intraoperative and postoperative CSF leaks, and need for reoperation during initial hospitalization in the pre and post-protocol groups.
| Pre-Protocol | Post-Protocol | p -Value | |
|---|---|---|---|
| Resection result | |||
| Gross total resection | 81 (64.3%) | 33 (73.3%) | 0.269 |
| Subtotal resection | 45 (35.7%) | 12 (26.7%) | |
| CSF leak during hospitalization | |||
| Intraoperative CSF leak | 62 (49.2%) | 25 (55.6%) | 0.465 |
| Intraoperative lumbar drain | 17 (13.5%) | 2 (4.4%) | 0.105 |
| Postoperative CSF leak | 8 (6.3%) | 1 (2.2%) | 0.448 |
| Postoperative lumbar drain | 8 (6.3%) | 2 (4.4%) | 1 |
| Reoperation during initial hospitalization | 7 (5.6%) | 4 (8.9%) | 0.43 |
| CSF leak | 4 (3.2%) | 0 (0%) | 0.15 |
| Residual tumor | 2 (1.6%) | 2 (4.4%) | |
| Other | 1 (0.8%) | 2 (4.4%) |
Abbreviation: CSF, cerebrospinal fluid.
Evaluation of laboratories sent for DI during the first 24 hours ( Table 3 ) demonstrated a significant decrease in the number of serum sodium blood tests (5.04 vs. 4.31, p = 0.002) and urinalyses (4.45 vs. 3.0 p <0.001). There was no significant difference in the number of patients that reached trigger values in terms of urine output ( p = 0.927 for UOP >400 mL/h × 1 hour and p = 0.149 for UOP >250 mL/h × 2 hours), sodium level ( p = 0.901), or specific gravity ( p = 0.915) during the first 24 hours. However, there was a significant decrease in the number of patients treated with DDAVP during the first 24 hours (11.9 vs. 2.22%, p = 0.043). Between 24 and 48 hours, the only significant difference was in the number of urinalyses sent (2.87 vs. 2.24, p = 0.015). Overall, there were less patients treated with DDAVP in the post-protocol group (21.4 vs. 8.89%, p = 0.044) during their hospitalization. However, there was no significant difference in patients ultimately discharged on DDAVP (12.7 vs. 4.44%, p = 0.161). All patients who ultimately required DDAVP upon discharge were given their first dose of DDAVP by 48 hours in the post-protocol group. Conversely, in the pre-protocol group, there were patients that did not receive their first dose of DDAVP until the fourth day postoperatively.
Table 3. Table depicting the criteria used to evaluate for diabetes insipidus (number of sodium draws, number of urinalyses sent, urine output, specific gravity, and doses of DDAVP administered) at 24 h, 48 h, and overall during the initial hospitalization between the pre protocol and post-protocol group.
| Pre-protocol | Post-protocol | p -Value | |
|---|---|---|---|
| First 24 h | |||
| Na draws (number) | 5.04 | 4.31 | 0.002 |
| Urinalysis (number) | 4.45 | 3.0 | 0.00 |
| Na >145 (patients) | 12 (9.5%) | 4 (8.89%) | 0.901 |
| Urine output >400 mL × 1 h (patients) | 50 (39.7%) | 20 (44.4%) | 0.927 |
| Urine output >2,500 × 2 h (patients) | 57 (45.2%) | 26 (57.8%) | 0.149 |
| Specific gravity <1.005 | 9 (7.14%) | 3 (6.67%) | 0.915 |
| DDAVP given (patients) | 15 (11.9%) | 1 (2.22%) | 0.043 |
| 24–48 h | |||
| Na draws (number) | 3.17 | 3.22 | 0.803 |
| Urinalysis (number) | 2.87 | 2.24 | 0.015 |
| Na >145 (patients) | 17 (13.5%) | 4 (8.89%) | 0.598 |
| Urine output >400 mL × 1 h (patients) | 78 (61.9%) | 26 (57.8%) | 0.626 |
| Urine output >2,500 × 2 h (patients) | 52 (41.3%) | 18 (40.0%) | 0.883 |
| Specific gravity <1.005 | 3 (2.38%) | 3 (6.67%) | 0.187 |
| DDAVP given (patients) | 18 (14.3%) | 3 (6.67% | 0.181 |
| DDAVP totals | |||
| Total patients treated DDAVP | 27 (21.4%) | 4 (8.89%) | 0.044 |
| Doses when DDAVP given | 3.41 | 4.25 | 0.596 |
| Discharged on DDAVP | 16 (12.7%) | 2 (4.44%) | 0.161 |
| Need for DDAVP at 6 mo | 5 (3.9%) | 0 (0.0%) | 0.51 |
Evaluation for adrenal insufficiency revealed no significant difference between the pre- and post-protocol in need for hydrocortisone at discharge, at 1 month, at 3 months, and at 6 months ( Fig. 1 ). There were no readmissions for hypoadrenalism or Addisonian crisis in the post-protocol group.
