Abstract
Background
Deutetrabenazine is approved in the USA, China, Australia, Israel, Brazil, and South Korea for the treatment of chorea associated with Huntington disease.
Objective
We aimed to evaluate the long-term safety and tolerability of deutetrabenazine for the treatment of Huntington disease.
Methods
This open-label, single-arm, multi-center study included patients who completed a double-blind study (Rollover) and patients who converted overnight from a stable tetrabenazine dose (Switch). Exposure-adjusted incidence rates (adverse events per person-year) were calculated. Efficacy was analyzed using a stable post-titration timepoint (8 weeks). Changes in the Unified Huntington’s Disease Rating Scale total motor score and total maximal chorea score from baseline to week 8, as well as those from week 8 to week 145 (or the last visit on the study drug if that occurred earlier), were evaluated as both efficacy and safety endpoints during the study.
Results
Of 119 patients (Rollover, n = 82; Switch, n = 37), 100 (84%) completed ≥ 1 year of treatment. End-of-study exposure-adjusted incidence rates for adverse events in Rollover and Switch, respectively, were: any, 2.57 and 4.02; serious, 0.11 and 0.14; leading to dose suspension, 0.05 and 0.04. Common adverse events (≥ 4% either cohort) included somnolence (Rollover, 20%; Switch, 30%), depression (32%; 22%), anxiety (27%; 35%), insomnia (23%; 16%), and akathisia (6%; 11%). Adverse events of interest included suicidality (9%; 5%) and parkinsonism (4%; 8%). Mean dose at week 8 was 38.1 mg (Rollover) and 36.5 mg (Switch). Mean dose across cohorts after titration was 37.6 mg; at the final visit, mean dose across cohorts was 45.7 mg. Patients showed minimal change in the Unified Huntington’s Disease Rating Scale total maximal chorea scores with stable dosing from weeks 8–145 or at the end of treatment, but total motor score increased versus week 8 (mean change [standard deviation]: 8.2 [11.9]). There were no unexpected adverse events upon drug withdrawal, and mean (standard deviation) total maximal chorea scores increased 4.7 (4.6) units from week 8 to 1-week follow-up.
Conclusions
Adverse events observed with long-term deutetrabenazine exposure were consistent with previous studies. Reductions in chorea persisted over time. Upon treatment cessation, there was no unexpected worsening of chorea.
Clinical Trial Registration
ClinicalTrials.gov identifier: NCT01897896.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40263-022-00956-8.
Key Points
| In this single-arm open-label extension study (Alternatives for Reducing Chorea in Huntington Disease [ARC-HD]), the safety and efficacy of deutetrabenazine were evaluated for the treatment of chorea associated with Huntington disease; compared to the pivotal double-blind study, no new safety concerns arose. |
| Conclusions related to efficacy were limited because of the study design, but there appeared to be continued chorea control throughout the study with an average daily end-of-study dose of 45.7 mg. |
| Overall, the use of twice-daily deutetrabenazine maintained and improved chorea control in patients with Huntington disease with favorable safety and tolerability. |
Introduction
Huntington disease (HD) is a neurodegenerative disorder characterized by motor impairment, cognitive function decline, and behavioral-emotional disturbances [1–5]. Chorea is the most common motor symptom of HD, affecting ~ 90% of adult patients [6–8]. Chorea can interfere with daily function, cause social isolation, and increase the risk of injury [2, 7, 8], leading to decreased quality of life [9, 10].
Abnormal dopaminergic signaling is a target of pharmacotherapy for chorea associated with HD [5, 11]. Tetrabenazine, the first treatment approved by the US Food and Drug Administration for chorea associated with HD [12], modulates dopamine by selectively inhibiting vesicular monoamine transporter type 2 [5]. Tetrabenazine pharmacokinetics are characterized by a substantial fluctuation in plasma concentrations, variable cytochrome P450 2D6 metabolism, and a short terminal half-life of active metabolites, such that three-times-daily administration is required. These variations may explain some adverse events (AEs; e.g., somnolence, insomnia, fatigue, depression, anxiety) [13–16].
Deutetrabenazine, a novel, highly selective, deuterium-containing vesicular monoamine transporter type 2 inhibitor, is approved in the USA, China, Australia, Israel, Brazil, and South Korea for the treatment of chorea associated with HD and tardive dyskinesia in adults [17–19]. Deuteration may increase the half-life of active metabolites, leading to more uniform systemic exposure with lower and less frequent dosing [15].
First-HD was a randomized, double-blind, placebo-controlled, 12-week trial (N = 90) in which deutetrabenazine improved motor signs, as well as patient-rated and clinician-rated impressions of change, with a favorable safety profile [20]. The present study includes patients who completed this double-blind study as well as patients who underwent overnight conversion from tetrabenazine to deutetrabenazine [16]. This analysis evaluated the long-term safety and efficacy of deutetrabenazine over the full extension period.
Methods
Study Design and Enrollment
Alternatives for Reducing Chorea in Huntington Disease (ARC-HD) was an open-label, single-arm, two-cohort study conducted at 37 sites in the USA, Canada, and Australia (ClinicalTrials.gov identifier: NCT01897896). A listing of ARC-HD investigators and coordinators is available in the Electronic Supplementary Material (ESM). The Rollover cohort had successfully completed First-HD, which evaluated the effect of deutetrabenazine on change from baseline using the Unified Huntington’s Disease Rating Scale (UHDRS) total maximal chorea (TMC) score and included a 1-week washout and follow-up evaluation [20]; this cohort included patients who had been receiving the placebo and those who were given the study drug for 12 weeks. The Switch cohort comprised patients with HD chorea who had been on a stable dose (≥ 8 weeks) of tetrabenazine that provided a therapeutic benefit. A total of 119 patients with HD chorea were enrolled (Rollover cohort, n = 82; Switch cohort, n = 37). The study was conducted from November 2013 to August 2017. Study drug dosage ranged from 6 mg daily up to 72 mg daily. Dosage increments were 6 mg per day per week until 48 mg daily. At the discretion of the site, the dosage was permitted to be increased by 12 mg per day per week to a maximum of 36 mg twice daily.
