Abstract
Autism spectrum disorder (ASD) is a neurodevelopmental disorder with a substantially increasing incidence rate. It is characterized by repetitive behavior, learning difficulties, deficits in social communication, and interactions. Numerous medications, dietary supplements, and behavioral treatments have been recommended for the management of this condition, however, there is no cure yet. Recent studies have examined the therapeutic potential of the sodium-glucose cotransporter 2 (SGLT2) inhibitors in neurodevelopmental diseases, based on their proved anti-inflammatory effects, such as downregulating the expression of several proteins, including the transforming growth factor beta (TGF-β), interleukin-6 (IL-6), C-reactive protein (CRP), nuclear factor κB (NF-κB), tumor necrosis factor alpha (TNF-α), and the monocyte chemoattractant protein (MCP-1). Furthermore, numerous previous studies revealed the potential of the SGLT2 inhibitors to provide antioxidant effects, due to their ability to reduce the generation of free radicals and upregulating the antioxidant systems, such as glutathione (GSH) and superoxide dismutase (SOD), while crossing the blood brain barrier (BBB). These properties have led to significant improvements in the neurologic outcomes of multiple experimental disease models, including cerebral oxidative stress in diabetes mellitus and ischemic stroke, Alzheimer’s disease (AD), Parkinson’s disease (PD), and epilepsy. Such diseases have mutual biomarkers with ASD, which potentially could be a link to fill the gap of the literature studying the potential of repurposing the SGLT2 inhibitors’ use in ameliorating the symptoms of ASD. This review will look at the impact of the SGLT2 inhibitors on neurodevelopmental disorders on the various models, including humans, rats, and mice, with a focus on the SGLT2 inhibitor canagliflozin. Furthermore, this review will discuss how SGLT2 inhibitors regulate the ASD biomarkers, based on the clinical evidence supporting their functions as antioxidant and anti-inflammatory agents capable of crossing the blood-brain barrier (BBB).
Keywords: autism spectrum disorder, sodium-glucose cotransporter 2 inhibitors, canagliflozin, neurological disorders, oxidative stress, anti-inflammatory
1. Introduction
Autism spectrum disorder (ASD) is a common neurodevelopmental disorder that affects about 1% of the population [1]. The reported global prevalence of ASD is one in 161 people, while in the UAE it affects one in 89 people [2,3]. The reported incidence of ASD has increased substantially in recent years, as it is estimated that one child is diagnosed with ASD every 20 min in the United Arab Emirates [2,3]. ASD is characterized by repetitive behavior, deficits in interactions, social communication, and activities [1]. Currently, there is no definitive cure for this disorder, and most therapies aim at ameliorating the symptoms, improving the children’s functioning and supporting their learning and development. In fact, various pharmacological agents, dietary therapies, and behavioral interventions have been utilized to benefit this condition [4]. Sodium-glucose cotransporter 2 (SGLT2) inhibitors are considered as oral glucose-lowering medication that work by inhibiting the reabsorption of the renal glucose [5]. The list of SGLT2 inhibitors that are Food and Drug Administration (FDA) approved, include dapagliflozin, ertugliflozin, empagliflozin, canagliflozin, ipragliflozin, tofogliflozin, luseogliflozin, and remogliflozin [6]. The available evidence from preclinical studies and clinical studies showed that the SGLT2 inhibitors have distinctive morbidity and mortality reduction benefits in patients with type 2 diabetes mellitus (T2DM) and heart failure (HF). Accordingly, the European Association of the Study for Diabetes (EASD) and the American Diabetes Association (ADA), recommend SGLT2 inhibitors as the mainstay treatment of T2DM and the first line of T2DM treatment, in case of heart failure (HF) [7,8], and as an independent treatment of HF with a reduced ejection fraction, regardless of the diabetes status, according to the European Society of Cardiology (ESC) [9].
In addition to their blood glucose lowering effect, SGLT2 inhibitors have several pleiotropic benefits (Table 1), such as improving the visceral adiposity, reduction of body weight, lowering blood pressure, anti-inflammatory, anti-oxidant, as well as normalizing the serum uric acid levels and lipid profile [10,11,12,13]. Based on these reported benefits of the SGLT2 inhibitors, researchers are tempted to further examine the utility of these agents in the management of other diseases that are characterized by abnormal elevated levels of inflammation and oxidative stress, and more specifically, in neurological disorders.
Table 1.
Comparison between the SGLT2 inhibitors affinity and pleotropic effects.
| Sotagliflozin | Canagliflozin | Dapagliflozin | Empagliflozin | Ertugliflozin | |
|---|---|---|---|---|---|
| Affinity for SGLT2 over SGLT1 | 20 fold [14] | 250 fold [14] (Dual inhibitor) |
1200 fold [14] | 2500 fold [14] | 2500 fold [14] |
| AChE inhibition | Ki 5.6 µM [15] | Ki 0.13 µM (most potent) [15] |
Ki 25.02 µM [15] | Ki 0.177 µM [15] | Ki 31.69 µM [15] |
| Anti-inflammatory | Not applicable | Yes [16] | Yes [17] | Yes [18] | No [19] |
| Oxidative stress inhibition | Yes [20] | Yes [21] | Yes [22] | Yes [23] | Yes [24] |
| Nervous system remodeling | Not applicable | Not applicable | Not applicable | Yes [25] | Not applicable |
| mTOR signaling reduction | Not applicable | Yes [26] | Yes [26] | Yes [27] | Yes [28] |
2. Etiology and the Pathophysiology of Autism
The etiology of ASD is complex in nature. It could be associated with genetic factors and/or environmental components, such as infection, toxins, or medications, which in turn might induce several epigenetic changes [2]. Moreover, recent findings indicated a positive correlation between the brain development and the intestinal microbiota [29]. This explains why infants fed on cow’s milk formula had a drastically increased plasma osmolality which affected the homeostasis hemodynamics of the brain development negatively, compared to the breast-fed infants, emphasizing the gut microbiota-brain axis association to neurodevelopmental disorders [30,31]. The microbiome is essential for the microglial maturation process and taking control of the CNS glial activation, thus regulating the inflammation in the CNS, as the gut dysbiosis impacts the immune system homeostasis, which leads to developmental delays and to the developmental pathway disruption [32].
The risk of developing drug- induced ASD is increased during the second trimester of the fetal development, when exposed to neurotoxic or teratogenic drugs of various pharmacological classes of interest [33]. The pathological process of ASD remains unclear, but the neurological findings, during the first year of the child’s life, confirm the premature brain overgrowth, as a result of the excessive neuron numbers, which leads to defects in the neural wiring and patterning, with short cortical interactions hindering the function of the long-distance interactions within the brain regions in a large-scale. These networks underlie the communication and socio-emotional functions, such early alterations in the brain might be linked to the ASD clinical manifestations [34]. According to the literature, medications such as anticonvulsants and antidepressants have the potential of causing ASD during pregnancy, for instance, valproic acid (VPA) is an anticonvulsant drug used primarily in bipolar disorder and epilepsy [35]. During pregnancy, and at a vital stage of the nervous system development (second trimester), it was found to increase the risk of developing intellectual disorders, including ASD in children [35]. Moreover, preclinical studies showed that when rodents are prenatally exposed to VPA, they displayed neurodevelopmental characteristics which are comparable to those observed in the human setting [36]. When pregnant rodents were injected intraperitoneally (IP) to this drug on gestational day (GD) 12.5, the delivered pups were found to display social interaction impairments, anxiety, and recognition memory deficits, which are categorized as typical ASD-like behaviors [37,38,39]. Furthermore, it has been reported that VPA was capable of inducing dendritic spine loss in the prefrontal complex and the CA1 region of the hippocampus in the mouse model [40]. Furthermore, in a rat preclinical model, VPA was reported to significantly decrease the number of positive Nissl bodies in the lower layers of somatosensory cortex, as well as in the middle and lower layers of the prefrontal cortex (PFC), while it increased the apoptotic cell death and the histone levels in the neocortex [41,42]. Other medications, such as selective serotonin reuptake inhibitors (SSRIs) have been controversial in this case. An investigation which included 117,737 patients detected a significant association between SSRIs exposure across all trimesters and offspring ASD development [43,44], while it has been challenged by a retrospective study of 35,906 births confirming no relation between SSRIs exposure and ASD [45], which concludes that SSRIs exposure solely is not a confounding variable in causing ASD [46]. In addition to the behavioral testing, several biochemical assays showed that autistic children display elevated levels of plasma lipid peroxidation, reactive oxygen species (ROS), and a significant inhibition of antioxidant enzymes and ATP levels [47,48]. The mechanistic target of rapamycin (mTOR) is a substantial signaling node that receives input from the regulatory type proteins to send signals from the nutrient stores, the growth factors, energy, and the ambient oxygen levels [49]. Such involvements make mTOR a sensor for cell survival and growth cellular resources. Furthermore, mTOR has been implicated in the pyrimidine and purine nucleotide biosynthesis, the phosphorylation of other protein substrates, and the DNA transcription [50]. The mTOR enzyme plays a vital role in the brain, to establish the spine morphology, the axon development, the dendritic arborization, and the synaptic flexibility [51]. Brain malformations are associated with the mTOR regulatory genes mutations as the activation of the mTOR pathway in the hippocampal neurons, elevates the branching and growth of the dendritic arbors, while their complexity was decreased in such cells by the mTOR removal in vitro [52]. Signals of the mTOR complex 1 and the mTOR complex 2 were found to be crucial for a healthy dendritic arbor development of the rat hippocampus in vitro [53]. In mice models, mTOR have shown to play role in regulating the axon outgrowth in the mouse dorsal root ganglion, as it is upregulated after injury, which leads to the increased capacity of the axonal growth [54]. Moreover, the mTOR pathway regulates the balance in the brain between the cellular activation and the inhibition, thus the supply network integrity, the synaptic plasticity, and the learning capability [55]. Hence, numerous neurodevelopmental disorders are associated with the deviant mTOR pathway activation. Particularly, a subset of the cortical development malformations is directly caused by the mTOR activity regulators’ genes mutations [56]. An abnormal mTOR activity has been identified in brain development disorders, including the defective connectivity or synaptogenesis, such as epilepsy and ASD, and can be associated with intellectual disability [57]. Since abnormalities and dysregulations of mTOR have been identified in ASD; establishing this association would be of a significance from a therapeutic point of view, as the mTOR inhibitors are clinically available [58]. Accordingly, interventions that target these mechanistic pathways would plausibly impact the progression of the disease, thus improve the overall behavioral symptoms. Additionally, the preceding studies proposed a relation between the neurogenic disorders, including ASD and maternal infections [59]. Pregnant mice and rats infected with human influenza led to offspring with autistic behaviors [60]. Other viruses, such as the mumps, cyto-megalovirus, and measles are unlikely to be associated with current ASD cases because of the vaccination programs that effectively reduced their prevalence [61], while there is no evidence for vaccination to increase the risk of causing autism [62]. Toxic exposures, such as to heavy metals, pesticides, air pollutants, persistent, and non-persistent organic pesticides demonstrated a neurotoxicity by interacting with the genetics factors, hence modifying the neurodevelopment of the synapses, increasing the oxidative stress and neuroinflammation [63,64]. Genetic research discovered that the ASD etiology is robustly heterogenic and multigenic by sequencing technology, as hundreds of peril genes were identified, mainly those involved in the transcriptional regulation, chromatin remodeling, and synapse formation [65,66].