Fig. 1.
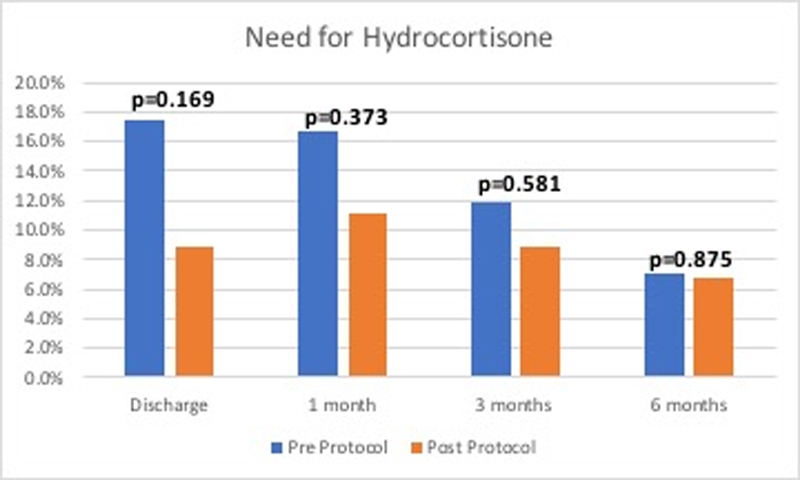
Bar graph demonstrating the need for new hydrocortisone supplementation after surgical resection over time.
There was a significant decrease in the total number of laboratories sent (55.66 vs. 18.82, p <0.001) including basic metabolic panels (7.91 vs. 3.53, p <0.001), urinalyses (10.21 vs. 7.27, p = 0.004), serum osmolality (11.14 vs. 0.22, p <0.001), urine osmolality (10.85 vs. 0.4, p <0.001), and urine sodium (10.54 vs. 0.04, p <0.001). The number of serum sodium sent increased significantly from 4.83 to 7.36 ( p = 0.008) ( Table 4 ). A cost analysis based on average cost of each laboratory test performed demonstrated significant reductions in the amount spent on each laboratory test, with the exception of serum sodium which was sent instead of basic metabolic panel in the post-protocol group. This resulted in a significant reduction in laboratory-related costs from an average of $361.56 per patient in the pre-protocol group to $105.61 per patient in the post-protocol group ( p <0.001) ( Table 4 ).
Table 4. Table illustrating the total laboratories sent and the associated cost for each patient between the pre-protocol and post-protocol group.