Standard Protocol Approvals, Registrations, and Patient Consents
This study (ClinicalTrials.gov identifier: NCT01897896) was approved by the ethics boards at all involved centers prior to subject enrollment. At enrollment, an independent qualified healthcare provider assessed all subjects for capacity to provide informed consent, and study personnel subsequently assessed subjects for capacity to continue in the study. Subjects or legally authorized representatives provided written informed consent, and subjects without capacity provided assent, if required by local regulations. All subjects were required to have daily contact with a caregiver; subjects with more advanced disease (total functional capacity [TFC] score of 5–7) at their original screening visit were required to have a live-in caregiver.
Study Treatment and Plan
Entry criteria into the double-blind placebo-controlled cohort enrolled prior to this study have been previously published [20]. All Rollover patients completed a 1-week washout of deutetrabenazine or placebo treatment from the First-HD trial to allow for a return to baseline chorea control and then started on 6 mg of deutetrabenazine the next morning (day 1). The treatment assignment for patients in the double-blind placebo-controlled cohort remained blinded throughout this study. Over an 8-week period, patients were titrated to an individualized response-driven dose, in weekly increments of ± 6 mg per day, to optimize chorea control and tolerability. After the baseline visit, in-clinic study visits were conducted at weeks 2, 4, 8, 15, 28, and every 13th week thereafter until the final treatment visit, and telephone study visits were conducted at weeks 1, 3, and 5.
Entry criteria into the overnight conversion cohort prior to this study have been previously published [16]. Switch patients were converted from their existing tetrabenazine regimen to a deutetrabenazine regimen using a protocol-specified algorithm that was hypothesized to provide comparable systemic exposure to total (α + β)-dihydrotetrabenazine metabolites (see Frank et al. [16] and data on file). Patients continued their tetrabenazine regimen through midnight of day 0 and switched to deutetrabenazine the next morning (day 1). Patients remained on their initial dose of deutetrabenazine for 1 week. After week 1, dose adjustments (weekly increments of ± 6 mg/day) were permitted through week 8 to optimize chorea control and tolerability. In-clinic study visits were conducted at baseline and weeks 1, 4, 8, 15, 28, and every 13th week thereafter until the final treatment visit, and telephone study visits were conducted at weeks 2, 3, and 7.
This open-label study concluded when deutetrabenazine became commercially available in the USA. At that time, subjects in both cohorts had a final assessment after drug washout, 1 week following the final visit on treatment.
If data for a scheduled visit were missing, data from an unscheduled visit could be reassigned according to a prespecified visit window algorithm.
Safety Assessments
Safety and tolerability were assessed from consent through to the end of the follow-up, defined as 4 weeks after the last treatment dose, and included AE monitoring, clinical laboratory testing, measurement of vital signs and weight, physical and neurological examinations, and 12-lead electrocardiograms (ECGs). To be conservative, AEs that occurred during the follow-up period were counted as events that occurred while on treatment. Adverse events with selected preferred terms were combined into AEs of interest. Exposure-adjusted incidence rates (EAIRs) were used to compare the frequency of AEs in this open-label extension study with those in the short-term First-HD study and were calculated by adjusting the incidence of AEs by the duration of treatment exposure (AEs per person-year) [21]. Safety assessments also included the following scales: UHDRS parkinsonism subscale, Unified Parkinson’s Disease Rating Scale dysarthria item, Hospital Anxiety and Depression Scale, Swallowing Disturbance Questionnaire, Columbia Suicide Severity Rating Scale, Barnes Akathisia Rating Scale, Montreal Cognitive Assessment, and the Epworth Sleepiness Scale.
Efficacy Assessments
Changes in the UHDRS total motor score (TMS) and TMC score from baseline to week 8, as well as those from week 8 to week 145 (or the last visit on study drug if that occurred earlier), were evaluated as both efficacy and safety endpoints during the study. For TMS and TMC, lower scores indicate better motor function and less chorea, respectively.
Statistical Analysis
Demographic and clinical characteristics are shown as mean (standard deviation [SD]) and 95% confidence interval (CI) or n (%), as appropriate. Because of the open-label single-arm study design, only descriptive statistical analyses were performed. Safety analyses included all patients who were administered any study drug in either the Rollover or Switch cohorts.
For the Switch cohort, baseline values were the most recent available values prior to the first administration of the study drug; the baseline values for TMC and TMS were the means of the values from the screening and baseline visits. For the Rollover cohort, baseline values were the most recent values obtained at least 4 weeks after the last administration of the First-HD study drug. Baseline values of TFC were obtained from the original baseline visit.
As there were dose adjustments for the Switch cohort and a titration for the Rollover cohort in the initial weeks, the week 8 (stable dose) visit was selected for comparison purposes. For efficacy analyses of the TMC and TMS, the Rollover and Switch cohorts were analyzed separately from baseline through week 8. The two cohorts were combined for the analysis of changes from week 8 to week 145 (or the last visit on the study drug, if that occurred earlier). The maximum duration of participation was 171 weeks (for safety assessments).
Data Availability Statement
Consistent with the policies of the Huntington Study Group, the corresponding author is confirmed as the guarantor of the work and all authors had full access to the data. All authors have the right to publish any and all data separate and apart from any sponsor, and we take full responsibility for the data, the analyses and interpretation, and the conduct of the research.
