Abstract
Exploring innovative methods to provide essential nutrients and reducing ruminant greenhouse gas emission is crucial for animal production and diminishing global warming. This study was conducted to examine the efficacy of Moringa oleifera leaves (ML) in ruminants at 0%, 5%, 10%, 15%, 20%, 30% and 40% level in different roughage (R) and concentrate (C) (80R:20C, 70R:30C and 60R:40C) under in vitro conditions. Chemical composition of ML, concentrate mixture and berseem were estimated. Rumen fermentation parameters of male goat kids viz., total gas production, CH4, true dry matter digestibility (TDMD), organic matter digestibility (TOMD), partial fraction (PF), microbial biomass (MBP), ammonia (N), acetate, propionate, butyrate and acetate propionate ratio were observed under in vitro conditions. Results revealed that crude protein, organic matter and ethyl ether content were higher in ML as compared to concentrate mixture and berseem. Magnesium and iron content were also higher in ML as compared to concentrate and berseem. Total gas production, digestibility of DM and OM, MBP, acetate and propionate level were improved (P < 0.05) upto 10–20% replacement. In contrast, decreased in CH4 (%) and CH4 (mL/100 mg dDM) was noted with increased levels of ML incorporation. There was no change observed in ammonia, acetate: propionate ratios at all the three planes of nutrition. In this study, it is concluded that mixing Moringa oleifera leaves in feed can be used as protein supplement and reduce the methane emission without causing any effect on digestibility and rumen fermentation parameters. However, ML can be suggested for widespread practice to attain the sustainable animal production (10–20%) and to alleviate the global warming.
Keywords: Moringa oleifera, Methane emission, Digestibility, Rumen fermentation
Introduction
Livestock rearing is important for global food production. Animal production in farm is typically reduced due to the low quality and scarcity of animal feeds in tropical countries. Feedstuffs especially protein sources such as legumes, cereals and grains essential for animal development, have become very expensive and limited in many regions of the world (Choudhary et al. 2022). Hence, it is required for searching a substitute source of feed which are edible, rich in protein and minerals, low-priced and fulfils the basic needs of small ruminants.
The ruminant cattle industry significantly contributes to greenhouse gas (GHG) emission that cause global warming (Eisen and Brown 2022). Methane is one of the main GHG and its potency is twenty-five times as that of CO2. Ruminants are one major causes of emission of methane (81–92 MT) produced per year worldwide which is equal to total anthropogenic methane (23–27%). Methane is produced by enteric fermentation process in ruminants and contributes about 13% of methane emission from livestock in India (Gupta et al. 2018). Cattle contribute 49.10% enteric methane emission followed by buffalo, goat, sheep and others as 42.80%, 5.38%, 2.59% and 0.73% within agriculture. Different sources such as amino acids, organic acids, essential oils and exogenous enzymes have been used to alleviate the ruminant methane emission (Benetal et al. 2022; Kholif et al. 2022). Numerous studies have reported a reduction in enteric methane emission by feeding of tree leaves to ruminants and many workers have advocated their use as an alternative protein source for livestock (Ku-Vera et al. 2020).
Moringa oleifera is a perennial tree feed and also called as “miracle tree”. It is a multipurpose and fast-growing tree with nutritional and therapeutic properties that can be planted in a variety of climates including drought and heat, and can be harvested numerous times (Abbas et al. 2018). Its leaves contain sufficient quantity of minerals, proteins and vitamins according to the nutritional demand in lactating goats (Afzal et al. 2022). It is also having antioxidant properties such phenols, vitamin C, carotene and flavonoids (Saleem et al. 2020). It is an inexpensive protein constituent as compared to soyabean and sesame feed meals used in livestock feeding. Moringa oleifera leaves (ML) meal contains 9 times extra protein as compared to yogurt having good feeding effect and can be used as protein substitute in animal feed (Su and Chen 2020). The normal crude protein content in ML was 180–270 g CP/kg DM as reported by Kholif et al. (2018). Application of Moringa foliage improved the feed consumption, metabolic profile and growth performance of goat kids (Wankhede et al. 2022). ML are natural feed supplement which produce secondary metabolites like tannins and saponins, modify the pathways of rumen fermentation and prevent the growth of methanogens effectively (Zeru et al. 2022). ML strengthened the immune system and reduced oxidative stress in goats due to the presence of bioactive compounds (Al-Juhaimi et al. 2020; Teclegeorgish et al. 2021). Dong et al. (2019) reported that supplementation of ML in goats food improved content of fat milk and decrease the M. ruminantium which involved in methane production. Application of Moringa oil (4%) along with 30–50% of roughage ration decreased the methanogens and protozoa population but increase the Provotella which involved in rumen acidosis (Ebeid et al. 2020). There has been less research on the effects of ML at various concentration on male goat kids to reduce enteric methane emission, and how it affects rumen fermentation kinetics and digestibility. Hence, the purpose of this study was to investigate the consequence of M. oleifera leaves on rumen fermentation and methane production under in vitro conditions with different plane of nutrition.
Materials and methods
Farm description and feed preparation
The present study was conducted in Animal Nutrition Division, National Dairy Research Institute (NDRI), Karnal, Haryana, India. This institution is located at 29° 42ʹN and 79° 54ʹE at 834 feet above the sea level. Moringa oleifera leaves was collected from NDRI farm, shade and oven dried, grind and powder were packed in airtight polythene bags, while berseem and concentrate mixture were oven dried at 60 ℃. Desiccated samples were crushed and sieve (1 mm) by using electrically operated Wiley mill. After complete drying, sample was grinded and placed in sample bottles for further use. Dried samples were used for analysis of DM, OM, CP and EE as per AOAC (2005), NDF and ADF (Van Soest et al. 1991).
Estimation of secondary metabolites in Moringa oleifera leaves
Tannin estimation was carried out using the method given by Nwinuka et al. (2005). Tannic acid (1 mg/mL) was used as the reference. Plant leaves extract (1 mL) was mixed with Folin-Ciocalteu reagent (0.5 mL) and sodium carbonate solution (1 mL). The total volume was made up to 5 mL. Tannins concentration was determined by measuring absorbance at 755 nm.
Methanolic extract of leaves (500 µL) and anisaldehyde reagent (500 µL, 0.5%) were mixed in a test tube and left for 10 min for saponins estimation. Sulphuric acid (5%, 2 mL) was added in tubes, mixed properly and kept in water bath at 60℃ for 10 min. The tubes were cooled and the absorbance was measured at 435 nm (Baccou et al. 1977).
Total phenolic content (TPC) in methanolic extract of ML was determined using the Folin-Ciocalteu reagent assay. Folin-Ciocalteu (750 mL), sodium carbonate (7.5%, 2 mL) and leaves methanolic extract (200 ml) were added in a tube. The mixture was diluted with deionized water to 7 mL, then left at room temperature in the dark for 2 h. The absorbance was measured at 765 nm using spectrophotometer (Folin and Ciocalteu 1927).
The total flavonoid content (TFC) was determined using the method described by Zhishen et al. (1999). Briefly, methanolic extract (1 mL) was added to a 10 mL volumetric flask containing water (4 mL). Sodium nitrate (0.3 mL, 5%) was added in flask followed by aluminum chloride (0.3 mL, 10%) at 5 min, sodium hydroxide (2 mL, 1 M) at 6 min. Then 2.4 mL water was added in flask and absorbance was measured at 510 nm.
Mineral composition of feed ingredients
Dried (0.5 g) dried and crushed feed sample was weighted into digestion tubes, tri acid mixture (10 mL) was added for digestion in Kelplus micro digestion assembly. The absence of white fumes and black particles in the residues suggested that samples had been completely digested. The digested samples were then filtered using filter paper. Filter paper was rinsed many times with double distilled water and Inductivity coupled plasma (ICP-Optical Emission Spectrometer was used for mineral analysis, while P was estimated using spectrophotometric method (Sultana 2020).
