Abstract
Autoimmune thyroid diseases (AITDs) are chronic autoimmune disorders that cause impaired immunoregulation, leading to specific immune responses against thyroid antigens. Graves’ disease (GD) and Hashimoto’s thyroiditis (HT) are the major forms of AITDs. Increasing evidence suggests a possible role of microbiota alterations in the pathogenesis and progression of AITDs. This systematic review was designed to address the following question: “Is microbiota altered in patients with AITDs?” After screening the selected studies using the inclusion and exclusion criteria, 16 studies were included in this review (in accordance with PRISMA statement guidelines). A meta-analysis revealed that patients with HT showed significantly higher values of diversity indices (except for the Simpson index) and that patients with GD showed significant tendencies toward lower values of all assessed indices compared with healthy subjects. However, the latter demonstrated a higher relative abundance of Bacteroidetes and Actinobacteria at the phylum level and thus Prevotella and Bifidobacterium at the genus level, respectively. Thyroid peroxidase antibodies showed the most significant positive and negative correlations between bacterial levels and thyroid functional parameters. In conclusion, significant alterations in the diversity and composition of the intestinal microbiota were observed in both GD and HT patients.
Keywords: autoimmune thyroid disease, Graves’ disease, Hashimoto’s thyroiditis, Graves’ orbitopathy, microbiota, microbiome, microbes
1. Introduction
Autoimmune thyroid diseases (AITDs) are the most common autoimmune diseases, manifesting primarily in the forms of Hashimoto’s thyroiditis (HT) and Graves’ disease (GD) [1]. Their pathogenesis refers to genetic predisposition, environmental factors, and disturbances in the functioning of the immune system [2]. Immune disorders lead to reactivity to thyroid autoantigens such as thyroid peroxidase (TPO), thyroglobulin (TG), and thyroid-stimulating hormone receptor. This causes an inflammatory infiltration of the thyroid gland and the production of cytokines, which impact the cells of the immune system and the follicle cells of the thyroid [1,3]. Despite sharing a common autoimmune cause, HT and GD show contrasting effects on thyroid function and thus contrasting clinical symptoms: HT, which determines hypothyroidism, is associated with weight gain, fatigue, weakness, dry skin [4], anemia [5], and predisposition to depressive conditions [6], even if euthyroidism is present, whereas GD, the most common cause of hyperthyroidism in iodine-sufficient areas, co-occurs with weight loss, heat intolerance, trembling, anxiety, tachycardia, and irritability [7]. Graves’ ophthalmopathy (GO) is a characteristic complication of GD, which in mild form affects 25–50% of patients with GD and manifests as eye pain, eyelid edema, excessive tearing, and light sensitivity. However, a minority of patients experience vision deterioration, corneal breakdown, and optic nerve neuropathy [8,9].
Recently, the role of microbiota in autoimmune diseases has gained much attention. Every human being has a unique composition of intestinal microbes, and there is no single optimal pattern. However, in most of the cases, Firmicutes and Bacteroidetes are the predominant phyla. The composition of the microbiome is variable and depends on several factors such as gender, age, lifestyle, physical activity, drug therapies, and diet [10,11].
