Abstract
Systemic sclerosis (SSc) is a chronic autoimmune disease, featuring fibrosis in multiple organs. The serum from SSc patients contain inflammatory mediators, contributing to SSc pathogenesis and could be used to develop cell culture models. Here, we compared the fibrotic effects of serum samples from SSc patients with TGFβ1 on human dermal fibroblasts (HDFs). HDF cells were cultured in four different culture media supplementations; 10% SSc serum, 10% healthy human serum, 10% fetal bovine serum or 10% FBS supplemented with 10 ng/Ml human TGFβ. The collagen content in cell layers was measured by spectrophotometric Picro-Sirius red staining. The mRNA expression of α-SMA, COL I and III, TGFβ1, arginase and E-Cadherin genes were determined by real time RT-PCR. TGF-β1 levels in cell culture supernatants were measured using ELISA. Cell layer collagen content was significantly increased following TGF-β1 treatment, compared with FBS group and SSc serum treatment in comparison with healthy controls. Although not statistically significant, the mRNA expression of α-SMA, COLI and III, TGFβ1, and arginase increased upon TGF-β1 treatment in comparison with FBS group, and in SSc serum treatment group in comparison with healthy controls. E-Cadherin decreased following TGF-β1 treatment and SSc serum treatment in comparison with their counterparts. TGF-β1 levels increased in cell culture supernatants of HDF cells exposed to TGF-β1 and SSc serum. An in vitro model of SSc serum-induced fibrosis using human HDF cells was evaluated in comparison to the TGF-β1 fibrosis induced model and data suggested that it may be used in documenting the role of pro-fibrotic factors in serum or plasma from SSc patients.
Key Words: Fibrosis, human dermal fibroblast, systemic sclerosis, autoimmune disease, TGF-β
Introduction
Systemic sclerosis (SSc, scleroderma) is an idiopathic, chronic autoimmune disease, characterized by immune system abnormalities including excessive autoantibodies (1) and abnormal inflammatory mediators (2). Typical representatives of SSc are vascular damage, endothelial dysfunction and excessive fibrosis, particularly affecting the skin but may progress to internal organs such as heart and lungs (3). Being more common in women (4), SSc has the highest mortality rate among rheumatic diseases (5). Based on the degree of skin involvement fibrosis, SSc is divided into two types: diffuse cutaneous SSc and limited cutaneous SSc (6).
Despite various suggested hypotheses on the pathogenetic mechanisms in SSc, the relationship between autoimmunity-mediated fibrosis and organ damage is not thoroughly understood. The immune system may promote fibrosis, while fibrosis may reciprocally activate the immune system (7). Sera obtained from SSc patients contain mediators of inflammation which possibly contribute to tissue injury and organ dysfunction (7). Thus, these inflamed serum or plasma could be considered as suitable sources of developing SSc models, either in vitro, ex vivo or in vivo (8).
Fibrosis arises from an imbalance between deposition and removal of extracellular matrix (ECM) components (9), and demonstrates active myofibroblasts producing excessive amounts of ECM proteins, including collagen; both are the hallmarks of SSc (10). Fibrosis occurs at different stages of the disease, depending on the stage of development, it has clinically different progression, and shows a poor response to treatment (3). Examinations of the skin and other internal organs show an increase in fibroblasts, resulting in dense deposits of collagen and other members of the ECM (11). The molecular mechanism of fibrosis in SSc is not fully understood, but extracellular and intracellular stimuli and processes, such as disturbing the balance of the ECM components, and overexpression of ECM proteins, especially collagens and fibronectin, play the most significant role (9).
Many pro-fibrotic cytokines, which were increased in SSc patients, are involved in the development and progression of fibrosis-associated SSc, including transforming growth factor (TGF)-β1 (12, 13), interleukin 4 (IL-4), IL- 6 (14), IL-13 (15), IL-17 (16), and IL-33 (17). Despite this complexity, TGF‐β is a potent inducer of tissue fibrosis and has been used for in vitro fibrosis models (18) by inducing collagen accumulation (19). The pro-fibrotic role of TGF‐β and its signaling through SMAD (suppressor of mothers against decapentaplegic) proteins has been also well studied (20). Although the TGF‐β-mediated in vitro fibrosis model replicates several aspects of SSc biology, it does have important limitations. The cytokine network in SSc is much more complex and the administration of one cytokine is unlikely to fully reproduce the SSc phenotype (21).