Results
Baseline Characteristics
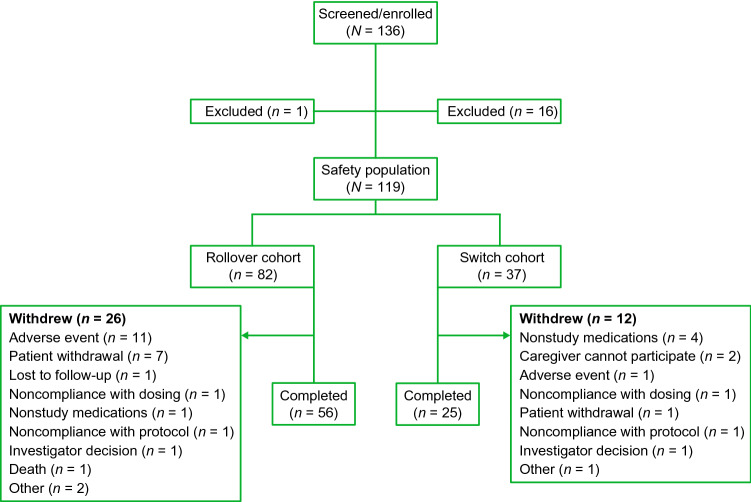
A total of 119 patients were enrolled in the ARC-HD trial: 82 in the Rollover cohort and 37 in the Switch cohort. In the Switch cohort, reasons for switching from tetrabenazine included inadequate control of chorea and limited dosing because of side effects. A total of 17 patients were excluded from enrollment (Fig. 1), and 38 withdrew during the study. Overall, 81 patients (68%) who received deutetrabenazine treatment completed the long-term follow-up. Demographic and clinical characteristics at baseline are shown in Table 1. One hundred patients (84%) completed at least 1 year of deutetrabenazine treatment; patients were followed for a mean (SD) of 116 (50) and 124 (44) weeks in the Rollover and Switch cohorts, respectively.
Fig. 1.
Patient disposition
Table 1.
Baseline characteristics by cohort
| Baseline characteristics | Rollover cohort (n = 82) | Switch cohort (n = 37) |
|---|---|---|
| Patient demographics | ||
| Age, years | 53.7 (12.3) | 52.4 (11.5) |
| Female, n (%) | 37 (45) | 15 (41) |
| White, n (%) | 76 (93) | 36 (97) |
| Education, years | 14.4 (2.6) | 14.5 (2.5) |
| Patient clinical characteristics | ||
| Body weight at screening, kg | 74.3 (15.0) | 71.4 (16.5) |
| BMI at screening, kg/m2 | 25.8 (4.6) | 23.9 (4.7) |
| UHDRS TFC | 9.6 (2.2) | 8.3 (2.1) |
| UHDRS TMCa | 12.0 (4.1) | 12.5 (5.3) |
| UHDRS TMSa | 34.7 (16.1) | 37.8 (18.6) |
| BARS: summary score | 1.1 (1.7) | 0.8 (1.3) |
| BARS: global clinical assessment | 0.5 (0.8) | 0.4 (0.7) |
| ESS: total score | 4.4 (3.7) | 6.0 (4.1) |
| HADS: anxiety subscale | 2.7 (3.0) | 4.3 (3.4) |
| HADS: depression subscale | 2.0 (2.5) | 3.4 (2.5) |
| MoCA: total score | 23.9 (4.4) | 21.9 (3.9) |
| SDQ: swallowing disturbance | 3.5 (3.1) | 4.7 (3.1) |
| UPDRS speech | 0.9 (0.8) | 1.1 (0.7) |
| UHDRS Parkinsonism | 10.5 (5.9) | 12.1 (6.9) |
| Stroop color | 46.8 (15.9) | 40.7 (12.9) |
| Stroop word | 58.4 (20.8) | 50.4 (16.2) |
| Stroop interference | 28.9 (15.1) | 21.7 (8.2) |
| Symbol digit modalities | 24.4 (8.9) | 22.7 (17.4) |
| Verbal fluency | 25.1 (11.0) | 21.5 (10.8) |
All values are mean (SD) unless otherwise noted
BARS Barnes Akathisia Rating Scale, BMI body mass index, ESS Epworth Sleepiness Scale, HADS Hospital Anxiety and Depression Scale, MoCA Montreal Cognitive Assessment, SD standard deviation, SDQ Swallowing Disturbance Questionnaire, TFC total functional capacity, TMC total maximal chorea, TMS total motor score, UHDRS Unified Huntington’s Disease Rating Scale, UPDRS Unified Parkinson’s Disease Rating Scale
aFor the Switch cohort, baseline values were the most recent available values prior to the first administration of the study drug; the baseline values for TMC and TMS were the means of the values from the screening and baseline visits. For the Rollover cohort, baseline values were the most recent available values obtained at least 4 days after the last administration of the First-HD study drug; baseline values for TFC were obtained from the original baseline visit, prior to the first administration of the First-HD study drug
Dosage
Patients who rolled over from First-HD completed a 1-week washout period and follow-up evaluation before restarting on deutetrabenazine. Those patients were re-titrated as some of them had been assigned to placebo, and treatment assignment remained blinded during the conduct of this study. At the week 8 visit, the mean (SD) daily dose of deutetrabenazine being taken by subjects was 38.1 (10.53) mg.
For the Switch cohort, the mean (SD) daily dose of tetrabenazine at baseline was 42.1 (19.6) mg; following the overnight conversion, the mean (SD) daily dose of deutetrabenazine was 20.3 (10.2) mg at week 1 (approximately 48% of the tetrabenazine dose) and 29.7 (10.3) mg at week 4. By week 8, the mean (SD) daily dose was 36.5 (12.9) mg. Across cohorts, the mean (SD) daily dose after titration was 37.6 (11.3) mg at week 8 and 45.7 (16.7) mg at the final visit.