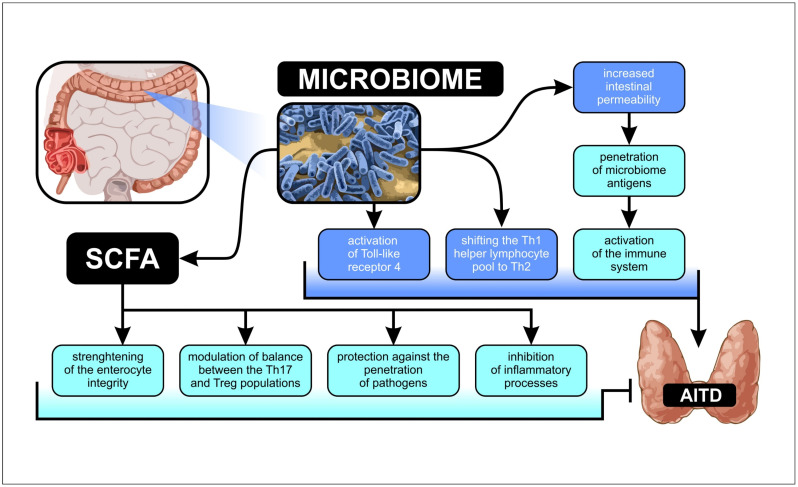
Studies have reported the association between dysbiosis and the onset of diseases such as type 1 diabetes, rheumatoid arthritis, inflammatory bowel disease, systemic lupus erythematosus, autoimmune dermatitis, and autoimmune neurological diseases [12]. In these diseases, interactions in the pathomechanism can be observed at the level of microbes and their metabolites. Several pathomechanisms focusing on AITDs are available in the literature. Firstly, alterations in the composition of intestinal bacteria can lead to increased intestinal permeability [13], which is associated with an increased level of zonulin, a protein responsible for the regulation of intercellular connection [14,15]. The decreased tightness of enterocytes enables the penetration of microbiome antigens and the activation of the immune system via molecular mimicry [16]. Some bacterial antigens in the intestine have structures similar to those of autoantigens. Due to this analogy, plasma cells may be activated to generate antibodies that bind to antigens expressed on thyroid follicle cells and orbital fibroblasts in GO [17]. Dysbiosis also results in an increased production of autoantibodies by posttranslational protein modification. Furthermore, it contributes to the development of AITDs by shifting the Th1 helper lymphocyte pool to Th2 and inducing Toll-like receptor 4’s activation [18,19].
Similarly, metabolites of the microbiome significantly affect thyroid function. Research studies have focused primarily on short-chain fatty acids (SCFAs), which strengthen the integrity of enterocytes, protect against the intrusion of pathological microbes, modulate the immune system, and inhibit inflammatory processes [20,21]. In addition, SCFAs are primarily involved in the modulation of the balance between Th17 and Treg populations, which are closely related to the development of autoimmune diseases [17,22].
Furthermore, the gut–thyroid axis includes the involvement of the microbiome in the metabolism of thyroid hormones. Previous studies have indicated the presence of deiodinases in the human intestine. Studies using animal models have reported the ability of intestinal bacteria to absorb deconjugated iodothyronine and even the competition to bind thyroid hormones to albumin [13,23]. In addition, some studies have reported that intestinal microbes contribute to the enterohepatic metabolism of thyroid hormones [24,25]. Furthermore, the microbiota influences the uptake of microelements necessary for the functioning of the thyroid gland, such as iodine, copper, iron, selenium, and zinc. Animal models have shown limited iodine uptake in individuals lacking the microbiome; however, no such association has been observed in parenterally fed humans with short bowel syndrome or after bariatric surgery [20,26,27]. In contrast, the competitive bacterial uptake of selenium has been observed, thereby reducing the bioavailability under reduced selenium conditions [26,28,29]. The diversity of reports on the interaction between the microbiota and the thyroid gland prompts an investigation into the involvement of the microbiome in thyroid disorders (Figure 1).
Figure 1.
Potential relationships between alterations in gut microbiota and autoimmune thyroid disease pathogenesis. (AITD, autoimmune thyroid disease; SCFA, short-chain fatty acid).
This systematic review was designed to answer the following question: “Is microbiota altered in patients with autoimmune thyroid diseases?”, which was formulated according to PICO (“Population”, “Intervention”, “Comparison” and “Outcome”).
