Abstract
Microsporidia are obligate intracellular protozoan parasites that cause a wide variety of opportunistic infection in patients with AIDS. Because it is able to grow in vitro, Encephalitozoon cuniculi is currently the best-studied microsporidian. T cells mediate protective immunity against this parasite. Splenocytes obtained from infected mice proliferate in vitro in response to irradiated parasites. A transient state of hyporesponsiveness to parasite antigen and mitogen was observed at day 17 postinfection. This downregulatory response could be partially reversed by addition of nitric oxide (NO) antagonist to the culture. Mice infected with E. cuniculi secrete significant levels of gamma interferon (IFN-γ). Treatment with antibody to IFN-γ or interleukin-2 (IL-12) was able to neutralize the resistance to the parasite. Mutant animals lacking the IFN-γ or IL-12 gene were highly susceptible to infection. However, mice unable to secrete NO withstood high doses of parasite challenge, similar to normal wild-type animals. These studies describe an IFN-γ-mediated protection against E. cuniculi infection that is independent of NO production.
Microsporidia are obligate intracellular parasites that infect an extremely wide range of hosts in the animal kingdom (4). They are distinct enough to be placed in a separate phylum, Microspora (5), and are characterized by the polar filament which is used to inject sporoplasm into the host cell (51). Species of microsporidia that infect mammals are unicellular, gram-positive organisms with mature spores 0.5 to 2 by 1 to 4 μm in diameter (10). Classification is based on size, nuclear arrangement, mode of division, and association of proliferative forms within the host cell.
Most of what is known about the biology of microsporidia is based on the microsporidian Encephalitozoon cuniculi, which commonly infects rodents and has been found in humans as well (54). Little is known regarding host immunity to E. cuniculi. E. cuniculi was the first mammalian microsporidian successfully grown in vitro (43). It infects epithelial and endothelial cells, fibroblasts, and macrophages in a variety of mammals, including rabbits, rodents, carnivores, monkeys, and humans (6, 8, 19). In an experimental model, normal mice infected with E. cuniculi usually express few clinical signs of disease (35). Conversely, immunodeficient hosts, such as athymic or SCID mice, develop lethal disease after experimental infection (26, 41). The studies conducted have shown that T cells are responsible for the prevention of lethal disease. Adoptive transfer of sensitized syngeneic T-cell-enriched spleen cells protected athymic or SCID mice against E. cuniculi challenges (18, 40). Transfer of naive lymphocytes or hyperimmune serum fail to protect or prolong the survival of these mice. Furthermore, E. cuniculi is increasingly being recognized an opportunistic infection in the individuals with AIDS (19, 50). Studies by Didier have shown that cytokines released by sensitized T cells activate macrophages to kill E. cuniculi in vitro (9). However, there are no in vivo data demonstrating the mechanism of T-cell-mediated protection against this emerging opportunistic pathogen.
The data herein demonstrate that E. cuniculi infection in the immunocompetent host induces a strong cellular immune response characterized by the production of gamma interferon (IFN-γ). Mice unable to produce this cytokine are susceptible to infection. Thus, protective immunity induced in the normal mice is dependent on Th1 type of immune response.
MATERIALS AND METHODS
Mice.
Maurice Gately (Hoffman-La Roche) kindly provided a breeding pair of p40−/− mice on a C57BL/6 background. These mice lack the gene for the p40 chain of interleukin-12 (IL-12) heterodimer and thus are unable to produce IL-12 (30). Inducible nitric oxide synthase-deficient (iNOS−/−) mice on a C57BL/6 × 129 background were provided by John Mackmicking and Carl Nathan (Cornell University Medical School, Ithaca, N.Y.). These mice were backcrossed for five generations to wild-type C57BL/6 as previously described (25). The mice were bred under conditions approved by the Animal Research Facilities at Dartmouth Medical School. Mice deficient in the IFN-γ gene and wild-type C57BL/6 mice were obtained from The Jackson Laboratory, Bar Harbor, Maine.
Parasites and infection.
A rabbit isolate of E. cuniculi, kindly provided by Elizabeth Didier (Tulane Medical Research Center), was used throughout the study. Parasites were grown in rabbit kidney (RK-13) cells (American Type Culture Collection) which were maintained in RPMI 1640 (Gibco BRL) containing 10% fetal calf serum (HyClone Laboratories). Organisms were collected from the culture medium and centrifuged at 325 × g for 10 min. After two washes with phosphate-buffered saline the parasites were resuspended and injected via the intraperitoneal (i.p.) route to mice. Unless stated otherwise, mice were challenged with 107 parasites.
T-cell proliferation.
Following euthanasia, the spleens from infected animals were removed and homogenized in a petri dish, and contaminating erythrocytes were lysed in RBC lysis buffer (Sigma Chemical Co., St. Louis, Mo.). Cells were suspended in RPMI 1640 with 10% fetal calf serum and centrifuged for 10 min at 500 × g. Cells were cultured at the concentration of 2 × 105/well in 96-well flat-bottomed plates in a 200-μl volume with 5 μg of concanavalin A (ConA; Sigma Chemical Co.) per ml or 5 × 103 irradiated spores. After 72 h at 37°C in 5% CO2, [3H]thymidine (0.5 μCi/well; Amersham, Arlington Heights, Ill.) was pulsed for 8 h to determine DNA synthesis. An automated cell harvester was used to harvest pulsed splenocytes onto glass filters. The filters were dried, and incorporation of radioactive thymidine was determined by liquid scintillation.
Detection of cytokine mRNA by quantitative PCR.