Animal models have been developed that reproduce some features of SSc; these include a bleomycin-induced model, a sclerodermatous graft-versus-host disease model (22, 23), and other murine models using reactive oxygen species (ROS) (24), DNA topoisomerase I antigen with complete Freund’s adjuvant (25), and angiotensin II (26). Nevertheless, there is increasing ethical concern about the use of animal models in medical research and so there is a pressing need to develop and validate more efficient in vitro models to study SSc. Here, we investigated the fibrotic effects of SSc patient serum on cultured human cutaneous fibroblast cells (HDFs) as a novel cellular model for SSc, in comparison to the fibrotic effects of TGF‐β.
Materials and Methods
Cell culture and treatments
The current study was approved by the committee of ethics at Golestan University of Medical Sciences (GoUMS) (code of ethics: IR.GOUMS.REC.1398.121). All subjects provided a written informed consent prior to research participation. Eight female SSc subjects and eight healthy age-matched healthy female volunteers were enrolled. Patients with symptoms overlapping with those of other autoimmune, rheumatic and/or connective tissue diseases were excluded. Venous blood samples were drawn from all subjects and allowed to clot for 30 min before centrifugation at 1,500 g for 15 min. Sera were stored in aliquots at –80 °C until use. Serum samples were heat-inactivated and filtered to remove aggregates, as previously described (27).
The effects of SSc patients sera on TGF-β1-induced fibrosis HDF cells was compared with those of healthy individuals’ sera. Briefly, human dermal fibroblast (HDF) cells (National Cell Bank of Iran (NCBI), Pasteur Institute, Iran) (NCBI code: C645) were cultured in Roswell Park Memorial Institute (RPMI) 1640 medium (Bioidea, Iran), containing 100 U/mL penicillin–streptomycin (Bioidea, Iran), and 10% fetal bovine serum (FBS) (Bioidea, Iran), and incubated at 37 °C in 5% CO2 humidified chamber. Cells were trypsinized using trypsin-EDTA (Bioidea, Iran), seeded into 96-well plates, and divided into four groups: SSc growth medium supplemented with 10% SSc serum, normal growth medium supplemented with 10% healthy human serum,10% FBS, and 10% FBS supplemented with 10 ng/mL recombinant human TGF-β1 (eBioscience, USA) (21), and cultured for 48 h under suitable condition.
Picro-Sirius red staining and spectrophotometric analysis
Picro-Sirius red, a strong anionic dye containing 6 sulfate groups, binds cationic collagen fibers. This is a simple and sensitive technique for collagen staining in tissues and cultures (28). Briefly, cells were cultured in 96-well plates and fixed in Kahle’s fixation solution for 10 min at room temperature. Kahle’s fixation solution contains 30 ml 95% ethanol, 12 ml 37% formaldehyde, 4 mL glacial acetic acid, and 60 mL distilled water (29). Cell layers were carefully washed with PBS and incubated in the Picro-Sirius red (Sigma-Aldrich, USA) (0.1% in PBS) at room temperature for 1 h. The staining solution was removed, cells were washed three times with 0.1% acetic acid, and carefully washed once with PBS. For spectrophotometric analysis, Picro-Sirius red was eluted in 0.1 N sodium hydroxide (NaOH), 200 mL/well, the plates were placed on a rocking platform at room temperature for 1 h, and the optical density at 540 nm was determined using a 4300 Chromate Manager spectrophotometer (Peachtree Corners, Georgia, USA) (21).
Total RNA extraction and quantitative gene expression analysis by real-time PCR
Total RNA from cultured HDFs was extracted using RNX-plus reagent (Cinnagen, Iran) according to the manufacturer’s instructions. Yield and purity of the extracted RNA was determined by spectrophotometry (Picodrop, Saffron Walden, UK). Total RNA was aliquoted and stored at -80 °C, immediately after extraction. One µg of extracted RNA was reverse transcribed using RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher, Waltham, MA, USA). Real-time polymerase chain reaction (RT-PCR) was used to quantify mRNA gene expression of TGFβ1, collagen type I and III, arginase, α-SMA, and E-cadherin. Glyceraldehyde 3-phosphate dehydro-genase (GAPDH) was used as an internal control to normalize gene expression levels. The sequences of specific primers are listed in Table 1. Real-time (RT)-PCR reaction was performed using 1 µL total cDNA, 0.5 µL of each primer and an RT-PCR kit with SYBR Green (Yekta Tajhiz, Tehran, Iran) according to the manufacturer’s protocols. Reactions were performed using ABI step one plus Cycler (Applied Biosystems, CA, USA) with the following reaction profile for cDNA synthesis: pre-denaturation for 3 min at 94 °C; PCR amplification for 40 cycles (each cycle consisting of 10 s at 94°C, 30 s at 60 °C and 40 s at 72 °C). The efficiency and specificity of each primer set was confirmed with standard curve and melting profile analysis.
