Abstract
Sirtuin 1 (SIRT1) regulates cellular processes by deacetylating non-histone targets, including transcription factors and intracellular signalling mediators; thus, its abnormal activation is closely linked to the pathophysiology of several diseases. However, its function in Toxoplasma gondii infection is unclear. We found that SIRT1 contributes to autophagy activation via the AMP-activated protein kinase (AMPK) and PI3K/AKT signalling pathways, promoting anti-Toxoplasma responses. Myeloid-specific Sirt1−/− mice exhibited an increased cyst burden in brain tissue compared to wild-type mice following infection with the avirulent ME49 strain. Consistently, the intracellular survival of T. gondii was markedly increased in Sirt1-deficient bone-marrow-derived macrophages (BMDMs). In contrast, the activation of SIRT1 by resveratrol resulted in not only the induction of autophagy but also a significantly increased anti-Toxoplasma effect. Notably, SIRT1 regulates the FoxO-autophagy axis in several human diseases. Importantly, the T. gondii-induced phosphorylation, acetylation, and cytosolic translocation of FoxO1 was enhanced in Sirt1-deficient BMDMs and the pharmacological inhibition of PI3K/AKT signalling reduced the cytosolic translocation of FoxO1 in BMDMs infected with T. gondii. Further, the CaMKK2-dependent AMPK signalling pathway is responsible for the effect of SIRT1 on the FoxO3a-autophagy axis and for its anti-Toxoplasma activity. Collectively, our findings reveal a previously unappreciated role for SIRT1 in Toxoplasma infection.
Keywords: Sirtuin 1, autophagy, AMP-activated protein kinase, PI3K/AKT signalling pathway, Toxoplasma gondii, bone-marrow-derived macrophages, Class O of forkhead box transcription factors
1. Introduction
Toxoplasma gondii is an opportunistic obligate intracellular protozoan parasite that is capable of infecting all nucleated cells of a variety of warm-blooded animals; it infects approximately one-third of the world’s population [1,2]. Toxoplasma infection of immunocompetent individuals is typically asymptomatic; however, >10% of infected individuals develop cervical lymphadenopathy and toxoplasmic chorioretinitis [3]. In addition, toxoplasmosis in immunocompromised patients, including those on immunosuppressive drugs or chemotherapy, those with AIDS [4], and congenitally infected individuals, can be life-threatening because of toxoplasmic encephalitis or pneumonitis [5,6]. During Toxoplasma infection, parasite proliferation in innate immune cells is promoted by the remodelling of the phagosomal compartments via parasitophorous vacuole (PV) membrane formation and the subsequent production of diverse antigens [7]. Moreover, T. gondii interferes with the activation of host immunity by suppressing the inflammatory response and the production of microbicidal nitric oxide and cytokines [8,9].
Autophagy is an essential biological process for the degradation of cytosolic components, including organelles and soluble macromolecules, by lysosomal digestion [10]. Autophagy-related genes (Atgs) and autophagy-regulating kinases are important components of the autophagy process. The UNC-51-like kinase 1/2 (ULK1/2) complex is a key protein in the initiation of autophagy and is regulated by AMP-activated protein kinase (AMPK) and mammalian target of rapamycin complex 1 (mTORC1) [10,11]. Together with maintaining cellular homeostasis, autophagy is implicated in activating an immune response to Toxoplasma infection [12,13,14]. T. gondii has various strategies to preserve PV integrity by attenuating host–cell signalling related to the autophagic targeting of PV and activating mTORC1 via the PI3K/AKT signalling axis [12]. Therefore, additional studies on their interactions are needed.
Sirtuin 1 (SIRT1) is a nicotinamide adenosine dinucleotide (NAD)-dependent protein deacetylase that participates in a wide range of biological processes, including metabolism, cell cycle, inflammation, neurodegeneration, and DNA repair [15,16]. SIRT1 deacetylates various histone and non-histone proteins, such as the transcription factor forkhead box class O (FoxO), the tumor suppressor p53, and nuclear factor κB (NF-κB), to regulate cellular physiology and energy expenditure [17]. SIRT1 directly and indirectly modulates autophagy. The SIRT1-mediated deacetylation of FOXO1 induces autophagy by inducing the expression of small GTPase Rab 7 [18], which is required for the fusion of autophagosomes and terminal endosomes/lysosomes [19]. Moreover, the SIRT1-dependant deacetylation of FOXO3 induces BCL2/adenovirus E1B 19 kDa protein-interacting protein 3 (Bnip3)-mediated autophagy [20]. SIRT1 also deacetylates Atg5, 7, and 8 [21]. The deacetylation of microtubule-associated protein 1 light-chain 3 (LC3) by SIRT1 results in relocation from the nucleus to autophagosomes in the cytoplasm under starvation conditions, followed by autophagy activation [22]. In addition, SIRT1 activation by resveratrol (RES) activated autophagy and attenuated apoptosis by modulating AMPK-dependent nutrient-sensing pathways, thus improving spinal cord injury [23]. Previously, we reported that 4-hydroxybenzaldehyde (4-HBA) suppresses the growth of intracellular T. gondii by SIRT1-dependent the activation of autophagy [24]. Although SIRT1 regulates diverse cellular processes, its role in the modulation of host protective immunity during Toxoplasma infection is unclear.
In this study, myeloid-specific Sirt1−/− mice (mSirt1−/− mice) had a markedly higher protozoan parasite load and T. gondii surface antigen 1 (Sag1) mRNA level in brain tissues. Moreover, a Sirt1 deficiency in primary macrophages increased the survival and proliferation of intracellular T. gondii. Conversely, the pharmacological activation of SIRT1 induced autophagy activation and then promoted the host protective immune response against Toxoplasma infection. In addition, RES-mediated autophagy induction, autophagosome maturation, and lysosomal fusion were diminished in Sirt1-deficient primary macrophages. Finally, the activities of FoxO1 and FoxO3a, key regulators of autophagy, were modulated by SIRT1 via the PI3K/AKT and CAMKK2/AMPK signalling pathways, respectively. These data indicate a previously unappreciated function for myeloid SIRT1 in the regulation of autophagy and anti-Toxoplasma responses in mouse primary macrophages.
2. Results
2.1. Myeloid SIRT1 Is Required for Antiparasitic Activity in Response to Toxoplasma Infection
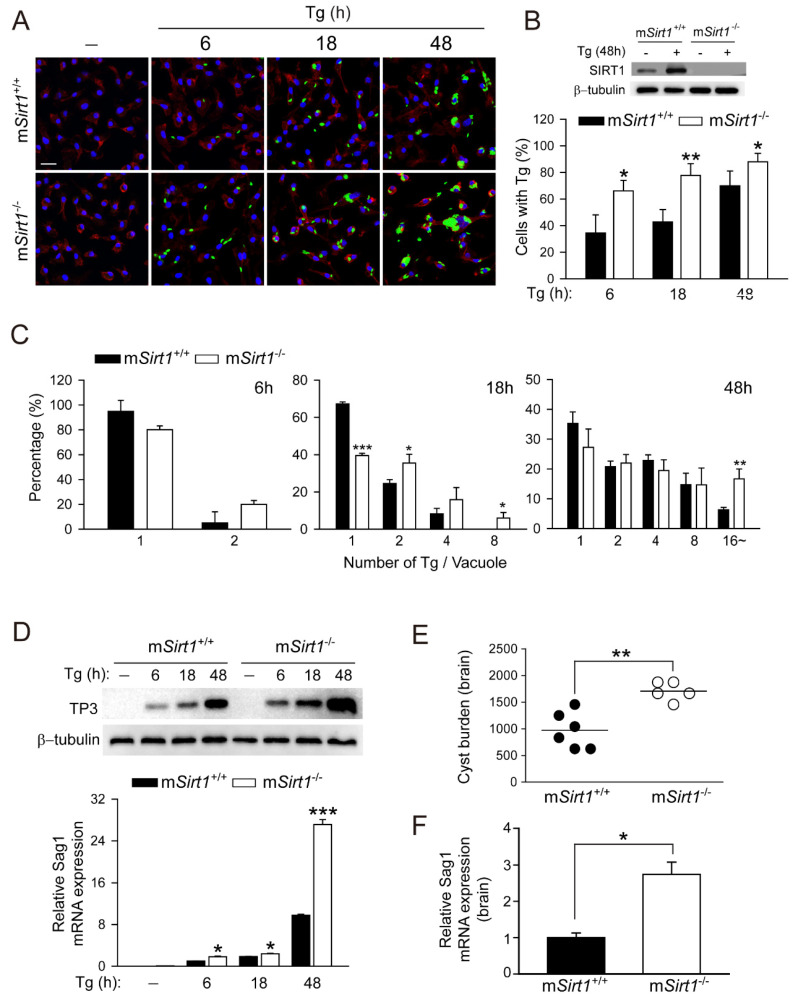
We reported that 4-HBA exerts an anti-Toxoplasma effect by inducing autophagy in a manner dependent on SIRT1 [24]. Herein, we examined whether SIRT1 contributes to the activation of host defences against T. gondii infection. To assess the role of myeloid-specific SIRT1, BMDMs were isolated from mSirt1+/+ or mSirt1−/− mice and infected with a GFP-expressing RH strain of T. gondii (GFP-Tg) for the indicated time periods (Figure 1A–C). The number of cells infected with T. gondii (Figure 1A,B) and the proliferation of intracellular T. gondii (Figure 1A,C) were significantly enhanced in Sirt1-deficient BMDMs compared to wild-type (WT) BMDMs. The levels of the Toxoplasma antigens p30 membrane protein (for TP3 protein; Figure 1D, top) and Sag1 mRNA (Figure 1D, bottom) were markedly increased in Sirt1-deficient BMDMs.