Fifty-six percent of patients had an increase in mean daily dose from week 8 (after dose adjustment) to the final assessment, with 9% having a decreased mean daily dose and 35% taking the same dose at the final assessment. At their final assessment, 15% had a mean daily dose of < 25 mg, 51% had a mean daily dose between 25 and 50 mg, and 34% had a mean daily dose > 50 mg. Overall, the median compliance from day 1 through the last dose was 97% for the Rollover cohort and 93% for the Switch cohort.
Safety
During this open-label study, 68% of patients in the Rollover cohort and 70% of patients in the Switch cohort experienced any AE that was assessed as being at least possibly related to the study drug. Exposure-adjusted incidence rates were generally lower than those for the deutetrabenazine and placebo arms in First-HD (Table 2). At the end of the study, the EAIRs (AEs per person-year) of patients who reported any AE were 2.57 and 4.02 (Rollover and Switch cohorts, respectively), serious AEs were 0.11 and 0.14, AEs leading to dose suspension were 0.05 and 0.04, and AEs leading to withdrawal from the study were 0.06 and 0.03. Common (≥ 4% in either cohort) AEs included fall (38 and 43%), depression (32 and 22%), anxiety (27 and 35%), insomnia (23 and 16%), somnolence (20 and 30%), and akathisia (6 and 11%) in the Rollover and Switch cohorts, respectively (Table 3; for a complete listing, see ESM). Rates of AEs of interest include suicidality (9 and 5%) and parkinsonism (4 and 8%), respectively. From baseline through week 15, mean (SD) body weight increased in the Rollover cohort (1.3 kg [3.3]) and changed minimally in the Switch cohort (− 0.03 kg [2.5]). From baseline to week 132, body weight decreased in the Rollover cohort (mean [SD], − 1.7 kg [8.0]) and the Switch cohort (− 2.3 kg [7.2]). Mean (SD) changes in body mass index were minimal from baseline to week 8 (Rollover, − 0.2 [2.4] kg/m2; Switch, − 0.2 [1.7] kg/m2) and remained small between weeks 8 and 132 (Rollover, − 0.8 [2.7] kg/m2; Switch, − 0.7 [2.4] kg/m2). There were no clinically relevant mean changes from baseline or shifts from normal to abnormal in any of the hematology and serum chemistry parameters assessed. At week 8, there were no increases in the incidence of any abnormal ECG parameter relative to baseline in either cohort. Among patients receiving citalopram or escitalopram at baseline, two patients had a QTcF > 450 ms in the Rollover cohort (1 at baseline and 1 at week 2) and no patients had a post-baseline QT or QTcF > 450 ms in the Switch cohort. Moreover, there were no notable differences between baseline and post-baseline assessments in the incidence of abnormal ECG parameters at any timepoint. There was one death in the Rollover cohort that was considered unlikely to be related to the study drug (sudden cardiac arrest). No notable changes in safety assessments were observed over the treatment period (ESM).
Table 2.
Exposure-adjusted incidence rates for AEs by study and cohort
| First-HD | Open-label extension | |||
|---|---|---|---|---|
| Deutetrabenazine (n = 45) | Placebo (n = 45) | Rollover cohort (n = 82) | Switch cohort (n = 37) | |
| AEs | EAIR (number of AEs/person-year) | |||
| Any AE | 4.32 (27/6.2) | 4.65 (27/5.8) | 2.57 (76/29.6) | 4.02 (35/8.7) |
| SAEs | 0.10 (1/10.4) | 0.10 (1/10.0) | 0.11 (17/158.6) | 0.14 (11/77.0) |
| Severe AEs | 0.19 (2/10.4) | 0.10 (1/10.1) | 0.10 (16/161.0) | 0.09 (7/79.9) |
| Treatment-related AEs | 2.54 (19/7.5) | 1.44 (12/8.4) | 0.69 (55/79.5) | 0.83 (26/31.2) |
| AEs leading to dose reduction | 0.30 (3/10.1) | 0.31 (3/9.8) | 0.13 (20/151.4) | 0.14 (10/72.4) |
| AEs leading to dose suspension | 0.10 (1/10.4) | 0.10 (1/10.1) | 0.05 (8/166.8) | 0.04 (3/83.3) |
| AEs leading to withdrawal | 0.10 (1/10.4) | 0.10 (1/10.1) | 0.06 (11/181.0) | 0.03 (3/87.3) |
| AEs of interest | ||||
| Fall | 0.20 (2/10.1) | 0.42 (4/9.6) | 0.24 (31/129.1) | 0.26 (16/60.6) |
| Somnolence | 0.53 (5/9.5) | 0.21 (2/9.7) | 0.11 (16/148.9) | 0.19 (11/59.0) |
| Depressiona | 0.19 (2/10.3) | 0.31 (3/9.8) | 0.19 (27/140.4) | 0.11 (8/74.8) |
| Anxiety | 0.10 (1/10.3) | 0.10 (1/9.9) | 0.14 (22/156.2) | 0.19 (13/68.5) |
| Insomnia | 0.30 (3/10.1) | 0.21 (2/9.7) | 0.13 (19/142.3) | 0.08 (6/78.9) |
| Akathisia and restlessness with agitationb | 0.19 (2/10.4) | 0.10 (1/10.1) | 0.05 (9/173.8) | 0.09 (7/80.8) |
| Suicidalityc | 0.10 (1/10.4) | 0 (0/10.1) | 0.04 (7/173.8) | 0.02 (2/84.6) |
| Dysphagiad | 0 (0/10.4) | 0.10 (1/9.9) | 0.03 (6/173.8) | 0.06 (5/78.6) |
| Parkinsonisme | 0 (0/10.4) | 0 (0/10.1) | 0.03 (5/173.7) | 0.05 (4/82.0) |
AE adverse event, EAIR exposure-adjusted incidence rate, PT preferred term, SAE serious adverse event
aAE of interest of depression included the following selected PTs: all PTs containing “depression”
bAE of interest of akathisia and restlessness with agitation included the following selected PTs: akathisia, hyperkinesia, psychomotor hyperactivity, restlessness, agitation
cAE of interest of suicidality included the following PTs: completed suicide, suicidal depression, intentional overdose, intentional self-injury, deliberate poisoning, self-injurious ideation, suicidal behavior, suicidal ideation, suicide attempt
dAE of interest of dysphagia included the following selected PTs: aphagia, dysphagia
eAE of interest of parkinsonism included the following selected PTs: akinesia, bradykinesia, cogwheel rigidity, freezing phenomenon, hypertonia, masked facies, muscle rigidity, on and off phenomenon, parkinsonian crisis, parkinsonian gait, parkinsonian rest tremor, parkinsonism, Parkinson’s disease, resting tremor
Table 3.