2. Results
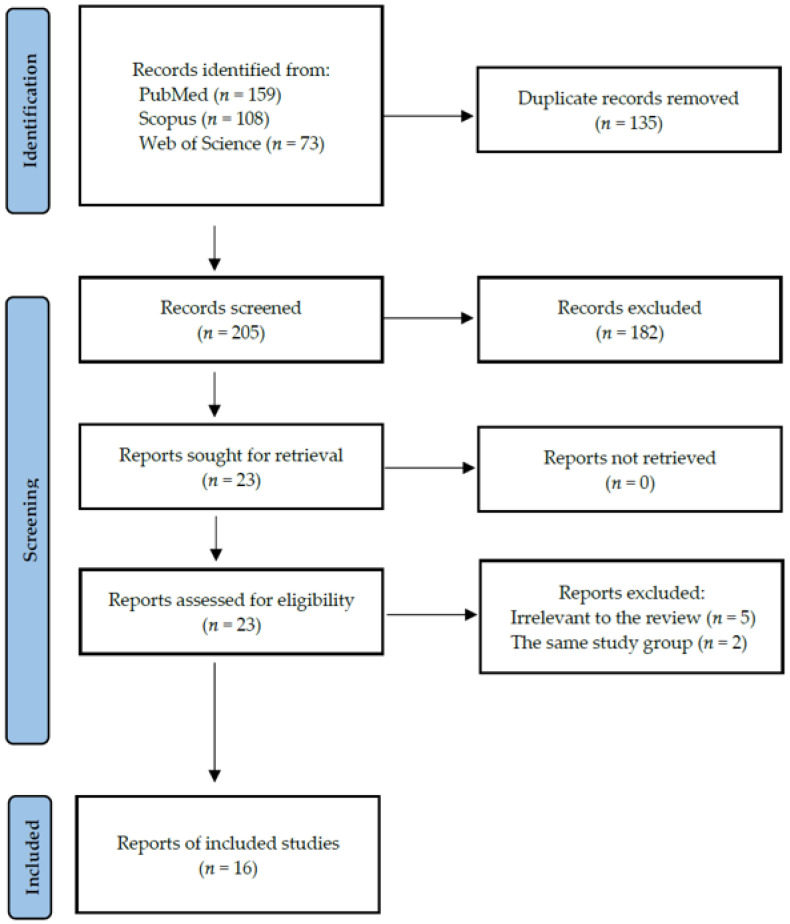
After screening the studies using the inclusion and exclusion criteria, 16 studies were included in this review, thus including data collected in five different countries from a total of 761 human participants with diagnosed AITDs (including 563 with GD and 198 with HT) and 488 controls. The detailed selection strategy of the studies is shown in Figure 2. The inclusion and exclusion criteria are presented in Section 4.
Figure 2.
PRISMA flow diagram representing the search strategy.
From each eligible study included in this systematic review, data on general characteristics were collected, such as the year of publication and setting, participants, AITD diagnosis, inclusion and exclusion criteria, thyroid parameters determined, and medications supplemented (Table 1). The detailed characteristics are presented in Table 2, such as the type of laboratory material, methods of microbiological analysis, altered microbiota composition (at the phylum and genus levels), and changes in richness (ACE and Chao1) and diversity (Simpson and Shannon) indices. All studies examined fecal samples, which were analyzed using 16S rRNA gene sequencing (except for one study that used 16S rDNA gene sequencing).
Table 1.
General characteristics of the included studies.