Table1.
List of specific primers used for quantitative real time RT-PCR
| Amplicon size (bp) | Tm (°C) | Sequence (5’>3’) |
Gene Bank
accession no. |
Genes |
|---|---|---|---|---|
| 259 | 58 | ACGAAGACATCCCACCAATC | NM_001100.4 | hCol1a1-F |
| ATGGTACCTGAGGCCGTTC | NM_001100.4 | hCol1a1-R | ||
| 70 | 58 | AGGATGGTTGCACGAAACAC | NM_000090.4 | hCOL3A1-F |
| ACAGCCTTGCGTGTTCGATA | NM_000090.4 | hCOL3A1-R | ||
| 79 | 59.5 | CCCTGGGGAACACTACATTTTG | NM_001244438.2 | ARG1-F |
| GCCAATTCCTAGTCTGTCCACTT | NM_001244438.2 | ARG1-R | ||
| 202 | 58 | CAGCAACAATTCCTGGCGAT | NM_000660.7 | TGFB1-F |
| GGTAGTGAACCCGTTGATGT | NM_000660.7 | TGFB1-R | ||
| 248 | 60 | ATTTTTCCCTCGACACCGAT | NM_Z35402.1 | E. Cadherin-F |
| TCCCAGGCGTAGACCAAGA | NM_Z35402.1 | E. Cadherin-R | ||
| 172 | 58 | GAAGGTGAAGGTCGGAGT | NM_001357943.2 | GAPDH-F |
| GAAGATGGTGATGGGATTTC | NM_001357943.2 | GAPDH-R |
Measurement of TGF-β1 levels in cell culture supernatant
Cell culture supernatants were collected and stored at -80 °C until assay. TGF-β1 levels were measured using a commercial human/mouse TGF-β1 ELISA kit (eBioscience, San Diego, USA). Reaction products in triplicate wells were measured at the wavelength of 570 nm according to the manufacturer's guidelines using a Stat Fax 4300 Chromate Manager spectrophotometer.
Statistical analyses
All biological and experimental assay were performed in triplicate and data were presented as mean ± SD. SPSS 26.0 and GraphPad Prism 8.4.3 were used for data analyses. The nonparametric test of Kruskal-Wallis was used to compare means of multiple samples. Mann-Whitney U-test and the independent samples t-test were used to compare mean differences between two groups. 2-∆∆Ct method was used to normalize results. P<0.05 were considered as statistically significant.
Results
Serum from SSc patients increased the production of collagen in HDF cells
In order to evaluate the amount of collagen production from HDF cells among four treatment groups, the Picro-Sirius red staining method was used. As shown in figure 1, the collagen secretion was significantly different between four different study groups including HDF cells incubated with serum of scleroderma patients (10%), serum of healthy individuals (10%), with FBS (10%) alone or incubated with FBS (10%) plus TGF-β1 (10 ng/mL). The red color (relating to collagen content) was more intense in cells incubated either with SSc serum or FBS+ TGF-β1 groups. The morphology of the cells treated with SSc serum or FBS+ TGF-β1 were different changing to more nodular and multilayer arrangement; however, the FBS and healthy serum-treated cells showed more fibroblast-like and single layer shape (Figure 1A). Quantitative measurements by spectrometry also indicated the significant elevation of collagen in SSc serum and FBS+ TGF-β1 groups. As shown in figure 1B, collagen content in the SSc serum treatment group was significantly higher than the healthy serum treatment group (P = 0.032), and also the collagen content of the TGF-β1 group was remarkably higher than the FBS group (P = 0.007).
Fig. 1.