| Pre-protocol | Post-protocol | p -Value | |
|---|---|---|---|
| Total labs | 55.66 | 18.82 | 0.00 |
| Basic metabolic panel | 7.91 | 3.53 | 0.00 |
| Serum Na | 4.83 | 7.36 | 0.008 |
| Urinalysis | 10.21 | 7.27 | 0.004 |
| Serum osmolality | 11.14 | 0.22 | 0.00 |
| Urine osmolality | 10.85 | 0.4 | 0.00 |
| Urine Na | 10.54 | 0.04 | 0.00 |
| Cost analysis (per patient) | |||
| Basic metabolic | $82.61 | $36.89 | 0.00 |
| Serum Na | $28.71 | $43.69 | 0.008 |
| Urinalysis | $28.29 | $20.13 | 0.004 |
| Serum osmolality | $81.85 | $1.63 | 0.00 |
| Urine osmolality | $82.12 | $3.03 | 0.00 |
| Urine sodium | $56.91 | $0.24 | 0.00 |
| Total laboratory cost | $361.56 | $105.61 | 0.00 |
A univariate analysis was performed to determine factors associated with the length of stay. There was no significant difference in the length of stay between the pre-protocol and post-protocol groups (4.88 vs. 4.98 days, p = 0.829). There was no difference in the length of stay associated with secretion status (4.78 vs. 5.33 days, p = 0.24) or whether the tumor was newly diagnosed or recurrent (4.81 vs. 5.33 days, p = 0.32). Macroadenomas were associated with a longer length of stay compared with microadenomas (5.01 vs. 3.88 days, p = 0.0). Other factors found to be significant on univariate analysis were reoperation during first hospitalization, intraoperative or postoperative CSF leak, intraoperative or postoperative lumbar drain, need for DDAVP, and new need for hydrocortisone at discharge ( Table 5 ). Linear regression analysis demonstrated that reoperation during first hospitalization, intraoperative lumbar drain, and need for DDAVP were independently associated with length of stay ( Table 6 ).
Table 5. Table illustrating the results of a univariate analysis of factors associated with a length of stay.
| Length of stay (days) | p -Value | |
|---|---|---|
| Pre-protocol | 4.88 | 0.829 |
| Post-protocol | 4.98 | |
| New tumor | 4.81 | 0.32 |
| Recurrent | 5.33 | |
| Secretor | 4.78 | 0.24 |
| Non-secretor | 5.33 | |
| Microadenoma | 3.88 | 0.002 |
| Macroadenoma | 5.01 | |
| Reoperation during 1 st hospitalization | ||
| No | 4.46 | 0.000 |
| Yes | 11.36 | |
| Intraoperative CSF leak | ||
| No | 4.45 | 0.023 |
| Yes | 5.34 | |
| Intraoperative lumbar drain | ||
| No | 4.66 | 0.000 |
| Yes | 7.0 | |
| Postoperative CSF leak | ||
| No | 4.72 | 0.000 |
| Yes | 8.33 | |
| Postoperative lumbar drain | ||
| No | 4.75 | 0.000 |
| Yes | 7.39 | |
| DDAVP given | ||
| No | 4.36 | 0.000 |
| Yes | 7.39 | |
| New need for hydrocortisone at discharge | ||
| No | 4.63 | 0.001 |
| Yes | 6.42 |
Table 6. Table illustrating the results of the multivariate regression analysis of factors associated with increased length of stay.
| Beta | p -Value | 95% CI | |
|---|---|---|---|
| Reoperation during 1 st hospitalization | 6.118 | 0.000 | 4.974–7.262 |
| Ddavp given | 1.810 | 0.000 | 1.006–2.554 |
| Intraoperative lumbar drain | 1.224 | 0.009 | 0.313–2.136 |
| Macroadenoma vs. microadenoma | 0.659 | 0.151 | -0.244 to 1.562 |
| New vs. recurrent | 0.240 | 0.493 | -0.450 to 0.931 |
| Intraoperative CSF leak | 0.309 | 0.258 | -0.229 to 0.848 |
| Postoperative CSF leak | 0.484 | 0.497 | -0.919 to 1.887 |
| Postoperative lumbar drain | −0.366 | 0.586 | −1.693 to 0.960 |
| New need for hydrocortisone | 0.290 | 0.454 | −0.474 to 1.055 |
Abbreviation: CSF, cerebrospinal fluid.
The 30-day readmission rates did not differ significantly between the pre- and post-protocol groups,8.7 vs. 8.8% [ p = 0.97]). Hyponatremia accounted for 27.3% of readmissions prior to implementation of our protocol while there none in the post-protocol cohort ( Table 7 ).
