Abstract
Angiogenesis is an essential process in the growth, development, and transition of tumors from dormancy to proliferating state. Resveratrol (RSV), as a natural polyphenolic compound, is claimed to be effective in regulating angiogenesis. This study aimed to evaluate the impact of RSV onthe angiogenesis process in HUVECs (human umbilical vein endothelial cells) alone and co-cultured with Jurkat cells. The effects of RSV on HUVECs and Jurkat cell viability and apoptosis were measured by MTT and Annexin-V/PI methods. HUVECs were co-cultured with pre-treated Jurkat cells and incubated for 24 h, 48 h and 72 h. The angiogenesis process in HUVECs and Jurkat cells alone and in co-culture models was investigated by analyzing the expression of VEGF, VEGFR-2, and Interleukin-8 (IL-8) employing qPCR and ELISA. RSV at low concentration (40 µM) had no significant effects on apoptosis rate of HUVECs, but higher concentrations (80-160 µM) increased apoptosis in co-culture method and HUVECs alone. RSV significantly reduced VEGFR2 and IL-8 gene expression also, IL-8 protein concentration in HUVECs, but the effects of this drug in the HUVECs-Jurkats co-culture were different. Expression of VEGF in Jurkat cells increased following treatment with RSV. RSV had direct anti-angiogenic effects on HUVECs. Unexpectedly its indirect effects were not significant on HUVECs-Jurkats co-culture. Results of our study showed, RSV may be effective in anti-angiogenesis therapy, but in some situations, it may induce angiogenesis. So, appropriate concentrations should achieve to minimize the unpredicted effects of RSV
Key Words: Angiogenesis Inhibitors, Resveratrol, co-culture, VEGFR2, VEGF, Interleukin-8
Introduction
Angiogenesis means the generation of new blood vessels from pre-existing vessels. Stimulation and inhibition of new blood vessels formation completely balance in normal circumstances. This process is controlled by pro - and anti-angiogenic factors (1). New blood vessels maturation and stabilization are normal in physiological angiogenesis, but tumor cells have abnormal vascular structures, irregular blood flow, increased vascular permeability, and late maturation. Pathologic angiogenesis occurs when the balance between angiogenesis-related factors is disrupted and causes uncontrolled angiogenesis (2). The high angiogenesis rate exacerbates pathological conditions in some diseases such as cancer, chronic inflammation, arthritis, psoriasis, and autoimmune diseases (3).
As bone marrow (BM) and lymphatic organs are the predominant sites of tumor accumulation in hematological malignancies, initially; scientists believed that angiogenesis would not be as relevant in these disorders as in solid tumors. Increased microvessel density (MVD) in BM and lymph nodes have been observed in various hematologic diseases such as leukemia, multiple myeloma and myelodysplastic syndromes. Angiogenesis is crucial in providing oxygen and nutrients for malignant cells. Additionally, the increased endothelial cell mass is essential for producing cytokines and growth factors that act on the malignant cells in a paracrine fashion, promoting their proliferation or survival (4).
Recently, accumulating studies have indicated that leukemia cells produce various angiogenic factors and express receptors for these factors, leading to regulate of their proliferation and differentiation by modulating gene expression (5). Recently, angiogenic factors become potential targets to control the development of hematological malignancies. The vascular endothelial growth factor (VEGF) family and its receptors are primary signaling mediators that play important roles in angiogenesis. Three types of VEGF receptors are: VEGFR-1or Fms-like tyrosine kinase 1 (Flt1), VEGFR-2 or Kinase Insert Domain Receptor (KDR) and VEGFR-3 or Fms-like tyrosine kinase 3 (Flt4). VEGFR-2 is the main VEGF signaling transmitter in angiogenesis and vascular permeability through its involvement in Phospholipase C (PLC)/ Protein kinase C (PKC)/ Mitogen activated protein kinase (MAPK) signaling pathway (6, 7). Binding of VEGF-A to the active site of its tyrosine kinase receptor induces dimerization and activation of VEGFR2 (8). VEGFR2 predominantly expressed on the surface of endothelial cells. However; osteoblasts, neuronal cells, pancreatic duct cells, retinal progenitor cells and megakaryocytes express it, at low concentrations (9). Incredibly; the VEGFR2 level is 3 to 5-fold higher in tumor vasculature compared with the normal vasculature (10).
IL-8 or C-X-C Motif Chemokine Ligand 8 (CXCL8) is another angiogenic factor that has two different angiogenesis-related functions.1). IL-8 binds to CXC chemokine receptor 2 (CXCR2) and acts as a pro-angiogenic factor. 2). It can bind to the Duffy antigen/receptor for chemokine (DARC) receptor, a suppressive receptor, inactivate (Glutamic acid-Leucine-Arginine) ELR+ angiogenic chemokines, thus it can prevent tumor growth and development (11). Generally, CXCL8/IL-8 chemokine family promotes angiogenesis in endothelial cells, and increases the proliferation, survival and migration in endothelial and cancer cells. Blocking of IL-8 activity may inhibit the growth and metastasis of several tumors (12, 13).
Resveratrol (RSV), also known as 3, 5, 4’-trihydroxy-trans-stilbene, is a polyphenolic non-flavonoid natural compound, mainly it is founded in grape skin, mulberry and peanut (14). RSV has many benefits such as anti-cancer, anti-inflammatory, anti-aging and anti-diabetic properties. This natural compound also plays an important role in regulating angiogenesis, apoptosis, oxidative stress, and inflammation (15). Studies have reported that RSV inhibits the development of various types of tumors. Anti-tumor properties of RSV are probably related to the inhibition of three main stages of carcinogenesis: tumor onset, stimulation and development (16, 17).
