Abstract
Both the ability to speak and to infer complex linguistic messages from sounds have been claimed as uniquely human phenomena. In schizophrenia, formal thought disorder (FTD) and auditory verbal hallucinations (AVHs) are manifestations respectively relating to concrete disruptions of those abilities. From an evolutionary perspective, Crow (1997) proposed that “schizophrenia is the price that Homo sapiens pays for the faculty of language”. Epidemiological and experimental evidence points to an overlap between FTD and AVHs, yet a thorough investigation examining their shared neural mechanism in schizophrenia is lacking. In this review, we synthesize observations from three key domains. First, neuroanatomical evidence indicates substantial shared abnormalities in language-processing regions between FTD and AVHs, even in the early phases of schizophrenia. Second, neurochemical studies point to a glutamate-related dysfunction in these language-processing brain regions, contributing to verbal production deficits. Third, genetic findings further show how genes that overlap between schizophrenia and language disorders influence neurodevelopment and neurotransmission. We argue that these observations converge into the possibility that a glutamatergic dysfunction in language-processing brain regions might be a shared neural basis of both FTD and AVHs. Investigations of language pathology in schizophrenia could facilitate the development of diagnostic tools and treatments, so we call for multilevel confirmatory analyses focused on modulations of the language network as a therapeutic goal in schizophrenia.
Subject terms: Schizophrenia, Diseases of the nervous system
“It seemed not improbable that the cortical centres that are the last organized, which are the most highly evolved and voluntary, and which are supposed to be located on the left side of the brain, might suffer first in insanity”.
James Crichton-Browne (1879)1, On The Weight of the Brain.
Introduction
Schizophrenia is a neuropsychiatric disorder involving several language disturbances2. Disrupted speech productions (e.g., derailment, tangentiality, and thought block), reduced verbal output (the negative symptom of alogia), and aberrant speech perceptions (e.g., hearing “voices” in the absence of acoustic-linguistic stimuli) are hallmark symptoms and diagnostic criteria for schizophrenia3,4. Delusions are also viewed as a disturbance in the referential use of language5. Besides, several pragmatic, semantic and syntactic processing deficits are seen across all stages of schizophrenia (for reviews, see6,7). While these phenomena appear ostensibly varied, they can be conceptually traced to a shared pathophysiological and neurocognitive substrate: the language system7–10.
The study of the language system in schizophrenia has a long tradition. By examining the relative weight of patients’ two cerebral hemispheres, Crichton-Browne suggested that, in the course of schizophrenia development, the left hemisphere may suffer first1, implying that cortical language-related brain regions might be affected. Kraepelin described one form of dementia praecox as “an unusually striking disorder of expression in speech, with relatively little impairment of the remaining psychic activities”11. Bleuler argued that “looseness of associations” in thought, speech and other psychological functions is a core feature of schizophrenia12. From an evolutionary perspective, Crow proposed that a “saltational genetic change” which occurred between 100 and 250 thousand years ago allowed the two cerebral hemispheres to develop somewhat independently, laying the foundation for language to evolve13. Crow further considered that, arising from this evolutionary event, schizophrenia could be seen as an epiphenomenon of a failure to establish hemispheric specialization for language8,13,14. Led by DeLisi and colleagues’ work on genetic susceptibility and language network8, and later by Kircher’s15 and Kuperberg’s6 studies on anatomy of FTD and psycholinguistics respectively, the interest on language as a core domain in psychosis continued over time.
More recent studies have supported the possibility that an evolutionary modification of the human brain lies at the core of the occurrence of psychotic disorders. Xu and colleagues found that schizophrenia-associated genetic loci are more likely to be found in human accelerated regions (HAR), i.e., genomic regions that are “highly conserved among nonhuman species but experienced accelerated substitutions in the human genome”16. This aligns with Erady and colleagues’ findings, who reported that certain genomic features (novel open reading frames proximal to HARs) contributing to schizophrenia arise, in part, from the divergence between humans and other primates17. Furthermore, studies have reported that unique human brain connectivity differentiating us from chimpanzee significantly overlaps with brain structural dysconnectivity in schizophrenia18,19.
Broadly, evidence indicating that language dysfunctions constitute part of the pathophysiological and neurocognitive substrate of schizophrenia comes from at least three different types of studies. First, neuroimaging studies have shown that patients with FTD and AVHs were characterized by structural and functional abnormalities in language-related brain regions20–29. Likewise, neuromodulation studies (e.g., repetitive transcranial magnetic stimulation, rTMS) targeting extended language regions have been shown to alleviate auditory hallucinations30–33, as well as to ameliorate gesture performance in schizophrenia34. Second, neurochemical studies have provided clues to link language-related disturbances with known deficits in dopaminergic and glutamatergic systems in schizophrenia35,36. Third, genetic studies have implicated the enrichment of language-related genes in schizophrenia37, which might contribute to brain dysconnectivity, especially in the early stages of the illness38. Also, a developmental speech-disorder related to the gene FOXP2 has been associated with cognitive dysfunction39, psychotic speech profiles40, and reduced grey matter density41 in patients with schizophrenia, albeit not consistently42.
Notwithstanding these accumulating indicators of a disruption in the neural substrates of language in schizophrenia, a comprehensive synthesis is still lacking across these various levels of evidence. To bring together FTD and AVHs (i.e., two major domains of psychosis) as concurrently language-system symptoms in schizophrenia, in this study we examined neuroimaging evidence and neurotransmitter-level observations that have been reported in the language brain regions in schizophrenia. Moreover, we considered candidate genes that have been involved in both schizophrenia and language functions. We argue that a dysfunction of the language system is a critical feature of schizophrenia, and that we could obtain better diagnostic and treatment options to alleviate these symptoms in patients by investigating FTD and AVHs’ underlying mechanisms across several levels. Note that, throughout the article, the perisylvian network (i.e., the left inferior frontal gyrus [BA44, BA45]) and (posterior) superior temporal regions (BA42, BA22) are referred to as language processing regions as a whole. Nonetheless, we acknowledge that language processing further involves brain regions such as the premotor cortex, the frontal operculum, the middle temporal gyrus, and the angular gyrus43–46.
Symptoms related to language and brain abnormalities
Formal thought disorder (FTD)
FTD comprises clusters of atypical language productions which are interpreted as reflecting alterations in the neurocognitive processes that structure thoughts. Loosening of associations, derailment, tangentiality and incoherence are all included under the umbrella term of “positive forms of FTD”, while poverty of speech and slowed thinking are commonly called “negative forms of FTD”. In this dichotomous categorization, the “positive form” is considered to reflect a disorganization of the cognitive processes underlying FTD, whereas the “negative form” would reflect a reduction of such processes. Both forms have been related to poor long-term clinical outcomes47,48.
The prevalence of FTD in schizophrenia varies across studies and across disease stages, ranging from 27% to 80%15, but in two large-scale studies, FTD was observed in more than half of patients with chronic schizophrenia (50.39% and 72.7%, respectively)49,50. FTD may also affect individuals without schizophrenia, such as those at high-risk for psychosis, patients’ non-psychotic relatives, and patients with affective disorders51–53, suggesting that FTD is a transdiagnostic feature that is not specific to schizophrenia.
Neuropsychological studies have linked FTD to alterations in executive functions54–58. McGrath et al.56 proposed that deficits in initiation and planning of speech may contribute to poverty of speech, while a failure at maintaining information and inhibiting distractions could lead to positive FTD symptoms such as looseness of associations and derailment. Xu et al. demonstrated that poor sustained attention and planning in first-episode patients predicted residual FTD symptom severity at one-year follow-up57. Inhibitory control has been found to be impaired in patients with FTD compared to non-FTD patients59. In a recent review, it has been proposed that an excitatory/inhibitory imbalance at the microscale level could result in linguistic disorganization and impoverishment in schizophrenia7.
Other studies have investigated semantic processing dysfunctions in patients with FTD60–65. Doughty and Done systematically reviewed semantic impairments in schizophrenia61. The authors found a large effect for naming and verbal fluency tests (both phonemic fluency and category fluency), suggesting an impairment in semantic knowledge, semantic memory, and executive function in patients. Interestingly, some studies using semantic priming tasks have reported that patients with FTD showed a hyper-priming effect, despite general deficits in processing speed and various cognitive abilities63–65. In this type of studies, subjects are required to decide whether a target is a word or not (i.e., implementing a lexical decision task) while a semantic priming effect is elicited in some trials. This semantic priming effect refers to the facilitation of processing a target stimulus (e.g., the word “boat”) by providing a meaningful stimulus that precedes the target and shares features of meaning with the target (e.g., the word “ship”). The priming effect can then be calculated as the difference in reaction time between trials with related prime-target pairs and trials with unrelated pairs. The hyper-priming hypothesis would suggest that positive forms of FTD symptoms such as derailment and tangentiality can be understood as a failure to inhibit loosely associated concepts or word meanings that are stored in an individual’s semantic network. A meta-analysis on semantic processing has shown that increased semantic priming is only observed in patients with thought disorder, but not in patients with schizophrenia as a whole group65. Moreover, it has been suggested that the hyper-priming effect is restricted to indirectly related prime-target word pairs66. Yet, the specific level of hierarchical linguistic processing wherein the abnormal priming effect occurs in schizophrenia is still unclear67. Recently, a higher degree of semantic similarity that may result from the hyper-priming effect has been related to a model of reduced synaptic gain in Broca’s area and temporal semantic hub62,68, suggesting that a glutamate-centered excitation/inhibition imbalance plays a role in the lexical-semantic deficits seen in schizophrenia.
FTD has also been related to impaired pragmatic abilities69–71. Broadly, pragmatics concerns the actual use of language in socio-cultural constrained contexts of communication, focusing on the interpretation of literal, figurative and implicated meanings72. Patients with schizophrenia show impairments in understanding proverbs, metaphors, and irony, as well as in inferring communicative intentions73,74. Kuperberg et al. found that, compared to non-FTD patients and controls, patients with schizophrenia and FTD are less sensitive to pragmatic violations70. The severity of FTD symptoms has also been associated with pragmatic task performances69,71, although pragmatic deficits in schizophrenia may also relate to Theory of Mind impairments75.
Auditory verbal hallucinations (AVHs)
AVHs can be loosely defined as speech-like perceptions of voices in the absence of external sources. Like FTD, AVHs constitute a transdiagnostic psychotic symptom, occurring in patients with affective disorders76, Alzheimer’s disease77, substance abuse78, and even in the healthy population79. The cross-sectional prevalence of AVHs in schizophrenia attains a range between 40% and 80% of chronic patients80,81, similar to what is reported for FTD. Studies have described that FTD and AVHs tend to co-occur in both patients with schizophrenia and non-psychotic subjects, suggesting that FTD and AVHs may share common neural abnormalities82,83.
Several neurocognitive models have been proposed to account for AVHs. To illustrate, here we mention only two. First, the source monitoring model suggests that AVHs occur when people experience self-generated inner speech as if it was externally generated84,85. In this account, the source monitoring abnormality would reflect disruptions in the corollary discharge86, which is a general mechanism to attenuate sensations from self-generated actions, thus distinguishing them from externally originated sensations87,88. Alternatively, the memory intrusion hypothesis proposes unwanted memories as the source of the hallucinations’ content89,90. In line with this, patients with auditory hallucinations exhibit deficits in inhibition of memories, and to have difficulties contextualizing them89. This is of relevance to the content of AVH often relating to traumatic experiences91.
