Abstract
Secretory immunoglobulin A (SIgA) antibodies reactive with the pioneer oral streptococci Streptococcus mitis biovar 1 and Streptococcus oralis, the late oral colonizer Streptococcus mutans, and the pioneer enteric bacterium Enterococcus faecalis in saliva samples from 10 human infants from birth to age 2 years were analyzed. Low levels of salivary SIgA1 and SIgA2 antibodies reactive with whole cells of all four species were detected within the first month after birth, even though S. mutans and E. faecalis were not recovered from the mouths of the infants during the study period. Although there was a fivefold increase in the concentration of SIgA between birth and age 2 years, there were no differences between the concentrations of SIgA1 and SIgA2 antibodies reactive with the four species over this time period. When the concentrations of SIgA1 and SIgA2 antibodies reactive with all four species were normalized to the concentrations of SIgA1 and SIgA2 in saliva, SIgA1 and SIgA2 antibodies reactive with these bacteria showed a significant decrease from birth to 2 years of age. Adsorption of each infant’s saliva with cells of one species produced a dramatic reduction of antibodies recognizing the other three species. Sequential adsorption of saliva samples removed all SIgA antibody to the bacteria, indicating that the SIgA antibodies were directed to antigens shared by all four species. The induction by the host of a limited immune response to common antigens that are likely not involved in adherence may be among the mechanisms that commensal streptococci employ to persist in the oral cavity.
The genus Streptococcus contains many species of commensal and pathogenic facultatively anaerobic, gram-positive cocci that colonize the skin and mucous membranes of the upper respiratory tract, alimentary canal, and genitourinary tract of humans and other mammals. Alpha- and nonhemolytic streptococci are pioneer species in the colonization of the human large bowel and mouth (9, 26, 29, 35). Throughout life viridans streptococci comprise a large proportion of the commensal microbiota of the oral cavity and pharynx (3), and different species of viridans streptococci are known to colonize distinct habitats within the mouth (14). For example, Streptococcus mitis biovar 1, Streptococcus oralis, and Streptococcus salivarius are the predominant species isolated from the mucous membranes and tongue of human neonates (26, 29, 35). However, Streptococcus mutans colonizes the mouth only after the provision of nonshedding surfaces by the eruption of teeth (7). In contrast to case for neonates, in adults S. oralis is observed almost exclusively on the teeth and Streptococcus sanguis is the predominant streptococcus isolated from the buccal mucosa (14).
Adaptive humoral immunity at mucosal surfaces is effected principally by secretory immunoglobulin A (SIgA) (23). SIgA antibodies are secreted by local plasma cells derived from IgA-committed B cells that originate in mucosa-associated lymphoid tissues that serve as IgA-inductive sites situated at the portals of entry to the body. Stimulation of these lymphoid tissues by bacterial antigen may cause SIgA antibodies to appear in secretions remote from the source of the antigenic stimulus (23).
The indigenous microbiota of the mouth and other mucosal surfaces exists in a state of homeostasis with the host except when the microbiota is perturbed, when the mucosal surface is damaged, or when the immune system is compromised (3). Mucosal SIgA antibodies are presumed to play a role in the regulation of commensal bacteria (3, 10, 16). However, despite the facts that saliva contains SIgA antibodies reactive with commensal bacteria (16) and commensal bacteria are coated with SIgA (4), these microorganisms colonize and persist on mucosal and tooth surfaces. This observation suggests that indigenous oral bacteria are unaffected by, are not subjected to, or are able to avoid immune elimination by mucosal antibodies (reviewed in references 3, 10, and 20).
As part of a longitudinal study of the relationships between oral colonization of infants by commensal bacteria and the development of the secretory immune response, we have examined the salivary immune response to S. mitis biovar 1, S. oralis, and S. mutans, viridans streptococci that colonize the soft and hard tissues of the mouth. These bacteria were selected in order to be able to compare the secretory immune response to bacteria (S. mitis biovar 1 and S. oralis) that colonize the mouth immediately postpartum and are among the first to be exposed to the immature salivary immune system with that to S. mutans, which colonizes the mouth much later and is therefore exposed to a more developed immune system. Furthermore, to study the specificity of bacterium-reactive salivary SIgA antibodies and to examine the secretory immune response to a commensal enteric bacterium at a remote site, we examined salivary SIgA antibodies reactive with Enterococcus faecalis (formally Streptococcus faecalis), a resident of the large bowel.
MATERIALS AND METHODS
Study population.
Ten healthy, full-term infants provided saliva and microbiological samples for study. Details on this population have been published previously (13, 26). The 10 infants included some that had been exclusively breast fed for the first 6 months postpartum and then formula fed, some that had been entirely formula fed, and some that had received mixed feeding.
Sample collection and processing. (i) Microbiological samples.
The composition of the oral microbiota of the infants was determined from swab samples (Vacutainer Anaerobic Specimen Collector; Becton-Dickinson Microbiology Systems, Cockeysville, Md.) taken at 1 to 3 days, 2 and 4 weeks, and 2, 4, 6, 8, 10, 12, and 24 months postpartum. The mucosal surfaces of the cheeks, buccal sulci, edentulous ridges, tongue, hard palate, and teeth, once erupted, were swabbed, and the sample was transported anaerobically to the laboratory within 1 h of collection. After the swab was placed in 2 ml of reduced transport fluid (34), bacteria were dispersed by ultrasound at 80 W for 10 s with a Sonifier 250 (Branson Ultrasonics Corp., Danbury, Conn.) equipped with a 3-mm-diameter microprobe. The dispersed sample was serially diluted in reduced transport fluid to 10−5.
(ii) Whole mouth saliva.
Whole saliva was collected at 1 to 3 days, 2 and 4 weeks, and 2, 4, 6, 8, 10, 12, and 24 months postpartum, using sterile 3-ml plastic transfer pipettes. Immediately after collection, EDTA was added to a final concentration of 5 mM to prevent formation of heterotypic calcium ion-dependent immunoglobulin-mucin complexes and to inhibit IgA1 protease activity in the sample (13). The saliva samples were held at −85°C until assay of SIgA.
Recovery and identification of oral streptococci.
The methods used to identify the Streptococcus species have been described in detail previously (11, 26).