Different studies showed that RSV inhibits angiogenesis via down regulation of VEGF/VEGFR2 in various cell types. RSV and other flavonoids could be suggested as potential inhibitors of VEGFR2 to modulate response to angiogenesis-related abnormalities. In addition , RSV leads to decreased expression of VEGF and CXCL8/IL-8 in various cell lines such as smooth muscle cells, fibroblasts, epithelial cells and co-culture of endothelial-melanoma cells (18). We investigated the effects of RSV on angiogenesis, either in HUVECs alone and HUVECs-Jurkats co-culture to examine its potential for regulation of neovascular formation in acute lymphoblastic leukemia (ALL).
Materials and Methods
Cell culture
HUVECs culture
HUVECs were purchased from the National Cell Bank of Iran (Pasteur Institute, Tehran, Iran). They were cultured in 75cm2 flasks according to the ATCC protocol. Cells were grown in Dulbecco Modified Eagle’s Medium (DMEM; GIBCO, Paisley, UK) , supplemented with 2 mmol/l L-Glutamine, 10% fetal bovine serum (FBS; GIBCO, Paisley,UK) and 1% pen/strep (100U/ml penicillin G sodium, 100µg/Ml streptomycin sulfate, Sigma-Aldrich, Germany). Then they were incubated at 37˚C in the presence of 5% CO2 and 95% humidity. Following nearly 80% confluency, HUVECs were collected with 0.25% Trypsin-EDTA 1X (GIBCO Life Technologies, Paisley, UK), according to the ATCC trypsinization protocol and centrifuged at 1200 rpm for 5 min. The supernatant was removed and resuspended in a fresh DMEM medium. Cells were counted and seeded at a density of 2500-5000 cells/cm2 at least 24-48 hours.
Jurkat cells culture
Jurkat cells (Pasteur Institute, Tehran, Iran) were plated in 75cm2 flasks (1-2×106 cells) with 3-5 mL RPMI 164 (GIBCO, Paisley, UK ) supplemented with 10% FBS and 1% pen/strep and incubated at 37˚C containing 5% CO2 and 95% humidity. The cells were regularly examined with an inverted microscope for morphological changes.
Co-culture of HUVECs with Resveratrol-treated Jurkat cell
HUVECs and Jurkats were co-cultured with direct contact in a ratio of 1:3 to determine intracellular interaction between endothelial and leukemia cells. First, HUVECs were cultured in 6-well plates at a density of 15 x104 cells per well and incubated 72 h to reach 70-80% confluency. About 45 x104 Jurkat cells (pre-treated with 80 µM RSV for eight hours) were added and incubated 24, 48 and 72 hours. HUVECs were also directly treated with 80 µM RSV (Sigma-Aldrich, Germany, CAS 501-36-0) and incubated 24 and 48 h. The cells were also treated with dimethyl sulfoxide (DMSO, GIBCO, UK) (0.1%) as control. Each plate was monitored daily for morphology, confluency and medium status. After incubation, the supernatant of all wells was collected and stored at -80˚C for subsequent ELISA assay.
Cell viability assay
To evaluate the effect of RSV on the proliferation of treated cells, HUVECs were grown in 96 well plates and treated with different concentrations of RSV (40, 80, 160 µM) for 24 h and 48 h. Next, 10 µL MTT [3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide] reagent (Sigma-Aldrich, Germany) (0.5 mg/mL) was added and incubated at 37 ˚C for 3-4 h. Consequently, 100 µL DMSO was added to dissolve violet crystals of formazan. Finally; the intensity of formazan crystal in each well was measured by an ELISA reader at 570nm.
Measurement of apoptotic cell death by Annexin V-PI staining
The apoptosis rate of HUVECs alone and co-cultured with Jurkat cell was determined by an apoptosis assay kit (Sigma-Aldrich, Germany) via Annexin-V/PI staining. HUVECs were cultured and treated with different concentrations of RSV (40, 80,160 µM) alone or co-cultured with Jurkat cells for 24 h at 37 ˚C. The cells were washed by Phosphate Buffered Saline (PBS, Sigma-Aldrich, Germany) and centrifuged at 1500 rpm for 5 min. Subsequently; 90 µL Annexin-V binding buffer 1X, 0.5 µL Annexin V-FITC and 0.5 µL Propidium iodide was added to the cells and incubated in the dark for 20 min. Then; 300 µL of binding buffer 1X was added and the cells were centrifuged at 400 rcf for 5 min. The supernatant was discarded and the cell pellet was dissolved in 200 µL PBS. Finally; the fluorescence intensity of samples was determined by a flow cytometer.
ELISA
Following co-culture of HUVECs and pre-treated Jurkat cells or treatment of HUVECs alone with RSV, supernatants were used for quantitative analysis by ELISA assay. Briefly; HUVECs were treated with RSV at a concentration of 80 μM and incubated 24 and 48 h. HUVECs were also concomitantly co-cultured with pre-treated Jurkat cells (80 μM RSV) and incubated at 37 °C 24, 48 and 72 h. Supernatants of each well were collected separately for further analysis. The IL-8, origin from HUVECs, was measured in all supernatants using an ELISA kit (IBL, Germany) according to the manufacturer's instructions.
RNA isolation and Real-time PCR
Following treatment with RSV (80 μM) for 24 and 48 h, total RNA of HUVECs (300000 cells) was extracted using TRIzol reagent (SinaClon BioScience, Iran). RNA quality was evaluated via measurement protein contamination (absorption ratio at 260 to 280) or contamination with organic compounds (absorption ratio 260 to 230) such as phenol, trisol and other aromatic compounds by nanodrop instrument. cDNA synthesis kit (Takara, Japan) was used to convert RNA (1 µL) to cDNA according to the manufacturer’s instructions. Amplification of synthesized cDNA using SYBER Green (Amplicon SYBER Green qPCR Master Mix, 2x) was performed by a light cycler instrument (Roche Diagnostics, Germany). The initiation activation step of qPCR (30 seconds at 95 ˚C) followed by 40 cycles, consisting of a denaturation phase for 15 min at 95 ˚C, an annealing phase for 15 seconds at 95 ˚C and finally an extension phase for 30 seconds at 60˚C. Fold change values were calculated based on the 2-∆∆Ct formula for relative expression in each sample (19) and GAPDH was used as the internal control. The amplification and melting curves were controlled to confirm the specificity of the product. The nucleotide sequence of the primers used is given in Table 1.