Neuroimaging evidence of impairments in the language circuits
Structural and functional abnormalities in language-processing regions are frequently found in neuroimaging studies of schizophrenia38,92,93. In schizophrenia, frontal and temporal lobe brain regions are the most affected areas, along with widespread cortical thinning and surface area reduction94. Cortical thinning in the language regions correlates with the severity of patients’ positive symptoms94,95. Previous brain-wide association studies (BWAS)96 identified that, in the early stages of schizophrenia, a functional dysconnectivity takes place in the inferior frontal gyrus92,93,97.
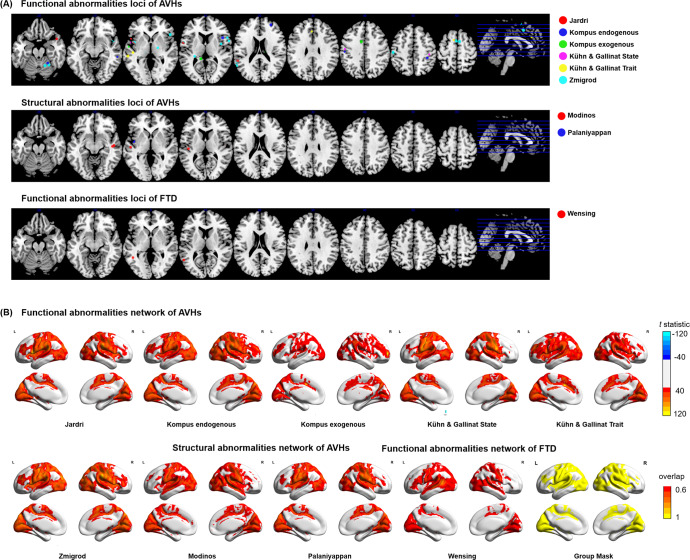
To provide a descriptive summary of brain abnormalities associated with FTD and AVHs, we searched PubMed for meta-analyses of neuroimaging studies on brain abnormalities in relation to FTD and AVHs (as of May 2022). Included meta-analyses are listed in Table 1. Reported significant loci from these meta-analyses are displayed in Fig. 1A, and listed in Supplementary Information (SI) Table 1.
Table 1.
Meta-analyses of formal thought disorder and auditory verbal hallucinationsa.
| Author | Year | Imaging modality | Num. of studies | Num. of subjects | Meta-analysis methods | Significant threshold |
|---|---|---|---|---|---|---|
| Formal thought disorder | ||||||
| Wensing et al.23 | 2017 | task-based fMRI, PET | 18 studies | 165 SZ-FTD, 15 HC-FTD, 25 SZ-nonFTD, 132 HC | Activation likelihood estimation | cluster FWE q < 0.05 (cluster-forming threshold at voxel p < 0.001) |
| Auditory verbal hallucinations | ||||||
| Modinos et al.28 | 2013 | sMRI (grey matter volume) | 9 studies | 307 SZ-AVHs, 131 SZ-nonAVHs, 307 HC | Parametric Voxel-based Meta-analysis | cluster-level q < 0.001 uncorrected |
| Palaniyappan et al.29 | 2012 | sMRI (grey matter volume) | 7 studies | 350 SZ | Signed Differential Mapping | p < 0.005, cluster extent 10 voxels |
| Jardri et al.24 | 2011 | task-based fMRI, PET, SPECT | 10 studies | 68 SZ-AVHs | Activation likelihood estimation | FDR q < 0.05, cluster threshold = 200 mm2 |
| Kühn and Gallinat27 | 2012 | task-based fMRI, PET | State studies:10; Trait studies: 8 | State: 85 SZ-AVHs; Trait: 81 SZ-AVHs, 39 SZ-nonAVHs, 69 HC | Activation likelihood estimation | FDR q < 0.01, cluster threshold = 100 mm2 |
| Kompus et al.25 | 2011 | task-based fMRI, PET | Endogenous studies: 12; Exogenous studies: 11 | Endogenous: 103 SZ-AVHs; Exogenous: 204 SZ-AVHs, 170 HC | Activation likelihood estimation | FDR q < 0.05, cluster threshold = 200 mm2 |
| Zmigrod et al.109 | 2016 | fMRI, PET | 13 studies | 190 AVHs (SZ, Psychosis, HC, Other diagnoses) | Activation likelihood estimation | FDR q < 0.05, cluster threshold = 200 mm2 |
aMeta-analyses were retrieved from PubMed search: (“formal thought disorder” OR “auditory hallucinations” OR “auditory verbal hallucinations”) AND (neuroimaging OR (“brain imaging”) OR (“magnetic resonance imaging”) OR MRI OR (“positron emission tomography”) OR PET). The article type was limited to meta-analyses published in English between January 1991 and August 2021.
Fig. 1. Brain abnormalities associated with formal thought disorders (FTD) and auditory verbal hallucinations (AVHs).
A Significant loci retrieved from previous meta-analyses of FTD23 and AVHs24,25,27–29,109. A four-millimeter sphere is created centering on the reported loci. Colors indicate different meta-analyses. B T-statistic maps of brain regions connected to the activation loci of each meta-analysis (T threshold = 40 for illustration). A group mask was created based on binarized T-statistic maps above 60% of studies.
Brain abnormalities in relation to FTD
Only one fMRI meta-analysis on FTD was found from the literature search23. The authors reported two significant loci showing abnormal activation: one in the left posterior middle temporal gyrus, and another in the left superior temporal gyrus (Fig. 1A and SI Table 1). Two systematic reviews implicated other brain functional abnormalities in FTD, including the bilateral inferior frontal gyri, anterior cingulate cortex, striatum and cerebellum20,22. Systematic reviews reported grey matter volume reduction in FTD, including bilateral superior temporal gyri, inferior frontal gyri, inferior parietal lobe, orbitofrontal cortex, cerebellum, nucleus accumbens and amygdala-hippocampal region20,21. Previous studies suggested that structural abnormalities in the language regions are associated with semantic task performances98 and the severity of FTD99.
The neuroimaging findings are consistent with the above-mentioned neuropsychological accounts of FTD, in that structural and functional abnormalities in the superior and middle temporal gyri constitute core deficits of FTD. Additional abnormalities in other brain regions subserving executive functions (anterior cingulate cortex and lateral prefrontal cortex) and motivation (orbitofrontal and medial prefrontal cortex) have also been related to positive FTD100,101, whereas insula, precuneus and frontocingulate abnormalities more closely relate to negative FTD symptoms21,99,102. Of notice, in the case of reduced connectivity between core language-processing regions, inefficient or maladaptive engagement of non-language regions may contribute to a more severe manifestation of FTD103.
A few diffusion-weighted imaging (DWI) studies have been conducted in schizophrenia patients with FTD. In line with the structural studies, fibers connecting the language regions, including the middle longitudinal fasciculus104, cingulum105 and uncinate fasciculus106, have been found to show reduced integrity, which is associated with FTD severity. Associations with other tracts, such as the corpus callosum107 and internal capsule108, have also been reported. Yet, in general, the results of DWI findings in FTD vary across studies20,105. Thus, at present, the sensitivity and specificity of white matter impairment in relation to FTD remains unclear.
Brain abnormalities in relation to AVHs
Two sMRI meta-analyses of AVHs were retrieved from the literature search28,29. The most commonly reported structural abnormalities involve the bilateral superior temporal gyri, followed by the right middle temporal gyrus and the right insula (Fig. 1A and SI Table 1). For the functional studies, four fMRI meta-analyses of AVHs were retrieved from the existing literature24,25,27,109 (Fig. 1A and SI Table 1). They all showed abnormal activation converging on the bilateral superior temporal gyri, the middle temporal gyri and the inferior frontal gyrus. Other functional abnormalities in AVHs included the post-central gyri, the supramarginal gyri, the insula, the anterior cingulate cortex, the thalamus, the hippocampus, and the cerebellum24,25,27,109. Some studies reported an activation of speech-production and speech-perception regions, as well as their right hemisphere homologues110–112. Other studies have suggested that the altered activity of the speech-perception regions is modulated by the inferior frontal gyrus, the anterior cingulate cortex and the insula (the salience network). An altered connectivity among these brain regions may then underlie patients’ impairment in source-monitoring and salience-detection functions, resulting in the misattribution of internal voices as externally generated110,112–114. It is worth noting that functional abnormalities in the superior temporal gyrus in patients occur across different imaging conditions, including the hallucination periods115, during language tasks performance25,27, and in the absence of external tasks116,117, suggesting that this abnormality might have a primary role in the occurrence of AVHs.
DWI studies generally reported fractional anisotropy (FA) changes in the left arcuate fasciculus, which is the major association tract connecting the inferior frontal gyrus and the superior temporal gyrus. Parallelly, both increased118,119 and decreased FA120–122 were reported in patients with AVHs, compared to non-AVHs patients and healthy controls. As mentioned in the section Auditory verbal hallucinations (AVHs) earlier, this impairment in the arcuate fasciculus may give rise to AVHs through disrupted corollary discharge. Other studies also implicate the corpus callosum in AVHs, especially the section connecting the bilateral auditory cortex120,123.
Brain abnormalities overlapping between FTD and AVHs
To quantify the degree of network-level overlap of brain abnormalities between FTD and AVHs, we conducted a recently proposed network mapping analysis approach124–126. This approach uses focal loci as seed regions to derive brain networks based on a normative human connectome (see Supplementary materials). To do this, first we retrieved focal loci from previous meta-analyses on FTD and AVHs23–25,27–29,109 (Table 1). Then, brain regions connected to the seed regions of each meta-analysis were determined by comparing the z-transformed connectivity with zeros using a one-sample t-test. T-statistic maps of each meta-analysis were set at a range of thresholds (T = 5–40, corresponding voxel-wise p = 1.7 × 10−6 ~ 1.8 × 10−215, df = 1095), and binarized to create a group mask (above 60% of studies) (Fig. 1B). This group mask represents the spatial distribution of a hypothetical connectome that has a high probability of being implicated in individuals with both AVHs and FTD (as in the case of schizophrenia). The results of this analysis revealed that functional networks of FTD and AVHs are indeed highly overlapping (Dice index ranging from 0.70 to 0.99, for T thresholds at 5–40, SI Table 2). When the overlap was restricted to a meta-analysis of functional imaging in AVHs109 and FTD23 only, we observed a 68.8% overlap. These results suggest that the neural substrate of FTD and AVHs overlaps considerably, affecting brain regions including the bilateral inferior frontal gyrus, the superior temporal gyrus, the pre- and post-central gyrus, the insula, the middle cingulate cortex and the occipital regions. Note that, since FTD and AVHs are simultaneously present in many patients, we could not determine if the shared neural abnormalities reported here partly relate to their concurrent presence in patients.
Brain abnormalities in the course of illness development
If abnormalities in the language-processing frontal and temporal gyri are central to the pathology of schizophrenia, it becomes critical to understand the developmental course of such aberrations. Meta-analyses of longitudinal studies in schizophrenia have shown that patients exhibit an excessive grey matter volume loss in the frontal cortex and superior temporal gyrus127,128, which is related to symptoms severity and clinical outcomes129,130. These neural abnormalities occur even before the illness onset. In parallel, studies on high-risk individuals131, childhood-onset schizophrenia132, and first-episode schizophrenia patients133,134 have reported volume reductions in the frontal and temporal cortex. Consistently, other studies have found that patterns of brain functional abnormalities differ between the early stage and the chronic phase of the illness: first-episode patients show more functional dysconnectivity in the left inferior frontal gyrus, especially in males38,92,93, whereas patients with longer illness durations exhibit widespread dysconnectivity96. A study of medication-naive patients with varying illness durations also showed an accelerated cortical thinning in the prefrontal and temporal areas in patients135. Overall, these results indicate that, in the course of schizophrenia, frontal and temporal language-processing brain regions are affected since the early stages of the illness.