Splenocytes from E. cuniculi-infected animals were collected at days 0, 10, 17, and 24 p.i. (post infection). RNA from spleen cells was collected by using Trizol (Life Technologies Inc., Gaithersburg, Md.) as instructed by the manufacturer. Reverse transcription was performed with Moloney murine leukemia virus reverse transcriptase (Life Technologies) and random hexamer primers (Promega, Madison, Wis.). Expression of mRNAs for IFN-γ, IL-10, IL-4, and iNOS was measured by quantitative PCR using the PQRS quantitative method (36). The splenocytes from uninfected mice were used to establish a baseline value of 1.0, against which the level of message for cytokine in the test mice was quantitated.
Cytokine detection in serum.
Serum was collected from the blood of infected mice and tested for the presence of IFN-γ and IL-4 (Endogen, Cambridge, Mass.) by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s instructions.
Measurement of cytokine production in spleen cell cultures.
Cytokine production in the culture supernatants of the ConA- and parasite-stimulated spleen cell cultures was measured. Levels of IFN-γ were determined by cytokine ELISA as described above. Nitrite production in the culture supernatants was assayed by the Greiss reaction (13). Briefly, 100 μl of the culture supernatant was added to a mixture of 1% sulfanilamide dihydrochloride in 2.5% phosphoric acid and 0.1% napthylenediamine hydrochloride in 2.5% H3PO4, then incubated for 10 min at room temperature, and read with a spectrometer (A570). Nitrite concentration was calculated from a NaNO2 standard curve.
Cytokine depletion assays.
Rat anti-mouse IL-10 (Endogen) was used at a concentration of 40 μg/ml. Control antibody was isotype-specific rat immunoglobulin G (IgG; Sigma). For IFN-γ depletion, mice were treated with rat anti-mouse IFN-γ (XMG6) (3 mg/mouse per week) i.p. starting 2 days prior to challenge. Control mice received equal amount of rat IgG. Endogenous IL-12 was neutralized by administering 0.5 mg of goat anti-mouse IL-12 (kindly provided by Maurice Gately) beginning 2 days prior to infection. The antibody treatment was continued twice weekly thereafter. Control mice were treated with equal quantity of goat IgG (Sigma).
Statistical analysis.
Statistical analysis of the data was performed by Student’s t test (34).
RESULTS
Antigen-specific proliferation of splenocytes from E. cuniculi-infected mice.
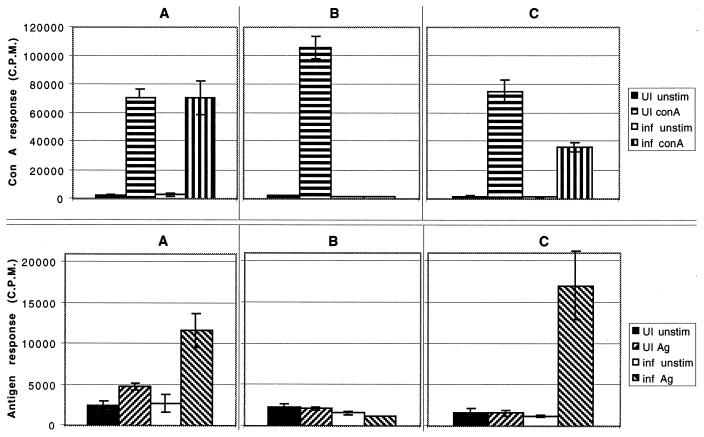
A time course studying antigen-specific T-cell proliferation was performed. Splenocytes from E. cuniculi-infected mice were isolated at days 10, 17, and 24 p.i., and the proliferative response to antigenic stimulation was determined. Antigen-specific proliferation of splenocytes from day 10-p.i. animals was significantly greater (P = 0.01) than for uninfected animals (Fig. 1A). Spleen cells from the infected mice showed a normal ConA response (Fig. 1A). At day 17 p.i., the splenocytes failed to proliferate in response to antigenic stimulation (Fig. 1B). To determine whether this was an antigen-specific downregulation, splenocytes from infected mice were stimulated with mitogen. As with parasite antigen, these splenocytes failed to proliferate with mitogen (Fig. 1B), possibly due to the generalized immunosuppression that has been observed during acute infections in other parasite infections (3, 24, 46). The immunosuppression was ablated at day 24 p.i., and splenocytes from the infected animals responded significantly (P = 0.001) to antigenic stimulation (Fig. 1C). However, the ConA response at this time point, although significantly improved (P = 0.01), was still significantly lower than for the uninfected controls (Fig. 1C) (P = 0.04).
FIG. 1.
Proliferation of antigen (Ag)-specific splenocytes following i.p. infection with 107 spores of E. cuniculi. Pooled splenocytes (n = 3) were collected at days 10 (A), 17 (B), and 24 (C) postchallenge. Spleen cells were cultured in quadruplicate in the presence of ConA or irradiated spores in 96-well plates. After 72 h of incubation, proliferation was measured by [3H]thymidine incorporation. The data are representative of two separate experiments. UI, uninfected; inf, infected.
NO mediates a role in reduced lymphoproliferative response.