Production of S. mitis biovar 1, S. oralis, S. mutans, and E. faecalis for antigen.
S. mitis biovar 1 NCTC 12261, S. oralis ATCC 35037, S. mutans NCTC 10449, and E. faecalis ATCC 19433 were grown to substrate exhaustion in the ultrafiltrate (10,000-molecular-weight cutoff) (Minitan Ultra filtration System; Millipore, Bedford, Mass.) of Todd-Hewitt broth (Difco) containing 1% glucose maintained at pH 7.0 in an atmosphere of 95% N2–5% CO2 at 37°C. The bacteria were harvested, washed by centrifugation at 16,000 × g for 20 min, and resuspended in phosphate-buffered saline (PBS) (pH 7.4) and finally in 10 mM HEPES buffer (pH 7.4) containing 5 mM EDTA.
Assay of SIgA1 and SIgA2 in saliva.
Concentrations of SIgA1 and SIgA2 in saliva were determined by enzyme-linked immunosorbent assay (ELISA) (13). All antibodies employed in the ELISA were affinity purified, and incubations were performed at room temperature for 1 h unless otherwise stated. The assay volume was 100 μl, and the wells were washed three times with PBS (pH 8.0) containing 0.1% Tween 20 between each addition of antibodies, samples or standards, and substrate. Briefly, the wells in one half of Immulon 2 plates (Dynatech, Chantilly, Va.) were coated overnight with 10 μg of a murine monoclonal antibody (MAb) to human secretory component (SC) (Hybridoma Labs, Baltimore, Md.) per ml. Unbound antibody was washed from the wells, which were then blocked with PBS (pH 8.0) containing 0.1% bovine serum albumin. After the wells were washed, dilutions of saliva or of colostral SIgA1 or SIgA2 purified in our laboratory as standards (12) were added in duplicate and incubated overnight with shaking. Murine α1 and α2 MAbs (Southern Biotechnology Associates, Inc., Birmingham, Ala.) at 0.5 μg/ml were used to detect bound SIgA1 and SIgA2, respectively. Following washing, bound MAbs α1 and α2 were amplified by using biotinylated rabbit anti-mouse gamma chain, at a concentration of 1.5 μg/ml, obtained from Jackson ImmunoResearch, West Grove, Pa. The wells were washed again, and streptavidin conjugated with horseradish peroxidase (BioSource International, Camarillo, Calif.) at 0.1 μg/ml was used to detect the biotinylated antibodies. Following a final wash, o-phenylenediamine (1 mg/ml) in citrate-phosphate buffer (pH 4.5) containing 0.012% hydrogen peroxide was added to each well. The optical density at 450 nm was measured with a model EL309 automated microplate reader (Bio-Tek Instruments, Inc., Winooski, Vt.). The coefficients of variation within and between individual ELISAs did not exceed 10%.
Assay of SIgA1 and SIgA2 antibodies reactive with S. mitis biovar 1, S. oralis, S. mutans, and E. faecalis in saliva.
Concentrations of SIgA1 and SIgA2 antibodies reactive with S. mitis biovar 1, S. oralis, S. mutans, or E. faecalis in saliva were determined by ELISA as described above except that the wells in the remaining half of the Immulon 2 plates (Dynatech) were coated overnight with 10 μg (dry weight) of formalin-killed S. mitis biovar 1, S. oralis, S. mutans, or E. faecalis per ml instead of anti-human SC MAb. Duplicates of each saliva sample were added to both the anti-human SC MAb-coated and the S. mitis biovar 1-, S. oralis-, S. mutans-, or E. faecalis-coated wells on a single 96-well plate. As murine α1 and α2 MAbs were used to detect bound SIgA1 and SIgA2 and bacterium-reactive SIgA1 and SIgA2, respectively, it was possible to interpolate absorbances from the bacterium-coated wells into the SIgA1 and SIgA2 standard curves.
Assay of protein in saliva.
In order to control for differences in salivary flow rates, the concentrations of SIgA were normalized to the protein content of the saliva. The total protein in saliva was determined by the bicinchoninic acid assay (Pierce Chemical Co., Rockford, Ill.) according to the manufacturer’s protocol.
Fine specificity of SIgA, SIgA1, and SIgA2 anti-Streptococcus antibodies in saliva.
Cell envelope preparations (CEPs) of S. mitis biovar 1, S. oralis, S. mutans, or E. faecalis were prepared as described previously (26). Briefly, a suspension of each bacterium in 10 mM HEPES buffer (pH 7.4) was held on ice and subjected to four 1-min cycles of ultrasound with a GE 50 micro-ultrasonic processor operated at a setting of 6 and equipped with a 3-mm-diameter microprobe (Daigger Corp., Wheeling, Ill.). After sonication, the bacteria were removed by centrifugation and the supernatant was stored at −80°C.
The fine specificity of S. mitis biovar 1-, S. oralis-, S. mutans-, or E. faecalis-reactive salivary total SIgA, SIgA1, and SIgA2 antibodies was determined by Western blotting. The CEPs together with molecular weight standards (Bio-Rad, Hercules Calif.) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis with the Mini Protean II system (Bio-Rad). The separating gel was 11% acrylamide, and the stacking gel was 5% acrylamide. The separated CEPs were transferred to polyvinylidene difluoride membranes (Immobilon-P; Millipore) by using a Trans Blot SD system (Bio-Rad). The membrane was blocked for 1 h in 4% bovine serum albumin in Tris-buffered saline (pH 8.3) containing 0.02% NaN3. Individual lanes were cut from the membrane, and three lanes of the CEPs were incubated overnight with each saliva sample. The strips were then subjected to three 10-min washes in Tris-buffered saline containing 0.1% Tween and 0.5 M NaCl. SIgA1 and SIgA2 antibodies were detected as described above for ELISA except that the substrate was 0.5 mg of 3′,3-diaminobenzidine per ml in 0.01 M PBS (pH 7.4) containing 0.01% H2O2. Bound total SIgA was detected by using a polyclonal goat anti-human α-chain antibody (Jackson ImmunoResearch) at 2.0 μg/ml. Bound goat anti-human α-chain antibody was amplified by using biotinylated rabbit anti-goat gamma chain antibodies.