Neurochemical and genetic basis of language abnormalities
Neurochemical abnormalities
Over the decades, a dominant theory of schizophrenia has posited that dopaminergic dysfunction is at the core of the disorder136–138. Specifically, it has been shown that patients with schizophrenia exhibit a higher level of synthesis, release and binding of dopamine in subcortical regions, which can be treated with antipsychotics blocking dopamine D2 receptors139,140. Though antipsychotics alleviate the severity of AVHs and FTD, the majority of patients still show residual symptoms. This suggests that dysfunction in other neurotransmitter systems may play a role as well in their maintenance141,142.
The high dopaminergic level in subcortical regions has been reported to correlate with a dysregulation of glutamate in cortical areas143. Glutamate has been recently posited to play key roles in the physiology of both typical language processing and its disorders144. Moreover, previous studies have suggested that alterations in the glutamate system in frontal and temporal regions may underlie the occurrence of AVHs and FTD35,36,145,146.
It has been reported that, compared to patients without hallucinations, patients with auditory hallucinations have a higher level of glutamate/glutamine in the superior temporal gyrus and lateral prefrontal cortex35. Besides, patients’ hallucinatory symptoms correlated with a higher level of glutamate in the left superior temporal gyrus, and a lower level of glutamate in the anterior cingulate cortex, suggesting an imbalanced glutamate interaction between cortical regions in hallucinating patients146.
In terms of an association between glutamate and FTD, a pharmaco-fMRI study on healthy subjects showed that ketamine (glutamate NMDA receptor antagonist) elicited transient FTD symptoms, disrupting participants’ lexical and semantic verbal fluency36, and their free flowing speech145. Also, in these healthy participants, ketamine induced a higher correlation between speech production and brain activation in the right middle and inferior temporal gyri, similar to the brain activation in the superior temporal cortex observed in patients with FTD147. Interestingly, ketamine produces a cortical disinhibitory effect that disrupts the selective pyramidal neuronal tuning, which is required for working memory maintenance148. Moreover, ketamine, when administered to patients with prior experience of psychosis, results in the re-emergence of AVHs and FTD features in particular149. More direct examinations of the glutamate dynamics and FTD in patients are needed150, since pharmaco-fMRI studies in healthy participants only provide indirect evidence of the involvement of glutamate in the occurrence of FTD.
Overall, growing evidence points to the possibility that a glutamatergic disruption underlies the occurrence of AVHs and FTD in schizophrenia. Yet, disentangling the specific role that glutamate plays in typical language processing, on the one hand, and the occurrence and maintenance of AVHs and FTD, on the other, warrants further research.
Genes related to schizophrenia and language
Schizophrenia’s heritability is estimated to be 79%151. Of notice, both FTD and AVHs are more prevalent in first-degree relatives of patients with schizophrenia than in first-degree relatives of healthy controls, suggesting that genetic variants contribute to the etiology of these symptoms152,153.
In conducting our review, we looked for studies examining schizophrenia-risk genetic variants that are parallelly implicated in both the development and dysfunction of language, and we also retrieved genetic studies specifically focused on FTD and AVHs in schizophrenia154–157. To systematically obtain the genetic variants that overlap between schizophrenia and language disorders, we first retrieved language-related genes from two recent reviews158,159. Schizophrenia-risk genes were subsequently identified from the genome-wide association studies (GWAS) catalog, including 1450 risk loci mapped genes. In total, we could identify 8 genes that overlap between and have been independently associated with both schizophrenia and language and its related disorders (Fig. 2). These include forkhead box P1, P2 (FOXP1, FOXP2), roundabout guidance receptor 1,2 (ROBO1, ROBO2), glutamate ionotropic receptor NMDA type subunit 2A (GRIN2A), BAF chromatin remodeling complex subunit (BCL11A), inactive phospholipase C-like 1 (PLCL1) and inner mitochondrial membrane peptidase subunit 2 (IMMP2L).
Fig. 2. Shared genetic risk variants for schizophrenia and language disorders.
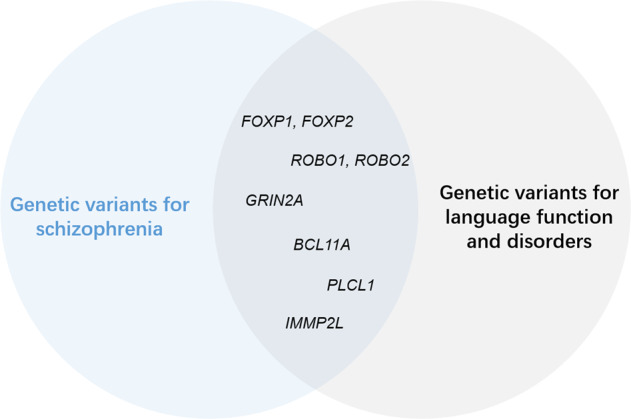
Schizophrenia risk genes were retrieved GWAS catalog database (https://www.ebi.ac.uk/gwas/) using trait label “schizophrenia” (EFO ID: 0000692). Language-related genes were collated from two recent reviews158,159.
FOXP1 and FOXP2 encode transcriptional factors of the forkhead box family. Both genes have been associated with neural development and language evolution160. Risk alleles of FOXP2 have been reported to contribute to auditory hallucinations in schizophrenia161–163. A recent study reported that a polygenic risk score obtained from FOXP2 genetic clusters correlated with functional connectivity in the inferior frontal gyrus in first-episode schizophrenia patients with short illness duration38. Another study found that the FOXP2 risk allele is associated with decreased gray matter volume in several brain regions, including language regions, in patients with schizophrenia164.
The ROBO gene family encodes proteins that are involved in axon guidance and cell migration, especially midline crossing and axons projections in the forebrain. The ROBO2 gene has been related to the size of expressive vocabulary during language acquisition165, schizophrenia166, and handedness167. A different gene, GRIN, encodes subunits of NMDA receptors, with GRIN2A specifically encoding glutamate-binding GluN2 subunit168. The GRIN2A has been associated with several neurodevelopmental disorders, including speech disorders, seizures, autism and schizophrenia169. Now, BCL11A, also known as B-cell CLL/lymphoma 11 A, is a zinc finger transcription factor which mediates effects of the glutamate neurotransmitters on axonal branching and neurite outgrowth170. BCL11A influences early neurodevelopment and has been implicated in schizophrenia, intellectual developmental disorder, and expressive language development171,172. Aside, the PLCL1 protein regulates efficacy of GABAergic neurotransmission173, and it has been associated with language development and risk for schizophrenia174,175. Finally, IMMP2L encodes the inner mitochondrial membrane peptidase subunit 2-like protein, which has been reported in schizophrenia, autism, and other neurodevelopmental disorders176. An animal study showed that IMMP2L knockdown mice exhibited sex-specific changes in locomotion activity and social interaction, which are symptoms of autism and Gilles de la Tourette syndrome177.
Overall, this accumulated evidence suggests that schizophrenia178–181 and language disorders144 share genetic variants that play a role in the early neural development and are likely associated with deficits in key neurotransmitter systems.
Potential clinical applications of the analysis of speech
Diagnostic and prognostic tools
In schizophrenia, features of distinctive language anomalies allow automatic linguistic analysis tools to discriminate between patients’ and healthy controls’ speech. Compared to control subjects, patients’ speech is characterized by longer pauses, reduced semantic coherence, lower sentence complexity and several anomalies at the phonetic, syntactic, semantic, and pragmatic levels182. Recent studies have shown that, implemented along with machine learning algorithms, automated speech analysis tools might help to reach a diagnosis of schizophrenia183 and to predict psychosis conversion among high-risk individuals184. A study focused on clinical high-risk individuals showed that a decrease in semantic coherence, greater variance in that coherence, and a reduction in usage of possessive pronouns were predictive features of psychosis conversion, reaching 79% accuracy in the test dataset184. Another study reported that patients with schizophrenia-spectrum disorders could be distinguished from healthy controls with 85% cross-validated accuracy using a set of measures related to connectedness across words185. Altogether, the existing evidence suggests that analyzing patients’ speech using automatic linguistic tools might help to reach clinical diagnoses and to predict conversion to psychosis, although a series of obstacles remain186.
Treatments targeting language-processing brain regions
As FTD and AVHs have been shown to be associated with abnormalities in language-processing brain regions, non-invasive treatment strategies targeting these regions have been proposed. One commonly applied method is repetitive transcranial magnetic stimulation (rTMS), which generates a brief, high-intensity magnetic field that stimulates the brain tissue187,188. Studies have just started revealing the neural mechanism of rTMS treatment. Vercammen et al. found that 1-Hz rTMS targeting the left temporoparietal cortex enhanced functional connectivity strength between the target site and the right insula189. Arterial spin labeling (ASL) studies have shown that an improvement in clinical symptoms after rTMS treatment was accompanied by a reduction in blood flow in language brain regions190. Blood flow in the superior temporal gyrus further distinguished responders and non-responders to rTMS191. All these studies demonstrated that rTMS to language brain regions would modulate brain activity and connectivity between the target site and other brain regions.
While the rTMS treatment for FTD has been poorly studied192, many studies have investigated the efficacy of the rTMS to reduce AVHs, normally targeting the left temporoparietal cortex (including the superior temporal gyrus and the temporoparietal junction)33. While targeting the left temporoparietal cortex, some studies have reported a positive effect of the rTMS to reduce AVHs severity32,33,193, whereas others reports showed no superior benefit of real stimulation over sham conditions194–196. The inconsistency may reflect the heterogeneity of sampled patients (for example, in the degree of resistance to antipsychotics and illness duration), and stimulation protocols (e.g., duration and frequency of the stimulation). Two meta-analyses found that low-frequency (1 Hz) rTMS may achieve better efficacy, as this frequency can reduce hyperactivity of temporal areas in patients with AVHs30,31. Importantly, when rTMS has been applied on the dorsolateral prefrontal cortex (DLPFC), it did not improve language fluency197 nor AVHs192, which might be expected, mainly considering that the DLPFC seems to be involved primarily in pragmatic language processing198.
Broca’s area, a major player in the language circuit critical for verbal and non-verbal communication, has also been a site targeted by rTMS in patients with schizophrenia and AVHs, although results have been negative194. Nevertheless, co-speech gestures (i.e., non-verbal communication components) that are impaired in patients199–201 and in high-risk individuals for psychosis202 could be improved after Broca’s area stimulation34. However, more studies are still needed to explore different stimulation strategies and distributed target sites to fully take advantage of rTMS to alleviate AVHs and FTD.
Future directions
Different lines of research have gathered evidence of language disturbances in schizophrenia (Fig. 3 and Table 2). In this review, we mainly focused on the pathology of the language system in schizophrenia, yet other higher-order brain networks (e.g., the default mode network, the executive control network, and the salience network) have also been involved in FTD and AVHs7. Neurocognitive studies have reported that semantic and pragmatic abnormalities, as well as executive dysfunctions, are related to both FTD and AVHs59,113. Pragmatic deficits have also been related to Theory of Mind impairments75,203 (but see204). Overall, the current evidence suggests that both abnormalities in language-related regions and connectivity between language regions and other higher-order brain networks are impaired in schizophrenia. To determine how regional abnormalities and dysconnectivity across higher-order brain networks may give rise to FTD and AVHs, longitudinal and intervention studies are needed. For instance, studies could focus on how rTMS targeting higher-order brain networks might affect the language system over time in patients with schizophrenia and AVHs and/or FTD. Of notice, collecting speech samples in a harmonized manner will be important to conduct a deep phenotyping of language in such studies.
Fig. 3. Multilevel evidence of language disturbances in schizophrenia.
