Abstract
Irrigation not only helps to improve food security but also creates numerous water bodies for mosquito production. This study assessed the effect of irrigation on malaria vector bionomics and transmission in a semi-arid site with ongoing malaria vector control program. The effectiveness of CDC light traps in the surveillance of malaria vectors was also evaluated relative to the human landing catches (HLCs) method. Adult mosquitoes were sampled in two study sites representing irrigated and non-irrigated agroecosystems in western Kenya using a variety of trapping methods. The mosquito samples were identified to species and assayed for host blood meal source and Plasmodium spp. sporozoite infection using polymerase chain reaction. Anopheles arabiensis was the dominant malaria vector in the two study sites and occurred in significantly higher densities in irrigated study site compared to the non-irrigated study site. The difference in indoor resting density of An. arabiensis during the dry and wet seasons was not significant. Other species, including An. funestus, An. coustani, and An. pharoensis, were collected. The An. funestus indoor resting density was 0.23 in irrigated study site while almost none of this species was collected in the non-irrigated study site. The human blood index (HBI) for An. arabiensis in the irrigated study site was 3.44% and significantly higher than 0.00% for the non-irrigated study site. In the irrigated study site, the HBI of An. arabiensis was 3.90% and 5.20% indoor and outdoor, respectively. The HBI of An. funestus was 49.43% and significantly higher compared to 3.44% for An. arabiensis in the irrigated study site. The annual entomologic inoculation rate for An. arabiensis in the irrigated study site was 0.41 and 0.30 infective bites/person/year indoor and outdoor, respectively, whereas no transmission was observed in the non-irrigated study site. The CDC light trap performed consistently with HLC in terms of vector density. These findings demonstrate that irrigated agriculture may increase the risk of malaria transmission in irrigated areas compared to the non-irrigated areas and highlight the need to complement the existing malaria vector interventions with novel tools targeting the larvae and both indoor and outdoor biting vector populations.
Graphical abstract

Supplementary Information
The online version contains supplementary material available at 10.1007/s00436-022-07678-2.
Keywords: Irrigation, Vector density, Vector bionomics, Malaria transmission, Anopheles
Introduction
In Africa, food insecurity and famine continue to affect millions of people (Baro and Deubel 2006). Given that nearly half of potential arable land in Africa occur in areas with irregular rainfall pattern, many countries have adopted irrigated agriculture as a key strategy to meet the rising demand for food (Blank et al. 2002). This effort has improved crop production by enabling the reclamation of arid and semi-arid lands, enhancing crop yield, extending the crop-growing season, and reducing the risk of crop failure (Oomen et al. 1988; Keiser et al. 2005; Yohannes et al. 2005). In addition, irrigation projects have led to improved nutrition and socioeconomic conditions for the vulnerable population (Bryan et al. 2019). Despite these socioeconomic benefits, irrigated agriculture creates numerous water bodies that may support large populations of mosquitoes including malaria vectors although this may not necessarily lead to increased risk of malaria transmission (Patz et al. 2004; Muturi et al. 2008a).
In Sudan, introduction of the Gezira-Managil scheme in the Nile River Valley led to an increased densities of An. arabiensis exacerbating malaria outbreaks (Oomen et al. 1988). Similarly, irrigation schemes increased vector densities and malaria incidences in South Central Sierra Leone (Gbakima 1994), Ethiopia (Yohannes et al. 2005), Cameroon (Robert et al. 1992), and Burundi (Coosemans 1985). In contrast, reduction in malaria transmission was reported in irrigated rice cultivations of Mali (Sissoko et al. 2004) and Lower Moshi Tanzania (Ijumba et al. 2002a) as compared to the adjacent non-irrigated areas. Reduced transmission could be attributed to increased wealth that was implicated in the increased acquisition and use of insecticide treated nets and anti-malarial drugs in irrigation projects leading to reduced malaria incidence (Ijumba et al. 2002a; Henry et al. 2003; Diuk-Wasser et al. 2005). However, in some cases, introduction of irrigation schemes like in Senegal River Delta had no impact on malaria transmission (Faye et al. 1995). Worthwhile noting is that in areas of stable malaria transmission, the introduction of irrigated agriculture has little or no impact on malaria transmission (Ijumba and Lindsay 2001; Ijumba et al. 2002b) nevertheless in semi-arid savannah zone of Africa irrigated rice cultivation can alter malaria transmission pattern from seasonal to perennial (Dolo et al. 2004; Sissoko et al. 2004). Hence, the impact of water development projects on malaria transmission is variable and likely depends on the ecology of local mosquito vectors, underlying ecological factors, epidemiologic setting, socioeconomic conditions, and existing malaria control measures (Keiser et al. 2005). Thus, its complexity can only be understood through site-specific evaluation of these parameters.
Insecticide-based vector control interventions mainly long-lasting insecticidal nets (LLINs) and indoor residual spraying (IRS) have been implemented to reduce malaria transmission with significant impacts. These tools have resulted in dramatic reduction in the proportion of endophagic and anthropophilic malaria vector species such as Anopheles gambiae, An. coluzzii, and An. funestus and a proportionate increase in An. arabiensis, which tend to be exophagic and less anthropophilic. However, previous studies indicate that vectors can develop resistance to insecticides or adapt to the presence of insecticides by becoming partially zoophilic and exophilic. Hence with the scale up of LLINs and widespread use of IRS, there is likely to be a shift in vector dominance from the highly endophilic An. gambiae/An. coluzzi and An. funestus to the more zoophilic and exophilic An. arabiensis (Bayoh et al. 2010; Futami et al. 2014; Abong’o et al. 2020).
There is a pressing need to enhance our understanding on the effect of irrigation in a site where there is malaria vector control. The study aims to assess the effect of a recently established irrigation scheme in Homa Bay, Kenya, on malaria vector bionomics and transmission. Vector control intervention using LLINs and IRS with organophosphate, pirimiphos-methyl (Actellic® 300CS) was being undertaken during the study period. Long-term success of the current malaria control efforts, ITNs and IRS, is dependent on continuous operational surveillance of the mosquito vectors, thus an effective mosquito sampling tool is required. Hence, the secondary goal was to compare the trap effectiveness of Centers for Disease Control and Prevention (CDC) light traps against the gold standard, human landing catches (HLCs). Results of this study will serve as the baseline vector bionomics and malaria transmission pattern for the evaluation of the success of core vector interventions and inform policymakers in planning and guiding future interventions especially in irrigated areas where there is scale up of LLINs distribution and application of IRS.
Material and methods
Study site
The study was conducted in Rangwe (0°35′24″S; 34°35′05″E) and Rachuonyo South (0°19′17″S; 34°07′22″E) sub-counties in Homa Bay County of western Kenya situated at an altitude of 1,202 m above sea level adjacent to the eastern shore of Lake Victoria (Fig. 1). The county is a semi-arid expansive lowland characterized by black cotton soils. The area experiences bimodal rainfall pattern with a mean annual of 1,226 mm. The long rainy season occur between April and June while short rainy season occur from October to November. The hot and dry season is from January to March. The mean annual temperature is 25.7 °C, with a minimum of 18.3 °C and maximum of 29 °C. Relative humidity varies from 52 to 67%. The main economic activities are fishing in Lake Victoria and irrigated and non-irrigated subsistence farming.
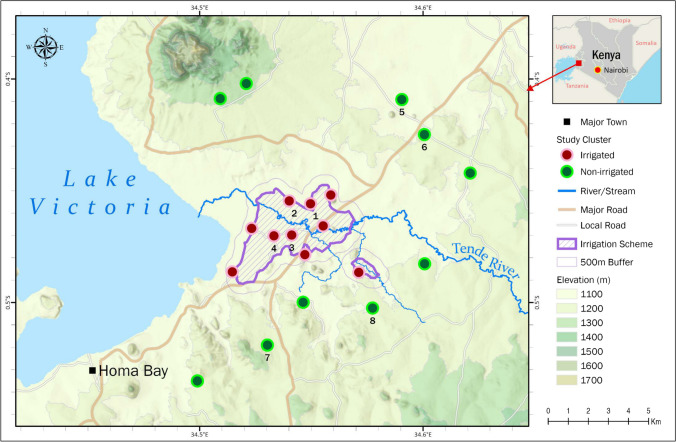
Fig. 1.
The site map indicates the study clusters in Homa Bay, Kenya. The red dots represent the clusters within the irrigated zone and the green dots represent the clusters within the non-irrigated zone. Clusters labeled with numbers 1–8 also have been surveyed monthly for malaria vectors population dynamics research
The study was conducted in Kimira-Oluch Irrigation Scheme (0°26′44″S; 34°31′28.0″E) and its vicinity, which lies in an area of 110 km2 and is located approximately 10 km north of the town of Homa Bay, Homa Bay County of western Kenya. The study site was stratified into irrigated and non-irrigated zones depending on proximity to irrigation scheme. Each zone consisted of 10 clusters (cluster radii vary from 0.25 to 1 km) and with populations ranging from 50 to 250 residents in each cluster. The irrigated zone is within a concrete canal and flood irrigation systems. The crops grown under this irrigation scheme mainly include maize, beans, kales, tomatoes, pawpaw, bananas, watermelons, and rice grown in paddies. The non-irrigated zone is located about 5–10 km from the irrigated zone.