Experimental design and rumen liquor collection
Rumen liquor was collected from the male goat kids with stomach tube and vaccum pump fitted with sterile pipe connected to it in early morning before feeding in pre-warmed thermos flask, filtered using four layers of muslin cloth and used as an inoculum for in vitro gas production in different treatments (Menke and Steingass 1988). Details of the experiment as follows: ML at different concentration (0, 5, 10, 15, 20, 30 and 40% replaced with concentrate mixture) along with three different ratios of roughage and compound feed (60R:40C, 70R:30C and 80R:20C) were prepared for in vitro experiment. This trial was conducted with three replications with 3 sub replicates of each treatment (total n = 9 samples). Different substrates (200 mg) were weighted separately and added in calibrated glass syringes (100 mL) at the bottom side. The piston was greased through petroleum jelly and hard-pressed inside the syringe. These syringes were incubated at 39 ℃ in water bath. Solution media was prepared separately which contains micro mineral solution (0.124 mL), macro mineral solution (250 mL), rumen buffer solution (250 mL), resazurin solution (1.25 mL) and distilled water (500 mL). This solution was prewarmed at 39 ℃ and fizzed with CO2, when this mixture become colorless rumen liquor was added. After proper mixing this mixture (30 mL) was injected in syringes through auto dispenser, shaken gently, outlet closed and incubated in water bath for 24 h at 39 ℃ for further use. Piston level was checked for initial reading and syringes were shaken after every 30 min.
Analysis of total gas production
After 24 h incubation of the above mixture, total gas production was estimated by deducting the initial reading as of final reading.
Estimation of in vitro methane production
The in vitro methane (CH4) production was estimated by taking the sample gas after 24 h of incubation from the head space in the air tight syringe and injected to gas chromatogram fitted with flame ionization detector and stainless-steel column packed with Porapak-Q (1.5 m × 3.2 mm × 2 mm). The temperature for injector column and detector were adjusted as 40, 50 and 100 ℃ and flow rate for nitrogen gas through column was 30, 300 and 30 mL/min. Gas sample (2 mL) from syringe was injected into GC through injection port. The standard gas used for methane estimation composed of methane and CO2 (50%).
Estimation of true dry and organic matter digestibility (TDMD/TOMD), partial fraction (PF) and microbial biomass (MBN)
After 24 h incubation, syringe samples from each replicate were transferred to centrifuge tubes and centrifuged for 10 min at 3000 rpm. Obtained pellet was used for the estimation of DM and OM. Pellets obtained after centrifugation were refluxed with neutral detergent solution, filtered using G1 crucibles and then residues were oven dried to evaluate the TOMD. For in vitro organic matter digestibility (IVTOMD) estimation the residue was ashed at 550–650 ℃ in Muffle furnace. Microbial biomass production and partial fraction were estimated using TDOM (Blummel et al. 1997).
Estimation of ammonia production
Aliquot gas sample was used for the assessment of ammonia nitrogen (NH3–N) by Kjeldahl process. Supernatant was acidified with equal volume of HCl (0.5 M) and placed at − 20 ℃. Acidified supernatant (5 mL) and NaOH (10 mL) steam distilled using KELPLUS-N analyzer (Pelican, India). The ammonia was collected in boric acid solution (20%) comprising of mixed indicators and then titrated with H2SO4 (Komolong et al. 2001).
Estimation of volatile fatty acids (VFA)
Supernatant (4 mL) was added in m-phosphoric acid (25%, 2 mL) and overnight placed at 4 ℃. The solution was centrifuged for 15 min at 3000 rpm and stored at 4 ℃ for VFA analysis. The individual VFA in different samples were estimated using gas chromatograph (Nucon 5700) equipped with flame ionization detector and stainless-steel column packed with chromosorb 101. Analytical conditions used for fractionation of VFA was injector port temperature (210 ℃), column temperature (180 ℃), detector temperature (230 ℃). Flow rate of carrier nitrogen gas was 40 mL/min (Bannink et al. 2006).
Statistical analysis
Results were statistically analyzed using SPSS software ver. 16.0 through one-way analysis of variance at P < 0.05. The values of above parameters were presented as mean ± standard error.
Results
Chemical composition of feed ingredients and total mixed ration (TMR)
The chemical composition of berseem fodder, concentrate and M. oleifera leaves are presented in the Fig. 1. DM content was higher in concentrate/compound feed (91.21%) followed by M. oleifera (29.26%) and berseem (23.61%). Organic matter, CP and EE content were higher in M. oleifera leaves (89.62, 23.37 and 7.13%) followed by compound feed (89.26, 20.14, 4.45%) and berseem (87.22, 17.56 and 2.98%), respectively. The content of NDF, ADF and TA were 59.29, 39.33 and 12.78% in berseem, 23.58, 11.69, 10.74% in compound feed and 26.86, 18.70 and 10.03% in M. oleifera, respectively.
Fig. 1.
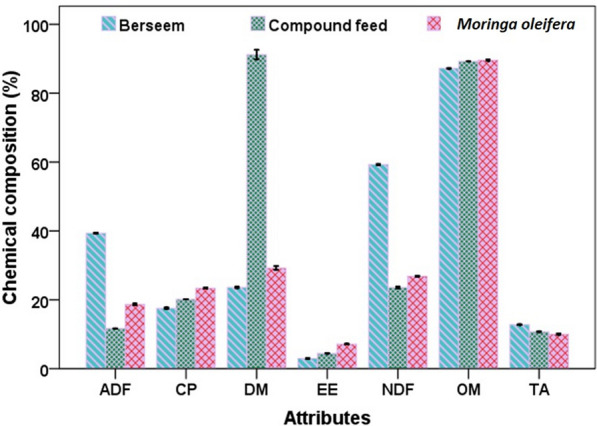
Chemical composition of berseem, compound feed and Moringa oleifera
The chemical composition of TMR is presented in Table 1. The CP (%) in 60R:40C was higher in 40% TMR (19.11) followed by 18.98 in 30%, 18.85 in 20%, 18.79% in 15% TMR, 18.72% in 10%, 18.66% in 5% and 18.59 in 0% TMR. In 70R:30C group, CP (%) was higher in 40% TMR (19.74%) followed by 18.86 in 20%, 18.62 in 30%, 18.48% in 15% TMR, 18.43% in 10%, 18.38% in 5% and 18.33 in 0% TMR. The CP (%) in 80R:20C group was higher in 40% TMR (18.33%) followed by 18.27 in 30%, 18.21 in 20%, 18.17% in 15% TMR, 18.14% in 10% and 0% and 18.11% in 5% TMR.
Table 1.