Cluster analysis of Western blot banding patterns.
Each lane of the CEPs from the different species and the molecular weight standards was scanned with an Ultroscan XL Laser Densitometer (Pharmacia-LKB, Piscataway, N.J.), and the densitograms were imported into an analytic software program (GelCompar 4.0; Applied Maths, Kortrijk, Belgium). Cluster analysis was performed by Ward’s method (37).
Adsorption of salivas.
In order to determine the specificity of SIgA antibodies, S. mitis biovar 1, S. oralis, S. mutans, and E. faecalis cells were used to adsorb pools of saliva from each of the 10 infants. Briefly, four aliquots of the saliva samples were diluted in PBS-Tween and mixed with an equal volume of packed, washed, formalin-killed bacteria of one of the test species. The suspension was incubated at 37°C for 2 h and then at 4°C overnight. After adsorption, the bacteria were removed by centrifugation and antibacterial antibodies in adsorbed and unabsorbed aliquots of the saliva samples were determined by ELISA as described above.
In order to determine whether any of the antibodies detected were uniquely specific for a single species, four saliva samples were adsorbed sequentially with washed formalin-killed cells of each test species. Antibody activities remaining after adsorption (percent) were determined by dividing the optical density at 450 nm of each adsorbed saliva by that of the corresponding unabsorbed saliva at the same dilution and multiplying by 100.
Statistical analysis.
The study was designed to examine the following: (i) changes over time in the salivary concentrations of (a) SIgA1 and SIgA2 and (b) S. mitis biovar 1-, S. oralis-, S. mutans, and E. faecalis-reactive SIgA1 and SIgA2 antibodies and (ii) interactions between time and (a) SIgA1 and SIgA2 and (b) S. mitis biovar 1-, S. oralis-, S. mutans-, and E. faecalis-reactive SIgA1 and SIgA2 antibodies. As differences within individuals over time would be expected to be more highly correlated than differences between individuals over time, a two-factor repeated-measures analysis-of-variance was used to analyze these data. The within-subjects factor was time (i.e., measurements of samples from the same subject at 1, 6, 12, and 24 months), and the between-subjects factors were (i) SIgA1 and SIgA2 and (ii) S. mitis biovar 1-, S. oralis-, S. mutans-, and E. faecalis-reactive SIgA1 and SIgA2 antibodies. The level of statistical significance was set at α = 0.05.
RESULTS
Viridans streptococci were the dominant pioneer bacteria during the first month postpartum, and S. mitis biovar 1, S. oralis, and S. salivarius comprised 80.3% of the isolates recovered from the oral swabs. Teeth began to erupt at 6 months postpartum in two infants, and all infants had erupted teeth by 12 months. Although S. mutans was rarely observed as a transient bacterium, this bacterium failed to colonize the oral cavity of any of the infants by 2 years of age. Furthermore, enterococci were not detected in any of the infants’ mouths throughout the study.
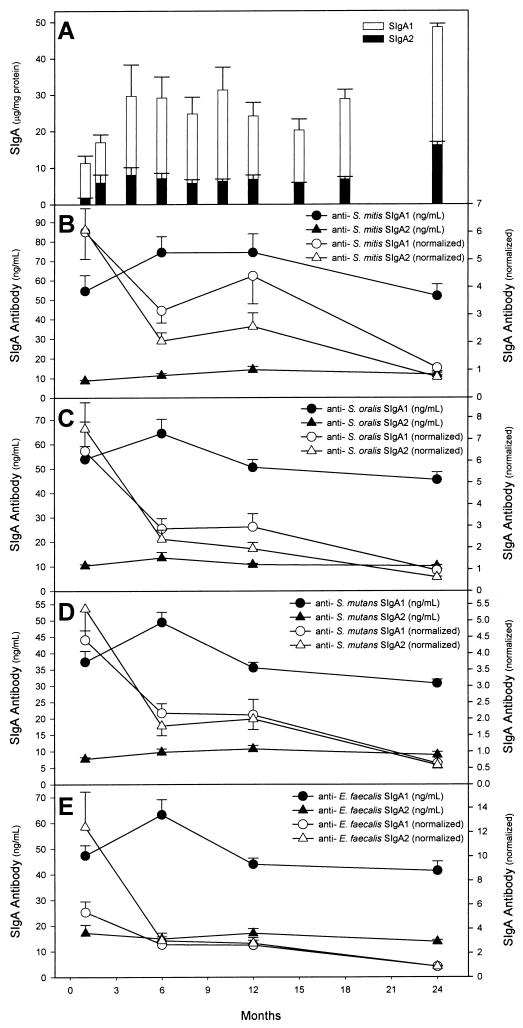
As salivary flow rates could not be obtained from the infants, the concentrations of salivary SIgA were normalized to the protein content of the saliva samples. SIgA, at a mean concentration of 13.0 μg/mg of protein, was detected in whole saliva from the neonates within 3 days postpartum (Fig. 1A). The concentrations of SIgA1 and SIgA2 showed significant increases (P < 0.0001) from birth to age 2 years. By 2 years of age, the concentration of SIgA in the infants’ saliva showed a fivefold increase, reaching a concentration of 64.7 μg/mg of protein. However, this constituted only 28% of the mean concentration of SIgA (230 μg/mg of protein) in their mothers’ saliva (13). SIgA1 was the dominant subclass in the infants’ saliva. SIgA2 represented as little as 12.4% of total SIgA at 2 weeks postpartum but reached 25% of total SIgA by 2 years of age, a value that approached the proportion (30.4%) of SIgA2 in the mothers’ saliva (13).
FIG. 1.
(A) Concentrations of salivary SIgA1 and SIgA2 from birth to 2 years of age. Results here and in panels B to E are expressed as means ± standard errors of the means. (B to E) Concentrations of SIgA1 and SIgA2 antibodies reactive with S. mitis biovar 1 (B), S. oralis (C), S. mutans (D), and E. faecalis (E) in saliva. The same data are also expressed as nanograms of antibody per microgram of SIgA1 or SIgA2 (normalized).