Table 7. Table comparing the readmission rates and the factors leading to readmission in the pre and post-protocol group.
| Pre-protocol | Post-protocol | p -Value | |
|---|---|---|---|
| Readmission | 11 (8.7%) | 4 (8.8%) | 0.9742 |
| Reason for readmission | |||
| Hyponatremia | 3 (27.3%) | – | |
| CSF leak | 3 (27.3%) | 1 (25%) | |
| Infection | 3 (27.3%) | – | |
| Other | 2 (18.2%) | 3 (75%) |
Abbreviation: CSF, cerebrospinal fluid.
Discussion
The evaluation, surgical, and medical management of patients with pituitary tumors are complex and involve a multidisciplinary team consisting of neurosurgeons, otolaryngologists endocrinologists, and neuro-intensivists. Patients with pituitary tumors can have variable clinical presentations, ranging from asymptomatic to having endocrinologic or neurological dysfunction. Rarely, this can be life threatening. Endoscopic endonasal transsphenoidal surgery has become the preferred treatment option for patients with symptoms, patients with uncontrolled endocrinopathy despite maximal medical therapy, or patients whose tumors have demonstrated radiographic progression over an observation period. The goal of surgery includes successful and safe tumor removal and maintenance of endocrinologic function. Postoperative endocrinopathy after pituitary surgery can be a source of significant resource utilization, a driver of length of stay, and a cause of 30-day unplanned readmissions. In the modern era of health care economics, multidisciplinary teams caring for patients with pituitary tumors must achieve the goals of surgery while taking into consideration hospital driven quality metrics. Ambulatory surgery protocols with shorter length of stays have been developed for endoscopic endonasal resection of pituitary adenomas in selective populations with safe and effective outcomes. 5 Other studies have explored the magnitude of cost reduction accompanied with implementation of a surgical step down unit, demonstrating a 12.5% reduction in cost for patients. 6 Araujo-Castro et al have delineated a very detailed, multidisciplinary protocol for enhancing the preoperative management of these medically complex patients as well based on recent international guidelines. 7 That being said, the data pertaining to postoperative managements of patients with specific attention to laboratory usage and diagnosis of endocrinopathies is sparse.
To do this effectively, we implemented an institutional protocol for both the inpatient and outpatient management of patients following endoscopic endonasal transsphenoidal surgery for pituitary adenomas. As expected, our analyses demonstrate that the implementation of the protocol did not have any effect on surgery related factors, such as extent of resection, rate of intraoperative or postoperative CSF leak, and need for re-operation during first hospitalization. Rather, the protocol made an impact on the quality and effectiveness of the postoperative care of these patients.
The reported rate of DI after endoscopic pituitary surgery varies widely, likely reflecting a variability in definition of DI from center to center. Urine outputs considered to be indicative of DI include 300 mL/h × 3 hours, 600 mL/h × 2 hours, 400 mL/h × 1 hour, amongst others. Given this variability, the rates of DI range from 1.6 to 45% for transient DI and 0.3 to 10% for permanent DI. In most patients, DI develops within the first 24 to 48 hours of surgery and tends to resolve by the fifth postoperative day. 8 9 10 Given this variability in both the timing of onset of DI as well as the duration of this process, this gives rise to different management strategies for these patients both within a single institution as well as among different institutions. Prior to our protocol implementation, diagnosis and treatment of DI at our institution involved an excess of unnecessary laboratory studies. By streamlining the process through a protocol that was agreed upon by the multidisciplinary pituitary team, we significantly reduced the number of laboratories sent. Additionally, we switched from sending the more expensive basic metabolic panel to a serum sodium, which was more cost efficient. By modifying the quantity as well as the type of laboratories sent, we were able to reduce cost in a statistically significant manner by $255.95 per patient ( p <0.001).