Chemical composition (%) of total mixture ration in different groups (60R:40C, 70R:30C and 80R:20C)
| Attributes (%) | 0% | 5% | 10% | 15% | 20% | 30% | 40% |
|---|---|---|---|---|---|---|---|
| DM (60R:40C) | 50.65 ± 0.35 | 49.41 ± 0.48 | 48.17 ± 0.53 | 46.93 ± 0.43 | 45.69 ± 0.30 | 43.22 ± 0.58 | 40.74 ± 0.52 |
| OM | 88.04 ± 0.15 | 88.04 ± 0.13 | 88.05 ± 0.25 | 88.06 ± 0.36 | 88.06 ± 0.26 | 88.08 ± 0.32 | 88.09 ± 0.20 |
| CP | 18.59 ± 0.53 | 18.66 ± 0.33 | 18.72 ± 0.27 | 18.79 ± 0.29 | 18.85 ± 0.15 | 18.98 ± 0.28 | 19.11 ± 0.11 |
| EE | 3.57 ± 0.21 | 3.62 ± 0.15 | 3.68 ± 0.12 | 3.73 ± 0.11 | 3.78 ± 0.10 | 3.89 ± 0.20 | 4.00 ± 0.12 |
| NDF | 45.01 ± 0.22 | 45.07 ± 0.44 | 45.14 ± 0.25 | 45.20 ± 0.42 | 45.27 ± 0.52 | 45.40 ± 0.40 | 45.53 ± 0.43 |
| ADF | 28.27 ± 0.28 | 28.41 ± 0.41 | 28.55 ± 0.35 | 28.69 ± 0.40 | 28.83 ± 0.27 | 29.12 ± 0.13 | 29.40 ± 0.40 |
| TA | 11.96 ± 0.08 | 11.95 ± 0.16 | 11.94 ± 0.18 | 11.92 ± 0.07 | 11.91 ± 0.21 | 11.88 ± 0.11 | 11.85 ± 0.15 |
| DM (70R:30C) | 43.89 ± 0.35 | 42.96 ± 0.44 | 42.03 ± 0.47 | 41.10 ± 0.54 | 39.41 ± 0.41 | 38.31 ± 0.58 | 45.58 ± 0.59 |
| OM | 87.83 ± 0.74 | 87.84 ± 0.40 | 87.84 ± 0.44 | 87.85 ± 0.63 | 86.14 ± 0.58 | 87.86 ± 0.54 | 96.80 ± 0.68 |
| CP | 18.33 ± 0.33 | 18.38 ± 0.38 | 18.43 ± 0.34 | 18.48 ± 0.28 | 18.86 ± 0.36 | 18.62 ± 0.37 | 19.74 ± 0.26 |
| EE | 3.42 ± 0.07 | 3.46 ± 0.09 | 3.50 ± 0.09 | 3.54 ± 0.05 | 3.28 ± 0.06 | 3.66 ± 0.06 | 4.19 ± 0.12 |
| NDF | 48.58 ± 0.53 | 48.63 ± 0.36 | 48.68 ± 0.31 | 48.72 ± 0.49 | 49.65 ± 0.46 | 48.86 ± 0.51 | 51.33 ± 0.55 |
| ADF | 31.04 ± 0.75 | 31.14 ± 0.60 | 31.25 ± 0.75 | 31.35 ± 0.68 | 31.99 ± 0.32 | 31.67 ± 0.77 | 33.05 ± 0.70 |
| TA | 12.17 ± 0.07 | 12.16 ± 0.16 | 12.15 ± 0.15 | 12.14 ± 0.07 | 12.06 ± 0.08 | 12.10 ± 0.10 | 13.16 ± 0.10 |
| DM (80R:20C) | 35.89 ± 0.50 | 36.51 ± 0.48 | 35.89 ± 0.60 | 35.27 ± 0.42 | 34.65 ± 0.65 | 33.41 ± 0.41 | 32.17 ± 0.64 |
| OM | 87.64 ± 0.64 | 87.63 ± 0.63 | 87.64 ± 0.35 | 87.64 ± 0.35 | 87.64 ± 0.38 | 87.65 ± 0.45 | 87.66 ± 0.51 |
| CP | 18.14 ± 0.14 | 18.11 ± 0.12 | 18.14 ± 0.06 | 18.17 ± 0.14 | 18.21 ± 0.12 | 18.27 ± 0.15 | 18.33 ± 0.12 |
| EE | 3.33 ± 0.11 | 3.30 ± 0.07 | 3.33 ± 0.11 | 3.35 ± 0.07 | 3.38 ± 0.04 | 3.43 ± 0.05 | 3.49 ± 0.06 |
| NDF | 52.21 ± 0.29 | 52.18 ± 0.18 | 52.21 ± 0.21 | 52.25 ± 0.54 | 52.28 ± 0.30 | 52.34 ± 0.45 | 52.41 ± 0.52 |
| ADF | 33.94 ± 0.53 | 33.87 ± 0.27 | 33.94 ± 0.74 | 34.01 ± 0.31 | 34.08 ± 0.65 | 34.22 ± 0.58 | 34.36 ± 0.63 |
| TA | 12.36 ± 0.10 | 12.36 ± 0.12 | 12.36 ± 0.14 | 12.35 ± 0.05 | 12.34 ± 0.05 | 12.33 ± 0.05 | 12.32 ± 0.10 |
Secondary metabolites in Moringa oleifera leaves
On the basis of % dry matter, the value of each chemical component such as tannin, saponin, TPC and TFC in M. oleifera leaves was calculated. The TPC content was higher (4.28%) followed by TFC (3.61%), tannin (2.02%) and saponin (1.01%), respectively.
Mineral composition of berseem fodder, concentrate and Moringa oleifera leaves
Calcium content was higher in berseem (1.92%) followed by M. oleifera (1.85%) and concentrate mixture (1.37%), while phosphorus content was higher in concentrate mixture (0.79%) followed by berseem fodder (0.38%) and M. oleifera (0.15%). Magnesium content was higher in M. oleifera (4.81%) followed by concentrate mixture (0.81%) and berseem fodder (0.40%). The Fe (ppm) content was 287 in concentrate mixture, berseem fodder (289 ppm) and M. oleifera (330 ppm). The level of Cu and Zn (ppm) in concentrate mixture (29.21, 49), berseem fodder (4.61, 16.41) and M. oleifera (9.21 and 26.72 ppm) presented in Table 2.
Table 2.
Proximate mineral composition of berseem fodder, concentrate and Moringa leaves
| Parameter | Concentrate mixture | Berseem fodder | Moringa oleifera |
|---|---|---|---|
| Ca (%) | 1.37 ± 0.05 | 1.92 ± 0.02 | 1.85 ± 0.12 |
| P (%) | 0.79 ± 0.10 | 0.38 ± 0.04 | 0.15 ± 0.05 |
| Mg (%) | 0.81 ± 0.03 | 0.40 ± 0.03 | 4.81 ± 0.14 |
| Fe (ppm) | 287.00 ± 0.50 | 289.53 ± 0.65 | 330 ± 0.33 |
| Cu (ppm) | 29.21 ± 0.23 | 4.61 ± 0.06 | 9.21 ± 0.23 |
| Zn (ppm) | 49.00 ± 0.45 | 16.41 ± 0.23 | 26.72 ± 0.31 |
Fermentation parameters
The total gas production increased (P < 0.05) at 10% replacement of concentrate feed in 60R:40C group. The values for total gas production in 60R:40C was 162.93 and 168.34 (mL/gDM) at 10% and 15% level, no further difference was observed after these replacement level (Table 3). IVDMD, IVOMD, acetate, MBN production were higher at 15% replacement in 60R:40C, while propionate was higher at 10% replacement level (P > 0.05) than other replacement groups. It was observed that with increased replacement of concentrate with ML, there was significant reduction (P < 0.05) in CH4 (%) and CH4 ml/100 mg of digestible dry matter (dDM). The CH4 (%) and CH4 (mL/100 mg) in 60R:40C were 30.22 and 7.50 at 10% level of replacement. Reduction in methane was observed at 10% replacement, however no further reduction (P < 0.05) in methane was observed above this level. A significant (P < 0.05) increase in the acetate (57.90% and 59.81%) and propionate (19.42% and 19.73%) production was observed upto 10% and 15% replacement in 60R:40C. The ammonia nitrogen (mg/dL) was similar (P < 0.05) in all planes of nutrition. The values for ammonia-N (mg/dL) in 60R:40C was 13.14 at 0%, 12.64 at 5%, 13.08 at 10%, 12.14 at 15%, 14.17 at 20%, 12.69 at 30% and 12.02 at 40% level, respectively. The values for partition factor in 60R:40C was 4.04 at 0%, 3.94 at 5%, 4.01 at 10%, 3.88 at 15%, 3.85 at 20%, 4.20 at 30% and 5.24 at 40% level, respectively. There was an increase (P < 0.05) in MBP (mg) at 15% level as compared other level (Table 3).
Table 3.