On the left, we list the focus of the current review ranging from behavioral symptoms, neuroanatomy, molecular-genetic mechanisms and potential clinical applications focused on language domain. On the right, from top to bottom are the outstanding questions pertinent to the overlap between formal thought disorder and auditory verbal hallucinations in schizophrenia, ranging from mechanistic aspects to treatments.
Table 2.
Summary of findings across levels of analyses.
| Levels of analyses | Formal thought disorder (FTD) | Auditory verbal hallucinations (AVHs) |
|---|---|---|
| Neurocognitive |
• A transdiagnostic symptom with a positive and negative form; • FTD is related to executive functions such as inhibitory control, sustained attention and planning; • FTD is also related to semantic and pragmatic deficits |
• A transdiagnostic symptom, tend to co-occur with FTD; • AVH is related to impaired monitoring function and likely failure to inhibit unwanted memories |
| Neuroanatomical |
• Functional abnormalities involve inferior frontal gyrus, medial orbital frontal cortex and middle temporal gyri; • Structural abnormalities in left posterior superior temporal gyrus; • Structural connectivity studies are less consistent, implicating fibers connecting the language regions and also other fibers |
• Abnormal activation during AVHs or auditory tasks in more widespread areas in bilateral superior and middle temporal gyri, inferior frontal gyri, post-central gyri, anterior cingulate cortex insula and cerebellum; • Structural abnormalities in the bilateral superior temporal gyri; • Structural connectivity studies mainly reported arcuate and uncinate fasciculus |
| Neurochemical |
• Partially treated by antipsychotics • NMDA antagonisms elicits transient FTD symptoms and brain activation in the right middle and inferior temporal gyri |
• Related to excessive dopamine in the striatum, • Partially treated by antipsychotics • Relates to higher level of glutamate/glutamine in the superior temporal gyrus and lateral prefrontal cortex |
| Genetic | • Genes implicated in both language function and schizophrenia mainly involve in early neural development and neurotransmission. Some genes are directly related to structure and function of language regions | |
Neuroimaging studies have shown that both structural and functional abnormalities underlie patients’ AVHs24–29 and FTD20–23. FTD has also been frequently found in non-clinical voice-hearers (i.e., individuals with AVHs but no diagnosable psychiatric conditions)82,83. However, an elucidation of the shared and distinct neural basis of these syndromes is still missing. Longitudinal developmental brain imaging studies from both clinical and non-clinical samples with thorough phenotyping of AVHs and FTD will be critical to unpack the brain-level mechanisms of these syndromes. A valid and reliable characterization of the mechanisms underlying schizophrenia would have to account for how AVHs and FTD ‘come together’ in a single individual.
Genetic studies have found hundreds of genetic risk loci and structural variants for the phenotype of schizophrenia205,206 and for the faculty of language207,208. However, few studies have directly examined whether there is a shared genetic basis underlying these behavioral traits37. Moreover, more work is needed to further understand how this genetic basis is influenced by environmental exposure across the lifespan209, and how the genetic knowledge about schizophrenia might be key to offering more effective treatments against this disorder210.
The innate capacity of large-scale brain networks involved in higher-order functions such as language to adaptively reorganize in the face of dysfunction is becoming increasingly clear with modern neuroimaging and neuromodulation studies211. In the case of the language network, this is likely to occur through interhemispheric interactions212, as well as through contributions from domain-general regions. Such adaptive network plasticity not only supports recovery after damage, but also forms the robust basis for rehabilitative treatment approaches. Considering this, further seminal work is needed for us to develop and test what might be called a “Language Network Modulation” therapy targeting FTD and AVHs in schizophrenia. The success of such an approach may relate to the neuroimaging, neurochemical, linguistic, and genetic profile of the affected individual.
Conclusion
Our review focused on current findings from different levels of analyses about language disturbances, FTD and AVHs among patients with schizophrenia. Neuropsychological studies indicate shared deficits in speech processing and its interaction with executive functions and self-monitoring. Neuroimaging studies indicate a shared reduction in grey matter volume and altered task-induced activations in the superior and middle temporal gyri, and the inferior frontal gyrus. Preliminary neurochemical studies indicate a shared glutamatergic dysfunction in language-related brain regions. Genome-wide association database indicates an overlap in genes involved in the risk for schizophrenia and language functions. Despite this overlap, further mechanistic studies are needed to explain how phenomenological divergence occurs at the level of clinical expression. Considering the potential utility of using speech analysis tools in clinical practice, we call for the development and testing of a “Language Network Modulation” treatment for schizophrenia especially targeting FTD and AVHs. Bridging different levels of evidence and conducting highly-controlled and reproducible experimental studies related to the language system will be critical to reaching this goal.
Supplementary information
Acknowledgements
J.F.F. is supported by the National Key R&D Program of China (2019YFA0709502), 111 Project (B18015), Shanghai Municipal Science and Technology Major Project (2018SHZDZX01), ZJLab and Shanghai Center for Brain Science and Brain-Inspired Technology. X.C. is sponsored by the Shanghai Sailing Program (21YF1402400), National Natural Science Foundation of China (82102138). L.P. is supported by the Monique H. Bourgeois Chair (McGill University) and his research program is supported by the Graham Boeckh Foundation (Centre for Youth Mental Health-Douglas) and a grant from Tannenbaum Open Science Institute for language-related research in psychosis. We would like to thank Professor Iris E.C. Sommer for her constructive advice on the manuscript.
Author contributions
J.F.F. conceptualized the review; X.C., L.P., W.Z., and J.J.K. drafted the manuscript; X.C., X.S.T., and X.C. performed the analysis in Section Brain abnormalities overlapping between FTD and AVHs; L.P. and H.C.H. critically reviewed and edited the manuscript; All authors approved the final version of the manuscript for submission.
Competing interests
L.P. reports personal fees from Janssen Canada, Otsuka Canada, SPMM Course Limited, UK, Canadian Psychiatric Association; book royalties from Oxford University Press; investigator-initiated educational grants from Janssen Canada, Sunovion and Otsuka Canada outside the submitted work. All authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Lena Palaniyappan, Email: lena.palaniyappan@mcgill.ca.
Jianfeng Feng, Email: jianfeng64@gmail.com.
Supplementary information
The online version contains supplementary material available at 10.1038/s41537-022-00308-x.
References
- 1.Crichton-Browne J. On the weight of the brain and its component parts in the insane. Brain. 1879;2:42–67. [Google Scholar]
- 2.Andreasen NC, Grove WM. Thought, language, and communication in schizophrenia: diagnosis and prognosis. Schizophr. Bull. 1986;12:348–359. doi: 10.1093/schbul/12.3.348. [DOI] [PubMed] [Google Scholar]
- 3.Andreasen NC. Symptoms, signs, and diagnosis of schizophrenia. Lancet (London, England). 1995;346:477–481. doi: 10.1016/s0140-6736(95)91325-4. [DOI] [PubMed] [Google Scholar]
- 4.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 5.Hinzen W, Rosselló J, McKenna P. Can delusions be understood linguistically. Cogn. Neuropsychiatry. 2016;21:281–299. doi: 10.1080/13546805.2016.1190703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuperberg GR. Language in schizophrenia Part 1: an introduction. Lang Linguist Compass. 2010;4:576–589. doi: 10.1111/j.1749-818X.2010.00216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palaniyappan, L. Dissecting the neurobiology of linguistic disorganisation and impoverishment in schizophrenia. Semin Cell Dev Biol. 10.1016/j.semcdb.2021.08.015 (2021). [DOI] [PubMed]
- 8.DeLisi LE. Speech disorder in schizophrenia: review of the literature and exploration of its relation to the uniquely human capacity for language. Schizophr Bull. 2001;27:481–496. doi: 10.1093/oxfordjournals.schbul.a006889. [DOI] [PubMed] [Google Scholar]
- 9.Hugdahl K, Sommer IE. Auditory verbal hallucinations in schizophrenia from a levels of explanation perspective. Schizophr Bull. 2018;44:234–241. doi: 10.1093/schbul/sbx142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li X, Branch CA, DeLisi LE. Language pathway abnormalities in schizophrenia: a review of fMRI and other imaging studies. Curr Opin Psychiatry. 2009;22:131–139. doi: 10.1097/YCO.0b013e328324bc43. [DOI] [PubMed] [Google Scholar]
- 11.Kraepelin, E. Psychiatrie: Ein Lehrbuch Für Studierende Und Aerzte. 8. Auflage. Barth: Leipzig: [trans. Barclay, R.M. and Robertson, G.M. 1919: Dementia Praecox and Paraphrenia. Krieger: Huntington, New York. Reprinted 1971]; 1909.
- 12.Bleuler, E. Dementia Praecox Oder Gruppe Der Schizophrenien. Deiticke: Leipzig: [trans. Zinkin, J., 1950. Dementia Praecox or the Group of Schizophrenias. International University Press, New York]; 1911.
