1. Introduction
Approximately 20-30% of adults suffer from chronic pain[19,26] and it is one of the most common reasons why patients seek medical care.[51] Results from many structural magnetic resonance imaging (sMRI) studies suggest that chronic pain is associated with alterations of brain structure. Indeed, chronic pain has been reported to impact gray matter volume and density[6-8,10,24,59,62,63,66,72,74], white matter volume[6,56,63], cortical thickness[33,34,43,49,50,60,65,103] and cortical surface area.[60,65,103] However, the heterogeneity of reported findings across chronic pain conditions, imaging features, and brain regions in individual studies makes integration and comprehensive assessment of results challenging. Neuroimaging meta-analyses that aggregate effects across diverse experiments are well-suited to quantitatively identify consistent structural brain alterations related to chronic pain.[58,95]
Prior meta-analyses of associations between chronic pain and brain structure have been limited to gray matter volume and gray matter density.[14,84,88] Furthermore, prior meta-analyses have often selectively focused on specific chronic pain conditions such as migraine [32,35], back pain [102], neuropathic pain[64] or fibromyalgia.[46,81] Those studies have found both convergent and divergent results. Specifically, prior meta-analyses have indicated decreased gray matter in a variety of cortical brain areas including several prefrontal regions[14,32,46,64,84,102], the cingulate cortex[35,46,82,84,102], the central gyrus[14,32,64,84], parahippocampal gyrus[36,82] and the cerebellum.[82] Furthermore, subcortical brain areas including the insula[14,64,84,102], thalamus[14,64,84] and amygdala[35] have been reported to have decreased gray matter in chronic pain. However, other meta-analyses have revealed gray matter increases in similar regions, including parahippocampal gyrus, medial frontal gyrus[64], thalamus[14], caudate[14], cerebellum[14,82], insula[64], anterior cingulate cortex (ACC)[64], and post-central gyrus.[14] The heterogeneity of meta-analytic findings may reflect focus on specific chronic pain conditions or the consideration of a limited set of imaging features. In addition, there have been no coordinate-based meta-analyses of white matter or cortical surface area, and only one meta-analysis has been conducted of cortical thickness (in migraine).[81] As such, there is a clear need for a comprehensive meta-analysis that spans across chronic pain conditions and integrates findings from multiple structural imaging features to identify common patterns of structural alterations across pain conditions.
Another source of heterogeneity of prior meta-analytical results may be inconsistent adherence to methodological best practices, which may have introduced bias and reduced generalizability. First, a sufficient sample of included studies (n ≥ 17) in meta-analyses on neuroimaging is necessary to have the power to detect small effects and to avoid results being driven by a small number of studies.[22] Second and relatedly, the sample size of healthy and patient groups included in individual experiments must be sufficient (n ≥ 10 per group). Notably, these two criteria have not been uniformly applied in prior work.[14,32,35,46,64,82,84,102] Third, coordinate-based meta-analyses test convergence between experiments against the null hypothesis of random spatial associations throughout the brain, where each voxel has an equal a priori chance of having an effect.[20,58,87] For this reason, it is critical that studies included in a neuroimaging meta-analysis employ a whole-brain analytical approach. The inclusion of experiments that only report region-of-interest (ROI) analyses may result in an over-representation of these regions.[20,58,87] Fourth, differing approaches to control for multiple comparisons in neuroimaging studies can introduce variability in the reported findings. In meta-analyses, this problem can be present at two levels. The inclusion of experiments that lack adequate control of type I error will inflate apparent structural differences attributed to chronic pain. Additionally, meta-analyses themselves need to rigorously control for multiple comparisons across locations in the brain. Current standards for meta-analyses recommend cluster-wise family-wise error (cFWE) correction as the gold standard.[22]
Responding to these gaps in the literature, we conducted a pre-registered, comprehensive anatomic likelihood estimate (ALE) meta-analysis of structural brain changes associated with chronic pain conditions. Rather than focusing on one disease or feature type, we evaluated a range of common imaging features derived from T1-weighted MRI (including gray matter, white matter, cortical thickness, and cortical surface area) across diverse chronic pain conditions. We reduced the potential for regional bias by requiring that all studies report the results of whole-brain analyses and employed adequate type I error control. As described below, this approach allowed us to evaluate if chronic pain is associated with convergent changes to brain structure across disorders and imaging features, in a large sample of 103 experiments.
2. Methods
We followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline[55] and field-standard guidelines for meta-analyses.[58,87] Procedures and analyses were preregistered on PROSPERO (https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=176498). Revisions that were made, such as the modification of authorship, can be seen in the document. This systematic review is the third in a series of meta-analyses[98,99] sponsored by the Analgesic, Anesthetic, and Addiction Clinical Trial Translations, Innovations, Opportunities, and Networks (ACTTION), a public-private partnership with the United States Food and Drug Administration.
2.1. Literature search and selection of studies
The current meta-analysis included all studies reporting structural differences between healthy controls and patients with chronic pain, regardless of the specific pain condition. The International Classification of Diseases (ICD-11)[96] represents a fairly major shift in pain diagnosis. Chronic pain is divided into seven subcategories.[66] In addition, the criterion for the duration from when episodic pain is defined as chronic has been reduced from six to at least three months.[96] However, a majority of the studies published to date were conducted before ICD-11 was published. As we included studies from 1999 onward in the current study, our inclusion criteria stipulated that patients had to have either a diagnosis of a pain condition considered chronic regardless of a time criterion (e.g. burning mouth syndrome, fibromyalgia) or have had a syndrome associated with pain that lasted for at least six months (e.g. headache, osteoarthritis; Table S1).
A comprehensive literature search was conducted using PubMed / MEDLINE, EMBASE, PsycINFO, Web of Science, Cochrane Library and SCOPUS to identify peer reviewed articles investigating neuroanatomical differences in chronic pain disorders. These databases were queried using the following search: (“MRI” OR “magnetic resonance imaging”) AND (“VBM” OR “voxel-based morphometry” OR “cortical thickness” OR “cortical volume” OR “cortical surface area” OR “grey matter density” OR “gray matter density”) AND (“pain” OR “neuropathy” OR “neuropathic” OR “hyperalgesia” OR “allodynia”) AND “brain”. Additionally, we screened reference lists of selected studies as well as systematic reviews and meta-analyses focusing on imaging of chronic pain. The final literature search took place on April 26, 2021 and was restricted to articles published from January 1, 1990 to April 26, 2021. Articles were limited to those published in English.
The search criteria yielded a total of 9,268 peer-reviewed published articles and 65 references from existing review articles and meta-analyses (Figure 1). After removing duplicates, we screened a total of 7,849 abstracts. Two independent reviewers (ATH, AX) determined whether the study should be selected for full-text evaluation. During abstract screening, we excluded abstracts based on the following criteria[58,87]: (1) sample size n < 10 participants per group; (2) studies of neonates, children, or adolescents; (3) healthy controls only or patients with chronic pain only; (4) neuroimaging modalities such as EEG without MRI; (5) animal only or cell or tissue only experiments; (6) postmortem studies. To avoid inappropriate exclusion, no papers reporting functional MRI data or diffusion imaging were excluded during abstract screening. This allowed us to evaluate sections of seemingly irrelevant papers that may have additionally studied structural changes. We evaluated the full-text of papers passing abstract screening to assess the following inclusion criteria [58,87]: (1) whole brain analysis (i.e. field of view not confined to a restricted region of interest); (2) analysis of structural brain features (i.e. gray matter, white matter, cortical thickness, cortical surface area; increased or decreased morphometric values); (3) results reported in stereotactic space (Talairach or Montreal Neurological Institute [MNI]) as standard coordinates (x, y, z); (4) results reported as comparisons between patients with chronic pain and healthy controls; (5) results thresholded at p < .001 uncorrected or cluster-level corrected p < .05; (6) n ≥ 10 participants per group; (7) participants aged 18 years or older. To find reproducible group differences in MRI studies, a sample size of about 20 participants is required.[18,86] This criterion was often not met, especially in older studies. To include valid studies in our ALE and at the same time to avoid excluding too many studies, we chose the criterion of at least 10 participants as a compromise. This threshold had been employed in two prior studies [98,99] and was preregistered accordingly. We included studies in which treatment or intervention was reported if relevant measurements were obtained and documented before treatment. In cases where the studies fulfilled all relevant inclusion criteria but did not report the results in the required format (e.g., coordinates missing), the corresponding authors were contacted via email and the results were included in the ALE if provided. All full-text articles from the included abstracts were reviewed by a reviewer (ATH) to determine whether they met inclusion criteria and final inclusion decisions were confirmed by at least one other independent reviewer (BL, LW, AX). In cases where reviewers disagreed or were uncertain, a senior third reviewer (TDS) evaluated the article. According to our inclusion criteria, we identified n = 98 peer-reviewed articles with case-control differences (including n = 14 null results; see Figure 1 and Table S2), jointly reporting on 5,075 participants from n = 103 experiments. Additionally, n = 15 studies fulfilling inclusion criteria without specifying exact pain duration were identified (Table S3).
Figure 1.

PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) flowchart depicting study inclusion / exclusion criteria and flowchart.