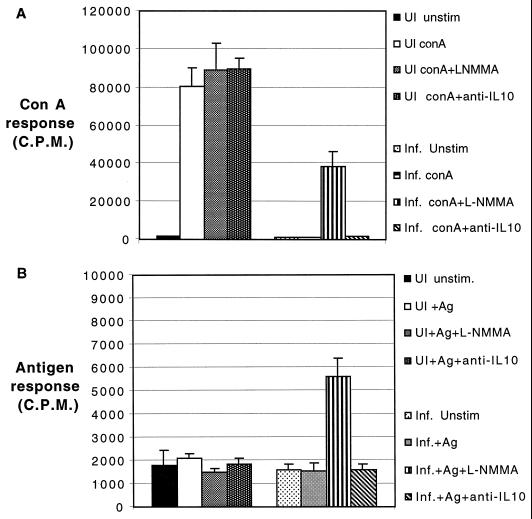
Nitric oxide (NO) is known to downregulate the host immune response in wide range of infections. The role of this molecule in the inhibition of lymphoproliferative response during E. cuniculi infection was studied. The NO synthase antagonist l-NMMA was added to the day 17-p.i. splenocyte cultures at concentrations known to antagonize NO. Addition of l-NMMA to the spleen cell cultures significantly neutralized the suppression in both mitogen (P = 0.001)- and antigen (P = 0.05)-stimulated cultures (Fig. 2). Although IL-10 has been reported to be involved in the immunosuppression in other models (22, 52), no reversal was observed with antibody to IL-10 in both the mitogen- and antigen-stimulated conditions. No difference was observed when rat IgG was added to cultures (data not shown). Culture supernatants obtained from splenocytes of infected mice at various time points p.i. were assayed for the production of IFN-γ and nitrites. As shown in Table 1, both IFN-γ and nitrite production was greatest in the culture supernatant at day 17 p.i. Interestingly, this is the time point at which the splenocytes from E. cuniculi-infected mice are unable to proliferate in response to both antigen and mitogen.
FIG. 2.
Reversal of E. cuniculi-mediated suppression by an NO synthase antagonist. Pooled splenocytes (n = 3) from mice infected 17 days earlier with E. cuniculi were cultured in the presence of ConA (A) or irradiated spores (B) and treated with either 0.5 mM l-NMMA or rat anti-murine IL-10 (40 μg/ml). After 72 h of incubation, lymphoproliferation was assayed by [3H]thymidine incorporation. The data are representative of two separate experiments. Abbreviations are as in Fig. 1.
TABLE 1.
Cytokine production by splenocytes from E. cuniculi-infected micea
| Day p.i. | Nitrite (nM)
|
IFN-γ (pg/ml)
|
||||
|---|---|---|---|---|---|---|
| Unstim-ulated | ConA stim-ulated | Antigen stimulated | Unstim-ulated | ConA stim-ulated | Antigen stimulated | |
| 0 | ND | ND | ND | ND | 1,050 ± 125 | 33 ± 12 |
| 10 | ND | 1.5 ± 0.2 | 2.0 ± 0.4 | 15 ± 2.6 | 1,164 ± 176 | 145 ± 32 |
| 17 | 10.5 ± 0.8 | 12.6 ± 1.5 | 11.5 ± 0.7 | 358 ± 38.6 | 1,560 ± 223 | 1,162 ± 120 |
| 24 | 2.6 ± 0.3 | 4.2 ± 1.1 | 5.0 ± 0.3 | 126 ± 7.5 | 1,283 ± 144 | 525 ± 65 |
A total of 106 pooled splenocytes from mice (n = 3) at various days p.i. were cultured in 24-well plates in the presence of ConA or irradiated parasites. After 72 h of incubation, supernatants were collected and stored at −70°C. Supernatants were assayed for nitrite production by the Griess reaction and for IFN-γ by ELISA. The data represent means ± standard deviations of triplicate cultures. Results of one of two similar experiment are shown. ND, not detected.
Cytokine profile of E. cuniculi-infected animals.
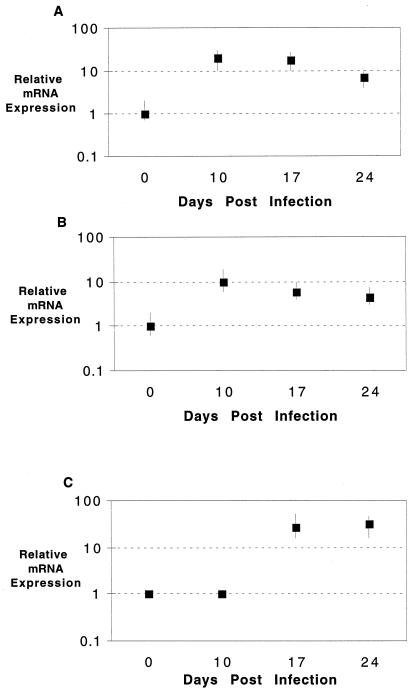
Cytokine analysis of splenocytes from infected animals was performed by quantitative PCR. Message for IFN-γ and IL-10 was increased at day 10 p.i. (Fig. 3A and B). Both cytokines are known to play an important immunoregulatory role in infectious diseases. The message levels for these cytokines remained more or less unchanged at day 24 p.i. The message for IL-4 could not be detected in the infected animals at any of the time points tested (data not shown). The iNOS message was undetectable at day 10 p.i. However, at day 17 p.i., we observed severalfold increase in the message for this molecule, which continued up to day 24 p.i. (Fig. 3C).
FIG. 3.
Cytokine mRNA expression of splenocytes following E. cuniculi infection. Splenocytes from E. cuniculi-infected mice (three per group) were harvested at various time points (days 0, 10, 17, and 24) p.i. mRNA expression for IFN-γ (A), IL-10 (B), and iNOS (C) was assayed by reverse transcription-PCR. The differences in transcriptional levels for the genes are expressed relative to day 0 (assigned as 1). The cDNA concentration examined at each time point was standardized to the HPRT mRNA level (not shown).
