Abstract
An impressive change in the epidemiology and severity of invasive group A streptococcal infections occurred in the 1980s, and the incidence of streptococcal toxic shock syndrome cases continues to rise. The reason for the resurgence of severe invasive cases remains a mystery—has there been a change in the pathogen or in host protective immunity? To address these questions, we have studied 33 patients with invasive infection caused by genotypically indistinguishable M1T1 strains of Streptococcus pyogenes who had different disease outcomes. Patients were classified as having severe (n = 21) and nonsevere (n = 12) invasive infections based on the presence or absence of shock and organ failure. Levels of anti-M1 bactericidal antibodies and of anti-streptococcal superantigen neutralizing antibodies in plasma were significantly lower in both groups than in age- and geographically matched healthy controls (P < 0.01). Importantly, the levels of these protective antibodies in plasma samples from severe and nonsevere invasive cases were not different. Together the data suggest that low levels of protective antibodies may contribute to host susceptibility to invasive streptococcal infection but do not modulate disease outcome. Other immunogenetic factors that regulate superantigen responses may influence the severity of systemic manifestations associated with invasive streptococcal infection.
After years of steadily declining morbidity and mortality due to group A streptococcal infections, a resurgence of severe, invasive disease has been ongoing since 1980 (9, 12, 17, 19–21, 24, 25, 31, 32, 49), leading to the recognition of streptococcal toxic shock syndrome (STSS) (52), the most severe form of invasive infection (10, 13, 49). STSS patients suffer from severe acute hypotension, multiorgan failure, and in some cases deep soft tissue destruction (31). The rise in STSS cases is persisting (reviewed in reference 31), and ongoing surveillance studies in Ontario, Canada, revealed a marked increase in the number of reported cases of invasive group A streptococcal infections from 1992 to the present (10, 13). The increased incidence of these infections has been accompanied by a remarkable vigor in virulence and severity, with numerous cases of STSS and necrotizing fasciitis (NF) (4, 7, 23). The reason for this impressive change in the epidemiology and clinical manifestation of group A streptococcal infections remains a mystery—have the bacteria acquired new virulence, or has the host susceptibility to factors produced by reemerging strains of Streptococcus pyogenes been compromised due to the lack of protective immunity against these strains?
These possibilities are not mutually exclusive, and there is little doubt that the disease outcome is determined by host-pathogen interplay. Group A streptococci produce a number of virulence factors that can contribute to the pathogenesis of invasive group A streptococcal disease. These include the surface M protein, hyaluronic capsule, proteases, DNases, lipotechoic acid, streptococcal toxins such as streptolysins O and S, and the streptococcal pyrogenic exotoxins (Spes) (1, 19, 22, 26, 33, 35, 42, 44, 51). As superantigens, the Spes can cause activation of large numbers of immune cells to synthesize and release massive amounts of inflammatory cytokines that have been shown to mediate many of the systemic manifestations associated with sepsis, including hypotension and organ failure (reviewed in references 26, 27, and 50). Although it may be hypothesized that the resurgence of invasive group A streptococcal infections is related to production or overproduction of specific virulence factors, studies of clusters and disease outbreaks revealed that the same streptococcal strain can be isolated from STSS cases, nonsevere invasive cases, and asymptomatic contacts, indicating a strong influence of host factors in disease pathogenesis (5, 8, 23, 24, 34, 36, 45, 47).
Patients with invasive group A streptococcal disease, including those infected with indistinguishable M1T1 strains, can be classified as having severe or nonsevere invasive disease based on the presence or absence, respectively, of shock and organ failure. Therefore, even if pathogen virulence products are contributing to the increase in invasive disease, host factors must play a pivotal role in determining the severity of the systemic manifestations.
Several host factors have been shown to increase the risk of severe invasive streptococcal disease. Differences in confounding factors such as age, underlying disease (10), and ongoing viral infections can be accounted for in multivariate analyses, thereby allowing studies to focus on the role of host immune defense mechanisms in modulating the severity of invasive streptococcal infections. We have reported that host immune responses to the various streptococcal virulence factors can vary (28, 40, 41), and we believe that this interindividual variation can potentially affect the severity of systemic manifestations associated with invasive infections.
The lack of protective immunity to specific virulence factors produced by the bacteria is likely to affect host susceptibility to infection. Previous studies have suggested that low levels of antibodies directed to specific Spes or to the M protein may render the host susceptible to invasive infections (21, 48). In fact, several investigators have proposed that low levels of anti-M1 protein in the general populations of the United States, Canada, and Scandinavian countries may have contributed to the remarkable change in the epidemiology of invasive group A streptococcal infections and may be responsible for the impressive rise in the number of STSS cases (14, 21, 48). However, in the majority of these studies, evaluation of the levels of protective antibodies was performed against isolates that were not necessarily recovered from the patients being evaluated, and thus the clinical relevance and immunological specificity of these antibodies could not be ascertained. Furthermore, the role of the antistreptococcal protective antibodies in modulating the severity of invasive streptococcal infections has not been addressed directly.
The goal of this study was to determine if differences in severity of the systemic manifestations of invasive group A streptococcal infections are associated with differences in levels in plasma of antibodies to the M serotype of the infecting isolate and/or antibodies that can neutralize the activity of superantigens produced by these isolates. We report that invasive cases had significantly low levels of protective antibodies compared to age-matched healthy controls; however, the levels were equally low in severe and nonsevere invasive cases. The data indicate that while the lack of this protective humoral immunity may confer risk of invasive infection, it is not a factor in determining the severity of systemic manifestation associated with these infections. Together the data suggest that other host immunogenetic factors, possibly those regulating cytokine responses to superantigens, may be more important in modulating disease outcome.
MATERIALS AND METHODS
Subjects, case definitions, and clinical material.
Patients were identified through ongoing surveillance for all invasive group A streptococcal infections in Ontario, Canada. Group A streptococcal infections were classified according to the scheme proposed by the Working Group on Streptococcal Infections (52). Patients were enrolled from 1994 to 1996, and only those who had invasive infection caused by indistinguishable M1T1 strains (as described below) were included in this study (n = 33). Invasive infection meant that the isolate was obtained from a normally sterile site. Invasive cases could be subdivided into severe and nonsevere depending on the clinical course of the infection. Severe invasive infection patients (n = 21) were those who had STSS, NF, or NF plus STSS. STSS was defined as invasive infection associated with shock and organ failure early in the course of infection. Patients with nonsevere invasive infections (n = 12) had no signs of hypotension or multiple organ failure; they included patients with bacteremia, cellulitis, and erysipelas.