Low levels of SIgA1 and SIgA2 antibodies reactive with whole cells of S. mitis biovar 1, S. oralis, S. mutans, and E. faecalis were detected within the first month after birth (Fig. 1B to E), at which time they each represented ∼1% of the total SIgA. SIgA antibodies reactive with S. mutans and E. faecalis were present in saliva despite the fact that S. mutans was recovered only rarely from oral swabs and E. faecalis was never recovered from the mouths of the infants.
Although there was a fivefold increase in the concentration of total SIgA between birth and age 2 years, there were no differences between the concentrations of SIgA1 and SIgA2 antibodies reactive with S. mitis biovar 1, S. oralis, S. mutans, and E. faecalis when the data were expressed as nanograms of antibody per milliliter of saliva. In addition, when the concentrations of SIgA1 and SIgA2 antibodies reactive with whole cells of these bacteria were normalized to the concentrations of SIgA1 and SIgA2 in saliva, the antibodies all showed a significant decrease from birth to 2 years of age (P < 0.001), even though there was a transient increase in the concentration of S. mitis biovar 1-reactive SIgA and SIgA2 antibodies between 6 and 12 months. Thus, overall the proportion of SIgA1 and SIgA2 represented by antibodies reactive with S. mitis biovar 1, S. oralis, S. mutans, and E. faecalis declined over time. Analysis showed that, with the single exception of the 1-month time point for E. faecalis (P = 0.03), there were no differences in the concentrations of S. mitis biovar 1, S. oralis, S. mutans, and E. faecalis whole-cell-reactive antibodies between the SIgA1 and SIgA2 subclasses.
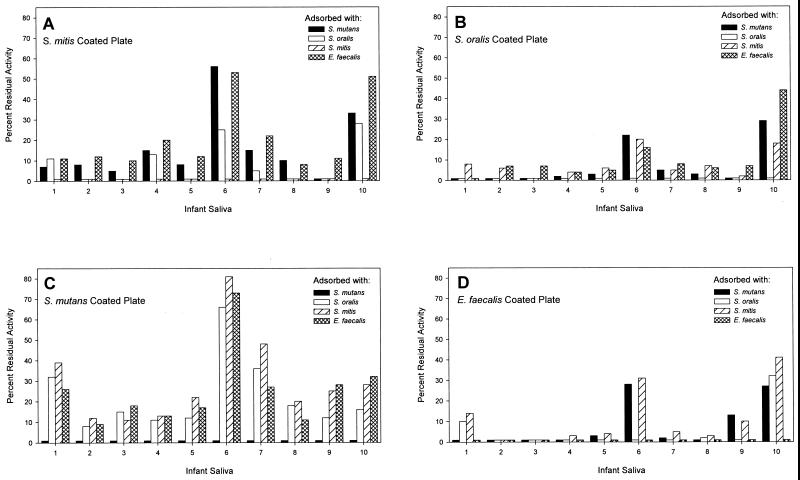
In order to determine the specificity of salivary SIgA antibodies reactive with S. mutans and E. faecalis, saliva samples were cross-adsorbed. The results (Fig. 2) showed that, in general, adsorption of each infant’s saliva with cells of one species produced a dramatic reduction of antibodies recognizing the three other species. SIgA antibody reactive with S. mutans (Fig. 2C) was reduced by between 52 and 92% in 9 of the 10 infants when the saliva samples were adsorbed with cells of S. mitis biovar 1, S. oralis, and E. faecalis. A similar effect of adsorption was seen when levels of antibodies to the other species were examined (Fig. 2A, B, and D). However, the saliva samples from infants 6 and 10 showed differences from the general trend. The saliva from infant 6 gave relatively high percentages of residual antibody for each bacterium, while infant 10 showed a similar effect for S. mitis biovar 1, S. oralis, and E. faecalis but not for S. mutans (Fig. 2A, B, and D). Nevertheless, sequential adsorption of saliva samples from infants 1, 2, 6, and 10 removed all SIgA antibody to the test bacteria (data not shown).
FIG. 2.
Assay of SIgA antibodies reactive with S. mitis biovar 1, S. oralis, S. mutans, and E. faecalis in aliquots of saliva from the cohort of 10 infants after adsorption with whole cells of these four species. Antibody in unabsorbed saliva was set to 100%. ELISA was run on wells coated with S. mitis biovar 1 (A), S. oralis (B), S. mutans (C), or E. faecalis (D).
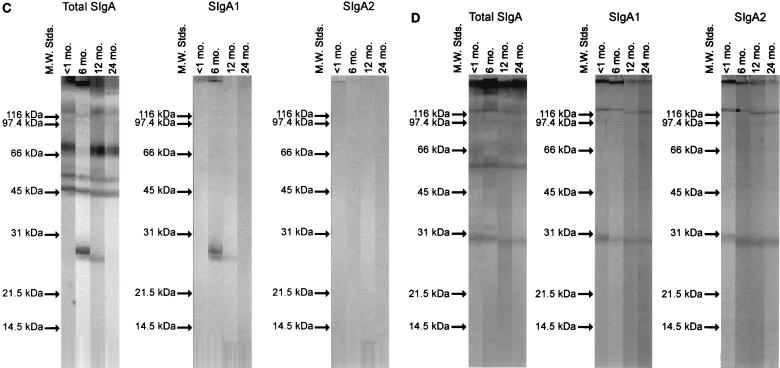
The range of envelope protein antigens of S. mitis biovar 1, S. oralis, S. mutans, and E. faecalis reactive with total SIgA, SIgA1, and SIgA2 antibodies was examined by Western blotting. The profiles of proteins recognized by salivary SIgA antibodies were then analyzed by cluster analysis with Ward’s method (37). Figure 3 shows Western blot profiles of CEPs developed with saliva samples collected from a single infant from birth to 2 years of age. The blots show the reactivity of total SIgA, SIgA1, and SIgA2 antibodies with envelope antigens of S. mitis biovar 1 (Fig. 3A), S. oralis (Fig. 3B), S. mutans (Fig. 3C), and E. faecalis (Fig. 3D). Within 1 month postpartum, SIgA1 and SIgA2 antibodies reactive with envelope proteins of between 100 and 20 kDa were detected (Fig. 3).
FIG. 3.