Circulating IFN-γ and IL-4 levels in the sera of infected animals were determined. Pooled serum from three E. cuniculi-infected mice assayed for these cytokines. The level of IFN-γ in the serum was elevated at day 10 p.i. (245 ± 12 pg/ml). The cytokine level peaked at day 17 p.i. (1,850 ± 65 pg/ml) and began to taper off by day 24 p.i. (1,235 ± 30 pg/ml). In contrast, no IL-4 was detected in sera from the infected animals. Sera from control uninfected animals lacked any of these cytokines.
In vivo role of IFN-γ in protection against E. cuniculi infection.
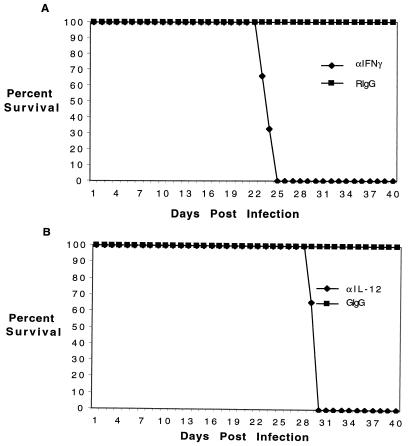
To determine whether the increased level of IFN-γ was important in host immunity, a depletion study was performed. Two days prior to infection, mice were depleted of either IL-12 or IFN-γ by treatment with a monoclonal antibody. Antibody treatment was continued throughout the study. The mice were infected with parasites via the i.p. route and assessed daily for evidence of morbidity (development of asciteis) and mortality. All mice depleted of IFN-γ died by day 25 p.i. (Fig. 4); mice treated with anti-IL-12 antibody died 4 days later. Depletion of IL-12 most likely resulted in decreased levels of IFN-γ. The delay in time to death between the two different test groups could be due to other immune modulators stimulating IFN-γ production when IL-12 is depleted.
FIG. 4.
Neutralization of IFN-γ and IL-12 in E. cuniculi-infected mice. (A) C57BL/6 mice (six per group) were infected i.p. with 107 spores of E. cuniculi and treated with antibody to IFN-γ (3 mg/mouse) weekly at 2 days prior to infection. The control animals were injected with an equal amount of rat IgG (RIgG). (B) C57BL/6 mice (six per group) were treated with 0.5 mg of goat anti-mouse IL-12 twice weekly beginning two days prior to infection or an equal volume of goat IgG (GIgG). The animals were observed daily for morbidity or mortality. The study was performed twice, with similar results.
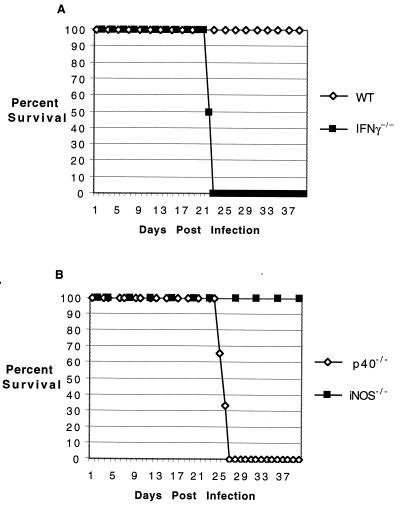
The importance of IFN-γ in the protective immune response is reported to be mediated by NO production (14, 27, 32). However, recent studies by others and us have shown a limited role for this molecule in protection against Toxoplasma gondii (23, 39). To evaluate the importance of NO in protection against E. cuniculi infection, iNOS−/− mice were infected as previously described. None of these animals died or exhibited any signs or symptoms of disease throughout the course of experiment and appeared clinically indistinct from the wild-type controls (Fig. 5). In contrast, mice lacking the p40 or IFN-γ gene succumbed to infection. These animals died at approximately the same time as the antibody-depleted mice. These results exclude a role of NO in E. cuniculi immunity.
FIG. 5.
Survival of gene knockout animals following E. cuniculi challenge. Knockout mice on a C57BL/6 background and the parental wild-type (WT) animals (six per group) were infected i.p. with 107 spores of E. cuniculi (A), and mortality was monitored on a daily basis (B). The study was performed twice, with similar findings.
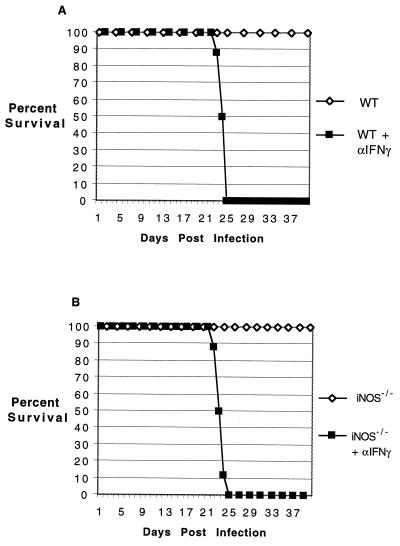
To determine if the susceptibility of the knockout mice was dose dependent, mice were challenged with a fivefold-higher dose of parasites. While the IFN-γ−/− and p40−/− mice succumbed to infection earlier than those challenged with lower dose, the increase in the size of inoculum made no difference in the susceptibility of iNOS−/− and parental wild-type mice (data not shown). These animals survive for the duration of the experiment. Gene knockout animals have been shown to be able to compensate for the loss by alternate redundant mechanisms (7, 29). Although less likely, it is possible that iNOS−/− mice are protected against E. cuniculi infection by an IFN-γ-independent mechanism. To rule out this possibility, both mutant and parental wild-type animals were infected with 107 parasites as described earlier. The infected animals were treated with anti-IFN-γ antibody starting 2 days prior to infection. Both the iNOS−/− and wild-type mice depleted of IFN-γ succumbed to infection at almost the same time after challenge (Fig. 6). None of the mice treated with control antibody died during the period of experimentation.