Plasma samples were collected from each consenting patient before the administration of adjunctive therapy, namely, intravenous administration of pooled immunoglobulin G (IVIG). Plasma samples and clinical isolates were frozen promptly and stored at −80°C until processed. Controls were age-matched healthy individuals who resided in the same geographical area as the study patients.
Characterization of bacterial isolates.
Clinical isolates were identified as S. pyogenes by standard methodology (13), and each was designated by patient number. M and T serotyping was performed at the National Reference Center for the Streptococcus, Edmonton, Canada. All M1T1 isolates included here were further typed by pulsed-field gel electrophoresis and by random amplified polymorphic DNA analysis. All had identical DNA banding patterns after digestion with two enzymes (SmaI and SfiI), indicating that they represent a genotypically indistinguishable strain of S. pyogenes (data not shown). The presence of the genes encoding SpeA, SpeB, SpeC, SpeF, and streptococcal superantigen (SSA) was detected by PCR with primer pairs specific for each gene, as previously described (33, 39). All M1T1 isolates studied here had the speA, speB, and speF genes.
Preparation of bacterial culture supernatant.
Group A streptococcal isolates recovered from sterile sites of patients with invasive disease were cultured overnight in 10 ml of Todd-Hewitt broth supplemented with 1.5% yeast extract (Difco, Detroit, Mich.). The bacteria were removed by centrifugation, and proteins in the culture supernatants were precipitated by addition of 95% ice-cold ethanol (1 part supernatant to 3 parts ethanol) and incubation for 24 h at −20°C. The precipitates were dissolved in 1 ml of distilled H2O and dialyzed for 24 h against multiple changes of distilled H2O. The dialysates were filter sterilized and stored at −20°C.
rSpe preparation and generation of rabbit polyclonal antibodies to SpeA and SpeF.
Recombinant SpeA (rSpeA) and rSpeF were expressed and purified as His fusion proteins according to the manufacturers’ (Novagen, Madison, Wis., and Qiagen Inc., Chatsworth, Calif.) recommendations. The speA clone was kindly provided by C. Collins, University of Miami, Miami, Fla. The N-terminal His tag of rSpeA was removed by digestion of the fusion protein with 1 U of thrombin/mg of rSpeA for 16 h at room temperature with the thrombin cleavage capture kit (Novagen). The purity of rSpeA was evaluated by silver staining after sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting with anti-SpeA antibodies (kindly provided by P. Schliervert, University of Minnesota) and later was confirmed by use of anti-rSpeA polyclonal antibodies generated in our laboratory. The mitogenic and cytokine-producing activities and T-cell receptor Vβ specificity of rSpeA were comparable to those of native SpeA, as previously indicated (37).
The speF gene was amplified from strain M1T1 isolate 5448 by PCR with 5′SF-EK (5′ CGC CCA TCC GAC GAT GAC GAT AAG GTT CAA ACA GAG GTC TCA AAT 3′) as the forward primer and 3′SF (5′ CGG GGT ACC TTA TTT TTG AGT AGG TGT 3′) as the reverse primer, which together generated a 1,193-bp product. The amplified product was purified and subcloned in PCRII vector (Invitrogen Corporation, San Diego, Calif.) according to the manufacturer’s instructions and was sequenced by using the fmol DNA sequencing kit (Promega Corporation, Madison, Wis.) to ensure that no sequence artifacts were introduced by the PCR. The cloned insert was digested with BamHI and KpnI and subcloned into the PQE30 vector (Qiagen Inc.), which was used to transform M15 Escherichia coli cells. The bacteria were grown to an optical density (OD) at 600 nm of 0.8, and then expression was induced with 1 to 2 mM IPTG (isopropyl-β-d-thiogalactopyranoside) for 5 h. Bacterial cells were harvested by centrifugation at 4,000 × g for 10 min, and the pellet was resuspended in sonication buffer (50 mM Na-phosphate [pH 7.8], 300 mM NaCl) and sonicated on ice (10-s burst, 20 s, 60 to 70 W). This was followed by centrifugation for 15 min at 10,000 × g, and the supernatant was collected and filtered through a 0.2-μm-pore-size filter. The His-tagged rSpeF protein was purified on an Ni-nitrilotriacetic acid column and stepwise eluted with a 10, 20, 50, and 300 mM imidazole. The purified protein was eluted in the 50 mM fraction and immediately dialyzed against distilled H2O overnight with several changes. The purity of the rSpeF was determined by silver staining after sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and the specific expression of the protein was determined by immunoblotting with rabbit polyclonal anti-SpeF antibodies (kindly provided by S. Holms, Umea University, Umea, Sweden) and later confirmed by use of anti-rSpeF polyclonal antibodies generated in our laboratory.
For initial immunization, 0.2 mg of rSpeA and 0.5 mg of rSpeF were emulsified in Freund’s complete adjuvant and injected into male New Zealand White rabbits. Boosting doses of rSpeA (0.1 mg) and rSpeF (0.25 mg) emulsified in Freund’s incomplete adjuvant were administrated every 2 weeks, for a total of five booster injections. The antibody titers in rabbit sera were monitored by enzyme-linked immunosorbent assay (ELISA) (as described below) with pure recombinant protein as the ELISA antigen. The rabbit antisera were adsorbed with an E. coli M15 strain to remove anti-E. coli reactive antibodies, and the specificity of the antisera and lack of cross-reactivity with other Spes were confirmed by immunoblotting.
Measurement of plasma anti-M1 antibodies by ELISA.
The presence of anti-M1 protein antibodies in plasma samples from patients was determined by ELISA with a peptide copying the N terminus of type M1 protein [SM1(1-26)c], provided by J. B. Dale, as the ELISA antigen. The sequence was deduced from the emm1.0 allele described by Haanes-Fritz et al. (18). Microtiter plates were coated with 0.2 μg of the SM1 peptide per ml in coating buffer (0.1 M carbonate buffer, pH 9.6 to 9.8) at 4°C for 18 h, rinsed with wash buffer (0.05% Tween 20 in phosphate-buffered saline [PBS]), and blocked with 1% bovine serum albumin in PBS for 60 min at 37°C. Fetal bovine serum (FBS), diluted 1:100 in PBS, was used as a negative control, and a rabbit anti-M1 antiserum, provided by J. B. Dale and generated as previously described (29), was serially diluted in PBS and used as a positive control. Different dilutions of plasma from patients or controls were added to duplicate coated wells and incubated for 2 h at room temperature. The plates were rinsed with wash buffer, and goat anti-human or goat anti rabbit immunoglobulin (Ig)–peroxidase conjugate diluted 1:1,000 was added to the appropriate wells. After 1 h of incubation, the plates were rinsed with wash buffer and freshly made peroxidase substrate solution (ABST; Kirkegaard-Perry, Gaithersburg, Md.) was added. The reaction was monitored at 415 nm, and the OD was used to determine antibody titers from a standard curve generated with serial dilutions of the control antibody. Results are expressed as mean ELISA titers ± standard errors of the means (SEMs).