Statistical analyses
The GraphPad Prism software version 8.4.2 was used to assess the gene expression changes after treatment with the drug. All the data were stated as a mean±standard deviation (SD) and three independent experiments were performed in triplicate. The two-tailed Student's t-test was performed to determine any difference between the two groups. The one-way ANOVA test was applied to compare significant differences between the control and the experimental group. For all experiments, the P- value < 0.05 was considered significant.
Results
Effects of Resveratrol on apoptosis
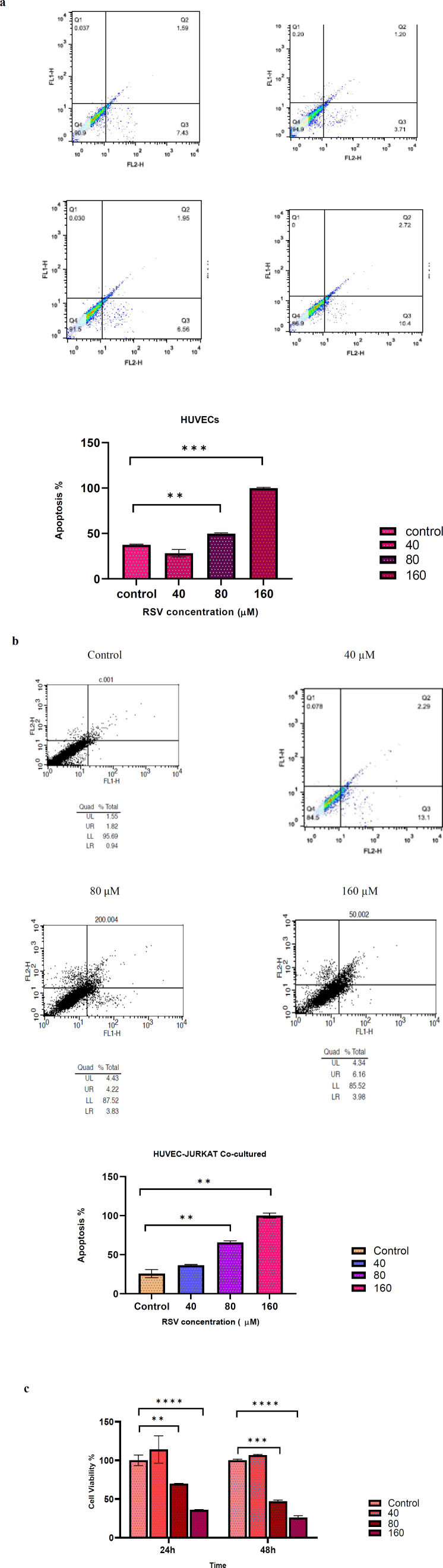
Annexin-V/PI method was performed to determine the effects of RSV on the apoptosis rate of HUVECs alone and co-cultured with pre-treated Jurkat cells. HUVECs were incubated 24 h with different concentrations of RSV (40, 80 and 160 μM) and the apoptosis rate was measured by a flow cytometer. The results showed that apoptosis at concentrations of 80 and 160 μM significantly increased (P=0.004, P= 0001 respectively) compared with the control group. However; there was no significant increase in apoptosis rate in response to 40 μM RSV. In addition; while the co-culture of HUVECs and pre-treated Jurkat cells did not show any significant changes in apoptosis rate at low concentration of RSV (40 μM), higher concentrations (80, 160 μM) significantly increased apoptosis compared with the control group (P=0.009, P=0.003 respectively) (Figure 1 a, b).
Fig. 1.
The Apoptosis rate of HUVECs alone and co-cultured with Jurkat cell. (a) HUVECs were treated with various concentrations of RSV (40, 80 and 160 µM). Flow cytometer results of each concentration showed respectively. (b) Jurkat cells in the co-culture model were treated with different concentrations of RSV (40, 80, 160 µM). Flow cytometer results of each concentration showed respectively. (c) Effects of RSV on cell viability of HUVECs. Cells were treated with different concentrations of RSV (40, 80 and 160 µM) 24 h and 48 h. The data are presented as mean±SD. The data were extracted from three separate experiments. * represents p<0.05, ** indicates p<0.01 and*** denotes p<0.001
Effects of Resveratrol on the viability of HUVECs
HUVECs were incubated 24 h and 48 h with different concentrations of RSV (40, 80 and 160 μM) and analyzed by MTT assay to determine the cytotoxic effect of RSV. As depicted in Fig.1c, the survival rate of HUVECs markedly decreased at a high concentration of RSV (80 and 160 μM) compared with the control after 24 h (P=0.011, P=0.0007 respectively) and 48 h (P=00001, P= 00001). However, a lower concentration of RSV (40 μM) had no significant effect on cell survival.