Figure 1.
Myeloid SIRT1 is essential for host protection against T. gondii infection in primary macrophages and mice. (A–C) BMDMs from mSirt1+/+ and mSirt1−/− mice were infected with GFP-conjugated T. gondii RH strain (MOI = 1) for the indicated periods. Cells were fixed and stained with Texas Red®-X phalloidin for F-actin in the cytoskeleton (red) and DAPI for nuclei (blue), respectively. (A) Fluorescent images showing the number of intracellular T. gondii. (B,C) Number of T. gondii RH-infected cells (for B) and T. gondii RH per vacuole (for C) were quantified. Scale bar = 25 µm (D) BMDMs from mSirt1+/+ and mSirt1−/− mice were infected with T. gondii RH strain (MOI = 1) for the indicated time periods. TP3 protein expression (top) and Sag1 mRNA expression (bottom) was examined by immunoblot and qPCR analysis, respectively. (E,F) mSirt1+/+ and mSirt1−/− mice (n = 5 per group) were infected with 40 cysts of the ME49 strain (i.p. injection) for 3 weeks. (E) The number of cysts in the brain was counted under a microscope. (F) Sag1 mRNA expression in brain homogenates was evaluated by real-time qPCR analysis. Data are representative of three independent experiments and are presented as means ± SD (for B–D) or ± SEM (for F). * p < 0.05, ** p < 0.01, *** p < 0.001, compared with control cultures or control mice infected with T. gondii (two-tailed Student’s t-test). Tg, Toxoplasma gondii.
We next examined the in vivo role of myeloid-specific SIRT1 in response to Toxoplasma infection. mSirt1+/+ and mSirt1−/− mice were intraperitoneally infected with strain type II ME49 (Figure 1E; 40 cyst/mouse), respectively. The parasite load in infected brain tissue was significantly greater in mSirt1−/− mice than in mSirt1+/+ mice (Figure 1E). In addition, the mRNA level of Sag1 was increased in the infected brains of mSirt1−/− mice (Figure 1F). Taken together, these data suggest that myeloid-specific SIRT1 plays a pivotal role in the antiparasitic response to Toxoplasma.
2.2. RES Restricts Intracellular Parasite Growth by Activating SIRT1 in Primary Macrophages
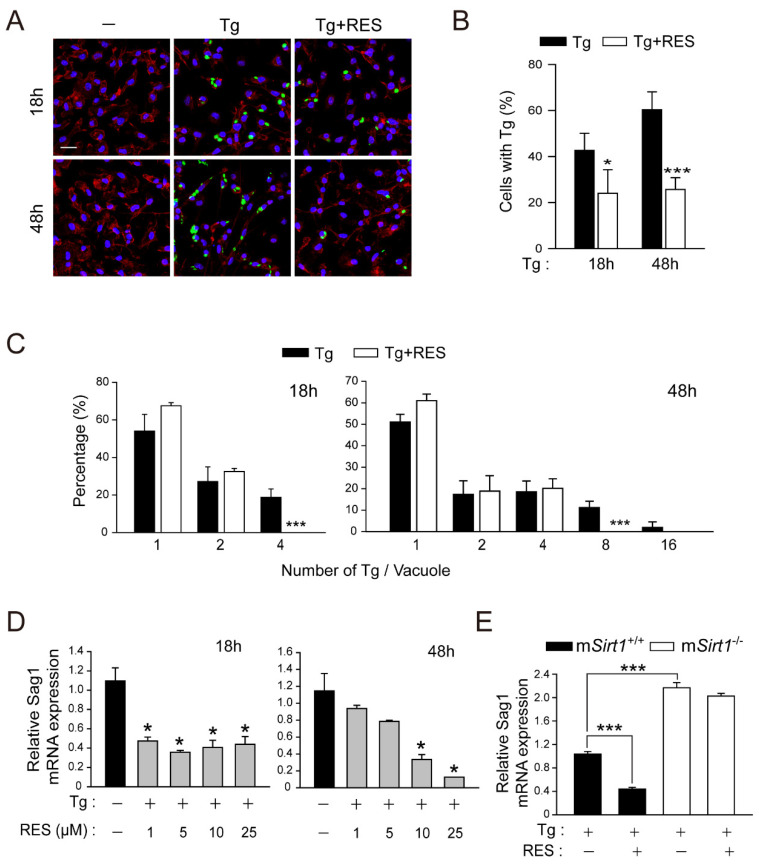
The stilbenoid polyphenol RES (trans 3,5,4′-trihydroxystilbene) exerts beneficial effects by regulating SIRT1 activity in a range of cellular functions [25]. To determine whether the activation of SIRT1 by RES induces antiparasitic responses in macrophages, we compared the number of cells infected with T. gondii (Figure 2A,B) and the intracellular proliferation of T. gondii (Figure 2A,C). Contrary to the effect of a myeloid-specific SIRT1 deficiency (Figure 1), the intracellular growth of T. gondii was markedly reduced in cells treated with RES compared to the solvent control. Similarly, RES significantly decreased the Sag1 mRNA level in BMDMs infected with T. gondii (Figure 2D). To investigate the role of SIRT1 in the anti-Toxoplasma effect of RES, we compared the mRNA level of Sag1 after RES treatment in mSirt1+/+ and mSirt1−/− BMDMs infected with T. gondii. RES inhibited T. gondii proliferation in mSirt1+/+ BMDMs (Figure 2E). However, RES did not exert an anti-Toxoplasma effect in Sirt1-deficient primary macrophages. Collectively, these data suggest that SIRT1 is required for the effect of RES on anti-Toxoplasma activity.
Figure 2.
RES treatment promotes the elimination of intracellular T. gondii in primary murine macrophages. (A–C) BMDMs were infected with a GFP-conjugated RH strain (MOI = 1) for 2 h, followed by the further treatment of RES (10 µM) for the indicated times. Cells were stained with Texas Red®-X phalloidin for F-actin in the cytoskeleton (red) and DAPI for nuclei (blue), respectively. (A) Fluorescent images showing the number of intracellular T. gondii. (B,C) Number of T. gondii RH-infected cells (for B) and T. gondii RH per vacuole were quantified. Scale bar = 25 µm (D,E) qPCR analysis for evaluating Sag1 mRNA expression. (D) T. gondii-infected BMDMs were stimulated with increasing concentrations of RES (1, 5, 10, or 25 µM) for 18 h (for left) or 48 h (for right). (E) BMDMs isolated from mSirt1+/+ and mSirt1−/− mice were infected with RH strain of T. gondii (MOI = 1) for 2 h and then further stimulated with RES (10 µM) for 48 h. Data are representative of three independent experiments and are presented as means ± SD. * p < 0.05 and *** p < 0.001, compared with control cells (two-tailed Student’s t-test). Tg, Toxoplasma gondii; RES, resveratrol.
2.3. Pharmacological Activation of SIRT1 by RES Is Essential for the Induction of Antiprotozoal Autophagy and the Colocalisation of PV with Autophagosomes/Lysosomes
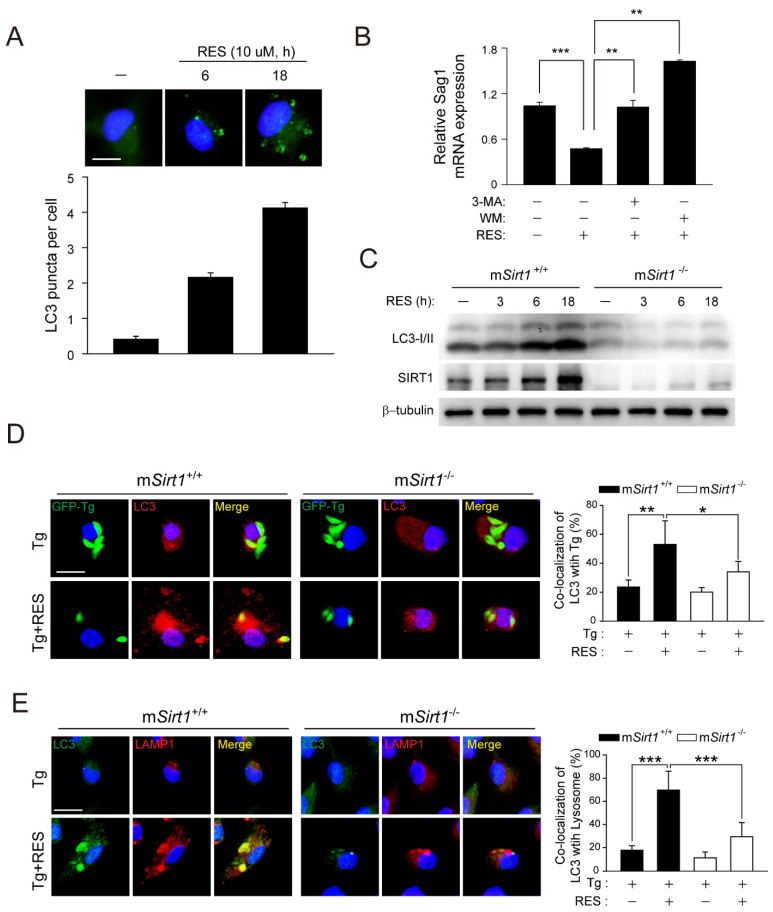
SIRT1 modulates health and disease by modulating autophagy activation via the transcriptional or epigenetic regulation of Atgs and autophagy regulators [26]. To determine whether RES induces autophagy, we enumerated LC3 punctate structures in BMDMs. RES significantly increased the number of LC3 aggregates in a time-dependent manner (Figure 3A). Moreover, the RES-mediated decrease in Sag1 mRNA levels was significantly attenuated by pretreatment with the autophagy inhibitors 3-methyladenine (3-MA) and wortmannin (WM) (Figure 3B). However, the RES-mediated increase in the levels of LC3-II proteins was suppressed in mSirt1-deficient BMDMs (Figure 3C).