2.2. Data extraction
The coordinates and information for each experiment were manually extracted by at least one author (ATH or VS) and independently checked for accuracy by the other author (ATH or VS). In cases where more than one chronic pain patient group (e.g. chronic headache and chronic low back pain[100]) was compared separately to a healthy control group in one paper, the subgroups were defined as distinct samples. An experiment was defined as a feature specific analysis (e.g. gray matter, cortical thickness) per sample. For each included sample, the following information was extracted: the chronic pain condition being studied, the duration of condition, medication use, and sample characteristics including the sample size, sex, and age distribution of participants (Table S4). In addition, and to generate the best possible overview of the included studies, the following MRI-related information was recorded: MRI model, field strength (Tesla), head coil, software used for analysis (e.g., SPM, FreeSurfer), coordinate space (MNI or Talairach), smoothing kernel, type I error correction, and structural imaging feature (Table S5).
2.3. Anatomic likelihood estimation (ALE) meta-analysis
The ALE was performed using an algorithm that calculates probability estimates of structural changes. In this process, the clusters reported in each study are declared as 3D Gaussian probability distributions to compensate for spatial uncertainty.[21] The full width at half-maximum (FWHM) of these 3D Gaussian distributions was determined by experimental data on the variance between-participants and between-template, i.e. the MNI or Talairach space coordinates. Experiments with larger sample sizes should provide more reliable results of true effects.[21] The ALE algorithm accounts for between-participant variance by applying narrower Gaussian distributions for experiments with larger sample sizes. Further, the algorithm takes between-template differences into account by transforming Talairach into MNI coordinates.[42] Subsequently, the probabilities for all effect foci in each experiment are calculated, resulting in an individually modeled effect map for each experiment. ALE scores are generated by integrating all effect maps and describe the convergence of results at each particular location.[21] This implies that the effect map values reflect data from each of the individual experiments, while the ALE values represent data from multiple experiments. P-values of ALE scores were defined as the proportion of values resulting from a null distribution reflecting a random spatial correlation between experiments. This distribution was calculated for each meta-analysis performed. Subsequently, nonparametric p-values were thresholded according to current best practice recommendations, with a voxel-wise threshold of p < .001.[22,23]
Cluster significance at this voxel height was estimated using 10,000 Monte Carlo simulations.[20,73] To correct for multiple comparisons, we applied cFWE correction, the current gold standard correction methodology in ALE meta-analyses.[20] Clusters were considered significant if they reached a cFWE corrected significance of p < .05. Additionally, for exploratory purposes, we performed a potentially more sensitive secondary cluster correction procedure using Threshold Free Cluster Enhancement (TFCE).[85] Using TFCE, the problem of selecting a cluster threshold in topological inference methods is avoided. The correction amplifies signal regions that exhibit some spatial contiguity without relying on hard threshold-based clustering. Excursion sets across all possible thresholds are integrated, resulting in a new set of values that reflect both, cluster height and support. TFCE correction is considered more sensitive and is reported to have better false positive rate control than other correction methods, with no significant difference in hit rate between methods.[30] However, TFCE is considered an exploratory approach because there are no known publications that directly validate its use for ALE meta-analyses. All analytic code for the ALE meta-analysis is publicly available at https://github.com/PennLINC/sMRI_ChronicPain.
2.3.1. Main Analysis.
In order to assess the directionality of the overall structural changes, we performed an undirected meta-analysis including all data that reported structural changes in patients compared to controls (n (healthy controls vs patients with chronic pain) = 153). These analyses thus included data showing both an increase or a decrease in structural features in patients with chronic pain and served to capture basic structural differences between the two groups. We also performed two directed meta-analyses that included all experiments that reported either an increase (n (patients with chronic pain > healthy controls) = 56) or a decrease in patients with chronic pain compared to healthy controls (n (healthy controls > patients with chronic pain) = 97).
2.3.2. Feature-specific analyses.
In addition to our pre-registered analysis that included diverse measurements of brain structure, we also performed secondary, feature-specific analyses that were not pre-registered. Specifically, we evaluated both, undirected (healthy controls vs patients with chronic pain) and directed analyses (patients with chronic pain > / < healthy controls) of studies of gray matter (VBM) and cortical thickness. The number of white matter (n = 4) and cortical surface area (n = 3) studies was insufficient to perform feature-specific analyses.
2.3.3. Sensitivity analyses.
Our pre-registered inclusion criteria stipulated that study participants have a defined chronic pain condition or that all study participants suffer from pain for at least six months. Our literature review revealed 15 studies that did not clearly indicate whether individual patients had suffered for at least six months from a pain condition not classified per se as a chronic pain condition (mostly headache syndromes). However, those studies reported a mean duration of pain lasting at least six months across the study sample. Accordingly, while these studies were not included in our primary analyses, we conducted sensitivity analyses including these 15 studies (Table S3). Finally, we refrained from performing sub-analyses regarding specific chronic diseases within the current meta-analysis, as the only condition meeting the minimum number of (i.e., at least 17 experiments) required for ALE was headache and there have been recent ALE meta-analyses of this condition.[32,35]
3. Results
A total of 103 experiments from 84 articles reporting 96 distinct chronic pain samples, with a total of 5,075 participants (n = 3,850 female) were evaluated with the aim of finding common brain structure alteration across chronic pain conditions. The most commonly represented pain conditions was episodic headache lasting for at least six months (23% of all samples across the included studies). Back pain, chronic headache, and fibromyalgia each represented 10.42% of all samples across the included studies; (primary) trigeminal neuralgia (9.38% of all samples across the included studies) and complex regional pain syndrome (7.29% of all samples across the included studies) were also well-represented. The composition of included pain disorders can be seen in Table S1. A total of 42 samples provided information on the average duration of illness, which was 127.92 months (SD = 76.07, average range 3 months - 320.52 months). The majority of experiments evaluated gray matter (n = 75), followed by cortical thickness (n = 20), white matter (n = 4), cortical surface area (n = 3), and cortical volume (n = 1). Further details on the included experiments are included in Tables S4 and S5. All extracted coordinates can be found at https://github.com/PennLINC/sMRI_ChronicPain.
3.1. Main analysis
Our primary analysis of all studies did not reveal significant differences in brain structure between patients with chronic pain and healthy controls. For a voxel height of z > 3.09, the required cluster size to achieve cFWE correction at p < .05 was not reached in either the directed or undirected analysis (undirected k > 99; healthy controls > patients with chronic pain k = 96; patients with chronic pain > healthy controls k = 90). This finding was consistent for both the undirected comparison (healthy controls vs. patients with chronic pain) as well as the directed comparisons (healthy controls > patients with chronic pain; patients with chronic pain > healthy controls).
However, follow-up exploratory analyses using TFCE revealed several effects. Specifically, the undirected group comparison yielded four significant clusters (Table 1, Figure 2a) including the right insula (cluster 1 and 2), right inferior frontal gyrus (IFG) (cluster 2), left hippocampus (cluster 3), and left thalamus (cluster 4). Significant clusters in the directed analysis (healthy controls > patients with chronic pain; Table 2 and Figure 2b) were observed in right (cluster 1) and left insula (cluster 3), right IFG (cluster 1), ACC (cluster 2), and left superior medial gyrus (cluster 2). In contrast, the opposite directed analysis did not reveal any significant clusters (patients with chronic pain > healthy controls; Table 2).
Table 1.
Overall structural differences for the undirected group contrast, reporting results both with cFWE and TFCE cluster correction including peak voxel MNI-coordinates and cluster sizes.
| Cluster | Region | MNI | Cluster size (voxels) |
n (experiments contributed to cluster) |
cFWE | TFCE | References (experiments contributed to cluster) |
||
|---|---|---|---|---|---|---|---|---|---|
| x | y | z | |||||||
| MAIN ANALYSIS | |||||||||
| Undirected effect: healthy controls vs. patients with chronic pain | |||||||||
| Cluster 1 | r insula lobe | 38 | 16 | −2 | 142 | 18 | * | Baliki et al.[10], Baliki et al.[10], Boehme et al.[14], Fritz et al.[29], Geha et al.[30], Nair et al.[79], Niddam et al.[83], Obermann et al.[78], Pomares et al.[91], Riederer et al.[95], Rodriguez-Raecke et al.[97], Schmidt-Wilcke et al.[102], Seminowicz et al.[109], Valet et al.[124], Yoon et al.[136], Younger et al.[137], Zhang et al.[140], Zhang et al.[140] | |
| r insula lobe | 42 | 4 | 6 | ||||||
| Cluster 2 | r IFG (p. orbitalis) | 36 | 28 | −18 | 73 | 19 | * | Baliki et al.[10], Boehme et al.[14], Geha et al.[30], Gustin et al.[36], Lai et al.[53], Lai et al.[53], Messina et al.[71], Messina et al.[69], Naegel et al.[78], Nair et al. [79], Nair et al.[79], Niddam et al.[83], Obermann et al.[78], Petrusic et al.[87], Schmidt-Wilcke et al.[102], Seminowicz et al.[107], Valet et al.[124], Yoon et al.[136], Zhang et al.[140] | |
| r insula lobe | 30 | 22 | −10 | * | |||||
| Cluster 3 | l hippocampus | −24 | −16 | −14 | 56 | 11 | * | Baliki et al.[10], Baliki et al.[10], Boehme et al.[14], Fayed et al.[27], Hubbard et al.[40], Niddam et al.[83], Palm-Meinders et al.[85], Schweinhardt et al.[105], vanVelzen et al.[125], Wu et al.[131], Yu et al.[138] | |
| Cluster 4 | l thalamus | −14 | −26 | 0 | 28 | 13 | * | As-Sanie et al.[9], Gustin et al.[36], Li et al.[58], Obermann et al.[78], Palm-Meinders et al.[85], Riederer et al.[95], Schmidt-Wilcke et al.[101], Schmidt-Wilcke et al.[103], Schweinhardt et al.[105], Seminowicz et al.[107], Tsai et al.[120], Wu et al.[131], Younger et al.[137] | |
Notes. cFWE = Family-wise error correction ; TFCE = Threshold Free Cluster Enhancement; MNI = Montreal Neurological Institute stereotaxic space; l = left; r = right; IFG = inferior frontal gyrus; ACC = anterior cingulate cortex.