3. SGLT2 Inhibitors Decrease Oxidative Stress
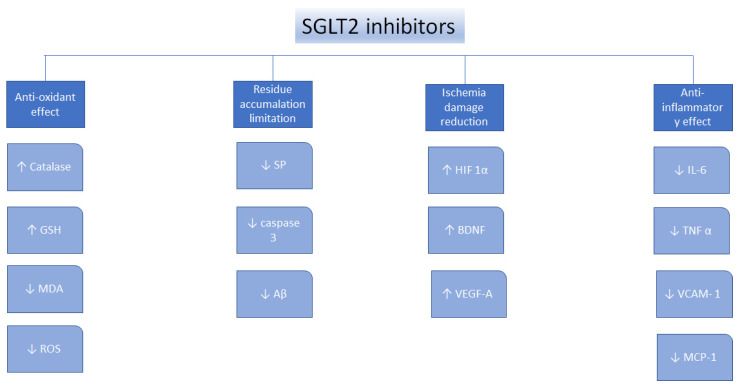
Oxidative stress, defined as the imbalance between the antioxidants production and the pro-oxidants levels, is a vital element underlying the pathogens of nephropathy, neural disorders, cardiovascular disorders, diabetes mellitus, liver conditions, and cancer [67]. SGLT2 inhibitors were found to perform as antioxidants indirectly because of their ability to reduce the generation of free radicals [68], to upregulate the antioxidant systems, such as glutathione (GSH) and superoxide dismutase (SOD) [69,70,71], to suppress pro-oxidants, such as thiobarbituric acid-reactive substances (TBARS), to reduce nicotinamide adenine dinucleotide phosphate oxidase 4 (NOX4) [72,73], and decrease the glucose-induced oxidative stress [74]. Moreover, canagliflozin, dapagliflozin, and empagliflozin were found to decrease oxidative stress in many types of cancer, by suppressing the cellular proliferation [75,76,77,78,79] (Figure 1).
Figure 1.
Mechanisms of action for the SGLT2 inhibitors. ↑—increase, ↓—decrease, glutathione—GSH, malondialdehyde—MDA, senile plaques—SP, amyloid β—Aβ, hypoxia-inducible factor 1α—HIF1α, brain-derived neurotrophic factor—BDNF, vascular endothelial growth factor A—VEGF-A, interleukin 6—IL-6, tumor necrosis factor α—TNFα, vascular cell adhesion protein—VCAM-1, reactive oxygen species—ROS, monocyte chemotactic protein-1—MCP-1 [75].
4. The Anti-Inflammatory Characteristics of the SGLT2 Inhibitors
The process of inflammation has a significant role in neurodevelopmental diseases, metabolic diseases, lifelong kidney disorder, liver disease, cardiovascular disease, and cancer [80,81]. SGLT2 inhibitors have shown anti-inflammatory effects in multiple preclinical disease models [82]. SGLT2 inhibitors have been reported to downregulate the pro-inflammatory mediators, including transforming the growth factor beta (TGF-β), interleukin-6 (IL-6), c-reactive protein (CRP), nuclear factor κB (NF-κB), tumor necrosis factor alpha (TNF-α), and the monocyte chemoattractant protein (MCP-1) [83,84]. The SGLT2 inhibitors were found to attenuate the inflammation by their capability to regulate the imbalanced redox state, the tissue hemodynamic alterations, and the renin-angiotensin system (RAS) [85,86]. Furthermore, the expression of proinflammatory cytokines could be the result of the activated transcription factors activated by oxidative stress, including the nuclear factor erythroid 2–related factor 2 (Nrf2), peroxisome proliferator-activated receptor (PPAR) γ, hypoxia-inducible factor (HIF)-1α, and NF-κB [87,88]. SGLT2 inhibitors reduced oxidative stress in many diseases, and they may also reduce inflammation caused by the chemokines regulation and cytokines transcription factors (Figure 2).
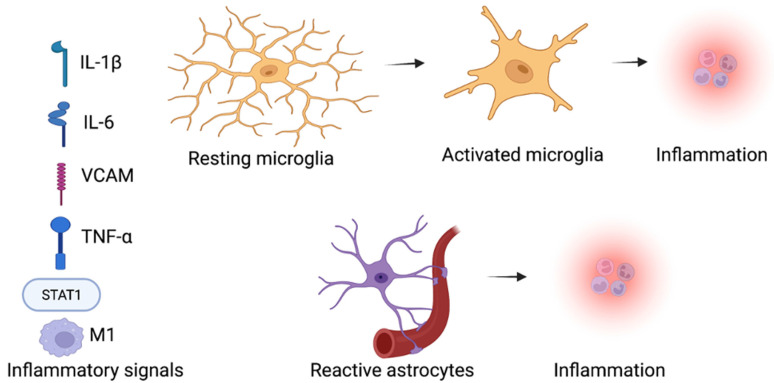
Figure 2.
Inflammatory signals promote inflammation by activating the microglia and astrocytes within the brain in ASD. SGLT2 inhibitors influence on the inflammation and neuroinflammation, SGLT2 inhibitors decrease the inflammatory factors levels, such as the M1 macrophages, STAT1 inflammatory transcription factor, cytokine interleukin-1β (IL-1β), tumor necrosis factor (TNF-α), and vascular cell adhesion protein (VCAM) in neurodevelopmental diseases [43,44]. (Created with BioRender.com).
5. Pleiotropic Perspective of the SGLT2 Inhibitors in ASD
The effects of the SGLT2 inhibitors have been extensively studied [6,10,13,89,90,91], and the assessment of the design, limitations, and outcomes of the SGLT2 inhibitors trials have been comprehensively discussed [6,92,93]. Different from other reviews on SGLT2 inhibitors, this review aims thoroughly at focusing on the studies that assessed the SGLT2 inhibitors benefits from a neurodevelopmental point-of-view. Databases, such as PubMed, MEDLINE, and Google Scholar have been searched using the following terms: SGLT2 inhibitors, antioxidants, oxidative stress, and neurodevelopmental diseases, up to 31 January 2022. This review will discuss how the SGLT2 inhibitors regulate the ASD biomarkers, based on the clinical evidence supporting their function as antioxidants and anti-inflammatories that can cross the blood-brain barrier (BBB). This disease-specific review will provide a better understanding of the potential of antioxidant and anti-inflammatory roles of the SGLT2 inhibitors in ASD. The potential therapeutic role of the SGLT2 inhibitors in ASD have not yet been studied. Therefore, this review aims at providing the theoretical evidence about their plausible efficacy in these disorders, which would encourage further research in this area.
6. Role of the SGLT2 Inhibitors in Neurodevelopmental Disorders
Glucose is the primary metabolic substrate of the neural function [94]. It is transported across the BBB into the brain and made available to the glial cells and neurons via several glucose transporters (GLUTs) [95]. Of notable importance in the CNS, are the GLUT1 which is expressed in the glial cells and BBB, and the GLUT3 which is expressed in the neurons [96]. SGLT receptors were originally found to be expressed in the intestines and kidneys, but later protein expression studies showed that the SGLT receptors are distributed in different brain areas, such as the hippocampus, putamen, frontal cortex, hypothalamus, parietal cortex, and brainstem [97]. Moreover, it has been shown that the SGLT2 inhibitors can cross the BBB as they are partially lipid soluble. Accordingly, numerous studies have underlined the protein expression of the SGLT2 receptors in the encephalon of APP/PS1xd/db mice, emphasizing their potential therapeutic benefits in neurodevelopmental disorders [98,99].
6.1. SGLT2 Inhibitors in Diabetes Mellitus and Ischemic Stroke
Diabetes mellitus and obesity are risk factors for cognitive disorders [100]. It has been reported that a dose of 10 mg/kg of empagliflozin given for a period of 22 weeks, notably improved the cognitive status of db/db mice, by increasing the cerebral brain derived neurotrophic factor and the reduction of the cerebral oxidative stress [101]. In addition, a previous study has shown that receiving of 10 mg/kg of empagliflozin 24 h following a reperfusion, has decreased the parenchymal microglial burden of APP/PS1xdb/db mice and db/db mice [102]. Another preclinical study showed that the exposure to 10 mg/kg of empagliflozin at 24-hour following the reperfusion, ameliorated the neurological obstruction in ischemic stroke rat model in a dose-dependent manner [103]. Similarly, a decrease in the malondialdehyde (MDA) levels, elevated the catalase activity, and increased the GSH levels were observed in rat brain cells, following the systemic administration of empagliflozin leading to the suppressed levels of inflammation and the cerebral oxidative stress [103].
6.2. SGLT2 Inhibitors’ Ameliorative Impact in AD and PD
Parkinson’s disease (PD) and Alzheimer’s disease (AD) are common age-related neurodegenerative conditions. Currently, the available pharmacotherapeutic options for AD and PD merely provide symptomatic relief without curing the process [104]. The main pathological characteristics of AD, are the intra- and extra-cellular plaques accumulation, which is composed of neurofibrillary tangles (NFTs) and beta-amyloid. Recent studies have stated that the inhibition of the SGLT receptors may provide a beneficial effect on this process. A previous APP/PS1xdb/db mouse model study reported that empagliflozin reduced the insoluble and soluble levels of amyloid β in the hippocampus and the cortex of tested mice, with an overall reduction of the senile plaque density [102]. Moreover, in the scopolamine-induced memory impairment in the rat model, canagliflozin was found to prevent the memory impairment [105]. Such observed therapeutic effects might be attributed to the inhibition of the acetylcholinesterase enzyme, which is a property of canagliflozin and other SGLT2 inhibitors [66]. Furthermore, several previous studies have shown that the oxidative stress-induced neuroinflammation can lead to a mitochondrial dysfunction and glial cell activation, causing multiple molecular events in the brain, including the neural cell dissolution in PD and AD [106]. In a recent preclinical study involving a rotenone-induced PD in a rat, it was shown that a dose of 150 mg/kg of dapagliflozin was able to enhance PD motor activity in the rotarod and open-field tests [106]. In addition, dapagliflozin at the same dose, was found to alleviate the neuronal oxidative stress, through the reduction of the lipid peroxides [106]. Furthermore, dapagliflozin was reported to upregulate the glial derived neurotrophic element and its phosphatidylinositol 3-kinase protein kinase, and the glycogen synthase kinase-3β (GSK-3β) pathway, which affects the regulation of numerous key cellular operations, such as proliferation, senescence, differentiation, motility, and survival [106].
6.3. Application of the SGLT2 Inhibitors in Epilepsy
Erdogan et al. 2018 illustrated that the activity of a pentylenetetrazol-induced seizure in a rat model was decreased significantly, following exposure to the SGLT2 inhibitor dapagliflozin in a dose-dependent manner (75–150 mg/kg, i.p.), which has been attributed to the reduction in the sodium transport across the neuronal membranes, and the decreased glucose availability, hence stabilizing against the depolarization and excitability [107]. Collectively, the impact of the SGLT2 inhibitors in the neural disorders have shown promising therapeutic potentials, and some of the most significant observations are summarized in Table 2.
Table 2.