In an effort to reduce malaria burden in the lake endemic zone, vector control interventions were instigated between 2006 and 2008 through the use of LLINs and IRS. The first mass LLIN distribution occurred in 2006 followed by successive rounds of distribution in 2011, 2014, 2017, and 2021 (Ministry of Health 2016; 2021a; Ng’ang’a et al. 2021). Insecticide residual spraying was first implemented in Rachuonyo district in 2008 followed by successive rounds in 2009 to 2012 and 2017 to 2021 in targeted areas (PMI 2013; Gimnig et al. 2016; 2021b). According to a recent study conducted by Orondo et al. (2021) in the study site, the use of LLINs and IRS in the irrigated and non-irrigated zones is similar.
Study design
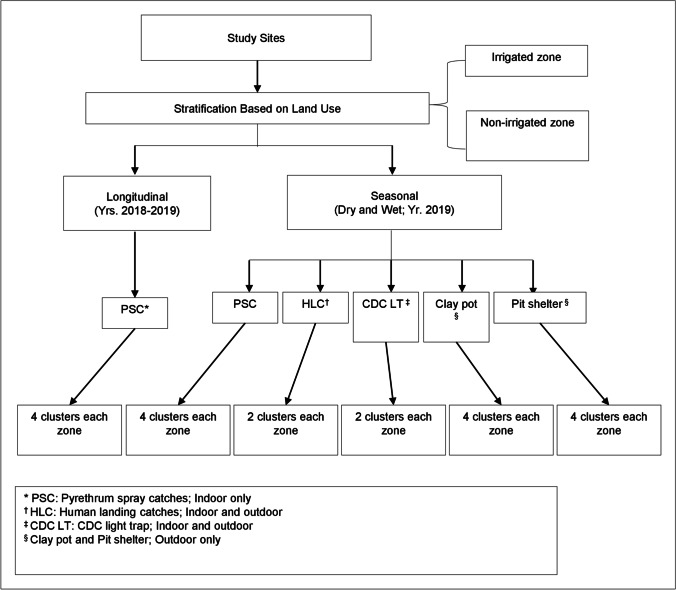
Seasonal surveys were conducted in the dry (Jan–Mar) and wet (Apr–Jun) seasons in 2019 using five different trapping methods (Fig. 2). Indoor and outdoor host-seeking vector collections using CDC light traps and HLCs were undertaken in two randomly selected clusters in each zone. There were 160 trap-nights for each trap. Indoor and outdoor resting vector collections using pyrethrum spray catches (PSCs) (indoor), clay pots (outdoor), and pit shelters (outdoor) were undertaken in four randomly selected clusters in each zone. There was a total of 320 trap-nights for each trap and 144 for pit shelters. Longitudinal adult vector surveillance was conducted using PSCs in four clusters in each zone for malaria vectors population dynamic research between 2018 and 2019 (Fig. 2).
Fig. 2.
Study design flow chart
Seasonal survey
CDC light traps
The CDC light traps were set both indoor and outdoor to assess vector host-seeking behavior (Fig. 3a, b) (WHO 1975). The indoor CDC light trap was set 1 m beside an occupied bed at a height of 1.5 m off-ground and the outdoor trap was set within 5 m away from the front door at a height of 1.5 m off-ground. Vector collections were undertaken in five randomly selected houses in each cluster from 6 p.m. to 6 a.m. for four consecutive nights once per season.
Fig. 3.
Vector sampling tools [a indoor CDC light trap, b outdoor CDC light trap, c indoor human landing catches, d outdoor human landing catches, e pyrethrum spray catches, f clay pot, g pit shelter] used for outdoor and/or indoor host-seeking/resting malaria vector (pictures captured in the field)
Human landing catches
Human landing catches were conducted both indoor and outdoor to assess vector host-seeking behavior (Gimnig et al. 2013; WHO 2013). In each compound, vectors were collected indoors (at the house entrance) and outdoors 5 m away from the sentinel indoor collection house (Fig. 3c, d). Collection was undertaken by four volunteers, two in each of the indoor and outdoor stations, who alternated after 6 h. Hourly collections were done from 6 p.m. to 6 a.m. each night, with 45 min of collection and a 15 min break per hour. Each hourly collection was placed in individually labeled paper cups and maintained with a 10% sugar solution pad and then placed in a cool box. The same collectors conducted HLCs every night and were rotated between positions (indoor vs. outdoor). All collections were supervised by a team leader. Vector collections were undertaken in five randomly selected houses in each cluster for four consecutive nights once per season. All collectors were provided with anti-malarial chemo-prophylaxis during the study period.
Pyrethrum spray catches
Indoor resting vector collections using PSCs (Fig. 3e) were undertaken in 20 randomly selected houses in each cluster once per season from 6 a.m. to 9 a.m. following WHO protocol (WHO 1975).
Clay pots
Outdoor resting vectors were assessed using clay pot outdoors (≤ 5 m away from the house) placed behind the house close to the bedroom where there is minimal human activities to minimize disturbance (Fig. 3f). The 20 l capacity clay pots were ∼0.5 m in height, 45 cm in diameter on wide base, and a 20 cm diameter opening as described by Odiere et al. with modifications (Odiere et al. 2007). During setting, the pots were filled with 2 l of rainwater to increase humidity (Ng’Habi et al. 2010) and tilted at 45° to the ground. Vector collections were undertaken in 20 randomly selected houses in each cluster once per season. One pot was set at 6 p.m. in each of the 20 houses and mosquito collected the following morning between 6 a.m. and 9 a.m. using a hand-held Prokopack aspirator.
Pit shelters
Pit shelters were dug (1.5 m in depth, 1.5 m in length, and 1 m in width) within 20 m of each selected house according to the method of Muirhead-Thomas (Fig. 3g) (Muirhead-Thomson 1958). In each of the four vertical sides, approximately 0.6 m from the bottom of the pit, cavities were dug to a depth of about 0.3 m. The mouth of the main pit was shaded from above using an artificial shelter. Vector collections were undertaken in one randomly selected house in each cluster for five consecutive nights monthly per season. Vector collection was undertaken between 6 a.m. and 9 a.m. inside the cavities by using a hand-held Prokopack aspirator according to WHO protocol (WHO 1975).
Longitudinal surveillance
Temporal indoor resting vector population abundance was determined by conducting monthly surveys by PSCs in five randomly selected houses in each cluster. Application of IRS was undertaken in the study area by the National Malaria Control Program (Kenya) during the dry seasons in February of 2018 and 2019.
Vector species identification
All adult mosquitoes collected were transferred to the International Center of Excellence for Malaria Research (ICEMR) laboratory in Homa Bay, sorted, and anophelines identified morphologically to species as previously described (Gillies and Coetzee 1987). Female Anopheles mosquitoes were physiologically classified according to their gonotrophic stages: unfed, blood-fed, half-gravid, and gravid. For species identification, DNA was extracted from the legs and wings of each specimen using the Chelex protocol by Musapa et al. (2013). Sibling species in An. gambiae s.l. and An. funestus were speciated by conventional polymerase chain reaction (PCR) as described by Scott et al. (1993) and Koekemoer et al. (2002), respectively.
Molecular detection of blood meal sources and sporozoite infections
The abdomen of Anopheles mosquito specimens was carefully separated from the head and thorax and DNA extracted (Musapa et al. 2013). The blood meal sources of each freshly fed Anopheles mosquitoes were analyzed by multiplexed PCR as described by Kent and Norris (2005).
The DNA extracted (Musapa et al. 2013) from the head and thorax of each mosquito specimen was used to determine sporozoite infections of Plasmodium spp. by using the multiplexed real-time quantitative PCR (qPCR) assay. The assay was performed using the published species-specific 18 s ribosomal RNA probes and primers for Plasmodium falciparum, P. malariae, and P. ovale (Shokoples et al. 2009; Veron et al. 2009).
Data management and analysis
Data were entered in Microsoft Excel 2010 datasheets and analyses were done using R statistical software (version 4.0.3; R foundation for statistical computing, Vienna, Austria). Mean density (95% confidence interval, CI) and proportions were calculated for vector populations. The density of adult anopheline mosquitoes was calculated as the average number of female mosquitoes per house per night (f/h/n). Several models were evaluated for the analysis of vector density, and the model with the lowest akaike information criterion (AIC) and variables of interest was selected as the best model (Additional file 1). In the analysis of seasonal data, a negative binomial mixed model (NBMM) was fitted to analyze Anopheles densities outdoor, indoor, and trapping method (HLC and CDC light trap) (Additional file 1; Table S1, Table S2, Table S3). Zone and trapping methods were fitted as the fixed variables in the outdoor and indoor models while zone, trapping methods, and location (indoor and outdoor) were considered as fixed variables in the trapping method model. In the indoor and trapping method models, house number and date were used as covariates whereas house number and cluster were used as covariates in the outdoor model. In the analysis of longitudinal data, a NBMM with repeated measures were fitted to compare Anopheles densities and seasonality in the two zones by adjusting for months (Additional file 1; Table S4). Zone and season were fitted as the fixed variables and year: (date: cluster), date: cluster, cluster considered as covariates. The chi-square test was used to compare differences in vector species gonotrophic stage proportions between indoor and outdoor collections and also the zones. The human blood index (HBI) for each mosquito species was calculated as the proportion of mosquito samples that had fed on humans out of the total number tested (Garrett-Jones 1964). Sporozoite rates were calculated as the proportion of Anopheles mosquito samples positive for Plasmodium spp. out of the total number tested. The human biting rate was calculated as the product of blood-fed females per person per night and the human blood index. Annual entomological inoculation rates (EIRs) were calculated as the product of the sporozoite rate and the human biting rates (Macdonald 1957).