Figure 2.
Displayed are TFCE corrected results from the ALE main analyses of structural differences between patients with chronic pain and healthy controls. A) Group comparison of structural brain tissue (r insula, r IFG, l hippocampus and l thalamus); B) Structural deficits in patients with chronic pain (l & r insula, r IFG, l & r ACC, and l superior medial gyrus).
Note. IFG = inferior frontal gyrus; ACC = anterior cingulate cortex; l = left; r = right.
Table 2.
Overall structural differences for the directed group contrasts, reporting results both with cFWE and TFCE cluster correction including peak voxel MNI-coordinates and cluster sizes.
| Cluster | Region | MNI | Cluster size (voxels) |
n (experiments contributed to cluster) |
cFWE | TFCE | References (experiments contributed to cluster) |
||
|---|---|---|---|---|---|---|---|---|---|
| x | y | z | |||||||
| MAIN ANALYSIS | |||||||||
| Healthy controls > patients with chronic pain | |||||||||
| Cluster 1 | r insula lobe | 38 | 14 | 0 | 372 | 25 | * | Baliki et al.[10], Baliki et al.[10], Boehme et al.[14], Fritz et al.[29], Geha et al.[30], Gustin et al.[36], Ikeda et al.[41], Lai et al.[53], Lai et al.[53], Luchtmann et al.[62], Messina et al.[71], Nair et al.[79], Niddam et al.[83], Obermann et al.[78], Petrusic et al.[87], Pomares et al.[91], Riederer et al.[95], Rodriguez-Raecke et al.[97], Schmidt-Wilcke et al.[102], Seminowicz et al.[107], Seminowicz et al.[109], Valet et al.[124], Yoon et al.[136], Younger et al.[137], Zhang et al.[140] | |
| r IFG (p. orbitalis) | 36 | 30 | −18 | * | |||||
| r insula lobe | 30 | 22 | −10 | * | |||||
| r IFG (p. orbitalis) | 44 | 12 | 4 | * | |||||
| r insula lobe | 42 | 4 | 6 | * | |||||
| r insula lobe | 34 | 12 | −16 | * | |||||
| Cluster 2 | l ACC | −6 | 36 | 12 | 339 | 25 | * | Absinta et al.[2], Agostini et al.[3], Barad et al.[11], Hubbard et al.[40], Jensen et al.[43], Luchtmann et al.[62], Mordasini et al.[74], Nair et al.[79], Nan et al.[80], Obermann et al.[78], Pomares et al.[91], Riederer et al.[95], Rocca et al.[96], Rodriguez-Raecke et al.[97], Ruscheweyh et al.[99], Schmidt-Wilcke et al.[102], Schmidt-Wilcke et al.[100], Seminowicz et al.[109], Sinding et al.[113], Valet et al.[124], Wang et al.[127], Wu et al.[131], Wu et al.[131], Yu et al.[138] | |
| l ACC | −2 | 38 | 4 | * | |||||
| l ACC | −4 | 26 | 30 | * | |||||
| r ACC | 12 | 34 | 28 | * | |||||
| l superior medial gyrus | 0 | 40 | 28 | * | |||||
| r ACC | 6 | 34 | 28 | * | |||||
| Cluster 3 | l insula lobe | −38 | 18 | 0 | 137 | 17 | * | Absinta et al.[2], Baliki et al.[10], Boehme et al.[14], Gustin et al.[36], Kuchinad et al.[47], Lai et al.[52], Naegel et al.[78], Nair et al.[79], Nair et al.[79], Niddam et al.[83], Obermann et al.[78], Riederer et al.[95], Rodriguez-Raecke et al.[97], Schmidt-Wilcke et al.[102], Seminowicz et al.[109], Wang et al.[128], Yoon et al.[136] | |
| l insula lobe | −32 | 14 | −12 | * | |||||
| l insula lobe | −34 | 16 | −10 | * | |||||
| l insula lobe | −30 | 22 | 2 | * | |||||
| Patients with chronic pain > healthy controls | |||||||||
| No suprathreshold cluster | |||||||||
Notes. cFWE = Family-wise error correction ; TFCE = Threshold Free Cluster Enhancement; MNI = Montreal Neurological Institute stereotaxic space; l = left; r = right; IFG = inferior frontal gyrus; ACC = anterior cingulate cortex.
3.2. Feature-specific analysis: gray matter
Similarly, the required cluster size applying cFWE at p < .05 was not reached within secondary undirected and directed analyses of gray matter features (undirected k > 93; healthy controls > patients with chronic pain k = 94; patients with chronic pain > healthy controls k = 92). However, secondary undirected analyses using TFCE revealed multiple significant clusters (Table 3, Figure 3a), including left hippocampus and left thalamus (both cluster 1), right (cluster 2) and left insula (cluster 4), right amygdala (cluster 2) as well as right ACC (cluster 3). Directed comparisons identified several areas of lower gray matter in patients with chronic pain compared to healthy controls (Table 4, Figure 3b), including left and right ACC (cluster 1), right (cluster 2 and 3) and left insula (cluster 4), and left superior medial gyrus (both cluster 2), right IFG (cluster 3), right IFG (cluster 3), and left superior medial gyrus (cluster 1 and 5). In contrast, there were no significant clusters of greater gray matter in patients with chronic pain compared to healthy controls (patients with chronic pain > healthy controls; Table 2).
Table 3.
Gray matter differences for undirected group comparisons, reporting results both with cFWE and TFCE cluster correction including peak voxel MNI-coordinates and cluster sizes.
| Cluster | Region | MNI | Cluster size (voxels) |
n (experiments contributed to cluster) |
cFWE | TFCE | References (experiments contributed to cluster) |
||
|---|---|---|---|---|---|---|---|---|---|
| x | y | z | |||||||
| GRAY MATTER | |||||||||
| Undirected effect: healthy controls vs. patients with chronic pain | |||||||||
| Cluster 1 | l hippocampus | −24 | −16 | −14 | 323 | 21 | * | As-Sanie et al.[9], Baliki et al.[10], Baliki et al.[10], Boehme et al.[14], Fayed et al.[27], Gustin et al.[36], Hubbard et al.[40], Li et al.[58], Niddam et al.[83], Obermann et al.[78], Palm-Meinders et al.[85], Riederer et al.[95], Schmidt-Wilcke et al.[101], Schmidt-Wilcke et al.[103], Schweinhardt et al.[105], Seminowicz et al.[107], Tsai et al.[120], vanVelzen et al.[125], Wu et al.[131], Younger et al.[137], Yu et al.[138] | |
| l thalamus | −14 | −26 | 0 | * | |||||
| Cluster 2 | r insula lobe | 30 | 22 | −10 | 150 | 17 | * | As-Sanie et al.[9], Baliki et al.[10], Boehme et al.[14], Geha et al.[30], Gustin et al.[36], Ikeda et al.[41], Messina et al.[69], Muthulingam et al.[77], Naegel et al.[78], Neeb et al.[81], Niddam et al.[83], Obermann et al.[78], Rodriguez-Raecke et al.[97], Schmidt-Wilcke et al.[102], Seminowicz et al.[107], Valet et al.[124], Yoon et al.[136] | |
| r amygdala | 32 | −2 | −14 | * | |||||
| r Insula Lobe | 34 | 12 | −16 | * | |||||
| Cluster 3 | r ACC | 8 | 18 | −12 | 29 | 8 | * | Boehme et al.[14], Geha et al.[30], Gustin et al.[36], Messina et al.[70], Riederer et al.[95], Tsai et al.[120], Wu et al.[131], Yoon et al.[136] | |
| Cluster 4 | l Insula Lobe | −38 | 18 | 0 | 23 | 11 | * | Absinta et al.[2], Baliki et al.[10], Boehme et al.[14], Gustin et al.[36], Kuchinad et al.[47], Niddam et al.[83], Riederer et al.[95], Rodriguez-Raecke et al.[97], Schmidt-Wilcke et al.[102], Wang et al.[127], Yoon et al.[136] | |
Notes. cFWE = Family-wise error correction ; TFCE = Threshold Free Cluster Enhancement; MNI = Montreal Neurological Institute stereotaxic space; l = left; r = right; IFG = inferior frontal gyrus; ACC = anterior cingulate cortex.