Collagen content assay using Picro-Sirius red staining. A: The amount of collagen in cultured human fibroblasts exposed to sera from four sources: SSc subjects, healthy individuals, FBS, and FBS supplemented TGF-β1 (10 ng/mL). Each condition was tested in triplicate. Collagen content was significantly different among the four groups (P =0.0001), as determined by the nonparametric Kruskal-Wallis with Dunn-Bonferroni post hoc testing. Each bar demonstrates means ± SEM for normally distributed values. The dots in the graph show value for each replicate. *P < 0.05, *** P < 0.001, **** P < 0.0001. B: The microscopic appearance of human dermal fibroblasts stained with Picro-Sirius red to demonstrate collagen accumulation
The effect of serum from SSc patients on the expression of fibrosis genes in comparison TGFβ1 fibrosis model
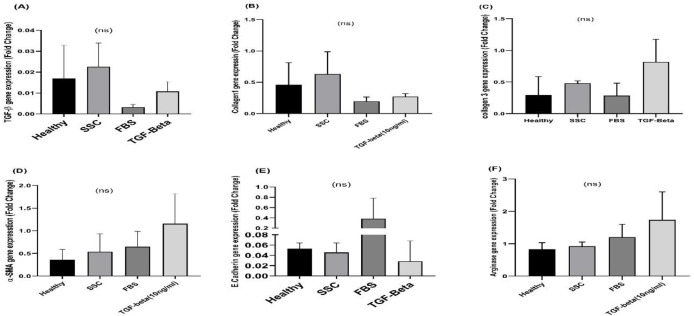
As shown in Figure 2A, the highest level of TGF-β1 gene expression was observed in the SSc serum incubated cells. We found that normalized TGF-β1 gene expression level in SSc serum-treated cells was higher than FBS+ TGF-β1 treated cells. In accordance to the Picro-Sirius staining results, figure 2B and 2C showed that the gene expression of collagen type I and III were higher in HDF cells treated with SSc serum than healthy controls and also in dermal cells treated with recombinant TGF-β1 compared to FBS alone; however, the difference were statistically non-significant. The maximum expression level of other fibrosis associated genes α-SMA and arginase were observed in HDF cells treated with FBS supplemented with human recombinant TGF-β1(not statistically significant, Figure 2D-E). As demonstrated in figure 2F, we showed that the mRNA expression level of E-Cadherin, as an epithelial associated gene, was decreased when HDF cells were exposed to TGF-β1+FBS or human serum from SSc patients compared to FBS or healthy control, respectively (not statistically significant).
Fig. 2.
The mRNA expression levels of fibrosis-associated genes among cultured cells exposed to four treatments. The differences in the mRNA expression levels of fibrosis-associated genes in fibroblasts, cultured with serum from healthy controls, serum from SSC patients, FBS alone and human TGFβ1 (10 ng/mL) + FBS, are demonstrated. A: TGFβ; B: Collagen I; C: Collagen III; D: α-SMA; E: E-cadherin; F: Arginase. Results are presented as mean ± SEM. Data were analyzed using the nonparametric Kruskal-Wallis test with Dunn-Bonferroni post hoc testing. NS: not statistically significant
Serum from SSc patients increased the secretion of TGF-β in HDF cells: As shown in Figure 3, the secretion of TGFβ1 was significantly different between treatment groups (P = 0.046), with increased levels in supernatant of human dermal fibroblast cells treated with both SSc serum and FBS+ TGF-β1. The highest level of TGF-β1 was detected in FBS+ TGF-β1 group. The TGF-β1 level in supernatant of the SSc serum treated HDF cells was higher than healthy serum treated cells, indicating the fibrotic effect of SSc serum in cell culture
Fig. 3.