Figure 3.
RES induces autophagy activation in a SIRT1-dependent manner. (A) BMDMs were stimulated with RES (10 µM) for various times and subjected to immunofluorescence microscopy for analysing LC3 puncta formation (top). Quantification of LC3 punctate foci per cell (bottom). Each experiment included a minimum of 100 cells scored in 5 random fields. Scale bar = 10 µm. (B) BMDMs were pre-treated with 3-MA (10 µM, 2 h) or WM (100 nM, 2 h) and further incubated with RES for 18 h. The mRNA expression of the Sag1 gene was evaluated by real-time qPCR analysis. (C) BMDMs from mSirt1+/+ and mSirt1−/− mice were stimulated with RES for the indicated time periods and subjected to immunoblot analysis of LC3 and β-tubulin. (D,E) BMDMs from mSirt1+/+ and mSirt1−/− mice were infected with a GFP-conjugated RH strain (for D) or a T. gondii RH strain (for E) for 2 h, followed by further treatment of RES (10 µM) for 18 h. (D) Cells were immunostained with an anti-LC3 antibody (Alexa Fluor 594-conjugated goat anti-rabbit IgG, red) and the level of colocalisation T. gondii with LC3 was quantified. Scale bar = 10 µm. (E) LC3 (Alexa Fluor 488-conjugated goat anti-rabbit IgG, green), Alexa Fluor 594-conjugated LAMP1 (red), and DAPI (blue) were detected by confocal microscopy. Immunofluorescence microscopy images were obtained from one representative of 3 independent samples, with each experiment containing a minimum of 50 cells scored in 7 random fields. Scale bar = 10 µm. Data are representative of three independent experiments and are presented as means ± SD. * p < 0.05, ** p < 0.01, and *** p < 0.001, compared with control cells (two-tailed Student’s t-test). Tg, Toxoplasma gondii; RES, resveratrol; 3-MA, 3-methyladenine; WM, wortmannin.
Based on the above, we next questioned whether the pharmacological activation of SIRT1 by RES treatment is involved in autophagosome maturation and lysosomal degradation in BMDMs infected with T. gondii. RES significantly enhanced the intracellular colocalisation of LC3-positive autophagic vacuoles with T. gondii-containing PVs (Figure 3D) and autophagolysosomal fusion (Figure 3E), which was significantly decreased in mSirt1-deficient BMDMs. Therefore, SIRT1 mediated RES-induced autophagy activation and antiparasitic responses in primary macrophages.
2.4. SIRT1 Suppresses T. gondii-Induced FoxO1 Activation Via the PI3K/AKT Signalling Pathway in T. gondii Infection
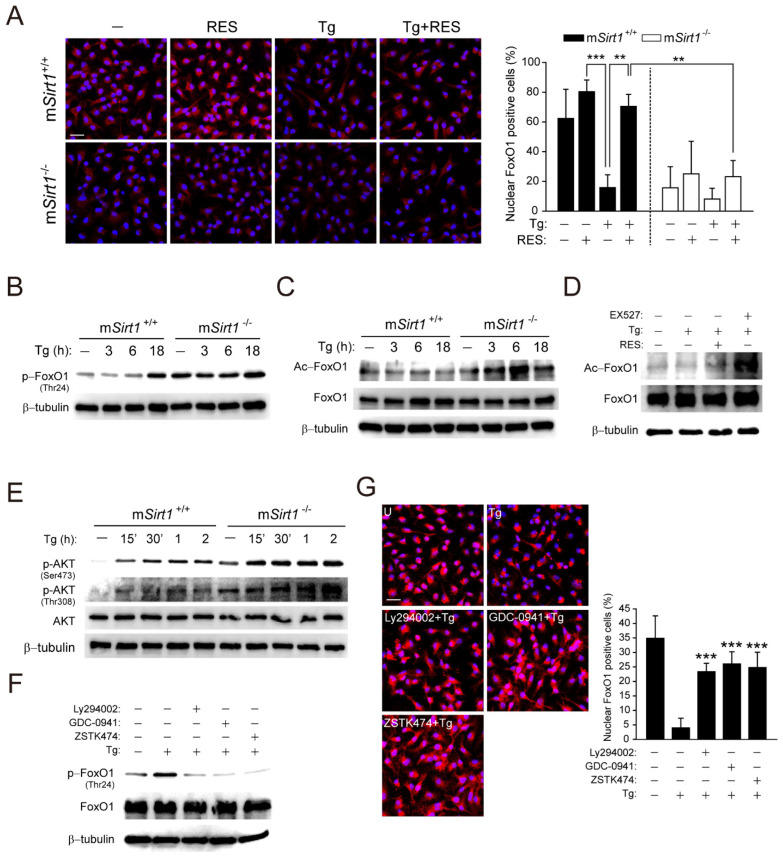
SIRT1 is involved in FoxO1-mediated autophagy regulation, which has been implicated in tissue homeostasis, cellular stress, carcinogenesis, and immunity [18,26,27]. To examine the effect of SIRT1 on FoxO1 activity during T. gondii infection, we evaluated cytosolic translocation, phosphorylation, and acetylation in mSirt1+/+ and mSirt1−/− BMDMs (Figure 4A–C). Toxoplasma gondii infection promoted FoxO1 cytoplasmic translocation in mSirt1+/+ BMDMs, but this was abolished by post-treatment with RES (Figure 4A). Moreover, the proportion of nuclear FoxO1-positive cells in normal mSirt1−/− BMDMs was similar to that in mSirt1+/+ BMDMs infected with T. gondii. The inhibition by RES of T. gondii-induced FoxO1 cytosolic translocation was not observed in mSirt1−/− BMDMs. Furthermore, T. gondii-induced phosphorylation (Figure 4B) and acetylation (Figure 4C) of FoxO1 were enhanced in mSirt1−/− compared to mSirt1+/+ BMDMs. Similarly, stimulation with EX527, a selective SIRT1 inhibitor, increased FoxO1 acetylation in BMDMs infected with T. gondii, but stimulation with RES had no effect (Figure 4D).
Figure 4.
SIRT1 deficiency leads to the excessive activation of PI3K/AKT-mediated FoxO1 upon T. gondii infection. (A) BMDMs from mSirt1+/+ and mSirt1−/− mice were infected with a RH strain (MOI = 1, for 2 h) and then further stimulated with RES (10 µM) for 18 h. Cells were immunostained using an anti-FoxO1 antibody (Alexa Fluor 568-conjugated goat anti-rabbit IgG, red) and DAPI for nuclei (blue). Cells were then subjected to immunofluorescence microscopy. Representative images (left) and quantitative data (right) showing the cytoplasmic translocation of FoxO1 are from one representative of 3 independent samples, with each experiment containing a minimum of 50 cells scored in 3 random fields. Scale bar = 25 µm. (B,C,E) BMDMs from mSirt1+/+ and mSirt1−/− mice were infected with T. gondii for the indicated time periods. The phosphorylation (for B) and acetylation (for C) of FoxO1 or AKT phosphorylation (Ser473 and Thr308, for E) were determined by immunoblot analysis. (D) BMDMs were infected with T. gondii for 18 h in the presence of RES (10 µM) or EX527 (4 µM). The level of FoxO1 acetylation was evaluated by immunoblot analysis. (F,G) BMDMs were infected with T. gondii for 18 h in the presence of LY294002 (10 µM), GDC0941 (250 nM), or ZSTK474 (10 nM). (F) The level of FoxO1 phosphorylation was evaluated by immunoblot analysis, and total protein was determined by monitoring β-tubulin (B–F), AKT (E), or FoxO1 (C,D,F) as a loading control. (G) Immunofluorescence microscopy analysis was assessed to determine the nuclear and cytoplasmic localisation of FOXO1, as described in Figure 4A. Scale bar = 25 µm. Data are representative of three independent experiments and are presented as means ± SD. ** p < 0.01 and *** p < 0.001, compared with mSirt1+/+ cells (for A) or control cells (for G) (two-tailed Student’s t-test). Tg, Toxoplasma gondii; RES, resveratrol.
AKT-induced phosphorylation of FoxO1 promotes its exclusion from the nucleus, leading to attenuated transcription of genes associated with autophagy [27,28]. Toxoplasma gondii rapidly induced the phosphorylation of AKT (Ser473 and Thr308) within 15 min in mSirt1+/+ BMDMs, and the expression level was greater in mSirt1−/− BMDMs (Figure 4E). Toxoplasma gondii-induced FoxO1 phosphorylation (Figure 4F) and cytosolic translocation (Figure 4G) and AKT phosphorylation (Figure S1) were attenuated by selective inhibitors of PI3K/AKT signalling, such as Ly294002, GDC-0941, or ZSTK474. These results indicate that a deficiency of myeloid SIRT1 promotes the T. gondii-induced hyperactivation of PI3K/AKT signalling, leading to impaired FoxO1 activation in primary murine macrophages.