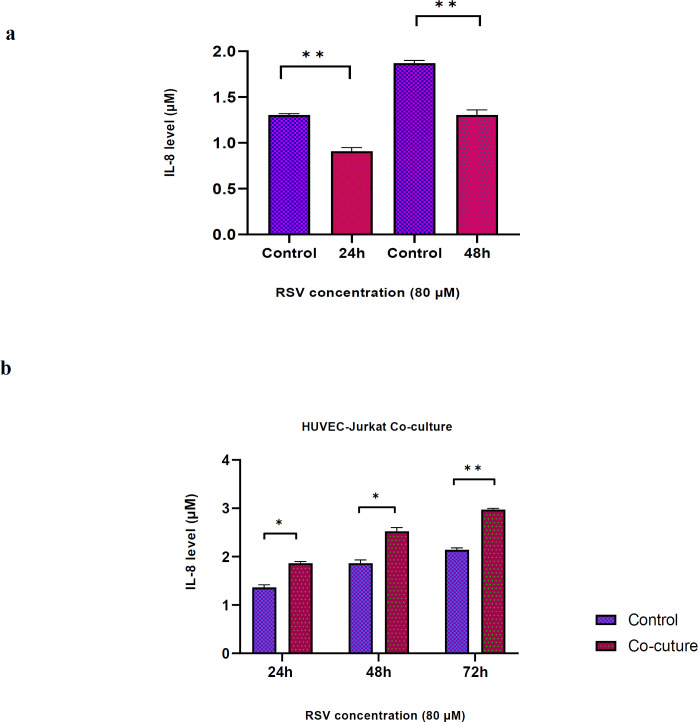
RSV effects on CXCL8/IL-8 secretion by HUVECs co-cultured with Jurkat cell
The results indicated that RSV (80 μM) significantly reduced the secretion of IL-8 by HUVECs after 24 and 48 h incubation compared with controls (P=0.011, P=0.012, respectively). However; the co-culture of HUVECs-Jurkats markedly enhanced secreted IL-8 levels after 24, 48 and 72 h incubation compared with controls (P=0.016, P=0.021 and P=0.003, respectively) (Figure 2).
Fig. 2.
Effects of RSV on IL-8 secretion in HUVECs. (a) Secretion of IL-8 was measured in HUVECs after 24 and 48 h incubation with RSV at a concentration of 80 μM. The data showed a significant reduction of IL-8 secretion compared to controls (P=0.011, P=0.012, respectively). (b) Secretion of IL-8 was measured in HUVECs-Jurkats co-culture after 24, 48 and 72 h incubation with RSV at the concentration of 80 μM. The results showed secreted IL-8 levels markedly enhanced compared with controls (P=0.016, P=0.021 and P=0.003, respectively). Data are presented as mean±SD of three independent experiments, where * and ** indicate P<0.05 and P<0.01, respectively
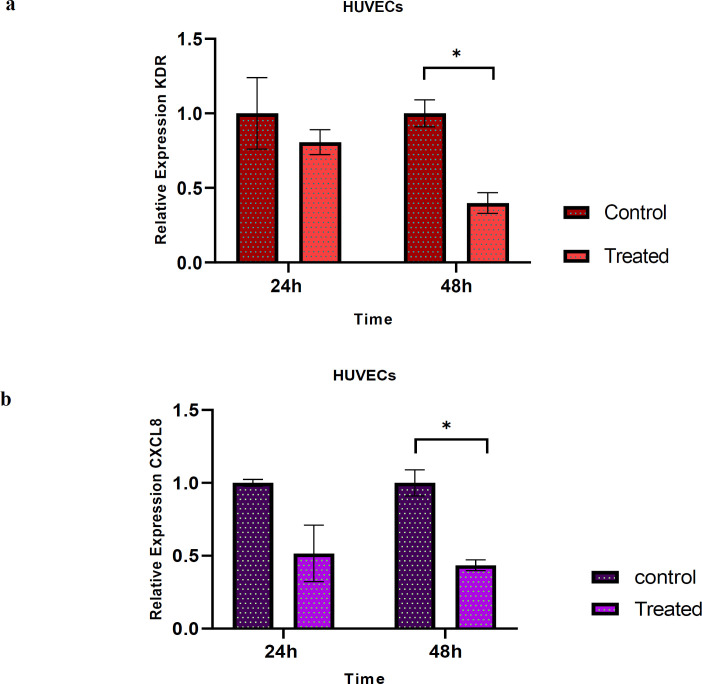
Resveratrol effect on VEGFR2 and CXCL8/IL-8 gene expression in HUVECs
Real time-PCR was employed to analyze the effects of RSV on the mRNA expression of VEGFR2 and IL-8 in HUVECs alone. As illustrated in Fig.3, the reduction of VEGFR2 and IL-8 mRNA expressions were not significant after 24 h. However; RSV markedly diminished the mRNA expressions of VEGFR2 and IL-8 after 48 h compared to controls (P=017, P=0.014, respectively) (Figure 3).
Fig. 3.