Measurement of levels of anti-M1 opsonic and bactericidal antibodies in plasma.
The levels of opsonic and bactericidal anti-M1 antibodies in patients’ plasma were determined by a neutrophil-mediated opsonophagocytosis assay by the method of Fischer et al. (15). Neutrophils were isolated from adult venous blood by dextran sedimentation and Ficoll-Hypaque density centrifugation. Bacteria were grown overnight to log phase in Todd-Hewitt broth containing 20% normal rabbit serum, and then 50 μl was added to 5 ml of Todd-Hewitt broth and allowed to grow at 37°C with occasional monitoring until the OD at 530 nm reached 0.05. Briefly, 10 μl of bacteria was incubated with 40 μl of 1:50-diluted plasma from patients or controls or of a 1:50 dilution of the rabbit anti-SM1(1-26)c antibody in 96-well round-bottom microtiter plates for 15 min at 37°C, followed by incubation on ice for 15 min. Neutrophils (2 × 105 per 40 μl of RPMI 1640 medium) were added to all wells, followed by 10 μl of newborn rabbit complement (Rockland Laboratories, Gilberstville, Pa.), and incubated at 37°C for 1 h with horizontal rocking. The percentage of neutrophils associated with streptococci (percent phagocytosis) was estimated by microscopic counts of Wright-stained (Sigma Chemical Co., St. Louis, Mo.) smears prepared from the assay mixture. Each assay was performed in triplicate with 300 to 400 neutrophils counted per slide, for a total of 900 to 1,000 neutrophils.
The levels of bactericidal anti-M1 antibodies in plasma of patients and controls were determined as described by Fischer et al. (15). Neutrophils and bacteria were treated as described above for the opsonic assay except that 10 μl of the mixture of bacteria and neutrophils was spread on blood agar plates immediately before and 1.5 h after the coincubation of the opsonized bacteria and neutrophils. The blood agar plates were incubated at 37°C overnight, the number of colonies in each plate was counted, and the percentage of bactericidal activity was calculated.
Measurement of anti-Spe antibodies by ELISA.
Microtiter plates were coated with 0.5 μg of rSpeA, SpeB (Toxin Technology), or rSpeF per ml in coating buffer (0.1 M carbonate buffer, pH 9.6 to 9.8) at 4°C for 18 h, rinsed with wash buffer (0.05% Tween 20 in PBS), and blocked with 1% bovine serum albumin in PBS for 60 min at 37°C. FBS (diluted 1:100) was used as negative control, and serially diluted rabbit anti-SpeA, -SpeB, and -SpeF antisera were used as positive controls. Different dilutions of plasma from patients and controls were added to duplicate coated wells and incubated for 2 h at room temperature. The plates were rinsed with wash buffer, and then goat anti-human or goat anti-rabbit Ig–peroxidase conjugate diluted 1:1,000 was added to the appropriate wells. After 1 h of incubation, the plates were rinsed with wash buffer and freshly made peroxidase substrate solution (azino-di[3-ethylbenzthiozoline sulfate] [ABST]; Kirkegaard-Perry) was added. The reaction was monitored at 415 nm, and the OD was used to determine antibody titers from a standard curve generated with serial dilutions of the control antibody. Results are expressed as mean ELISA titers ± SEMs.
Measurement of levels of anti-streptococcal superantigen neutralizing antibodies in plasma.
Assessment of neutralizing activity in patients’ plasma was performed by measuring the ability of plasma to inhibit the proliferation of peripheral blood mononuclear cells (PBMC) in response to either pure Spe proteins or the mixture of superantigens present in the culture supernatants of bacterial isolates as described previously (38). Plasma from each patient was tested for the ability to neutralize the mitogenic activity elicited by supernatants of the patient’s own isolate. In the case of healthy controls, plasma from each control was tested separately against six representative randomly selected isolates (three from severe cases and three from nonsevere cases). Briefly, PBMC were isolated from healthy donors by Ficoll-Hypaque gradient centrifugation, and 1.5 × 106 cells/ml were cultured in RPMI 1640 medium supplemented with 25 mM HEPES, 4 mM l-glutamine, and 100 U of penicillin-streptomycin per ml (RPMI complete medium) and incubated at 37°C with 5% CO2 and 95% humidity. The cells were cultured with the various stimuli (Spe or M1T1 culture supernatants) in the presence of either heat-inactivated patient plasma (1% plasma plus 4% FBS) or 5% FBS. After 3 days, the cells were pulsed for 6 h with 1 μCi of [3H]thymidine (specific activity, 6.7 Ci/mmol; DuPont, Wilmington, Del.) per well and harvested onto glass fiber filters, and radioactivity was counted in a Packard liquid scintillation counter. All samples were assayed in triplicate, and the data are presented as mean counts per minute ([3H]thymidine uptake) ± SEM or as percent inhibition of toxin mitogenicity as calculated by the equation (38). 1 − [(cpmpp + stimulus − cpmpp)/(cpmFBS + stimulus − cpmFBS)] × 100, where pp is patient plasma.
Statistical evaluation of data.
Evaluation of statistical differences was performed with the two-sample t test, assuming unequal variances.
RESULTS
Cases and controls.
A cohort of patients who were infected with genotypically indistinguishable M1T1 strains of S. pyogenes (5a) were studied. All patients had invasive infection and were classified as having severe or nonsevere infection, based primarily on the presence or absence of shock and organ failure. The goal was to determine whether differences in the severity of the clinical manifestations are related to differences in levels of protective antibodies in plasma between the two groups. The sex distributions were similar in both groups (38% female and 62% male for the severe cases and 42% female and 58% male for the nonsevere cases). The mean ages were 51 ± 6 and 41 ± 5 years for severe and nonsevere cases, respectively and there was no significant difference in underlying diseases between the groups (P ≥ 0.62). Inasmuch as the level of protective antibodies may vary with age (30) (see below), patient values were compared, throughout the study, to those for age-matched healthy controls who resided in the same geographical area and therefore were likely to have been exposed to similar strains of group A streptococci.
Anti-M1 antibodies in plasma specimens of patients with severe and nonsevere invasive group A streptococcal infections.