The protocol was also effective at accurately and efficiently identifying DI and treating only when necessary. First, we demonstrated a significant reduction in the number of patients requiring DDAVP, proving that the protocol correctly differentiated patients who truly went into DI from patients who had transient postoperative mobilization of fluid. Furthermore, all patients requiring DDAVP at discharge were identified within the first 48 hours after surgery. Prior to the protocol implementation, patients requiring DDAVP at discharge were identified as late as the fourth postoperative day. Univariate analysis comparing length of stay in the pre-protocol and post-protocol group did not reveal a statistically significant difference between the two ( p = 0.829). However, we suspect that with a larger sample size, correctly identifying patients that truly need DDAVP earlier will likely have implications on the length of stay in the future as multivariate regression analysis identified the need for DDAVP as a variable associated with length of stay ( p <0.001). More specifically, the need for DDAVP was associated with a length of stay increase of 3 days. This is similar to published data by Harsh et al which suggests at least a 1 day increase in length of stay associated with DI. 5 We hypothesize that the early and accurate identification of DI requiring treatment with DDAVP will lead to a reduction in the length of stay. Other factors that contributed to the length of stay in the multivariate analysis were need for intraoperative lumbar drain and need for reoperation during the first hospitalization (most commonly for CSF leak repair).
Interestingly, some series have identified large tumor size and intraoperative CSF leak as risk factors for developing long-term DI. 5 11 12 It is postulated that this is due to the need for more aggressive intraoperative manipulation of the pituitary gland and/or the pituitary stalk, thus affecting function. It is difficult to discern whether the effects on length of stay are due to the CSF leak (requiring intervention such as lumbar drainage or re-operation) or whether they are due to the time required for accurate identification and treatment of DI (time required to determine appropriate dosing and interval of DDAVP).
There is often a lack of consensus between neurosurgeons and endocrinologists in the postoperative management of corticosteroids in patients undergoing surgery for pituitary adenomas. 1 Nonspecific symptoms such as fatigue often leads to intracranial imaging and the diagnosis of pituitary adenomas and patients with borderline levels of low cortisol are often treated with steroids preoperatively. Those patients then require stress-dose steroids in the immediate postoperative period. It is important to have a strategy to test the cortisol response postoperatively to determine which patients require long-term steroid use. Our protocol outlines an algorithm for the corticotroph axis evaluation, even for patients who are on steroid therapy preoperatively. This allows for discontinuation of corticosteroid therapy in patients with an intact hypothalamic-pituitary-axis and an appropriate cortisol response, which avoids the well described negative effects of long-term steroid use. While there was a higher overall percentage of patients discharged on hydrocortisone in the pre-protocol group, this did not reach statistical significance. As there were no set parameters in the pre-protocol group, and the tendency was to have a low threshold for supplementation, there were no cases of missed need for hydrocortisone. However, there was one case of readmission secondary to hypoadrenalism which was inadequately supplemented. Utilizing a set threshold for morning cortisol in the protocol resulted in a reduction in the number of patients being discharged on hydrocortisone supplementation. Despite a much higher threshold for supplementation, there was only one patient in the post-protocol group who was started on hydrocortisone after discharge, but the reasons for this were unclear. The hydrocortisone for this patient was subsequently discontinued within 2 months of initiation, thus supporting it was not necessary to begin with. There were no patients in the post-protocol group with readmission or complications secondary to a missed diagnosis of cortisol insufficiency.
In terms of readmission, there was no statistically significant difference in the pre-protocol and post-protocol groups. This suggests the relative safety of the protocol by demonstrating that the changes made did not lead to missed or delayed diagnoses of postoperative endocrine dysfunction. Hyponatremia, CSF leak, and infection, were leading causes of readmission in the pre-protocol cohort, which is consistent with other published series. The incidence of postoperative hyponatremia quoted in the literature ranges from 2.3 to 16%. 13 14 15 16 Interestingly, no readmissions for hyponatremia were noted in the post-protocol cohort. This is an important finding as data has shown that postoperative, delayed hyponatremia care pathway/protocol implementation has not necessarily decreased readmission rates or clinical outcomes. 17 In most cases, hyponatremia tends to occur approximately 1 week after transsphenoidal surgery and can last up to 2 weeks or more. The purpose of the outpatient component of the protocol is to obtain a sodium level one week postoperatively so that hyponatremia, even if mild, can be identified early on. This way, it can be managed in the outpatient setting with simple measures such as fluid restriction and sodium chloride tablets when necessary. Severe hyponatremia (typically less than 120 mmol/L), even when asymptomatic, can require readmission for concern for and prevention of extreme symptoms such as seizures, coma, and death. Symptoms tend to be associated with the rate of drop in serum sodium concentration rather than the actual value alone. 14 18 19 20 Severe hyponatremia may require more aggressive therapy than cannot be performed in the outpatient setting such as intravenous hyperosmolar therapy. Therefore, early identification is key.