FIG. 6.
Depletion of IFN-γ in iNOS−/− animals infected with E. cuniculi. Parental wild-type (WT) C57BL/6 (A) and iNOS−/− (B) mice (six per group) were treated with anti-IFN-γ antibody 2 days prior to challenge as described for Fig. 5. The control animals were injected with an equal amount of rat IgG. The animals were monitored daily for survival.
DISCUSSION
E. cuniculi, previously observed in laboratory animals, is considered a zoonotic infection (8). Complications due to E. cuniculi infection have been found in immunocompromised patients (44). Human immunodeficiency virus (HIV)-infected patients coinfected with E. cuniculi have a wide range of organ involvement, including liver failure, pneumonitis, sinusitis, and granulomatous liver necrosis (6). E. cuniculi is closely related to other microsporidia, including Encephalitozoon intestinalis and Encephalitozoon hellum, which are also associated with disseminated infection in HIV-infected individuals (5).
The immune responses generated during natural E. cuniculi infection are not well studied. Currently available literature suggests that T cells play a very important role in protection against the parasite (18, 41). In this report, induction of strong cellular immune response in E. cuniculi-infected host is demonstrated. T-cell proliferation and production of immune cytokines p.i. characterize this response.
The role of T-cell immunity against E. cuniculi infection has been described by other laboratories (26, 40). In the present study, antigen-specific proliferation of splenocytes from mice infected 10 days prior was observed. This response was followed by a period of transient immunosuppression, characterized by a low proliferative index to both antigen and mitogen. Lymphocyte proliferation was restored by day 24 postchallenge. Earlier studies by Didier and Shadduck have demonstrated that spleen cells from mice infected 1 week earlier express a significantly lower mitogenic response (11). However, downregulation of both mitogenic and antigenic responses at day 17 p.i. is reported here. The differences between our findings and those of Didier and Shadduck could be attributed to the different mouse strains used in these studies. Variation in immune response, depending on the strain of mice, has been reported with other infectious disease models (28, 33). Transient periods of lymphocyte hyporesponsiveness have also been described for other parasitic infections (22, 46). During murine T. gondii infection, the period of lymphocyte hyporesponsiveness persists from days 7 to 14 p.i. (17). Infection with Neospora caninum results in immunosuppression which is shorter in duration.
The principal mechanism for the downregulatory response appears to be via induction of NO. Interestingly, maximal immunosuppression is observed at day 17 p.i., when levels of both IFN-γ and nitrite production are highest in spleen cell cultures. This also coincides with the high serum IFN-γ levels in infected animals. No IL-4 was detected in sera of infected mice. Our findings are similar to those reported recently for murine salmonellosis infection (42). Bacterial challenge was followed by NO-mediated immunosuppression which was neutralized by IFN-γ depletion. NO has also been demonstrated to be an important immunoregulatory molecule in, for example, plasmodium (38), T. gondii (3, 22), N. caninum (24), and Trypanosoma brucei (46) infections. Treatment of spleen cell cultures with l-NMMA, an inhibitor of NO synthase, restores approximately 50% of the proliferative response by day 17-p.i. mice. The reversal of transient lymphocyte hyporesponsiveness was observed under both mitogen- and antigen-stimulated conditions.
Apart from its downregulatory role in lymphoproliferation, the microbicidal activity of NO against intracellular pathogens is well documented (15, 21). The release of NO is mediated by a key Th1-type cytokine, IFN-γ (20, 37). This cytokine plays a critical role in protective immunity against wide variety of intracellular pathogens, including viruses, bacteria, and parasites (47, 49, 53). In the present study, antibody depletion of IFN-γ or IL-12, the cytokine important for the induction of IFN-γ, resulted in mortality of E. cuniculi-infected mice. Studies have shown that IFN-γ knockout mice challenged with E. intestinalis, a parasite closely related to E. cuniculi, develop a chronic disease, whereas parental wild-type animals are able to clear the infection (1, 12). The IFN-γ-mediated protection is believed to be primarily dependent on the cytokine’s ability to activate macrophages (52). Following a cascade of complex molecular events, including costimulation with tumor necrosis factor, activated macrophages release NO (14). In vitro killing of E. cuniculi by IFN-γ-activated macrophages has been recently reported by Didier (9). Addition of l-NMMA to the cultures blocked the killing of the parasites, suggesting that the effect was mediated by NO. Based on these findings, it can be postulated that the absence of NO would result in loss of protection against the parasite. However, iNOS−/− mice, which are unable to make NO through the inducible pathway, survived a very high dose of challenge, similar to the wild-type animals. In contrast, mice lacking the IFN-γ or IL-12 gene were unable to survive infection with a fivefold-lower dose.
The mechanism of host protection against E. cuniculi, as for other intracellular pathogens, appears to be dependent on IL-12 and IFN-γ. However, while in other infectious agents IFN-γ-mediated immunity is dependent on the production of NO, such does not seem to be the case with E. cuniculi. These findings are similar to those presented in a recent report on murine toxoplasmosis, where vaccine-based immunity was found to be independent of NO (23). Although macrophage activation and subsequent release of NO are among the important parasiticidal effects of IFN-γ, other considerations are the enhancement of class I and class II expression on antigen-presenting cells, resulting in greater proliferation of T cells (2, 31). A role of IFN-γ in the induction of CD8+ T-cell responses has been reported for viral systems (45, 48). It is likely that in the absence of this cytokine, T-cell responses are compromised, leading to inappropriate protection against infection. Further studies exploring the mechanism of T-cell protection against the parasite are under way. Alternatively, iNOS−/− mice may be protected by an IFN-γ-dependent redundant pathway as has been recently described for influenza virus (16). Identification of these pathways will be beneficial in understanding the host immune response against this emerging opportunistic infection in the HIV-infected population (6).