Figure 3.
Displayed are TFCE corrected results from the ALE feature-specific analysis of gray matter change. A) undirected effect (l hippocampus, l thalamus, l & r insula lobe, r amygdala, r olfactory cortex); B) Gray matter deficits in patients with chronic pain (l & r insula, r IFG, l & r ACC, l superior medial gyrus).
Note. r = right; l = left; IFG = inferior frontal gyrus; ACC = anterior cingulate cortex.
Table 4.
Gray matter differences for the directed group contrasts, reporting results both with cFWE and TFCE cluster correction including peak voxel MNI-coordinates and cluster sizes.
| Cluster | Region | MNI | Cluster size (voxels) |
n (experiments contributed to cluster) |
cFWE | TFCE | References (experiments contributed to cluster) |
||
|---|---|---|---|---|---|---|---|---|---|
| x | y | z | |||||||
| GRAY MATTER | |||||||||
| Healthy controls > patients with chronic pain | |||||||||
| Cluster 1 | l ACC | −4 | 26 | 30 | 144 | 13 | * | Absinta et al.[2], Agostini et al.[3], Mordasini et al.[74], Nan et al.[80], Obermann et al.[78], Pomares et al.[91], Riederer et al.[95], Rocca et al.[96], Rodriguez-Raecke et al.[97], Ruscheweyh et al.[99], Schmidt-Wilcke et al.[102], Schmidt-Wilcke et al.[100], Valet et al.[124] | |
| r ACC | 12 | 34 | 28 | * | |||||
| l superior medial gyrus | 0 | 40 | 28 | * | |||||
| r ACC | 6 | 34 | 28 | * | |||||
| l ACC | −4 | 40 | 16 | * | |||||
| l ACC | −6 | 40 | 22 | * | |||||
| Cluster 2 | r insula lobe | 30 | 20 | −10 | 116 | 14 | * | Baliki et al[10], Boehme et al.[14], Geha et al.[30], Gustin et al.[36], Ikeda et al.[41], Luchtmann et al.[62], Muthulingam et al.[77], Niddam et al. [83], Obermann et al.[78], Rodriguez-Raecke et al.[97], Schmidt-Wilcke et al.[102], Seminowicz et al.[107], Valet et al.[124], Yoon et al.[136] | |
| r insula lobe | 34 | 12 | −16 | * | |||||
| Cluster 3 | r insula lobe | 44 | 2 | 6 | 62 | 12 | * | Absinta et al.[2], Baliki et al.[10], Baliki et al.[10], Boehme et al.[14], Fritz et al.[29], Ivo et al.[42], Pomares et al.[91], Riederer et al.[95], Rodriguez-Raecke et al.[97], Schmidt-Wilcke et al.[102], Valet et al.[124], Younger et al.[137] | |
| r IFG (p. opercularis) | 46 | 12 | 4 | * | |||||
| r insula lobe | 40 | 14 | −2 | * | |||||
| Cluster 4 | l insula lobe | −38 | 18 | 0 | 26 | 11 | * | Absinta et al.[2], Baliki et al.[10], Boehme et al.[14], Gustin et al.[36], Kuchinad et al.[47], Niddam et al.[83], Riederer et al.[95], Rodriguez-Raecke et al.[97], Schmidt-Wilcke et al.[102], Wang et al.[127], Yoon et al.[136] | |
| Cluster 5 | l superior medial gyrus | −4 | 62 | 12 | 20 | 10 | * | Ceko et al.[19], Fritz et al.[29], Kuchinad et al.[47], Lai et al.[53], Pomares et al.[91], Rodriguez-Raecke et al.[97], Seminowicz et al.[107], Valet et al.[124] | |
| Patients with chronic pain > healthy controls | |||||||||
| No suprathreshold cluster | |||||||||
Notes. cFWE = Family-wise error correction ; TFCE = Threshold Free Cluster Enhancement; MNI = Montreal Neurological Institute stereotaxic space; l = left; r = right; IFG = inferior frontal gyrus; ACC = anterior cingulate cortex.
3.3. Feature-specific analysis: cortical thickness
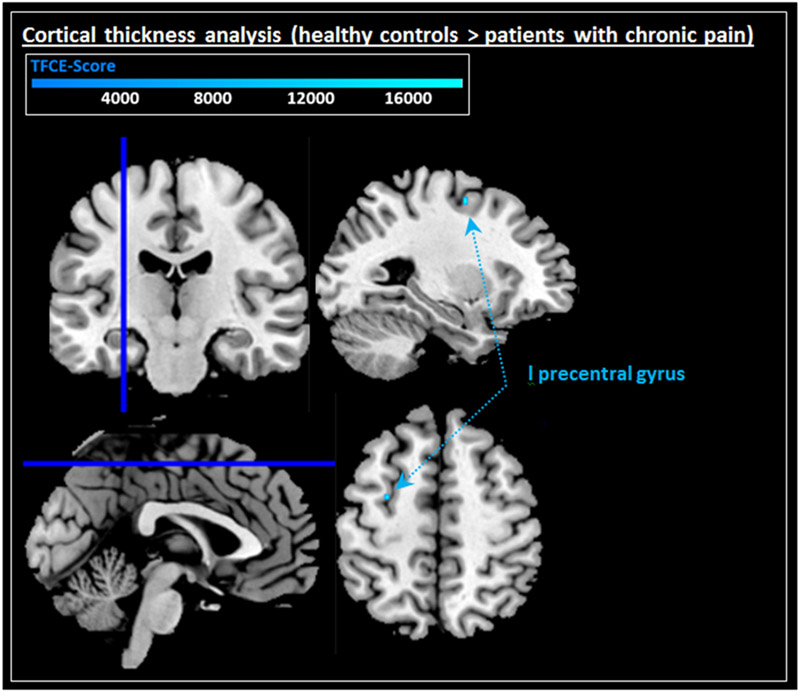
Specific analyses of studies examining cortical thickness did not reveal any significant effects for either the undirected comparison (healthy controls vs. patients with chronic pain) or the directed comparisons (undirected k > 91; healthy controls > patients with chronic pain; k = 87; patients with chronic pain > healthy controls k = 70; Table 5). Similarly, undirected secondary analyses using TFCE did not reveal significant effects. However, secondary directed analyses (healthy controls > patients with chronic pain) using TFCE discovered reduced cortical thickness within the left precentral gyrus in patients with chronic pain (cluster 1; Figure 4 and Table 5). In contrast, no increases in cortical thickness were seen in patients with chronic pain when TFCE was used (patients with chronic pain > healthy controls).
Table 5.
Cortical thickness differences resulting from the ALE feature-specific meta-analysis, reporting results both with cFWE and TFCE cluster correction including peak voxel MNI-coordinates and cluster sizes.
| Cluster | Region | MNI | Cluster size (voxels) |
n (experiments contributed to cluster) |
cFWE | TFCE | References (experiments contributed to cluster) |
||
|---|---|---|---|---|---|---|---|---|---|
| x | y | z | |||||||
| CORTICAL THICKNESS | |||||||||
| Undirected effect: healthy controls vs. patients with chronic pain | |||||||||
| No suprathreshold cluster | |||||||||
| Healthy controls > patients with chronic pain | |||||||||
| Cluster 1 | l precentral gyrus | −28 | −4 | 54 | 2 | NA | * | NA | |
| Patients with chronic pain > healthy controls | |||||||||
| No suprathreshold cluster | |||||||||
Notes. cFWE = Family-wise error correction ; TFCE = Threshold Free Cluster Enhancement; MNI = Montreal Neurological Institute stereotaxic space; l = left.
Figure 4.
Displayed are TFCE corrected significant results from the feature-specific analysis of cortical thickness changes: cortical thickness deficits in patients with chronic pain (l precentral gyrus).
Note. l = left.
3.4. Sensitivity analyses
As noted above, 15 studies did not explicitly specify inclusion criteria regarding a duration of greater than six months of chronic pain and were thus not included in the main analyses. However, in order to ensure that these studies did not influence our observed results, we re-analyzed the data with these studies included. Notably, inclusion of these studies did not substantially impact results; while specific clusters similarly survived TFCE, no significant results were observed for our primary analysis using cFWE (Table S6) or specific analyses of gray matter (Table S7) or cortical thickness (Table S8).
4. Discussion
This comprehensive, pre-registered ALE-based meta-analysis of chronic pain examined structural brain alterations across imaging features derived from T1-weighted MRI (including gray matter, white matter, cortical thickness, and cortical surface area). Consequently, the analyses of the current study investigated whether there are consistent patterns of structural alterations associated with chronic pain that span across conditions and structural imaging metrics. Our results were based on a total of 103 (main analysis), 75 (gray matter), and 20 (cortical thickness) neuroimaging experiments. Consequently, these were well-powered ALE meta-analyses, which included considerably more studies than previous analyses.[14,84,88] We did not detect any significant structural brain changes associated with chronic pain conditions across or within specific structural imaging features when using cFWE correction. However, in a subsequent analysis using TFCE correction, we detected several clusters of alterations (including both gray matter and cortical thickness) in chronic pain. These subtle structural differences largely overlapped with previously identified pain networks.[17,38] The failure to achieve significance using the more conservative cFWE correction suggests that chronic pain may be associated with subtle, spatially diffuse alterations of brain structure.