- 13.Crow TJ. Schizophrenia as failure of hemispheric dominance for language. Trends Neurosci. 1997;20:339–343. doi: 10.1016/s0166-2236(97)01071-0. [DOI] [PubMed] [Google Scholar]
- 14.Crow TJ. Nuclear schizophrenic symptoms as a window on the relationship between thought and speech. Br. J. Psychiatry. 1998;173:303–309. doi: 10.1192/bjp.173.4.303. [DOI] [PubMed] [Google Scholar]
- 15.Kircher T, Bröhl H, Meier F, Engelen J. Formal thought disorders: from phenomenology to neurobiology. Lancet Psychiatry. 2018;5:515–526. doi: 10.1016/S2215-0366(18)30059-2. [DOI] [PubMed] [Google Scholar]
- 16.Xu K, Schadt EE, Pollard KS, Roussos P, Dudley JT. Genomic and network patterns of schizophrenia genetic variation in human evolutionary accelerated regions. Mol. Biol. Evol. 2015;32:1148–1160. doi: 10.1093/molbev/msv031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Erady C, et al. Novel open reading frames in human accelerated regions and transposable elements reveal new leads to understand schizophrenia and bipolar disorder. Mol. Psychiatry. 2022;27:1455–1468. doi: 10.1038/s41380-021-01405-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wei Y, et al. Genetic mapping and evolutionary analysis of human-expanded cognitive networks. Nat. Commun. 2019;10:1–110. doi: 10.1038/s41467-019-12764-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van den Heuvel MP, et al. Evolutionary modifications in human brain connectivity associated with schizophrenia. Brain. 2019;142:3991–4002. doi: 10.1093/brain/awz330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cavelti M, Kircher T, Nagels A, Strik W, Homan P. Is formal thought disorder in schizophrenia related to structural and functional aberrations in the language network? A systematic review of neuroimaging findings. Schizophr. Res. 2018;199:2–16. doi: 10.1016/j.schres.2018.02.051. [DOI] [PubMed] [Google Scholar]
- 21.Sumner PJ, Bell IH, Rossell SL. A systematic review of the structural neuroimaging correlates of thought disorder. Neurosci. Biobehav. Rev. 2018;84:299–315. doi: 10.1016/j.neubiorev.2017.08.017. [DOI] [PubMed] [Google Scholar]
- 22.Sumner PJ, Bell IH, Rossell SL. A systematic review of task-based functional neuroimaging studies investigating language, semantic and executive processes in thought disorder. Neurosci Biobehav Rev. 2018;94:59–75. doi: 10.1016/j.neubiorev.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 23.Wensing T, et al. Neural correlates of formal thought disorder: an activation likelihood estimation meta‐analysis. Hum. Brain Mapp. 2017;38:4946–4965. doi: 10.1002/hbm.23706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jardri R, Pouchet A, Pins D, Thomas P. Cortical activations during auditory verbal hallucinations in schizophrenia: a coordinate-based meta-analysis. Am. J. Psychiatry. 2011;168:73–81. doi: 10.1176/appi.ajp.2010.09101522. [DOI] [PubMed] [Google Scholar]
- 25.Kompus K, Westerhausen R, Hugdahl K. The “paradoxical” engagement of the primary auditory cortex in patients with auditory verbal hallucinations: a meta-analysis of functional neuroimaging studies. Neuropsychologia. 2011;49:3361–3369. doi: 10.1016/j.neuropsychologia.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 26.Köse G, Jessen K, Ebdrup BH, Nielsen MØ. Associations between cortical thickness and auditory verbal hallucinations in patients with schizophrenia: a systematic review. Psychiatry Res. Neuroimaging. 2018;282:31–39. doi: 10.1016/j.pscychresns.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 27.Kühn S, Gallinat J. Quantitative meta-analysis on state and trait aspects of auditory verbal hallucinations in schizophrenia. Schizophr. Bull. 2012;38:779–786. doi: 10.1093/schbul/sbq152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Modinos G, et al. Neuroanatomy of auditory verbal hallucinations in schizophrenia: a quantitative meta-analysis of voxel-based morphometry studies. Cortex. 2013;49:1046–1055. doi: 10.1016/j.cortex.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 29.Palaniyappan L, Balain V, Radua J, Liddle PF. Structural correlates of auditory hallucinations in schizophrenia: a meta-analysis. Schizophr. Res. 2012;137:169–173. doi: 10.1016/j.schres.2012.01.038. [DOI] [PubMed] [Google Scholar]
- 30.Slotema CW, Blom JD, van Lutterveld R, Hoek HW, Sommer IEC. Review of the efficacy of transcranial magnetic stimulation for auditory verbal hallucinations. Biol. Psychiatry. 2014;76:101–110. doi: 10.1016/j.biopsych.2013.09.038. [DOI] [PubMed] [Google Scholar]
- 31.He H, et al. Repetitive transcranial magnetic stimulation for treating the symptoms of schizophrenia: a PRISMA compliant meta-analysis. Clin. Neurophysiol. 2017;128:716–724. doi: 10.1016/j.clinph.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 32.Kennedy NI, Lee WH, Frangou S. Efficacy of non-invasive brain stimulation on the symptom dimensions of schizophrenia: a meta-analysis of randomized controlled trials. Eur. Psychiatry. 2018;49:69–77. doi: 10.1016/j.eurpsy.2017.12.025. [DOI] [PubMed] [Google Scholar]
- 33.Li J, Cao X, Liu S, Li X, Xu Y. Efficacy of repetitive transcranial magnetic stimulation on auditory hallucinations in schizophrenia: a meta-analysis. Psychiatry Res. 2020;290:1–6. doi: 10.1016/j.psychres.2020.113141. [DOI] [PubMed] [Google Scholar]
- 34.Walther S, et al. Single session transcranial magnetic stimulation ameliorates hand gesture deficits in schizophrenia. Schizophr. Bull. 2020;46:286–293. doi: 10.1093/schbul/sbz078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hugdahl K, et al. Glutamate as a mediating transmitter for auditory hallucinations in schizophrenia: a 1H MRS study. Schizophr. Res. 2015;161:252–260. doi: 10.1016/j.schres.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 36.Nagels A, Kirner-Veselinovic A, Krach S, Kircher T. Neural correlates of S-ketamine induced psychosis during overt continuous verbal fluency. Neuroimage. 2011;54:1307–1314. doi: 10.1016/j.neuroimage.2010.08.021. [DOI] [PubMed] [Google Scholar]
- 37.Murphy E, Benítez-Burraco A. Bridging the gap between genes and language deficits in schizophrenia: an oscillopathic approach. Front Hum. Neurosci. 2016;10:1–15. doi: 10.3389/fnhum.2016.00422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Du J, et al. The genetic determinants of language network dysconnectivity in drug-naïve early stage schizophrenia. npj Schizophr. 2021;7:1–10. doi: 10.1038/s41537-021-00141-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lang X, et al. FOXP2 contributes to the cognitive impairment in chronic patients with schizophrenia. Aging (Albany NY). 2019;11:6440–6448. doi: 10.18632/aging.102198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tolosa A, et al. FOXP2 gene and language impairment in schizophrenia: association and epigenetic studies. BMC Med. Genet. 2010;11:1–8. doi: 10.1186/1471-2350-11-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sanjuán J, et al. FOXP2 expression and gray matter density in the male brains of patients with schizophrenia. Brain Imaging Behav. 2021;15:1403–1411. doi: 10.1007/s11682-020-00339-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McCarthy NS, Clark ML, Jablensky A, Badcock JC. No association between common genetic variation in FOXP2 and language impairment in schizophrenia. Psychiatry Res. 2019;271:590–597. doi: 10.1016/j.psychres.2018.12.016. [DOI] [PubMed] [Google Scholar]
- 43.Braga RM, DiNicola LM, Becker HC, Buckner RL. Situating the left-lateralized language network in the broader organization of multiple specialized large-scale distributed networks. J. Neurophysiol. 2020;124:1415–1448. doi: 10.1152/jn.00753.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hickok G, Poeppel D. The cortical organization of speech processing. Nat Rev Neurosci. 2007;8:393–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- 45.Friederici AD. The brain basis of language processing: from structure to function. Physiol. Rev. 2011;91:1357–1392. doi: 10.1152/physrev.00006.2011. [DOI] [PubMed] [Google Scholar]
- 46.Fedorenko E, Thompson-Schill SL. Reworking the language network. Trends Cogn. Sci. 2014;18:120–126. doi: 10.1016/j.tics.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peralta V, Cuesta MJ, de Leon J. Formal thought disorder in schizophrenia: a factor analytic study. Compr. Psychiatry. 1992;33:105–110. doi: 10.1016/0010-440x(92)90005-b. [DOI] [PubMed] [Google Scholar]
- 48.Harvey PD, Docherty NM, Serper MR, Rasmussen M. Cognitive deficits and thought disorder: II. An 8-month followup study. Schizophr. Bull. 1990;16:147–156. doi: 10.1093/schbul/16.1.147. [DOI] [PubMed] [Google Scholar]
- 49.Breier A, Berg PH. The psychosis of schizophrenia: prevalence, response to atypical antipsychotics, and prediction of outcome. Biol. Psychiatry. 1999;46:361–364. doi: 10.1016/s0006-3223(99)00040-2. [DOI] [PubMed] [Google Scholar]
- 50.Cuesta MJ, Peralta V. Testing the hypothesis that formal thought disorders are severe mood disorders. Schizophr. Bull. 2011;37:1136–1146. doi: 10.1093/schbul/sbr092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morgan CJ, et al. Thought disorder in schizophrenia and bipolar disorder probands, their relatives, and nonpsychiatric controls. Schizophr. Bull. 2017;43:523–535. doi: 10.1093/schbul/sbx016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Remberk B, Namysłowska I, Rybakowski F. Cognitive impairment and formal thought disorders in parents of early-onset schizophrenia patients. Neuropsychobiology. 2012;65:206–215. doi: 10.1159/000337001. [DOI] [PubMed] [Google Scholar]
- 53.DeVylder JE, et al. Symptom trajectories and psychosis onset in a clinical high-risk cohort: the relevance of subthreshold thought disorder. Schizophr. Res. 2014;159:278–283. doi: 10.1016/j.schres.2014.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stirling J, Hellewell J, Blakey A, Deakin W. Thought disorder in schizophrenia is associated with both executive dysfunction and circumscribed impairments in semantic function. Psychol Med. 2006;36:475–484. doi: 10.1017/S0033291705006884. [DOI] [PubMed] [Google Scholar]
- 55.Barrera A, McKenna PJ, Berrios GE. Formal thought disorder in schizophrenia: an executive or a semantic deficit? Psychol. Med. 2005;35:121–132. doi: 10.1017/s003329170400279x. [DOI] [PubMed] [Google Scholar]
- 56.McGrath J, Scheldt S, Hengstberger P, Dark F. Thought disorder and executive ability. Cogn. Neuropsychiatry. 1997;2:303–314. doi: 10.1080/135468097396306. [DOI] [PubMed] [Google Scholar]
- 57.Xu J-Q, et al. Executive function as predictors of persistent thought disorder in first-episode schizophrenia: a one-year follow-up study. Schizophr Res. 2014;159:465–470. doi: 10.1016/j.schres.2014.08.022. [DOI] [PubMed] [Google Scholar]
- 58.Nagels A, et al. Distinct neuropsychological correlates in positive and negative formal thought disorder syndromes: the thought and language disorder scale in endogenous psychoses. Neuropsychobiology. 2016;73:139–147. doi: 10.1159/000441657. [DOI] [PubMed] [Google Scholar]
- 59.Tan EJ, Rossell SL. Building a neurocognitive profile of thought disorder in schizophrenia using a standardized test battery. Schizophr Res. 2014;152:242–245. doi: 10.1016/j.schres.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 60.Bora E, Yalincetin B, Akdede BB, Alptekin K. Neurocognitive and linguistic correlates of positive and negative formal thought disorder: a meta-analysis. Schizophr Res. 2019;209:2–11. doi: 10.1016/j.schres.2019.05.025. [DOI] [PubMed] [Google Scholar]
- 61.Doughty OJ, Done DJ. Is semantic memory impaired in schizophrenia? A systematic review and meta-analysis of 91 studies. Cogn Neuropsychiatry. 2009;14:473–509. doi: 10.1080/13546800903073291. [DOI] [PubMed] [Google Scholar]
- 62.Manschreck TC, et al. Semantic priming in thought disordered schizophrenic patients. Schizophr. Res. 1988;1:61–66. doi: 10.1016/0920-9964(88)90041-2. [DOI] [PubMed] [Google Scholar]
- 63.Moritz S, et al. “Hyper-priming” in thought-disordered schizophrenic patients. Psychol. Med. 2001;31:221–229. doi: 10.1017/s0033291701003105. [DOI] [PubMed] [Google Scholar]
- 64.Spitzer M, Braun U, Hermle L, Maier S. Associative semantic network dysfunction in thought-disordered schizophrenic patients: direct evidence from indirect semantic priming. Biol Psychiatry. 1993;34:864–877. doi: 10.1016/0006-3223(93)90054-h. [DOI] [PubMed] [Google Scholar]
- 65.Pomarol-Clotet, et al. Semantic priming in schizophrenia: systematic review and meta-analysis. Br. J. Psychiatry. 2008;192:92–97. doi: 10.1192/bjp.bp.106.032102. [DOI] [PubMed] [Google Scholar]
- 66.Kuperberg GR, et al. Multimodal neuroimaging evidence for looser lexico-semantic networks in schizophrenia: evidence from masked indirect semantic priming. Neuropsychologia. 2019;124:337–349. doi: 10.1016/j.neuropsychologia.2018.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Alonso-Sánchez MF, et al. Progressive changes in descriptive discourse in First Episode Schizophrenia: a longitudinal computational semantics study. Schizophrenia. 2022;8:1–9. doi: 10.1038/s41537-022-00246-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Alonso-Sánchez, M. F., Limongi, R., Gati, J., Palaniyappan, L. Language network self-inhibition and semantic similarity in first-episode schizophrenia: a computational-linguistic and effective connectivity approach. Schizophr. Res. 10.1016/j.schres.2022.04.007 (2022). [DOI] [PubMed]
- 69.Salavera C, Puyuelo M, Antoñanzas JL, Teruel P. Semantics, pragmatics, and formal thought disorders in people with schizophrenia. Neuropsychiatr. Dis. Treat. 2013;9:177–183. doi: 10.2147/NDT.S38676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kuperberg GR, McGuire PK, David AS. Reduced sensitivity to linguistic context in schizophrenic thought disorder: evidence from on-line monitoring for words in linguistically anomalous sentences. J. Abnorm Psychol. 1998;107:423–434. doi: 10.1037//0021-843x.107.3.423. [DOI] [PubMed] [Google Scholar]
- 71.Langdon R, Coltheart M, Ward PB, Catts SV. Disturbed communication in schizophrenia: the role of poor pragmatics and poor mind-reading. Psychol. Med. 2002;32:1273–1284. doi: 10.1017/s0033291702006396. [DOI] [PubMed] [Google Scholar]
- 72.Mey, J. L. Pragmatics: Overview. In: Encyclopedia of Language & Linguistics. 51–62 (Elsevier, 2006) 10.1016/B0-08-044854-2/00306-0.