Preclinical evidence of the reparative implications of the SGLT2 inhibitors in neurological disorders.
| Disorder | Animal Species | Medication | Results | References |
|---|---|---|---|---|
| Cognitive impairment | Mice | Empagliflozin | Increase the cerebral brain derived neurotrophic factor and reduce the cerebral oxidative stress. | [61,62] |
| AD | Rats | Canagliflozin | Reduce amyloid β levels, plaque density, and acetylcholinesterase. | [66] |
| PD | Rats | Dapagliflozin | Upregulate the GDNF/PI3K/AKT/GSK-3β pathway and reduce the ROS-dependent neuronal apoptosis. | [67] |
| Epilepsy | Rats | Dapagliflozin | Reduce sodium and glucose transported across the neurons. | [68] |
| Stroke | Rats | Empagliflozin | Upregulate VEGF and HIF-1α; decreased MDA, elevated GSH and activity of catalase. | [63,64] |
7. SGLT Receptors as a Potential Target for Neurological Disorders
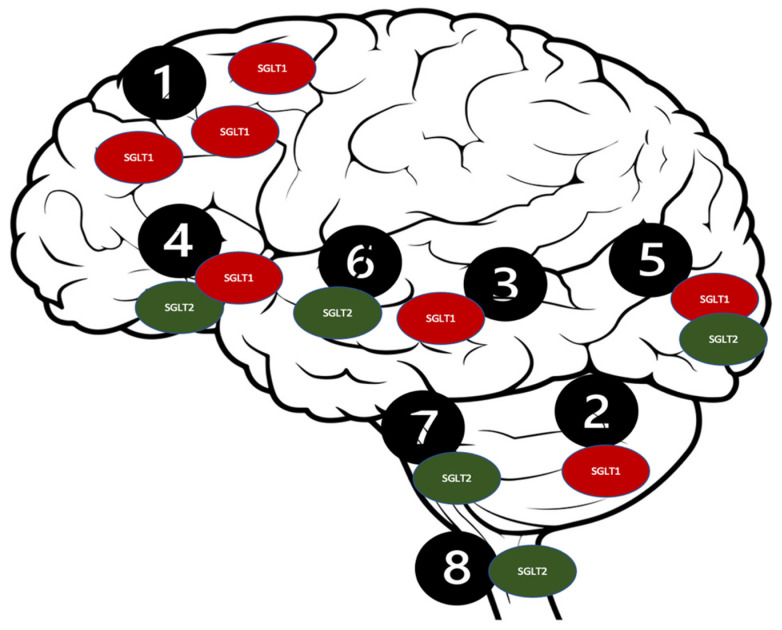
SGLT co-transporters contain spanning monomer proteins, including a single N-glycosylation site and 14 transmembrane domains. They transport galactose and glucose against a concentration gradient alongside the simultaneous Na+ ions transportation [108]. Numerous reports have stated the presence of the SGLT receptors in a mammalian central nervous system (CNS) [97,109]. Furthermore, studies have proven that the SGLT2 receptors are significantly expressed in the cerebellum, the BBB endothelial cells, and the hippocampus, while the SGLT1 receptors are expressed in the CA1 and CA3 regions of the hippocampus [110,111,112,113] (Figure 3).
Figure 3.
Distribution of the SGLT receptors in the CNS. 1. Brain cortex (pyramidal cells); 2. Purkinje neurons; 3. Hippocampus; 4. Hypothalamus; 5. Micro vessels; 6. Amygdala cells; 7. Periaqueductal gray; 8. Dorsomedial medulla.
Such a distribution of the SGLT2 receptors [114] could potentially be responsible for their intriguing neuroprotective qualities, which could be beneficial in several neurological disorders, including ASD [99]. The SGLT2 inhibitors’ proposed mechanisms are presented in Figure 3. The antioxidant effect of the SGLT2 inhibitors can be attributed to their stimulatory action on the nuclear factor erythroid 2 (Nrf2)- related factor 2 pathway [115]. This displays the antioxidant activity because of their genetic expression of the antioxidant proteins, including glutathione-s-transferase (GST), SOD, and NADPH quinone dehydrogenase-1 to protect against cellular apoptosis [116]. The anti-inflammatory characteristics of the SGLT2 inhibitors could be accredited to the downregulation of NF-KB, which decreases IL-1β and the TNF-α expression [117]. Empagliflozin has the highest selectivity for the SGLT2 receptors (2500-fold) when compared to dapagliflozin which has (1200-fold) selectivity, and canagliflozin (250-fold) [118,119]. Therefore, in the context of the neuroprotective effects associated with the SGLT1 and SGLT2 receptors’ inhibition, canagliflozin was hypothetically preferred over other SGLT2 inhibitors, due to its dual SGLT1/SGLT2 inhibition capability [120].
8. Mutual Oxidative Biomarkers of ASD and the Potential Therapeutic Utility of the SGLT2 Inhibitors
ASD is a neurodevelopmental disorder characterized by diverse range of the impaired social abilities and communication, stereotypic and repetitive behaviors [121]. ASD could be diagnosed in early childhood, with a female-to-male ratio of 1:4, and an increasing prevalence over the past 20 years, as the current estimate is one in 160 children worldwide has an ASD [2,122]. There is no definitive curative medication for ASD yet, as the currently available pharmacological or behavioral therapies cannot enhance the core parameters of ASD [123,124,125]. Early detection of the autism symptoms is crucially impacting children’s adaptive skills development, and social intelligence, which have been reported in many studies practicing advanced imaging methods [126]. Finding validated biomarkers for the ASD screening or treatment follow-up have been attempted in many prospective studies, as the Autism Birth Cohort, has considered the proteomic, genetic, metagenomic, microbiological, and immunological parameters to utilize in a case-control study [127]. Moreover, the 1-Year Well-Baby Check-up Approach investigated various endpoints in autistic patients, including the gene expression, the immune system functionality, early brain growth, and cerebellar functions, to identify the biomarkers of the disease [128]. In neurodevelopmental diseases, such as ASD, oxidative stress was found to change the intracellular balance between the antioxidant defense mechanisms and the reactive oxygen species (ROS) [129]. Accordingly, both the enzymatic and nonenzymatic mechanisms of protection have been reported to exist. Enzymes include catalase (CAT), SOD, ceruloplasmin, and glutathione peroxidase (GPX) [130]. In contrast, other systems include phenolic compounds, glutathione (GSH), and the antioxidant nutrients (vitamins, A C, E, B6; folate) [131]. Moreover, elevated oxidative stress was found to trigger the activation of the mast cell, which in turn increases the production of the proinflammatory, neuro-sensitizing, and vasoactive molecules connected to ASD, such as histamine, IL-6, bradykinin, tryptase, and the vascular endothelial growth factor (VEGF) [131]. These factors interrupt the BBB [132], permitting the entero-toxic molecules into the brain inducing neuroinflammation [133]. Oxidative stress has a central function in the ASD pathogenesis as it upregulates the neural deterioration in the genetically susceptible patients. The mammalian brain reacts to oxidative stress deterioration, as it accounts for 20% of the basal oxygen consumption, whereas it is responsible for a few percent of body weight [134]. Numerous animal studies have assessed the impact of the oxidative stress on the CNS in different models [135,136,137]. The imbalance between the antioxidant and oxidant systems involved in the neurodegenerative pathologies, e.g., AD [138], and mental disorders, including bipolar disorder and schizophrenia [139,140,141]. In a postmortem analysis study, brain tissues from autistic patients were found to display higher levels of oxidative stress biomarkers than healthy individuals [142,143,144,145,146]. The association between ASD and oxidative stress was disclosed in various studies with different biomarkers, as illustrated in Table 3, head-to-head with the mutual antioxidative ability of the SGLT2 inhibitors [147,148].
Table 3.
Comparison of the oxidative stress biomarkers’ blood levels in autistic patients with a mutual therapeutic biomarker impact of the SGLT2 inhibitors. ↑—increase, ↓—decrease.
| ASD Biomarkers | Result | Reference | SGLT2 Inhibitor Name/Subject of Study | Result | Reference Number |
|---|---|---|---|---|---|
| GSH | Statistically significantly lower level of GSH in the ASD group than in the control group. | [148,149,150,151] | Empagliflozin/Wistar rats | ↑ GSH | [103] |
| CAT | Lower CAT activity in the erythrocytes of autistic patients than in the healthy controls. | [152,153] | Empagliflozin/Wistar rats | ↑ CAT | [103] |
| GPX | GPX activity in the erythrocytes is significantly lower in the ASD group than in the control group after the meta-analysis. | [152,154,155,156,157] | Dapagliflozin/Wistar rats | ↑ GPX | [158] |
| TNF-α | ASD children produced more TNF-α than those obtained from the control. | [159,160,161] | Empagliflozin/ApoE-/-mice | ↓ TNF-α | [83] |
| IL-6 | Autistic mice displayed elevated IL-6 in the brain. | [155,156,157,158,159,160,161,162] | Empagliflozin/ApoE-/-mice | ↓ IL-6 | [83] |
| Caspase-3 | Assessed the active caspase-3 levels and determined the significant elevation in children with ASD. | [156,157,158,159,160,161,162,163,164,165,166] | Empagliflozin/Wistar rats | ↓ caspase 3 | [102] |
| HIF-1α | Serum HIF-1α levels were borderline significantly lower in the ASD group. | [167] | Empagliflozin/Wistar rats | ↑ HIF-1α, | [102] |
| Aβ | Severe ASD patients produced beta-amyloid at twice more than the control group and four times more than the mild ASD group. | [168,169] | Empagliflozin/APP/PS1xdb/db mice | ↓ Aβ | [101] |
SGLT2 inhibitors have the potential to improve ASD patients’ behavioral and brain disruptions by increasing the cerebral brain derived neurotrophic factor and reducing the cerebral oxidative stress, including elevated the GSH and catalase activity, reduced MDA, amyloid β levels, plaque density, and acetylcholinesterase [101,102,103,104].
9. Conclusions
ASD remains a global health dilemma, as it is a chronic condition, and is incurable, leading to a reduced quality of life. It is crucial to find the mutual molecular mechanisms of ASD and redefine the indications for the well-studied medication with numerous pleiotropic effects to find a solution. This review has disclosed the impact of the SGLT2 inhibitors in neurological diseases, which could relate to ASD as it shares multiple pathways and mutual biomarkers. SGLT2 inhibitors display several neuroprotective properties, highlighting their therapeutic potential for ASD patients, as these agents have the capability to inhibit the acetylcholinesterase enzyme, reduce the elevated levels of the oxidative stress in the brain, and restore the anabolism and catabolism balance. Moreover, clinical intervention studies are vital to determine whether the displayed methods are useful as the SGLT2 inhibitors have never been tested on ASD directly. Currently, our research team is conducting a preclinical experiment to assess the effects of canagliflozin on the VPA-induced ASD in Wistar rats.
Acknowledgments
We would like to thank our colleagues Ali Saad and Petrilla Jayaprakash for helping us with creating the figures.