Results
Seasonal survey
Vector species composition
A total of 3,556 female Anopheles mosquitoes belonging to four species were collected using the five trapping methods during the study period. Anopheles gambiae s.l. was the predominant anopheline species accounting for 79.2%, followed by An. coustani (15.6%), An. pharoensis (4.6%), and An. funestus group (0.6%). In addition, 1,140 male Anopheles mosquitoes and 17,387 Culex species were collected (males, n = 3,776; females, n = 13,611). A total of 958 specimens (941 An. gambiae s.l. and 17 An. funestus) were analyzed for sibling species identification. Of these, 765 (81.3%) An. gambiae s.l. and 7 (41.2%) An. funestus were successfully amplified and all were confirmed as An. arabiensis and An. funestus s.s., respectively.
Indoor and outdoor vector density
The mean density of the female An. arabiensis mosquitoes varied by zone and collection method (Table 1, Fig. 4, and Additional file 1). Only few An. funestus were collected in the trapping methods, and the mean density was not analyzed.
Table 1.
Densities of resting female Anopheles mosquito collected using pyrethrum spray catches (PSCs) (indoor), clay pot (outdoor), and pit shelter (outdoor) from irrigated and non-irrigated zones pooled of dry (Jan–Mar, 2019) and wet (Apr–Jun, 2019) seasons (n = 320 trap-nights for each trap; n = 144 trap-nights for pit shelter) [mean (95% CI)]
| Study site and species | Dry season | Wet season | ||||
|---|---|---|---|---|---|---|
| Indoor | Outdoor | Indoor | Outdoor | |||
| PSC | Clay pot | Pit shelter | PSC | Clay pot | Pit shelter | |
| Irrigated zone | ||||||
| An. arabiensis | 4.36 | 3.34 | 9.75 | 2.08 | 1.88 | 11.42 |
| (2.89, 5.84) | (2.29, 4.39) | (5.26, 14.24) | (0.88, 3.27) | (1.27, 2.48) | (8.50, 14.34) | |
| An. funestus | 0.01 | 0.03 | 0 | 0 | 0 | 0.04 |
| (0, 0.04) | (0, 0.06) | (0, 0.09) | ||||
| An. coustani | 0 | 0 | 0.15 | 0.01 | 0.01 | 0.04 |
| (0, 0.32) | (0, 0.04) | (0, 0.04) | (0, 0.09) | |||
| Non-irrigated zone | ||||||
| An. arabiensis | 0.06 | 0.11 | 0.30 | 0.35 | 0.21 | 0.77 |
| (0.01, 0.12) | (0.02, 0.21) | (0, 0.73) | (0.15, 0.55) | (0.09, 0.33) | (0.48, 1.05) | |
| An. funestus | 0 | 0 | 0 | 0 | 0 | 0.04 |
| (0, 0.09) | ||||||
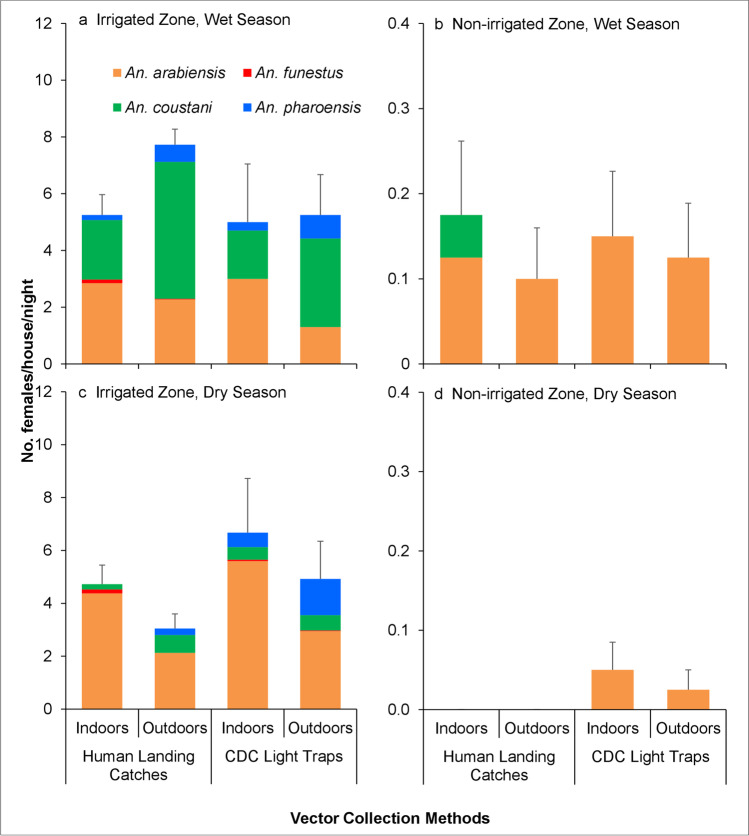
Fig. 4.
Host-seeking female Anopheles mosquito densities collected using human landing catches and CDC light traps indoors and outdoors from irrigated and non-irrigated zones in dry (Jan–Mar) and wet (Apr–Jun) seasons in 2019. Error bars were for the standard error for the total Anopheles mosquitoes collected. (n = 160 trap-nights for each trap)
In the irrigated zone, the outdoor mean density of An. arabiensis was significantly higher compared to the non-irrigated zone (Z = − 8.276, df = 776, P < 0.001) (Table 1, Fig. 4, Additional file 1: Table S1). Similarly, in the irrigated zone, the indoor mean density of An. arabiensis was significantly higher compared to the non-irrigated zone (Z = − 9.403, df = 628, P < 0.001) (Table 1, Fig. 4, Additional file 1: Table S2).
Pit shelters (Z = 6.433, df = 776, P < 0.001) and clay pots (Z = 3.117, df = 776, P < 0.01) yielded a significantly higher outdoor density of An. arabiensis than CDC light traps, whereas the difference in outdoor mean density of An. arabiensis between HLC and CDC light trap was not significant (Z = 0.966, df = 776, P = 0.334) (Additional file 1: Table S1). There was no significant difference in the indoor mean density of An. arabiensis from PSC and HLC compared to CDC light traps (all, P > 0.001) (Additional file 1: Table S2).
Overall, the mean density of An. arabiensis was higher in the irrigated zone than in the non-irrigated zone. There was no significant difference in the mean density of An. arabiensis during the dry and wet seasons (all, P > 0.001) (Additional file 1: Table S1, Table S2, and Table S3).
HLC and CDC light trap comparison
The HLC and CDC light trap yielded a significantly higher host-seeking density of An. arabiensis in the irrigated zone than non-irrigated zone (Z = − 9.841, df = 631, P < 0.001) (Fig. 4, Additional file 1: Table S3). There was no significant difference between HLC and CDC light traps in terms of the An. arabiensis mean host-seeking density (Z = 0.351, df = 631, P = 0.725) (Fig. 4, Additional file 1: Table S3). The results indicated that CDC light trap performed consistently with HLC in terms of vector density. The mean indoor and outdoor host-seeking density of An. arabiensis from HLC and CDC light traps collections varied significantly (Z = − 3.175, df = 631, P < 0.01) with the highest mean host-seeking density collected indoors (Fig. 4, Additional file 1: Table S3).
Gonotrophic status of female Anopheles mosquitoes
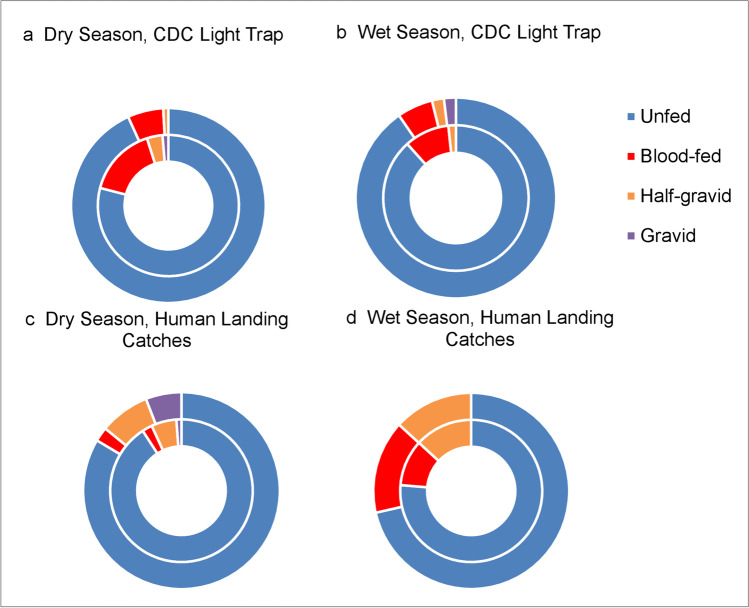
The gonotrophic status of An. arabiensis variation was significantly higher indoor compared to outdoor collections using CDC light trap during the dry season (χ2 = 11.94, df = 3, P = 0.03). In contrast, there was no significant difference during the wet season (χ2 = 3.08, df = 3, P = 0.38) (Fig. 5). There was also no significant difference in the gonotrophic status of An. arabiensis between indoor and outdoor collections using HLC during the dry (χ2 = 5.68, df = 3, P = 0.13) and wet seasons (χ2 = 1.11, df = 3, P = 0.78) (Fig. 5). Most of the An. arabiensis collected by HLC and CDC light trap were unfed (Fig. 5). Due to the small number of mosquito collections in HLC and CDC light traps in non-irrigated zone, the gonotrophic status was not analyzed.