When multiple comparisons were corrected with cFWE, we found null results when looking both within and across structural imaging features. In contrast to the current findings, previous meta-analyses have identified convergent alterations in several cortical and subcortical brain regions associated with chronic pain. The majority of results indicated lower gray matter in patients with chronic pain compared to healthy controls within the insula, thalamus, ACC, middle frontal gyrus, pre- and post-central gyri, IFG and SFG.[14,32,35,52] However, other studies have reported higher gray matter in similar areas such as thalamus, pre- and post-central gyri, inferior parietal lobule, and hippocampus.[14,84]
In contrast to these cFWE corrected null results, we observed several clusters associated with chronic pain as part of our exploratory analysis using TFCE.[17,38] The ACC, insula, IFG, superior medial gyrus, thalamus, and hippocampus all showed structural reductions in patients with chronic pain in both our main and gray matter-specific analyses. The positive TFCE results are somewhat surprising, as TFCE has been previously reported to reduce the false alarm rate while achieving an equivalent hit rate to cFWE.[30] Therefore, we had expected TFCE to yield similar results to cFWE. However, on closer inspection, the regions identified by TFCE only narrowly missed the cluster defining initial threshold for cFWE. This reflected an unique data structure in which we observed larger, spatially diffuse clusters with a flat effect size (e.g. plateau rather than a peak; see schematic in Figure 5). These clusters were just below the significance threshold for cFWE. It should be noted that TFCE has only recently begun to be used in neuroimaging meta-analyses.[25,45,67,68] Ongoing work comparing the statistical properties of TFCE and cFWE in meta-analyses may provide useful data on the relative strengths and weaknesses of the two approaches.
Figure 5.
A) Schematic representation of a plateau versus focal cluster and the influence on significant results. B) Significant TFCE cluster from main analysis (undirected) showing the distribution of Z-values ranging from Z = 1.96 (p < .05) till Z =3.09 (p < .001).
Although the clusters detected using TFCE were not observed when applying our pre-registered correction using cFWE, they may still provide useful information regarding structural changes associated with chronic pain. Specifically, results based on TFCE suggested that chronic pain is associated with subtle effects on the structure of the ACC, insula, and IFG. Together with the insula, the ACC is part of the salience network[78] and is essential for pain processing.[54] In this context, it is postulated that the insula transmits exteroceptive and interoceptive information to consciousness[16,40,41] particularly through functional connections to the cingulate cortex[89], and elicits feelings in relation to this salient information. The salience network contributes to a variety of complex functions, including self-awareness, through the integration of sensory, emotional, and cognitive information. Chronic pain may disrupt the synchronization of this mechanism by affecting a number of brain regions involved in self-observation, pain and emotion processing.[4,14,38,44,57] In addition, the IFG is thought to be involved in pain catastrophizing[27,76], and pain perception.[29] A decrease in pain catastrophizing after cognitive behavioral therapy was associated with an increase in gray matter in the IFG and ACC.[80]
We also observed clusters that survived correction with TFCE in emotional-affective and modulatory components of the pain network[11], including amygdala, (hypo)thalamus and (para)hippocampus. The amygdala plays a relevant role in cognitive control over affective pain components.[13,15,61,90] Within multidimensional pain perception the amygdala is thought to serve as a mediator between chronic pain or acute pain and affect, cognition, as well as body sensation.[61,83] In doing so, it integrates polymodal information from the thalamus and cingulate cortex with nociception-specific information from the spinal cord and brainstem. The thalamus processes and relays nociceptive information between the spinal cord and cortex. [1,101] While the hypothalamus is involved in neuroendocrine and autonomic pain processing, the thalamus and ACC are essential for conscious, cognitive processing of pain. Additionally, the parahippocampal cortex modulates pain experience and pain sensitivity[28] and specifically associated anxiety, fear, and aversive pain content.[48] Reduced gray matter density in the parahippocampal cortex has been reported with longer pain syndrome persistence in patients with pain disorder.[94] The emotional interplay between learning and memory, primary functions of the hippocampus[9], is considered an important factor in the transition from acute to chronic pain.[3,93] The hippocampus is furthermore thought to be a sex-dependent biomarker for chronic pain[70], given its hormone-dependent sex-specific responses to stress.[31,53,71]
It is important to note that structural changes can occur at different anatomical and temporal levels (for a comprehensive overview see Kuner[39]). Structural brain changes in the context of chronic pain may be induced by nociceptive activity leading to changes in the density of synaptic spines, the degeneration or regeneration of axons, degeneration of neurons, and the proliferation of astrocytes and microglia which may modulate nociceptive processing.[39] It should be noted that reported associations from imaging studies of structural brain changes in chronic pain do not allow us to disentangle whether structural changes are the consequence or the cause of chronic pain. However, one informative study by Rodriguez-Raecke et al.[72] reported that in patients with primary hip osteoarthritis, decreased gray matter did not result in chronic pain but rather was a reversible consequence of chronic nociceptive transmission that normalizes after appropriate treatment. Moreover, the duration and severity of pain may have a crucial impact on the extent of change in brain anatomy.[79]
Although the TFCE corrected results were in line with previous study results, it is important to consider that cFWE correction remains the gold standard for ALE meta-analyses.[22] Methodological differences in terms of both inclusion criteria and meta-analytic techniques may have led our cFWE corrected findings to differ from those of prior meta-analyses. First and most importantly, the current study considered a broad range of chronic pain conditions rather than focusing on a single condition. It should be noted that the inclusion criteria for chronic pain was fairly stringent, requiring study participants to have a diagnosis of a chronic pain disorder or have chronic pain for at least six months. However, sensitivity analyses that included studies not fully meeting this definition of chronic pain indicated that this criterion did not drive our observed results. Second, guidelines for best practices in neuroimaging meta-analyses[58] emphasize that experiments included in ALE should be based on whole brain analyses. Notably, meta-analyses that included ROI analyses may have be biased towards these predefined regions.[20,58,87]
Third, our approach was consistent with other contemporary recommendations for neuroimaging meta-analyses, including the requirement that component experiments be of sufficient size and that appropriate type I error control be used. These criteria, as well as the use of cFWE type I error correction to control for multiple comparisons in the meta-analysis itself, may partially explain the divergence of results in prior meta-analyses. The observed null results with our primary cFWE analysis suggest that the evidence is less consistent than previous research may have suggested. In particular, our results did not support strong meta-analytic evidence for substantial structural changes associated with chronic pain across conditions.
Differences between our results and prior findings may also be due to the diversity of chronic pain conditions evaluated. While this meta-analysis provides a broad synthesis of chronic pain, it is possible that the brain structures impacted may differ depending on the chronic pain condition. Baliki et al.[7] found that chronic low back pain, knee osteoarthritis, and complex regional pain syndrome are associated with distinct gray matter changes that may reflect unique maladaptive physiology for various chronic pain conditions. The multidimensional construct of pain can be categorized differently depending on the categorization system (for example, DSM or ICD) or edition (for example, ICD10[97] and ICD11[96]). For instance pain can be divided into nociceptive pain, caused by inflamed or damaged tissue, and neuropathic pain, caused by damage or lesions of the nervous system. The specific type of pain may differentially affect associations with brain structure. For example, one study reported that patients with neuropathic back pain were more affected than non-neuropathic patients.[5] Further, the somatotopic representations of chronic pain also vary across conditions (from the head to the back and abdomen to the limbs). It is possible that structural changes associated with chronic pain are organized according to the somatotopic representation, leading to diffuse results across patients and studies.
Heterogeneity driven by differences regarding sample demographics, including gender[2,91], age[2] or medication status[12], may have further contributed to inconsistent results across experiments. Generally, a higher prevalence of chronic pain conditions can be found among women and elderly people.[91] While endometriosis or pelvic pain syndrome are female specific pain disorders, the prevalence for other diseases such as migraine[47] or fibromyalgia[69] are skewed with a female-to-male ratio of 3:1. Furthermore, medication, such as opioids, nonsteroidal anti-inflammatory drugs, antiepileptic drugs, or antidepressants, can alter patients' brain function[12,37] and structure.[37,77] However, the large heterogeneity in medication usage and inclusion criteria across studies make it difficult to evaluate and control for within an ALE meta-analysis.
Limitations
Several limitations of our study should be noted. It is important to note that associations of structural changes and chronic pain do not allow us to disentangle whether any observed structural alterations are the consequence or the cause of chronic pain. Additionally, due to heterogeneity of the studies included, we could not analyze the influence of medication status or comorbidities. Furthermore, it should be noted that although ALE is field-standard, it has several important limitations. First, the data used in ALE is based only on the coordinates of the reported peaks, rather than complete maps, resulting in the inability of the algorithm to take into account relative effect sizes of corresponding results. We would therefore note that an image-based meta-analysis combining whole-brain statistical data, rather than using exclusively significant peak coordinates, could offer important advantages.[75] Second, the method does not allow covariates to be controlled for within the analysis, thereby limiting the algorithm’s ability to detect the influence of potential confounders. However, considering the large variability in how potentially interesting covariates such as age or disease duration were reported, inclusion of these covariates would be difficult even independent of the ALE algorithm. Furthermore, previous studies suggested that it is unlikely that an individual study could significantly bias the results of ALE meta-analyses [20,21,92] Third, a known limitation of ALE is the inability to include experiments without significant results. This limitation biases meta-analyses toward significant results, but also strengthens confidence in the reported null cFWE results of the current pre-registered analyses.