Abbreviations
| ADA | American Diabetes Association |
| AD | Alzheimer’s disease |
| APP/PS1 | Amyloid precursor protein double transgenic mice expressing a chimeric mouse/human (Mo/HuAPP695swe) and a mutant human presenilin 1 |
| ASD | Autism spectrum disorder |
| BBB | Blood-brain barrier |
| CNS | Central nervous system |
| CRP | C-reactive protein |
| db/db | Diabetes mellitus most widely used mouse model |
| EASD | European Association of the Study for Diabetes |
| ESC | European Society of Cardiology |
| FDA | Food and Drug Administration |
| GD | Gestational day |
| GLUTs | Glucose transporters |
| GSH | Glutathione |
| GST | Glutathione-s-transferase |
| GSK-3β | Glycogen synthase kinase-3β |
| HF | Heart failure |
| HIF-1α | Hypoxia-inducible factor |
| IL-6 | Interleukin-6 |
| i.p | Intraperitoneally |
| MDA | Malondialdehyde |
| mTOR | Mechanistic target of rapamycin |
| MCP-1 | Monocyte chemoattractant protein |
| NFTs | Neurofibrillary tangles |
| NADPH | Nicotinamide adenine dinucleotide phosphate-reduced |
| NOX4 | Nicotinamide adenine dinucleotide phosphate oxidase 4 |
| Nrf2 | Nuclear factor erythroid 2–related factor 2 |
| NF-κB | Nuclear factor κB |
| PD | Parkinson’s disease |
| PFC | Prefrontal cortex |
| PPAR | Peroxisome proliferator-activated receptor |
| ROS | Reactive oxygen species |
| RAS | Renin-angiotensin system |
| SSRIs | Selective serotonin uptake inhibitors |
| SGLT2 | Sodium-glucose cotransporter 2 |
| SOD | Superoxide dismutase |
| TBARS | Thiobarbituric acid-reactive substances |
| TNF-α | Tumor necrosis factor alpha |
| T2DM | Type 2 diabetes mellitus |
| VPA | Valproic acid |
| VEGF | Vascular endothelial growth factor |
Author Contributions
A.A. and B.S. created the study concept and design, as well as revising the work critically for publishing approval. M.M.N. drafted the work and substantially contributed to the analysis and interpretation of the data. S.A. critically revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data in this study were not created nor analyzed by the authors. Data sharing is not applicable to this article.
Conflicts of Interest
Authors have no conflict of interest to declare.
Funding Statement
This research was funded by the UAE University’s Office of Graduate Studies and Research, as well as the Zayed-Center for Health Sciences, respectively (21M132 and 12M099) and the APC was funded by UAE University’s Office of Graduate studies and Research (21M132).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jones E.J., Gliga T., Bedford R., Charman T., Johnson M.H. Developmental pathways to autism: A review of prospective studies of infants at risk. Neurosci. Biobehav. Rev. 2013;39:1–33. doi: 10.1016/j.neubiorev.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elsabbagh M., Divan G., Koh Y.J., Kim Y.S., Kauchali S., Marcín C., Montiel-Nava C., Patel V., Paula C.S., Wang C., et al. Global prevalence of autism and other pervasive developmental disorders. Autism Res. 2012;5:160–179. doi: 10.1002/aur.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tom D. Autism Facts in The UAE—Autism 101 [Internet]. NAET Dubai. 2022. [(accessed on 3 January 2022)]. Available online: https://www.naetdubai.com/autism-facts-in-the-uae/
- 4.Medavarapu S., Marella L.L., Sangem A., Kairam R. Where is the Evidence? A Narrative Literature Review of the Treatment Modalities for Autism Spectrum Disorders. Cureus. 2019;11:e3901. doi: 10.7759/cureus.3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verma S., McMurray J.J.V. SGLT2 inhibitors and mechanisms of cardiovascular benefit: A state-of-the-art review. Diabetologia. 2018;61:2108–2117. doi: 10.1007/s00125-018-4670-7. [DOI] [PubMed] [Google Scholar]
- 6.Cowie M.R., Fisher M. Sglt2 inhibitors: Mechanisms of cardiovascular benefit beyond glycaemic control. Nat. Rev. Cardiol. 2020;17:761–772. doi: 10.1038/s41569-020-0406-8. [DOI] [PubMed] [Google Scholar]
- 7.Cannon C.P., McGuire D.K., Pratley R., Dagogo-Jack S., Mancuso J., Huyck S., Charbonnel B., Shih W.J., Gallo S., Ma- siukiewicz U., et al. Design and baseline characteristics of the evaluation of ertugliflozin efficacy and safety cardiovascular outcomes trial (vertis-cv) Am. Heart J. 2018;206:11–23. doi: 10.1016/j.ahj.2018.08.016. [DOI] [PubMed] [Google Scholar]
- 8.Buse J.B., Wexler D.J., Tsapas A., Rossing P., Mingrone G., Mathieu C., D’Alessio D.A., Davies M.J. 2019 update to: Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2020;43:487–493. doi: 10.2337/dci19-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kolesnik E., Scherr D., Rohrer U., Benedikt M., Manninger M., Sourij H., von Lewinski D. SGLT2 Inhibitors and Their Antiarrhythmic Properties. Int. J. Mol. Sci. 2022;23:1678. doi: 10.3390/ijms23031678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alicic R.Z., Johnson E.J., Tuttle K.R. Sglt2 inhibition for the prevention and treatment of diabetic kidney disease: A review. Am. J. Kidney Dis. 2018;72:267–277. doi: 10.1053/j.ajkd.2018.03.022. [DOI] [PubMed] [Google Scholar]
- 11.Bailey C.J. Uric acid and the cardio-renal effects of sglt2 inhibitors. Diabetes Obes. Metab. 2019;21:1291–1298. doi: 10.1111/dom.13670. [DOI] [PubMed] [Google Scholar]
- 12.Thomas M.C., Cherney D.Z.I. The actions of sglt2 inhibitors on metabolism, renal function and blood pressure. Diabetologia. 2018;61:2098–2107. doi: 10.1007/s00125-018-4669-0. [DOI] [PubMed] [Google Scholar]
- 13.Saisho Y. Sglt2 inhibitors: The star in the treatment of type 2 diabetes? Diseases. 2020;8:14. doi: 10.3390/diseases8020014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shakil S. Molecular Interaction of Anti-Diabetic Drugs with Acetylcholinesterase and Sodium Glucose Co-Transporter 2. J. Cell. Biochem. 2017;118:3855–3865. doi: 10.1002/jcb.26036. [DOI] [PubMed] [Google Scholar]
- 15.Shaikh S., Rizvi S.M., Suhail T., Shakil S., Abuzenadah A., Anis R., Naaz D., Dallol A., Haneef M., Ahmad A., et al. Prediction of Anti-Diabetic Drugs as Dual Inhibitors Against Acetylcholinesterase and Beta-Secretase: A Neuroinformatics Study. CNS Neurol Disord Drug Targets. 2016;15:1216–1221. doi: 10.2174/1871527315666161003125752. [DOI] [PubMed] [Google Scholar]
- 16.Xue L., Yuan X., Zhang S., Zhao X. Investigating the Effects of Dapagliflozin on Cardiac Function, Inflammatory Response, and Cardiovascular Outcome in Patients with STEMI Complicated with T2DM after PCI. Evid. Based Complement. Altern. Med. 2021;2021:9388562. doi: 10.1155/2021/9388562. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Ganbaatar B., Fukuda D., Shinohara M., Yagi S., Kusunose K., Yamada H., Soeki T., Hirata K.-I., Sata M. Empagliflozin ameliorates endothelial dysfunction and suppresses atherogenesis in diabetic apolipoprotein E-deficient mice. Eur. J. Pharm. 2020;875:173040. doi: 10.1016/j.ejphar.2020.173040. [DOI] [PubMed] [Google Scholar]
- 18.Liu H., Sridhar V.S., Lovblom L.E., Lytvyn Y., Burger D., Burns K., Brinc D., Lawler P.R., Cherney D.Z.I. Markers of Kidney Injury, Inflammation, and Fibrosis Associated with Ertugliflozin in Patients With CKD and Diabetes. Kidney Int. Rep. 2021;6:2095–2104. doi: 10.1016/j.ekir.2021.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bode D., Semmler L., Wakula P., Hegemann N., Primessnig U., Beindorff N., Powell D., Dahmen R., Ruetten H., Oeing C., et al. Dual SGLT-1 and SGLT-2 inhibition improves left atrial dysfunction in HFpEF. Cardiovasc. Diabetol. 2021;20:7. doi: 10.1186/s12933-020-01208-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hasan R., Lasker S., Hasan A., Zerin F., Zamila M. Canagliflozin ameliorates renal oxidative stress and inflammation by stimulating AMPK–Akt–eNOS pathway in the isoprenaline—Induced oxidative stress model. Sci. Rep. 2020;10:14659. doi: 10.1038/s41598-020-71599-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zaibi N., Li P., Xu S.Z. Protective effects of dapagliflozin against oxidative stress-induced cell injury in human proximal tubular cells. PLoS ONE. 2021;16:e0247234. doi: 10.1371/journal.pone.0247234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iannantuoni F., De Marañon A.M., Diaz-morales N., Falcon R., Hernandez-mijares A., Rovira-llopis S. The SGLT2 Inhibitor Empagliflozin Ameliorates the Inflammatory Profile in Type 2 Diabetic Patients and Promotes an Antioxidant Response in Leukocytes. J. Clin. Med. 2019;8:1814. doi: 10.3390/jcm8111814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Croteau D., Luptak I., Chambers J.M., Hobai I., Panagia M., Pimentel D.R., Siwik D.A., Qin F., Colucci W.S. Effects of sodium-glucose linked transporter 2 inhibition with ertugliflozin on mitochondrial function, energetics, and metabolic gene expression in the presence and absence of diabetes mellitus in mice. J. Am. Heart Assoc. 2021;10:e019995. doi: 10.1161/JAHA.120.019995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hayden M.R., Grant D.G., Aroor A.R., Demarco V.G. Empagliflozin Ameliorates Type 2 Diabetes-Induced Ultrastructural Remodeling of the Neurovascular Unit and Neuroglia in the Female db/db Mouse. Brain Sci. 2019;9:57. doi: 10.3390/brainsci9030057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou J., Zhu J., Yu S., Ma H., Chen J., Ding X., Chen G., Liang Y., Zhang Q. Sodium-glucose co-transporter-2 (SGLT-2) inhibition reduces glucose uptake to induce breast cancer cell growth arrest through AMPK/mTOR pathway. Biomed. Pharm. 2020;132:110821. doi: 10.1016/j.biopha.2020.110821. [DOI] [PubMed] [Google Scholar]
- 26.Sun X., Han F., Lu Q., Li X., Ren D., Zhang J., Han Y., Xiang Y.K., Li J. Empagliflozin Ameliorates Obesity-Related Cardiac Dysfunction by Regulating Sestrin2-Mediated AMPK-mTOR Signaling and Redox Homeostasis in High-Fat Diet—Induced Obese Mice. Diabetes. 2020;69:1292–1305. doi: 10.2337/db19-0991. [DOI] [PubMed] [Google Scholar]