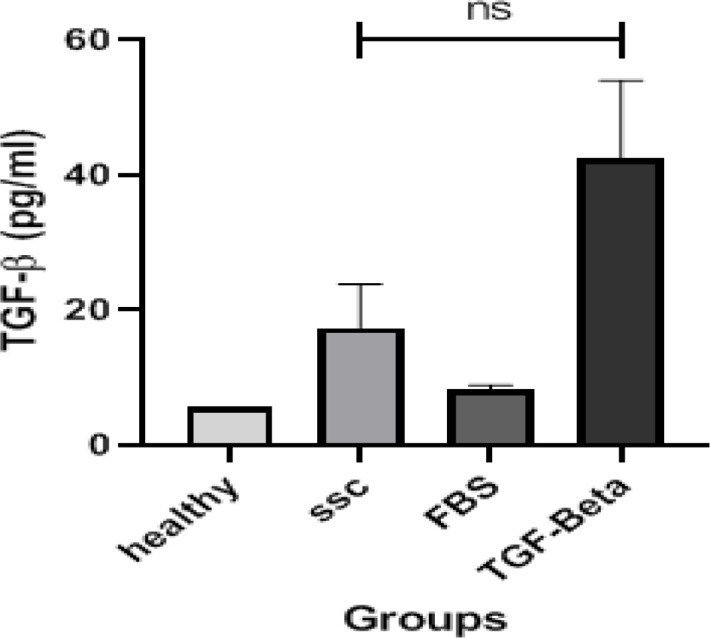
TGF-β1 levels in cell culture supernatants. The differences in the TGF-β1 levels in fibroblasts, cultured with serum from healthy controls, serum from SSC patients, FBS alone and human TGFβ1 (10 ng/mL) + FBS, are demonstrated. Kruskal-Wallis with Dunn-Bonferroni post hoc test was used to compare the means of multiple samples. All of the experiments were repeated in duplicate for each sample. Data of each bar demonstrates means ± SD for all values. NS: not statistically significant
Discussion
SSc is a complex autoimmune disease defined by fibrosis-associated complications (30, 31). Among the developed in vitro fibrosis models, previous models have been characterized by TGF-β induction (21), but there is no specific cellular model for SSc that can be similar to the clinical conditions of scleroderma (21, 32). Accordingly, we investigated the fibrosis effects of serum from SSc patients on human dermal fibroblast cells (HDFs), in comparison with the fibrosis model induced on human fibroblast cells by TGF-β1. In this study, in order to evaluate the accuracy of the fibrosis model induced by growth factor TGF-β1, a comparison of this group with the FBS group was conducted.
We also performed a comparison between serum from SSc patients and healthy individuals to evaluate the effect of inducing fibrosis. In the current study, we used 10ng/ml of TGF-β1 for fibrosis induction in HDF cells and found higher collagen content, higher fibrosis-associated gene expression (TGF-β1, collagen I and III, α-SMA) and higher TGF-β1 secretion. Although both SSc serum treated and TGF-β1 treated fibroblasts demonstrated elevated levels of TGF-β1, the lower levels of TGF-β1 among cytokine-treated counterparts could be due to the negative regulation of the TGF-β signaling, during the addition of excessive, exogenous cytokines to the culture media (33). The α-SMA is the main marker of active fibroblast cells named myofibroblasts (34), which increases in fibrotic conditions (35). The α-SMA is the actin protein isoform and plays an important role in the fibrosis process. The expression of α-SMA is higher in myofibroblasts and their activation plays an important role in the fibrosis promotion (36). As mentioned, we found higher expression of the fibrosis-associated genes in the FBS+TGF-β1 group compared to FBS group. Similar changes in the gene expression were seen in the group of HDF cells treated with serum from SSc patients in comparison with the serum from healthy individuals, which may be due to the high level of several inflammatory factors and fibrosis stimulants in the serum of these individuals (37). These inflammatory factors including pro-inflammatory cytokines such as IL-6, IL1β, and IL-8 are not only attributed to fibrosis, but also related to several other fibrosis-related diseases (38).
Both TGF-β1 and SSc patient serum, compared to the other two groups (FBS and normal serum), non-significantly increased the gene expression of arginase, the enzyme involved in production of polyamines. Polyamines as ingredient of extracellular matrix elements can play an important role in cell migration and are indirectly related to the fibrotic conditions (39). Although, TGF-β1 treatment markedly increased the mRNA expression of arginase in HDF cells, compared to other counterparts, this elevation was not noticeable among HDF cells treated with SSc serum, which might be due to the lack of other necessary ECM components to elicit its expression (40).
Collagen secretion is also increased by the induction of TGF-β1 in the studies using Picro-Sirius staining according to the previous studies (21). In the current study, the comparison between TGF-β1 and the FBS groups, as well as the SSc serum group in comparison with the normal serum group demonstrated a significant increase in collagen content of the two first groups in comparison to their counterparts. This observation is in accordance with the results of the previous studies (21), TGF-β1 at a concentration of 1 ng/mL is able to induce fibrosis and thus increase the mRNA expression levels of collagen type I and III, which are important components of the ECM repair following injury. Although collagen type I is generally introduced as a major fibrosis-associated protein, the increase in thin fibrils of SSc skin is most likely due to an increase in type III collagen (41). Accordingly, the higher levels of both collagen types among SSc treated samples, could demonstrate the indisputable effect of SSc serum on the expression of fibrosis-associated genes. To the best of our knowledge, this is the first study investigating the effect of sera from SSc patients on fibrosis induction in HDF fibroblasts and also proposing an in vitro/ex vivo model. This model is probably suitable for investigating the effect of anti-fibrotic and anti-inflammatory agents in pre-clinical studies.