Fig. 5.
Gonotrophic status of female An. arabiensis mosquitoes collected using human landing catches (HLCs) and CDC light traps indoor and outdoor in irrigated zones in 2019. Outer rings referred to outdoor collection; inner rings referred to indoor collection
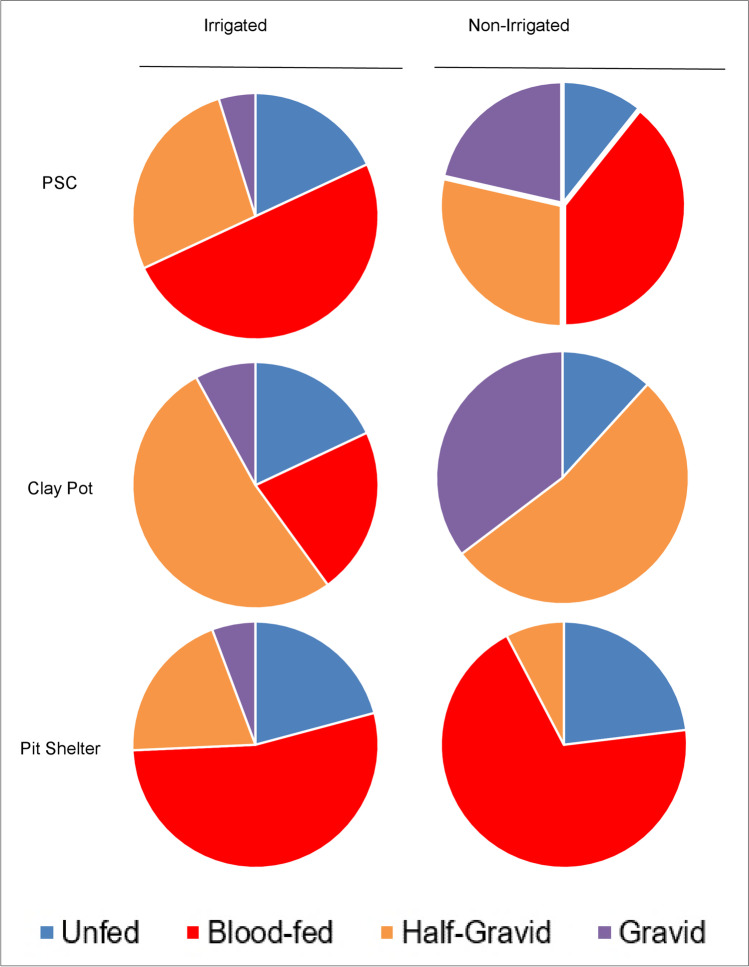
The gonotrophic status of An. arabiensis variation was significantly higher in the irrigated zone than in the non-irrigated zone in PSC (χ2 = 10.51, df = 3, P = 0.02) and clay pot (χ2 = 14.64, df = 3, P = 0.01) collections whereas there was no significant difference in pit shelter (χ2 = 6.87, df = 3, P = 0.08) collections (Fig. 6). Pit shelters and PSC yielded a higher proportion of blood-fed An. arabiensis compared to clay pots that captured mostly half-gravid An. arabiensis (Fig. 6).
Fig. 6.
Gonotrophic status of resting female An. arabiensis mosquitoes collected using pyrethrum spray catches (PSCs) (indoor), clay pot (outdoor), and pit shelter (outdoor) from irrigated and non-irrigated zones in 2019
Blood meal indices
The majority of the blood meals were of bovine origin (71.6%). Only 0.6% of mosquito samples had human blood meals and less than 1% of the samples had blood meals of goat, pig, or dog origin (Table 2). In the irrigated zone, outdoor HBI of An. arabiensis was almost twofold higher (outdoor, 5.20%; indoor, 3.90%) than indoor whereas in the non-irrigated zone, the blood meals were of bovine origin (Table 2).
Table 2.
The host feeding preference of An. arabiensis mosquitoes collected indoor and outdoor by different collection methods from irrigated and non-irrigated zones from seasonal sampling in 2019
| Blood-meal origins | Indoor | Outdoor | ||||
|---|---|---|---|---|---|---|
| PSCa (%) | Clay pot (%) | Pit shelter (%) | ||||
| Irrigated zone | Non-irrigated zone | Irrigated zone | Non-irrigated zone | Irrigated zone | Non-irrigated zone | |
| No. tested | 205 | 13 | 114 | 1 | 136 | 6 |
| Human | 2 (1.0) | 0 (0) | 1 (0.9) | 0 (0) | 0 (0) | 0 (0) |
| Bovine | 157 (76.6) | 10 (76.9) | 67 (58.8) | 1 (100.0) | 101 (74.3) | 4 (66.7) |
| Human + bovine | 4 (2.0) | 0 (0) | 0 (0) | 0 (0) | 8 (5.9) | 0 (0) |
| Human + dog | 2 (1.0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Bovine + dog | 1 (0.5) | 0 (0) | 1 (0.9) | 0 (0) | 0 (0) | 0 (0) |
| Pig | 1 (0.5) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Human + goat | 0 (0) | 0 (0) | 1 (0.9) | 0 (0) | 0 (0) | 0 (0) |
| Human + bovine + dog | 0 (0) | 0 (0) | 2 (1.8) | 0 (0) | 0 (0) | 0 (0) |
| Human + dog + goat | 0 (0) | 0 (0) | 1 (0.9) | 0 (0) | 0 (0) | 0 (0) |
| Goat | 0 (0) | 0 (0) | 2 (1.8) | 0 (0) | 0 (0) | 0 (0) |
| Dog | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (0.7) | 0 (0) |
| Bovine + goat | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 3 (2.2) | 0 (0) |
| Bovine + pig | 0 (0) | 0 (0) | 1 (0.9) | 0 (0) | 0 (0) | 0 (0) |
| Unknown | 38 (18.5) | 3 (23.1) | 38 (33.3) | 0 (0) | 23 (16.9) | 2 (33.3) |
| HBIb | 3.90% | 0.00% | 4.39% | 0.00% | 5.88% | 0.00% |
aPSC, pyrethrum spray catches
bHuman blood index (HBI) was calculated as the number of mosquito positive for human blood meal (including mixed blood meal) divided by the total number tested
Sporozoite rate and entomological inoculation rate
Sporozoite-positive An. arabiensis samples were detected in the irrigation zone only and the sporozoite rate was twofold higher indoors than outdoors (Table 3). None of the An. funestus samples tested positive for sporozoites (Table 3). The annual EIR for An. arabiensis in irrigated zone was 0.71 infective bites/person/year (ib/p/year) and was higher indoors than outdoors (Table 4). Malaria transmission was not detected in the non-irrigated zone (Table 4).
Table 3.
The sporozoite rate of An. arabiensis and An. funestus mosquitoes collected indoor and outdoor by different collection methods from irrigated and non-irrigated zones in 2019
| Zone | Location | Method | An. arabiensis | An. funestus | ||||
|---|---|---|---|---|---|---|---|---|
| Mosquito tested | No. positive | Sporozoite rate (%) | Mosquito tested | No. positive | Sporozoite rate (%) | |||
| Irrigated zone | Indoors | PSCa | 170 | 5 | 2.9 | 1 | 0 | 0 |
| CDC LTb | 228 | 1 | 0.4 | 2 | 0 | 0 | ||
| HLCc | 246 | 4 | 1.6 | 11 | 0 | 0 | ||
| Sub-total | 644 | 10 | 1.6 | 14 | 0 | 0 | ||
| Outdoors | Clay pot | 238 | 3 | 1.3 | 1 | 0 | 0 | |
| Pit shelter | 198 | 0 | 0 | 1 | 0 | 0 | ||
| CDC LT | 101 | 1 | 1 | 1 | 0 | 0 | ||
| HLC | 150 | 2 | 1.3 | 1 | 0 | 0 | ||
| Sub-total | 687 | 6 | 0.8 | 4 | 0 | 0 | ||
| Non-irrigated zone | Indoors | PSC | 14 | 0 | 0 | 0 | 0 | - |
| CDC LT | 8 | 0 | 0 | 0 | 0 | - | ||
| HLC | 3 | 0 | 0 | 0 | 0 | - | ||
| Sub-total | 25 | 0 | 0 | 0 | 0 | - | ||
| Outdoors | Clay pot | 18 | 0 | 0 | 0 | 0 | - | |
| Pit shelter | 24 | 0 | 0 | 2 | 0 | 0 | ||
| CDC LT | 5 | 0 | 0 | 0 | 0 | - | ||
| HLC | 5 | 0 | 0 | 0 | 0 | - | ||
| Sub-total | 52 | 0 | 0 | 2 | 0 | 0 | ||
aPSC, pyrethrum spray catches
bCDC LT, CDC light trap
cHLC, human landing catches
- Not tested
Table 4.