- 27.Moellmann J., Mann P., Krueger K., Klinkhammer B., Boor P., Marx N., Lehrke M. The SGLT2 inhibitor ertugliflozin causes a switch of cardiac substrate utilization leading to reduced cardiac mTOR-signaling, unfolded protein response and apoptosis. Eur. Heart J. 2021;42:3289. doi: 10.1093/eurheartj/ehab724.3289. [DOI] [Google Scholar]
- 28.Malhotra A., Kudyar S., Gupta A., Kudyar R., Malhotra P. Sodium glucose co-transporter inhibitors—A new class of old drugs. Int. J. Appl. Basic Med. Res. 2015;5:161. doi: 10.4103/2229-516X.165363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maiuolo J., Gliozzi M., Musolino V., Carresi C., Scarano F., Nucera S., Scicchitano M., Oppedisano F., Bosco F., Ruga S., et al. The Contribution of Gut Microbiota–Brain Axis in the Development of Brain Disorders. Front. Neurosci. 2021;15:616883. doi: 10.3389/fnins.2021.616883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hahr J.Y. Iatrogenic autism. Med. Hypotheses. 2013;81:251–252. doi: 10.1016/j.mehy.2013.04.037. [DOI] [PubMed] [Google Scholar]
- 31.Fattorusso A., Di Genova L., Dell’Isola G.B., Mencaroni E., Esposito S. Autism Spectrum Disorders and the Gut Microbiota. Nutrients. 2019;11:521. doi: 10.3390/nu11030521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dash S., Syed Y.A., Khan M.R. Understanding the Role of the Gut Microbiome in Brain Development and Its Association With Neurodevelopmental Psychiatric Disorders. Front. Cell Dev. Biol. 2022;10:880544. doi: 10.3389/fcell.2022.880544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nicolini C., Fahnestock M. The valproic acid-induced rodent model of autism. Exp. Neurol. 2018;299 Pt A:217–227. doi: 10.1016/j.expneurol.2017.04.017. [DOI] [PubMed] [Google Scholar]
- 34.Courchesne E., Pierce K., Schumann C.M., Redcay E., Buckwalter J.A., Kennedy D.P., Morgan J. Mapping Early Brain Development in Autism. Neuron. 2007;56:399–413. doi: 10.1016/j.neuron.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 35.Zhu M.-M., Li H.-L., Shi L.-H., Chen X.-P., Luo J., Zhang Z.-L. The pharmacogenomics of valproic acid. J. Hum. Genet. 2017;62:1009–1014. doi: 10.1038/jhg.2017.91. [DOI] [PubMed] [Google Scholar]
- 36.Kim K.C., Kim P., Go H.S., Choi C.S., Yang S.-I., Cheong J.H., Shin C.Y., Ko K.H. The critical period of valproate exposure to induce autistic symptoms in Sprague–Dawley rats. Toxicol. Lett. 2011;201:137–142. doi: 10.1016/j.toxlet.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 37.Takuma K., Hara Y., Kataoka S., Kawanai T., Maeda Y., Watanabe R., Takano E., Hayata-Takano A., Hashimoto H., Ago Y., et al. Chronic treatment with valproic acid or sodium butyrate attenuates novel object recognition deficits and hippocampal dendritic spine loss in a mouse model of autism. Pharm. Biochem. Behav. 2014;126:43–49. doi: 10.1016/j.pbb.2014.08.013. [DOI] [PubMed] [Google Scholar]
- 38.Barendse E.M., Schreuder L.J., Thoonen G., Hendriks M.P.H., Kessels R.P.C., Backes W.H., Aldenkamp A.P., Jansen J.F.A. Working memory network alterations in high-functioning adolescents with an autism spectrum disorder. Psychiatry Clin. Neurosci. 2017;72:73–83. doi: 10.1111/pcn.12602. [DOI] [PubMed] [Google Scholar]
- 39.Wang Z., Jing J., Igarashi K., Fan L., Yang S., Li Y., Jin Y. Executive function predicts the visuospatial working memory in autism spectrum disorder and attention-deficit/hyperactivity disorder. Autism Res. 2018;11:1148–1156. doi: 10.1002/aur.1967. [DOI] [PubMed] [Google Scholar]
- 40.Mahmood U., Ahn S., Yang E.-J., Choi M., Kim H., Regan P., Cho K., Kim H.-S. Dendritic spine anomalies and PTEN alterations in a mouse model of VPA-induced autism spectrum disorder. Pharm. Res. 2018;128:110–121. doi: 10.1016/j.phrs.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 41.Codagnone M.G., Podestá M.F., Uccelli N.A., Reinés A. Differential Local Connectivity and Neuroinflammation Profiles in the Medial Prefrontal Cortex and Hippocampus in the Valproic Acid Rat Model of Autism. Dev. Neurosci. 2015;37:215–231. doi: 10.1159/000375489. [DOI] [PubMed] [Google Scholar]
- 42.Chomiak T., Hu B. Alterations of neocortical development and maturation in autism: Insight from valproic acid exposure and animal models of autism. Neurotoxicol. Teratol. 2013;36:57–66. doi: 10.1016/j.ntt.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 43.Mezzacappa A., Lasica P.-A., Gianfagna F., Cazas O., Hardy P., Falissard B., Sutter-Dallay A.-L., Gressier F. Risk for Autism Spectrum Disorders According to Period of Prenatal Antidepressant Exposure: A Systematic Review and Meta-analysis. JAMA Pediatr. 2017;171:555–563. doi: 10.1001/jamapediatrics.2017.0124. [DOI] [PubMed] [Google Scholar]
- 44.Rai D., Lee B.K., Dalman C., Newschaffer C., Lewis G., Magnusson C. Antidepressants during pregnancy and autism in offspring: Population based cohort study. BMJ. 2017;358:j2811. doi: 10.1136/bmj.j2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brown H.K., Ray J.G., Wilton A.S., Lunsky Y., Gomes T., Vigod S.N. Association between Serotonergic Antidepressant Use during Pregnancy and Autism Spectrum Disorder in Children. JAMA. 2017;317:1544–1552. doi: 10.1001/jama.2017.3415. [DOI] [PubMed] [Google Scholar]
- 46.Viktorin A., Uher R., Reichenberg A., Levine S.Z., Sandin S. Autism risk following antidepressant medication during pregnancy. Psychol. Med. 2017;47:2787–2796. doi: 10.1017/S0033291717001301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matsuo K., Yabuki Y., Fukunaga K. 5-aminolevulinic acid inhibits oxidative stress and ameliorates autistic-like behaviors in prenatal valproic acid-exposed rats. Neuropharmacology. 2020;168:107975. doi: 10.1016/j.neuropharm.2020.107975. [DOI] [PubMed] [Google Scholar]
- 48.Mirza R., Sharma B. Beneficial effects of pioglitazone, a selective peroxisome proliferator-activated receptor-c agonist in prenatal valproic acid-induced behavioral and biochemical autistic like features in Wistar rats. Int. J. Dev. Neurosci. 2019;76:6–16. doi: 10.1016/j.ijdevneu.2019.05.006. [DOI] [PubMed] [Google Scholar]
- 49.Ma X.M., Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nat. Rev. Mol. Cell Biol. 2009;10:307–318. doi: 10.1038/nrm2672. [DOI] [PubMed] [Google Scholar]
- 50.Renna M., Bento C.F., Fleming A., Menzies F.M., Siddiqi F.H., Ravikumar B., Puri C., Garcia-Arencibia M., Sadiq O., Corrochano S., et al. IGF-1 receptor antagonism inhibits autophagy. Hum. Mol. Genet. 2013;22:4528–4544. doi: 10.1093/hmg/ddt300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tavazoie S.F., Alvarez V.A., Ridenour D.A., Kwiatkowski D.J., Sabatini B.L. Regulation of neuronal morphology and function by the tumor suppressors Tsc1 and Tsc2. Nat. Neurosci. 2005;8:1727–1734. doi: 10.1038/nn1566. [DOI] [PubMed] [Google Scholar]
- 52.Jaworski J., Spangler S., Seeburg D.P., Hoogenraad C.C., Sheng M. Control of Dendritic Arborization by the Phosphoinositide-3′-Kinase–Akt–Mammalian Target of Rapamycin Pathway. J. Neurosci. 2005;25:11300–11312. doi: 10.1523/JNEUROSCI.2270-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Angliker N., Rüegg M.A. In vivo evidence for mTORC2-mediated actin cytoskeleton rearrangement in neurons. BioArchitecture. 2013;3:113–118. doi: 10.4161/bioa.26497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Abe N., Borson S.H., Gambello M.J., Wang F., Cavalli V. Mammalian Target of Rapamycin (mTOR) Activation Increases Axonal Growth Capacity of Injured Peripheral Nerves *. J. Biol. Chem. 2010;285:28034–28043. doi: 10.1074/jbc.M110.125336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.LaSarge C., Danzer S. Mechanisms regulating neuronal excitability and seizure development following mTOR pathway hyperactivation. Front. Mol. Neurosci. 2014;7:18. doi: 10.3389/fnmol.2014.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Crino P.B. mTOR Signaling in Epilepsy: Insights from Malformations of Cortical Development. Cold Spring Harb. Perspect. Med. 2015;5:a022442. doi: 10.1101/cshperspect.a022442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huber K.M., Klann E., Costa-Mattioli M., Zukin R.S. Dysregulation of Mammalian Target of Rapamycin Signaling in Mouse Models of Autism. J. Neurosci. 2015;35:13836–13842. doi: 10.1523/JNEUROSCI.2656-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Winden K.D., Ebrahimi-Fakhari D., Sahin M. Abnormal mTOR Activation in Autism. Annu. Rev. Neurosci. 2018;41:1–23. doi: 10.1146/annurev-neuro-080317-061747. [DOI] [PubMed] [Google Scholar]
- 59.Atladóttir H.Ó., Thorsen P., Østergaard L., Schendel D.E., Lemcke S., Abdallah M., Parner E.T. Maternal Infection Requiring Hospitalization during Pregnancy and Autism Spectrum Disorders. J. Autism Dev. Disord. 2010;40:1423–1430. doi: 10.1007/s10803-010-1006-y. [DOI] [PubMed] [Google Scholar]
- 60.Patterson P.H. Maternal infection and immune involvement in autism. Trends Mol. Med. 2011;17:389–394. doi: 10.1016/j.molmed.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zerbo O., Iosif A.-M., Walker C., Ozonoff S., Hansen R.L., Hertz-Picciotto I. Is Maternal Influenza or Fever During Pregnancy Associated with Autism or Developmental Delays? Results from the CHARGE (CHildhood Autism Risks from Genetics and Environment) Study. J. Autism Dev. Disord. 2013;43:25–33. doi: 10.1007/s10803-012-1540-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bölte S., Girdler S., Marschik P.B. The contribution of environmental exposure to the etiology of autism spectrum disorder. Cell. Mol. Life Sci. 2019;76:1275–1297. doi: 10.1007/s00018-018-2988-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grandjean P., Landrigan P.J. Neurobehavioural effects of developmental toxicity. Lancet Neurol. 2014;13:330–338. doi: 10.1016/S1474-4422(13)70278-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kalkbrenner A.E., Schmidt R.J., Penlesky A.C. Environmental Chemical Exposures and Autism Spectrum Disorders: A Review of the Epidemiological Evidence. Curr. Probl. Pediatr. Adolesc. Health Care. 2014;44:277–318. doi: 10.1016/j.cppeds.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.De Rubeis S., He X., Goldberg A.P., Poultney C.S., Samocha K., Ercument Cicek A., Kou Y., Liu L., Fromer M., Walker S., et al. Synaptic, transcriptional and chromatin genes disrupted in autism. Nature. 2014;515:209–215. doi: 10.1038/nature13772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Woodbury-Smith M., Scherer S.W. Progress in the genetics of autism spectrum disorder. Dev. Med. Child Neurol. 2018;60:445–451. doi: 10.1111/dmcn.13717. [DOI] [PubMed] [Google Scholar]