We also showed that E-cadherin was decreased among HDF cells treated with serum from SSc patients compared to the groups treated with serum from the healthy individuals, and in the TGF-β1 treated cells in comparison with the FBS group. According to previous studies, the expression of E-cadherin gene is decreased in fibrotic conditions (42), which was in accordance with our findings. The downregulation of E-cadherin could be responsible for the loss of cellular adhesion and the overexpression of β-catenin for the induction of fibrosis (43). It also can explain the morphological changes and nodular re-arrangement of the TGF-β1 and SSc serum treated HDF cells observed in Figure 1. It was previously reported that TGF-β1 induction can increase the collagen secretion using Picro-Sirius staining method (21). Our investigation was also associated with limitations, including the diverse clinical symptoms of included SSc patients. In spite of our significant efforts to match the confounding variables between the patients and the healthy subjects, due to the origin of SSc which encompasses diverse inflammatory manifestations, the sera might have been different, regarding the inflammatory background. In order to overcome this limitation, we pooled the sera from patients and increased the biological replicates. Moreover, we studied the effects of sera from SSc patients in monolayer cell culture, which is suggested to be studied in 3D culture, in future studies to better evaluate the effects of ECM components. In the current study, the comparison between TGF-β1 and the FBS groups, as well as the SSc serum group in comparison with the normal serum group demonstrated a significant increase in collagen content of the two first groups in comparison to their counterparts. Accordingly, the SSc serum-induced fibrosis assessment in HDF cell culture could potentially be a suitable model for investigating the effect of anti-fibrotic and anti-inflammatory agents in pre-clinical studies.
In conclusion, we developed and characterized an ex vivo/ in vitro cellular model of SSc by applying serum from SSc patients and inducing fibrosis in human dermal fibroblast (HDF) culture, in comparison to a previously confirmed induction model by TGF-β1. We showed that serum from SSc patients, due to the ability of inducing pro-fibrotic state in the HDF cells, could be an effective alternative for TGF-β1 induced fibrosis model and could be used to study the effects of anti-fibrotic agents in SSc; similar approaches could be used for other diseases.
Conflicts of Interest
Authors declare no conflict of interest.
Acknowledgment
We would like to appreciate the scientific and technical consults from Professor Jeffrey kopp. This article was derived from a M.Sc. thesis in the field of Medical Immunology which was financially supported by the Deputy of Research and Technology at GoUMS (Grant code: 110609)
References
- 1.Furue M, Mitoma C, Mitoma H, et al. Pathogenesis of systemic sclerosis-current concept and emerging treatments. Immunol Res. 2017;65:790–7. doi: 10.1007/s12026-017-8926-y. [DOI] [PubMed] [Google Scholar]
- 2.Doridot L, Jeljeli M, Chene C, et al. Implication of oxidative stress in the pathogenesis of systemic sclerosis via inflammation, autoimmunity and fibrosis. Redox Biol. 2019;25:101122. doi: 10.1016/j.redox.2019.101122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Korman B. Evolving insights into the cellular and molecular pathogenesis of fibrosis in systemic sclerosis. Transl Res. 2019;209:77–89. doi: 10.1016/j.trsl.2019.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Desbois AC, Cacoub P. Systemic sclerosis: An update in 2016. Autoimmun Rev. 2016;15:417–26. doi: 10.1016/j.autrev.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 5.Almeida C, Almeida I, Vasconcelos C. Quality of life in systemic sclerosis. Autoimmun Rev. 2015;14:1087–96. doi: 10.1016/j.autrev.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 6.Yanaba K. Strategy for treatment of fibrosis in systemic sclerosis: Present and future. J Dermatol. 2016;43:46–55. doi: 10.1111/1346-8138.13026. [DOI] [PubMed] [Google Scholar]