Conclusion
This comprehensive meta-analysis quantitatively synthesized structural MRI studies of chronic pain while adhering to best practices for neuroimaging meta-analyses. Our pre-registered approach did not find evidence of significant differences in brain structure between patients with chronic pain and healthy controls. However, exploratory secondary analyses suggested that limited structural deficits may be present in patients with chronic pain. Overall, our results suggest that if alterations in brain structure are present in chronic pain, they are likely subtle and spatially diffuse.
Supplementary Material
Acknowledgments
This study was funded by the Analgesic, Anesthetic, and Addiction Clinical Trial Translations, Innovations, Opportunities, and Networks (ACTTION) public-private partnership with the United States Food and Drug Administration. This work was supported by the International Research Training Group (IRTG2150) “The neuroscience of Modulating Aggression and Impulsivity in Psychopathology” of the German Research Foundation (DFG – Project number 269953372/GRK2150). Additional support was provided by NIH R01-MH074457, NIH R00-DA043609, NIH DP2-GM140923, DFG HA 3202/7-4, DFG Jo 1453/1-1 441023175, DFG HA 3202/7-4, DFG HA 3202/26-1, DLR VSF1_2021-076, EFRE.NRW, Apic P4, BMBF and R35 NS097306.
Footnotes
Conflict of interest declaration
Robert H. Dworkin, PhD, has received in the past 5 years research grants and contracts from the US Food and Drug Administration and the US National Institutes of Health, and compensation for serving on advisory boards or consulting on clinical trial methods from Abide, Acadia, Adynxx, Analgesic Solutions, Aptinyx, Aquinox, Asahi Kasei, Astellas, Biogen, Biohaven, Boston Scientific, Braeburn, Cardialen, Celgene, Centrexion, Chiesi, Chromocell, Clexio, Collegium, Concert, Confo, Decibel, Editas, Eli Lilly, Endo, Ethismos (equity), Eupraxia, Exicure, Glenmark, Gloriana, Grace, Hope, Lotus, Mainstay, Merck, Mind Medicine (also equity), Neumentum, Neurana, NeuroBo, Novaremed, Novartis, OliPass, Pfizer, Q-State, Reckitt Benckiser, Regenacy (also equity), Sangamo, Sanifit, Scilex, Semnur, SIMR Biotech, Sinfonia, SK Biopharmaceuticals, Sollis, SPRIM, Teva, Theranexus, Toray, Vertex, Vizuri, and WCG.
References
- [1].Ab Aziz CB, Ahmad AH. The role of the thalamus in modulating pain. Malaysian J Med Sci 2006;13:11–18. [PMC free article] [PubMed] [Google Scholar]
- [2].Aloisi AM, Bonifazi M. Sex hormones, central nervous system and pain. Horm Behav 2006;50:1–7. [DOI] [PubMed] [Google Scholar]
- [3].Apkarian AV. Pain perception in relation to emotional learning. Curr Opin Neurobiol 2008;18:464–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain 2005;9:463. [DOI] [PubMed] [Google Scholar]
- [5].Apkarian AV, Sosa Y, Sonty S, Levy RM, Harden RN, Parrish TB, Gitelman DR. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J Neurosci 2004;24:10410–10415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Arkink EB, Schmitz N, Schoonman GG, Van Vliet JA, Haan J, Van Buchem MA, Ferrari MD, Kruit MC. The anterior hypothalamus in cluster headache. Cephalalgia 2017;37:1039–1050. [DOI] [PubMed] [Google Scholar]
- [7].Baliki MN, Schnitzer TJ, Bauer WR, Apkarian AV. Brain morphological signatures for chronic pain. PLoS One 2011;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Barad MJ, Ueno T, Younger J, Chatterjee N, Mackey S. Complex regional pain syndrome is associated with structural abnormalities in pain-related regions of the human brain. J Pain 2014;15:197–203. doi: 10.1016/j.jpain.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Barkus C, McHugh SB, Sprengel R, Seeburg PH, Rawlins JNP, Bannerman DM. Hippocampal NMDA receptors and anxiety: At the interface between cognition and emotion. Eur J Pharmacol 2010;626:49–56. doi: 10.1016/j.ejphar.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Bishop JH, Shpaner M, Kubicki A, Clements S, Watts R, Naylor MR. Structural network differences in chronic muskuloskeletal pain: Beyond fractional anisotropy. Neuroimage 2018;182:441–455. [DOI] [PubMed] [Google Scholar]
- [11].Borsook D, Becerra L. Phenotyping central nervous system circuitry in chronic pain using functional MRI: Considerations and potential implications in the clinic. Curr Pain Headache Rep 2007;11:201–207. [DOI] [PubMed] [Google Scholar]
- [12].Borsook D, Becerra LR. Breaking down the barriers: fMRI applications in pain, analgesia and analgesics. Mol Pain 2006;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bushnell MC, Čeko M, Low LA. Cognitive and emotional control of pain and its disruption in chronic pain. Nat Rev Neurosci 2013;14:502–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Cauda F, Palermo S, Costa T, Torta R, Duca S, Vercelli U, Geminiani G, Torta DME. Gray matter alterations in chronic pain: A network-oriented meta-analytic approach. NeuroImage Clin 2014;4:676–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Costafreda SG, Brammer MJ, David AS, Fu CHY. Predictors of amygdala activation during the processing of emotional stimuli: A meta-analysis of 385 PET and fMRI studies. Brain Res Rev 2008;58:57–70. [DOI] [PubMed] [Google Scholar]
- [16].Craig AD, Craig AD. How do you feel--now? The anterior insula and human awareness. Nat Rev Neurosci 2009;10:2009. [DOI] [PubMed] [Google Scholar]
- [17].Davis KD, Flor H, Greely HT, Iannetti GD, MacKey S, Ploner M, Pustilnik A, Tracey I, Treede RD, Wager TD. Brain imaging tests for chronic pain: Medical, legal and ethical issues and recommendations. Nat Rev Neurol 2017;13:624–638. doi: 10.1038/nrneurol.2017.122. [DOI] [PubMed] [Google Scholar]
- [18].Desmond JE, Glover GH. Estimating sample size in functional MRI (fMRI) neuroimaging studies: Statistical power analyses. J Neurosci Methods 2002;118:115–128. [DOI] [PubMed] [Google Scholar]
- [19].Dueñas M, Ojeda B, Salazar A, Mico JA, Failde I. A review of chronic pain impact on patients, their social environment and the health care system. J Pain Res 2016;9:457–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Eickhoff SB, Bzdok D, Laird AR, Kurth F, Fox PT. Activation likelihood estimation meta-analysis revisited. Neuroimage 2012;59:2349–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: A random-effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp 2009;30:2907–2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Eickhoff SB, Nichols TE, Laird AR, Hoffstaedter F, Amunts K, Fox PT, Bzdok D, Eickhoff CR. Behavior, sensitivity, and power of activation likelihood estimation characterized by massive empirical simulation. Neuroimage 2016;137:70–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Flandin G, Friston KJ. Analysis of family-wise error rates in statistical parametric mapping using random field theory. Hum Brain Mapp 2019;40:2052–2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Fritz HC, McAuley JH, Wittfeld K, Hegenscheid K, Schmidt CO, Langner S, Lotze M. Chronic Back Pain Is Associated with Decreased Prefrontal and Anterior Insular Gray Matter: Results from a Population-Based Cohort Study. J Pain 2016;17:111–118. [DOI] [PubMed] [Google Scholar]
- [25].Giehl K, Tahmasian M, Eickhoff SB, van Eimeren T. Imaging executive functions in Parkinson’s disease: An activation likelihood estimation meta-analysis. Park Relat Disord 2019;63:137–142. doi: 10.1016/j.parkreldis.2019.02.015. [DOI] [PubMed] [Google Scholar]
- [26].Goldberg DS, McGee SJ. Pain as a global public health priority. BMC Public Health 2011;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Gracely RH, Geisser ME, Giesecke T, Grant MAB, Petzke F, Williams DA, Clauw DJ. Pain catastrophizing and neural responses to pain among persons with fibromyalgia. Brain 2004;127:835–843. [DOI] [PubMed] [Google Scholar]
- [28].Grant JA, Courtemanche J, Duerden EG, Duncan GH, Rainville P. Cortical Thickness and Pain Sensitivity in Zen Meditators. Emotion 2010;10:43–53. [DOI] [PubMed] [Google Scholar]
- [29].Gu X, Han S. Attention and reality constraints on the neural processes of empathy for pain. Neuroimage 2007;36:256–267. [DOI] [PubMed] [Google Scholar]