Select common (≥ 4%) adverse events by cohorta
| Preferred term, n (%) | Rollover cohort (n = 82) | Switch cohort (n = 37) |
|---|---|---|
| Fall | 31 (38) | 16 (43) |
| Depression | 26 (32) | 8 (22) |
| Anxiety | 22 (27) | 13 (35) |
| Insomnia | 19 (23) | 6 (16) |
| Somnolence | 16 (20) | 11 (30) |
| Urinary tract infection | 12 (15) | 4 (11) |
| Weight decreased | 11 (13) | 9 (24) |
| Diarrhea | 11 (13) | 7 (19) |
| Irritability | 11 (13) | 4 (11) |
| Nasopharyngitis | 8 (10) | 6 (16) |
| Nausea | 8 (10) | 3 (8) |
| Chorea | 7 (9) | 7 (19) |
| Dysphagia | 7 (9) | 6 (16) |
| Constipation | 7 (9) | 5 (14) |
| Laceration | 7 (9) | 5 (14) |
| Akathisia | 5 (6) | 4 (11) |
| Dry mouth | 4 (5) | 4 (11) |
| Pneumonia | 1 (1) | 7 (19) |
| Decreased appetite | 1 (1) | 4 (11) |
aA complete listing of common adverse events (≥ 4%) is available in the ESM
Efficacy
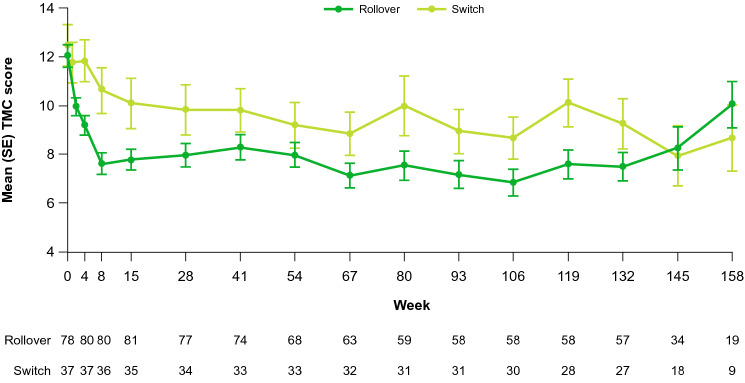
The mean (standard error) TMC score at baseline was 12.0 (4.1) units and 12.5 (5.3) units in the Rollover and Switch cohorts, respectively (Fig. 2). Mean chorea scores decreased from baseline to week 8 by − 4.5 (SD: 3.1; 95% CI − 5.2, − 3.7) and − 2.1 (SD: 3.3; 95% CI − 3.1, − 1.0) in both the Rollover and Switch cohorts, respectively. In both the Rollover and Switch cohorts, mean motor scores decreased from baseline to week 8 by − 7.1 (SD: 7.3; 95% CI − 8.8, − 5.5) and − 2.4 (SD: 8.7; 95% CI − 5.4, 0.5), respectively (Table 4). Patients had minimal change in TMC from week 8 to week 145 (or to the end of treatment, whichever was earlier; mean change, − 0.5 [SD: 5.2; 95% CI − 1.9, 1.0]), but there was an increase in the TMS over this period (mean change: 8.2 [SD: 11.9; 95% CI 4.8, 11.5]). There were no unexpected AEs upon drug withdrawal, and chorea scores increased by a mean (SD) of 4.7 (4.6) units from week 8 to the 1-week follow-up.
Fig. 2.
Chorea by study cohorta. SE standard error, TMC total maximal chorea. aTreatment was initiated on day 1 for the Switch cohort and day 8 for the Rollover cohort. Dose adjustments were permitted through week 8
Table 4.
Changes in chorea and motor scores
| Change in UHDRS TMC score | Change in UHDRS TMS | |
|---|---|---|
| Change from baseline to week 8 | ||
| Rollover cohort | − 4.4 (3.1) | − 7.1 (7.3) |
| Switch cohort | − 2.1 (3.3) | − 2.4 (8.7) |
| Change from week 8 to week 145a | ||
| All subjects | − 0.5 (5.2) | 8.2 (11.9) |
Values shown are mean (SD)
SD standard deviation, TMC total maximal chorea, TMS total motor score, UHDRS Unified Huntington’s Disease Rating Scale
aThe Rollover and Switch cohorts were analyzed separately from baseline through week 8. The two cohorts were combined for the analysis of changes from week 8 to week 145 (or the last visit on the study drug, if that occurred earlier)
Discussion
The ARC-HD open-label extension was designed to assess the long-term safety and efficacy of deutetrabenazine in patients who completed the First-HD trial or converted overnight from taking tetrabenazine. Deutetrabenazine demonstrated a favorable safety and tolerability profile, with compliance rates greater than 90% over the open-label extension period. Although the incidence of AEs may increase over time, rates of AEs leading to dose reduction, suspension, or withdrawal were all lower during the open-label extension than in either the placebo or deutetrabenazine arm of the 12-week First-HD trial. Low rates of depression, suicidality, and parkinsonism were observed, and the rates of AEs of special interest were comparable to or lower than those observed in First-HD. There were no increases in ECG parameters relative to baseline in either cohort. No new safety concerns arose.