| Author, Year | Setting | Study Group (F/M; Age) | Control Group (F/M; Age) | AITD Diagnosis |
Inclusion Criteria | Exclusion Criteria | Thyroid Parameters Determined | Treatment |
|---|---|---|---|---|---|---|---|---|
| Chang et al., 2021 [30] | Taiwan | 55 (35/20); 45.09 ± 12.08 | 48 (30/18); 42.60 ± 9.78 | GD | Previously diagnosed with GD | Pregnancy, gastrointestinal disorders, gout, stroke, cancer, autoimmune diseases, history of gastrointestinal surgery, use of antibiotics, probiotics, prebiotics or symbiotics (<2 months), hormonal medication, Chinese herbal medicine (<3 months), pure vegetarians | TSH, FT4, TPOAb | PTU, MMI, CBZ |
| Chen et al., 2021 [31] | China | 15 (8/7); 28.87 ± 6.79 | 14 (8/6); 27.29 ± 5.73 | GD | Previously diagnosed GD | Untreated, transient hyperthyroidism, autoimmune diseases, long-term hormone treatment, diabetes, metabolic diseases, constipation, chronic diarrhea, inflammatory bowel disease, cancer, familial genetic disease, severe dysfunction of multiple organs, use of antibiotics, microbiological preparations (<1 month), vegetarians, pregnancy, lactation, history of gastrointestinal surgery, addiction to alcohol or drugs | TSH, FT3, FT4, T3, T4, TGAb, TPOAb, TRAb | MMI |
| Ishaq et al., 2018 [32] | China | 27 (17/10); range: 35–50 | 11 (7/4); age-matched | GD | Previously diagnosed GD | GD treatment (<6 months), gastrointestinal diseases, use of prebiotics, probiotics, or antibiotics (<2 months) | TSH, FT3, FT4, T3, T4, TGAb, TPOAb, TRAb | No treatment (<6 months) |
| Jiang et al., 2021 [33] | China | 45 (33/12); 37 (range: 16–65) | 59 (37/22); 43 (range: 22–71) | GD | Previously diagnosed GD | Malignancies, gastrointestinal diseases, endocrine system diseases, use of antibiotics, probiotics, or prebiotics (<1 month) | TSH, FT3, FT4, T3, T4, TGAb, TPOAb, TRAb, TMAb | Untreated |
| Shi et al., 2019 [34] | China | 33 (16/17); 46.0 ± 11.71 | 32 (16/16); 43.4 ± 9.7 | GO | Diagnosed with Graves’ orbitopathy, CAS ≥ 3/7, and NOSPECS score ≥IV | Age < 18 or >65 years, use of antibiotics or probiotics (<4 weeks), use of hormonal medication, Chinese herbal medicine (<3 months), chronic diarrhea or constipation, inflammatory bowel disease, acute infections, diabetes, stroke, heart diseases, renal or hepatic dysfunction, cancer, autoimmune diseases, gastrointestinal surgery, pure vegetarians, pregnancy, lactation, alcohol or substance addiction | TSH, FT3, FT4, T3, T4, TGAb, TPOAb, TRAb | MMI |
| Shi et al., 2021 [35] | China | GO: 33 (16/17); 46.0 ± 11.7; GD: 30 (20/10); 45.0 ± 12.8 |
32 (16/16); 43.4 ± 9.7 | GD/GO | Diagnosed with GD/GO | Age < 18 or >65 years, use of antibiotics or probiotics (<4 weeks), use of hormonal medication, Chinese herbal medicine (<3 months), chronic diarrhea or constipation, inflammatory bowel disease, acute infections, diabetes, stroke, heart diseases, renal or hepatic dysfunction, cancer, autoimmune diseases, gastrointestinal surgery, pure vegetarians, pregnancy, lactation, alcohol or substance addiction | TSH, FT3, FT4, T3, T4, TGAb, TPOAb, TRAb | MMI |
| Su et al., 2020 [36] | China | 58 (35/23); 42.07 ± 10.22 | 63 (35/28); 43.86 ± 9.20 | GD | Diagnosed with GD | Pregnancy, smoking, alcohol addiction, diarrhea, hypertension, diabetes, lipid dysregulation, BMI > 27, use of antibiotics, probiotics, prebiotics, symbiotics, hormonal medication, laxatives, proton pump inhibitors, insulin sensitizers, Chinese herbal medicine (<3 months), autoimmune diseases, malignancy, history of gastrointestinal surgery | TSH, FT3, FT4, TGAb, TPOAb | Untreated |
| Yan et al., 2020 [37] | China | 39 (28/11); 37.49 ± 12.95 | 17 (11/6); 33.42 ± 9.13 | GD | Diagnosed with GD | Pregnancy, smoking, alcohol addiction, use of antibiotics, hormonal medication, Chinese herbal medicine (3 months), use of medicine for the treatment of GD (<6 months), gastrointestinal diseases | TSH, T3, T4, TGAb, TPOAb, TRAb | No treatment (<6 months) |