- 73.Parola A, Brasso C, Morese R, Rocca P, Bosco FM. Understanding communicative intentions in schizophrenia using an error analysis approach. npj Schizophr. 2021;7:1–9. doi: 10.1038/s41537-021-00142-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pawełczyk A, Łojek E, Żurner N, Gawłowska-Sawosz M, Pawełczyk T. Higher-order language dysfunctions as a possible neurolinguistic endophenotype for schizophrenia: Evidence from patients and their unaffected first degree relatives. Psychiatry Res. 2018;267:63–72. doi: 10.1016/j.psychres.2018.05.070. [DOI] [PubMed] [Google Scholar]
- 75.Schnell Z, et al. Neuropragmatics and irony processing in schizophrenia–possible neural correlates of the meta-module of pragmatic meaning construction. J. Pragmat. 2016;92:74–99. [Google Scholar]
- 76.Baethge C, et al. Hallucinations in bipolar disorder: characteristics and comparison to unipolar depression and schizophrenia. Bipolar Disord. 2005;7:136–145. doi: 10.1111/j.1399-5618.2004.00175.x. [DOI] [PubMed] [Google Scholar]
- 77.Bassiony MM, Lyketsos CG. Delusions and hallucinations in Alzheimer’s disease: review of the brain decade. Psychosomatics. 2003;44:388–401. doi: 10.1176/appi.psy.44.5.388. [DOI] [PubMed] [Google Scholar]
- 78.Perälä J, et al. Alcohol-induced psychotic disorder and delirium in the general population. Br. J. Psychiatry. 2010;197:200–206. doi: 10.1192/bjp.bp.109.070797. [DOI] [PubMed] [Google Scholar]
- 79.Sommer IE, et al. Healthy individuals with auditory verbal hallucinations; Who are they? psychiatric assessments of a selected sample of 103 subjects. Schizophr. Bull. 2010;36:633–641. doi: 10.1093/schbul/sbn130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Waters F, et al. Visual hallucinations in the psychosis spectrum and comparative information from neurodegenerative disorders and eye disease. Schizophr. Bull. 2014;40:S233–S245. doi: 10.1093/schbul/sbu036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lim A, et al. Prevalence and classification of hallucinations in multiple sensory modalities in schizophrenia spectrum disorders. Schizophr. Res. 2016;176:493–499. doi: 10.1016/j.schres.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 82.Sommer IE, et al. Formal thought disorder in non-clinical individuals with auditory verbal hallucinations. Schizophr. Res. 2010;118:140–145. doi: 10.1016/j.schres.2010.01.024. [DOI] [PubMed] [Google Scholar]
- 83.Strik W, Dierks T, Hubl D, Horn H. Hallucinations, thought disorders, and the language domain in schizophrenia. Clin. EEG Neurosci. 2008;39:91–94. doi: 10.1177/155005940803900214. [DOI] [PubMed] [Google Scholar]
- 84.Jones SR, Fernyhough C. Neural correlates of inner speech and auditory verbal hallucinations: a critical review and theoretical integration. Clin. Psychol Rev. 2007;27:140–154. doi: 10.1016/j.cpr.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 85.Allen P, Aleman A, McGuire PK. Inner speech models of auditory verbal hallucinations: evidence from behavioural and neuroimaging studies. Int. Rev. Psychiatry. 2007;19:407–415. doi: 10.1080/09540260701486498. [DOI] [PubMed] [Google Scholar]
- 86.Shergill SS, et al. Functional magnetic resonance imaging of impaired sensory prediction in schizophrenia. JAMA Psychiatry. 2014;71:28–35. doi: 10.1001/jamapsychiatry.2013.2974. [DOI] [PubMed] [Google Scholar]
- 87.Feinberg I. Efference copy and corollary discharge: implications for thinking and its disorders. Schizophr. Bull. 1978;4:636–640. doi: 10.1093/schbul/4.4.636. [DOI] [PubMed] [Google Scholar]
- 88.Frith CD, Done DJ. Towards a neuropsychology of schizophrenia. Br. J. Psychiatry. 1988;153:437–443. doi: 10.1192/bjp.153.4.437. [DOI] [PubMed] [Google Scholar]
- 89.Waters F, Badcock J, Michie P, Maybery M. Auditory hallucinations in schizophrenia: intrusive thoughts and forgotten memories. Cogn Neuropsychiatry. 2006;11:65–83. doi: 10.1080/13546800444000191. [DOI] [PubMed] [Google Scholar]
- 90.Jones SR. Do we need multiple models of auditory verbal hallucinations? Examining the phenomenological fit of cognitive and neurological models. Schizophr. Bull. 2010;36:566–575. doi: 10.1093/schbul/sbn129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Read J, Os J, Morrison AP, Ross CA. Childhood trauma, psychosis and schizophrenia: a literature review with theoretical and clinical implications. Acta Psychiatr. Scand. 2005;112:330–350. doi: 10.1111/j.1600-0447.2005.00634.x. [DOI] [PubMed] [Google Scholar]
- 92.Wang Q, et al. Brain connectivity deviates by sex and hemisphere in the first episode of schizophrenia-a route to the genetic basis of language and psychosis? Schizophr Bull. 2019;45:484–494. doi: 10.1093/schbul/sby061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liu Z, et al. Distinguishable brain networks relate disease susceptibility to symptom expression in schizophrenia. Hum. Brain Mapp. 2018;39:3503–3515. doi: 10.1002/hbm.24190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.van Erp TGM, et al. Cortical brain abnormalities in 4474 individuals with schizophrenia and 5098 control subjects via the Enhancing Neuro Imaging Genetics Through Meta Analysis (ENIGMA) Consortium. Biol Psychiatry. 2018;84:644–654. doi: 10.1016/j.biopsych.2018.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Walton E, et al. Positive symptoms associate with cortical thinning in the superior temporal gyrus via the ENIGMA Schizophrenia consortium. Acta Psychiatr Scand. 2017;135:439–447. doi: 10.1111/acps.12718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cheng W, et al. Voxel-based, brain-wide association study of aberrant functional connectivity in schizophrenia implicates thalamocortical circuitry. NPJ Schizophr. 2015;1:1–8. doi: 10.1038/npjschz.2015.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li T, et al. Brain-wide analysis of functional connectivity in first-episode and chronic stages of schizophrenia. Schizophr. Bull. 2017;43:436–448. doi: 10.1093/schbul/sbw099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nestor PG, et al. Neuropsychological correlates of MRI temporal lobe abnormalities in schizophrenia. Am. J. Psychiatry. 1993;150:1849–1855. doi: 10.1176/ajp.150.12.1849. [DOI] [PubMed] [Google Scholar]
- 99.Sans-Sansa B, et al. Association of formal thought disorder in schizophrenia with structural brain abnormalities in language-related cortical regions. Schizophr. Res. 2013;146:308–313. doi: 10.1016/j.schres.2013.02.032. [DOI] [PubMed] [Google Scholar]
- 100.Palaniyappan L, Al-Radaideh A, Gowland PA, Liddle PF. Cortical thickness and formal thought disorder in schizophrenia: an ultra high-field network-based morphometry study. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2020;101:1–8. doi: 10.1016/j.pnpbp.2020.109911. [DOI] [PubMed] [Google Scholar]
- 101.Chen J, et al. Neurobiological substrates of the positive formal thought disorder in schizophrenia revealed by seed connectome-based predictive modeling. NeuroImage Clin. 2021;30:1–11. doi: 10.1016/j.nicl.2021.102666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Palaniyappan L, et al. Structural correlates of formal thought disorder in schizophrenia: an ultra-high field multivariate morphometry study. Schizophr. Res. 2015;168:305–312. doi: 10.1016/j.schres.2015.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dey A, et al. Conceptual disorganization and redistribution of resting-state cortical hubs in untreated first-episode psychosis: a 7T study. NPJ Schizophr. 2021;7:1–9. doi: 10.1038/s41537-020-00130-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Asami T, et al. Abnormalities of middle longitudinal fascicle and disorganization in patients with schizophrenia. Schizophr. Res. 2013;143:253–259. doi: 10.1016/j.schres.2012.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pan Y, et al. Acute conceptual disorganization in untreated first-episode psychosis: a combined magnetic resonance spectroscopy and diffusion imaging study of the cingulum. J. Psychiatry Neurosci. 2021;46:E337–E346. doi: 10.1503/jpn.200167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Szeszko PR, et al. Clinical and neuropsychological correlates of white matter abnormalities in recent onset schizophrenia. Neuropsychopharmacology. 2008;33:976–984. doi: 10.1038/sj.npp.1301480. [DOI] [PubMed] [Google Scholar]
- 107.Kubicki M, et al. Reduced interhemispheric connectivity in schizophrenia-tractography based segmentation of the corpus callosum. Schizophr. Res. 2008;106:125–131. doi: 10.1016/j.schres.2008.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Arnedo J, et al. Decomposition of brain diffusion imaging data uncovers latent schizophrenias with distinct patterns of white matter anisotropy. Neuroimage. 2015;120:43–54. doi: 10.1016/j.neuroimage.2015.06.083. [DOI] [PubMed] [Google Scholar]
- 109.Zmigrod L, Garrison JR, Carr J, Simons JS. The neural mechanisms of hallucinations: a quantitative meta-analysis of neuroimaging studies. Neurosci. Biobehav. Rev. 2016;69:113–123. doi: 10.1016/j.neubiorev.2016.05.037. [DOI] [PubMed] [Google Scholar]
- 110.Sommer IEC, et al. Auditory verbal hallucinations predominantly activate the right inferior frontal area. Brain. 2008;131:3169–3177. doi: 10.1093/brain/awn251. [DOI] [PubMed] [Google Scholar]
- 111.Shergill SS, Brammer MJ, Williams SCR, Murray RM, McGuire PK. Mapping auditory hallucinations in schizophrenia using functional magnetic resonance imaging. Arch. Gen. Psychiatry. 2000;57:1033–1038. doi: 10.1001/archpsyc.57.11.1033. [DOI] [PubMed] [Google Scholar]
- 112.Raij TT, et al. Reality of auditory verbal hallucinations. Brain. 2009;132:2994–3001. doi: 10.1093/brain/awp186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mechelli A, et al. Misattribution of speech and impaired connectivity in patients with auditory verbal hallucinations. Hum. Brain Mapp. 2007;28:1213–1222. doi: 10.1002/hbm.20341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hunter MD, et al. Neural activity in speech-sensitive auditory cortex during silence. Proc. Natl Acad. Sci. USA. 2006;103:189–194. doi: 10.1073/pnas.0506268103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hoffman RE, Pittman B, Constable RT, Bhagwagar Z, Hampson M. Time course of regional brain activity accompanying auditory verbal hallucinations in schizophrenia. Br. J. Psychiatry. 2011;198:277–283. doi: 10.1192/bjp.bp.110.086835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Alderson-Day B, McCarthy-Jones S, Fernyhough C. Hearing voices in the resting brain: a review of intrinsic functional connectivity research on auditory verbal hallucinations. Neurosci. Biobehav. Rev. 2015;55:78–87. doi: 10.1016/j.neubiorev.2015.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Northoff G. Are Auditory Hallucinations related to the brain’s resting state activity? A neurophenomenal resting state hypothesis. Clin. Psychopharmacol. Neurosci. 2014;12:189–195. doi: 10.9758/cpn.2014.12.3.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hubl D, et al. Pathways that make voices. Arch. Gen. Psychiatry. 2004;61:658. doi: 10.1001/archpsyc.61.7.658. [DOI] [PubMed] [Google Scholar]