2.5. The CaMKK2-, but Not the LKB1-, Dependent AMPK Signalling Pathway Contributes to the Activation of SIRT1-Mediated Autophagy and anti-Toxoplasma Effects in Primary Murine Macrophages
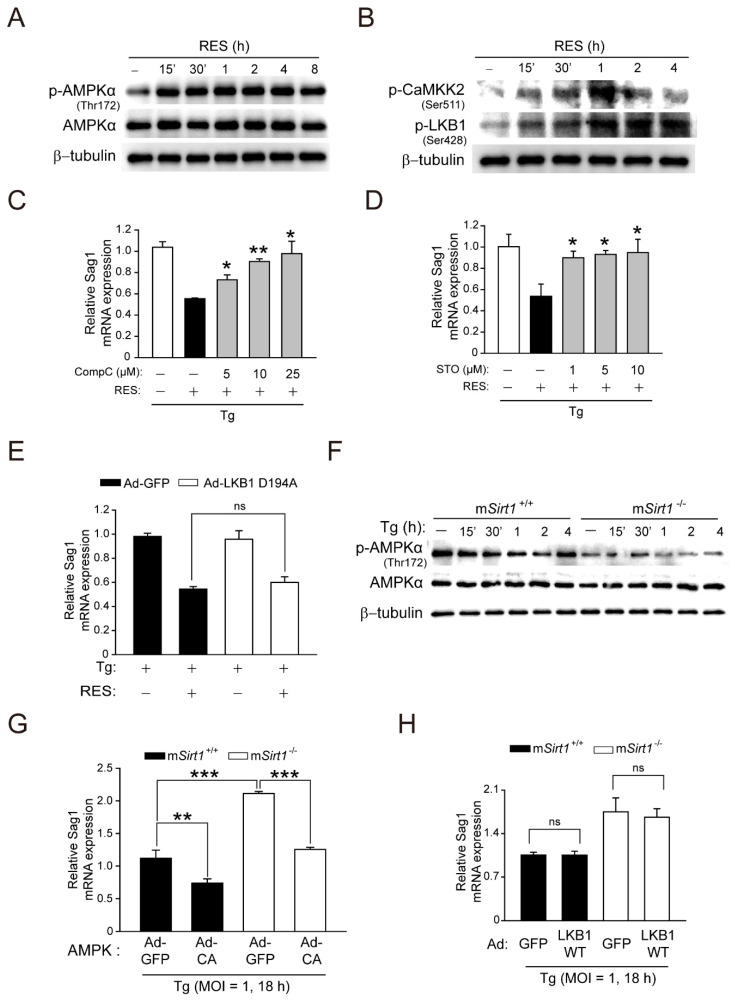
CaMKK2-dependent AMPK signalling is essential for the docosahexaenoic acid (DHA)-induced activation of antimicrobial autophagy in T. gondii infection [13]. Importantly, SIRT1 and AMPK regulate each other’s activities, an imbalance in which is implicated in human diseases, such as cardiovascular disease, type 2 diabetes, inflammatory disease, and cancer [29]. We investigated whether AMPK is required for SIRT1-mediated autophagy activation and anti-Toxoplasma responses in primary macrophages. RES activated AMPKα within 15 min, an effect sustained for 8 h (Figure 5A). Moreover, the phosphorylation of CaMKK2 (Ser511) and LKB1 (Ser428), upstream kinases of AMPKα, was increased within 15 min of RES stimulation, which peaked at 1 h, in BMDMs (Figure 5B). Interestingly, the pretreatment of BMDMs with dorsomorphin compound C (CompC, a pharmacological AMPK inhibitor) or STO-609 (a selective inhibitor of CaMKK) significantly attenuated the RES-mediated increase in LC3 aggregates (Figure S2A). Moreover, the pretreatment of T. gondii-infected BMDMs with CompC (Figure 5C) or STO-609 (Figure 5D) significantly increased the mRNA level of T. gondii Sag1. However, the adenoviral transduction of a catalytically inactive form of LKB1 (Ad-LKB1 D194A; aspartic acid to alanine at residue 194) did not affect the RES-induced decrease in the T. gondii Sag1 mRNA level (Figure 5E) or the RES-induced LC3-II protein level (Figure S2B).
Figure 5.
CaMKK2-dependent AMPK signalling is crucial for the activation of SIRT1-mediated antiparasitic responses against Toxoplasma infection in primary murine macrophages. (A,B) BMDMs were stimulated with RES (10 µM) for the various time periods, and then immunoblot analysis was assessed to evaluate the phosphorylation of AMPKα (for A) or CaMKK2 and LKB1 (for B). (C,D) BMDMs were pretreated with increasing concentrations of CompC (5, 10, or 25 μM, for C) or STO (1, 5, or 10 μM, for D) for 45 min, then infected with T. gondii RH strain (MOI = 1) for 18 h in the presence or absence of RES. Sag1 mRNA expression was examined by qPCR analysis. (E) BMDMs transduced with Ad-GFP, Ad-LKB1 WT, or Ad-LKB1 D194A for 36 h (MOI of 10), respectively, were infected with a T. gondii RH strain (MOI = 1) for 2 h, then further incubated with RES (10 µM) for 22 h. Sag1 mRNA expression was evaluated by qPCR analysis. (F) BMDMs from mSirt1+/+ and mSirt1−/− mice were infected with T. gondii for various time periods. Cell lysates were subjected to immunoblot analysis using antibodies against p-AMPKα (Thr172), AMPKα, and β-tubulin. (G,H) BMDMs from mSirt1+/+ and mSirt1−/− mice were transduced for 36 h with adenovirus-expressing GFP (Ad-GFP, for G,H), constitutively active AMPK (Ad-CA, for G), or WT LKB1 (Ad-LKB1 WT, for H), at a MOI of 10, and each cell then was infected with a T. gondii RH strain (MOI = 1) for 18 h. The mRNA expression of the Sag1 gene was evaluated by real-time qPCR analysis. Data are representative of three independent experiments and are presented as means ± SD. * p < 0.05, ** p < 0.01, *** p < 0.001 (two-tailed Student’s t-test). Tg, Toxoplasma gondii; RES, resveratrol; CompC, Compound C; STO, STO-609; adenovirus-expressing GFP, Ad-GFP; adenovirus-expressing WT LKB1, Ad-LKB1 WT; adenovirus-expressing LKB1 D194A mutant, Ad-LKB1 D194A; ns, not significant.
We next examined whether a myeloid SIRT1 deficiency affects AMPK activation in response to T. gondii infection. Sirt1−/− BMDMs exhibited the reduced phosphorylation of AMPKα under normal conditions and from 15 min to 4 h after T. gondii infection compared to mSirt1+/+ BMDMs (Figure 5F). The overexpression of adenovirus containing a constitutively active AMPK reduced the mRNA level of T. gondii Sag1 in mSirt1+/+ and mSirt1−/− BMDMs (Figure 5G). However, adenoviral transduction with WT LKB1 in mSirt1+/+ and mSirt1−/− BMDMs did not alter the mRNA level of T. gondii Sag1. Collectively, these results implicate CaMKK2-AMPK, but not LKB1, signalling in SIRT1-mediated autophagy activation and anti-Toxoplasma responses.
2.6. SIRT1 Positively Regulates the Activity of FoxO3a Via AMPK Signalling Pathways
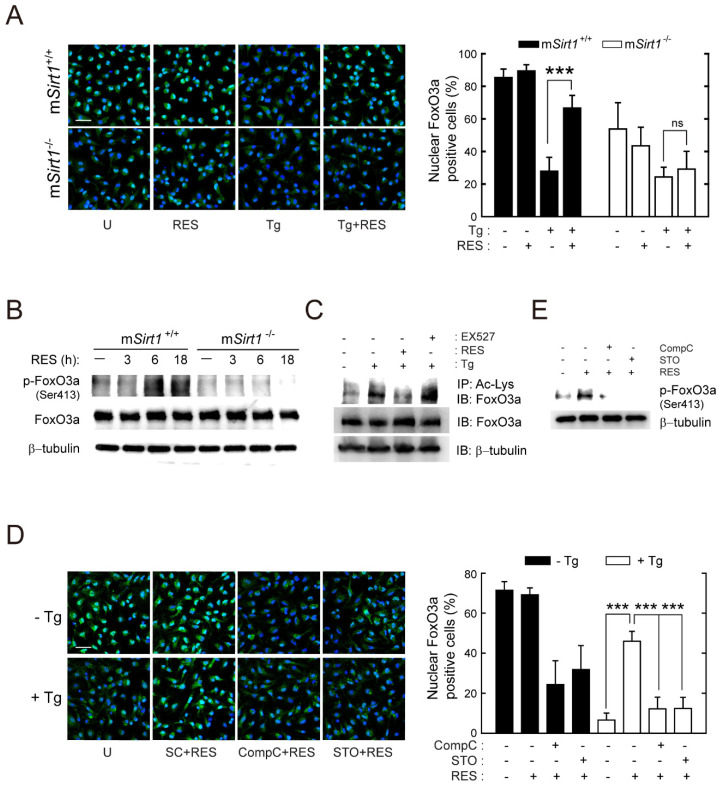
The AMPK-mediated phosphorylation of FoxO3a transactivation domain sites results in the induction of FoxO3a nuclear accumulation and, subsequently, the transcription of target genes associated with autophagy activation [27,30]. To examine the role of SIRT1 in the regulation of FoxO3a activity during T. gondii infection, we enumerated cells with nuclear FoxO3a in RES-stimulated mSirt1+/+ and mSirt1−/− BMDMs. Toxoplasma gondii infection induced the cytosolic translocation of FoxO3a in mSirt1+/+ BMDMs, which was suppressed by RES (Figure 6A). Although FoxO3a cytoplasmic translocation after T. gondii infection in mSirt1−/− BMDMs was similar to that in normal cells, no inhibitory effect of RES was observed (Figure 6A). Indeed, RES induced the phosphorylation of FoxO3a (Ser413) within 6 h in mSirt1+/+ BMDMs, but not in mSirt1-deficient BMDMs (Figure 6B). Moreover, T. gondii-induced FoxO3a acetylation was abolished by post-treatment with RES but was enhanced by the selective SIRT1 inhibitor EX-527 (Figure 6C).