- 67.Sies H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015;4:180–183. doi: 10.1016/j.redox.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Steven S., Oelze M., Hanf A., Kröller-Schön S., Kashani F., Roohani S., Welschof P., Kopp M., Gödtel-Armbrust U., Xia N., et al. The sglt2 inhibitor empagliflozin improves the primary diabetic complications in zdf rats. Redox Biol. 2017;13:370–385. doi: 10.1016/j.redox.2017.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yaribeygi H., Atkin S.L., Butler A.E., Sahebkar A. Sodium-glucose cotransporter inhibitors and oxidative stress: An update. J. Cell Physiol. 2019;234:3231–3237. doi: 10.1002/jcp.26760. [DOI] [PubMed] [Google Scholar]
- 70.Oshima H., Miki T., Kuno A., Mizuno M., Sato T., Tanno M., Yano T., Nakata K., Kimura Y., Abe K., et al. Empagliflozin, an sglt2 inhibitor, reduced the mortality rate after acute myocardial infarction with modification of cardiac metabolomes and antioxidants in diabetic rats. J. Pharm. Exp. 2019;368:524–534. doi: 10.1124/jpet.118.253666. [DOI] [PubMed] [Google Scholar]
- 71.Osorio H., Coronel I., Arellano A., Pacheco U., Bautista R., Franco M., Escalante B. Sodium-glucose cotransporter inhibition prevents oxidative stress in the kidney of diabetic rats. Oxid. Med. Cell. Longev. 2012;2012:542042. doi: 10.1155/2012/542042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Terami N., Ogawa D., Tachibana H., Hatanaka T., Wada J., Nakatsuka A., Eguchi J., Horiguchi C.S., Nishii N., Yamada H., et al. Long-term treatment with the sodium glucose cotransporter 2 inhibitor, dapagliflozin, ameliorates glucose homeostasis and diabetic nephropathy in db/db mice. PLoS ONE. 2014;9:e100777. doi: 10.1371/journal.pone.0100777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tahara A., Kurosaki E., Yokono M., Yamajuku D., Kihara R., Hayashizaki Y., Takasu T., Imamura M., Li Q., Tomiyama H., et al. Effects of sodium-glucose cotransporter 2 selective inhibitor ipragliflozin on hyperglycaemia, oxidative stress, inflammation and liver injury in streptozotocin-induced type 1 diabetic rats. J. Pharm. Pharm. 2014;66:975–987. doi: 10.1111/jphp.12223. [DOI] [PubMed] [Google Scholar]
- 74.Tsai K.F., Chen Y.L., Chiou T.T., Chu T.H., Li L.C., Ng H.Y., Lee W.C., Lee C.T. Emergence of SGLT2 inhibitors as powerful antioxidants in human diseases. Antioxidants. 2021;10:1166. doi: 10.3390/antiox10081166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Scafoglio C.R., Villegas B., Abdelhady G., Bailey S.T., Liu J., Shirali A.S., Wallace W.D., Magyar C.E., Grogan T.R., Elashoff D., et al. Sodium-glucose transporter 2 is a diagnostic and therapeutic target for early-stage lung adenocarcinoma. Sci. Transl. Med. 2018;10:eaat5933. doi: 10.1126/scitranslmed.aat5933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Komatsu S., Nomiyama T., Numata T., Kawanami T., Hamaguchi Y., Iwaya C., Horikawa T., Fujimura-Tanaka Y., Hamanoue N., Motonaga R., et al. Sglt2 inhibitor ipragliflozin attenuates breast cancer cell proliferation. Endocr. J. 2020;67:99–106. doi: 10.1507/endocrj.EJ19-0428. [DOI] [PubMed] [Google Scholar]
- 77.Kaji K., Nishimura N., Seki K., Sato S., Saikawa S., Nakanishi K., Furukawa M., Kawaratani H., Kitade M., Moriya K., et al. Sodium glucose cotransporter 2 inhibitor canagliflozin attenuates liver cancer cell growth and angiogenic activity by inhibiting glucose uptake. Int. J. Cancer. 2018;142:1712–1722. doi: 10.1002/ijc.31193. [DOI] [PubMed] [Google Scholar]
- 78.Koepsell H. The Na(+)-D-glucose cotransporters sglt1 and sglt2 are targets for the treatment of diabetes and cancer. Pharmacol. Ther. 2017;170:148–165. doi: 10.1016/j.pharmthera.2016.10.017. [DOI] [PubMed] [Google Scholar]
- 79.Villani L.A., Smith B.K., Marcinko K., Ford R.J., Broadfield L.A., Green A.E., Houde V.P., Muti P., Tsakiridis T., Steinberg G.R. The diabetes medication canagliflozin reduces cancer cell proliferation by inhibiting mitochondrial complex-i supported respiration. Mol. Metab. 2016;5:1048–1056. doi: 10.1016/j.molmet.2016.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Han V.X., Patel S., Jones H.F., Nielsen T.C., Mohammad. S.S., Hofer M.J., Gold W., Brilot F., Lain S.J., Nassar N., et al. Maternal acute and chronic inflammation in pregnancy is associated with common neurodevelopmental dis-orders: A systematic review. Transl. Psychiatry. 2021;11:1–2. doi: 10.1038/s41398-021-01198-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liu Y.Z., Wang Y.X., Jiang C.L. Inflammation: The common pathway of stress-related diseases. Front. Hum. Neurosci. 2017;11:316. doi: 10.3389/fnhum.2017.00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ojima A., Matsui T., Nishino Y., Nakamura N., Yamagishi S. Empagliflozin, an inhibitor of sodium-glucose cotransporter 2 exerts anti-inflammatory and antifibrotic effects on experimental diabetic nephropathy partly by suppressing ages-receptor axis. Horm. Metab. Res. 2015;47:686–692. doi: 10.1055/s-0034-1395609. [DOI] [PubMed] [Google Scholar]
- 83.Han J.H., Oh T.J., Lee G., Maeng H.J., Lee D.H., Kim K.M., Choi S.H., Jang H.C., Lee H.S., Park K.S., et al. The beneficial effects of empagliflozin, an sglt2 inhibitor, on atherosclerosis in apoe (-/-) mice fed a western diet. Diabetologia. 2017;60:364–376. doi: 10.1007/s00125-016-4158-2. [DOI] [PubMed] [Google Scholar]
- 84.Vallon V., Rose M., Gerasimova M., Satriano J., Platt K.A., Koepsell H., Cunard R., Sharma K., Thomson S.C., Rieg T. Knockout of na-glucose transporter sglt2 attenuates hyperglycemia and glomerular hyperfiltration but not kidney growth or injury in diabetes mellitus. Am. J. Physiol. Ren. Physiol. 2013;304:F156–F167. doi: 10.1152/ajprenal.00409.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yaribeygi H., Butler A.E., Atkin S.L., Katsiki N., Sahebkar A. Sodium-glucose cotransporter 2 inhibitors and inflammation in chronic kidney disease: Possible molecular pathways. J. Cell Physiol. 2018;234:223–230. doi: 10.1002/jcp.26851. [DOI] [PubMed] [Google Scholar]
- 86.Shin S.J., Chung S., Kim S.J., Lee E.M., Yoo Y.H., Kim J.W., Ahn Y.B., Kim E.S., Moon S.D., Kim M.J., et al. Effect of sodium-glucose co-transporter 2 inhibitor, dapagliflozin, on renal renin-angiotensin system in an animal model of type 2 diabetes. PLoS ONE. 2016;11:e0165703. doi: 10.1371/journal.pone.0165703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Reuter S., Gupta S.C., Chaturvedi M.M., Aggarwal B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010;49:1603–1616. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dandekar A., Mendez R., Zhang K. Cross talk between er stress, oxidative stress, and inflammation in health and disease. Methods Mol. Biol. 2015;1292:205–214. doi: 10.1007/978-1-4939-2522-3_15. [DOI] [PubMed] [Google Scholar]
- 89.Singh M., Kumar A. Risks associated with sglt2 inhibitors: An overview. Curr. Drug Saf. 2018;13:84–91. doi: 10.2174/1574886313666180226103408. [DOI] [PubMed] [Google Scholar]
- 90.Filippas-Ntekouan S., Filippatos T.D., Elisaf M.S. Sglt2 inhibitors: Are they safe? Postgrad. Med. 2018;130:72–82. doi: 10.1080/00325481.2018.1394152. [DOI] [PubMed] [Google Scholar]
- 91.Vardeny O. The sweet spot: Heart failure prevention with sglt2 inhibitors. Am. J. Med. 2020;133:182–185. doi: 10.1016/j.amjmed.2019.08.013. [DOI] [PubMed] [Google Scholar]
- 92.Chao E.C., Henry R.R. SGLT2 inhibition—A novel strategy for diabetes treatment. Nat. Rev. Drug Discov. 2020;9:551–559. doi: 10.1007/s00125-019-05039-w. [DOI] [PubMed] [Google Scholar]
- 93.Lytvyn Y., Bjornstad P., Udell J.A., Lovshin J.A., Cherney D.Z.I. Sodium glucose cotransporter-2 inhibition in heart failure: Potential mechanisms, clinical applications, and summary of clinical trials. Circulation. 2017;136:1643–1658. doi: 10.1161/CIRCULATIONAHA.117.030012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Siesjö B.K. Brain energy metabolism and catecholaminergic activity in hypoxia, hypercapnia and ischemia. Journal of neural transmission. J. Neural. Transm. Suppl. 1978;1:17–22. [PubMed] [Google Scholar]
- 95.Duelli R., Kuschinsky W. Brain glucose transporters: Relationship to local energy demand. Physiology. 2001;16:71–76. doi: 10.1152/physiologyonline.2001.16.2.71. [DOI] [PubMed] [Google Scholar]
- 96.Wright E.M., Turk E. The sodium/glucose cotransport family SLC5. Pflügers Arch. 2004;447:510–518. doi: 10.1007/s00424-003-1202-0. [DOI] [PubMed] [Google Scholar]