Western blot of envelope antigens incubated with saliva collected from a single infant at 1, 6, 12, and 24 months (mo.) and probed to detect total SIgA, SIgA1, and SIgA2 antibodies. (A) S. mitis biovar 1; (B) S. oralis; (C) S. mutans; (D) E. faecalis. M.W. Stds., molecular weight standards.
Cluster analysis was used to determine whether there were similarities in the profiles of the antibody responses to the CEPs both within and between infants over time. For example, if at a particular time point salivary antibodies from each infant recognized a similar set of CEPs, then blots from each infant at that time point should be included in the same cluster. Conversely, if salivary SIgA antibodies from each infant recognized a unique set of CEPs, then blots from each infant over time should form a separate cluster.
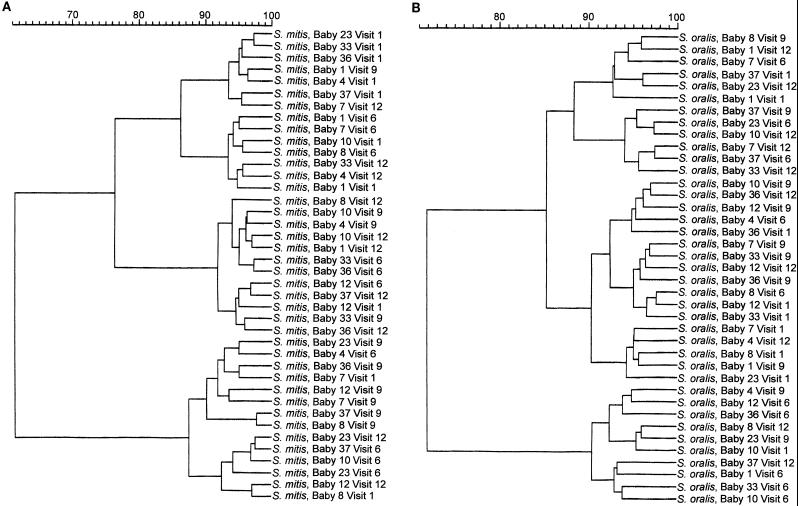
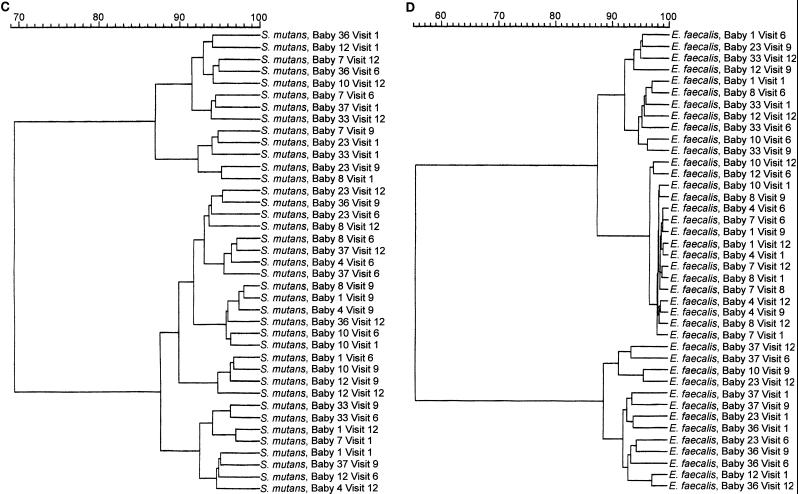
Examination of the blot profiles among the infants showed that there were common antigens recognized by the majority of saliva samples. However, despite these common responses, it was apparent from cluster analysis of the CEPs recognized by total SIgA, SIgA1, and SIgA2 antibodies, both within and between subjects over time, that there was pattern variability. The results of cluster analysis by Ward’s method (37) for total SIgA antibodies are shown in Fig. 4. On the whole, neither blots from individual infants nor blots from individual time points grouped together in single clusters with high similarities. Comparisons of similarity between the profiles of envelope antigens from among the four different species were also made. Again, the results showed no specific clusters which included all time points for a single infant or a single collection time, except in the case of E. faecalis. Analysis of this bacterium gave one cluster of 14 isolates with a similarity of 98%, which included the saliva profiles of all four time points for infants 4 and 7 and three of four time points for infant 8 (Fig. 4D).
FIG. 4.
Individual Western blot strips of S. mitis biovar 1, S. oralis, S. mutans, and E. faecalis envelope antigens were incubated with saliva collected from 10 infants (babies 1, 4, 7, 8, 10, 12, 23, 33, 36, and 37) at 1, 6, 12, and 24 months (visits 1, 6, 9, and 12) and probed to detect total SIgA, SIgA1, and SIgA2 antibodies. For each IgA subclass, the percent similarities of the bands recognized by the salivary antibodies both within and among the infants were examined by cluster analysis with Ward’s method (37). Only data for total SIgA antibodies are shown. The data are displayed as dendrograms. (A) Anti-S. mitis biovar 1 total SIgA antibodies; (B) anti-S. oralis total SIgA antibodies; (C) anti-S. mutans total SIgA antibodies; (D) anti-E. faecalis total SIgA antibodies.
DISCUSSION
The genus Streptococcus includes a number of species that are autochthonous to the mucosal surfaces of humans and other mammals (19). In the mouth viridans streptococci comprise a large fraction of the facultative commensal microbiota (3), and in the large bowel enterococci, mainly E. faecalis, Enterococcus faecium, and Streptococcus bovis, are numerically significant (9). Certain species of viridans streptococci, notably S. mitis biovar 1, S. oralis, and S. salivarius, are among the first bacteria to colonize the mucosal surfaces of the mouth of the human neonate. In our study (26) and others (14, 29, 35), these three species comprised over 80% of the total recoverable microbiota. In contrast, S. mutans does not colonize the oral cavity until after the eruption of teeth provides a nonshedding surface (7). S. mutans did not colonize the mouths of the infants in our study by 24 months of age. Our data are consistent with those obtained by Caufield et al. (8) in a longitudinal study of 38 infants and their mothers. These workers described a median age of 26 months, with a window of infectivity of between 19 and 31 months, for the colonization by S. mutans, although eight infants (21%) were not colonized with S. mutans when the study ended at 56 months.