Effect of Moringa oleifera leaf on in vitro digestibility, total gas, methane, PF and MBP along with roughage and concentrate feed (60R:40C)
| Parameters | 0% | 5% | 10% | 15% | 20% | 30% | 40% | SEM | P value |
|---|---|---|---|---|---|---|---|---|---|
| Total gas (mL/gDM) | 150.43 ± 3.89c | 156.41 ± 4.76bc | 162.93 ± 0.10ab | 168.34 ± 4.88ab | 173.29 ± 0.07a | 171.15 ± 5.23a | 171.65 ± 0.24a | 2.274 | 0.020 |
| IVTDMD (%) | 68.83 ± 0.43c | 70.06 ± 0.47c | 74.12 ± 0.18b | 75.20 ± 1.35ab | 75.42 ± 0.52ab | 77.92 ± 1.93a | 77.94 ± 0.22a | 0.797 | < 0.001 |
| IVTOMD (%) | 71.21 ± 0.40c | 72.75 ± 0.39bc | 76.38 ± 1.18b | 76.36 ± 0.72ab | 76.73 ± 0.65ab | 78.54 ± 0.25ab | 79.28 ± 1.74a | 0.671 | < 0.001 |
| CH4 (%) | 31.81 ± 0.14a | 30.70 ± 0.23b | 30.22 ± 0.25b | 29.13 ± 0.35c | 28.27 ± 0.30d | 27.64 ± 0.31d | 27.48 ± 0.25d | 0.351 | < 0.001 |
| CH4 (mL/100 mg) | 7.97 ± 0.14 | 7.78 ± 0.17 | 7.50 ± 0.37 | 7.34 ± 0.02 | 7.31 ± 0.52 | 7.03 ± 1.46 | 6.95 ± 0.26 | 0.341 | 0.99 |
| Acetate(mM) | 50.89 ± 0.73d | 54.39 ± 1.31cd | 57.90 ± 2.14bc | 59.81 ± 2.58abc | 62.60 ± 2.16ab | 64.48 ± 3.26ab | 65.50 ± 1.18a | 1.292 | 0.002 |
| Propionate (mM) | 17.55 ± 0.67c | 18.32 ± 0.99bc | 19.42 ± 0.37abc | 19.73 ± 0.69abc | 20.13 ± 0.64ab | 20.68 ± 1.21ab | 21.18 ± 0.49a | 0.360 | 0.05 |
| Butyrate (mM) | 10.08 ± 0.10 | 10.21 ± 0.72 | 11.35 ± 0.16 | 11.07 ± 0.51 | 10.33 ± 0.81 | 9.90 ± 0.78 | 9.42 ± 0.45 | 0.227 | 0.29 |
| AP ratio | 2.91 ± 0.12 | 2.98 ± 0.09 | 2.99 ± 0.14 | 3.03 ± 0.06 | 3.12 ± 0.19 | 3.12 ± 0.11 | 3.09 ± 0.02 | 0.040 | 0.79 |
| PF | 4.04 ± 0.06 | 3.94 ± 0.13 | 4.01 ± 0.23 | 3.88 ± 0.07 | 3.85 ± 0.33 | 4.20 ± 0.87 | 5.24 ± 2.27 | 0.308 | 0.936 |
| MBP (mg) | 49.91 ± 1.41d | 52.31 ± 2.69cd | 55.03 ± 1.07bcd | 56.87 ± 1.91abc | 58.61 ± 1.16ab | 60.60 ± 0.36ab | 61.52 ± 2.77a | 1.056 | 0.004 |
| Ammonia N (mg/dl) | 13.14 ± 0.53 | 12.64 ± 0.84 | 13.08 ± 0.45 | 12.14 ± 1.33 | 14.17 ± 1.48 | 12.69 ± 1.47 | 12.02 ± 0.41 | 0.36 | 0.808 |
Means with different superscripts a, b, c and d in the same row differ significantly (P < 0.05)
The total gas production increased (P < 0.05) at 10% replacement of concentrate feed with ML in 70R:30C group. The total gas production in forage to concentrate 70R:30C ratio was 157.26(mL/gDM) at 10% level. The IVDMD (73.19%) and IVOMD (74.07%) production was higher at 20% replacement in 70R:30C than other level of replacement feed. The percentage of CH4 in 70R:30C was 32.16, 31.03, 30.55, 29.44, 28.57, 27.94 and 27.78% at 0, 5, 10, 15, 20, 30 and 40%, respectively. The values of CH4 (mL/100 mg dDM) were 8.12 at 0%, 7.80 at 5%, 7.87 at 10%, 7.44 at 15%, 6.90 at 20%, 6.79 at 30% and 6.88 at 40% level respectively. Reduction in methane level was observed upto 15–20% in 70R:30C. A significant (P < 0.05) increase in the acetate (%) and propionate (%) production were observed upto 20% replacement in 70R:30C and no further change was observed. No difference was observed in butyrate production and acetate butyrate ratios in all the groups at all levels of supplementation. Ammonia-N (mg/dL) in 70R:30C was 13.23 at 0%, 12.65 at 5%, 12.76 at 10%, 12.29 at 15%, 13.76 at 20%, 11.97 at 30% and 11.97 at 40% level respectively. The PF ratio in 70R:30C was 3.90 at 0%, 3.84 at 5%, 3.92 at 10%, 3.84 at 15%, 3.91 at 20%, 3.98 at 30% and 3.81 at 40% level, respectively (P > 0.05). There was significant increase (P < 0.05) in MBP (mg) at 20% level (53.17%) as compared to others, data presented in Table 4.
Table 4.
Effect of replacement of Moringa oleifera leaf on in vitro digestibility, total gas, methane, PF and MBP along with roughage and concentrate feed (70R:30C)
| Parameters | 0% | 5% | 10% | 15% | 20% | 30% | 40% | SEM | P value |
|---|---|---|---|---|---|---|---|---|---|
| Total gas (mL/gDM) | 146.43 ± 3.89c | 151.41 ± 4.76bc | 157.26 ± 0.80abc | 163.68 ± 4.92ab | 165.95 ± 4.62ab | 168.48 ± 5.46a | 171.32 ± 1.85a | 2.42 | 0.02 |
| IVTDMD (%) | 62.64 ± 0.67e | 64.25 ± 0.43e | 66.56 ± 0.69d | 69.58 ± 1.54c | 73.19 ± 0.37b | 74.52 ± 0.48ab | 75.85 ± 0.16a | 1.10 | < 0.001 |
| IVTOMD (%) | 64.69 ± 0.14c | 66.25 ± 0.40c | 69.89 ± 1.31b | 71.01 ± 1.46b | 74.07 ± 0.81a | 75.07 ± 0.47a | 75.68 ± 0.01a | 0.93 | < 0.001 |
| CH4 (%) | 32.16 ± 0.15a | 31.03 ± 0.24b | 30.55 ± 0.25b | 29.44 ± 0.35c | 28.57 ± 0.30d | 27.94 ± 0.31d | 27.78 ± 0.25d | 0.35 | < 0.001 |
| CH4 (mL/100 mg) | 8.12 ± 0.13a | 7.80 ± 0.15ab | 7.87 ± 0.29ab | 7.44 ± 0.31bc | 6.90 ± 0.17c | 6.79 ± 0.18c | 6.88 ± 0.09c | 0.13 | 0.001 |
| Acetate (mM) | 49.05 ± 1.23c | 52.62 ± 0.68bc | 56.83 ± 2.10ab | 58.53 ± 2.46ab | 61.01 ± 2.35a | 62.98 ± 3.36a | 63.47 ± 1.19a | 1.30 | 0.002 |
| Propionate (mM) | 16.62 ± 0.52c | 17.36 ± 1.22bc | 18.69 ± 0.28abc | 19.25 ± 0.86ab | 19.66 ± 0.45ab | 20.11 ± 1.19a | 20.73 ± 0.38a | 0.39 | 0.02 |
| Butyrate (mM) | 9.99 ± 0.30 | 10.04 ± 1.04 | 11.45 ± 0.16 | 10.87 ± 0.61 | 10.04 ± 0.91 | 9.14 ± 1.16 | 9.42 ± 0.53 | 0.29 | 0.41 |
| AP ratio | 2.96 ± 0.15 | 3.05 ± 0.17 | 3.04 ± 0.12 | 3.04 ± 0.04 | 3.11 ± 0.17 | 3.14 ± 0.11 | 3.06 ± 0.02 | 0.04 | 0.97 |
| MBP (mg) | 46.61 ± 0.46d | 47.82 ± 1.91cd | 49.94 ± 2.95bcd | 50.17 ± 3.28bcd | 53.17 ± 1.35abc | 55.33 ± 1.27ab | 56.85 ± 0.11a | 1.005 | 0.018 |
| PF | 3.90 ± 0.17 | 3.84 ± 0.20 | 3.92 ± 0.06 | 3.84 ± 0.17 | 3.91 ± 0.13 | 3.98 ± 0.09 | 3.81 ± 0.09 | 0.047 | |
| Ammonia N (mg/dl) | 13.23 ± 0.48 | 12.65 ± 0.81 | 12.76 ± 0.83 | 12.29 ± 1.71 | 13.76 ± 0.56 | 11.97 ± 1.18 | 11.97 ± 0.49 | 0.33 | 0.821 |
Means with different superscripts a, b, c, d and e in the same row differ significantly (P < 0.05)
The values for gas production in 80R:20C was 143.10, 151.08, 154.26, 163.68, 163.95, 166.15, 177.32 (mL/gDM) at 0, 5, 10, 15, 20, 30 and 40% level, respectively. The IVDMD (70.52%) and IVOMD (70.18%) production was higher at 20% and 15% replacement in 80R:20C than other level of feed. The percentage of CH4 in 80R:20C was 34.23, 33.03, 32.25, 30.53, 28.56, 27.94 and 26.78% at 0, 5, 10, 15, 20, 30 and 40% respectively. The values of CH4 (mL/100 mg dDM) in 80R:20C was 8.89 at 0%, 8.61 at 5%, 8.40 at 10%, 7.90 at 15%, 7.05 at 20%, 6.86 at 30% and 6.56 at 40% level respectively (Table 5). Reduction in methane level was observed up to 15–20% in 80R:20C. A significant (P < 0.05) increase in the acetate (55.76%) at 10% and propionate (19.37%) production were observed at 20% replacement in 80R:20C. No difference was observed in butyrate production and acetate butyrate ratios in all the groups at all levels of supplementation. Ammonia nitrogen (mg/dL) was similar (P < 0.05) in all the planes of nutrition. There was no significant increase in PF ration in different plane of nutrition. The PF ratio in 80R:20C was 2.90 at 0%, 2.84 at 5%, 2.92 at 10%, 2.84 at 15%, 2.91 at 20%, 2.98 at 30% and 2.81 at 40% level, respectively. There was significant increase (P < 0.05) in MBP (mg) at 15% level (51.34%) was observed as compared to other level. The values for MBP (mg) in 80R:20C was 42.65 at 0%, 45.38 at 5%, 47.63 at 10%, 51.34 at 15%, 53.28 at 20%, 54.43 at 30% and 56.82 at 40% level respectively.