Figure 6.
RES treatment promotes the activation of FoxO3a, which is mediated by SIRT1 and AMPK. (A) BMDMs from mSirt1+/+ and mSirt1−/− mice were infected with the RH strain (MOI = 1, for 2 h) and then further stimulated with RES (10 µM) for 18 h. Cells were fixed and immunostained with an anti-FoxO3 antibody (Alexa Fluor 488-conjugated goat anti-rabbit IgG, green) and DAPI for nuclei (blue). Cells were then subjected to immunofluorescence microscopy. Representative images (left) and quantitative data (right) showing the cytoplasmic translocation of FoxO3a were obtained from one representative of 3 independent samples, with each experiment containing a minimum of 50 cells scored in 7 random fields. Scale bar = 25 µm. (B) BMDMs from mSirt1+/+ and mSirt1−/− mice were stimulated with RES (10 µM) for the indicated time periods. The phosphorylation of FoxO3 (Ser413) was determined by immunoblot analysis. (C) BMDMs were infected with T. gondii for 18 h in the presence of RES (10 µM) or EX527 (4 µM). Cell lysates were subjected to immunoprecipitation analysis using an anti-acetylated-lysine antibody (Ac-Lys), and then the immunoprecipitates were analysed by Western blot using an anti-FoxO3a antibody. Cell lysates were harvested and subjected to Western blot analysis for FoxO3a and β-tubulin, as loading controls. (D) BMDMs were pretreated with Compound C (25 µM) or STO-609 (10 µM) for 45 min, then infected with RH strain (MOI = 1) for 18 h in the presence or absence of RES (10 µM). Cells were fixed, immunostained, and quantified as described in Figure 6A. Representative images (left) and quantitative data (right) showing the cytoplasmic translocation of FoxO3a. Scale bar = 25 µm. (E) BMDMs were pretreated with Compound C (25 µM) or STO-609 (10 µM, 45 min) and then stimulated with RES (10 µM, 18 h). The phosphorylation of FoxO3 (Ser413) was determined by immunoblot analysis. Data are representative of three independent experiments and are presented as means ± SD. *** p < 0.001 (two-tailed Student’s t-test). Tg, Toxoplasma gondii; RES, resveratrol; CompC, Compound C; STO, STO-609; ns, not significant.
Next, we investigated the involvement of AMPK signalling in SIRT1-mediated FoxO3a activation. Confocal images showed that RES-induced FoxO3a nuclear accumulation in T. gondii-infected cells was abolished by CompC or STO-609 (Figure 6D). Furthermore, CompC or STO-609 pretreatment significantly decreased FoxO3a nuclear accumulation (Figure 6D) and FoxO3a phosphorylation (Figure 6E) in BMDMs treated with RES (without infection by T. gondii). These findings indicate that CaMKK2/AMPK activation is essential for SIRT1-mediated FoxO3a activation during T. gondii infection.
3. Discussion
SIRT proteins are important players in human health and disease by regulating the cellular stress response [17,25]. Neospora caninum infection of caprine endometrial epithelial cells leads to mitochondrial dysfunction by downregulating SIRT1 expression, promoting the proliferation of intracellular protozoa [31]. In addition, SIRT3 activates the antimicrobial response to promote host survival by coordinating mitochondrial function and autophagy activation in Mycobacterium tuberculosis infection [32]. However, the mechanisms by which SIRT1 regulates the immune response to T. gondii are unclear.
Obligate intracellular parasites have several strategies to survive and proliferate in host immune and non-immune cells. During T. gondii infection, host-cell signalling cascades that inhibit autophagy targeting of PV can be activated, thereby preventing the constitutive autophagy-mediated lysosomal degradation of PV [14]. Therefore, drug candidates or endogenous factors capable of inducing autophagy have therapeutic potential for Toxoplasma infection. The correlation between SIRT1 activity and autophagy induction has been described [17]. We found that the pharmacological activation of SIRT1 by 4-HBA resulted in autophagy induction, which restricts the growth of intracellular parasites in primary murine macrophages [24]. In this study, a myeloid Sirt1 deficiency increased susceptibility to Toxoplasma infection in mice and primary murine macrophages (Figure 1). Moreover, SIRT1 activation by RES suppressed intracellular parasite growth (Figure 2), the formation of autophagy vacuoles, the lipidation of the autophagy protein LC3, and autophagosome–lysosome fusion (Figure 3). However, the effect of RES on T. gondii infection was attenuated in mSirt1−/− BMDMs. Therefore, endogenous SIRT1 is important for the activation of a host immune response by regulating autophagy activity during Toxoplasma infection. Additional studies on the role of SIRT1 in various infection routes and strains of T. gondii and in immune cells important in Toxoplasma infection (e.g., neutrophils and dendritic cells) are needed.
SIRT1 modulates autophagy activity by deacetylating the transcription factors that regulate the expression of genes associated with the autophagy machinery, as well as by directly deacetylating Atg5, 7, and 8 in the cytoplasm [17,27]. FoxO transcription factors regulate autophagy at multiple levels, including transcription, by binding to the promoters of autophagy genes [33], directly interacting with autophagy proteins in the cytoplasm [34], and epigenetic modulation [35]. The transactivation activity of FoxO proteins is modulated by post-translational modification. Indeed, the AKT-dependent phosphorylation and acetylation of FoxO proteins promote their nuclear exclusion, whereas their AMPK-dependent phosphorylation and deacetylation lead to nuclear accumulation [27]. Herein, T. gondii infection of mSirt1+/+ BMDMs slightly increased the nuclear exclusion, phosphorylation, and acetylation of FoxO1, as well as the phosphorylation of AKT, compared to mSirt1−/− BMDMs. Moreover, T. gondii-induced nuclear exclusion of FoxO1 was attenuated by RES and PI3K/AKT inhibitor pretreatment in primary macrophages (Figure 4). Nuclear and cytoplasmic FoxO1 can induce autophagy, for which SIRT1 is essential. However, the mechanism of autophagy activation and cell fate (survival or autophagic death) differs depending on FoxO1 cellular localisation [17]. Specifically, the RES-induced pharmacological activation of SIRT1 in diabetic mice promoted the deacetylation of FoxO1, resulting in autophagy activation by binding to the Rab7 promoter [36]. By contrast, stresses such as starvation and oxidation strongly induce FoxO1 acetylation by p300 histone acetyltransferase and its dissociation from SIRT1. Furthermore, FoxO1 binding to Atg7 in the cytoplasm results in autophagic cell death [34,37]. Therefore, our results suggest that SIRT1 suppresses aberrant post-translational phosphorylation and acetylation and the nuclear exclusion of FoxO1 via PI3K-dependent AKT signalling during T. gondii infection, ultimately inducing autophagy.
Previous studies demonstrated that AMPK is an essential intermediator in the regulation of cellular energy homeostasis and the activation of host defenses against intracellular pathogens [38,39]. In Toxoplasma infection, CaMKK2-dependent AMPK signalling is required for CD40-mediated autophagic clearance [40]. Omega-3 polyunsaturated fatty acids promote the CaMKK2/AMPK-mediated induction of autophagy, which is crucial for protection against T. gondii infection [13]. In this study, RES rapidly activated CaMKK2-dependent AMPK signalling, which is required for autophagy activation and the suppression of intracellular parasitic growth. Moreover, T. gondii-induced AMPK phosphorylation was decreased in mSirt1−/− compared to mSirt1+/+ BMDMs. These data indicate that CaMKK2-dependent AMPK signalling is essential to the SIRT1-mediated activation of antiparasitic autophagy (Figure 5 and Figure S2). Because AMPK signalling is associated with the SIRT1-FoxO3 axis in autophagy activation and other physiological processes, we investigated the roles of these mediators in Toxoplasma infection. Toxoplasma gondii infection promoted the nuclear exclusion of FoxO3a in primary macrophages, but this effect was significantly inhibited by RES. Additionally, RES induced FoxO3a phosphorylation within 6 h in mSirt1+/+ BMDMs, but the effect was negligible in mSirt1−/− BMDMs. Importantly, the RES-induced nuclear accumulation of FoxO3a was suppressed by CompC or STO-609 pretreatment in primary macrophages, irrespective of T. gondii infection. AMPK activates FoxO3 phosphorylation at Ser413, promoting its stabilisation and nuclear accumulation and leading to the transcriptional or epigenetic activation of autophagy genes [35,41]. Our results suggest a correlation between AMPK and FoxO3a in the activation of the SIRT1-mediated antiparasitic response (Figure 6).