| Yang et al., 2022 [38] | China | 191 (116/75); mean: 45.8 | 30 (NR); NR | GD | Newly diagnosed with GD, without receiving any treatment, participating voluntarily with compliance, and written informed consent | Use of probiotics, prebiotics, antibiotics, Chinese herbal medicine (<1 month), complications of infection-associated diseases or other diseases (<1 month), chronic stress | TSH, FT3, FT4, TPOAb, TGAb TRAb | Untreated |
| Yang et al., 2019 [39] | China | 15 (NR); range: 46–55 | 15 (age- and sex-matched) | GD | Diagnosed with GD | Osteoporosis, pregnancy, autoimmune diseases, infectious diseases, use of antibiotics, probiotics, metformin, acarbose, herbal preparations, use of any antithyroid treatment, chronic stress | NR | NR |
| Cornejo-Pareja et al., 2020 [40] | Spain | GD: 9 (7/2); 46.2 ± 8.6; HT: 9 (9/0); 40.3 ± 9.6 |
11 (7/4); 48.8 ± 6.2 | GD and HT | Diagnosed with GD or HT | Pregnancy, diabetes, autoimmune diseases, gastrointestinal disorders, extreme diets, use of antibiotics, probiotics (<3 months), nonacceptance of informed consent | TSH, FT3, FT4, TPOAb, TSIAb | GD: CBZ; HT: LT4 |
| El-Zawawy et al., 2021 [41] | Egypt | GD: 13 (4/9); 38.2; HT: 7 (6/1); 39.4 |
30 (17/13); 39.7 ± 10.9 | GD and HT | Newly diagnosed and uncontrolled AITD (GD or HT) | Malignancy, recent gastrointestinal surgery (<6 months), recent hospitalization, use of antibiotics, nonsteroidal anti-inflammatory drugs, corticosteroids (<3 months), infectious diarrhea, other comorbidities, autoimmune diseases, pregnancy, severe burn, sepsis, pure vegetarians, smoking, alcohol or substance addiction, unable to give consent as mentally challenged, children | TSH, FT3, FT4, TPOAb, TRAb | NR |
| Cayres et al., 2021 [42] | Brazil | 40 (36/4); 48.9 ± 13.3 | 53 (NR); 45.6 ± 16.7 | HT | Diagnosed with HT | Use of anti-inflammatories, immunosuppressant drugs, antibiotics, vaccination (<30 days), gastrointestinal surgeries, inflammatory bowel diseases, chronic diarrhea | TSH, FT4, TPOAb, TGAb | LT4 |
| Ishaq et al., 2017 [43] | China | 29 (20/9); range: 40–60 | 12 (8/4); range: 40–60 | HT | Diagnosed with HT | Gastrointestinal diseases, use of antibiotics, probiotics, and prebiotics (<60 days) | TSH, T3, T4, TPOAb, TGAb | NR |
| Liu et al., 2020 [44] | China | HTE: 45 (45/0); 34.6 ± 1.0 HTH: 18 (18/0); 36.3 ± 2.1 |
34 (34/0); 29.6 ± 0.6 | HT | Diagnosed with HT | Autoimmune diseases, thyroid surgeries, pregnancy, use of antibiotics (<1 week) | TSH, FT3, FT4, T3, T4, TPOAb, TGAb | LT4 |
| Zhao et al., 2018 [45] | China | Exploration cohort: 28 (25/3); 44.29 ± 12.25; validation cohort: 22 (19/3); 45.82 ± 10.7 |
Exploration cohort: 16 (14/2); 44.63 ± 10.33; validation cohort: 11 (9/2); 43.73 ± 10.95 |
HT | Diagnosed with HT, the presence of euthyroidism | Pregnancy, lactation, smoking, alcohol addiction, hypertension, diabetes, lipid dysregulation, BMI > 27, use of antibiotics, probiotics, prebiotics, symbiotics, hormonal medication, laxatives, proton pump inhibitors, insulin sensitizers, Chinese herbal medicine (<3 months), autoimmune diseases, malignancy, history of gastrointestinal surgery | TSH, FT3, FT4, TPOAb, TGAb | NR |
Legend: NR, not reported; F, females; M, males; AITD, autoimmune thyroid disease; GD, Graves’ disease; GO, Graves’ orbitopathy; HT, Hashimoto’s thyroiditis; HTH, Hashimoto’s thyroiditis with hypothyroidism; HTE, Hashimoto’s thyroiditis with euthyroidism; BMI, body mass index; TPOAb, thyroid peroxidase antibody; TRAb, TSH-receptor antibody; TGAb, thyroglobulin antibody; TSH, thyroid-stimulating hormone; TSIAb, thyroid-stimulating immunoglobulin antibody; TMAb, thyroid microsomal antibody; FT3, free triiodothyronine; FT4, free thyroxine; T3, triiodothyronine; T4, thyroxine; PTU, propylthiouracil; MMI, methimazole; CBZ, carbimazole; LT4, levothyroxine; CAS, clinical activity score.