- 119.Psomiades M, et al. Integrity of the arcuate fasciculus in patients with schizophrenia with auditory verbal hallucinations: a DTI-tractography study. NeuroImage Clin. 2016;12:970–975. doi: 10.1016/j.nicl.2016.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ćurčić-Blake B, et al. Not on speaking terms: hallucinations and structural network disconnectivity in schizophrenia. Brain Struct. Funct. 2015;220:407–418. doi: 10.1007/s00429-013-0663-y. [DOI] [PubMed] [Google Scholar]
- 121.Geoffroy PA, et al. The arcuate fasciculus in auditory-verbal hallucinations: a meta-analysis of diffusion-tensor-imaging studies. Schizophr. Res. 2014;159:234–237. doi: 10.1016/j.schres.2014.07.014. [DOI] [PubMed] [Google Scholar]
- 122.Catani M, et al. Altered integrity of perisylvian language pathways in schizophrenia: relationship to auditory hallucinations. Biol. Psychiatry. 2011;70:1143–1150. doi: 10.1016/j.biopsych.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 123.Mulert C, et al. Hearing voices: a role of interhemispheric auditory connectivity? World J. Biol. Psychiatry. 2012;13:153–158. doi: 10.3109/15622975.2011.570789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Darby RR, Joutsa J, Fox MD. Network localization of heterogeneous neuroimaging findings. Brain. 2019;142:70–79. doi: 10.1093/brain/awy292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Boes AD, et al. Network localization of neurological symptoms from focal brain lesions. Brain. 2015;138:3061–3075. doi: 10.1093/brain/awv228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Peng, S., Xu, P., Jiang, Y., Gong, G. Activation network mapping for integration of heterogeneous fMRI findings. Nat. Hum. Behav. 10.1038/s41562-022-01371-1 (2022). [DOI] [PubMed]
- 127.Haijma SV, et al. Brain volumes in schizophrenia: a meta-analysis in over 18,000 subjects. Schizophr. Bull. 2013;39:1129–1138. doi: 10.1093/schbul/sbs118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Radua J, et al. Multimodal meta-analysis of structural and functional brain changes in first episode psychosis and the effects of antipsychotic medication. Neurosci. Biobehav. Rev. 2012;36:2325–2333. doi: 10.1016/j.neubiorev.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 129.Vita A, De Peri L, Deste G, Sacchetti E. Progressive loss of cortical gray matter in schizophrenia: a meta-analysis and meta-regression of longitudinal MRI studies. Transl. Psychiatry. 2012;2:e190–e190. doi: 10.1038/tp.2012.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.van Haren NEM. Changes in cortical thickness during the course of illness in schizophrenia. Arch. Gen. Psychiatry. 2011;68:871. doi: 10.1001/archgenpsychiatry.2011.88. [DOI] [PubMed] [Google Scholar]
- 131.Wood SJ, Reniers RLEP, Heinze K. Neuroimaging findings in the at-risk mental state: a review of recent literature. Can. J. Psychiatry. 2013;58:13–18. doi: 10.1177/070674371305800104. [DOI] [PubMed] [Google Scholar]
- 132.Taylor JL, et al. Superior temporal gyrus differences in childhood-onset schizophrenia. Schizophr. Res. 2005;73:235–241. doi: 10.1016/j.schres.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 133.Mwansisya TE, et al. Task and resting-state fMRI studies in first-episode schizophrenia: a systematic review. Schizophr. Res. 2017;189:9–18. doi: 10.1016/j.schres.2017.02.026. [DOI] [PubMed] [Google Scholar]
- 134.Sheng J, et al. Altered volume and lateralization of language-related regions in first-episode schizophrenia. Schizophr. Res. 2013;148:168–174. doi: 10.1016/j.schres.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 135.Zhang W, et al. Brain structural abnormalities in a group of never-medicated patients with long-term schizophrenia. Am. J. Psychiatry. 2015;172:995–1003. doi: 10.1176/appi.ajp.2015.14091108. [DOI] [PubMed] [Google Scholar]
- 136.Davis KL, Kahn RS, Ko G, Davidson M. Dopamine in schizophrenia: a review and reconceptualization. Am. J. Psychiatry. 1991;148:1474–1486. doi: 10.1176/ajp.148.11.1474. [DOI] [PubMed] [Google Scholar]
- 137.Kapur S, Mizrahi R, Li M. From dopamine to salience to psychosis—linking biology, pharmacology and phenomenology of psychosis. Schizophr Res. 2005;79:59–68. doi: 10.1016/j.schres.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 138.Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III–the final common pathway. Schizophr Bull. 2009;35:549–562. doi: 10.1093/schbul/sbp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.McCutcheon R, Beck K, Jauhar S, Howes OD. Defining the locus of dopaminergic dysfunction in schizophrenia: a meta-analysis and test of the mesolimbic hypothesis. Schizophr. Bull. 2018;44:1301–1311. doi: 10.1093/schbul/sbx180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Howes OD, et al. Elevated striatal dopamine function linked to prodromal signs of schizophrenia. Arch Gen Psychiatry. 2009;66:13–20. doi: 10.1001/archgenpsychiatry.2008.514. [DOI] [PubMed] [Google Scholar]
- 141.Millan MJ, et al. Altering the course of schizophrenia: progress and perspectives. Nat Rev Drug Discov. 2016;15:485–515. doi: 10.1038/nrd.2016.28. [DOI] [PubMed] [Google Scholar]
- 142.Kahn RS, Sommer IE. The neurobiology and treatment of first-episode schizophrenia. Mol Psychiatry. 2015;20:84–97. doi: 10.1038/mp.2014.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Jauhar S, et al. The relationship between cortical glutamate and striatal dopamine in first-episode psychosis: a cross-sectional multimodal PET and magnetic resonance spectroscopy imaging study. The Lancet Psychiatry. 2018;5:816–823. doi: 10.1016/S2215-0366(18)30268-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Li W, Kutas M, Gray JA, Hagerman RH, Olichney JM. The role of glutamate in language and language disorders—evidence from ERP and pharmacologic studies. Neurosci. Biobehav. Rev. 2020;119:217–241. doi: 10.1016/j.neubiorev.2020.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Nagels A, et al. S-ketamine-induced NMDA receptor blockade during natural speech production and its implications for formal thought disorder in schizophrenia: a pharmaco-fMRI study. Neuropsychopharmacology. 2018;43:1324–1333. doi: 10.1038/npp.2017.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hjelmervik H, et al. Intra-regional Glu-GABA vs inter-regional Glu-Glu imbalance: a 1H-MRS study of the neurochemistry of auditory verbal hallucinations in schizophrenia. Schizophr. Bull. 2020;46:633–642. doi: 10.1093/schbul/sbz099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kircher TTJ, et al. Reversed lateralization of temporal activation during speech production in thought disordered patients with schizophrenia. Psychol. Med. 2002;32:439–449. doi: 10.1017/s0033291702005287. [DOI] [PubMed] [Google Scholar]
- 148.Roussy M, et al. Ketamine disrupts naturalistic coding of working memory in primate lateral prefrontal cortex networks. Mol. Psychiatry. 2021;26:6688–6703. doi: 10.1038/s41380-021-01082-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lahti A. Effects of ketamine in normal and schizophrenic volunteers. Neuropsychopharmacology. 2001;25:455–467. doi: 10.1016/S0893-133X(01)00243-3. [DOI] [PubMed] [Google Scholar]
- 150.Liang, L. et al. Widespread cortical thinning, excessive glutamate and impaired linguistic functioning in schizophrenia: a cluster analytic approach. Front. Hum. Neurosci. 2022;16 (in press). 10.3389/fnhum.2022.954898. [DOI] [PMC free article] [PubMed]
- 151.Hilker R, et al. Heritability of schizophrenia and schizophrenia spectrum based on the nationwide danish twin register. Biol. Psychiatry. 2018;83:492–498. doi: 10.1016/j.biopsych.2017.08.017. [DOI] [PubMed] [Google Scholar]
- 152.Gooding DC, et al. Thought disorder in offspring of schizophrenic parents: findings from the new york high-risk project. Schizophr. Bull. 2012;38:263–271. doi: 10.1093/schbul/sbq061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Brunelin J, et al. Impaired verbal source monitoring in schizophrenia: an intermediate trait vulnerability marker. Schizophr. Res. 2007;89:287–292. doi: 10.1016/j.schres.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 154.Shao X, Liao Y, Gu L, Chen W, Tang J. The etiology of auditory hallucinations in schizophrenia: from multidimensional levels. Front Neurosci. 2021;15:1–15. doi: 10.3389/fnins.2021.755870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Levy DL, et al. The genetic basis of thought disorder and language and communication disturbances in schizophrenia. J. Neurolinguistics. 2010;23:176–192. doi: 10.1016/j.jneuroling.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Legge, S. E. et al. Associations between schizophrenia polygenic liability, symptom dimensions, and cognitive ability in schizophrenia. JAMA Psychiatry10.1001/jamapsychiatry.2021.1961 (2021). [DOI] [PMC free article] [PubMed]
- 157.Wang, K. S., Zhang, Q., Liu, X., Wu, L., Zeng, M. PKNOX2 is associated with formal thought disorder in schizophrenia: a meta-analysis of two genome-wide association studies. J. Mol. Neurosci. 10.1007/s12031-012-9787-4 (2012). [DOI] [PubMed]
- 158.Graham SA, Fisher SE. Understanding language from a genomic perspective. Annu Rev Genet. 2015;49:131–160. doi: 10.1146/annurev-genet-120213-092236. [DOI] [PubMed] [Google Scholar]
- 159.Doust C, et al. The association of dyslexia and developmental speech and language disorder candidate genes with reading and language abilities in adults. Twin Res. Hum. Genet. 2020;23:23–32. doi: 10.1017/thg.2020.7. [DOI] [PubMed] [Google Scholar]
- 160.Güntürkün O, Ocklenburg S. Ontogenesis of Lateralization. Neuron. 2017;94:249–263. doi: 10.1016/j.neuron.2017.02.045. [DOI] [PubMed] [Google Scholar]
- 161.Lai CSL, Fisher SE, Hurst JA, Vargha-Khadem F, Monaco AP. A forkhead-domain gene is mutated in a severe speech and language disorder. Nature. 2001;413:519–523. doi: 10.1038/35097076. [DOI] [PubMed] [Google Scholar]
- 162.McCarthy-Jones S, et al. Preliminary evidence of an interaction between the FOXP2 gene and childhood emotional abuse predicting likelihood of auditory verbal hallucinations in schizophrenia. J Psychiatr Res. 2014;50:66–72. doi: 10.1016/j.jpsychires.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 163.Sanjuán J, et al. Association between FOXP2 polymorphisms and schizophrenia with auditory hallucinations. Psychiatr. Genet. 2006;16:67–72. doi: 10.1097/01.ypg.0000185029.35558.bb. [DOI] [PubMed] [Google Scholar]
- 164.Španiel F, et al. Genetic variation in FOXP2 alters grey matter concentrations in schizophrenia patients. Neurosci. Lett. 2011;493:131–135. doi: 10.1016/j.neulet.2011.02.024. [DOI] [PubMed] [Google Scholar]