The outpatient component of the protocol is difficult to ensure and is dependent on patient's adherence to the recommended 1L fluid restriction (four glasses of water per day) upon discharge. Thorough review of these instructions on discharge is important as is a post-discharge follow-up phone call as a reminder to patients and their caretakers. Postoperative fluid restriction has been studied as an effective method in decreasing the risk of postoperative hyponatremia and readmission. 21
Limitations of this study include the fact that it is a single institution study and that data obtained for the pre-protocol group was done so in a retrospective fashion. Additionally, while there was no significant difference in gender and primary versus recurrent tumors between the two cohorts, this was not a matched cohort. The post-protocol group was younger, and had a significantly higher proportion of microadenomas and secreting tumors. This heterogeneity in the cohorts can be a confounding factor and must be taken into consideration.
Conclusion
Implementation of a postoperative management protocol results in a more efficient and cost-effective diagnosis and management of endocrinopathy after pituitary adenoma surgery.
Conflict of Interest None declared.
Note
Portion of this work was presented at the NASBS Meeting in Orlando, Florida.
Supplementary Material
References
- 1.Prete A, Corsello S M, Salvatori R. Current best practice in the management of patients after pituitary surgery. Ther Adv Endocrinol Metab. 2017;8(03):33–48. doi: 10.1177/2042018816687240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McLaughlin N, Cohan P, Barnett P, Eisenberg A, Chaloner C, Kelly D F. Early morning cortisol levels as predictors of short-term and long-term adrenal function after endonasal transsphenoidal surgery for pituitary adenomas and Rathke's cleft cysts. World Neurosurg. 2013;80(05):569–575. doi: 10.1016/j.wneu.2012.07.034. [DOI] [PubMed] [Google Scholar]
- 3.Cote D J, Dasenbrock H H, Muskens I S. Readmission and other adverse events after transsphenoidal surgery: prevalence, timing, and predictive factors. J Am Coll Surg. 2017;224(05):971–979. doi: 10.1016/j.jamcollsurg.2017.02.015. [DOI] [PubMed] [Google Scholar]
- 4.Burke W T, Cote D J, Iuliano S I, Zaidi H A, Laws E R. A practical method for prevention of readmission for symptomatic hyponatremia following transsphenoidal surgery. Pituitary. 2018;21(01):25–31. doi: 10.1007/s11102-017-0843-5. [DOI] [PubMed] [Google Scholar]
- 5.Liu Y, Zheng T, Lv W. Ambulatory surgery protocol for endoscopic endonasal resection of pituitary adenomas: a prospective single-arm trial with initial implementation experience. Sci Rep. 2020;10(01):9755. doi: 10.1038/s41598-020-66826-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hunsaker J C, Khan M, Gamblin A, Karsy M, Couldwell W T. Use of a surgical stepdown protocol for cost reduction after transsphenoidal pituitary adenoma resection: a case series. World Neurosurg. 2021;152:e476–e483. doi: 10.1016/j.wneu.2021.05.126. [DOI] [PubMed] [Google Scholar]