In conclusion, myeloid-specific SIRT1 activates autophagy and the host response to T. gondii infection by controlling FoxO1 and FoxO3a transactivation activity via PI3K/AKT and CaMKK2/AMPK signalling, respectively. Moreover, the RES-dependent pharmacological activation of SIRT1 promotes antiparasitic autophagy, restricting the proliferation of T. gondii in primary murine macrophages (Figure 7). Our findings implicate SIRT1, in conjunction with the PI3K/AKT-FoxO1 and CaMKK2/AMPK-FoxO3a axis, in the activation of antiparasitic autophagy and will facilitate the development of novel therapeutics for Toxoplasma infection.
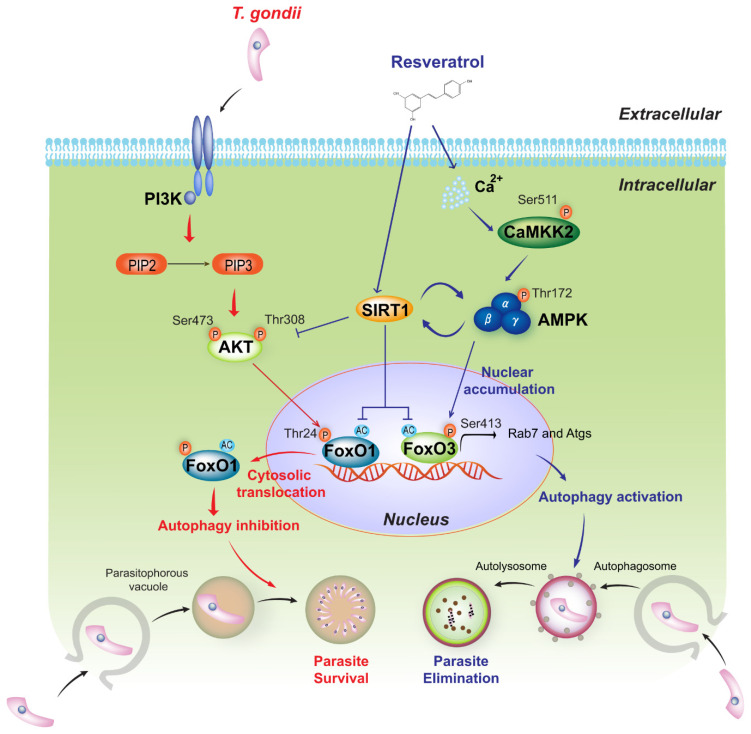
Figure 7.
Essential function and molecular mechanism of SIRT1 in modulating the host immune response to T. gondii infection. In macrophages, myeloid-specific SIRT1 increases autophagy activity during T. gondii infection, thereby eliminating intracellular T. gondii by inducing the colocalisation of parasitophorous vacuoles with autophagosomes/lysosomes. As a key regulatory mechanism of autophagy, SIRT1 attenuates the acetylation of the transcription factor FoxO1 and its PI3K/AKT-dependent phosphorylation (Thr24) in response to T. gondii, thus regulating FoxO1 transactivation activity by preventing its nuclear exclusion. Moreover, SIRT1 promotes FoxO3a deacetylation and its CaMKK2/AMPK-dependent phosphorylation (Ser413), leading to nuclear accumulation and the transactivation of FoxO3a. Pharmacological activation of SIRT1 by RES induces antiprotozoal autophagy by regulating FoxO1 and FoxO3a activity via the PI3K/AKT and CaMKK2/AMPK signalling pathways, respectively.
4. Materials and Methods
4.1. Mice and Ethics Statement
C57BL/6 and BALB/c mice were purchased from Koatech (Gyeonggi-do, Korea), and mSirt1−/− mice were kindly provided by Prof. Byung-Hyun Park (Department of Biochemistry, Chonbuk National University Medical School, Korea). Animal-related experimental procedures were approved by the Institutional Animal Care and Use Committee, Chungnam National University (202012A-CNU-200; Daejeon, Korea), and followed National Institutes of Health guidelines. Animals were housed in sawdust-covered cages in an air-conditioned environment under a 12 h light/dark cycle (5 animals per cage) with free access to standard rodent food and water. Animal breeding was provided by the staffs of IACUC and the Animal Core Facility under the guidance of supervisors who were certified animal technologists. Veterinary care was provided by the IACUC faculty and veterinary residents at the Chungnam National University School of Medicine.
4.2. Preparation of Cell and Parasite
BMDMs were differentiated over 5–7 days in a medium with a recombinant macrophage colony-stimulating factor (M-CSF), as described previously [42]. The culture medium consisted of Dulbecco’s modified Eagle’s medium (DMEM; Welgene, Gyeongsan, Korea) supplemented with 10% fetal bovine serum (FBS, Gibco BRL, Waltham, MA, USA) and 1% antibiotic–antimycotic (Gibco™ antibiotic–antimycotic (100X); Gibco BRL, Waltham, MA, USA). Human retinal pigment epithelial ARPE-19 cells (American Type Culture Collection, Manassas, VA, USA) were cultured in DMEM/F-12 (Welgene, Gyeongsan, Korea) with 10% FBS and 1% antibiotic–antimycotic.
The T. gondii RH strain was grown in ARPE-19 cells (MOI = 5) for 2–3 days at 37 °C and 5% CO2. Host cells and parasites were washed with phosphate-buffered saline (PBS) after spontaneous host cell disruption. Protozoans were suspended in cold medium and passed through a 26-gauge needle and a 5.0 μm pore filter (Millipore, Billerica, MA, USA). The GFP-RH strain was kindly provided by Dr. Yoshifumi Nishikawa (Obihiro University of Agriculture and Veterinary Medicine, Japan). The T. gondii ME49 strain was obtained from the brain tissue of BALB/c mice that infected 50 cysts and were maintained every 3 weeks.
4.3. Reagents and Antibodies
Resveratrol (RES; R5010), 3-methyladenine (3-MA; M9281), EX-527 (E7034), wortmannin (WM; W1628), and STO-609 were purchased from Sigma-Aldrich (Saint Louis, MO, USA), and Compound C (171260) was purchased from Calbiochem (San Diego, CA, USA). SYBR Green reagents were purchased from Applied Biosystems (Waltham, MA, USA). Dimethyl sulfoxide (DMSO; Sigma-Aldrich, Saint Louis, MO, USA) was used at 0.1% (v/v) as a solvent control.
Specific antibodies against AMPKα (2532), FoxO3a (75D8), FoxO1 (C29H4), phospho-Fox01 (9464), phospho-AMPK (2535), phospho-CAMKK2 (12818), phospho-LKB1 (3482), and acetylated-Lysine (9441) were purchased from Cell Signaling (Danvers, MA, USA). Phospho-Fox03a (AF2343) was purchased from Affinity Biosciences. Anti-LC3 (L8918) was obtained from Sigma-Aldrich. Anti-β-tubulin (Ab6046) and anti-SIRT1 (Ab28170) were purchased from Abcam (Burlingame, CA, USA). Anti-TP3 (sc-52255) and anti-LAMP1 (sc-17768) were purchased from Santa Cruz (Dallas, TX, USA). All other reagents were obtained from Sigma-Aldrich, unless otherwise indicated.
4.4. Immunoprecipitation and Western Blot Analysis
For immunopreciptiation, cells were washed twice with ice-cold PBS and lysed in NP-40 buffer (20 mM Tris (pH 7.5), 135 mM NaCl, 2 mM EDTA, 10% glycerol, 1% NP-40) with protease inhibitors and phosphatase inhibitor cocktail (Roche) for 30 min. Each lysate were centrifugated at 15,000× g at 4 °C for 15 min and proteins (500 mg) were immunoprecipitated using specific antibodies. Protein G sepharose beads (GE Healthcare, Piscataway, NJ, USA) were then included in each sample and incubated for 2 h at 4 °C. Samples were subjected to Western blot analysis.
For Western blot analysis, cells were collected and lysed in PRO-PREP (iNtRON Biotechnology) with various protease inhibitors. Protein concentrations were evaluated with a BCA assay kit. Proteins (30 µg) were immediately heated at 100 °C for 5 min, separated by SDS-PAGE, transferred to polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA). Membranes were developed using a chemiluminescence assay kit (Millipore) and analysed by Alliance Mini HD6 (UVitec, Cambridge, MA, USA).
4.5. RNA Extraction, Semi-Quantitative RT-PCR, and Real-Time Quantitative PCR
Total RNA was isolated using Trizol reagent (Invitrogen, Waltham, MA, USA) according to the manufacturer’s instructions, and semi-quantitative RT-PCR and real-time quantitative PCR were performed as described previously [42]. Briefly, cDNA was synthesised using Reverse Transcriptase Premix and amplified using SolgTM 2X Taq PCR Pre-Mix (Solgent, Daejeon, Korea). The products were resolved on 1% agarose gel for semi-quantitative RT-PCR or were analysed using the StepOne™ and StepOnePlus™ Software (Applied Biosystems, Waltham, MA, USA) for real-time quantitative PCR. All primers used in this study were follows: Sag1 (forward: 5′-ATCGCCTGAGAAGCATCACT-3′; reverse: 5′- GCGAAAATGGAAACGTGACT-3′), β-actin (forward: 5′- TCATGAAGTGTGACGTTGACATCCGT-3′; reverse: 5′-CCTAGAAGCATTTGCGGTGCACGATG-3′).
4.6. Experimental Murine Toxoplasmosis Model
To establish an in vivo toxoplasmosis model, mice were intraperitoneally (i.p.) infected with 40 cysts of strain ME49. Cysts in the mice brain homogenate were obtained at 3 weeks. The number of tissue cysts was counted under a light microscopy. To analyse the mRNA expression of the Sag1 gene, cDNA synthesis was carried out using Reverse Transcriptase Premix. cDNA was subjected to amplification using a real-time PCR instrument.