- 165.St Pourcain B, et al. Common variation near ROBO2 is associated with expressive vocabulary in infancy. Nat. Commun. 2014;5:1–9. doi: 10.1038/ncomms5831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Potkin SG, et al. A genome-wide association study of schizophrenia using brain activation as a quantitative phenotype. Schizophr. Bull. 2009;35:96–108. doi: 10.1093/schbul/sbn155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Cuellar-Partida G, et al. Genome-wide association study identifies 48 common genetic variants associated with handedness. Nat. Hum. Behav. 2021;5:59–70. doi: 10.1038/s41562-020-00956-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Myers SJ, et al. Distinct roles of GRIN2A and GRIN2B variants in neurological conditions. F1000Research. 2019;8:1–14. doi: 10.12688/f1000research.18949.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Strehlow V, et al. GRIN2A -related disorders: genotype and functional consequence predict phenotype. Brain. 2019;142:80–92. doi: 10.1093/brain/awy304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kuo T-Y, Chen C-Y, Hsueh Y-P. Bcl11A/CTIP1 mediates the effect of the glutamate receptor on axon branching and dendrite outgrowth. J. Neurochem. 2010;114:1381–1392. doi: 10.1111/j.1471-4159.2010.06852.x. [DOI] [PubMed] [Google Scholar]
- 171.Le Hellard S, et al. Identification of gene loci that overlap between schizophrenia and educational attainment. Schizophr. Bull. 2016;43:654–664. doi: 10.1093/schbul/sbw085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Peter B, Matsushita M, Oda K, Raskind W. De novo microdeletion of BCL11A is associated with severe speech sound disorder. Am. J. Med. Genet. Part A. 2014;164:2091–2096. doi: 10.1002/ajmg.a.36599. [DOI] [PubMed] [Google Scholar]
- 173.Zhu G, et al. Dysfunction of extrasynaptic GABAergic transmission in phospholipase C-related, but catalytically inactive protein 1 knockout mice is associated with an epilepsy phenotype. J. Pharmacol. Exp. Ther. 2012;340:520–528. doi: 10.1124/jpet.111.182386. [DOI] [PubMed] [Google Scholar]
- 174.Notaras, M. et al. Schizophrenia is defined by cell-specific neuropathology and multiple neurodevelopmental mechanisms in patient-derived cerebral organoids. Mol. Psychiatry10.1038/s41380-021-01316-6 (2021). [DOI] [PMC free article] [PubMed]
- 175.Namjou B, et al. Phenome-wide association study (PheWAS) in EMR-linked pediatric cohorts, genetically links PLCL1 to speech language development and IL5-IL13 to Eosinophilic Esophagitis. Front Genet. 2014;5:1–12. doi: 10.3389/fgene.2014.00401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Zhang Y, et al. Association of IMMP2L deletions with autism spectrum disorder: a trio family study and meta-analysis. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2018;177:93–100. doi: 10.1002/ajmg.b.32608. [DOI] [PubMed] [Google Scholar]
- 177.Kreilaus F, Chesworth R, Eapen V, Clarke R, Karl T. First behavioural assessment of a novel Immp2l knockdown mouse model with relevance for Gilles de la Tourette syndrome and Autism spectrum disorder. Behav. Brain Res. 2019;374:1–10. doi: 10.1016/j.bbr.2019.112057. [DOI] [PubMed] [Google Scholar]
- 178.Fromer M, et al. De novo mutations in schizophrenia implicate synaptic networks. Nature. 2014;506:179–184. doi: 10.1038/nature12929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Hall J, Trent S, Thomas KL, O’Donovan MC, Owen MJ. Genetic risk for schizophrenia: convergence on synaptic pathways involved in plasticity. Biol. Psychiatry. 2015;77:52–58. doi: 10.1016/j.biopsych.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 180.Trubetskoy V, et al. Mapping genomic loci implicates genes and synaptic biology in schizophrenia. Nature. 2022;604:502–508. doi: 10.1038/s41586-022-04434-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Singh T, et al. Rare coding variants in ten genes confer substantial risk for schizophrenia. Nature. 2022;604:509–516. doi: 10.1038/s41586-022-04556-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Corcoran CM, Cecchi GA. Using language processing and speech analysis for the identification of psychosis and other disorders. Biol. Psychiatry Cogn. Neurosci. Neuroimaging. 2020;5:770–779. doi: 10.1016/j.bpsc.2020.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.de Boer JN, et al. Language in schizophrenia: relation with diagnosis, symptomatology and white matter tracts. npj Schizophr. 2020;6:10. doi: 10.1038/s41537-020-0099-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Corcoran CM, et al. Prediction of psychosis across protocols and risk cohorts using automated language analysis. World Psychiatry. 2018;17:67–75. doi: 10.1002/wps.20491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Voppel A, de Boer J, Brederoo S, Schnack H, Sommer I. Quantified language connectedness in schizophrenia-spectrum disorders. Psychiatry Res. 2021;304:1–8. doi: 10.1016/j.psychres.2021.114130. [DOI] [PubMed] [Google Scholar]
- 186.Chandler C, Foltz PW, Elvevåg B. Improving the applicability of AI for psychiatric applications through human-in-the-loop methodologies. Schizophr. Bull. 2022;48:949–957. doi: 10.1093/schbul/sbac038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Lefaucheur, J.-P. et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): an update (2014–2018. Clin. Neurophysiol. 131, 474–528 (2020). [DOI] [PubMed]
- 188.Vercammen A, et al. Functional connectivity of the temporo-parietal region in schizophrenia: effects of rTMS treatment of auditory hallucinations. J Psychiatr Res. 2010;44:725–731. doi: 10.1016/j.jpsychires.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 189.Vercammen A, Knegtering H, den Boer JA, Liemburg EJ, Aleman A. Auditory hallucinations in schizophrenia are associated with reduced functional connectivity of the temporo-parietal area. Biol Psychiatry. 2010;67:912–918. doi: 10.1016/j.biopsych.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 190.Kindler J, et al. Reduced neuronal activity in language-related regions after transcranial magnetic stimulation therapy for auditory verbal hallucinations. Biol Psychiatry. 2013;73:518–524. doi: 10.1016/j.biopsych.2012.06.019. [DOI] [PubMed] [Google Scholar]
- 191.Homan P, Kindler J, Hauf M, Hubl D, Dierks T. Cerebral blood flow identifies responders to transcranial magnetic stimulation in auditory verbal hallucinations. Transl. Psychiatry. 2012;2:1–6. doi: 10.1038/tp.2012.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Marzouk T, Winkelbeiner S, Azizi H, Malhotra AK, Homan P. Transcranial magnetic stimulation for positive symptoms in schizophrenia: a systematic review. Neuropsychobiology. 2020;79:384–396. doi: 10.1159/000502148. [DOI] [PubMed] [Google Scholar]
- 193.Hoffman RE, et al. Transcranial magnetic stimulation of left temporoparietal cortex in three patients reporting hallucinated “voices”. Biol. Psychiatry. 1999;46:130–132. doi: 10.1016/s0006-3223(98)00358-8. [DOI] [PubMed] [Google Scholar]
- 194.Kim E-J, et al. Bilateral repetitive transcranial magnetic stimulation for auditory hallucinations in patients with schizophrenia: a randomized controlled, cross-over study. Clin. Psychopharmacol. Neurosci. 2014;12:222–228. doi: 10.9758/cpn.2014.12.3.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Dollfus S, et al. High-frequency neuronavigated rTMS in auditory verbal hallucinations: a pilot double-blind controlled study in patients with schizophrenia. Schizophr. Bull. 2018;44:505–514. doi: 10.1093/schbul/sbx127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Wagner E, et al. Repetitive transcranial magnetic stimulation (rTMS) for schizophrenia patients treated with clozapine. World J. Biol. Psychiatry. 2021;22:14–26. doi: 10.1080/15622975.2020.1733080. [DOI] [PubMed] [Google Scholar]
- 197.Jiang Y, et al. Effects of high-frequency transcranial magnetic stimulation for cognitive deficit in schizophrenia: a meta-analysis. Front. Psychiatry. 2019;10:1–11. doi: 10.3389/fpsyt.2019.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Hertrich, I., Dietrich, S., Blum, C., Ackermann, H. The role of the dorsolateral prefrontal cortex for speech and language processing. Front. Hum. Neurosci. 15, 10.3389/fnhum.2021.645209 (2021). [DOI] [PMC free article] [PubMed]
- 199.Walther S, Mittal VA, Stegmayer K, Bohlhalter S. Gesture deficits and apraxia in schizophrenia. Cortex. 2020;133:65–75. doi: 10.1016/j.cortex.2020.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Walther S, et al. Nonverbal social communication and gesture control in schizophrenia. Schizophr. Bull. 2015;41:338–345. doi: 10.1093/schbul/sbu222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Dutschke LL, et al. Gesture impairments in schizophrenia are linked to increased movement and prolonged motor planning and execution. Schizophr. Res. 2018;200:42–49. doi: 10.1016/j.schres.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 202.Gupta, T., Osborne, K. J., Mittal, V. A. Abnormal gesture perception and clinical high-risk for psychosis. Schizophr Bull. 10.1093/schbul/sbab056 (2021). [DOI] [PMC free article] [PubMed]
- 203.Li X, et al. Pragmatic ability deficit in schizophrenia and associated theory of mind and executive function. Front Psychol. 2017;8:1–11. doi: 10.3389/fpsyg.2017.02164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Parola A, et al. Pragmatics, theory of mind and executive functions in schizophrenia: disentangling the puzzle using machine learning. PLoS ONE. 2020;15:1–11. doi: 10.1371/journal.pone.0229603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Ripke S, et al. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–427. doi: 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Lam M, et al. Comparative genetic architectures of schizophrenia in East Asian and European populations. Nat. Genet. 2019;51:1670–1678. doi: 10.1038/s41588-019-0512-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Gialluisi A, et al. Genome-wide screening for DNA variants associated with reading and language traits. Genes Brain Behav. 2014;13:686–701. doi: 10.1111/gbb.12158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Reader RH, Covill LE, Nudel R, Newbury DF. Genome-wide studies of specific language impairment. Curr. Behav. Neurosci. Rep. 2014;1:242–250. doi: 10.1007/s40473-014-0024-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Smigielski L, Jagannath V, Rössler W, Walitza S, Grünblatt E. Epigenetic mechanisms in schizophrenia and other psychotic disorders: a systematic review of empirical human findings. Mol. Psychiatry. 2020;25:1718–1748. doi: 10.1038/s41380-019-0601-3. [DOI] [PubMed] [Google Scholar]
- 210.Bodkin JA, et al. Targeted treatment of individuals with psychosis carrying a copy number variant containing a genomic triplication of the glycine decarboxylase gene. Biol. Psychiatry. 2019;86:523–535. doi: 10.1016/j.biopsych.2019.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Hartwigsen G, Volz LJ. Probing rapid network reorganization of motor and language functions via neuromodulation and neuroimaging. Neuroimage. 2021;224:1–14. doi: 10.1016/j.neuroimage.2020.117449. [DOI] [PubMed] [Google Scholar]
- 212.Hartwigsen G, et al. Short-term modulation of the lesioned language network. Elife. 2020;9:1–19. doi: 10.7554/eLife.54277. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.