The annual entomological inoculation rate (EIR) of Anopheles mosquitoes sampled in irrigated and non-irrigated zones in 2019
| Study site and species | Indoors | Outdoors | ||||||
|---|---|---|---|---|---|---|---|---|
| HBIa (%) | Sporozoite rate (%) | Mosquito density | Annual EIR | HBI (%) | Sporozoite rate (%) | Mosquito density | Annual EIR | |
| Irrigated zone | ||||||||
| An. arabiensis | 3.90 | 1.60 | 3.58 | 0.41 | 5.20 | 0.80 | 3.96 | 0.30 |
| An. funestus | - | 0.00 | 0.04 | - | - | 0.00 | 0.02 | - |
| Non-irrigated zone | ||||||||
| An. arabiensis | 0.00 | 0.00 | 0.14 | 0.00 | 0.00 | 0.00 | 0.21 | 0.00 |
| An. funestus | - | - | 0.00 | - | - | 0.00 | 0.01 | - |
aHBI, human biting index
- Not tested
Longitudinal surveillance
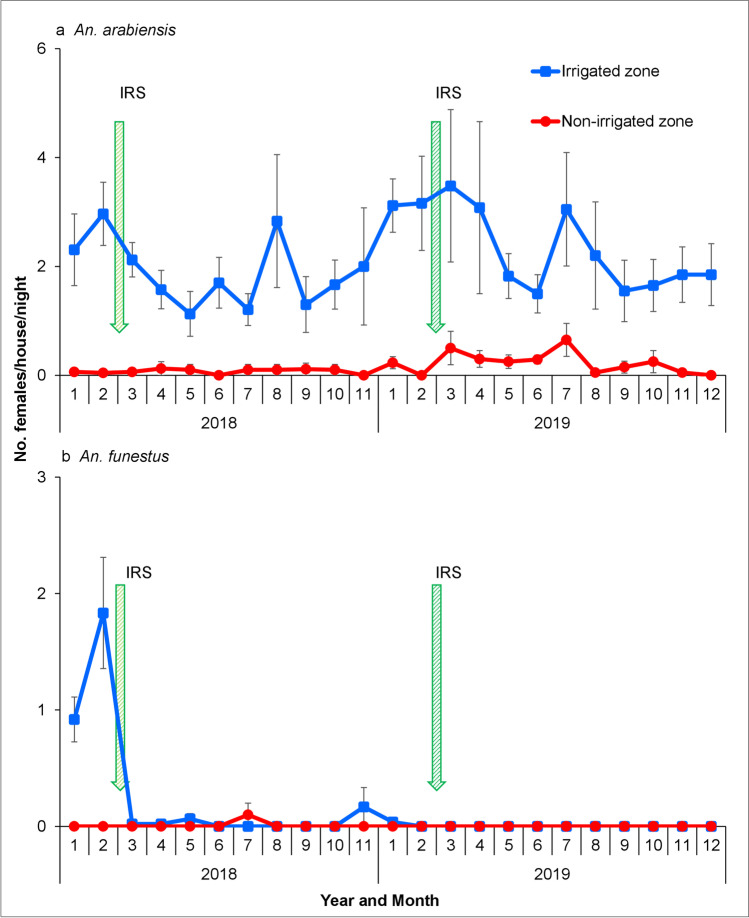
A total of 2,474 female anophelines were collected between January 1st, 2018, and December 31st, 2019, consisting of 2,248 (90.9%) An. gambiae s.l., 225 (9.1%) An. funestus, and 1 (0.04%) An. coustani. 760 specimens (621 An. gambiae s.l. and 139 An. funestus) were analyzed for sibling species identification. For the An. gambiae s.l. specimens, PCR results indicated that 99.7% were An. arabiensis and 0.3% An. gambiae s.s. All the An. funestus subjected to species identification were confirmed as An. funestus s.s. Overall, An. arabiensis was the dominant vector of malaria in the study sites. After adjusting for month, Anopheles arabiensis indoor resting density was 2.19 in irrigated zone and significantly higher than 0.21 in the non-irrigated zone (Z = − 4.690, df = 1540, P < 0.001) (Fig. 7, Additional file 1: Table S4). The difference in indoor resting density of An. arabiensis during the dry and wet seasons was not significant (Z = − 1.055, df = 1540, P = 0.292) (Additional file 1: Table S4). The An. funestus indoor resting density was 0.23 in irrigated zone while only few An. funestus were collected in the non-irrigated zone (Fig. 7). The study clearly indicated that the malaria vector species were more abundant in the irrigated zone than in the non-irrigated zone. In the irrigated zone, the HBI of An. funestus (49.43%) was 14-fold higher than An. arabiensis (3.44%) whereas in the non-irrigated zone, none of the An. arabiensis tested positive for human blood (Table 5).
Fig. 7.
Indoor resting density of female a An. arabiensis and b An. funestus mosquitoes collected using pyrethrum spray catches. Error bars were for the standard error for the Anopheles mosquitoes collected. Abbreviations: IRS, indoor residual spraying
Table 5.
Host feeding preference of An. arabiensis and An. funestus mosquitoes collected indoors using pyrethrum spray catches from irrigated and non-irrigated zone from longitudinal sampling in 2018 and 2019
| Blood-meal origins | An. arabiensis (%) | An. funestus (%) | ||
|---|---|---|---|---|
| Irrigated zone | Non-irrigated zone | Irrigated zone | Non-irrigated zone | |
| No. tested | 494 | 27 | 87 | 0 |
| Human | 7 (1.6) | 0 (0) | 39 (44.8) | 0 (0) |
| Bovine | 390 (78.8) | 22 (81.5) | 30 (34.5) | 0 (0) |
| Human + bovine | 5 (1.0) | 0 (0) | 3 (3.4) | 0 (0) |
| Human + dog | 4 (0.8) | 0 (0) | 0 (0) | 0 (0) |
| Bovine + dog | 4 (0.8) | 0 (0) | 0 (0) | 0 (0) |
| Pig + dog | 3 (0.6) | 0 (0) | 0 (0) | 0 (0) |
| Goat | 2 (0.4) | 0 (0) | 0 (0) | 0 (0) |
| Dog | 2 (0.4) | 0 (0) | 0 (0) | 0 (0) |
| Bovine + goat | 2 (0.4) | 0 (0) | 0 (0) | 0 (0) |
| Pig | 1 (0.2) | 1 (3.7) | 1 (1.1) | 0 (0) |
| Human + bovine + pig | 1 (0.2) | 0 (0) | 0 (0) | 0 (0) |
| Bovine + dog + pig | 1 (0.2) | 0 (0) | 0 (0) | 0 (0) |
| Human + pig | 0 (0) | 0 (0) | 1 (1.1) | 0 (0) |
| Unknown | 72 (14.5) | 4 (14.8) | 13 (14.9) | 0 (0) |
| HBIa | 3.44% | 0.00% | 49.43% | 0.00% |
aHuman blood index (HBI) was calculated as the number of mosquito positive for human blood meal (including mixed blood meal) divided by the total number tested
Discussion
This study investigated the effect of concrete canal and flood irrigation systems on species composition, malaria vector abundance and their seasonality, vector behavior, and malaria transmission in irrigated and non-irrigated sites where vector control using LLINs and IRS was being undertaken during the study period. Findings of the study demonstrated that An. arabiensis was the dominant anopheline species and was more abundant in irrigated zone compared to the non-irrigated zone. The high vector density of An. arabiensis together with their potential to transmit P. falciparum confirms the significant risk of malaria transmission in populations living within the irrigation scheme. The secondary aim of the study was to compare the trap effectiveness of CDC light traps against the gold standard, HLC. The results indicated that CDC light traps were equally effective in sampling malaria vectors as the HLC. This indicates the usefulness of this tool for continuous operational surveillance of the mosquito vectors within the study site.
The distribution of An. arabiensis is generally concentrated in the drier savannah environments where rainfall is < 1000 mm (Coetzee et al. 2000). Anopheles funestus was scarcely collected in the study site. Other anopheline species that were reported, and occurred only in the irrigated zone, were An. coustani and An. pharoensis. These two mosquito species have previously been considered as secondary vectors of malaria in Africa (Afrane et al. 2016) but recent studies have shown that they could play an important role in malaria transmission (Kerah-Hinzoumbé et al. 2009; Kibret et al. 2012; Mwangangi et al. 2013). Thus, it is prudent to integrate them in malaria vector surveillance and control strategies particularly where they are abundant.
A significant variation in vector density was observed in the irrigated and non-irrigated zones which is consistent with previous studies that the introduction of irrigation schemes leads to an increase in vector density and abundance (Ijumba et al. 2002a; Briet et al. 2003; Diuk-Wasser et al. 2005; Muturi et al. 2008b). In the irrigated zone, the irrigated canals, seepage areas, and flooded irrigated fields serve as the main larval habitats and provide stable mosquito breeding habitats during the dry season when other larval habitats dry up. In contrast, the low An. arabiensis density in the non-irrigated zone may be due to the temporary and parched nature of aquatic habitats (rain pools, rice fields, and edges of seasonal swamps) during the dry season. These observations have also been reported in similar studies in the Mwea Irrigation Scheme and the neighboring non-irrigated agroecosystems (Muturi et al. 2006). The indoor resting density of An. funestus was generally low; however, their indoor abundance was relatively high during the dry season of 2018 in the irrigated zone prior to the application of the IRS in the study site. Thereafter, An. funestus was rarely collected from our study. This can be attributed to the application of Actellic® 300CS IRS which has been shown to significantly reduce the indoor resting density of An. funestus in the same area (Abong’o et al. 2020). Indoor residual spraying is known to be highly effective on endophilic and anthropophilic mosquito species such as An. funestus due to high exposure to the wall sprayed insecticides.