- 97.Yu A.S., Hirayama B.A., Timbol G., Liu J., Diez Sampedro A., Kepe V., Satyamurthy N., Huang S.C., Wright E.M., Barrio J.R. Regional distribution of sglt activity in rat brain in vivo. Am. J. Physiol. Cell Physiol. 2013;304:C240–C247. doi: 10.1152/ajpcell.00317.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.O’Malley D., Reimann F., Simpson A.K., Gribble F.M. Sodium-coupled glucose cotransporters contribute to hypothalamic glucose sensing. Diabetes. 2006;55:3381–3386. doi: 10.2337/db06-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wiciński M., Wódkiewicz E., Górski K., Walczak M., Malinowski B. Perspective of SGLT2 inhibition in treatment of conditions connected to neuronal loss: Focus on Alzheimer’s disease and ischemia-related brain injury. Pharmaceuticals. 2020;13:379. doi: 10.3390/ph13110379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lin B., Koibuchi N., Hasegawa Y., Sueta D., Toyama K., Uekawa K., Ma M., Nakagawa T., Kusaka H., Kim-Mitsuyama S. Glycemic control with empagliflozin, a novel selective sglt2 inhibitor, ameliorates cardiovascular injury and cognitive dysfunction in obese and type 2 diabetic mice. Cardiovasc. Diabetol. 2014;13:148. doi: 10.1186/s12933-014-0148-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hierro-Bujalance C., Infante-Garcia C., DelMarco A., Herrera M., Carranza-Naval M.J., Suarez J., Alves-Martinez P., Lubian-Lopez S., Garcia-Alloza M. Empagliflozin reduces vascular damage and cognitive impairment in a mixed murine model of alzheimer’s disease and type 2 diabetes. Alzheimers Res. 2020;12:40. doi: 10.1186/s13195-020-00607-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Abdel-Latif R.G., Rifaai R.A., Amin E.F. Empagliflozin alleviates neuronal apoptosis induced by cerebral ischemia/reperfusion injury through HIF-1α/VEGF signaling pathway. Arch. Pharmacal Res. 2020;43:514–525. doi: 10.1007/s12272-020-01237-y. [DOI] [PubMed] [Google Scholar]
- 103.Amin E.F., Rifaai R.A., Abdel-Latif R.G. Empagliflozin attenuates transient cerebral ischemia/reperfusion injury in hyperglycemic rats via repressing oxidative-inflammatory-apoptotic pathway. Fundam. Clin. Pharm. 2020;34:548–558. doi: 10.1111/fcp.12548. [DOI] [PubMed] [Google Scholar]
- 104.Brookmeyer R., Johnson E., Ziegler-Graham K., Arrighi H.M. Forecasting the global burden of Alzheimer’s disease. Alzheimer’s Dement. 2007;3:186–191. doi: 10.1016/j.jalz.2007.04.381. [DOI] [PubMed] [Google Scholar]
- 105.Arafa N.M., Ali E.H., Hassan M.K. Canagliflozin prevents scopolamine-induced memory impairment in rats: Comparison with galantamine hydrobromide action. Chem.-Biol. Interact. 2017;277:195–203. doi: 10.1016/j.cbi.2017.08.013. [DOI] [PubMed] [Google Scholar]
- 106.Arab H.H., Safar M.M., Shahin N.N. Targeting ros-dependent akt/gsk-3beta/nf-kappab and dj-1/nrf2 pathways by da- pagliflozin attenuates neuronal injury and motor dysfunction in rotenone-induced parkinson’s disease rat model. ACS Chem. Neurosci. 2021;12:689–703. doi: 10.1021/acschemneuro.0c00722. [DOI] [PubMed] [Google Scholar]
- 107.Erdogan M.A., Yusuf D., Christy J., Solmaz V., Erdogan A., Taskiran E., Erbas O. Highly selective SGLT2 inhibitor dapagliflozin reduces seizure activity in pentylenetetrazol-induced murine model of epilepsy. BMC Neurol. 2018;18:81. doi: 10.1186/s12883-018-1086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shah K., DeSilva S., Abbruscato T. The Role of Glucose Transporters in Brain Disease: Diabetes and Alzheimer’s Disease. Int. J. Mol. Sci. 2012;13:12629–12655. doi: 10.3390/ijms131012629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Poppe R., Karbach U., Gambaryan S., Wiesinger H., Lutzenburg M., Kraemer M., Witte O.W., Koepsell H. Expression of the Na+-D-glucose cotransporter SGLT1 in neurons. J. Neurochem. 1997;69:84–94. doi: 10.1046/j.1471-4159.1997.69010084.x. [DOI] [PubMed] [Google Scholar]
- 110.Yu A.S., Hirayama B.A., Timbol G., Liu J., Basarah E., Kepe V., Satyamurthy N., Huang S.-C., Wright E.M., Barrio J.R. Functional expression of SGLTs in rat brain. Am. J. Physiol. Physiol. 2010;299:C1277–C1284. doi: 10.1152/ajpcell.00296.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Enerson B.E., Drewes L.R. The Rat Blood—Brain Barrier Transcriptome. Br. J. Pharm. 2005;26:959–973. doi: 10.1038/sj.jcbfm.9600249. [DOI] [PubMed] [Google Scholar]
- 112.Jurcovicova J. Glucose transport in brain—Effect of inflammation. Endocr. Regul. 2014;48:35–48. doi: 10.4149/endo_2014_01_35. [DOI] [PubMed] [Google Scholar]
- 113.Sa-Nguanmoo P., Tanajak P., Kerdphoo S., Jaiwongkam T., Pratchayasakul W., Chattipakorn N., Chattipakorn S. SGLT2-inhibitor and DPP-4 inhibitor improve brain function via attenuating mitochondrial dysfunction, insulin resistance, inflammation, and apoptosis in HFD-induced obese rats. Toxicol. Appl. Pharm. 2017;333:43–50. doi: 10.1016/j.taap.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 114.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th ed. American Psychiatric Association; Washington, DC, USA: 2000. [Google Scholar]
- 115.Hasan W., Kori R.K., Jain J., Yadav R.S., Jat D. Neuroprotective effects of mitochondria-targeted curcumin against rotenone-induced oxidative damage in cerebellum of mice. J. Biochem. Mol. Toxicol. 2020;34:e22416. doi: 10.1002/jbt.22416. [DOI] [PubMed] [Google Scholar]
- 116.Ahmed S.A., Mohammed W.I. Carvedilol induces the antiapoptotic proteins Nrf2 and Bcl2 and inhibits cellular apoptosis in aluminum-induced testicular toxicity in male Wistar rats. Biomed. Pharm. 2021;139:111594. doi: 10.1016/j.biopha.2021.111594. [DOI] [PubMed] [Google Scholar]
- 117.Abdelhamid A.M., Elsheakh A.R., Abdelaziz R.R., Suddek G.M. Empagliflozin ameliorates ethanol-induced liver injury by modulating NF-κB/Nrf-2/PPAR-γ interplay in mice. Life Sci. 2020;256:117908. doi: 10.1016/j.lfs.2020.117908. [DOI] [PubMed] [Google Scholar]
- 118.Ndefo U.A., Anidiobi N.O., Basheer E., Eaton A.T. Empagliflozin (Jardiance): A Novel SGLT2 Inhibitor for the Treatment of Type-2 Diabetes. P T A Peer-Rev. J. Formul. Manag. Pharm. 2015;40:364–368. [PMC free article] [PubMed] [Google Scholar]
- 119.Cinti F., Moffa S., Impronta F., Cefalo C.M., Sun V.A., Sorice G.P., Mezza T., Giaccari A. Spotlight on ertugliflozin and its potential in the treatment of type 2 diabetes: Evidence to date. Drug Des. Devel. 2017;11:2905–2919. doi: 10.2147/DDDT.S114932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pawlos A., Broncel M., Woźniak E., Gorzelak-Pabiś P. Neuroprotective Effect of SGLT2 Inhibitors. Molecules. 2021;26:7213. doi: 10.3390/molecules26237213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rice C. Prevalence of autism spectrum disorders—Autism and develop- mental disabilities monitoring network, United States, 2006. MMWR Surveill. Summ. 2009;58:1–20. [PubMed] [Google Scholar]
- 122.Mosharov E., Cranford M.R., Banerjee R. The quantitatively important relationship between homocysteine metabolism and glutathione synthesis by the transsulfuration pathway and its regulation by redox changes. Biochemistry. 2000;39:13005–13011. doi: 10.1021/bi001088w. [DOI] [PubMed] [Google Scholar]
- 123.Majumdar S., Mukherjee S., Maiti A., Karmakar S., Das A.S., Mukherjee M., Nanda A., Mitra C. Folic acid or combination of folic acid and vitamin B(12) prevents short-term arsenic trioxide-induced systemic and mito- chondrial dysfunction and DNA damage. Environ. Toxicol. 2009;24:377–387. doi: 10.1002/tox.20442. [DOI] [PubMed] [Google Scholar]
- 124.Bagnyukova T.V., Powell C.L., Pavliv O., Tryndyak V.P., Pogribny I.P. Induction of oxidative stress and DNA damage in rat brain by a folate/ methyl-deficient diet. Brain Res. 2008;1237:44–51. doi: 10.1016/j.brainres.2008.07.073. [DOI] [PubMed] [Google Scholar]
- 125.Schumann C.M., Bloss C.S., Barnes C.C., Wideman G.M., Carper R.A., Akshoomoff N., Pierce K., Hagler D., Schork N., Lord C., et al. Longitudinal magnetic resonance imaging study of cortical development through early childhood in autism. J. Neurosci. 2010;30:4419–4427. doi: 10.1523/JNEUROSCI.5714-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Stoltenberg C., Schjølberg S., Bresnahan M., Hornig M., Hirtz D., Dahl C., Lie K.K., Reichborn-Kjennerud T., Schreuder P., Alsaker E., et al. The autism birth cohort: A paradigm for gene-environment-timing research. Mol. Psychiatry. 2010;15:676–680. doi: 10.1038/mp.2009.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pierce K., Glatt S.J., Liptak G.S., McIntyre L.L. The power and promise of identifying autism early: Insights from the search for clinical and biological markers. Ann. Clin. Psychiatry. 2009;21:132–147. [PMC free article] [PubMed] [Google Scholar]
- 128.Abrahams B.S., Geschwind D.H. Advances in autism genetics: On the threshold of a new neurobiology. Nat. Rev. Genet. 2008;9:341–355. doi: 10.1038/nrg2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Abrahams B.S., Geschwind D.H. Connecting genes to brain in the autism spectrum disorders. Arch. Neurol. 2010;67:395–399. doi: 10.1001/archneurol.2010.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.McGinnis W.R. Oxidative stress in autism. Altern. Ther. Health Med. 2004;10:22–36. [PubMed] [Google Scholar]
- 131.Frossi B., de Carli M., Daniel K.C., Rivera J., Pupillo C. Oxidative stress stimulates IL-4 and IL-6 production in mast cells by APE/Ref-1-dependent pathway. Eur. J. Immunol. 2003;33:2168–2177. doi: 10.1002/eji.200323995. [DOI] [PubMed] [Google Scholar]
- 132.Theoharides T.C., Kempuraj D., Redwood L. Autism: An emerging neuroimmune disorder in search of therapy. Expert Opin. Pharm. 2009;10:2127–2143. doi: 10.1517/14656560903107789. [DOI] [PubMed] [Google Scholar]
- 133.Halliwell B. Oxidative stress and neurodegeneration: Where are we now? J. Neurochem. 2006;97:1634–1658. doi: 10.1111/j.1471-4159.2006.03907.x. [DOI] [PubMed] [Google Scholar]
- 134.Abdel-Salam O.M.E., Khadrawy Y.A., Salem N.A., Sleem A.A. Oxidative stress in a model of toxic demyelination in rat brain: The effect of piracetam and vinpocetine. Neurochem. Res. 2011;36:1062–1072. doi: 10.1007/s11064-011-0450-1. [DOI] [PubMed] [Google Scholar]
- 135.Perluigi M., Di Domenico F., Goorgi A., Schinnà M.E., Coccia R., Cini C., Bellia F., Cambria M.T., Cornelius C., Butterfield D.A., et al. Redox proteomics in aging rat brain: Involvement of mitochondrial reduced glutathione status and mitochondrial protein oxidation in the aging process. J. Neurosci. Res. 2010;88:3498–3507. doi: 10.1002/jnr.22500. [DOI] [PubMed] [Google Scholar]