- [30].Han H, Glenn AL, Dawson KJ. Evaluating alternative correction methods for multiple comparison in functional neuroimaging research. Brain Sci 2019;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hillerer KM, Slattery DA, Pletzer B. Neurobiological mechanisms underlying sex-related differences in stress-related disorders: Effects of neuroactive steroids on the hippocampus. Front Neuroendocrinol 2019;55:100796. doi: 10.1016/j.yfrne.2019.100796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Hu W, Guo J, Chen N, Guo J, He L. A meta-analysis of voxel-based morphometric studies on migraine. 2015. Available: www.ijcem.com/. [PMC free article] [PubMed] [Google Scholar]
- [33].Hubbard CS, Khan SA, Keaser ML, Seminowicz DA, Mathur VA, Goyal M. Altered brain structure and function correlate with disease severity and pain catastrophizing in migraine patients. eNeuro 2014;1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Jensen KB, Srinivasan P, Spaeth R, Tan Y, Kosek E, Petzke F, Carville S, Fransson P, Marcus H, Williams SCR, Choy E, Vitton O, Gracely R, Ingvar M, Kong J. Overlapping structural and functional brain changes in patients with long-term exposure to fibromyalgia pain. Arthritis Rheum 2013;65:3293–3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Jia Z, Yu S. Grey matter alterations in migraine: A systematic review and meta-analysis. NeuroImage Clin 2017;14:130–140. doi: 10.1016/j.nicl.2017.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Jia Z, Yu S. Grey matter alterations in migraine: A systematic review and meta-analysis. NeuroImage Clin 2017;14:130–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Kaindl AM, Asimiadou S, Manthey D, Hagen MVD., Turski L, Ikonomidou C. Antiepileptic drugs and the developing brain. Cell Mol Life Sci 2006;63:399–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kucyi A, Davis KD. The dynamic pain connectome. Trends Neurosci 2015;38:86–95. doi: 10.1016/j.tins.2014.11.006. [DOI] [PubMed] [Google Scholar]
- [39].Kuner R Central mechanisms of pathological pain. Nat Med 2010;16:1258–1266. doi: 10.1038/nm.2231. [DOI] [PubMed] [Google Scholar]
- [40].Kurth F, Eickhoff SB, Schleicher A, Hoemke L, Zilles K, Amunts K. Cytoarchitecture and probabilistic maps of the human posterior insular cortex. Cereb Cortex 2010;20:1448–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Kurth F, Zilles K, Fox PT, Laird AR, Eickhoff SB. A link between the systems: functional differentiation and integration within the human insula revealed by meta-analysis. Brain Struct Funct 2010;214:519–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Lancaster JL, Tordesillas-Gutiérrez D, Martinez M, Salinas F, Evans A, Zilles K, Mazziotta JC, Fox PT. Bias between MNI and talairach coordinates analyzed using the ICBM-152 brain template. Hum Brain Mapp 2007;28:1194–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Lee DH, Lee KJ, Cho KIK, Noh EC, Jang JH, Kim YC, Kang DH. Brain alterations and neurocognitive dysfunction in patients with complex regional pain syndrome. J Pain 2015;16:580–586. doi: 10.1016/j.jpain.2015.03.006. [DOI] [PubMed] [Google Scholar]
- [44].Legrain V, Iannetti GD, Plaghki L, Mouraux A. The pain matrix reloaded: A salience detection system for the body. Prog Neurobiol 2011;93:111–124. [DOI] [PubMed] [Google Scholar]
- [45].Lenzen LM, Donges MR, Eickhoff SB, Poeppl TB. Exploring the neural correlates of (altered) moral cognition in psychopaths. Behav Sci Law 2021;39:731–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Lin C, Lee SH, Weng HH. Gray Matter Atrophy within the Default Mode Network of Fibromyalgia: A Meta-Analysis of Voxel-Based Morphometry Studies. Biomed Res Int 2016;2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Lipton RB, Bigal ME, Diamond M, Freitag F, Reed ML, Stewart WF. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology 2007;68:343–349. [DOI] [PubMed] [Google Scholar]
- [48].Liu MG, Chen J. Roles of the hippocampal formation in pain information processing. Neurosci Bull 2009;25:237–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Magon S, May A, Stankewitz A, Goadsby PJ, Schankin C, Ashina M, Amin FM, Seifert CL, Mallar Chakravarty M, Müller J, Sprenger T. Cortical abnormalities in episodic migraine: A multi-center 3T MRI study. Cephalalgia 2019;39:665–673. [DOI] [PubMed] [Google Scholar]
- [50].Magon S, Sprenger T, Otti A, Papadopoulou A, Gündel H, Noll-Hussong M. Cortical Thickness Alterations in Chronic Pain Disorder: An Exploratory MRI Study. Psychosom Med 2018;80:592–598. [DOI] [PubMed] [Google Scholar]
- [51].Mäntyselkä P, Kumpusalo E, Ahonen R, Kumpusalo A, Kauhanen J, Viinamäki H, Halonen P, Takala J. Pain as a reason to visit the doctor: a study in Finnish primary health care. Pain 2001;89:175–180. Available: www.elsevier.nl/locate/pain. [DOI] [PubMed] [Google Scholar]
- [52].May A. Chronic pain may change the structure of the brain. Pain 2008;137:7–15. [DOI] [PubMed] [Google Scholar]
- [53].McEwen BS, Akil H. Revisiting the stress concept: Implications for affective disorders. J Neurosci 2020;40:12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct 2010;214:655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Moher D, Liberati A, Tetzlaff J, Altman DG. Reprint—Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Phys Ther 2009;89:873–880. [PubMed] [Google Scholar]
- [56].Mole TB, Maciver K, Sluming V, Ridgway GR, Nurmikko TJ. Specific brain morphometric changes in spinal cord injury with and without neuropathic pain. NeuroImage Clin 2014;5:28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Mouraux A, Diukova A, Lee MC, Wise RG, Iannetti GD. A multisensory investigation of the functional significance of the “pain matrix.” Neuroimage 2011;54:2237–2249. doi: 10.1016/j.neuroimage.2010.09.084. [DOI] [PubMed] [Google Scholar]
- [58].Müller VI, Cieslik EC, Laird AR, Fox PT, Radua J, Mataix-Cols D, Tench CR, Yarkoni T, Nichols TE, Turkeltaub PE, Wager TD, Eickhoff SB. Ten simple rules for neuroimaging meta-analysis. Neurosci Biobehav Rev 2018;84:151–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Naegel S, Holle D, Desmarattes N, Theysohn N, Diener HC, Katsarava Z, Obermann M. Cortical plasticity in episodic and chronic cluster headache. NeuroImage Clin 2014;6:415–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Nair VA, Beniwal-Patel P, Mbah I, Young BM, Prabhakaran V, Saha S. Structural imaging changes and behavioral correlates in patients with Crohn’s disease in remission. Front Hum Neurosci 2016;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Neugebauer V, Li W, Bird GC, Han JS. The amygdala and persistent pain. Neuroscientist 2004;10:221–234. [DOI] [PubMed] [Google Scholar]
- [62].Obermann M, Rodriguez-Raecke R, Naegel S, Holle D, Mueller D, Yoon MS, Theysohn N, Blex S, Diener HC, Katsarava Z. Gray matter volume reduction reflects chronic pain in trigeminal neuralgia. Neuroimage 2013;74:352–358. [DOI] [PubMed] [Google Scholar]
- [63].Palm-Meinders IH, Arkink EB, Koppen H, Amlal S, Terwindt GM, Launer LJ, Van Buchem MA, Ferrari MD, Kruit MC. Volumetric brain changes in migraineurs from the general population. Neurology 2017;89:2066–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Pan PL, Zhong JG, Shang HF, Zhu YL, Xiao PR, Dai ZY, Shi HC. Quantitative meta-analysis of grey matter anomalies in neuropathic pain. Eur J Pain (United Kingdom) 2015;19:1224–1231. [DOI] [PubMed] [Google Scholar]
- [65].Petrusic I, Dakovic M, Kacar K, Zidverc-Trajkovic J. Migraine with aura: Surface-based analysis of the cerebral cortex with magnetic resonance imaging. Korean J Radiol 2018;19:767–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Pleger B, Draganski B, Schwenkreis P, Lenz M, Nicolas V, Maier C, Tegenthoff M. Complex regional pain syndrome type I affects brain structure in prefrontal and motor cortex. PLoS One 2014;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Poeppl TB, Donges MR, Mokros A, Rupprecht R, Fox PT, Laird AR, Bzdok D, Langguth B, Eickhoff SB. A view behind the mask of sanity: meta-analysis of aberrant brain activity in psychopaths. Mol Psychiatry 2019;24:463–470. doi: 10.1038/s41380-018-0122-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Poeppl TB, Langguth B, Laird AR, Eickhoff SB. Meta-analytic Evidence for Neural Dysactivity Underlying Sexual Dysfunction. J Sex Med 2019;16:614–617. doi: 10.1016/j.jsxm.2019.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Queiroz LP. Worldwide epidemiology of fibromyalgia topical collection on fibromyalgia. Curr Pain Headache Rep 2013;17. [DOI] [PubMed] [Google Scholar]