The indoor and outdoor host-seeking density of An. arabiensis varied significantly with the highest biting densities collected indoors. Studies conducted over three decades ago by Githeko et al. showed that An. arabiensis was more likely to bite indoor than outdoor before the scale up of vector control intervention in western Kenya (Githeko et al. 1994a, 1996). In the present study, the endophagic tendency of An. arabiensis was still observed despite the high LLINs coverage and application of IRS in the study sites. This could be attributed to behavior of this species whereby it may enter a house protected with malaria vector control interventions in search of unprotected host, but exit without fatal exposure to insecticide-treated surfaces (Kitau et al. 2012; Okumu et al. 2013; Asale et al. 2014). Nonetheless, outdoor biting behavior was observed for this mosquito species which is consistent with other studies within its distribution range (Gatton et al. 2013).
It is worth mentioning that when comparing the traps deployed in the study, the mean vector density varied significantly between traps. The outdoor density of An. arabiensis was significantly higher in pit shelters and clay pots than for CDC light traps; in contrast, the indoor density of An. arabiensis was not significantly different between traps. Such variations were likely driven by differences in vector behavior, vector species composition, and history of malaria interventions rather than differences in the efficiency between the traps. Wide variation in the vector density of each trapping method has also been observed by Degefa et al. in a study conducted in western and attributed this variations to vector behavior (Degefa et al. 2019).
Human landing catches have been considered the gold standard method for estimating mosquito-human contact. However, it is a labor-intensive procedure requiring highly trained collectors, extensive supervision, variation in the skill of the collectors or their individual attractiveness to mosquitoes, and ethical concerns associated with potential exposure to infectious mosquito bites (Knols et al. 1995; WHO 2013). Our results indicate that CDC light trap is an effective trapping alternative to HLC for continuous operational surveillance of mosquito vectors within the study sites. In a recent study conducted in western Kenya and southwestern Ethiopia, human-odor-baited CDC light traps (HBLT) collected twice the number of outdoor host-seeking An. arabiensis and An. funestus compared to non-baited CDC light traps (Degefa et al. 2020). Thus, it will be important to evaluate the effectiveness of this tool in the study sites that could be a better outdoor surveillance tool than the non-baited CDC light trap.
Anthropophily was highest in An. funestus compared to An. arabiensis in the irrigated zone. These findings are consistent with previous studies that have reported An. funestus s.s. to exhibit anthropophagic behavior in Kenya (Githeko et al. 1994b; Mwangangi et al. 2003) and in other parts of Africa (Tanga et al. 2011; Mzilahowa et al. 2012; Dadzie et al. 2013). However, in recent reports, they have been shown to also feed on bovine (Degefa et al. 2017; Ogola et al. 2018) in the presence of LLINs. This plasticity of the feeding behavior of the vector may influence malaria transmission, leading to residual transmission after the densities of endophilic and endophagic vectors have been reduced by the interventions (Durnez and Coosemans 2013; Afrane et al. 2016). The life histories of An. arabiensis population of southern Tanzania were simulated in a model by Killeen et al. and estimated that two-thirds of the vector feeds outdoor in an area where bednet usage is high (Killeen et al. 2016). Studies have indicated that An. arabiensis exhibits behavior that mediates residual transmission such as feeding outdoors on humans or cattle and rapidly exiting houses without fatal exposure to insecticide-treated surfaces (Killeen et al. 2016). Findings of the present study demonstrated that An. arabiensis fed on humans both indoors and outdoors with a higher HBI outdoors and predominantly fed on bovine. However, it remains capable of transmitting malaria whenever it can feed on humans.
There was a significant difference in the risk of malaria transmission by An. arabiensis in the two zones, with higher transmission risk in the irrigated zone. These results show that irrigation has an effect on malaria transmission and An. arabiensis played a significant role in transmission. In addition, this species contributed almost equally to both indoor and outdoor transmission. In many studies, irrigated areas have been associated with increased malaria transmission than neighboring non-irrigated areas (Oomen et al. 1988; Gbakima 1994; Yohannes et al. 2005); however, in some cases, introduction of irrigation schemes reduces (Ijumba et al. 2002a; Sissoko et al. 2004) or has no impact on malaria transmission (Faye et al. 1995). Hence, the impact of water development projects on malaria transmission is variable and the transmission dynamic likely depends on the local epidemiological setting. Our data also suggest that the zoophagic behavior of An. arabiensis could be accounting for the low transmission in the irrigated zone whereas the low vector densities limited transmission in the non-irrigated zone. The zoophagic tendency of An. arabiensis indicates zooprophylaxis may be a potential strategy for malaria control.
The limitation of our study is the lack of information on the movement of endophagic mosquitoes as they exit the house after feeding and/or resting. This information would have improved the understanding of the effect of insecticide-based vector control interventions in the houses on the normal movement, density, and reticence feeding of endophilic species (WHO 1975; Müller et al. 2017).
Conclusion
Anopheles arabiensis was the dominant vector of malaria in the study sites. Our study demonstrated that there is a difference in malaria transmission by An. arabiensis between the two zones with higher transmission risk in the irrigated zone and the reason attributing to this is the high vector densities in the irrigated zone. The density of An. funestus was generally low nonetheless the anthropophily was highest in An. funestus compared to An. arabiensis. While most of the malaria transmission by An. arabiensis occurred indoors, transmission also occurred outdoors. The irrigation scheme should therefore incorporate additional vector management strategies to complement the LLINs and IRS to control outdoor malaria transmission. Larval source management to reduce vector density and new tools for protecting human exposed outdoor will probably be needed to control outdoor seeking mosquitoes. This is among the first few studies conducted in this newly established irrigation scheme in western Kenya and the findings will guide the Ministry of Health, Ministry of Agriculture, Livestock, Fisheries and Irrigation, and Ministry of Environment and Forestry in developing strategies that promote crop production in irrigated areas while limit proliferation of mosquito vector populations.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We acknowledge Dr. Ephantus J. Muturi for the data analysis and review of the manuscript. The authors would also like to acknowledge Dr. John Githure for his comments on the manuscript. The authors thank the field and laboratory team of the ICEMR project in Kenya more specific Fredrick Odongo, Enock Juma, and Sally Mongoi for providing technical support during the study. In addition, we thank the community in Homa Bay for their permission to collect mosquitoes in their houses.
Abbreviations
- AIC
Akaike information criterion
- CDC
Centers for Disease Control and Prevention
- DNA
Deoxyribonucleic acid
- EIRs
Entomological inoculation rates
- HBI
Human biting index
- HLCs
Human landing catches
- ICEMR
International Center of Excellence for Malaria Research
- IRS
Indoor residual spraying
- LLINs
Long-lasting insecticidal nets
- PCR
Polymerase chain reaction
- PSCs
Pyrethrum spray catches
- qPCR
Quantitative polymerase chain reaction
- RNA
Ribonucleic acid
- s.l.
Sensu lato
- s.s.
Sensu stricto
- WHO
World Health Organization
Author contribution
B.M.O., H.A., G.Z., J.K., A.K.G., and G.Y. conceived and developed the study. B.M.O., H.A., M-C.L., D.Z., X.W., P.W.O., K.O.O., C.J.O., W.O.O., S.M.M., D.O.O., and H.O. participated in the design and implementation of entomological studies. M-C.L. generated the map. B.M.O., D.Z., P.W.O., K.O.O., C.J.O., and W.O.O. carried out the laboratory analysis. B.M.O., X.W., and G.Z. did data analysis and interpretation. B.M.O. wrote the first draft of the manuscript. The final manuscript was edited by G.Y. and A.K.G. All authors read and approved the final manuscript.
Funding
This research is supported by grants from the National Institutes of Health (U19 AI129326 and D43 TW001505).
Data availability
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Declarations
Ethics approval and consent to participate
Prior to beginning of the study, ethical approval was obtained from the Maseno University Ethics Review Committee (MUERC Protocol No. 00456) and the University of California, Irvine Institutional Review Board (UCI IRB). Permission was obtained from household heads and chiefs of the study site. Written informed consent was obtained from household heads and all collectors. The collection methods used in this study were undertaken in accordance with the principles of the Declaration of Helsinki.
Consent for publication
The authors affirm that human research participants provided informed consent for publication of the images in Fig. 3c, d, e, f, and the graphical abstract.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Benyl M. Ondeto, Email: muyomabo@gmail.com
Xiaoming Wang, Email: xiaomiw1@uci.edu.
Harrysone Atieli, Email: etemesi2012@yahoo.com.
Pauline Winnie Orondo, Email: paulineorondo@gmail.com.
Kevin O. Ochwedo, Email: kevinochwedo@gmail.com
Collince J. Omondi, Email: cjomosh@yahoo.com
Wilfred O. Otambo, Email: oumaotambo@gmail.com
Daibin Zhong, Email: dzhong@uci.edu.
Guofa Zhou, Email: zhoug@uci.edu.
Ming-Chieh Lee, Email: mingchil@uci.edu.
Simon M. Muriu, Email: s.muriu@pu.ac.ke
David O. Odongo, Email: david.odongo@uonbi.ac.ke
Horace Ochanda, Email: hochanda@uonbi.ac.ke.
James Kazura, Email: jxk14@case.edu.
Andrew K. Githeko, Email: githeko@yahoo.com
Guiyun Yan, Email: guiyuny@uci.edu.