ACKNOWLEDGMENTS
We thank Julie Weiss for help with statistical analysis.
This work was supported by National Institutes of Health grant AI43693.
REFERENCES
- 1.Achbarou A, Ombrouck C, Gneragbe T, Charlotte F, Renia L, Desportes-Livage I, Mazier D. Experimental model for human intestinal microsporidiosis in interferon gamma receptor knockout mice infected by Encephalitozoon intestinalis. Parasite Immunol. 1996;18:387–392. doi: 10.1046/j.1365-3024.1996.d01-128.x. [DOI] [PubMed] [Google Scholar]
- 2.Belich M P, Glynne R J, Senger G, Sheer D, Trowsdale J. Proteasome components with reciprocal expression to that of the MHC-encoded LMP proteins. Curr Biol. 1994;4:769–776. doi: 10.1016/s0960-9822(00)00174-3. [DOI] [PubMed] [Google Scholar]
- 3.Candolfi E, Hunter C A, Remington J S. Roles of gamma interferon and other cytokines in suppression of the spleen cell proliferative response to concanavalin A and toxoplasma antigen during acute toxoplasmosis. Infect Immun. 1995;63:751–756. doi: 10.1128/iai.63.3.751-756.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Canning E, Lom J. The microsporidia of vertebrates. New York, N.Y: Academic Press; 1986. [Google Scholar]
- 5.Coyle C M, Wittner M, Kotler D P, Noyer C, Orenstein J M, Tanowitz H B, Weiss L M. Prevalence of microsporidiosis due to Enterocytozoon bieneusi and Encephalitozoon (Septata) intestinalis among patients with AIDS-related diarrhea: determination by polymerase chain reaction to the microsporidian small-subunit rRNA gene. Clin Infect Dis. 1996;23:1002–1006. doi: 10.1093/clinids/23.5.1002. [DOI] [PubMed] [Google Scholar]
- 6.De Groote M A, Visvesvara G, Wilson M L, Pieniazek N J, Slemenda S B, daSilva A J, Leitch G J, Bryan R T, Reves R. Polymerase chain reaction and culture confirmation of disseminated Encephalitozoon cuniculi in a patient with AIDS: successful therapy with albendazole. J Infect Dis. 1995;171:1375–1378. doi: 10.1093/infdis/171.5.1375. [DOI] [PubMed] [Google Scholar]
- 7.Denkers E Y, Scharton-Kersten T, Barbieri S, Caspar P, Sher A. A role for CD4+ NK1.1+ T lymphocytes as major histocompatibility complex class II independent helper cells in the generation of CD8+ effector function against intracellular infection. J Exp Med. 1996;184:131–139. doi: 10.1084/jem.184.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deplazes P, Mathis A, Baumgartner R, Tanner I, Weber R. Immunologic and molecular characteristics of Encephalitozoon-like microsporidia isolated from humans and rabbits indicate that Encephalitozoon cuniculi is a zoonotic parasite. Clin Infect Dis. 1996;22:557–559. doi: 10.1093/clinids/22.3.557. [DOI] [PubMed] [Google Scholar]
- 9.Didier E S. Reactive nitrogen intermediates implicated in the inhibition of Encephalitozoon cuniculi (phylum Microspora) replication in murine peritoneal macrophages. Parasite Immunol. 1995;17:405–412. doi: 10.1111/j.1365-3024.1995.tb00908.x. [DOI] [PubMed] [Google Scholar]
- 10.Didier E S, Didier P J, Friedberg D N, Stenson S M, Orenstein J M, Yee R W, Tio F O, Davis R M, Vossbrinck C, Millichamp N, et al. Isolation and characterization of a new human microsporidian, Encephalitozoon hellem (n. sp.), from three AIDS patients with keratoconjunctivitis. J Infect Dis. 1991;163:617–621. doi: 10.1093/infdis/163.3.617. [DOI] [PubMed] [Google Scholar]
- 11.Didier E S, Shadduck J A. Modulated immune responsiveness associated with experimental Encephalitozoon cuniculi infection in BALB/c mice. Lab Anim Sci. 1988;38:680–684. [PubMed] [Google Scholar]
- 12.El Fakhry Y, Achbarou A, Desportes-Livage I, Mazier D. Encephalitozoon intestinalis: humoral responses in interferon-gamma receptor knockout mice infected with a microsporidium pathogenic in AIDS patients. Exp Parasitol. 1998;89:113–121. doi: 10.1006/expr.1998.4267. [DOI] [PubMed] [Google Scholar]
- 13.Green L C, Wagner D A, Glogowski J, Skipper P L, Wishnok J S, Tannenbaum S R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 14.Green S J, Nacy C A, Meltzer M S. Cytokine-induced synthesis of nitrogen oxides in macrophages: a protective host response to Leishmania and other intracellular pathogens. J Leukoc Biol. 1991;50:93–103. doi: 10.1002/jlb.50.1.93. [DOI] [PubMed] [Google Scholar]
- 15.Gregory S H, Wing E J, Hoffman R A, Simmons R L. Reactive nitrogen intermediates suppress the primary immunologic response to Listeria. J Immunol. 1993;150:2901–2909. [PubMed] [Google Scholar]