The starting dose of deutetrabenazine for patients previously treated with tetrabenazine in the Switch cohort was hypothesized to maintain, but not necessarily improve, baseline levels of chorea. However, on deutetrabenazine, these patients achieved improvement in TMC scores compared with baseline, with no observed worsening of chorea after cessation of the study drug, beyond what was expected at this stage of disease. In the Rollover cohort, after a 1-week washout of the blinded investigational medication that returned chorea control to the natural history of their disease, on titration of deutetrabenazine, the TMC improved. It is important to note that the differences in TMC reductions between both cohorts may be attributed, in part, to the 1-week washout of deutetrabenazine or placebo treatment at the end of First-HD. The washout could potentially have raised chorea intensity above what would be considered “baseline”; thus, regression towards the mean may have contributed to the greater reduction in TMC score observed in the Rollover cohort.
Conclusions about efficacy are limited as this study was an open-label design that intentionally mimicked clinical practice. However, there appeared to be continued chorea control over the course of the study. Similarly, it is difficult to make conclusions comparing this study to a double-blind study, but it is encouraging that both cohorts had lower EAIRs of treatment-related AEs than First-HD (EAIR 0.70 and 0.88 for the Rollover and Switch cohorts, respectively vs EAIR 2.58 and 1.44 for the deutetrabenazine and placebo arms of First-HD, respectively). Adverse events leading to dose reduction, suspension, or withdrawal were generally lower in both cohorts than in both treatment arms of First-HD. In addition to the limitations of an open-label study design, the Switch cohort results may not be fully representative of patients taking tetrabenazine, as patients were required to be receiving a stable dose of tetrabenazine providing therapeutic benefit but were not optimized on therapy. Furthermore, as doses were individualized for each patient using a response-driven dosing regimen, this study does not provide set dose equivalences for overnight conversion from tetrabenazine to deutetrabenazine or vice versa. At the end of the study, the EAIRs of patients who reported any AE were lower in the Rollover cohort (2.57) than in the Switch cohort (4.02); however, the frequencies of serious AEs and AEs leading to dose suspension were similar. Last, although a few patients receiving citalopram or escitalopram experienced elevated QTcF values, the long-term consequences are unknown and these events were not connected to the one death from cardiac arrest in the Rollover cohort. According to the US prescribing information, deutetrabenazine should be avoided in patients with congenital long QT syndrome or a history of cardiac arrhythmias [17].
Conclusions
Results of this open-label extension add to the safety, tolerability, and efficacy profile of deutetrabenazine from the First-HD trial. For patients with HD, including those who previously benefited from tetrabenazine, chorea control can be maintained and improved through long-term use of twice-daily deutetrabenazine, with favorable safety and tolerability. This study demonstrates that long-term treatment with deutetrabenazine reduces chorea in patients with HD and is generally well tolerated.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the patients, families, and site personnel involved with this study. Michael Howell, PhD (Chameleon Communications International with funding from Teva Pharmaceuticals) wrote the first draft of the manuscript based on input from the authors, and Alaina Mitsch, PhD (Lumanity Communications Inc. with funding from Teva Pharmaceuticals) revised and styled the manuscript per journal requirements.
Huntington Study Group/ARC-HD Investigators and Coordinators: Samuel Frank, Claudia Testa, David Stamler, Elise Kayson, Mary C. Edmondson, Blair R. Leavitt, David Oakes, Christine O’Neill, Christina Vaughan, Jody Goldstein, Margaret Bockus, Stephanie Leyva, Victoria Snively, Jacquelyn Whaley, Cynthia Wong, William M. Mallonee, Gregory Suter, Joseph Jankovic, Joohi Jimenez-Shahed, Christine Hunter, Daniel O. Claassen, Lauren West, Olivia Roman, Victor Sung, Jenna Smith, Ronda Clouse, Marie Saint-Hilaire, Denyse Turpin, Raymond James, Ramon Rodriguez, Kyle Rizer, Karen Anderson, Hope Heller, Alexis Ahmad, Susan Criswell, Brad A. Racette, Frederick C. Nucifora Jr, Gregory Churchill, MaryJane Ong, Tilak Mendis, Neila Mendis, Carlos Singer, Jane S. Paulsen, Jane Kerr, Richard Dubinsky, Carolyn Gray, Stewart A. Factor, Elaine Sperin, Eric Molho, Sharon Evans, Breanna Nickels, Courtney von Bergen, Jessica Jaynes, Christina Reeves, Vicki Segro, Ali Samii, Emily Christopher, Debra Del Castillo, Sylvain Chouinard, Peggy Perry-Trice, Sherali Esmail, Wai Lun Alan Fung, Clare Gibbons, Amy Colcher, Cory Hackmyer, Andrew McGarry, Kevin Klos, Mark Gudesblatt, Daniel Schneider, Rohit Dhall, Edith Simpson, Joanne Wojcieszek, Andrea Hurt, Kathrin LaFaver, Annette Robinson, Fredy J. Revilla, Andrew P. Duker, Erin Neefus, Hilary Wilson-Perez, David Shprecher, Tyler Hohnholt, Paola Wall, James Boyd, Emily Houston, Eric S. Farbman, Shamine Poynor, Pinky Agarwal, Julissa Leon, Shirley Eberly, Arthur Watts, Pierre Tariot, Andrew Feigin, Scott R. Evans, Christopher A. Beck.
Declarations
Funding
This study and its open access publication was funded by Teva Pharmaceutical Instrustries, Tel Aviv, Israel.