The expression of VEGFR2 (KDR) and IL-8 (CXCL8) in HUVECs after treatment with RSV. (a) The relative fold-change of KDR after 24 h and 48 h treatment with RSV (80 µM) was measured. The mRNA expression of KDR decreased but not significantly compared to untreated control after 24 h incubation, but markedly decreased after 48 h. (b) Relative fold-change of CXCL8 after 24 h and 48 h treatment with RSV (80 µM) was measured. The results showed that the mRNA expression of CXCL8 decreased, but not significantly compared to untreated control, and the expression of it markedly reduced after 48 h compared to control. Data are presented as mean±SD of three independent experiments and * indicates p< 0.05
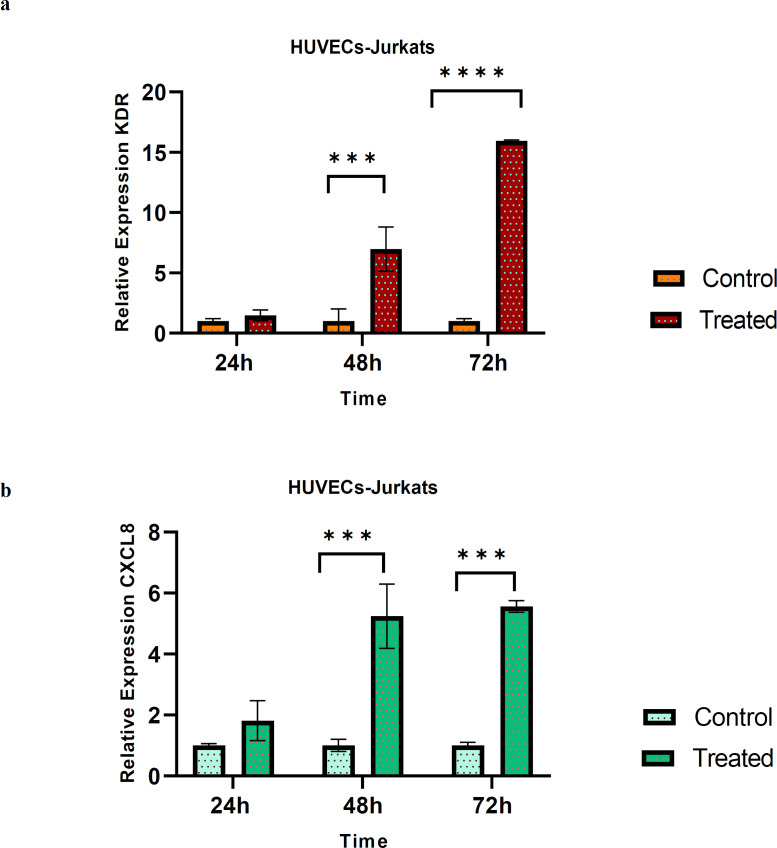
Resveratrol effect on VEGFR2 and CXCL8/IL-8 gene expression in HUVECs-Jurkat co-culture
HUVECs-Jurkat co-culture was incubated 24, 48, 72 h and the mRNA expression was evaluated. Our data revealed that 24 h exposure to the pre-treated Jurkat cell had no significant effect on the mRNA expression of VEGFR2 and IL-8 in HUVECs. However; a remarkably increased expression level of the genes mentioned above, was evident after 48 h (P-values of 0.007 and 0.002 respectively) and 72 h (P-values of 0.0001 and 0.001 respectively) in the co-culture model (Figure 4).
Fig. 4.
The expression levels of VEGFR2 (KDR) and IL-8 (CXCL8) in co-culture model. (a) The relative fold-change of KDR after 24 h, 48 h and 72 h treatment with RSV (80 μM) in the co-culture model were evaluated. The mRNA expression of KDR increased, but not significant after 24 h, and the expression of the gene increased dramatically after 48 and 72 hours compared to control. (b) The relative fold-change of CXCL8 after 24 h, 48 h and 72 h in the co-culture model were measured. The results showed that the mRNA expression of CXCL8 increased and it was significant after 48 h and 72 h compared to control. Data are presented as mean±SD of three independent experiments. * indicates p<0.05 and ** indicates p<0.01
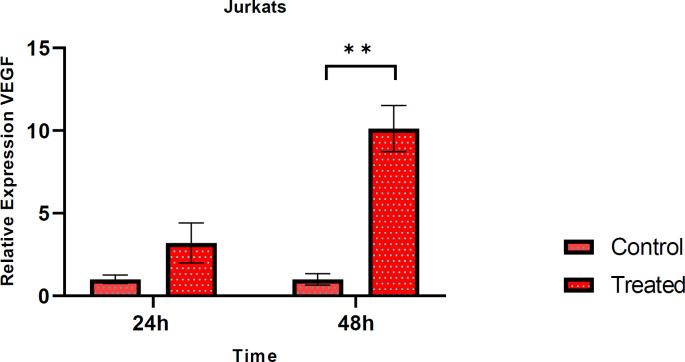
Resveratrol effect on VEGF gene expression in Jurkat cell
Jurkats were treated with 80 μM RSV for 24 h and 48 h to explore the effect of RSV on VEGF gene expression. Total RNAs of the cells were extracted and following reverse transcription reaction, cDNA was subjected to qPCR. As shown in Fig.5, VEGF expression increased after 24 h compared to control but not significant and its expression increased dramatically after 48 h compared to control (P-value= 0.012) (Figure 5).
Fig. 5.
The expression level of VEGF in Jurkat cells. Relative fold-change of VEGF was evaluated after 24 h and 48 h treatment with RSV. The mRNA expression of VEGF increased markedly compared to untreated control after 48 h, but the increased expression of it after 24 h was not significant. Data are presented as mean±SD of three independent experiments and ** indicates p<0.01
Discussion
Although there are various chemotherapy agents for the treatment of patients suffering from hematological malignancies, side effects and the resistance created in some patients have highlighted the necessity of attention to therapies that target other pathways involved in the progression of leukemia. Previous studies have demonstrated increased bone marrow microvessel density (MVD) and increased plasma levels of angiogenic factors in patients with hematologic malignancies, supporting the fact that angiogenesis plays a role in their pathogenesis and progression (4). It is also declared that neovascularization in BM is correlated with disease burden, prognosis, or treatment outcome (20).
During angiogenesis, endothelial cells are activated by angiogenic growth factors. These cells proliferate, migrate and form a new vascular network. VEGF-A ,one of the significant stimulants of angiogenesis, controlled by several factors, such as hypoxia, hormones, cytokines and other growth factors (21, 22). VEGF-A binds to VEGFR2 and leads to its dimerization and consequently phosphorylation of several downstream regulatory elements such as PKC, phosphatidylinositol 3-kinase (PI3K), PLC-y and serine/threonine- protein kinase B (PKB). This process causes endothelial cells survival, development and vascular formation (23). VEGFR2 is expressed on endothelial cells, T-lymphocytes and the Jurkat cell line (9, 24).
IL-8 is a chemokine that inhibits apoptosis, amplifies proliferation and promotes angiogenesis tumor development and metastasis (25). Several previous studies have demonstrated that IL-8 expression significantly elevated in hematologic malignancies such as ALL and played an important role in leukemia progression. Moreover; increased IL-8 expression may associate with increased survival of malignant cells in chronic lymphocytic leukemia (CLL) (26).