Although the primary habitat of enterococci is the large intestine (reviewed in references 9 and 25), these bacteria have been recovered in low numbers from the oral cavity, albeit largely from the dental plaque of individuals with underlying disease or from hospital personnel, where nosocomial transmission may occur (6, 18, 33). We did not observe enterococci in the mouths of the infants in our study. The rationale for selecting S. mitis biovar 1, S. oralis, S. mutans, and E. faecalis for study was to allow a comparison between the salivary immune response to early colonizers (S. mitis biovar 1 and S. oralis) and that to a late colonizer (S. mutans). The inclusion of E. faecalis further allowed a comparison of the specificity of salivary SIgA antibodies reactive with commensal oral bacteria to that of antibodies reactive with a commensal nonoral streptococcus.
To date the data have been equivocal as to whether SIgA antibodies reactive with commensal oral bacteria are induced by these bacteria and are, therefore, specific for them or whether they are induced by cross-reactive bacteria in the mouth, in the gut, or in food. For example, Smith et al., in a cross-sectional study (30–32), examined the induction of salivary IgA antibodies to culture supernatant antigens of S. mitis and S. salivarius in groups of infants between 3 and 27 weeks of age. Reactivity with these antigens was observed only after the isolation of the respective streptococcal species from the mouths of the infants. Furthermore, the infants did not harbor S. mutans, and, except for two subjects, no S. mutans-reactive SIgA antibodies were detected in their saliva. These data argued that the S. mitis- and S. salivarius-reactive SIgA antibodies were induced by these bacteria and were specific for them. In contrast, Bamman and Gibbons (2) detected SIgA antibodies reactive with S. mutans in saliva of predentate infants who did not harbor S. mutans. These antibodies were shown to be cross-reactive, as they were significantly reduced by adsorption with mixed bacterial growth from common dairy products (2).
In the present study, low levels of SIgA1 and SIgA2 antibodies reactive with S. mutans and E. faecalis, as well as S. mitis biovar 1 and S. oralis, were detected within 1 week postpartum by using a sensitive ELISA, even though S. mutans and E. faecalis did not colonize the oral cavity. It is possible that these antibodies were induced by S. mutans and E. faecalis that were transient bacteria in the oral cavity or that the salivary SIgA S. mutans- and E. faecalis-reactive antibodies were produced without antigenic exposure as the result of anti-idiotype induction (22). The results of cross-adsorption showed that, with the exception of saliva from two infants, each of the four bacteria was able to adsorb SIgA antibodies to the other three bacteria to a significant extent. The elimination of SIgA antibodies reactive with the test species following sequential adsorption of four saliva samples with each streptococcal species supports the conclusion that the antibodies were cross-reactive rather than species specific. Antigenic cross-reactivity among oral streptococci is well described, and preparation of species-specific antisera usually requires extensive adsorption of polyclonal sera raised against a single species (21, 36).
Therefore, overall these data suggest that S. mutans- and E. faecalis-reactive antibodies in saliva were directed largely to epitopes that are shared by S. oralis and S. mitis biovar 1 and likely by other members of the resident oral or intestinal microbiota of the infants or, perhaps, by bacterial antigens in food. Although we have not identified the common antigens recognized by the salivary SIgA1 and SIgA2 antibodies, it is likely that they include peptidoglycan, teichoic and lipoteichoic acids, and other wall and envelope polysaccharides, among others. The detection of antibodies against antigens that are ubiquitous in gram-positive bacteria may explain why cluster analysis of cell envelope proteins from the non-oral bacterium E. faecalis recognized by salivary SIgA antibodies grouped 14 profiles (all four time points for infants 4 and 7 and three of four time points for infant 8) with a similarity of 98%.
An alternative hypothesis that must be considered, however, is that S. oralis-, S. mitis biovar 1-, S. mutans-, and E. faecalis-reactive SIgA antibodies in saliva represent polyreactive SIgA antibodies (27). Quan et al. (27) described polyreactive SIgA antibodies in human saliva and colostrum that reacted with actin, myosin, tubulin, and spectrin but also with a large variety of surface and secreted antigens from Streptococcus pyogenes. Those authors proposed that these polyspecific SIgA antibodies are involved in plurispecific protection at mucosal surfaces.
The concentrations (nanograms per milliliter) of S. mitis biovar 1-, S. oralis-, S. mutans-, and E. faecalis-reactive SIgA1 and SIgA2 antibodies reached a peak at 6 months of age, paralleling the concentrations of SIgA1 and SIgA2 in the infants’ saliva during this period. However, thereafter these antibodies declined even as SIgA1 and SIgA2 continued to increase, so that by 24 months, the concentrations of SIgA1 and SIgA2 antibodies returned to levels observed 1 month postpartum. This decline over time of S. mitis biovar 1-, S. oralis-, S. mutans-, and E. faecalis-reactive SIgA1 and SIgA2 antibodies was even more marked when the antibody concentrations were normalized to SIgA1 and SIgA2 concentrations and was highly statistically significant (P < 0.001). Thus, SIgA antibodies reactive with S. mitis biovar 1, S. oralis, S. mutans, and E. faecalis constituted a decreasing proportion of salivary SIgA over time. These data confirm the findings of others (28) that commensal oral bacteria elicit a limited secretory immune response. As it is known that oral bacteria are coated with SIgA (4), it is possible that the presence of S. mitis biovar 1 and S. oralis in the mouth served to adsorb the SIgA streptococcus- and enterococcus-reactive antibodies, rendering them unavailable for assay. However, Widerström et al. (38) observed that Western blots of SIgA antibodies reactive with mutans streptococci in parotid and submandibular ductal saliva showed a high degree of similarity to those for whole mouth saliva, arguing against adsorption of antibody by the oral microbiota. In accordance with the results of our study, Gleeson and coworkers (15) showed that only low levels of SIgA antibodies reactive with Escherichia coli O antigen were detected in saliva of infants and children during the first 4 years of life, despite the colonization of the large intestines of the subjects with E. coli. Furthermore, consistent with our findings, Gleeson et al. (15) observed that the E. coli-reactive SIgA antibodies declined significantly during the period from birth to 1 year of age, a time when the total SIgA level in saliva was essentially constant.