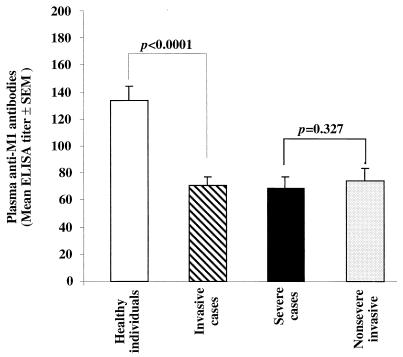
The levels of anti-M1 antibodies in plasma specimens of patients with severe and nonsevere invasive infections that were caused by indistinguishable M1T1 strains were first determined by ELISA. As shown in Fig. 1, the levels of anti-M1 antibodies were significantly lower in patients with invasive disease than in the age- and geographically matched healthy controls (P < 0.0001). The levels of anti-M1 antibodies in plasma specimens of patients with severe and nonsevere invasive disease were not significantly different from each other (P = 0.3), but both were significantly lower than those in controls (P < 0.0001) (Fig. 1).
FIG. 1.
Low levels of anti-M1 antibodies in patients with severe and nonsevere invasive infections caused by genotypically indistinguishable M1T1 strains of group A streptococci. Antibodies against the streptococcal M1 protein in acute-phase plasma specimens from 33 patients (21 with severe cases and 12 with nonsevere cases) and in 50 age-matched healthy controls from the same geographical area were determined by ELISA. Plasma specimens diluted 1:500 and 1:100 were added to duplicate wells coated with 0.2 μg of SM1(1-26)c peptide per ml. Rabbit antiserum specific for the M1 peptide (diluted 1:100, 1:500, and 1:1,000) was used to generate a standard curve, and FBS (diluted 1:100) served as negative control. Peroxidase-conjugated goat anti-human IgG and goat anti-rabbit IgG were used as secondary antibodies. After addition of peroxidase substrate, the reaction was monitored at 415 nm.
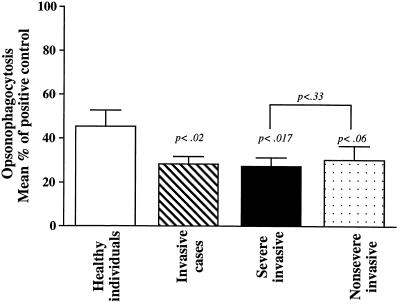
Next, we investigated whether the levels of protective anti-M1 opsonic and bactericidal antibodies in plasma specimens from patients with severe and nonsevere invasive infections were different. Neutrophil-mediated opsonophagocytosis and bactericidal assays were performed as described in Materials and Methods. The levels of anti-M1 opsonic antibodies in plasma specimens of patients with invasive infections, expressed as percentages of the positive control (rabbit anti-M1 antibody) level, were significantly lower than those in the control group (P < 0.02) (Fig. 2). Bactericidal activity in plasma specimens from invasive cases was significantly different from that for healthy controls (P < 0.007) (Table 1). Both severe and nonsevere invasive cases showed twofold-lower levels of anti-M1 opsonic antibodies compared to the controls, although this difference between the controls and the nonsevere cases was not significant (P = 0.06). However, the bactericidal activity in both the severe and nonsevere cases was significantly lower than that in the healthy controls (P = 0.04 and P = 0.006, respectively) (Table 1). Importantly, the difference in either opsonic or bactericidal activity between the severe and nonsevere cases was insignificant (P = 0.33 and 0.14, respectively).
FIG. 2.
Low levels of M1-specific opsonophagocytic antibodies in patients with severe and nonsevere invasive group A streptococcal infections. Neutrophil-mediated opsonophagocytosis was performed as detailed in Materials and Methods. Opsonophagocytic activity in acute-phase plasma specimens from 33 patients infected with M1T1 strains (21 with severe cases and 12 with nonsevere cases) and in 20 age-matched healthy controls from the same geographical area was determined as detailed in Materials and Methods. Rabbit antiserum specific for M1 protein (diluted 1:50) was used as a positive control, and FBS was used as a negative control. Plasma specimens from either controls or patients were tested at a 1:50 dilution. The percentage of neutrophils containing phagocytozed bacteria was determined by direct microscopy.
TABLE 1.
Levels of anti-M1 serotype bactericidal antibodies in plasmaa
| Study group (n) | % Bactericidal activity (mean ± SEM) | Pb |
|---|---|---|
| Healthy individuals (20) | 84 ± 5 | |
| All invasive cases (33) | 68 ± 5 | 0.0074 |
| Severe invasive cases (21) | 72 ± 5 | 0.04c |
| Nonsevere invasive cases (12) | 63 ± 5 | 0.006c |
A bactericidal assay was conducted to assess the bactericidal anti-M1 antibodies in plasma specimens from either patients or controls. The assay was performed as described in Materials and Methods, the mean percentage of bactericidal activity was calculated, and the results were compared by using the Student t test (one tailed).
Versus results for healthy controls.
The difference between results for severe and nonsevere cases was not significant (P = 0.14).
Levels of anti-streptococcal superantigen antibodies in plasma specimens from patients with severe and nonsevere invasive group A streptococcal infections.
Inasmuch as the levels of anti-M1 antibodies in plasma were equally low in the patients with the severe and nonsevere invasive infections, it was of interest to determine if their levels of anti-Spe antibodies in plasma were different. As indicated above, all patients were infected with indistinguishable M1T1 strains that harbored the speA, speB, and speF genes. Levels (determined by ELISA) of anti-SpeA, -SpeB, and -SpeF antibodies in plasma specimens of patients with invasive disease were significantly lower than those in controls (P > 0.003) (Table 2). Equally low levels of anti-Spe antibodies were found in plasma specimens of patients with severe and nonsevere invasive infections, with no significant difference between them (P = 0.1), but both groups had levels that were significantly lower than those in controls for all three Spes (Table 2).
TABLE 2.
Anti-Spe antibodies in patients with severe and nonsevere invasive group A streptococcal infections
| ELISA antigen | Level of anti-Spe antibodies in plasma (mean ELISA titer ± SEM) for:
|
|||
|---|---|---|---|---|
| Healthy individuals (n = 20) | All invasive cases (n = 33) | Severe invasive cases (n = 21) | Nonsevere invasive cases (n = 12) | |
| SpeA | 114 ± 14 | 72 ± 6 (P < 0.003a) | 67 ± 8 (P < 0.002a) | 80 ± 10 (P < 0.025ab; P = 0.15ab) |
| SpeB | 60 ± 6 | 28 ± 6 (P < 0.0002a) | 23 ± 7 (P < 0.0001a) | 37 ± 8 (P < 0.004ab; P = 0.111ab) |
| SpeF | 153 ± 16 | 90 ± 10 (P < 0.001a) | 83 ± 13 (P < 0.0008a) | 103 ± 15 (P < 0.016ab; P = 0.15ab) |
Versus results for age-matched healthy individuals.
Versus results for severe invasive cases.
Levels of neutralizing anti-streptococcal superantigen antibodies in plasma specimens from patients with severe and nonsevere invasive group A streptococcal infections.