Angiogenesis suppression can be a potential therapeutic approach and can evaluate its effectiveness in human leukemia investigations. recently; numerous anti-angiogenic agents have been confirmed to manage hematologic disease (27). RSV is a natural therapeutic compound that prevents angiogenesis progression by targeting various mediators. Therefore; we designed experiments to evaluate the effects of RSV on the expression or secretion of angiogenic factors, including VEGFR2 and IL-8 in both endothelial and leukemia cells.
The results obtained from the present study showed that RSV could not induce cytotoxic and apoptotic effects at low. Similarly; the induction of apoptosis increased at higher concentrations and longer incubation time with RSV (28). The current study demonstrated that RSV decreased the mRNA expression of VEGFR2, IL-8 and the secretion of IL-8 in HUVECs alone. The results were consistent with Huiming Zhang et al study. Who found that RSV induces a suppressive effect on expression and phosphorylation of VEGFR2 via SIRT1 (Sirtuin 1), a histone deacetylase mediator regulating angiogenesis (29).
Moreover; high doses of RSV can inhibit VEGF and VEGFR2 phosphorylation (30). Hu W-H et al. declared that Piceatannol, a natural analog of RSV, inhibited the angiogenic function by reducing of VEGF - VEGFR2 interaction (31). In another study; Mikuła-Pietrasik J et al. suggested that endothelial cell growth and proliferation were inhibited by RSV, which may be associated with decreased secretion of VEGF and IL-8 in RSV-treated cells (18). In contrast; when we examined the effects of RSV in a co-culture model of normal endothelial cells and acute T-lymphoblastic leukemia cells, we found that RSV increased the expression of VEGFR2 and IL-8. Consistently; the secretion of IL-8 from HUVECs in the co-culture model was increased dramatically by RSV-treated Jurkat cells. The reasons for the conflicting results might be related to using different concentrations of RSV or genotypic/ phenotypic differences of the cell lines.
Nuclear factor kappa B (NFkB) transcription factor may play a bilateral role due to RSV effects. RSV can prevent the stimulation and activation of the NFkB through various pathways such as inhibition of phosphorylation and degradation of the IkB (inhibitor of NFkB), thus preventing the placement and activation of the NFkB in the nucleus. On the other hand; low concentrations of the RSV increases the presence of NFkB subunits (p50, p65) in the nucleus (32). Moreover; several studies suggested that RSV can augment the level of MAPK , such as Extracellular signal-regulated kinase 1/2 (ERK1/2), which are necessary for tube network formation and microvessel growth (33). Some reports showed that RSV upregulates the ERK1/2 signaling pathway in various cell lines, such as K562 (erythroleukemia cell line), so it can induce proliferation (34). Gerald Thiel et al. suggested that RSV stimulates the expression of pro-inflammatory cytokine IL-8 in vitro ,which could be attributed to ERK1/2 protein kinase (35). According to a report by Kamaleddin et al. the effects of RSV may be dose-dependent, it may impose pro- or anti-angiogenic activity depending on its dosage. This study suggested that the physical and chemical environment of cells, also the type of cell line may influence RSV effects (36).
Recently, co-culture techniques have been widely used to study intercellular interactions (37). In this regard; the presence of extracellular vesicles (EVs), plays a pivotal role in the interaction of cells with each other. EVs are small spherical bodies surrounded by cell membranes and secreted directly by all cells. Since these mediators contain numerous cellular components, including RNA, DNA, proteins and active lipids, they can affect various physiological and pathological conditions, such as angiogenesis, inflammation and immune system functions (38, 39). We found that RSV increased the expression of angiogenic factors in both Jurkat cells and co-culture of Jurkat cells with HUVECs, which is probably related to the transfer of Jurkat cellular contents via EVs and hence changing the behavior of the endothelial cells. Umezu et al. showed that co-culture of HUVECs with leukemia cell line K562 resulted in the transfer of MicroRNA (miR)-92a, involving in tumor angiogenesis, from leukemic cells to the endothelial cells, thereby affecting the formation of new blood vessels by endothelial cells (40). Furthermore; IM Toaldo et al. reported that the metabolism of the endothelial cell was altered during co-culture with RSV-treated Caco-2 (enterocyte-like cells) (41).
Although our in vitro results indicated no significant anti-angiogenic effect of RSV on Jurkat cells, additional experiments are required to elucidate the present data. Overall; we suggest that RSV may have different biological effects in various cell types and may modulate pathological angiogenesis in endothelial cells by regulating some angiogenic factors. Since; multiple factors are involved in the development of blood vessels, regulating the angiogenesis is complicated and detailed characterization of these mediators is required in each separate leukemia and cell lines for an effective anti-angiogenic therapy. In addition; RSV has different targets and as hematological malignancies are heterogenous, a more comprehensive analysis is essential to solve the mechanism of RSV in regulating of angiogenesis.
There are several studies related to the properties of RSV. It is surprising that the present results showed the different effects of RSV on the expression and secretion of angiogenic factors at the same concentration and timing in Jurkat cells and HUVECs as two different cell types. This may make us to be more cautious in describing the effects of RSV in different situations. Although RSV regulates several critical cellular processes, its biological effects might be depend on the cell type, chemical and physical conditions of the target cell, pharmacological dose and preparation methods, etc. Therefore; further in vitro and in vivo investigations are needed to clarify the effects of RSV in various circumstances.
Conflicts of Interest
The authors declare that they have no conflict of interest.
Acknowledgment
We wish to thank all our colleagues in the Research and Enterprise Committee and the School of Allied Medicine, Iran University of Medical Science. The study was approved by the Medical Ethics Committee of Iran University of Medical Sciences and conformed to the provisions of the Declaration of Helsinki (IR.IUMS.REC.1398.289).