4.7. Adenovirus Production
Adenoviral vectors were used for target gene overexpression or mutation. Ad-GFP, Ad-DN-AMPK, and Ad-CA-AMPK were kindly provided by Dr. Hueng-Sik Choi (Chonnam National University, Gwangju, Korea). Ad-LKB1 and Ad-LKB1 D194A were kindly provided by Dr. Don-kyu Kim (Chonnam National University) [43]. Large-scale adenovirus production was achieved as described previously [42,44]. Briefly, HEK293A cells were transfected with adenovirus (MOI = 10), and the adenovirus-containing cells and media were subjected to three freezing (–196 °C) and thawing (37 °C) cycles. After centrifugation at 3000 rpm for 15 min, Ad-DN-AMPK and Ad-CA-AMPK were concentrated by CsCl gradient ultracentrifugation, and Ad-DN-LKB1 and Ad-LKB1 were purified using Adeno-X Maxi Purification Kit (Clontech) per the manufacturer’s protocol. Then, the viral titers were evaluated using an Adeno-X Rapid Titer Kit (Clontech, Mountain View, CA, USA).
4.8. Quantification of Intracellular T. gondii
BMDMs were seeded on 22-mm glass coverslips and then infected with the GFP-RH strain for the indicated time periods. The coverslips were washed using warmed PBS, fixed with 4% paraformaldehyde in PBS for 10 min, and permeabilised with 0.25% Triton X-100 in PBS for 10 min. Texas Red®-X phalloidin (Life Technologies Corporation, Carlsbad, CA, USA) for cytosol and 4′6-diamidino-2-phenylindole (DAPI, Sigma) for nucleus were used. Cover slides were analysed by confocal laser scanning microscopy (Leica TCS SP8, Leica microsystems).
4.9. Immunofluorescence Analyses
Immunofluorescence analysis of endogenous LC3 puncta and nuclear translocation analysis of FoxO1 and FoxO3 was performed as described previously [45]. Briefly, after fixation and permeabilisation, cells in coverslips were stained with a specific antibody for LC3 (1:400; MBL International, PM036), FoxO3a, or FoxO1 for 2 h at room temperature. After washing excess primary antibody with PBS, cells were incubated with Alexa Fluor 488-conjugated goat anti-rabbit IgG (molecular probes) or Alexa Fluor 568-conjugated goat anti-rabbit IgG (molecular probes) for 2 h.
4.10. Analysis of Autophagosome Maturation and Lysosomal Fusion with PV
For analysis, the autophagosome maturation and lysosomal fusion with PV, BMDMs were cultured on 22 mm glass coverslips and infected with the GFP-RH strain. The cells were fixed with 4% paraformaldehyde in PBS for 10 min and permeabilised with 0.25% Triton X-100 in PBS for 15 min. Cells in coverslips then were stained with specific antibody for LC3 (1:400; MBL International, PM036) and LAMP1 (1:400) overnight at 4 °C. After washing the samples with PBS, it were incubated with Alexa Fluor 488-conjugated goat anti-rabbit IgG or Alexa Fluor 594-conjugated goat anti-rabbit IgG for 2 h. After mounting, fluorescence images were acquired with a confocal laser-scanning microscope.
4.11. Statistical Analysis
A two-tailed Student’s t test was used to analyse differences between independent experimental data (means ± standard deviation [46] or the means ± standard error of the mean [47]). Differences were deemed significant at p value under 0.05.
Acknowledgments
We thank Don-Kyu Kim and Hueng-Sik CHoi (Chonnam National University) for providing the adenoviral construct expressing LKB1 WT, LKB1 D194A, CA-AMPK, and DN-AMPK; Eun-Kyeong Jo (Chungnam National University) for technical assistance; and Hae-Sung Nam (Chungnam National University) for statistics analysis.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms232113578/s1.
Author Contributions
Conceptualisation, B.-H.P. and J.-M.Y.; formal analysis, Y.-H.L., J.K. and J.L.; funding acquisition, E.-K.J. and J.-M.Y.; investigation, J.K., J.L., J.-H.L. and Y.M.C.; methodology, J.L., J.K., H.C., H.-D.C. and J.-H.L.; project administration, J.-M.Y.; resources, B.-H.P.; supervision, G.-H.C. and Y.-H.L.; visualisation, J.L. and J.-M.Y.; writing—original draft, J.-M.Y.; writing—review and editing, B.-H.P. and J.-M.Y. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted according to the guidelines of National Institutes of Health and approved by the Institutional Animal Care and Use Committee, Chungnam National University (202012A-CNU-200; Daejeon, Korea). Animal breeding and veterinary care was provided by the staffs of IACUC and the Animal Core Facility under the guidance of supervisors who were certified animal technologists.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (NRF-2019R1C1C1004431, NRF-2017R1A5A2015385, and NRF-2022R1C1C1004346).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Elmore S.A., Jones J.L., Conrad P.A., Patton S., Lindsay D.S., Dubey J.P. Toxoplasma gondii: Epidemiology, feline clinical aspects, and prevention. Trends Parasitol. 2010;26:190–196. doi: 10.1016/j.pt.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 2.Harker K.S., Ueno N., Lodoen M.B. Toxoplasma gondii dissemination: A parasite’s journey through the infected host. Parasite Immunol. 2015;37:141–149. doi: 10.1111/pim.12163. [DOI] [PubMed] [Google Scholar]
- 3.Montoya J.G., Liesenfeld O. Toxoplasmosis. Lancet. 2004;363:1965–1976. doi: 10.1016/S0140-6736(04)16412-X. [DOI] [PubMed] [Google Scholar]
- 4.Sasai M., Pradipta A., Yamamoto M. Host immune responses to Toxoplasma gondii. Int. Immunol. 2018;30:113–119. doi: 10.1093/intimm/dxy004. [DOI] [PubMed] [Google Scholar]
- 5.Luft B.J., Hafner R., Korzun A.H., Leport C., Antoniskis D., Bosler E.M., Bourland D.D., 3rd, Uttamchandani R., Fuhrer J., Jacobson J., et al. Toxoplasmic encephalitis in patients with the acquired immunodeficiency syndrome. Members of the ACTG 077p/ANRS 009 Study Team. N. Engl. J. Med. 1993;329:995–1000. doi: 10.1056/NEJM199309303291403. [DOI] [PubMed] [Google Scholar]
- 6.Mariuz P., Bosler E.M., Luft B.J. Toxoplasma pneumonia. Semin. Respir. Infect. 1997;12:40–43. [PubMed] [Google Scholar]
- 7.Sacks D., Sher A. Evasion of innate immunity by parasitic protozoa. Nat. Immunol. 2002;3:1041–1047. doi: 10.1038/ni1102-1041. [DOI] [PubMed] [Google Scholar]
- 8.Buzoni-Gatel D., Werts C. Toxoplasma gondii and subversion of the immune system. Trends Parasitol. 2006;22:448–452. doi: 10.1016/j.pt.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 9.Denkers E.Y. From cells to signaling cascades: Manipulation of innate immunity by Toxoplasma gondii. FEMS Immunol. Med. Microbiol. 2003;39:193–203. doi: 10.1016/S0928-8244(03)00279-7. [DOI] [PubMed] [Google Scholar]
- 10.Levine B., Mizushima N., Virgin H.W. Autophagy in immunity and inflammation. Nature. 2011;469:323–335. doi: 10.1038/nature09782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shibutani S.T., Saitoh T., Nowag H., Munz C., Yoshimori T. Autophagy and autophagy-related proteins in the immune system. Nat. Immunol. 2015;16:1014–1024. doi: 10.1038/ni.3273. [DOI] [PubMed] [Google Scholar]
- 12.Zhu W., Li J., Pappoe F., Shen J., Yu L. Strategies Developed by Toxoplasma gondii to Survive in the Host. Front. Microbiol. 2019;10:899. doi: 10.3389/fmicb.2019.00899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi J.W., Lee J., Lee J.H., Park B.J., Lee E.J., Shin S., Cha G.H., Lee Y.H., Lim K., Yuk J.M. Omega-3 Polyunsaturated Fatty Acids Prevent Toxoplasma gondii Infection by Inducing Autophagy via AMPK Activation. Nutrients. 2019;11:2137. doi: 10.3390/nu11092137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Subauste C.S. Recent Advances in the Roles of Autophagy and Autophagy Proteins in Host Cells During Toxoplasma gondii Infection and Potential Therapeutic Implications. Front. Cell Dev. Biol. 2021;9:673813. doi: 10.3389/fcell.2021.673813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haigis M.C., Guarente L.P. Mammalian sirtuins—Emerging roles in physiology, aging, and calorie restriction. Genes Dev. 2006;20:2913–2921. doi: 10.1101/gad.1467506. [DOI] [PubMed] [Google Scholar]
- 16.Blander G., Guarente L. The Sir2 family of protein deacetylases. Annu. Rev. Biochem. 2004;73:417–435. doi: 10.1146/annurev.biochem.73.011303.073651. [DOI] [PubMed] [Google Scholar]
- 17.Ng F., Tang B.L. Sirtuins’ modulation of autophagy. J. Cell. Physiol. 2013;228:2262–2270. doi: 10.1002/jcp.24399. [DOI] [PubMed] [Google Scholar]