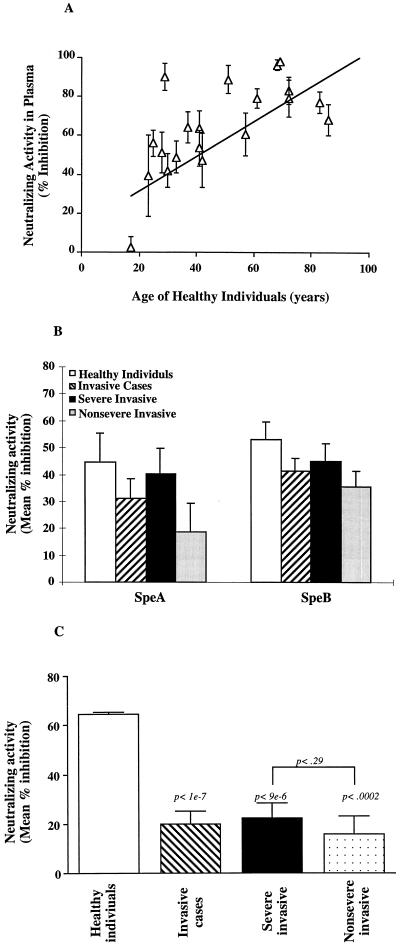
Previous studies have indicated that the levels of neutralizing anti-Spe antibodies do not always correlate with the total amount of binding antibodies as determined by ELISA or immunoblotting (37, 38, 42). Further, our studies have suggested that the quality (neutralizing activity) rather than the quantity (binding activity) of anti-streptococcal superantigen antibodies is more clinically relevant (38). Here we compared the levels of neutralizing antibodies in plasma specimens of the patients with the severe and nonsevere invasive M1T1 infections. The ability of plasma from patients or controls to neutralize the mitogenic activity of either the pure Spe proteins or the mixture of superantigens in the partially purified culture supernatants of the patient’s M1T1 isolates was tested. PBMC from a healthy responder were incubated with partially purified supernatant from M1T1 isolates in the presence of either FBS, plasma of the patient from whom the isolate was obtained, or plasma from age-matched healthy controls residing in the same area. The importance of matching the ages of patients and controls is illustrated in Fig. 3A, where it can be seen that the neutralizing activity increases with age.
FIG. 3.
Low levels of anti-streptococcal superantigen neutralizing antibodies in patients with severe and nonsevere invasive group A streptococcal infections. Neutralizing antibodies against the mixture of superantigens produced by the M1T1 isolates in plasma specimens of patients infected with indistinguishable M1T1 strains were evaluated. PBMC (106 cells/ml) from a healthy donor were stimulated either with phytohemagglutinin (1 μg/ml) or with the partially purified culture supernatant from M1T1 isolates (diluted 1:100 in RPMI) in the presence of 5% FBS or 4% FBS plus 1% plasma. (A) Neutralizing activity in plasma specimens from healthy individuals (n = 20) of different ages. (B) Neutralizing activity in plasma specimens from patients with severe (n = 21) and nonsevere (n = 12) infections and healthy controls (n = 20) against pure superantigens rSpeA and SpeB. Proliferation was assessed after 3 days of culture, and the mean counts per minute ([3H]thymidine uptake) ± SEM for triplicate cultures was calculated. Neutralizing activity is expressed as percent inhibition of mitogenic activity. (C) Results for patients with severe (n = 21) and nonsevere (n = 12) invasive disease or for age-matched healthy individuals who reside in the same area. Each patient plasma was tested for neutralizing activity against the isolate from that patient, or, in the case of the age-matched healthy individuals, supernatants from six representative isolates (three from severe cases and three from nonsevere cases) were used for stimulation. Proliferation was assessed after 3 days of culture, and the mean counts per minute ([3H]thymidine uptake) ± SEM for triplicate cultures was calculated. Neutralizing activity is expressed as percent inhibition of mitogenic activity.
No significant difference in the levels of neutralizing activity against pure Spe proteins was found between severe and nonsevere invasive cases or between the invasive cases and healthy controls (Fig. 3B). By contrast, significantly lower levels of isolate-specific neutralizing antibodies were found in plasma specimens from patients with severe and nonsevere invasive infections compared to the healthy controls (Fig. 3C) (P < 0.0002). The lack of concordance between the levels of neutralizing antibodies to a pure Spe and the mixture of superantigens produced by the isolate is consistent with the argument that most of the clinical group A streptococcal isolates produce a mixture of known and novel superantigens. This underscores the importance of evaluating the plasma neutralizing activity of the patient against the mixture of superantigens produced by their respective isolate. For example, 4 of 21 patients with severe invasive infections had high levels of SpeA-neutralizing antibodies (>80% inhibition of SpeA mitogenicity) but low levels against the mixture of superantigens in the isolate supernatant (<25% inhibition of supernatant mitogenicity) (data not shown). Importantly, there was no difference in the levels of neutralizing antibodies against the mixture of superantigens produced by the M1T1 isolates between the severe and nonsevere M1T1 cases (22 ± 6 versus 16 ± 7; P > 0.29), and both were lower than those of the healthy controls (Fig. 3C).
DISCUSSION
The recent resurgence of invasive group A streptococcal infections has puzzled the scientific community for the past decade. Although, there is no clear explanation for this change in epidemiology of streptococcal infections, it is becoming clear that both pathogen and host factors should be considered when attempting to elucidate the pathogenesis of these infections. The strongest indication for the central role of host factors in invasive group A streptococcal infections is derived from the fact that the same bacterial strain can be isolated from individuals who differ considerably in the spectrum of clinical symptoms, ranging from being asymptomatic to having STSS or NF (5, 8, 24, 34, 36, 46). In fact, the cohort of patients studied here were infected with genetically indistinguishable (by pulsed-field gel electrophoresis and random amplified polymorphic DNA analysis) M1T1 strains yet had very different disease outcomes and could be subclassified as having severe or nonsevere invasive infections (5a).
Inasmuch as invasive group A streptococcal disease can be caused by a number of distinct serotypes that produce distinct superantigens (6, 10, 23, 24), and since it has been shown that the response of an individual to different serotypes can be very different (40), it follows that host specific immune responses to the infecting strain are more clinically relevant than those to an unrelated serotype. In addition, previous studies (11) have shown that protective immunity to S. pyogenes may distinguish between clones of the same serotype. Accordingly, to understand the contribution of host factors to disease, it was important to conduct our studies with patients who were infected with the same streptococcal strain in order to normalize, as much as possible, for variations in disease severity that could be simply attributed to differences in the virulence and spectrum of superantigens produced by distinct serotypes or even subclones of the same serotype. To this end, we studied patients with severe and nonsevere infections who were all infected with the same M1T1 strain. How can infection with the same organism cause starkly different symptoms in different people?