References
- 1.Melincovici CS, Bosca AB, Susman S, et al. Vascular endothelial growth factor (VEGF) - key factor in normal and pathological angiogenesis. Rom J Morphol Embryol. 2018;59:455–67. [PubMed] [Google Scholar]
- 2.AbdElAal Asmaa A, Afify RA, Zaher AE, et al. Study of prognostic significance of marrow angiogenesis assessment in patients with de novo acute leukemia. Hematology. 2015;20:504–10. doi: 10.1179/1607845415Y.0000000012. [DOI] [PubMed] [Google Scholar]
- 3.Schmidt T, Carmeliet P. Angiogenesis: a target in solid tumors, also in leukemia? Hematology Am Soc Hematol Educ Program. 2011;2011:1–8. doi: 10.1182/asheducation-2011.1.1. [DOI] [PubMed] [Google Scholar]
- 4.Negaard HF, Iversen N, Bowitz-Lothe IM, et al. Increased bone marrow microvascular density in haematological malignancies is associated with differential regulation of angiogenic factors. Leukemia. 2009;23:162–9. doi: 10.1038/leu.2008.255. [DOI] [PubMed] [Google Scholar]
- 5.Martinelli S, Kanduri M, Maffei R, et al. ANGPT2 promoter methylation is strongly associated with gene expression and prognosis in chronic lymphocytic leukemia. Epigenetics. 2013;8:720–9. doi: 10.4161/epi.24947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shibuya M. Vascular Endothelial Growth Factor (VEGF) and Its Receptor (VEGFR) Signaling in Angiogenesis: A Crucial Target for Anti- and Pro-Angiogenic Therapies. Genes Cancer. 2011;2:1097–105. doi: 10.1177/1947601911423031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deng M, Zha J, Zhao H, et al. Apatinib exhibits cytotoxicity toward leukemia cells by targeting VEGFR2-mediated prosurvival signaling and angiogenesis. Exp Cell Res. 2020;390:111934. doi: 10.1016/j.yexcr.2020.111934. [DOI] [PubMed] [Google Scholar]
- 8.Modi SJ, Kulkarni VM. Vascular endothelial growth factor receptor (VEGFR-2)/KDR inhibitors: medicinal chemistry perspective. Medicine in Drug Discovery. 2019;2:100009. [Google Scholar]
- 9.Shibuya M. Differential roles of vascular endothelial growth factor receptor-1 and receptor-2 in angiogenesis. J Biochem Mol Biol. 2006;39:469–78. doi: 10.5483/bmbrep.2006.39.5.469. [DOI] [PubMed] [Google Scholar]
- 10.Lu RM, Chiu CY, Liu IJ, et al. Novel human Ab against vascular endothelial growth factor receptor 2 shows therapeutic potential for leukemia and prostate cancer. Cancer Sci. 2019;110:3773–87. doi: 10.1111/cas.14208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bie Y, Ge W, Yang Z, et al. The Crucial Role of CXCL8 and Its Receptors in Colorectal Liver Metastasis. Dis Markers. 2019;2019:8023460. doi: 10.1155/2019/8023460. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Russo RC, Garcia CC, Teixeira MM, et al. The CXCL8/IL-8 chemokine family and its receptors in inflammatory diseases. Expert Rev Clin Immunol. 2014;10:593–619. doi: 10.1586/1744666X.2014.894886. [DOI] [PubMed] [Google Scholar]
- 13.Yoo JY, Kim JH, Kim J, et al. Short hairpin RNA-expressing oncolytic adenovirus-mediated inhibition of IL-8: effects on antiangiogenesis and tumor growth inhibition. Gene Ther. 2008;15:635–51. doi: 10.1038/gt.2008.3. [DOI] [PubMed] [Google Scholar]
- 14.Li H, Xia N, Hasselwander S, et al. Resveratrol and Vascular Function. Int J Mol Sci. 2019:20. doi: 10.3390/ijms20092155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farzanegan A, Shokuhian M, Jafari S, et al. Anti-histaminic Effects of Resveratrol and Silymarin on Human Gingival Fibroblasts. Inflammation. 2019;42:1622–9. doi: 10.1007/s10753-019-01023-z. [DOI] [PubMed] [Google Scholar]
- 16.Gautam SC, Xu YX, Dumaguin M, et al. Resveratrol selectively inhibits leukemia cells: a prospective agent for ex vivo bone marrow purging. Bone Marrow Transplant. 2000;25:639–45. doi: 10.1038/sj.bmt.1702189. [DOI] [PubMed] [Google Scholar]
- 17.Xiao Y, Qin T, Sun L, et al. Resveratrol Ameliorates the Malignant Progression of Pancreatic Cancer by Inhibiting Hypoxia-induced Pancreatic Stellate Cell Activation. Cell Transplant. 2020;29:963689720929987. doi: 10.1177/0963689720929987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mikula-Pietrasik J, Kuczmarska A, Kucinska M, et al. Resveratrol and its synthetic derivatives exert opposite effects on mesothelial cell-dependent angiogenesis via modulating secretion of VEGF and IL-8/CXCL8. Angiogenesis. 2012;15:361–76. doi: 10.1007/s10456-012-9266-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 20.Rajkumar SV, Mesa RA, Tefferi A. A review of angiogenesis and anti-angiogenic therapy in hematologic malignancies. J Hematother Stem Cell Res. 2002;11:33–47. doi: 10.1089/152581602753448522. [DOI] [PubMed] [Google Scholar]