Table 5.
Effect of Moringa oleifera leaf on digestibility, total gas, methane, PF and MBP along with different roughage and concentrate feed (80R:20C)
| Parameters | 0% | 5% | 10% | 15% | 20% | 30% | 40% | SEM | P value |
|---|---|---|---|---|---|---|---|---|---|
| Total gas (mL/gDM) | 143.10 ± 3.78c | 151.08 ± 4.12bc | 154.26 ± 1.95abc | 163.68 ± 3.51ab | 163.95 ± 3.89ab | 166.15 ± 4.66a | 167.32 ± 1.87a | 2.307 | 0.01 |
| IVTDMD (%) | 60.31 ± 0.44e | 61.59 ± 0.75de | 63.90 ± 0.56d | 66.58 ± 1.54c | 70.52 ± 0.21b | 71.85 ± 0.68ab | 73.19 ± 0.37a | 1.089 | < 0.001 |
| IVTOMD (%) | 62.86 ± 0.70d | 64.42 ± 0.07d | 67.73 ± 1.01c | 70.18 ± 0.21b | 72.24 ± 0.48ab | 74.24 ± 1.21a | 73.85 ± 0.67a | 0.962 | < 0.001 |
| CH4 (%) | 34.23 ± 0.58a | 33.03 ± 0.10ab | 32.25 ± 0.21b | 30.53 ± 0.85c | 28.56 ± 0.57d | 27.94 ± 0.31de | 26.78 ± 0.17e | 0.601 | < 0.001 |
| CH4 (mL/100 mg) | 8.89a ± 0.50a | 8.61 ± 0.50a | 8.40 ± 0.12a | 7.90 ± 0.50ab | 7.05 ± 0.31bc | 6.86 ± 0.18bc | 6.56 ± 0.11c | 0.223 | 0.001 |
| Acetate (mM) | 47.92d ± 1.24d | 51.70 ± 0.20cd | 55.76 ± 1.46bc | 57.46 ± 1.98ab | 57.23 ± 1.51ab | 61.90 ± 2.89a | 62.68 ± 1.54a | 1.217 | < 0.002 |
| Propionate (mM) | 16.11 ± 0.77c | 16.80 ± 1.15bc | 18.30 ± 0.33ab | 18.79 ± 0.55ab | 19.37 ± 0.39a | 19.57 ± 0.87a | 20.19 ± 0.07a | 0.377 | 0.01 |
| Butyrate (mM) | 9.71 ± 0.43 | 9.58 ± 0.98 | 11.12 ± 0.07 | 10.31 ± 0.50 | 9.82 ± 0.76 | 8.97 ± 1.12 | 9.24 ± 0.48 | 0.267 | 0.45 |
| AP ratio | 2.99 ± 0.20 | 3.10 ± 0.20 | 3.05 ± 0.08 | 3.06 ± 0.04 | 2.95 ± 0.03 | 3.16 ± 0.09 | 3.10 ± 0.07 | 0.041 | 0.90 |
| MBP (mg) | 42.65 ± 1.25d | 45.38 ± 0.95cd | 47.63 ± 3.2bcd | 51.34 ± 0.20abc | 53.28 ± 2.59ab | 54.43 ± 2.48a | 56.82 ± 1.08a | 1.234 | 0.001 |
| PF | 2.90 ± 0.17 | 2.84 ± 0.20 | 2.92 ± 0.06 | 2.84 ± 0.17 | 2.91 ± 0.13 | 2.98 ± 0.09 | 2.81 ± 0.07 | 0.04 | 0.910 |
| Ammonia N (mg/dl) | 13.07 ± 0.46 | 12.60 ± 0.80 | 12.60 ± 0.80 | 12.13 ± 1.68 | 13.67 ± 0.54 | 11.90 ± 1.212 | 11.83 ± 0.46 | 0.331 | 0.814 |
Means with different superscripts a, b, c, d and e in the same row differ significantly (P < 0.05)
Discussion
Chemical composition of ML
ML assists as a healthy and affordable source of micronutrients and proteins. It helps to alleviate the feeding trouble and act as alternative source for ruminants with high biological value. In this study, enhancement in OM and CP with ML addition were observed. Kholif et al. (2019) revealed that ML extract supplementation in Nubian goats enhanced the digestibility of OM, dry matter and NDF. Fadiyimu et al. (2010) also revealed that feeding concentrate along with ML (25%) significantly enhanced the intake of CP, DM, weight gain and nutrient digestibility in sheep. This may be due the production of phenolics, tannins and saponins by ML which can be used as energy sources in low amounts by rumen microbes during rumen fermentation (Bodas et al. 2012). CP fraction of ML was 264, 435 g/kg DM (Gupta et al. 1989; Makkar and Becker 1996). Sultana et al. (2015) reported that ML feed (100%) significantly improved the CP and NDF content in Bengal goats.
Secondary metabolites in ML
Secondary metabolites found in ML include tannins, flavonoids, phenolics and saponins may also contribute to improvements in nutrient digestibility. Rumen microbes can utilize them as energy sources without negatively affecting rumen fermentation (Abdel-Raheem and Hassan 2021). They also have antibacterial and protozoal effects that help to reduce methane production while increasing acetate production, which improves carbohydrate digestion in ruminants (Parra-Garcia et al. 2019). SM also have an effect on ruminal cellulolytic and ammonia producing bacteria and limit the production of gases required for methanogenesis (Goel and Makkar 2012; Kholif and Olafadehan 2021).
Mineral profile of different feeds
Mineral content varied as the feed available. Supplementation of plant derived feed having appropriate nourishing sources and one of the finest methods to improve the nutritional status in ruminants (Abou-Elkhair et al. 2020). ML are appropriate for animal feed not only for nutrients it also having low quantities of antinutrients. In this study magnesium and iron content were higher in ML as compared to berseem and concentrate mixture. Many plants are deficient in minerals like iron but ML contains calcium, phosphorus, iron, magnesium and zinc about 24,700, 4,400, 318.81, 190 and 22.05 (mg/kg) on dry matter basis (Teixeira et al. 2014). Calcium is the utmost plentiful mineral vital for the animal body involved in skeleton and teeth formation. Keeping the optimum level of minerals such as calcium and phosphorus is crucial for normal functioning of the body. Trace minerals also play an important function for animal body and required in small amount such as zinc, iron, manganese and copper which involved in tissue repairing, protein metabolism, improved immune status and had positive outcome on milk production of animals (Brisibe et al. 2009).