- 18.Hariharan N., Maejima Y., Nakae J., Paik J., Depinho R.A., Sadoshima J. Deacetylation of FoxO by Sirt1 Plays an Essential Role in Mediating Starvation-Induced Autophagy in Cardiac Myocytes. Circ. Res. 2010;107:1470–1482. doi: 10.1161/CIRCRESAHA.110.227371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gutierrez M.G., Munafo D.B., Beron W., Colombo M.I. Rab7 is required for the normal progression of the autophagic pathway in mammalian cells. J. Cell Sci. 2004;117:2687–2697. doi: 10.1242/jcs.01114. [DOI] [PubMed] [Google Scholar]
- 20.Kume S., Uzu T., Horiike K., Chin-Kanasaki M., Isshiki K., Araki S., Sugimoto T., Haneda M., Kashiwagi A., Koya D. Calorie restriction enhances cell adaptation to hypoxia through Sirt1-dependent mitochondrial autophagy in mouse aged kidney. J. Clin. Investig. 2010;120:1043–1055. doi: 10.1172/JCI41376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee I.H., Cao L., Mostoslavsky R., Lombard D.B., Liu J., Bruns N.E., Tsokos M., Alt F.W., Finkel T. A role for the NAD-dependent deacetylase Sirt1 in the regulation of autophagy. Proc. Natl. Acad. Sci. USA. 2008;105:3374–3379. doi: 10.1073/pnas.0712145105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang R., Xu Y., Wan W., Shou X., Qian J., You Z., Liu B., Chang C., Zhou T., Lippincott-Schwartz J., et al. Deacetylation of nuclear LC3 drives autophagy initiation under starvation. Mol. Cell. 2015;57:456–466. doi: 10.1016/j.molcel.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 23.Zhao H., Chen S., Gao K., Zhou Z., Wang C., Shen Z., Guo Y., Li Z., Wan Z., Liu C., et al. Resveratrol protects against spinal cord injury by activating autophagy and inhibiting apoptosis mediated by the SIRT1/AMPK signaling pathway. Neuroscience. 2017;348:241–251. doi: 10.1016/j.neuroscience.2017.02.027. [DOI] [PubMed] [Google Scholar]
- 24.Lee J., Choi J.W., Han H.Y., Kim W.S., Song H.Y., Byun E.B., Byun E.H., Lee Y.H., Yuk J.M. 4-Hydroxybenzaldehyde Restricts the Intracellular Growth of Toxoplasma gondii by Inducing SIRT1-Mediated Autophagy in Macrophages. Korean J. Parasitol. 2020;58:7–14. doi: 10.3347/kjp.2020.58.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chung S., Yao H., Caito S., Hwang J.W., Arunachalam G., Rahman I. Regulation of SIRT1 in cellular functions: Role of polyphenols. Arch. Biochem. Biophys. 2010;501:79–90. doi: 10.1016/j.abb.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lapierre L.R., Kumsta C., Sandri M., Ballabio A., Hansen M. Transcriptional and epigenetic regulation of autophagy in aging. Autophagy. 2015;11:867–880. doi: 10.1080/15548627.2015.1034410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng Z. The FoxO-Autophagy Axis in Health and Disease. Trends Endocrinol. Metab. 2019;30:658–671. doi: 10.1016/j.tem.2019.07.009. [DOI] [PubMed] [Google Scholar]
- 28.Tan S.H., Shui G., Zhou J., Shi Y., Huang J., Xia D., Wenk M.R., Shen H.M. Critical role of SCD1 in autophagy regulation via lipogenesis and lipid rafts-coupled AKT-FOXO1 signaling pathway. Autophagy. 2014;10:226–242. doi: 10.4161/auto.27003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruderman N.B., Xu X.J., Nelson L., Cacicedo J.M., Saha A.K., Lan F., Ido Y. AMPK and SIRT1: A long-standing partnership? Am. J. Physiol. Endocrinol. Metab. 2010;298:E751–E760. doi: 10.1152/ajpendo.00745.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chiacchiera F., Simone C. The AMPK-FoxO3A axis as a target for cancer treatment. Cell Cycle. 2010;9:1091–1096. doi: 10.4161/cc.9.6.11035. [DOI] [PubMed] [Google Scholar]
- 31.Tao D.L., Zhao S.S., Chen J.M., Chen X., Yang X., Song J.K., Liu Q., Zhao G.H. Neospora caninum infection induced mitochondrial dysfunction in caprine endometrial epithelial cells via downregulating SIRT1. Parasites Vectors. 2022;15:274. doi: 10.1186/s13071-022-05406-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim T.S., Jin Y.B., Kim Y.S., Kim S., Kim J.K., Lee H.M., Suh H.W., Choe J.H., Kim Y.J., Koo B.S., et al. SIRT3 promotes antimycobacterial defenses by coordinating mitochondrial and autophagic functions. Autophagy. 2019;15:1356–1375. doi: 10.1080/15548627.2019.1582743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Webb A.E., Brunet A. FOXO transcription factors: Key regulators of cellular quality control. Trends Biochem. Sci. 2014;39:159–169. doi: 10.1016/j.tibs.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao Y., Yang J., Liao W., Liu X., Zhang H., Wang S., Wang D., Feng J., Yu L., Zhu W.G. Cytosolic FoxO1 is essential for the induction of autophagy and tumour suppressor activity. Nat. Cell Biol. 2010;12:665–675. doi: 10.1038/ncb2069. [DOI] [PubMed] [Google Scholar]
- 35.Shin H.J., Kim H., Oh S., Lee J.G., Kee M., Ko H.J., Kweon M.N., Won K.J., Baek S.H. AMPK-SKP2-CARM1 signalling cascade in transcriptional regulation of autophagy. Nature. 2016;534:553–557. doi: 10.1038/nature18014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang B., Yang Q., Sun Y.Y., Xing Y.F., Wang Y.B., Lu X.T., Bai W.W., Liu X.Q., Zhao Y.X. Resveratrol-enhanced autophagic flux ameliorates myocardial oxidative stress injury in diabetic mice. J. Cell. Mol. Med. 2014;18:1599–1611. doi: 10.1111/jcmm.12312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang B., Ding W., Zhang M., Li H., Guo H., Lin L., Chen J., Gu Y. Role of FOXO1 in aldosterone-induced autophagy: A compensatory protective mechanism related to podocyte injury. Oncotarget. 2016;7:45331–45351. doi: 10.18632/oncotarget.9644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Silwal P., Kim J.K., Yuk J.M., Jo E.K. AMP-Activated Protein Kinase and Host Defense against Infection. Int. J. Mol. Sci. 2018;19:3495. doi: 10.3390/ijms19113495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jeon S.M. Regulation and function of AMPK in physiology and diseases. Exp. Mol. Med. 2016;48:e245. doi: 10.1038/emm.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu E., Lopez Corcino Y., Portillo J.A., Miao Y., Subauste C.S. Identification of Signaling Pathways by Which CD40 Stimulates Autophagy and Antimicrobial Activity against Toxoplasma gondii in Macrophages. Infect. Immun. 2016;84:2616–2626. doi: 10.1128/IAI.00101-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sanchez A.M.J., Candau R., Bernardi H. AMP-activated protein kinase stabilizes FOXO3 in primary myotubes. Biochem. Biophys. Res. Commun. 2018;499:493–498. doi: 10.1016/j.bbrc.2018.03.176. [DOI] [PubMed] [Google Scholar]
- 42.Yuk J.M., Shin D.M., Lee H.M., Kim J.J., Kim S.W., Jin H.S., Yang C.S., Park K.A., Chanda D., Kim D.K., et al. The orphan nuclear receptor SHP acts as a negative regulator in inflammatory signaling triggered by Toll-like receptors. Nat. Immunol. 2011;12:742–751. doi: 10.1038/ni.2064. [DOI] [PubMed] [Google Scholar]
- 43.Misra J., Chanda D., Kim D.K., Li T., Koo S.H., Back S.H., Chiang J.Y., Choi H.S. Curcumin differentially regulates endoplasmic reticulum stress through transcriptional corepressor SMILE (small heterodimer partner-interacting leucine zipper protein)-mediated inhibition of CREBH (cAMP responsive element-binding protein H) J. Biol. Chem. 2011;286:41972–41984. doi: 10.1074/jbc.M111.274514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Salem N., Jr., Litman B., Kim H.Y., Gawrisch K. Mechanisms of action of docosahexaenoic acid in the nervous system. Lipids. 2001;36:945–959. doi: 10.1007/s11745-001-0805-6. [DOI] [PubMed] [Google Scholar]
- 45.Yang C.S., Kim J.J., Lee H.M., Jin H.S., Lee S.H., Park J.H., Kim S.J., Kim J.M., Han Y.M., Lee M.S., et al. The AMPK-PPARGC1A pathway is required for antimicrobial host defense through activation of autophagy. Autophagy. 2014;10:785–802. doi: 10.4161/auto.28072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barish G.D., Downes M., Alaynick W.A., Yu R.T., Ocampo C.B., Bookout A.L., Mangelsdorf D.J., Evans R.M. A Nuclear Receptor Atlas: Macrophage activation. Mol. Endocrinol. 2005;19:2466–2477. doi: 10.1210/me.2004-0529. [DOI] [PubMed] [Google Scholar]
- 47.Semenza G.L. Hypoxia-inducible factor 1 (HIF-1) pathway. Sci. Signal. 2007;2007:cm8. doi: 10.1126/stke.4072007cm8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.