- 7.Brown M, O'Reilly S. The immunopathogenesis of fibrosis in systemic sclerosis. Clin Exp Immunol. 2019;195:310–21. doi: 10.1111/cei.13238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Codullo V, Baldwin HM, Singh MD, et al. An investigation of the inflammatory cytokine and chemokine network in systemic sclerosis. Ann Rheum Dis. 2011;70:1115–21. doi: 10.1136/ard.2010.137349. [DOI] [PubMed] [Google Scholar]
- 9.Wick G, Grundtman C, Mayerl C, et al. The immunology of fibrosis. Annu Rev Immunol. 2013;31:107–35. doi: 10.1146/annurev-immunol-032712-095937. [DOI] [PubMed] [Google Scholar]
- 10.Serrati S, Chilla A, Laurenzana A, et al. Systemic sclerosis endothelial cells recruit and activate dermal fibroblasts by induction of a connective tissue growth factor (CCN2)/transforming growth factor beta-dependent mesenchymal-to-mesenchymal transition. Arthritis Rheum. 2013;65:258–69. doi: 10.1002/art.37705. [DOI] [PubMed] [Google Scholar]
- 11.Leask A. Matrix remodeling in systemic sclerosis. Semin Immunopathol. 2015;37:559–63. doi: 10.1007/s00281-015-0508-2. [DOI] [PubMed] [Google Scholar]
- 12.Varga J, Pasche B. Transforming growth factor beta as a therapeutic target in systemic sclerosis. Nat Rev Rheumatol. 2009;5:200–6. doi: 10.1038/nrrheum.2009.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mohammadi S, Seyedhoseini FS, Asadi J, et al. Effects of berberine on the secretion of cytokines and expression of genes involved in cell cycle regulation in THP-1 monocytic cell line. Iran J Basic Med Sci. 2017;20:530–7. doi: 10.22038/IJBMS.2017.8677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O'Reilly S, Hugle T, van Laar JM. T cells in systemic sclerosis: a reappraisal. Rheumatology (Oxford) 2012;51:1540–9. doi: 10.1093/rheumatology/kes090. [DOI] [PubMed] [Google Scholar]
- 15.O'Reilly S. Role of interleukin-13 in fibrosis, particularly systemic sclerosis. Biofactors. 2013;39:593–6. doi: 10.1002/biof.1117. [DOI] [PubMed] [Google Scholar]
- 16.Mohammadi S, Sedighi S, Memarian A. IL-17 is Aberrantly Overexpressed Among Under-treatment Systemic Lupus Erythematosus Patients. Iran J Pathol. 2019;14:236–42. doi: 10.30699/ijp.2019.94878.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vettori S, Cuomo G, Iudici M, et al. Early systemic sclerosis: serum profiling of factors involved in endothelial, T-cell, and fibroblast interplay is marked by elevated interleukin-33 levels. J Clin Immunol. 2014;34:663–8. doi: 10.1007/s10875-014-0037-0. [DOI] [PubMed] [Google Scholar]
- 18.Branton MH, Kopp JB. TGF-beta and fibrosis. Microbes Infect. 1999;1:1349–65. doi: 10.1016/s1286-4579(99)00250-6. [DOI] [PubMed] [Google Scholar]
- 19.Liu Y. Renal fibrosis: new insights into the pathogenesis and therapeutics. Kidney Int. 2006;69:213–7. doi: 10.1038/sj.ki.5000054. [DOI] [PubMed] [Google Scholar]
- 20.Lafyatis R. Transforming growth factor beta--at the centre of systemic sclerosis. Nat Rev Rheumatol. 2014;10:706–19. doi: 10.1038/nrrheum.2014.137. [DOI] [PubMed] [Google Scholar]
- 21.Xu Q, Norman JT, Shrivastav S, et al. In vitro models of TGF-beta-induced fibrosis suitable for high-throughput screening of antifibrotic agents. Am J Physiol Renal Physiol. 2007;293:F631–40. doi: 10.1152/ajprenal.00379.2006. [DOI] [PubMed] [Google Scholar]
- 22.Asano Y. Recent advances in animal models of systemic sclerosis. J Dermatol. 2016;43:19–28. doi: 10.1111/1346-8138.13185. [DOI] [PubMed] [Google Scholar]
- 23.Ruzek MC, Jha S, Ledbetter S, et al. A modified model of graft-versus-host-induced systemic sclerosis (scleroderma) exhibits all major aspects of the human disease. Arthritis Rheum. 2004;50:1319–31. doi: 10.1002/art.20160. [DOI] [PubMed] [Google Scholar]
- 24.Servettaz A, Goulvestre C, Kavian N, et al. Selective oxidation of DNA topoisomerase 1 induces systemic sclerosis in the mouse. J Immunol. 2009;182:5855–64. doi: 10.4049/jimmunol.0803705. [DOI] [PubMed] [Google Scholar]