- 16.Gunsegaran K, Mahalingam C J H, S, Nathan C F, MacMicking J D. Rapid interferon-γ-dependent clearance of influenza A virus and protection from consolidating pneumonitis in nitric oxide synthase2-deficient mice. J Exp Med. 1998;188:1541–1546. doi: 10.1084/jem.188.8.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haque S, Khan I, Haque A, Kasper L. Impairment of the cellular immune response in acute murine toxoplasmosis: regulation of interleukin-2 production and macrophage-mediated inhibitory effects. Infect Immun. 1994;62:2908–2916. doi: 10.1128/iai.62.7.2908-2916.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hermanek J, Koudela B, Kucerova Z, Ditrich O, Travnicek J. Prophylactic and therapeutic immune reconstitution of SCID mice infected with Encephalitozoon cuniculi. Folia Parasitol. 1993;40:287–291. [PubMed] [Google Scholar]
- 19.Hollister W S, Canning E U, Colbourn N I, Aarons E J. Encephalitozoon cuniculi isolated from the urine of an AIDS patient, which differs from canine and murine isolates. J Eukaryot Microbiol. 1995;42:367–372. doi: 10.1111/j.1550-7408.1995.tb01595.x. [DOI] [PubMed] [Google Scholar]
- 20.Igietseme J U. Molecular mechanism of T-cell control of Chlamydia in mice: role of nitric oxide in vivo. Immunology. 1996;88:1–5. [PMC free article] [PubMed] [Google Scholar]
- 21.James S L, Cheever A W, Caspar P, Wynn T A. Inducible nitric oxide synthase-deficient mice develop enhanced type 1 cytokine-associated cellular and humoral responses after vaccination with attenuated Schistosoma mansoni cercariae but display partially reduced resistance. Infect Immun. 1998;66:3510–3518. doi: 10.1128/iai.66.8.3510-3518.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khan I A, Matsuura T, Kasper L H. IL-10 mediates immunosuppression following primary infection with Toxoplasma gondii in mice. Parasite Immunol. 1995;17:185–195. doi: 10.1111/j.1365-3024.1995.tb00888.x. [DOI] [PubMed] [Google Scholar]
- 23.Khan I A, Matsuura T, Kasper L H. Inducible nitric oxide synthase is not required for long term vaccine based immunity against Toxoplasma gondii. J Immunol. 1998;161:2994–3000. [PubMed] [Google Scholar]
- 24.Khan I A, Schwartzman J D, Fonseka S, Kasper L H. Neospora caninum: role for immune cytokines in host immunity. Exp Parasitol. 1997;85:24–34. doi: 10.1006/expr.1996.4110. [DOI] [PubMed] [Google Scholar]
- 25.Khan I A, Schwartzman J D, Matsuura T, Kasper L H. A dichotomous role for nitric oxide during acute Toxoplasma gondii infection in mice. Proc Natl Acad Sci USA. 1997;94:13955–13960. doi: 10.1073/pnas.94.25.13955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koudela B, Vitovec J, Kucerova Z, Ditrich O, Travnicek J. The severe combined immunodeficient mouse as a model for Encephalitozoon cuniculi microsporidiosis. Folia Parasitol. 1993;40:279–286. [PubMed] [Google Scholar]
- 27.Kreil T R, Eibl M M. Nitric oxide and viral infection: NO antiviral activity against a flavivirus in vitro, and evidence for contribution to pathogenesis in experimental infection in vivo. Virology. 1996;219:304–306. doi: 10.1006/viro.1996.0252. [DOI] [PubMed] [Google Scholar]
- 28.Launois P, Louis J A, Milon G. The fate and persistence of Leishmania major in mice of different genetic backgrounds: an example of exploitation of the immune system by intracellular parasites. Parasitology. 1997;115:S25–S32. doi: 10.1017/s0031182097001777. [DOI] [PubMed] [Google Scholar]
- 29.Locksley R M, Reiner S L, Hatam F, Littman D R, Killeen N. Helper T cells without CD4: control of leishmaniasis in CD4-deficient mice. Science. 1993;261:1448–1451. doi: 10.1126/science.8367726. [DOI] [PubMed] [Google Scholar]
- 30.Magram J, Connaughton S E, Warrier R R, Carvajal D M, Wu C Y, Ferrante J, Stewart C, Sarmiento U, Faherty D A, Gately M K. IL-12-deficient mice are defective in IFN gamma production and type 1 cytokine responses. Immunity. 1996;4:471–481. doi: 10.1016/s1074-7613(00)80413-6. [DOI] [PubMed] [Google Scholar]
- 31.Mattsson R, Mattsson A, Holmdahl R, Scheynius A, Van der Meide P H. In vivo treatment with interferon-gamma during early pregnancy in mice induces strong expression of major histocompatibility complex class I and II molecules in uterus and decidua but not in extra-embryonic tissues. Biol Reprod. 1992;46:1176–1186. doi: 10.1095/biolreprod46.6.1176. [DOI] [PubMed] [Google Scholar]
- 32.McCabe R E, Luft B J, Remington J S. Effect of murine interferon gamma on murine toxoplasmosis. J Infect Dis. 1984;150:961–962. doi: 10.1093/infdis/150.6.961. [DOI] [PubMed] [Google Scholar]
- 33.McLeod R, Eisenhauer P, Mack D, Brown C, Filice G, Spitalny G. Immune responses associated with early survival after peroral infection with Toxoplasma gondii. J Immunol. 1989;142:3247–3255. [PubMed] [Google Scholar]
- 34.Neter J, Wasserman W, Kuter M H. Applied linear statistical models. Homewood, Ill: Irwin; 1985. [Google Scholar]