Gas production and rumen fermentation parameters
Animal feed composition having critical aspect to control the methane emission. Recently ruminant methane reduction approaches involved the addition of some inhibitors such as chemical, biological and natural animal feed to inhibit the growth of methanogenic microbes in gut of animals. ML are effective methanogen inhibitors and thus considered alternatives for rumen fermentation pathways. In this study increase in total gas production was observed upto 10% level. Gas production is mainly due to liberation of acetate, propionate and butyrate by the fermentation of carbohydrates. In the present study it is revealed that as the roughage contents increased methane production also augmented, but addition of ML reduces the methane production at 0, 5, 10, 15, 20, 30 and 40% levels. This might be due to the presence of ɑ-linolenic, tannins and saponins in ML (Machmüller et al. 2000). Presence of tannins and phenolics had antimicrobial effects which can be a main cause for methane reduction (Goel and Makkar 2012). Reduction in methane (17%) was also observed in ML treated ruminants over soyabean meal (Soliva et al. 2015). Supplementation of ML by replacing soyabean meal significantly reduces methane production, ammonia-N in steers and goats, but increased the production of CO2 reported by Elghandour et al. (2017a, b). ML feed decreased enteric methane emission and increased milk production in dairy cows as reported by Bashar et al. (2020). ML feeding may reduce the energy loss including methane and urinary nitrogen without having an effect on beef cattle production (Sultana et al. 2021).
Higher ruminal digestibility of fibers and other constituents in ML reportedly contributes to its considerably high energy concentration. Dey et al. (2014) also reported increased in the TDMD and TOMD contents on supplementation of M. oleifera leaves to wheat straw. Supplementation of M. oleifera improve digestibility, sustained outstanding situation and confirm better feeding value (Cohen-Zinder et al. 2016). Therefore, improvement in TDMD and TDMO in the present study might be due to higher degradability of Moringa leaves as both the parameters improved with incremental levels of concentrate replacement with ML. Li et al. (2019) reported that ML diet can enhance nutrient intake, nutritional digestibility and rumen fermentation in dairy Holsteins cows. Aregheore (2020) reported that ML supplementation (20%) in growing goats improved digestibility and weight gain.
The increasing level of ML in the experimental did not affect ammonical nitrogen concentration in any of the TMR. Ammonia-N ratio of ruminal in this study reached from 12.02 to 13.14 (mg/dl). This could be due to the total dietary nitrogen level was at par (iso-nitrogenous) or with a small difference, and thus nitrogen degradation in the rumen occurred in a similar fashion among the R:C ratio or within the same ratio in different level of ML replacement. Elghandour et al. (2017a, b) reported that ML supplementation decreased the ruminal ammonia-N and protozoal population. Application of ML decreased ruminal ammonia-N due to presence of tannins and phenols help retain dietary proteins and slow down the degradability of rumen proteins (Kholif et al. 2015, 2016). Rumen protozoa are thought to be the primary source of rumen ammonia due to bacterial protein consumption and proteolysis (Bhatta et al. 2012). Reduction in ammonia-N also may be due to the decrease in the protozoal and bacterial population which involved in degradation of proteins in ruminants (Newbold et al. 2004). ML may play a function in limiting ammonia by reducing ruminal protein breakdown and deamination and as well as rumen ammonia. Higher VFA and lower ruminal ammonia during ML feed showed increased in consumption of dietary nitrogen (Babiker et al. 2017). Increase in propionate production also represents a change in rumen fermentation to reduce methane emission (Polyorach et al. 2014). Moringa leaves silage increased the total gas production, acetate, propionate while reduced the ruminal protozoa population and methane production (Morsy et al. 2022).
PF which is the ratio of in vitro substrate truly digested to gas volume (Blümmel et al. 1997) theoretically varies from 2.75 to 4.41 the values of PF of all the three groups with ML were within the normal range indicating proper portioning of nutrient for microbial mass production. The increase in MBP (mg) in the current study might be due to higher fraction of CP, in concurrence with greater ruminal degradability of ML protein (Makkar and Becker 1997). It might also be due to the improvement of the rumen microbiome and stimulation of fermentation process by the fermentable N and available carbohydrates supplied by M. oleifera leaves. ML supplementation altered ruminal fermentation and reduced in vitro greenhouse gases production (Kholif et al. 2022). Present study revealed that supplementation of ML improved protein content, digestibility rate, microbial biomass and partial fraction and reduces methane gas emission.
Moringa oleifera leaves can be used as a protein basis in diet of goats under in vitro conditions. Supplementation of ML improved the nutrient digestibility, rumen fermentation parameters and corresponding decrease in methane production. Consequently, it can be concluded that M. oleifera leaf powder can be replaced up to 10–20% of concentrate as a protein source and for methane mitigation in ruminants. Still, further study on different animals with different concentration of ML is needed to validate/expand the results under in vitro and in vivo conditions.
Acknowledgements
We thank the Director, National Dairy Research Institute, Karnal, Haryana, India for providing facilities to conduct this research.
Abbreviations
- ML
Moringa leaves
- TDMD
True dry matter digestibility
- PF
Partial fraction
- CP
Crude protein
- EE
Ether extract
- OM
Organic matter
- MBP
Microbial biomass
- TMR
Total mixed ration
Author contributions
VKL performed all experiments and data analysis, PC wrote and editing the manuscript, MK helped in data analysis, MM conceptualization and editing the manuscript, GM experimental concept, design and final editing of the manuscript. All authors read and approved the final manuscript.
Funding
Not applicable.
Availability of data and materials
All data are presented in tables and figures within this manuscript.
Declarations
Ethics approval and consent to participate
Approval was obtained from the Institute Animal Ethics committee with IAEC number 92/16.
Consent for publication
Not applicable.