Table 2.
Detailed characteristics of the included studies considering microbiological analysis and microbiota alterations.
| Study | AITD Diagnosis |
Type of Laboratory Material | Methods of Microbiological Analysis | Altered Microbiota Composition | Richness | Diversity | ||
|---|---|---|---|---|---|---|---|---|
| ACE | Chao1 | Simpson | Shannon | |||||
| Chang et al., 2021 [30] | GD | Fecal samples collected in a clean container, aliquoted, immediately frozen, and stored at −80 °C | 16S rRNA gene sequencing | up: Bacteroidetes, Actinobacteria/Bacteroides, Prevotella_9; down: Firmicutes/Faecalibacterium, Lachnospiraceae_NK4A136_group | ↑ | ↑ | ↑ | ↑ |
| Chen et al., 2021 [31] | GD | Fecal samples collected on dry and clean paper, placed in sterile containers, transported at <4 °C, divided into portions, frozen, and stored at −80 °C | 16S rDNA gene sequencing | up: Lactobacillus, Veillonella, Streptococcus; down: Proteobacteria, Synergistetes | - | ↓ | ↓ * | ↓ * |
| Ishaq et al., 2018 [32] | GD | Fecal samples collected in an icebox, transported within 1 h of defecation, and stored at −80 °C | 16S rRNA gene sequencing | up: Prevotella_9, Haemophilus; down: Alistipes, Faecalibacterium, Dialister, Bifidobacterium, Lactobacillus | ↓ * | ↓ * | ↓ | ↓ |
| Jiang et al., 2021 [33] | GD | Fecal samples collected and stored at −80 °C | 16S rRNA gene sequencing | up: Bacteroidetes/Bacteroides, Lactobacillus; down: Firmicutes/Blautia, Eubacterium_hallii_group, Anaerostipes, Collinsella, Dorea, unclassified_f_Peptostreptococcaceae, Ruminococcus_torques_group | ↓ * | ↓ * | ↑ * | ↓ * |
| Shi et al., 2019 [34] | GO | Fecal samples (2.5 g) collected in tubes prefilled with fecal DNA stabilizer and stored at −80 °C | 16S rRNA gene sequencing | up: Bacteroidetes/unidentified_Prevotellaceae; down: Firmicutes/Blautia, Fusicatenibacter, Butyricicoccus, Anaerostipes, Collinsella | ns | ns | ↓ * | ↓ * |
| Shi et al., 2021 [35] | GD/GO | Fecal samples (2.5 g) collected in tubes prefilled with fecal DNA stabilizer and stored at −80 °C | 16S rRNA gene sequencing | GO vs. GD: up: Subdoligranulum, Bilophila; down: Deinococcus-Thermus, Chloroflexi/Blautia, Anaerostipes, Dorea, Butyricicoccus, Romboutsia, Fusicatenibacter, unidentified_Lachnospiraceae, unidentified_Clostridiales, Collinsella, Intestinibacter, Phascolarctobacterium | ns | ns | - | ↓ * |
| Su et al., 2020 [36] | GD | Fecal samples stored at −80 °C after liquid nitrogen freezing | 16S rRNA gene sequencing | up: Spirochaetae, Saccharibacteria, Bacteroidetes; down: Firmicutes, Proteobacteria, Synergistetes, Tenericutes, Verrucomicrobia | ↓ * | ↓ * | ↓ * | ↓ * |