- 25.Yoshizaki A, Yanaba K, Ogawa A, et al. Immunization with DNA topoisomerase I and Freund's complete adjuvant induces skin and lung fibrosis and autoimmunity via interleukin-6 signaling. Arthritis Rheum. 2011;63:3575–85. doi: 10.1002/art.30539. [DOI] [PubMed] [Google Scholar]
- 26.Stawski L, Han R, Bujor AM, et al. Angiotensin II induces skin fibrosis: a novel mouse model of dermal fibrosis. Arthritis Res Ther. 2012;14:R194. doi: 10.1186/ar4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saghaeian JM, Mohammadi S, Sedighi S. Culture and differentiation of monocyte derived macrophages using human serum: an optimized method. Zahedan J Res Med Sci. 2016;18:e7362. [Google Scholar]
- 28.Lattouf R, Younes R, Lutomski D, et al. Picrosirius red staining: a useful tool to appraise collagen networks in normal and pathological tissues. J Histochem Cytochem. 2014;62:751–8. doi: 10.1369/0022155414545787. [DOI] [PubMed] [Google Scholar]
- 29.Souza CM, Lima CG, Alves MJ Jr, et al. Standardization of histological procedures for the detection of toxic substances by immunohistochemistry in Dipteran larvae of forensic importance. J Forensic Sci. 2013;58:1015–21. doi: 10.1111/1556-4029.12140. [DOI] [PubMed] [Google Scholar]
- 30.Allanore Y, Simms R, Distler O, et al. Systemic sclerosis. Nat Rev Dis Primers. 2015;1 doi: 10.1038/nrdp.2015.2. [DOI] [PubMed] [Google Scholar]
- 31.Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Basalova N, Sagaradze G, Arbatskiy M, et al. Secretome of Mesenchymal Stromal Cells Prevents Myofibroblasts Differe-ntiation by Transferring Fibrosis-Associated microRNAs within Extracellular Vesicles. Cells. 2020:9. doi: 10.3390/cells9051272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen YG, Meng AM. Negative regulation of TGF-beta signaling in development. Cell Res. 2004;14:441–9. doi: 10.1038/sj.cr.7290246. [DOI] [PubMed] [Google Scholar]
- 34.Hinz B, Celetta G, Tomasek JJ, et al. Alpha-smooth muscle actin expression upregulates fibroblast contractile activity. Mol Biol Cell. 2001;12:2730–41. doi: 10.1091/mbc.12.9.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carpino G, Morini S, Ginanni Corradini S, et al. Alpha-SMA expression in hepatic stellate cells and quantitative analysis of hepatic fibrosis in cirrhosis and in recurrent chronic hepatitis after liver transplantation. Dig Liver Dis. 2005;37:349–56. doi: 10.1016/j.dld.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 36.Cherng S, Young J, Ma H. Alpha-smooth muscle actin (α-SMA) J Am Sci. 2008;4:7–9. [Google Scholar]
- 37.Atamas SP, White B. Cytokine regulation of pulmonary fibrosis in scleroderma. Cytokine Growth Factor Rev. 2003;14:537–50. doi: 10.1016/s1359-6101(03)00060-1. [DOI] [PubMed] [Google Scholar]
- 38.Mack M. Inflammation and fibrosis. Matrix Biol. 2018;68-69:106–21. doi: 10.1016/j.matbio.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 39.Prunotto M, Compagnone A, Bruschi M, et al. Endocellular polyamine availability modulates epithelial-to-mesenchymal transition and unfolded protein response in MDCK cells. Lab Invest. 2010;90:929–39. doi: 10.1038/labinvest.2010.65. [DOI] [PubMed] [Google Scholar]
- 40.Sennello JA, Flozak AS, Misharin AV, et al. Arginase 1 is negatively regulated by β-catenin signaling in the lung. bioRxiv . 2017:137539. [Google Scholar]
- 41.Perlish JS, Lemlich G, Fleischmajer R. Identification of collagen fibrils in scleroderma skin. J Invest Dermatol. 1988;90:48–54. doi: 10.1111/1523-1747.ep12462561. [DOI] [PubMed] [Google Scholar]
- 42.Chen MJ, Shih SC, Wang HY, et al. Caffeic Acid phenethyl ester inhibits epithelial-mesenchymal transition of human pancreatic cancer cells. Evid Based Complement Alternat Med. 2013;2013:270906. doi: 10.1155/2013/270906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Veerasamy M, Nguyen TQ, Motazed R, et al. Differential regulation of E-cadherin and alpha-smooth muscle actin by BMP 7 in human renal proximal tubule epithelial cells and its implication in renal fibrosis. Am J Physiol Renal Physiol. 2009;297:F1238–48. doi: 10.1152/ajprenal.90539.2008. [DOI] [PubMed] [Google Scholar]