- 35.Niederkorn J Y, Shadduck J A, Schmidt E C. Susceptibility of selected inbred strains of mice to Encephalitozoon cuniculi. J Infect Dis. 1981;144:249–253. doi: 10.1093/infdis/144.3.249. [DOI] [PubMed] [Google Scholar]
- 36.Reiner S L, Zheng S, Corry D B, Locksley R M. Constructing polycompetitor cDNAs for quantitative PCR. J Immunol Methods. 1993;165:37–46. doi: 10.1016/0022-1759(93)90104-f. . (Errata, 173:133 and 175:275, 1994.) [DOI] [PubMed] [Google Scholar]
- 37.Roach T I, Barton C H, Chatterjee D, Liew F Y, Blackwell J M. Opposing effects of interferon-gamma on iNOS and interleukin-10 expression in lipopolysaccharide- and mycobacterial lipoarabinomannan-stimulated macrophages. Immunology. 1995;85:106–113. [PMC free article] [PubMed] [Google Scholar]
- 38.Rockett K A, Awburn M M, Rockett E J, Cowden W B, Clark I A. Possible role of nitric oxide in malarial immunosuppression. Parasite Immunol. 1994;16:243–249. doi: 10.1111/j.1365-3024.1994.tb00346.x. [DOI] [PubMed] [Google Scholar]
- 39.Scharton-Kersten T M, Yap G, Magram J, Sher A. Inducible nitric oxide is essential for host control of persistent but not acute infection with the intracellular pathogen Toxoplasma gondii. J Exp Med. 1997;185:1261–1273. doi: 10.1084/jem.185.7.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schmidt E C, Shadduck J A. Mechanisms of resistance to the intracellular protozoan Encephalitozoon cuniculi in mice. J Immunol. 1984;133:2712–2719. [PubMed] [Google Scholar]
- 41.Schmidt E C, Shadduck J A. Murine encephalitozoonosis model for studying the host-parasite relationship of a chronic infection. Infect Immun. 1983;40:936–942. doi: 10.1128/iai.40.3.936-942.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schwacha M G, Eisenstein T K. Interleukin-12 is critical for induction of nitric oxide-mediated immunosuppression following vaccination of mice with attenuated Salmonella typhimurium. Infect Immun. 1997;65:4897–4903. doi: 10.1128/iai.65.12.4897-4903.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shadduck J A. Nosema cuiculi: in vitro isolation. Science. 1969;166:516–517. doi: 10.1126/science.166.3904.516. [DOI] [PubMed] [Google Scholar]
- 44.Shadduck J A, Greeley E. Microsporidia and human infections. Clin Microbiol Rev. 1989;2:158–165. doi: 10.1128/cmr.2.2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sharma D P, Ramsay A J, Maguire D J, Rolph M S, Ramshaw I A. Interleukin-4 mediates down regulation of antiviral cytokine expression and cytotoxic T-lymphocyte responses and exacerbates vaccinia virus infection in vivo. J Virol. 1996;70:7103–7107. doi: 10.1128/jvi.70.10.7103-7107.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sternberg J, McGuigan F. Nitric oxide mediates suppression of T cell responses in murine Trypanosoma bruceii infection. Eur J Immunol. 1992;22:2741–2744. doi: 10.1002/eji.1830221041. [DOI] [PubMed] [Google Scholar]
- 47.Suzuki Y, Conley F K, Remington J S. Importance of endogenous IFN-gamma for prevention of toxoplasmic encephalitis in mice. J Immunol. 1989;143:2045–2050. [PubMed] [Google Scholar]
- 48.Utermohlen O, Dangel A, Tarnok A, Lehmann-Grube F. Modulation by gamma interferon of antiviral cell-mediated immune responses in vivo. J Virol. 1996;70:1521–1526. doi: 10.1128/jvi.70.3.1521-1526.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Von Herrath M G, Coon B, Oldstone M B. Low-affinity cytotoxic T-lymphocytes require IFN-gamma to clear an acute viral infection. Virology. 1997;229:349–359. doi: 10.1006/viro.1997.8442. [DOI] [PubMed] [Google Scholar]
- 50.Weber R, Deplazes P, Flepp M, Mathis A, Baumann R, Sauer B, Kuster H, Luthy R. Cerebral microsporidiosis due to Encephalitozoon cuniculi in a patient with human immunodeficiency virus infection. N Engl J Med. 1997;336:474–478. doi: 10.1056/NEJM199702133360704. [DOI] [PubMed] [Google Scholar]
- 51.Wittner M, Tanowitz H B, Weiss L M. Parasitic infections in AIDS patients. Cryptosporidiosis, isosporiasis, microsporidiosis, cyclosporiasis. Infect Dis Clin North Am. 1993;7:569–586. [PubMed] [Google Scholar]
- 52.Woods M L D, Mayer J, Evans T G, Hibbs J B., Jr Antiparasitic effects of nitric oxide in an in vitro murine model of Chlamydia trachomatis infection and an in vivo murine model of Leishmania major infection. Immunol Ser. 1994;60:179–195. [PubMed] [Google Scholar]
- 53.Yang J, Mitsuyama M. An essential role for endogenous interferon-gamma in the generation of protective T cells against Mycobacterium bovis BCG in mice. Immunology. 1997;91:529–535. doi: 10.1046/j.1365-2567.1997.00288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zender H O, Arrigoni E, Eckert J, Kapanci Y. A case of Encephalitozoon cuniculi peritonitis in a patient with AIDS. Am J Clin Pathol. 1989;92:352–356. doi: 10.1093/ajcp/92.3.352. [DOI] [PubMed] [Google Scholar]