- 136.Spencer W.A., Jeyabalan J., Kichambre S., Gupta R.C. Oxidatively generated DNA damage after Cu(II) catalysis of dopamine and related catecholamine neurotransmitters and neurotoxins: Role of reactive oxygen species. Free Radic. Biol. Med. 2011;50:139–147. doi: 10.1016/j.freeradbiomed.2010.10.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sultana R., Perluigi M., Butterfield D.A. Protein oxidation and lipid peroxidation in brain of subjects with Alzheimer’s disease: Insight into mechanism of neurodegeneration from redox proteomics. Antioxid. Redox Signal. 2010;8:2021–2037. doi: 10.1089/ars.2006.8.2021. [DOI] [PubMed] [Google Scholar]
- 138.Andreazza A.C., Kauer-Sant’Anna M., Frey B.N., Bond D.J., Kapczinski F., Young L.T., Yatham L.N. Oxidative stress markers in bipolar disorder: A meta-analysis. J. Affect. Disord. 2008;111:135–144. doi: 10.1016/j.jad.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 139.Evans T.A., Siedlak S.A., Lu L., Fu X., Wang Z., McGinnis W.R., Fakhoury E., Castellani R.J., Hazen S.L., Walsh W.J., et al. The autistic phenotype exhibits a remarkably localized modification of brain protein by products of free radical-induced lipid oxidation. Am. J. Biochem. Biotechnol. 2008;4:61–72. doi: 10.3844/ajbbsp.2008.61.72. [DOI] [Google Scholar]
- 140.Tsaluchidu S., Cocchi M., Tonello L., Puri B.K. Fatty acids and oxidative stress in psychiatric disorders. BMC Psychiatry. 2008;8((Suppl. S1)):S1–S5. doi: 10.1186/1471-244X-8-S1-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ng F., Berk M., Dean O., Bush A.I. Oxidative stress in psychiatric disorders: Evidence base and therapeutic implications. Int. J. Neuropsychopharmacol. 2008;11:851–876. doi: 10.1017/S1461145707008401. [DOI] [PubMed] [Google Scholar]
- 142.Chauhan A., Gu F., Essa M.M., Wegiel J., Kaur K., Brown W.T., Chauhan V. Brain region-specific deficit in mitochondrial electron transport chain complexes in children with autism. J. Neurochem. 2011;117:209–220. doi: 10.1111/j.1471-4159.2011.07189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Bradstreet J.J., Smith S., Baral M., Rossignol D.A. Biomarker-guided interventions of clinically relevant conditions associated with autism spectrum disorders and attention deficit hyperactivity disorder. Altern. Med. Rev. 2010;15:15–32. [PubMed] [Google Scholar]
- 144.Sajdel-Sulkowska E.M., Lipinski B., Windom H., Audhya T., McGinnis W. Oxidative stress in autism: Elevated cerebellar 3-nitrotyrosine levels. Am. J. Biochem. Biotechnol. 2008;4:73–84. [Google Scholar]
- 145.Sajdel-Sulkowska E.M., Xu M., Koibuchi N. Increase in cerebellar neurotrophin-3 and oxidative stress markers in autism. Cerebellum. 2009;8:366–372. doi: 10.1007/s12311-009-0105-9. [DOI] [PubMed] [Google Scholar]
- 146.Sajdel-Sulkowska E.M., Xu M., McGinnis W., Koibuchi N. Brain region- specific changes in oxidative stress and neurotrophin levels in autism spectrum disorders (ASD) Cerebellum. 2011;10:43–48. doi: 10.1007/s12311-010-0223-4. [DOI] [PubMed] [Google Scholar]
- 147.Geier D.A., Geier M.R. Autism spectrum disorder-associated biomarkers for case evaluation and management by clinical geneticists. Expert Rev. Mol. Diagn. 2008;8:671–674. doi: 10.1586/14737159.8.6.671. [DOI] [PubMed] [Google Scholar]
- 148.Adams J.B., Audhya T., McDonough-Means S., Rubin R.A., Quig D., Geis E., Gehn E., Loresto M., Mitchell J., Atwood S., et al. Nutritional and metabolic status of children with autism vs. neurotypical children, and the association with autism severity. Nutr. Metab. 2011;8:34. doi: 10.1186/1743-7075-8-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Melnyk S., Fuchs G.J., Schulz E., Lopez M., Kahler S.G., Fussell J.J., Bellando J., Pavliv O., Rose S., Seidel L., et al. Metabolic imbalance associated with methylation dysregulation and oxidative damage in children with autism. J. Autism Dev. Disord. 2012;42:367–377. doi: 10.1007/s10803-011-1260-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Geier D.A., Kern J.K., Garver C.R., Adams J.B., Audhya T., Geier M.R. A prospective study of transsulfuration biomarkers in autistic disorders. Neurochem. Res. 2009;34:386–393. doi: 10.1007/s11064-008-9782-x. [DOI] [PubMed] [Google Scholar]
- 151.James S.J., Melnyk S., Jernigan S., Cleves M.A., Halsted C.H., Wong D.H., Cutler P., Bock K., Boris M., Bradstreet J.J., et al. Metabolic endophenotype and related genotypes are associated with oxidative stress in children with autism. Am. J. Med. Genet B Neuropsychiatr. Genet. 2006;141B:947–956. doi: 10.1002/ajmg.b.30366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Vergani L., Lanza C., Rivaro P., Abelmoschi M.L., Genti S., Veneselli E., Minniti G., Grasselli E., Canesi L., Voci A. Metals, metallothioneins and oxidative stress in blood of autistic children. Res. Autism Spectr. Disord. 2011;5:286–293. doi: 10.1016/j.rasd.2010.04.010. [DOI] [Google Scholar]
- 153.Zoroglu S.S., Armutcu F., Ozen S., Gurel A., Sivasli E., Yetkin O., Meram I. Increased oxidative stress and altered activities of erythrocyte free radical scavenging enzymes in autism. Eur. Arch. Psychiatry Clin. Neurosci. 2004;254:143–147. doi: 10.1007/s00406-004-0456-7. [DOI] [PubMed] [Google Scholar]
- 154.Yorbik O., Sayal A., Akay C., Akbiyik D.I., Sohmen T. Investigation of antioxidant enzymes in children with autistic disorder. Prostaglandins Leukot. Essent Fat. Acids. 2002;67:341–343. doi: 10.1054/plef.2002.0439. [DOI] [PubMed] [Google Scholar]
- 155.Pasca S.P., Dronca E., Kaucsár T., Craciun E.C., Endreffy E., Ferencz B.K., Iftene F., Benga I., Cornean R., Banerjee R., et al. One carbon metabolism disturbances and the C677T MTHFR gene polymorphism in children with autism spectrum disorders. J. Cell Mol. Med. 2009;10:4229–4238. doi: 10.1111/j.1582-4934.2008.00463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Meguid N.A., Dardir A.A., Abdel-Raouf E.R., Hashish A. Evaluation of oxidative stress in autism: Defective antioxidant enzymes and increased lipid peroxidation. Biol. Trace Elem. Res. 2011;143:58–65. doi: 10.1007/s12011-010-8840-9. [DOI] [PubMed] [Google Scholar]
- 157.Golse B., Debray-Ritzen P., Durosay P., Puget K., Michelson A.M. Alterations in two enzymes: Superoxide dismutase and glutathion peroxidase in developmental infantile psychosis (infantile autism) (author’s transl) Rev. Neurol. 1978;134:699–705. [PubMed] [Google Scholar]
- 158.Abdel-Wahab A.F., Bamagous G.A., Al-Harizy R.M., ElSawy N.A., Shahzad N., Ibrahim I.A., Al Ghamdi S.S. Renal protective effect of SGLT2 inhibitor dapagliflozin alone and in combination with irbesartan in a rat model of diabetic nephropathy. Biomed. Pharm. 2018;103:59–66. doi: 10.1016/j.biopha.2018.03.176. [DOI] [PubMed] [Google Scholar]
- 159.Xie J., Huang L., Li X., Li H., Zhou Y., Zhu H., Pan T., Kendrick K.M., Xu W. Immunological cytokine profiling identifies TNF-α as a key molecule dysregulated in autistic children. Oncotarget. 2017;8:82390. doi: 10.18632/oncotarget.19326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Jyonouchi H., Geng L., Ruby A., Reddy C., Zimmerman-Bier B. Evaluation of an association between gastrointestinal symptoms and cytokine production against common dietary proteins in children with autism spectrum disorders. J. Pediatr. 2005;146:605–610. doi: 10.1016/j.jpeds.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 161.Jyonouchi H., Sun S., Le H. Proinflammatory and regulatory cytokine production associated with innate and adaptive immune responses in children with autism spectrum disorders and developmental regression. J. Neuroimmunol. 2001;120:170–179. doi: 10.1016/S0165-5728(01)00421-0. [DOI] [PubMed] [Google Scholar]
- 162.Wei H., Chadman K.K., McCloskey D.P., Sheikh A.M., Malik M., Brown W.T., Li X. Brain IL-6 elevation causes neuronal circuitry imbalances and mediates autism-like behaviors. Biochim. Et Biophys. Acta (Bba)-Mol. Basis Dis. 2012;1822:831–842. doi: 10.1016/j.bbadis.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 163.Nadeem A., Ahmad S.F., Attia S.M., Al-Ayadhi L.Y., Al-Harbi N.O., Bakheet S.A. Dysregulation in IL-6 receptors is associated with upregulated IL-17A related signaling in CD4+ T cells of children with autism. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2020;97:109783. doi: 10.1016/j.pnpbp.2019.109783. [DOI] [PubMed] [Google Scholar]
- 164.Tsilioni I., Taliou A., Francis K., Theoharides T.C. Children with autism spectrum disorders, who improved with a luteolin-containing dietary formulation, show reduced serum levels of TNF and IL-6. Transl. Psychiatry. 2015;5:e647. doi: 10.1038/tp.2015.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ayaydın H., Kirmit A., Çelik H., Akaltun İ., Koyuncu İ., Ulgar Ş.B. High serum levels of serum 100 beta protein, neuron-specific enolase, Tau, active caspase-3, M30 and M65 in children with autism spectrum disorders. Clin. Psychopharmacol. Neurosci. 2020;18:270. doi: 10.9758/cpn.2020.18.2.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Siniscalco D., Sapone A., Giordano C., Cirillo A., de Novellis V., de Magistris L., Rossi F., Fasano A., Maione S., Antonucci N. The expression of caspases is enhanced in peripheral blood mononuclear cells of autism spectrum disorder patients. J. Autism Dev. Disord. 2012;42:1403–1410. doi: 10.1007/s10803-011-1373-z. [DOI] [PubMed] [Google Scholar]
- 167.Şimşek F., Işık Ü., Aktepe E., Kılıç F., Şirin F.B., Bozkurt M. Comparison of serum VEGF, IGF-1, and HIF-1α levels in children with autism spectrum disorder and healthy controls. J. Autism Dev. Disord. 2021;51:3564–3574. doi: 10.1007/s10803-020-04820-w. [DOI] [PubMed] [Google Scholar]
- 168.Sokol D.K., Chen D., Farlow M.R., Dunn D.W., Maloney B., Zimmer J.A., Lahiri D.K. High levels of Alzheimer beta-amyloid precursor protein (APP) in children with severely autistic behavior and aggression. J. Child Neurol. 2006;21:444–449. doi: 10.1177/08830738060210062201. [DOI] [PubMed] [Google Scholar]
- 169.El-Ansary A., Al-Ayadhi L. Neuroinflammation in autism spectrum disorders. J. Neuroinflammation. 2012;9:265. doi: 10.1186/1742-2094-9-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data in this study were not created nor analyzed by the authors. Data sharing is not applicable to this article.