Conflict of interest
Samuel Frank and Claudia Testa served as co-principal investigators of First-HD. Claudia Testa also served as a consultant for Lundbeck, with honoraria donated to the Medical College of Virginia Foundation. Mary C. Edmondson reports serving on a Wave Life Sciences Clinical Advisory Committee, funding from uniQure, and ENROLL-HD DSMB involvement. Blair R. Leavitt is currently employed as a Professor in the Department of Medical Genetics, and Division of Neurology, Department of Medicine and as a senior scientist at the Centre for Molecular Medicine and Therapeutics, The University of British Columbia, and BC Children’s Hospital. In the last 5 years, he has held or applied for research grants in the area of neurodegenerative disease from the Canadian Institutes of Health Research, The Huntington Society of Canada, Weston Brain Foundation, Brain Canada, uniQure, Triplet, and the Nanomedicines Innovations Network. He has served on scientific advisory boards for sRNAlytics, the Huntington’s Disease Society of America, and the Huntington Society of Canada. In addition, he has acted as a paid consultant to Roche, uniQure, Novartis, PTC Therapeutics, Triplet Therapeutics, Genentech, Takeda, and Ionis. He is also a shareholder, co-founder, and CEO of Incisive Genetics Inc., a biotech start-up company developing therapies for genetic brain diseases. David Oakes received research support from Auspex (for the First-HD and related studies), Vaccinex Inc., Prana Pharmaceuticals, Biogen, Inc., and the National Institutes of Health; and received honoraria from Raptor Pharmaceuticals and Voyager Inc. Christina Vaughan received an emergency fast-track COVID-19 response grant from the Adira Foundation. Nicholas Gross and Mark Forrest Gordon are employees of Teva Pharmaceuticals, and Juha-Matti Savola is a former employee of Teva Pharmaceuticals. Jody Goldstein, Elise Kayson, Christine O’Neill, and Jacquelyn Whaley report no disclosures.
Ethics approval
This study (ClinicalTrials.gov identifier: NCT01897896) was approved by the ethics boards at all involved centers prior to subject enrollment and conducted in accordance with the Helsinki Declaration and the International Council for Harmonisation Good Clinical Practice Guidelines.
Consent to participate
At enrollment, an independent qualified healthcare provider assessed all subjects for capacity to provide informed consent, and study personnel subsequently assessed subjects for capacity to continue in the study. Subjects or legally authorized representatives provided written informed consent, and subjects without capacity provided assent, if required by local regulations.
Consent for publication
Not applicable.
Availability of data and material
Qualified researchers may request access to patient-level data and related study documents, including the study protocol and the statistical analysis plan. Requests will be reviewed for scientific merit, product approval status, and conflicts of interest. Patient-level data will be de-identified and study documents will be redacted to protect the privacy of trial participants and to protect commercially confidential information. Please e-mail USMedInfo@tevapharm.com to make your request.
Code availability
Not applicable.
Author contributions
SF and MFG contributed to the drafting/revision of the manuscript for content, including medical writing for content, and had a major role in the acquisition of data, study concept or design, and the analysis or interpretation of data. CT, JG, EK, CV, and JW contributed to the drafting/revision of the manuscript for content, including medical writing for content, and had a major role in the acquisition of data and the analysis or interpretation of data. MCE, BRL, CO, and J-MS had a major role in the acquisition of data. DO and NG contributed to the drafting/revision of the manuscript for content, including medical writing for content, and the analysis or interpretation of data. All authors read and approved the final manuscript.
Footnotes
The members of “The Huntington Study Group/ARC-HD Investigators and Coordinators” are listed in the Acknowledgements section.
Contributor Information
Samuel Frank, Email: sfrank2@bidmc.harvard.edu.
The Huntington Study Group/ARC-HD Investigators and Coordinators:
Samuel Frank, Claudia Testa, David Stamler, Elise Kayson, Mary C. Edmondson, Blair R. Leavitt, David Oakes, Christine O’Neill, Christina Vaughan, Jody Goldstein, Margaret Bockus, Stephanie Leyva, Victoria Snively, Jacquelyn Whaley, Cynthia Wong, William M. Mallonee, Gregory Suter, Joseph Jankovic, Joohi Jimenez-Shahed, Christine Hunter, Daniel O. Claassen, Lauren West, Olivia Roman, Victor Sung, Jenna Smith, Ronda Clouse, Marie Saint-Hilaire, Denyse Turpin, Raymond James, Ramon Rodriguez, Kyle Rizer, Karen Anderson, Hope Heller, Alexis Ahmad, Susan Criswell, Brad A. Racette, Frederick C. Nucifora Jr, Gregory Churchill, MaryJane Ong, Tilak Mendis, Neila Mendis, Carlos Singer, Jane S. Paulsen, Jane Kerr, Richard Dubinsky, Carolyn Gray, Stewart A. Factor, Elaine Sperin, Eric Molho, Sharon Evans, Breanna Nickels, Courtney Bergen, Jessica Jaynes, Christina Reeves, Vicki Segro, Ali Samii, Emily Christopher, Debra Del Castillo, Sylvain Chouinard, Peggy Perry-Trice, Sherali Esmail, Wai Lun Alan Fung, Clare Gibbons, Amy Colcher, Cory Hackmyer, Andrew McGarry, Kevin Klos, Mark Gudesblatt, Daniel Schneider, Rohit Dhall, Edith Simpson, Joanne Wojcieszek, Andrea Hurt, Kathrin LaFaver, Annette Robinson, Fredy J. Revilla, Andrew P. Duker, Erin Neefus, Hilary Wilson-Perez, David Shprecher, Tyler Hohnholt, Paola Wall, James Boyd, Emily Houston, Eric S. Farbman, Shamine Poynor, Pinky Agarwal, Julissa Leon, Shirley Eberly, Arthur Watts, Pierre Tariot, Andrew Feigin, Scott R. Evans, and Christopher A. Beck