| Yan et al., 2020 [37] | GD | Fecal samples stored at −80 °C | 16S rRNA gene sequencing | up: Bacilli, Lactobacillales, Prevotella, Megamonas, Veillonella; down: Ruminococcus, Rikenellaceae, Alistipes | - | ns | ns | ↓ * |
| Yang et al., 2022 [38] | GD | Fecal samples (10 g) immediately stored in sterile iceboxes and stored at −80 °C | 16S rRNA gene sequencing | up: Actinobacteria/Bifidobacterium, Collinsella, Pediococcus; down: Firmicutes/Roseburia, Dialister | ns | ns | ns | ns |
| Yang et al., 2019 [39] | GD | Fecal samples (2 g) stored at −80 °C | 16S rRNA gene sequencing | up: Firmicutes, Proteobacteria, Actinobacillus/Oribacterium, Mogibacterium, Lactobacillus, Aggregatibacter; down: Bacteroidetes | ↓ | ↓ | ↓ | ↓ |
| Cornejo-Pareja et al., 2020 [40] | GD | Fecal samples immediately refrigerated and stored at −80 °C | 16S rRNA gene sequencing | up: Fusobacterium, Sutterella; down: Faecalibacterium | - | - | - | ns |
| HT | up: Victivallaceae | - | - | - | ns | |||
| El-Zawawy et al., 2021 [41] | GD | Fecal samples kept at −20 °C upon defecation at home and stored at −80 °C | 16S rRNA gene sequencing | up: Bacteroidetes/Prevotella; down: Firmicutes | - | - | - | ↓ |
| HT | - | - | - | ↓ | ||||
| Cayres et al., 2021 [42] | HT | Fecal samples (200 mg) | 16S rRNA gene sequencing | up: Bacteroides; down: Bifidobacterium | - | - | - | - |
| Ishaq et al., 2017 [43] | HT | Fecal samples collected in a sterile cup, transported within 4 h of defecation, and stored at −80 °C | 16S rRNA gene sequencing | down: Bifidobacterium, Lactobacillus, Dialister | ↑ * | ↑ * | ↑ | ↑ |
| Liu et al., 2020 [44] | HT | Fecal samples collected in tubes prefilled with fecal DNA stabilizer and stored at −80 °C | 16S rRNA gene sequencing | HTH: up: Phascolarctobacterium; HTE: up: Lachnospiraceae_incertae_sedis, Lactonifactor, Alistipes, Subdoligranulum | - | - | - | ↓ * |
| Zhao et al., 2018 [45] | HT | Fecal samples immediately divided into aliquots, frozen on dry ice, and stored at −80 °C | 16S rRNA gene sequencing | up: Firmicutes/Blautia, Roseburia, Ruminococcus_torques_group, Romboutsia, Dorea, Fusicatenibacter, Eubacterium_hallii_group; down: Bacteroidetes/Faecalibacterium, Bacteroides, Prevotella_9, Lachnoclostridium | ↑ | ↑ | ↓ | ↑ |
Legend: AITD, autoimmune thyroid disease; GD, Graves’ disease; GO, Graves’ orbitopathy; HT, Hashimoto’s thyroiditis; -, not reported; ns, nonsignificant difference; *, significant difference; ↓, lowered value; ↑, increased value; ACE, abundance-based coverage estimator; HTH, Hashimoto’s thyroiditis with hypothyroidism; HTE, Hashimoto’s thyroiditis with euthyroidism.