- 21.Tong Q, Zheng L, Lin L, et al. VEGF is upregulated by hypoxia-induced mitogenic factor via the PI-3K/Akt-NF-kappaB signaling pathway. Respir Res. 2006;7:37. doi: 10.1186/1465-9921-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ikhapoh IA, Pelham CJ, Agrawal DK. Atherogenic Cytokines Regulate VEGF-A-Induced Differentiation of Bone Marrow-Derived Mesenchymal Stem Cells into Endothelial Cells. Stem Cells Int. 2015;2015:498328. doi: 10.1155/2015/498328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dvorak HF. Angiogenesis: update 2005. J Thromb Haemost. 2005;3:1835–42. doi: 10.1111/j.1538-7836.2005.01361.x. [DOI] [PubMed] [Google Scholar]
- 24.Basu A, Hoerning A, Datta D, et al. Cutting edge: Vascular endothelial growth factor-mediated signaling in human CD45RO+ CD4+ T cells promotes Akt and ERK activation and costimulates IFN-gamma production. J Immunol. 2010;184:545–9. doi: 10.4049/jimmunol.0900397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li A, Dubey S, Varney ML, et al. IL-8 directly enhanced endothelial cell survival, proliferation, and matrix metalloproteinases production and regulated angiogenesis. J Immunol. 2003;170:3369–76. doi: 10.4049/jimmunol.170.6.3369. [DOI] [PubMed] [Google Scholar]
- 26.Scupoli MT, Donadelli M, Cioffi F, et al. Bone marrow stromal cells and the upregulation of interleukin-8 production in human T-cell acute lymphoblastic leukemia through the CXCL12/CXCR4 axis and the NF-kappaB and JNK/AP-1 pathways. Haematologica. 2008;93:524–32. doi: 10.3324/haematol.12098. [DOI] [PubMed] [Google Scholar]
- 27.Dong X, Han ZC, Yang R. Angiogenesis and antiangiogenic therapy in hematologic malignancies. Crit Rev Oncol Hematol. 2007;62:105–18. doi: 10.1016/j.critrevonc.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 28.Wu H, Chen L, Zhu F, et al. The Cytotoxicity Effect of Resveratrol: Cell Cycle Arrest and Induced Apoptosis of Breast Cancer 4T1 Cells. Toxins (Basel) 2019:11. doi: 10.3390/toxins11120731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang H, He S, Spee C, et al. SIRT1 mediated inhibition of VEGF/VEGFR2 signaling by Resveratrol and its relevance to choroidal neovascularization. Cytokine. 2015;76:549–52. doi: 10.1016/j.cyto.2015.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu WH, Duan R, Xia YT, et al. Binding of Resveratrol to Vascular Endothelial Growth Factor Suppresses Angiogenesis by Inhibiting the Receptor Signaling. J Agric Food Chem. 2019;67:1127–37. doi: 10.1021/acs.jafc.8b05977. [DOI] [PubMed] [Google Scholar]
- 31.Hu WH, Dai DK, Zheng BZ, et al. Piceatannol, a Natural Analog of Resveratrol, Exerts Anti-angiogenic Efficiencies by Blockage of Vascular Endothelial Growth Factor Binding to Its Receptor. Molecules. 2020:25. doi: 10.3390/molecules25173769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu L, Botchway BOA, Zhang S, et al. Inhibition of NF-kappaB Signaling Pathway by Resveratrol Improves Spinal Cord Injury. Front Neurosci. 2018;12:690. doi: 10.3389/fnins.2018.00690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qin SH, Lau ATY, Liang ZL, et al. Resveratrol Promotes Tumor Microvessel Growth via Endoglin and Extracellular Signal-Regulated Kinase Signaling Pathway and Enhances the Anticancer Efficacy of Gemcitabine against Lung Cancer. Cancers (Basel) 2020:12. doi: 10.3390/cancers12040974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cucciolla V, Borriello A, Oliva A, et al. Resveratrol: from basic science to the clinic. Cell Cycle. 2007;6:2495–510. doi: 10.4161/cc.6.20.4815. [DOI] [PubMed] [Google Scholar]
- 35.Thiel G, Ulrich M, Mukaida N, et al. Resveratrol stimulation induces interleukin-8 gene transcription via NF-kappaB. Pharmacol Res. 2018;134:238–45. doi: 10.1016/j.phrs.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 36.Kamaleddin MA. The paradoxical pro- and antiangiogenic actions of resveratrol: therapeutic applications in cancer and diabetes. Ann N Y Acad Sci. 2016;1386:3–15. doi: 10.1111/nyas.13283. [DOI] [PubMed] [Google Scholar]
- 37.Goers L, Freemont P, Polizzi KM. Co-culture systems and technologies: taking synthetic biology to the next level. J R Soc Interface. 2014:11. doi: 10.1098/rsif.2014.0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pando A, Reagan JL, Quesenberry P, et al. Extracellular vesicles in leukemia. Leuk Res. 2018;64:52–60. doi: 10.1016/j.leukres.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 39.Shahidi M, Mohsen Razavi S, Hayat P. Induction of endothelial cell proliferation and von Willebrand factor expression and secretion by leukemic plasma of patients with chronic lymphocytic leukemia before and after inhibition of NF-kappaB. Blood Coagul Fibrinolysis. 2016;27:711–6. doi: 10.1097/MBC.0000000000000502. [DOI] [PubMed] [Google Scholar]
- 40.Umezu T, Ohyashiki K, Kuroda M, et al. Leukemia cell to endothelial cell communication via exosomal miRNAs. Oncogene. 2013;32:2747–55. doi: 10.1038/onc.2012.295. [DOI] [PubMed] [Google Scholar]
- 41.Toaldo IM, Van Camp J, Gonzales GB, et al. Resveratrol improves TNF-alpha-induced endothelial dysfunction in a coculture model of a Caco-2 with an endothelial cell line. J Nutr Biochem. 2016;36:21–30. doi: 10.1016/j.jnutbio.2016.07.007. [DOI] [PubMed] [Google Scholar]