SIgA1 was the dominant subclass in saliva throughout the first 2 years of life. SIgA2 comprised less than 15% of total SIgA immediately postpartum and represented only 30% of total SIgA by 2 years of age. The subclass distribution of antibodies directed against commensal oral bacteria may be important because the cleavage of colonization-inhibitory SIgA1 antibodies by IgA1 protease in the oral cavity may subvert host defense (1, 17, 24). It has been reported that naturally occurring salivary SIgA antibodies against S. sanguis, S. oralis, Streptococcus gordonii, and S. mitis biovar 2 are found predominantly in the IgA1 subclass (1). However, in the present study, S. mitis biovar 1, S. oralis, E. faecalis, and S. mutans whole-cell antibody activity was equally divided between the subclasses even though SIgA2 comprised less than one-third of salivary SIgA.
Brown and Mestecky (5) observed that human parotid saliva IgA antibodies to glucosyltransferase from Streptococcus sobrinus and to cell wall carbohydrate and protein antigen I/II (antigen B) from S. mutans occur predominantly in the IgA1 subclass. However, in contrast, SIgA antibodies to antigen I/II and cell wall carbohydrate have been shown to be almost equally divided between the IgA1 and IgA2 subclasses in whole saliva from caries-resistant and caries-susceptible human subjects (16).
Except in cases of immune deficiency, where some commensal bacteria may become opportunistically pathogenic, the indigenous microbiota exists in a state of homeostasis with the host and plays an important role in host defense by excluding colonization of allochthonous pathogenic microorganisms (3). The coevolution of commensal bacteria and the mucosal immune system has doubtless led to the development of mechanisms used by these bacteria to avoid immune elimination (10).
ACKNOWLEDGMENTS
This work was supported by Public Health Service grant DE08178 from the National Institute of Dental Research. G.H.W.B. is supported by grant MT 7611 from the Medical Research Council of Canada.
REFERENCES
- 1.Ahl T, Reinholdt J. Subclass distribution of salivary secretory immunoglobulin A antibodies to oral streptococci. Infect Immun. 1991;59:3619–3625. doi: 10.1128/iai.59.10.3619-3625.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bamman L L, Gibbons R J. Immunoglobulin A antibodies reactive with Streptococcus mutans in the saliva of adults, children, and predentate infants. J Clin Microbiol. 1979;10:538–543. doi: 10.1128/jcm.10.4.538-543.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bowden G H W, Ellwood D C, Hamilton I R. Microbial ecology of the oral cavity. Adv Microb Ecol. 1979;3:135–217. [Google Scholar]
- 4.Brandtzaeg P, Fjellanger I, Gjeruldsen S T. Adsorption of immunoglobulin A onto oral bacteria in vivo. J Bacteriol. 1968;96:242–249. doi: 10.1128/jb.96.1.242-249.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown T A, Mestecky J. Immunoglobulin A subclass distribution of naturally occurring salivary antibodies to microbial antigens. Infect Immun. 1985;49:459–462. doi: 10.1128/iai.49.2.459-462.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campbell J, McGowan D A, Macfarlane T W. The prevalence of enterococci in the dental plaque of chronic hospital patients. Br J Oral Surg. 1983;21:171–174. doi: 10.1016/0007-117x(83)90038-0. [DOI] [PubMed] [Google Scholar]
- 7.Catalanotto F A, Shklair I L, Keene H J. Prevalence and localization of Streptococcus mutans in infants and children. J Am Dent Assoc. 1975;91:606–609. doi: 10.14219/jada.archive.1975.0398. [DOI] [PubMed] [Google Scholar]
- 8.Caufield P W, Cutter G R, Dasanayake A P. Initial acquisition of mutans streptococci by infants: evidence for a discrete window of infectivity. J Dent Res. 1993;72:37–45. doi: 10.1177/00220345930720010501. [DOI] [PubMed] [Google Scholar]
- 9.Chenoweth C, Schaberg D. The epidemiology of enterococci. Eur J Clin Microbiol Infect Dis. 1990;9:80–89. doi: 10.1007/BF01963631. [DOI] [PubMed] [Google Scholar]
- 10.Cole M F. Influence of secretory immunoglobulin A on the ecology of oral bacteria. In: Mergenhagen S E, Rosan B, editors. Molecular basis of oral microbial adhesion. Washington, D.C: American Society for Microbiology; 1985. pp. 131–135. [Google Scholar]
- 11.Cole M F, Evans M, Fitzsimmons S, Johnson J, Pearce C, Sheridan M J, Wientzen R, Bowden G. Pioneer oral streptococci produce immunoglobulin A1 protease. Infect Immun. 1994;62:2165–2168. doi: 10.1128/iai.62.6.2165-2168.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cole M F, Hale C A, Sturzenegger S. Identification of two subclasses of IgA in the chimpanzee (Pan troglodytes) J Med Primatol. 1992;21:275–278. [PubMed] [Google Scholar]
- 13.Fitzsimmons S P, Evans M K, Pearce C L, Sheridan M J, Weintzen R, Cole M F. Immunoglobulin A subclasses in infants’ saliva and in saliva and milk from their mothers. J Pediatr. 1994;124:566–573. doi: 10.1016/s0022-3476(05)83135-x. [DOI] [PubMed] [Google Scholar]
- 14.Frandsen E V G, Pedrazzoli V, Kilian M. Ecology of viridans streptococci in the oral cavity and pharynx. Oral Microbiol Immunol. 1991;6:129–133. doi: 10.1111/j.1399-302x.1991.tb00466.x. [DOI] [PubMed] [Google Scholar]
- 15.Gleeson M, Cripps A W, Clancy R L, Wlodarczyk J H, Dobson A J, Hensley J H. The development of IgA-specific antibodies to Escherichia coli O antigen in children. Scand J Immunol. 1987;26:639–643. doi: 10.1111/j.1365-3083.1987.tb02299.x. [DOI] [PubMed] [Google Scholar]