One of the main objectives of our studies over the past few years is to identify host factors that modulate the severity of invasive streptococcal infections. A variety of host factors can potentially affect disease outcome. These include age, underlying disease, or a preceding viral infection (9, 10, 43, 48); the presence of protective humoral immunity specific to the infecting isolate; and immunogenetic factors that regulate immune responses to streptococcal virulence factors, such as the Spes and other superantigens produced by these isolates. It is well established that the presence of M type-specific antibodies can protect the host from infection, as these antibodies opsonize the bacteria and enhance their elimination by phagocytic cells (3, 16). Our data seem to support this notion, as we have found that patients with invasive disease have significantly lower levels of binding, opsonic, and bactericidal anti-M1 antibodies compared to age- and geographically matched healthy controls. The low levels of anti-M1 antibodies were not due to nonspecific effects of sepsis, since levels of antibodies to other streptococcal components were comparable to those in controls (Fig. 3B). However, we have shown that there was no correlation between low levels of anti-M1 antibodies and disease severity: both the patients with the severe and nonsevere invasive infections had significantly lower levels of these antibodies in plasma than controls, and there was no significant difference between the patients with the severe and nonsevere infections.
Similarly, levels of antibodies to SpeA, SpeB, and SpeF (determined by ELISA) were significantly lower in plasma specimens of patients with invasive infections than in those of healthy controls, and equally low anti-Spe antibody levels were found for the severe and nonsevere invasive cases. An important role for anti-Spe antibodies in invasive streptococcal infections has been suggested (30, 37–39, 42), and we show here that the low levels of these antibodies are not a factor in disease severity. Furthermore, we and others (38, 42) have shown that the quality (neutralizing activity) rather than the quantity of anti-Spe antibodies is more relevant to disease pathogenesis. Thus, in addition to determining the levels of anti-Spe antibodies by ELISA, we also assessed the levels of antibodies that can neutralize the mixture of superantigens produced by these isolates. Although there was no statistical difference in the levels of neutralizing antibodies to pure Spe proteins between patient and control groups (Fig. 3B), we found a significant difference between patients and controls with respect to levels of neutralizing antibodies against the mixture of superantigens produced in the supernatants of the patients’ isolates (Fig. 3C). The findings illustrate the point that streptococcal isolates produce a mixture of known and novel superantigens. Patients who had high levels of neutralizing antibodies to a specific pure Spe but not to the mixture of superantigens produced by their infecting isolate may lack protective humoral immunity against the novel superantigens produced by these M1T1 isolates. This underscores the clinical relevance of evaluating the plasma neutralizing activity of the patient against the mixture of superantigens produced by the respective isolate.
Importantly, we found no difference in the levels of isolate-specific neutralizing antibodies between the patients with severe and nonsevere invasive infections; both were significantly lower than those in controls. The data suggest that the low levels of isolate-specific neutralizing antibodies may have contributed to the risk of invasive group A streptococcal disease but that they are not a major factor in determining disease severity. The mechanism by which lack of these antibodies may contribute to increased invasiveness of the organism is at present not clear. However, the superantigens are known to cause tissue damage and are capable of activating resident macrophages to produce inflammatory mediators and chemotactic factors; in the absence of neutralizing antibodies, the superantigen-mediated inflammatory reactions may facilitate bacterial invasion of host tissue.
Recent work from our laboratory has demonstrated that pooled human Ig (IVIG) preparations contain high levels of opsonic antibodies to several M serotypes (2), including M1T1 strains, as well as high levels of antibodies that can neutralize the immune stimulatory activity of a wide variety of streptococcal superantigens (37–39). Importantly, these protective antibodies were transferred to patient plasma, and their presence appeared to help halt disease progression (2, 23, 37). Patients who lack protective antibodies may benefit from IVIG adjunctive therapy, since these antibodies appear to help in the elimination of the bacterium and the neutralization of its toxins.
ACKNOWLEDGMENTS
This work was supported by grants from the U.S. Veterans Administration/Department of Defense Joint Fund on Emerging Pathogens (to M.K.), the National Institutes of Health (NIAID grant AI40198 to M.K.), the U.S.-Egypt Channel Scholarships Fund (to H.B.), and the Medical Research Council and The Swedish Society of Medicine (to A.N.-T.).
We are grateful to J. B. Dale (VA Medical Center, Memphis, Tenn.) for providing the SM1(1-26)C peptide and the control rabbit anti-M1 antibodies and to C. Collins (University of Miami, Miami, Fla.) for providing the SpeA clone from which we purified the rSpeA. Special thanks go to the Ontario Streptococcal Study Group for their help in collection of clinical material and patients’ records.
REFERENCES
- 1.Alouf J E, Knoll H, Kohler W. The family of mitogenic, shock-inducing and superantigenic toxins from staphylococci and streptococci. In: Alouf J E, Freer J H, editors. Sourcebook of bacterial protein toxins. London, United Kingdom: Academic Press; 1991. pp. 367–414. [Google Scholar]
- 2.Basma H, Norrby-Teglund A, Low D E, McGeer A, El-Ahmady O, Kotb M. Opsonic antibodies to the surface M protein of group A streptococci in pooled normal immunoglobulin G (IVIG): potential impact on the clinical efficacy of IVIG in severe invasive group A streptococcal infections. Infect Immun. 1998;66:2279–2283. doi: 10.1128/iai.66.5.2279-2283.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beachey E H, Seyer J M. Primary structure and immunochemistry of group A streptococcal M proteins. Semin Infect Dis. 1982;4:401–410. [Google Scholar]
- 4.Bisno A, Stevens D. Streptococcal infections of skin and soft tissues. N Engl J Med. 1996;334:240–245. doi: 10.1056/NEJM199601253340407. [DOI] [PubMed] [Google Scholar]
- 5.Campbell J R, Arango C A, Garcia-Prats J A, Baker C J. An outbreak of M serotype 1 group A streptococcus in a neonatal intensive care unit. J Pediatr. 1996;129:396–402. [PubMed] [Google Scholar]
- 5a.Chatellier et al. Unpublished data.