Competing interests
Authors have no competing interests to declare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abbas RK, Elsharbasy FS, Fadlelmula AA. Nutritional values of Moringa oleifera, total protein. amino acid, vitamins, minerals, carbohydrates, total fat and crude fiber, under the semi-arid conditions of Sudan. J Microb Biochem Technol. 2018;10:56–58. doi: 10.4172/1948-5948.1000396. [DOI] [Google Scholar]
- Abdel-Raheem SM, Hassan EH. Effects of dietary inclusion of Moringa oleifera leaf meal on nutrient digestibility, rumen fermentation, ruminal enzyme activities and growth performance of buffalo calves. Saudi J Biol Sci. 2021;28:4430–4436. doi: 10.1016/j.sjbs.2021.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abou-Elkhair R, Mahboub H, Sadek K, Ketkat S. Effect of prepartum dietary energy source on goat maternal metabolic profile, neonatal performance, and economic profitability. J Adv Vet Anim Res. 2020;7:566–574. doi: 10.5455/javar.2020.g454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afzal A, Hussain T, Hameed A, Shahzad M, Mazhar MU, Yang G. Dietary Moringa oleifera alters periparturient plasma and milk biochemical indicators and promotes productive performance in goats. Front Vet Sci. 2022;8:787719. doi: 10.3389/fvets.2021.787719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Juhaimi FY, Alsawmahi ON, Abdoun KA, Ghafoor K, Babiker EE. Antioxidant potential of Moringa leaves for improvement of milk and serum quality of Aardi goats. South Afr J Bot. 2020;129:134–137. doi: 10.1016/j.sajb.2019.03.022. [DOI] [Google Scholar]
- Aregheore EM. Intake and digestibility of Moringa oleifera–batiki grass mixtures by growing goats. Small Rumin Res. 2020;46:23–28. doi: 10.1016/S0921-4488(02)00178-5. [DOI] [Google Scholar]
- Association of Official Analytical Chemist . Official methods of analysis. 21. Washington, DC: Association of Official Analytical Chemists; 2005. [Google Scholar]
- Babiker EE, Juhaimia FAL, Ghafoora K, Abdoun KA. Comparative study on feeding value of Moringa leaves as a partial replacement for alfalfa hay in ewes and goats. Livest Sci. 2017;195:21–26. doi: 10.1016/j.livsci.2016.11.010. [DOI] [Google Scholar]
- Baccou JC, Lambert F, Sauvaire Y. Spectrophotometric method for the determination of total steroidal sapogenin. Analyst. 1977;102:458–465. doi: 10.1039/an9770200458. [DOI] [PubMed] [Google Scholar]
- Bannink A, Kogut J, Dijkstra J, France J, Kebreab E, Van Vuuren AM, Tamminga S. Estimation of the stoichiometry of volatile fatty acid production in the rumen of lactating cows. J Theor Biol. 2006;7(238):36–51. doi: 10.1016/j.jtbi.2005.05.026. [DOI] [PubMed] [Google Scholar]
- Bashar MK, Huque KS, Sarker NR, Sultana N. Quality assessment and feeding impact of Moringa feed on intake, digestibility, enteric CH4 emission, rumen fermentation, and milk yield. J Adv Vet Anim Res. 2020;7:521–529. doi: 10.5455/javar.2020.g449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benetel G, Silva TDS, Fagundes GM, Welter KC, Melo FA, Lobo AAG, Muir JP, Bueno ICS. Essential oils as in vitro ruminal fermentation manipulators to mitigate methane emission by beef cattle grazing tropical grasses. Molecules. 2022;27:2227. doi: 10.3390/molecules27072227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatta R, Saravanan M, Baruah KTL. Nutrient content, in vitro ruminal fermentation characteristics and methane reduction potential of tropical tannin-containing leaves. J Sci Food Agric. 2012;92:2929–2935. doi: 10.1002/jsfa.5703. [DOI] [PubMed] [Google Scholar]
- Blümmel M, Makkar HPS, Becker K. In vitro gas production—a technique revisited. J Anim Physiol Anim Nutr. 1997;77:24–34. doi: 10.1111/j.1439-0396.1997.tb00734.x. [DOI] [Google Scholar]
- Bodas R, Prieto N, García-González R, Andrés S, Giráldez FJ, López S. Manipulation of rumen fermentation and methane production with plant secondary metabolites. Anim Feed Sci Technol. 2012;176:78–93. doi: 10.1016/j.anifeedsci.2012.07.010. [DOI] [Google Scholar]
- Brisibe EA, Umoren UE, Brisibe F, Magalhaes PM, Ferreira JFS, Luthria D, Wu X, Prior RL. Nutritional characterization and antioxidant capacity of different tissues of Artemisia annua L. Food Chem. 2009;115:1240–1246. doi: 10.1016/j.foodchem.2009.01.033. [DOI] [Google Scholar]
- Choudhary S, Santra A, Muwel N, Sarkar S, Mandal A, Das SK. Screening of forest tree leaves from North Eastern Himalayan region as feed additives for modulating in vitro rumen fermentation and methanogenesis from total mixed ration. Agroforest Syst. 2022;96:359–374. doi: 10.1007/s10457-021-00724-5. [DOI] [Google Scholar]
- Cohen-Zinder M, Leibovich H, Vaknin Y, Sagi G, Shabtay A, Ben-Meir Y. Effect of feeding lactation cows with ensiled mixture of M. oleifera, wheat hay and molasses, on digestibility and efficiency of milk production. Anim Feed Sci Technol. 2016;211:75–83. doi: 10.1016/j.anifeedsci.2015.11.002. [DOI] [Google Scholar]
- Dey A, Paul SS, Pandey P, Rathore R. Potential of Moringa oleifera leaves in modulating in vitro methanogenesis and fermentation of wheat straw in buffalo. Indian J Anim Sci. 2014;84:533–538. [Google Scholar]
- Dong L, Zhang T, Diao Q. Effect of dietary supplementation of Moringa oleifera on the production performance and fecal methanogenic community of lactating dairy cows. Animals. 2019;9:262. doi: 10.3390/ani9050262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebeid HM, Mengwei L, Kholif AE, Hassan FU, Lijuan P, Xin L, Chengjian Y. Moringa oleifera oil modulates rumen microflora to mediate in vitro fermentation kinetics and methanogenesis in total mix rations. Curr Microbiol. 2020 doi: 10.1007/s00284-020-01935-2. [DOI] [PubMed] [Google Scholar]
- Eisen MB, Brown PO. Rapid global phaseout of animal agriculture has the potential to stabilize greenhouse gas levels for 30 years and offset 68 percent of CO2 emissions this century. PLOS Clim. 2022;1:e0000010. doi: 10.1371/journal.pclm.0000010. [DOI] [Google Scholar]
- Elghandour MMY, Vallejo LH, Salem AZM, Mellado M, Camacho LM, Cipriano M, Olafadehan OA, Olivares J, Rojas S. Moringa oleifera leaf meal as an environmental friendly protein source for ruminants: biomethane and carbon dioxide production, and fermentation characteristics. J Clean Prod. 2017 doi: 10.1016/j.jclepro.2017.07.151. [DOI] [Google Scholar]
- Elghandour MMY, Vázquez JC, Salem AZM, Kholif AE, Cipriano MM, Camacho LM, Márquez O. In vitro gas and methane production of two mixed rations influenced by three different cultures of Saccharomyces cerevisiae. J Appl Anim Res. 2017;45:389–395. doi: 10.1080/09712119.2016.1204304. [DOI] [Google Scholar]
- Fadiyimu AA, Alokan JA, Fajemisin AN. Digestibility, nitrogen balance and haematological profile of West African dwarf sheep fed dietary levels of Moringa oleifera as supplement to Panicum maximum. J Am Sci. 2010;6:634–643. [Google Scholar]
- Folin O, Ciocalteu V. On tyrosine and tryptophane determinations in proteins. J Biol Chem. 1927;73:627–650. doi: 10.1016/S0021-9258(18)84277-6. [DOI] [Google Scholar]
- Goel G, Makkar HPS. Methane mitigation from ruminants using tannins and saponins. Trop Anim Health Prod. 2012;4:729–739. doi: 10.1007/s11250-011-9966-2. [DOI] [PubMed] [Google Scholar]
- Gupta K, Barat GK, Wagle DS, Chawla HKL. Nutrient contents and antinutritional factors in conventional and non-conventional leafy vegetables. Food Chem. 1989;31:105–116. doi: 10.1016/0308-8146(89)90021-6. [DOI] [Google Scholar]
- Gupta S, Mohini M, Thakur SS, Mondal G. Effect of dietary monensin supplementation on nitrogen utilization and plasma metabolites in lactating Murrah buffaloes. Int J Curr Microbiol App Sci. 2018;7:3838–3845. doi: 10.20546/ijcmas.2018.707.446. [DOI] [Google Scholar]
- Kholif AE, Olafadehan OA. Essential oils and phytogenic feed additives in ruminant diet: chemistry, ruminal microbiota and fermentation, feed utilization and productive performance. Phytochem Rev. 2021;20:1087–1108. doi: 10.1007/s11101-021-09739-3. [DOI] [Google Scholar]