- 16.Hocini H, Iscaki S, Bouvet J-P, Pillot J. Unexpectedly high levels of some presumably protective secretory immunoglobulin A antibodies to dental plaque bacteria in salivas of both caries-resistant and caries-susceptible subjects. Infect Immun. 1993;61:3597–3604. doi: 10.1128/iai.61.9.3597-3604.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kilian M, Reinholdt J, Lomholt H, Poulsen K, Frandsen E V G. Biological significance of IgA1 protease in bacterial colonization and pathogenesis: critical evaluation of experimental evidence. APMIS. 1996;104:321–338. doi: 10.1111/j.1699-0463.1996.tb00724.x. [DOI] [PubMed] [Google Scholar]
- 18.Kurrie E, Bhaduri S, Krieger D, Gaus W, Heimpel H, Pfieger H, Arnold R, Vanek V. Risk factors for infections of the oropharynx and the respiratory tree in patient with acute leukemia. J Infect Dis. 1981;144:128–136. doi: 10.1093/infdis/144.2.128. [DOI] [PubMed] [Google Scholar]
- 19.Linton A H, Hinton M A. The normal microbiota of the body. In: Parker M T, Collier L H, editors. Topley and Wilson’s principles of bacteriology, virology and immunity, 8th ed., vol. 1. General microbiology and immunity. Philadelphia, Pa: B. C. Decker Inc.; 1990. pp. 312–329. [Google Scholar]
- 20.Marcotte H, Lavoie M C. Oral microbial ecology and the role of salivary immunoglobulin A. Microbiol Mol Biol Rev. 1998;62:71–109. doi: 10.1128/mmbr.62.1.71-109.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McKinney R M, Thacker L. Improvement in specificity of immunofluorescent reagents for identifying Streptococcus mutans by DEAE-cellulose-bacterial cell column immunosorption methods. J Dent Res. 1976;55A:A50–A57. doi: 10.1177/002203457605500121011. [DOI] [PubMed] [Google Scholar]
- 22.Mellander L, Carlsson B, Hanson L A. Secretory IgA and IgM antibodies to E. coli O and poliovirus type I antigens occur in amniotic fluid, meconium, and saliva from newborns. A neonatal immune response without antigenic exposure: a result of anti-idiotype induction? Clin Exp Immunol. 1986;63:555–561. [PMC free article] [PubMed] [Google Scholar]
- 23.Mestecky J, McGhee J R. Immunoglobulin A (IgA): molecular and cellular interactions involved in IgA biosynthesis and immune response. Adv Immunol. 1987;40:153–245. doi: 10.1016/s0065-2776(08)60240-0. [DOI] [PubMed] [Google Scholar]
- 24.Mestecky, J., and M. W. Russell. IgA subclasses. Monogr. Allergy 19:277–301. [PubMed]
- 25.Murray B E. The life and times for the enterococcus. Clin Microbiol Rev. 1990;3:46–65. doi: 10.1128/cmr.3.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pearce C, Bowden G H, Evans M, Fitzsimmons S P, Johnson J, Sheridan M J, Wientzen R, Cole M F. Identification of pioneer viridans streptococci in the oral cavity of human neonates. J Med Microbiol. 1995;42:67–72. doi: 10.1099/00222615-42-1-67. [DOI] [PubMed] [Google Scholar]
- 27.Quan C P, Bernman A, Pires R, Avrameas S, Bouvet J-P. Natural polyreactive secretory immunoglobulin A autoantibodies as a possible barrier to infection in humans. Infect Immun. 1997;65:3997–4004. doi: 10.1128/iai.65.10.3997-4004.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shroff K E, Meslin K, Cebra J J. Commensal enteric bacteria engender a self-limiting humoral mucosal immune response while permanently colonizing the gut. Infect Immun. 1995;63:3904–3913. doi: 10.1128/iai.63.10.3904-3913.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith D J, Anderson J M, King W F, van Houte J, Taubman M A. Oral streptococcal colonization of infants. Oral Microbiol Immunol. 1993;8:1–4. doi: 10.1111/j.1399-302x.1993.tb00535.x. [DOI] [PubMed] [Google Scholar]
- 30.Smith D J, King W F, Taubman M A. Salivary IgA antibody to oral streptococcal antigens in predentate infants. Oral Microbiol Immunol. 1990;5:57–62. doi: 10.1111/j.1399-302x.1990.tb00228.x. [DOI] [PubMed] [Google Scholar]
- 31.Smith D J, Taubman M A. Ontogeny of immunity to oral microbiota in humans. Crit Rev Oral Biol Med. 1992;3:109–133. doi: 10.1177/10454411920030010201. [DOI] [PubMed] [Google Scholar]
- 32.Smith D J, Taubman M A. Emergence of immune competence in saliva. Crit Rev Oral Biol Med. 1993;4:335–341. doi: 10.1177/10454411930040031101. [DOI] [PubMed] [Google Scholar]
- 33.Smyth C J, Docent M A, Halpenny M K, Ballagh S J. Carriage rates of enterococci in the dental plaque of haemodialysis patients in Dublin. Br J Oral Maxillofacial Surg. 1987;25:21–33. doi: 10.1016/0266-4356(87)90153-7. [DOI] [PubMed] [Google Scholar]
- 34.Syed S A, Loesche W J. Survival of human dental plaque bacteria in various transport media. Appl Microbiol. 1972;24:638–644. doi: 10.1128/am.24.4.638-644.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tappuni A R, Challacombe S J. Distribution and isolation frequency of eight streptococcal species in saliva from predentate and dentate children and adults. J Dent Res. 1993;72:31–36. doi: 10.1177/00220345930720010401. [DOI] [PubMed] [Google Scholar]
- 36.Thomson L A, Little W, Hageage G J. Application of fluorescent antibody methods in the analysis of plaque samples. J Dent Res. 1976;55A:A80–A86. doi: 10.1177/002203457605500126011. [DOI] [PubMed] [Google Scholar]
- 37.Ward J H. Hierarchical grouping to optimize an objective function. J Am Stat Assoc. 1963;58:236–344. [Google Scholar]
- 38.Widerström L, Bratthall D, Hamberg K. Immunoglobulin A antibody activity to mutans streptococci in parotid, submandibular and whole saliva. Oral Microbiol Immunol. 1992;7:326–331. doi: 10.1111/j.1399-302x.1992.tb00631.x. [DOI] [PubMed] [Google Scholar]