- 6.Chaussee M S, Liu J, Stevens D L, Ferretti J J. Genetic and phenotypic diversity among isolates of Streptococcus pyogenes from invasive infections. J Infect Dis. 1996;173:901–908. doi: 10.1093/infdis/173.4.901. [DOI] [PubMed] [Google Scholar]
- 7.Chelsom J, Halstensen A, Haga T, Hoiby A. Necrotizing fasciitis due to group A streptococci in western Norway: incidence and clinical features. Lancet. 1994;344:1111–1115. doi: 10.1016/s0140-6736(94)90629-7. [DOI] [PubMed] [Google Scholar]
- 8.Cockerill F R R, MacDonald K L, Thompson R L. An outbreak of invasive group A streptococcal disease associated with high carriage rates of the invasive clone among school-aged children. JAMA. 1997;277:38–43. [PubMed] [Google Scholar]
- 9.Cone L A, Woodard D R, Schlievert P M, Tomory G S. Clinical and bacteriological observations of a toxic shock-like syndrome due to Streptococcus pyogenes. N Engl J Med. 1987;317:146–149. doi: 10.1056/NEJM198707163170305. [DOI] [PubMed] [Google Scholar]
- 10.Davies H D, McGeer A, Schwartz B, Green K, Cann D, Simor A E, Low D E the Ontario Group A Streptococcal Study Group. Invasive group A streptococcal infections in Ontario, Canada. N Engl J Med. 1996;335:547–554. doi: 10.1056/NEJM199608223350803. [DOI] [PubMed] [Google Scholar]
- 11.de Malmanche S, Martin D. Protective immunity to the group A streptococcus may be only strain specific. Med Microbiol Immunol. 1994;183:299–306. doi: 10.1007/BF00196680. [DOI] [PubMed] [Google Scholar]
- 12.Demers B, Low D E, Simor A E, Vellend H, Jamieson F, Walmsley S, Green K, Krishnan C. Severe group A streptococcal disease—southern Ontario. Ontario Streptococcal Study Group. Can Dis Weekly Rep. 1991;17:192–194. [PubMed] [Google Scholar]
- 13.Demers B, Simor A-E, Vellend H, Schlievert P M, Byrne S, Jamieson F, Walmsley S, Low D E. Severe invasive group A streptococcal infections in Ontario, Canada: 1987–1991. Clin Infect Dis. 1993;16:792–800. doi: 10.1093/clind/16.6.792. [DOI] [PubMed] [Google Scholar]
- 14.Ferrieri P. Microbiological features of current virulent strains of group A streptococci. Pediatr Infect Dis J. 1991;10(Suppl.):S20–S24. doi: 10.1097/00006454-199110001-00005. [DOI] [PubMed] [Google Scholar]
- 15.Fischer G W, Wilson S R, Hunter K W. Functional characteristics of a modified immunoglobulin preparation for intravenous administration: summary of studies of opsonic and protective activity against group B streptococci. J Clin Immunol. 1982;2:31–35. doi: 10.1007/BF00918364. [DOI] [PubMed] [Google Scholar]
- 16.Fischetti V A. Streptococcal M protein. Sci Am. 1991;264:58–65. doi: 10.1038/scientificamerican0691-58. [DOI] [PubMed] [Google Scholar]
- 17.Gaworzewska E, Colman G. Changes in the pattern of infection caused by Streptococcus pyogenes. Epidemiol Infect. 1988;100:257–269. doi: 10.1017/s095026880006739x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haanes-Fritz E, Kraus W, Burdett V, Dale J B, Beachey E H, Cleary P P. Comparison of the leader sequences of 4 group A streptococcal M protein genes. Nucleic Acid Res. 1988;10:4667–4677. doi: 10.1093/nar/16.10.4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoge C W, Schwartz B, Talkington D F, Breiman R F, MacNeill E M, Englender S J. The changing epidemiology of invasive group A streptococcal infections and the emergence of streptococcal toxic shock-like syndrome. A retrospective population-based study. JAMA. 1993;269:384–389. [PubMed] [Google Scholar]
- 20.Hoiby E A. Streptococcal serogroup A epidemic in Norway 1987–1988. Scand J Infect Dis. 1990;22:421–429. doi: 10.3109/00365549009027073. [DOI] [PubMed] [Google Scholar]
- 21.Holm S E, Norrby A, Bergholm A M, Norgren M. Aspects of pathogenesis of serious group A streptococcal infections in Sweden, 1988–1989. J Infect Dis. 1992;166:31–37. doi: 10.1093/infdis/166.1.31. [DOI] [PubMed] [Google Scholar]
- 22.Kamezawa Y, Nakahara T, Nakano S, Abe Y, Nozaki-Renard J, Isono T. Streptococcal mitogenic exotoxin Z, a novel acidic superantigenic toxin. Infect Immun. 1997;65:3828–3833. doi: 10.1128/iai.65.9.3828-3833.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaul R, McGeer A, Low D E, Green K, Schwartz B. Population-based surveillance for group A streptococcal necrotizing fasciitis: clinical features, prognostic indicators, and microbiologic analysis of seventy-seven cases. Ontario Group A Streptococcal Study. Am J Med. 1997;103:18–24. doi: 10.1016/s0002-9343(97)00160-5. [DOI] [PubMed] [Google Scholar]
- 24.Kiska D L, Thiede B, Caracciolo J, Jordan M, Johnson D, Kaplan E L, Gruninger R P, Lohr J A, Gilligan P H, Denny F W., Jr Invasive group A streptococcal infections in North Carolina: epidemiology, clinical features, and genetic and serotype analysis of causative organisms. J Infect Dis. 1997;176:992–1000. doi: 10.1086/516540. [DOI] [PubMed] [Google Scholar]
- 25.Kohler W. Epidemiology and pathogenesis of streptococcal infection. Immun Infekt. 1992;20:92–98. [PubMed] [Google Scholar]
- 26.Kotb M. Bacterial exotoxins as superantigens. Clin Microbiol Rev. 1995;8:411–426. doi: 10.1128/cmr.8.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kotb M. Role of superantigens in the pathogenesis of infectious diseases and their sequelae. Curr Opin Infect Dis. 1992;5:364–374. [Google Scholar]
- 28.Kotb M, Ohnishi H, Majumdar G, Hackett S, Bryant A, Higgins G, Stevens D. Temporal relationship of cytokine release by peripheral blood mononuclear cells stimulated by the streptococcal superantigen pep M5. Infect Immun. 1993;61:1194–1201. doi: 10.1128/iai.61.4.1194-1201.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kraus W, Dale J B, Beachey E H. Identification of an epitope of type 1 streptococcal M protein that is shared with a 43-kD protein of human myocardium and renal glomeruli. J Immunol. 1990;145:4089–4093. [PubMed] [Google Scholar]
- 30.Kuwahata M, Imanaka H, Takei S, Masuda K. Age-related occurrence of inhibitory antibodies to streptococcal pyrogenic superantigens. Acta Paediatr Jpn. 1996;38:1–7. doi: 10.1111/j.1442-200x.1996.tb03425.x. [DOI] [PubMed] [Google Scholar]