- Kholif AE, Gouda GA, Morsy TA, Salem AZM, Lopez S, Kholif AM. Moringa oleifera leaf meal as a protein source in lactating goat's diets: feed intake, digestibility, ruminal fermentation, milk yield and composition, and its fatty acids profile. Small Rumin Res. 2015;129:129–137. doi: 10.1016/j.smallrumres.2015.05.007. [DOI] [Google Scholar]
- Kholif AE, Morsy TA, Gouda GA, Anele UY, Galyean ML. Effect of feeding diets with processed Moringa oleifera meal as protein source in lactating Anglo-Nubian goats. Anim Feed Sci Technol. 2016;217:45–55. doi: 10.1016/j.anifeedsci.2016.04.012. [DOI] [Google Scholar]
- Kholif AE, Gouda GA, Anele UY, Galyean ML. Extract of Moringa oleifera leaves improves feed utilization of lactating Nubian goats. Small Rumin Res. 2018;158:69–75. doi: 10.1016/j.smallrumres.2017.10.014. [DOI] [Google Scholar]
- Kholif AE, Gouda GA, Galyean ML, Anele UY, Morsy TA. Extract of Moringa oleifera leaves increases milk production and enhances milk fatty acid profile of Nubian goats. Agroforest Syst. 2019;93:1877–1886. doi: 10.1007/s10457-018-0292-9. [DOI] [Google Scholar]
- Kholif AE, Gouda GA, Morsy TA, Matloup OH, Fahmy M, Gomaa AS, Patra AK. Dietary date palm leaves ensiled with fibrolytic enzymes decreased methane production, and improved feed degradability and fermentation kinetics in a ruminal in vitro system. Waste Biomass Valor. 2022;13:3475–3488. doi: 10.1007/s12649-022-01752-7. [DOI] [Google Scholar]
- Komolong MK, Barber DG, McNeill DM. Post-ruminal protein supply and N retention of weaner sheep fed on a basal diet of lucerne hay (Medicago sativa) with increasing levels of quebracho tannins. Anim Feed Sci Technol. 2001;92:59–72. doi: 10.1016/S0377-8401(01)00246-2. [DOI] [Google Scholar]
- Ku-Vera JC, Jiménez-Ocampo R, Valencia-Salazar SS, Montoya-Flores MD, Molina-Botero IC, Arango J, Gómez-Bravo CA, Aguilar-Pérez CF, Solorio-Sánchez FJ. Role of secondary plant metabolites on enteric methane mitigation in ruminants. Front Vet Sci. 2020;7:584. doi: 10.3389/fvets.2020.00584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Zhang G-N, Xu H-J, Zhou S, Dou X-Z, Lin C, Zhang X-Y, Zhao H-B, Zhang Y-G. Effects of replacing alfalfa hay with Moringa oleifera leaves and peduncles on intake, digestibility, and rumen fermentation in dairy cows. Livest Sci. 2019;220:211–216. doi: 10.1016/j.livsci.2019.01.005. [DOI] [Google Scholar]
- Machmüller A, Ossowski DA, Kreuzer M. Comparative evaluation of the effects of coconut oil, oilseeds and crystalline fat on methane release, digestion and energy balance in lambs. Anim Feed Sci Technol. 2000;85:41–60. doi: 10.1016/S0377-8401(00)00126-7. [DOI] [Google Scholar]
- Makkar HPS, Becker K. Nutritional value and antinutritional components of whole and ethanol extracted Moringa oleifera leaves. Anim Feed Sci Technol. 1996;63:211–228. doi: 10.1016/S0377-8401(96)01023-1. [DOI] [Google Scholar]
- Makkar HPS, Becker K. Nutrients and antiquality factors in different morphological parts of the Moringa oleifera tree. J Agric Sci. 1997;128:311–322. doi: 10.1017/S0021859697004292. [DOI] [Google Scholar]
- Menke KH, Steingass H. Estimation of the energetic feed value obtained from chemical analysis and in vitro gas production using rumen fluid. Anim Res Dev. 1988;28:7–55. [Google Scholar]
- Morsy TA, Gouda GA, Kholif AE. In vitro fermentation and production of methane and carbon dioxide from rations containing Moringa oleifera leave silage as a replacement of soybean meal: in vitro assessment. Environ Sci Pollut Res. 2022 doi: 10.1007/s11356-022-20622-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbold CJ, McIntosh FM, Williams P, Losa R, Wallace RJ. Effects of a specific blend of essential oil compounds on rumen fermentation. Anim Feed Sci Technol. 2004;114:105–112. doi: 10.1016/j.anifeedsci.2003.12.006. [DOI] [Google Scholar]
- Nwinuka NM, Ibeh GO, Ekeke GI. Proximate composition and levels of some toxicants in four commonly consumed spices. J Appl Sci Environ Manag. 2005;9:150–155. [Google Scholar]
- Parra-Garcia A, Elghandour MMY, Greiner R, Barbabosa-Pliego A, Camacho-Diaz LM, Salem AZM. Effects of Moringa oleifera leaf extract on ruminal methane and carbon dioxide production and fermentation kinetics in a steer model. Environ Sci Pollut Res. 2019;26:15333–15344. doi: 10.1007/s11356-019-04963-z. [DOI] [PubMed] [Google Scholar]
- Polyorach S, Wanapat M, Cherdthong A. Influence of yeast fermented cassava chip protein (YEFECAP) and roughage to concentrate ratio on ruminal fermentation and microorganisms using in vitro gas production technique. Asian Australas J Anim Sci. 2014;27:36–46. doi: 10.5713/ajas.2013.13298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleem A, Saleem M, Akhtar MF. Antioxidant, anti-inflammatory and antiarthritic potential of Moringa oleifera Lam: an ethnomedicinal plant of Moringaceae family. South Afr J Bot. 2020;128:246–256. doi: 10.1016/j.sajb.2019.11.023. [DOI] [Google Scholar]
- Soliva CR, Kreuzer M, Foidl N, Foidl G, Machmüller A, Hess HD. Feeding value of whole and extracted Moringa oleifera leaves for ruminants and their effects on ruminal fermentation in vitro. Anim Feed Sci Tech. 2015;118(2):47–62. doi: 10.1016/j.anifeedsci.2004.10.005. [DOI] [Google Scholar]
- Su B, Chen X. Current status and potential of Moringa oleifera leaf as an alternative protein source for animal feeds. Front Vet Sci. 2020;7:53. doi: 10.3389/fvets.2020.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultana S. Nutritional and functional properties of Moringa oleifera. Metabol Open. 2020;8:100061. doi: 10.1016/j.metop.2020.100061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultana N, Alimon A, Huque K, Sazili A, Yaakub H, Hossain J, Baba M. The feeding value of Moringa (Moringa oleifera) foliage as replacement to conventional concentrate diet in Bengal goats. Adv Anim Vet Sci. 2015;3:164–173. doi: 10.14737/journal.aavs/2015/3.3.164.173. [DOI] [Google Scholar]
- Sultana N, Das NG, Kabir MA, Deb GK, Islam MT. Metabolic benefit of bulls being fed Moringa leaves twigs and branches as a major concentrate ingredient. Front Anim Sci. 2021;2:712919. doi: 10.3389/fanim.2021.712919. [DOI] [Google Scholar]
- Teclegeorgish ZW, Aphane YM, Mokgalaka NS, Steenkamp P, Tembu VJ. Nutrients, secondary metabolites and anti-oxidant activity of Moringa oleifera leaves and moringa-based commercial products. South Afr J Bot. 2021;142:409–420. doi: 10.1016/j.sajb.2021.07.008. [DOI] [Google Scholar]
- Teixeira EM, Carvalho MR, Neves VA, Silva MA, Arantes-Pereira L. Chemical characteristics and fractionation of proteins from Moringa oleifera Lam. Leaves. Food Chem. 2014;147:51–54. doi: 10.1016/j.foodchem.2013.09.135. [DOI] [PubMed] [Google Scholar]
- Van Soest PJ, Robertson JB, Lewis BA. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J Dairy Sci. 1991;74:3583–3597. doi: 10.3168/jds.S0022-0302(91)78551-2. [DOI] [PubMed] [Google Scholar]
- Wankhede SD, Dutta N, Tambe MB, Kaur N, Jadhav SE, Pattanaik AK. Effect of dietary inclusion of Moringa oleifera foliage on nutrient metabolism, metabolic profile, immunity and growth performance of goat kids. Emerging Animal Species. 2022;3:100005. doi: 10.1016/j.eas.2022.100005. [DOI] [Google Scholar]
- Zeru AE, Hassen A, Apostolides Z, Tjelele J. Screening of candidate bioactive secondary plant metabolite ion-features from Moringa oleifera accessions associated with high and low enteric methane inhibition from ruminants. Metabolites. 2022;12:501. doi: 10.3390/metabo12060501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhishen J, Mengcheng T, Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999;64:555–559. doi: 10.1016/S0308-8146(98)00102-2. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are presented in tables and figures within this manuscript.