- 7.Araujo-Castro M, Pascual-Corrales E, Martínez San Millan J. Multidisciplinary protocol of preoperative and surgical management of patients with pituitary tumors candidates to pituitary surgery. Ann Endocrinol (Paris) 2021;82(01):20–29. doi: 10.1016/j.ando.2020.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Ajlan A M, Abdulqader S B, Achrol A S. Diabetes insipidus following endoscopic transsphenoidal surgery for pituitary adenoma. J Neurol Surg B Skull Base. 2018;79(02):117–122. doi: 10.1055/s-0037-1604363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dumont A S, Nemergut E C, II, Jane J A, Jr, Laws E R., Jr Postoperative care following pituitary surgery. J Intensive Care Med. 2005;20(03):127–140. doi: 10.1177/0885066605275247. [DOI] [PubMed] [Google Scholar]
- 10.Sigounas D G, Sharpless J L, Cheng D M, Johnson T G, Senior B A, Ewend M G.Predictors and incidence of central diabetes insipidus after endoscopic pituitary surgery Neurosurgery 2008620171–78., discussion 78–79 [DOI] [PubMed] [Google Scholar]
- 11.Cappabianca P, Cavallo L M, Colao A, de Divitiis E. Surgical complications associated with the endoscopic endonasal transsphenoidal approach for pituitary adenomas. J Neurosurg. 2002;97(02):293–298. doi: 10.3171/jns.2002.97.2.0293. [DOI] [PubMed] [Google Scholar]
- 12.Gondim J A, Almeida J P, Albuquerque L A. Endoscopic endonasal approach for pituitary adenoma: surgical complications in 301 patients. Pituitary. 2011;14(02):174–183. doi: 10.1007/s11102-010-0280-1. [DOI] [PubMed] [Google Scholar]
- 13.Cusick J F, Hagen T C, Findling J W. Inappropriate secretion of antidiuretic hormone after transsphenoidal surgery for pituitary tumors. N Engl J Med. 1984;311(01):36–38. doi: 10.1056/NEJM198407053110107. [DOI] [PubMed] [Google Scholar]
- 14.Kelly D F, Laws E R, Jr, Fossett D. Delayed hyponatremia after transsphenoidal surgery for pituitary adenoma. Report of nine cases. J Neurosurg. 1995;83(02):363–367. doi: 10.3171/jns.1995.83.2.0363. [DOI] [PubMed] [Google Scholar]
- 15.Taylor S L, Tyrrell J B, Wilson C B.Delayed onset of hyponatremia after transsphenoidal surgery for pituitary adenomas Neurosurgery 19953704649–653., discussion 653–654 [DOI] [PubMed] [Google Scholar]
- 16.Whitaker S J, Meanock C I, Turner G F. Fluid balance and secretion of antidiuretic hormone following transsphenoidal pituitary surgery. A preliminary series. J Neurosurg. 1985;63(03):404–412. doi: 10.3171/jns.1985.63.3.0404. [DOI] [PubMed] [Google Scholar]
- 17.Bohl M A, Ahmad S, White W L, Little A S. Implementation of a postoperative outpatient care pathway for delayed hyponatremia following transsphenoidal surgery. Neurosurgery. 2018;82(01):110–117. doi: 10.1093/neuros/nyx151. [DOI] [PubMed] [Google Scholar]
- 18.Arieff A I. Hyponatremia associated with permanent brain damage. Adv Intern Med. 1987;32:325–344. [PubMed] [Google Scholar]
- 19.Arieff A I, Llach F, Massry S G. Neurological manifestations and morbidity of hyponatremia: correlation with brain water and electrolytes. Medicine (Baltimore) 1976;55(02):121–129. doi: 10.1097/00005792-197603000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Vingerhoets F, de Tribolet N.Hyponatremia hypo-osmolarity in neurosurgical patients. “Appropriate secretion of ADH” and “cerebral salt wasting syndrome.” Acta Neurochir (Wien) 198891(1-2):50–54. [DOI] [PubMed] [Google Scholar]
- 21.Perez-Vega C, Tripathi S, Domingo R A. Fluid restriction after transsphenoidal surgery for the prevention of delayed hyponatremia: a systematic review and meta-analysis. Endocr Pract. 2021;27(09):966–972. doi: 10.1016/j.eprac.2021.07.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