- 31.Low D E, Schwartz B, McGeer A. The reemergence of severe group A streptococcal disease: an evolutionary perspective. In: Scheld W M, Armston D, Hughes J M, editors. Emerging infections I. Washington, D.C: American Society for Microbiology; 1997. pp. 93–123. [Google Scholar]
- 32.Martin P R, Hoiby E A. Streptococcal serogroup A epidemic in Norway 1987–1988. Scand J Infect Dis. 1990;22:421–429. doi: 10.3109/00365549009027073. [DOI] [PubMed] [Google Scholar]
- 33.Mollick J, Miller G, Musser J, Cook R, Grossman D, Rich R. A novel superantigen isolated from pathogenic strains of Streptococcus pyogenes with aminoterminal homology to staphylococcal enterotoxins B and C. J Clin Invest. 1993;92:710–719. doi: 10.1172/JCI116641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muotiala A, Saxen H, Vuopio-Varkila J. Group A streptococcal outbreak of perinatal infection in a day-care centre. Adv Exp Med Biol. 1997;418:211–215. doi: 10.1007/978-1-4899-1825-3_50. [DOI] [PubMed] [Google Scholar]
- 35.Musser J M, Kapur V, Kanjilal S, Shah U, Musher D M, Barg N L, Johnston K H, Schlievert P M, Henrichsen J, Gerlach D, et al. Geographic and temporal distribution and molecular characterization of two highly pathogenic clones of Streptococcus pyogenes expressing allelic variants of pyrogenic exotoxin A (scarlet fever toxin) J Infect Dis. 1993;167:337–346. doi: 10.1093/infdis/167.2.337. [DOI] [PubMed] [Google Scholar]
- 36.Norgren M, Norrby A, Holm S E. Genetic diversity in T1M1 group A streptococci in relation to clinical outcome of infection. J Infect Dis. 1992;166:1014–1020. doi: 10.1093/infdis/166.5.1014. [DOI] [PubMed] [Google Scholar]
- 37.Norrby-Teglund A, Basma H, Low D E, McGeer A, Kotb M. Varying titers of neutralizing antibodies to streptococcal superantigens in different preparations of normal polyspecific immunoglobulin G (IVIG): implications for therapeutic efficacy. J Clin Infect Dis. 1998;26:631–641. doi: 10.1086/514588. [DOI] [PubMed] [Google Scholar]
- 38.Norrby-Teglund A, Kaul R, Low D E, McGeer A, Andersson J P, Andersson U G, Kotb M. Evidence for the presence of streptococcal superantigen neutralizing antibodies in normal polyspecific immunoglobulin G. Infect Immun. 1996;64:5395–5398. doi: 10.1128/iai.64.12.5395-5398.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Norrby-Teglund A, Kaul R, Low D E, McGeer A, Andersson J P, Andersson U G, Kotb M. Plasma from patients with severe invasive group A streptococcal infections treated with normal polyspecific IgG inhibits streptococcal superantigen-induced T cell proliferation and cytokine production. J Immunol. 1996;156:3057–3064. [PubMed] [Google Scholar]
- 40.Norrby-Teglund A, Lustig R, Kotb M. Differential induction of Th1 versus Th2 cytokines by group A. Infect Immun. 1997;65:5209–5215. doi: 10.1128/iai.65.12.5209-5215.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Norrby-Teglund A, Norgren M, Holm S E, Andersson U, Andersson J. Similar cytokine induction profiles of a novel streptococcal exotoxin, MF, and pyrogenic exotoxins A and B. Infect Immun. 1994;62:3731–3738. doi: 10.1128/iai.62.9.3731-3738.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Norrby-Teglund A, Pauksen K, Holm S E, Norgren M. Relation between low capacity of human sera to inhibit streptococcal mitogens and serious manifestation of disease. J Infect Dis. 1994;170:585–591. doi: 10.1093/infdis/170.3.585. [DOI] [PubMed] [Google Scholar]
- 43.Peterson C L, Vugia D J, Meyers H B, Chao S M, Vogt J, Lanson J, Brunell P A, Kim K S, Mascola L. Risk factors for invasive group A streptococcal infections in children with varicella: a case-control study. Pediatr Infect Dis J. 1996;15:151–156. doi: 10.1097/00006454-199602000-00012. [DOI] [PubMed] [Google Scholar]
- 44.Schlievert P M. Role of superantigens in human diseases. J Infect Dis. 1993;167:997–1002. doi: 10.1093/infdis/167.5.997. [DOI] [PubMed] [Google Scholar]
- 45.Schwartz B, Elliott J A, Butler J C, Simon P A, Jameson B L, Welch G E, Facklam R R. Clusters of invasive group A streptococcal infections in family, hospital, and nursing home settings. Clin Infect Dis. 1992;15:277–284. doi: 10.1093/clinids/15.2.277. [DOI] [PubMed] [Google Scholar]
- 46.Shanley T, Schrier D, Kapur V, Kehoe M, Musser J. Streptococcal cysteine protease augments lung injury induced by products of group A streptococci. Infect Immun. 1996;64:870–877. doi: 10.1128/iai.64.3.870-877.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stanley J, Desai M, Efstratiou A, George R. High-resolution genotyping of Streptococcus pyogenes. Application to outbreak studies and population genetics. Adv Exp Med Biol. 1997;418:313–316. [PubMed] [Google Scholar]
- 48.Stevens D L. Invasive group A streptococcus infections. Clin Infect Dis. 1992;14:2–13. doi: 10.1093/clinids/14.1.2. [DOI] [PubMed] [Google Scholar]
- 49.Stevens D L, Tanner M H, Winship J, Swarts R, Ries K M, Schlievert P M, Kaplan E. Severe group A streptococcal infections associated with a toxic shock-like syndrome and scarlet fever toxin A. N Engl J Med. 1989;321:1–7. doi: 10.1056/NEJM198907063210101. [DOI] [PubMed] [Google Scholar]
- 50.Sullivan G W, Mandell G L. The role of cytokines in infection. Curr Opin Infect Dis. 1991;4:344–349. [Google Scholar]
- 51.Watanabe-Ohnishi R, Low D E, McGeer A, Stevens D L, Schlievert P M, Schwartz B, Kreiwwirth B, Kotb M. Selective depletion of Vβ-Bearing T cells in patients with invasive group A streptococcal disease and streptococcal toxic shock syndrome: implications for a novel superantigen. J Infect Dis. 1994;171:74–84. doi: 10.1093/infdis/171.1.74. [DOI] [PubMed] [Google Scholar]
- 52.Working Group on Severe Streptococcal Infections. Defining the group A streptococcal toxic shock syndrome. Rationale and consensus definition. JAMA. 1993;269:390–391. [PubMed] [Google Scholar]