References
- Abong’o B, Gimnig JE, Torr SJ et al (2020) Impact of indoor residual spraying with pirimiphos-methyl (Actellic 300CS ) on entomological indicators of transmission and malaria case burden in Migori County, western Kenya. Sci Rep 10:4518. 10.1038/s41598-020-61350-2 [DOI] [PMC free article] [PubMed]
- Afrane YA, Bonizzoni M, Yan G. Secondary malaria vectors of sub-Saharan Africa: threat to malaria elimination on the continent? In: Current topics in malaria. In: Rodriguez-Morales AJ, editor. IntechOpen. 2016. pp. 473–490. [Google Scholar]
- Asale A, Getachew Y, Hailesilassie W, et al. Evaluation of the efficacy of DDT indoor residual spraying and long-lasting insecticidal nets against insecticide resistant populations of Anopheles arabiensis Patton (Diptera: Culicidae) from Ethiopia using experimental huts. Parasit Vectors. 2014;7:131. doi: 10.1186/1756-3305-7-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baro M, Deubel TF. Persistent hunger: perspectives on vulnerability, famine, and food security in sub-Saharan Africa. Annu Rev Anthr. 2006;35:521–538. doi: 10.1146/annurev.anthro.35.081705.123224. [DOI] [Google Scholar]
- Bayoh NM, Mathias DK, Odiere MR et al (2010) Anopheles gambiae: historical population decline associated with regional distribution of insecticide-treated bed nets in western Nyanza Province, Kenya. Malar J 9:62. 10.1186/1475-2875-9-62 [DOI] [PMC free article] [PubMed]
- Blank HG, Mutero CM, Murray-Rust H, editors. The changing face of irrigation in Kenya: opportunities for anticipating change in eastern and southern Africa. Colombo, Sri Lanka: International Water Management Institute (IWMI); 2002. [Google Scholar]
- Briet OJ, Dossou-yovo J, Akodo E, et al. The relationship between Anopheles gambiae density and rice cultivation in the savannah zone and forest zone of Cote d’Ivoire. Trop Med Int Heal. 2003;8:439–448. doi: 10.1046/j.1365-3156.2003.01054.x. [DOI] [PubMed] [Google Scholar]
- Bryan E, Chase C, Schulte M. Nutrition-sensitive irrigation and water management. Washington, DC: World Bank; 2019. [Google Scholar]
- Coetzee M, Craig M, le Sueur D. Distribution of African malaria mosquitoes belonging to the Anopheles gambiae complex. Parasitol Today. 2000;16:74–77. doi: 10.4269/ajtmh.2004.70.103. [DOI] [PubMed] [Google Scholar]
- Coosemans MH. Comparison of malarial endemicity in a rice-growing area and a cotton-growing area of the Rusizi plain. Burundi Ann Soc Belg Med Trop. 1985;65(Suppl 2):187–200. [PubMed] [Google Scholar]
- Dadzie SK, Brenyah R, Appawu MA. Role of species composition in malaria transmission by the Anopheles funestus group (Diptera: Culicidae) in Ghana. J Vector Ecol. 2013;38:105–110. doi: 10.1111/j.1948-7134.2013.12015.x. [DOI] [PubMed] [Google Scholar]
- Degefa T, Yewhalaw D, Zhou G, et al. Evaluation of the performance of new sticky pots for outdoor resting malaria vector surveillance in western Kenya. Parasit Vectors. 2019;12(1):278. doi: 10.1186/s13071-019-3535-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degefa T, Yewhalaw D, Zhou G, et al. Indoor and outdoor malaria vector surveillance in western Kenya: implications for better understanding of residual transmission. Malar J. 2017;16:443. doi: 10.1186/s12936-017-2098-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degefa T, Yewhalaw D, Zhou G, et al. Evaluation of human-baited double net trap and human-odour-baited CDC light trap for outdoor host-seeking malaria vector surveillance in Kenya and Ethiopia. Malar J. 2020;19:174. doi: 10.1186/s12936-020-03244-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diuk-Wasser MA, Toure MB, Dolo G, et al. Vector abundance and malaria transmission in rice-growing villages in Mali. Am J Trop Med Hyg. 2005;72:725–731. doi: 10.4269/ajtmh.2005.72.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolo G, Briët OJT, Dao A, et al. Malaria transmission in relation to rice cultivation in the irrigated Sahel of Mali. Acta Trop. 2004;89:147–159. doi: 10.1016/j.actatropica.2003.10.014. [DOI] [PubMed] [Google Scholar]
- Durnez L, Coosemans M (2013) Residual transmission of malaria: an old issue for new approaches. In: Manguin S (ed) Anopheles mosquitoes — new insights into malaria vectors. IntechOpen, 671–704. http://www.intechopen.com/articles/show/title/residual-transmission-of-malaria-an-old-issue-for-new-approaches
- Faye O, Fontenille D, Gaye O, et al. Malaria and rice growing in the Senegal River delta (Senegal) Ann Soc Belg Med Trop. 1995;75:179–189. [PubMed] [Google Scholar]
- Futami K, Dida GO, Sonye GO et al (2014) Impacts of insecticide treated bed nets on Anopheles gambiae s.l. populations in Mbita District and Suba District, western Kenya. Parasit Vectors 7:63. 10.1186/1756-3305-7-63 [DOI] [PMC free article] [PubMed]
- Garrett-Jones C. The human blood index of malaria vectors in relation to epidemiological assessment. Bull World Heal Organ. 1964;30:241–261. [PMC free article] [PubMed] [Google Scholar]
- Gatton ML, Chitnis N, Churcher T, et al. The importance of mosquito behavioral adaptations to malaria control in Africa. Evolution. 2013;67:1218–1230. doi: 10.1111/evo.12063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gbakima A. Inland valley swamp rice development: malaria, schistosomiasis, onchocerciasis in south central Sierra Leone. Public Health. 1994;108:149–157. doi: 10.1016/S0033-3506(05)80020-4. [DOI] [PubMed] [Google Scholar]
- Gillies M, Coetzee M. A supplement to the Anophelinae of Africa south of the Sahara. Sth Afr Inst Med Res. 1987;55:1–143. [Google Scholar]
- Gimnig JE, Walker ED, Otieno P, et al. Incidence of malaria among mosquito collectors conducting human landing catches in Western Kenya. Am J Trop Med Hyg. 2013;88:301–308. doi: 10.4269/ajtmh.2012.12-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimnig JE, Otieno P, Were V, et al. The effect of indoor residual spraying on the prevalence of malaria parasite infection, clinical malaria and anemia in an area of perennial transmission and moderate coverage of insecticide treated nets in western Kenya. PLoS One. 2016;11(1):e0145282. doi: 10.1371/journal.pone.0145282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Githeko AK, Service MW, Mbogo CM, et al. Sampling Anopheles arabiensis, A. gambiae sensu lato and A. funestus (Diptera: Culicidae) with CDC light-traps near a rice irrigation area and a sugarcane belt in western Kenya. Bull Entomol Res. 1994;84:319–324. doi: 10.1017/S0007485300032430. [DOI] [Google Scholar]
- Githeko AK, Service MW, Mbogo CM, et al. Origin of blood meals in indoor and outdoor resting malaria vectors in western Kenya. Acta Trop. 1994;84:319–324. doi: 10.1016/0001-706x(94)90024-8. [DOI] [PubMed] [Google Scholar]
- Githeko AK, Adungo NI, Karanja DM, et al. Some observations on the biting behavior of Anopheles gambiae s.s., Anopheles arabiensis, and Anopheles funestus and their implications for malaria control. Exp Parasitol. 1996;82:306–315. doi: 10.1006/expr.1996.0038. [DOI] [PubMed] [Google Scholar]
- Henry MC, Rogier C, Nzeyimana I, et al. Inland valley rice production systems and malaria infection and disease in the savannah of Côte d’Ivoire. Trop Med Int Heal. 2003;8:449–458. doi: 10.1046/j.1365-3156.2003.01053.x. [DOI] [PubMed] [Google Scholar]
- Ijumba JN, Lindsay SW. Impact of irrigation on malaria in Africa: paddies paradox. Med Vet Entomol. 2001;15:1–11. doi: 10.1046/j.1365-2915.2001.00279.x. [DOI] [PubMed] [Google Scholar]
- Ijumba J, Mosha F, Lindsay S. Malaria transmission risk variations derived from different agricultural practices in an irrigated area of northern Tanzania. Med Vet Entomol. 2002;16:28–38. doi: 10.1046/j.0269-283x.2002.00337.x. [DOI] [PubMed] [Google Scholar]
- Ijumba JN, Shenton FC, Clarke SE, et al. Irrigated crop production is associated with less malaria than traditional agricultural practices in Tanzania. Trans R Soc Trop Med Hyg. 2002;96:476–480. doi: 10.1016/S0035-9203(02)90408-6. [DOI] [PubMed] [Google Scholar]
- Keiser J, De Castro MC, Maltese MF, et al. Effect of irrigation and large dams on the burden of malaria on a global and regional scale. Am J Trop Med Hyg. 2005;72:392–406. doi: 10.4269/ajtmh.2005.72.392. [DOI] [PubMed] [Google Scholar]