References
- 1.Foroud T, Gray J, Ivashina J, Conneally PM. Differences in duration of Huntington’s disease based on age at onset. J Neurol Neurosurg Psychiatry. 1999;66(1):52–56. doi: 10.1136/jnnp.66.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jankovic J. Treatment of hyperkinetic movement disorders. Lancet Neurol. 2009;8(9):844–856. doi: 10.1016/S1474-4422(09)70183-8. [DOI] [PubMed] [Google Scholar]
- 3.Testa CM, Jankovic J. Huntington disease: a quarter century of progress since the gene discovery. J Neurol Sci. 2019;396:52–68. doi: 10.1016/j.jns.2018.09.022. [DOI] [PubMed] [Google Scholar]
- 4.Carlozzi NE, Schilling SG, Lai JS, Paulsen JS, Hahn EA, Perlmutter JS, et al. HDQLIFE: development and assessment of health-related quality of life in Huntington disease (HD) Qual Life Res. 2016;25(10):2441–2455. doi: 10.1007/s11136-016-1386-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwab LC, Garas SN, Drouin-Ouellet J, Mason SL, Stott SR, Barker RA. Dopamine and Huntington’s disease. Expert Rev Neurother. 2015;15(4):445–458. doi: 10.1586/14737175.2015.1025383. [DOI] [PubMed] [Google Scholar]
- 6.Anderson KE. Huntington’s disease. Handb Clin Neurol. 2011;100:15–24. doi: 10.1016/B978-0-444-52014-2.00002-1. [DOI] [PubMed] [Google Scholar]
- 7.Burgunder JM, Guttman M, Perlman S, Goodman N, van Kammen DP, Goodman L. An international survey-based algorithm for the pharmacologic treatment of chorea in Huntington’s disease. PLoS Curr. 2011;3:RRN1260. doi: 10.1371/currents.RRN1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jankovic J, Roos RAC. Chorea associated with Huntington’s disease: to treat or not to treat? Move Disord. 2014;29(11):1414–1418. doi: 10.1002/mds.25996. [DOI] [PubMed] [Google Scholar]
- 9.Helder DI, Kaptein AA, van Kempen GM, van Houwelingen JC, Roos RA. Impact of Huntington’s disease on quality of life. Mov Disord. 2001;16(2):325–330. doi: 10.1002/mds.1056. [DOI] [PubMed] [Google Scholar]
- 10.Ho AK, Gilbert AS, Mason SL, Goodman AO, Barker RA. Health-related quality of life in Huntington’s disease: which factors matter most? Mov Disord. 2009;24(4):574–578. doi: 10.1002/mds.22412. [DOI] [PubMed] [Google Scholar]
- 11.Coppen EM, Roos RA. Current pharmacological approaches to reduce chorea in Huntington’s disease. Drugs. 2017;77(1):29–46. doi: 10.1007/s40265-016-0670-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xenazine™ (tetrabenazine) [prescribing information]. Deerfield: Lundbeck; 2015.
- 13.Huntington Study Group Tetrabenazine as antichorea therapy in Huntington disease: a randomized controlled trial. Neurology. 2006;66(3):366–372. doi: 10.1212/01.wnl.0000198586.85250.13. [DOI] [PubMed] [Google Scholar]
- 14.Bashir H, Jankovic J. Deutetrabenazine for the treatment of Huntington’s chorea. Expert Rev Neurother. 2018;18(8):625–631. doi: 10.1080/14737175.2018.1500178. [DOI] [PubMed] [Google Scholar]
- 15.Schneider F, Stamler D, Bradbury M, Loupe PS, Hellriegel E, Cox DS, et al. Pharmacokinetics of deutetrabenazine and tetrabenazine: dose proportionality and food effect. Clin Pharmacol Drug Dev. 2021;10(6):647–659. doi: 10.1002/cpdd.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frank S, Stamler D, Kayson E, Claassen DO, Colcher A, Davis C, et al. Safety of converting from tetrabenazine to deutetrabenazine for the treatment of chorea. JAMA Neurol. 2017;74(8):977–982. doi: 10.1001/jamaneurol.2017.1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Austedo® (deutetrabenazine) tablets [prescribing information]. Parsippany: Teva Pharmaceuticals USA, Inc.; 2021.
- 18.China approves Austedo® for treating chorea associated with Huntington’s disease and tardive dyskinesia in adults. Teva Pharmaceutical Industries Ltd. 2020. Available from: https://www.tevapharm.com/news-and-media/latest-news/china-approves-austedo-for-treating-chorea-associated-with-huntingtons-disease-and-tardive-dyskinesia-i/#:~:text=(NYSE%20and%20TASE%3A%20TEVA),adults%2C%20after%20a%20priority%20review. Accessed 2 Aug 2022.
- 19.Austedo. Therapeutic goods administration. 2021. Available from: https://www.tga.gov.au/apm-summary/austedo. Accessed 2 Aug 2022.
- 20.Huntington Study Group. Frank S, Testa CM, Stamler D, Kayson E, Davis C, et al. Effect of deutetrabenazine on chorea among patients with Huntington disease: a randomized clinical trial. JAMA. 2016;316(1):40–50. doi: 10.1001/jama.2016.8655. [DOI] [PubMed] [Google Scholar]
- 21.Liu GF, Wang J, Liu K, Snavely DB. Confidence intervals for an exposure adjusted incidence rate difference with applications to clinical trials. Stat Med. 2006;25(8):1275–1286. doi: 10.1002/sim.2335. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Consistent with the policies of the Huntington Study Group, the corresponding author is confirmed as the guarantor of the work and all authors had full access to the data. All authors have the right to publish any and all data separate and apart from any sponsor, and we take full responsibility for the data, the analyses and interpretation, and the conduct of the research.