- [70].Reckziegel D, Tétreault P, Ghantous M, Wakaizumi K, Petre B, Huang L, Jabakhanji R, Abdullah T, Vachon-Presseau E, Berger S, Baria A, Griffith JW, Baliki MN, Schnitzer TJ, Apkarian AV. Sex-Specific Pharmacotherapy for Back Pain: A Proof-of-Concept Randomized Trial. Pain Ther 2021;10:1375–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Rehbein E, Hornung J, Sundström Poromaa I, Derntl B. Shaping of the Female Human Brain by Sex Hormones: A Review. Neuroendocrinology 2021;111:183–206. [DOI] [PubMed] [Google Scholar]
- [72].Rodriguez-Raecke R, Niemeier A, Ihle K, Ruether W, May A. Brain gray matter decrease in chronic pain is the consequence and not the cause of pain. J Neurosci 2009;29:13746–13750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Rottschy C, Langner R, Dogan I, Reetz K, Laird AR, Schulz JB, Fox PT, Eickhoff SB. Modelling neural correlates of working memory: A coordinate-based meta-analysis. Neuroimage 2012;60:830–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Ruscheweyh R, Deppe M, Lohmann H, Stehling C, Flöel A, Ringelstein EB, Knecht S. Pain is associated with regional grey matter reduction in the general population. Pain 2011;152:904–911. [DOI] [PubMed] [Google Scholar]
- [75].Salimi-Khorshidi G, Smith SM, Keltner JR, Wager TD, Nichols TE. Meta-analysis of neuroimaging data: A comparison of image-based and coordinate-based pooling of studies. Neuroimage 2009;45:810–823. doi: 10.1016/j.neuroimage.2008.12.039. [DOI] [PubMed] [Google Scholar]
- [76].Schmidt-Wilcke T, Hierlmeier S, Leinisch E. Altered regional brain morphology in patients with chronic facial pain. Headache 2010;50:1278–1285. [DOI] [PubMed] [Google Scholar]
- [77].Schwedt TJ, Chong CD. Medication Overuse Headache: Pathophysiological Insights from Structural and Functional Brain MRI Research. Headache 2017;57:1173–1178. [DOI] [PubMed] [Google Scholar]
- [78].Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 2007;27:2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Seminowicz DA, Labus JS, Bueller JA, Tillisch K, Naliboff BD, Bushnell MC, Mayer EA. Regional Gray Matter Density Changes in Brains of Patients With Irritable Bowel Syndrome. Gastroenterology 2010;139:48–57.e2. doi: 10.1053/j.gastro.2010.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Seminowicz DA, Shpaner M, Keaser ML, Krauthamer GM, Mantegna J, Dumas JA, Newhouse PA, Filippi CG, Keefe FJ, Naylor MR. Cognitive-behavioral therapy increases prefrontal cortex gray matter in patients with chronic pain. J Pain 2013;14:1573–1584. doi: 10.1016/j.jpain.2013.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Sheng LQ, Ma HR, Shi YY, Dai ZY, Zhong JG, Chen F, Pan PL. Cortical Thickness in Migraine: A Coordinate-Based Meta-Analysis. Front Neurosci 2021;14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Shi HC, Yuan CH, Dai ZY, Ma HR, Sheng LQ. Gray matter abnormalities associated with fibromyalgia: A meta-analysis of voxel-based morphometric studies. Semin Arthritis Rheum 2016;46:330–337. [DOI] [PubMed] [Google Scholar]
- [83].Simons LE, Elman I, Borsook D. Psychological processing in chronic pain: A neural systems approach. Neurosci Biobehav Rev 2014;39:61–78. doi: 10.1016/j.neubiorev.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Smallwood RF, Laird AR, Ramage AE, Parkinson AL, Lewis J, Clauw DJ, Williams DA, Schmidt-Wilcke T, Farrell MJ, Eickhoff SB, Robin DA. Structural brain anomalies and chronic pain: A quantitative meta-analysis of gray matter volume. J Pain 2013;14:663–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Smith SM, Nichols TE. Threshold-free cluster enhancement: Addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage 2009;44:83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- [86].Szucs D, Ioannidis JP. Sample size evolution in neuroimaging research: An evaluation of highly-cited studies (1990–2012) and of latest practices (2017–2018) in high-impact journals. Neuroimage 2020;221:117164. doi: 10.1016/j.neuroimage.2020.117164. [DOI] [PubMed] [Google Scholar]
- [87].Tahmasian M, Sepehry AA, Samea F, Khodadadifar T, Soltaninejad Z, Javaheripour N, Khazaie H, Zarei M, Eickhoff SB, Eickhoff CR. Practical recommendations to conduct a neuroimaging meta-analysis for neuropsychiatric disorders. Hum Brain Mapp 2019;40:5142–5154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Tatu K, Costa T, Nani A, Diano M, Quarta DG, Duca S, Apkarian AV, Fox PT, Cauda F. How do morphological alterations caused by chronic pain distribute across the brain? A meta-analytic co-alteration study. NeuroImage Clin 2018;18:15–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Taylor KS, Seminowicz DA, Davis KD. Two systems of resting state connectivity between the insula and cingulate cortex. Hum Brain Mapp 2009;30:2731–2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Thompson JM, Neugebauer V. Amygdala Plasticity and Pain. Pain Res Manag 2017;2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Tsang A, Von Korff M, Lee S, Alonso J, Karam E, Angermeyer MC, Borges GLG, Bromet EJ, de Girolamo G, de Graaf R, Gureje O, Lepine JP, Haro JM, Levinson D, Oakley Browne MA, Posada-Villa J, Seedat S, Watanabe M. Common Chronic Pain Conditions in Developed and Developing Countries: Gender and Age Differences and Comorbidity With Depression-Anxiety Disorders. J Pain 2008;9:883–891. [DOI] [PubMed] [Google Scholar]
- [92].Turkeltaub PE, Eickhoff SB, Laird AR, Fox M, Wiener M, Fox P. Minimizing within-experiment and within-group effects in activation likelihood estimation meta-analyses. Hum Brain Mapp 2012;33:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Vachon-Presseau E, Tétreault P, Petre B, Huang L, Berger SE, Torbey S, Baria AT, Mansour AR, Hashmi JA, Griffith JW, Comasco E, Schnitzer TJ, Baliki MN, Apkarian AV. Corticolimbic anatomical characteristics predetermine risk for chronic pain. Brain 2016;139:1958–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Valet M, Gündel H, Sprenger T, Sorg C, Mühlau M, Zimmer C, Henningsen P, Tölle TR. Patients with pain disorder show gray-matter loss in pain-processing structures: A voxel-based morphometric study. Psychosom Med 2009;71:49–56. [DOI] [PubMed] [Google Scholar]
- [95].Wager TD, Lindquist MA, Nichols TE, Kober H, Van Snellenberg JX. Evaluating the consistency and specificity of neuroimaging data using meta-analysis. Neuroimage 2009;45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].World Health Organization. International Statistical Classification of Diseases and Related Health Problems - 10th Revision. 2019:2019. Available: https://icd.who.int/browse10/2019/en. [Google Scholar]
- [97].World Health Organization. International statistical classification of diseases and related health problems (10th ed.). 2016. [Google Scholar]
- [98].Xu A, Larsen B, Baller EB, Scott JC, Sharma V, Adebimpe A, Basbaum AI, Dworkin RH, Edwards RR, Woolf CJ, Eickhoff SB, Eickhoff CR, Satterthwaite TD. Convergent neural representations of experimentally-induced acute pain in healthy volunteers: A large-scale fMRI meta-analysis. Neurosci Biobehav Rev 2020;112:300–323. doi: 10.1016/j.neubiorev.2020.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Xu A, Larsen B, Henn A, Baller EB, Scott JC, Sharma V, Adebimpe A, Basbaum AI, Corder G, Dworkin RH, Edwards RR, Woolf CJ, Eickhoff SB, Eickhoff CR, Satterthwaite TD. Brain Responses to Noxious Stimuli in Patients With Chronic Pain: A Systematic Review and Meta-analysis. JAMA Netw open 2021;4:e2032236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Yang Q, Wang Z, Yang L, Xu Y, Chen LM. Cortical thickness and functional connectivity abnormality in chronic headache and low back pain patients. Hum Brain Mapp 2017;38:1815–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Yen CT, Lu PL. Thalamus and pain. Acta Anaesthesiol Taiwanica 2013;51:73–80. doi: 10.1016/j.aat.2013.06.011. [DOI] [PubMed] [Google Scholar]
- [102].Yuan CH, Shi HC, Pan PL, Dai ZY, Zhong JG, Ma HR, Sheng LQ. Gray Matter Abnormalities Associated with Chronic Back Pain: A Meta-Analysis of Voxel-based Morphometric Studies. Clin J Pain 2017;33:983–990. [DOI] [PubMed] [Google Scholar]
- [103].Zhang Y, Qu M, Yi X, Zhuo P, Tang J, Chen X, Zhou G, Hu P, Qiu T, Xing W, Mao Y, Chen BT, Wu J, Zhang Y, Liao W. Sensorimotor and pain-related alterations of the gray matter and white matter in Type 2 diabetic patients with peripheral neuropathy. Hum Brain Mapp 2020;41:710–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.