- Kent RJ, Norris DE. Identification of mammalian blood meals in mosquitoes by a multiplexed polymerase chain reaction targeting cytochrome B. Am J Trop Med Hyg. 2005;73:336–342. doi: 10.4269/ajtmh.2005.73.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerah-Hinzoumbé C, Péka M, Antonio-Nkondjio C, et al. Malaria vectors and transmission dynamics in Goulmoun, a rural city in south-western Chad. BMC Infect Dis. 2009;9:71. doi: 10.1186/1471-2334-9-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kibret S, Lautze J, Boelee E, McCartney M. How does an Ethiopian dam increase malaria? Entomological determinants around the Koka reservoir. Trop Med Int Heal. 2012;17:1320–1328. doi: 10.1111/j.1365-3156.2012.03077.x. [DOI] [PubMed] [Google Scholar]
- Killeen GF, Govella NJ, Lwetoijera DW, Okumu FO (2016) Most outdoor malaria transmission by behaviourally-resistant Anopheles arabiensis is mediated by mosquitoes that have previously been inside houses. Malar J 15:225. 10.1186/s12936-016-1280-z [DOI] [PMC free article] [PubMed]
- Kitau J, Oxborough RM, Tungu PK, et al. Species shifts in the Anopheles gambiae complex: do LLINs successfully control Anopheles arabiensis? PLoS One. 2012;7(3):e31481. doi: 10.1371/journal.pone.0031481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knols B, de Jong R, Takken W. Differential attractiveness of isolated humans to mosquitoes in Tanzania. Trans R Soc Trop Med Hyg. 1995;89:604–606. doi: 10.1016/0035-9203(95)90406-9. [DOI] [PubMed] [Google Scholar]
- Koekemoer LL, Kamau L, Hunt RH, Coetzee M. A cocktail polymerase chain reaction assay to identify members of the Anopheles funestus (Diptera: Culicidae) group. Am J Trop Med Hyg. 2002;6:804–811. doi: 10.4269/ajtmh.2002.66.804. [DOI] [PubMed] [Google Scholar]
- Macdonald G. The epidemiology and control of malaria. London: Oxford University Press; 1957. [Google Scholar]
- Ministry of Health (2016) The epidemiology and control profile of malaria in Kenya: reviewing the evidence to guide the future vector control. https://dhsprogram.com/pubs/pdf/MIS36/MIS36.pdf. Accessed 19 Oct 2021
- Muirhead-Thomson R. A pit shelter for sampling outdoor mosquito populations. Bull World Heal Organ. 1958;19:1116–1118. [PMC free article] [PubMed] [Google Scholar]
- Müller GC, Junnila A, Traore MM, et al. A novel window entry/exit trap for the study of endophilic behavior of mosquitoes. Acta Trop. 2017;167:137–141. doi: 10.1016/j.actatropica.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musapa M, Kumwenda T, Mkulama M, et al. A simple chelex protocol for DNA extraction from Anopheles spp. J Vis Exp. 2013;9(71):e3281. doi: 10.3791/3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muturi EJ, Shililu J, Jacob B, et al. Mosquito species diversity and abundance in relation to land use in a riceland agroecosystem in Mwea, Kenya. J Vector Ecol. 2006;31:129–137. doi: 10.3376/1081-1710(2006)31[129:msdaai]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Muturi EJ, Muriu S, Shililu J, et al. Effect of rice cultivation on malaria transmission in central Kenya. Am J Trop Med Hyg. 2008;78:270–275. doi: 10.4269/ajtmh.2008.78.270. [DOI] [PubMed] [Google Scholar]
- Muturi EJ, Shililu JI, Jacob BG, et al. Diversity of riceland mosquitoes and factors affecting their occurrence and distribution in Mwea, Kenya. J Am Mosq Control Assoc. 2008;24:349–358. doi: 10.2987/5675.1. [DOI] [PubMed] [Google Scholar]
- Mwangangi JM, Mbogo CM, Nzovu JG, et al. Blood-meal analysis for anopheline mosquitoes sampled along the Kenyan coast. J Am Mosq Control Assoc. 2003;19:371–375. [PubMed] [Google Scholar]
- Mwangangi JM, Muturi EJ, Muriu SM, et al. The role of Anopheles arabiensis and Anopheles coustani in indoor and outdoor malaria transmission in Taveta District Kenya. Parasit Vectors. 2013;6:114. doi: 10.1186/1756-3305-6-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mzilahowa T, Hastings IM, Molyneux ME, McCall PJ. Entomological indices of malaria transmission in Chikhwawa District, southern Malawi. Malar J. 2012;11:380. doi: 10.1186/1475-2875-11-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Malaria Control Programme- Kenya [Internet]. [cited 2022 Jan 11]. Available from: https://web.facebook.com/nmcpkenya?_rdc=1&_rdr
- Ng’ang’a PN, Aduogo P, Mutero CM. Long lasting insecticidal mosquito nets (LLINs) ownership, use and coverage following mass distribution campaign in Lake Victoria basin, western Kenya. BMC Public Heal. 2021;21:1046. doi: 10.1186/s12889-021-11062-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng’Habi KRN, Mwasheshi D, Knols BGJ, Ferguson HM. Establishment of a self-propagating population of the African malaria vector Anopheles arabiensis under semi-field conditions. Malar J. 2010;9:356. doi: 10.1186/1475-2875-9-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odiere M, Bayoh MN, Gimnig J, et al. Sampling outdoor, resting Anopheles gambiae and other mosquitoes (Diptera : Culicidae) in western Kenya with clay pots. J Med Entomol. 2007;44:14–22. doi: 10.1093/jmedent/41.5.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogola EO, Fillinger U, Ondiba IM, et al. Insights into malaria transmission among Anopheles funestus mosquitoes Kenya. Parasit Vectors. 2018;11:577. doi: 10.1186/s13071-018-3171-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okumu FO, Mbeyela E, Lingamba G, et al. Comparative field evaluation of combinations of long-lasting insecticide treated nets and indoor residual spraying, relative to either method alone, for malaria prevention in an area where the main vector is Anopheles arabiensis. Parasit Vectors. 2013;6:46. doi: 10.1186/1756-3305-6-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oomen JMV, de Wolf J, Jobin WR. Health and irrigation: incorporation of disease-control measures in irrigation, a multi-faceted task in design, construction, operation. Wageningen, The Netherlands: International Institute for Land Reclamation and Improvement; 1988. [Google Scholar]
- Orondo PW, Nyanjom SG, Atieli H, et al. Insecticide resistance status of Anopheles arabiensis in irrigated and non-irrigated areas in western Kenya. Parasit Vectors. 2021;14:335. doi: 10.1186/s13071-021-04833-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patz JA, Daszak P, Tabor GM, et al. Unhealthy landscapes: policy recommendations on land use change and infectious disease emergence. Env Heal Perspect. 2004;112:1092–1098. doi: 10.1289/ehp.6877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PMI (2013) Indoor residual spraying 2 (IRS 2) Kenya: task order 2 final report. https://pdf.usaid.gov/pdf_docs/pdacy423.pdf. Accessed 22 Jul 2021
- Robert V, van den Broek A, Stevens P, et al. Mosquitoes and malaria transmission in irrigated rice-fields in the Benoue valley of northern Cameroon. Acta Trop. 1992;52:201–204. doi: 10.1016/0001-706X(92)90036-W. [DOI] [PubMed] [Google Scholar]
- Scott JA, Brogdon WG, Collins FH. Identification of single specimens of the Anopheles gambiae complex by the polymerase chain reaction. Am J Trop Med Hyg. 1993;49:520–529. doi: 10.4269/ajtmh.1993.49.520. [DOI] [PubMed] [Google Scholar]
- Shokoples SE, Ndao M, Kowalewska-Grochowska K, Yanow SK. Multiplexed real-time PCR assay for discrimination of Plasmodium species with improved sensitivity for mixed infections. J Clin Microbiol. 2009;47:975–980. doi: 10.1128/JCM.01858-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sissoko MS, Dicko A, Briët OJT, et al. Malaria incidence in relation to rice cultivation in the irrigated Sahel of Mali. Acta Trop. 2004;89:161–170. doi: 10.1016/j.actatropica.2003.10.015. [DOI] [PubMed] [Google Scholar]
- Tanga MC, Ngundu WI, Tchouassi PD. Daily survival and human blood index of major malaria vectors associated with oil palm cultivation in Cameroon and their role in malaria transmission. Trop Med Int Heal. 2011;16:447–457. doi: 10.1111/j.1365-3156.2011.02726.x. [DOI] [PubMed] [Google Scholar]
- The PMI VectorLink Kenya project [Internet]. [cited 2021 Feb 3]. Available from: https://pmivectorlink.org/where-we-work/kenya/
- Veron V, Simon S, Carme B. Multiplex real-time PCR detection of P. falciparum, P. vivax and P. malariae in human blood samples. Exp Parasitol. 2009;121:346–351. doi: 10.1016/j.exppara.2008.12.012. [DOI] [PubMed] [Google Scholar]
- WHO (1975) Manual on practical entomology in malaria. Part II. methods and techniques. https://apps.who.int/iris/handle/10665/42481. Accessed 1 May 2020
- WHO . Malaria entomology and vector control: guide for participants. Geneva: World Health Organization; 2013. [Google Scholar]
- Yohannes M, Haile M, Ghebreyesus TA, et al. Can source reduction of mosquito larval habitat reduce malaria transmission in Tigray, Ethiopia? Trop Med Int Heal. 2005;10:1274–1285. doi: 10.1111/j.1365-3156.2005.01512.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article [and its supplementary information files].