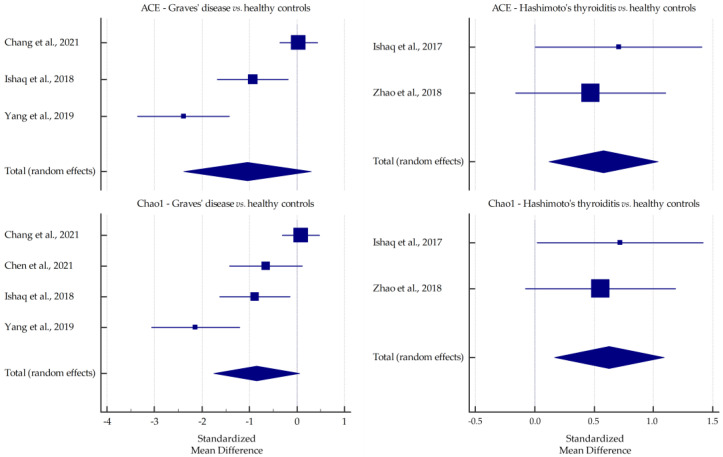
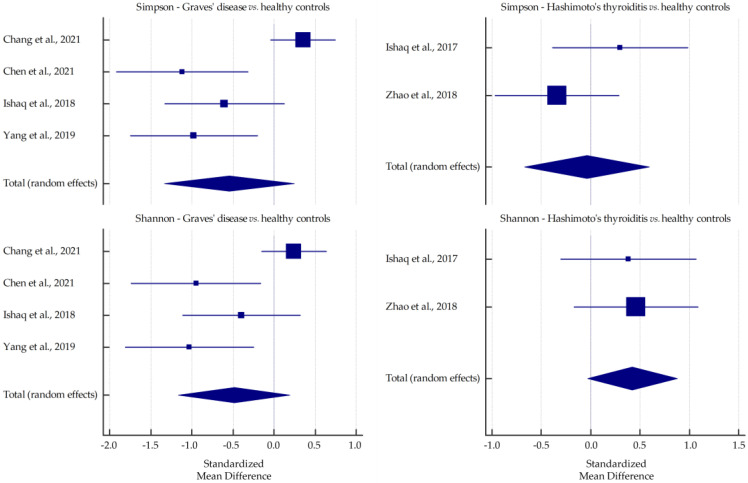
Pooled standardized mean differences in richness (ACE and Chao1) and diversity (Simpson and Shannon) indices for GD and HT are plotted in Figure 3 and Figure 4. All values were lower in GD patients than in healthy subjects, especially Chao1 at the borderline of significance (p-value = 0.068). However, in HT patients, significantly higher mean values of ACE and Chao1 indices were observed than in healthy controls (p-value = 0.014 and p-value = 0.008, respectively) and the Shannon index was increased at the margin of significance (p-value = 0.068).
Figure 3.
Pooled standardized mean differences in ACE and Chao1, shown separately for Graves’ disease and Hashimoto’s thyroiditis [30,31,32,39,43,45].
Figure 4.
Pooled standardized mean differences in Simpson and Shannon indices, shown separately for Graves’ disease and Hashimoto’s thyroiditis [30,31,32,39,43,45].
Significant differences in the relative abundance (at the phylum and genus levels) between the included studies (which reported p-values for comparisons) are presented in Table 3.
Table 3.
Relative abundance of significantly altered microbiota in autoimmune thyroid disease patients.
| Study | AITD Diagnosis |
Bacterial Phylum | Bacterial Genus | Relative Abundance | ||
|---|---|---|---|---|---|---|
| Study | Control | p-Value | ||||
| Chang et al., 2021 [30] | GD | Bacteroidetes | 0.4210 | 0.2690 | <0.01 | |
| Actinobacteria | 0.0288 | 0.0111 | <0.01 | |||
| Firmicutes | 0.5020 | 0.6630 | <0.01 | |||
| Collinsella | 0.0052 | 0.0014 | <0.01 | |||
| Parabacteroides | 0.0110 | 0.0009 | <0.01 | |||
| Prevotella_9 | 0.0767 | 0.0017 | <0.01 | |||
| Chen et al., 2021 [31] | GD | Synergistetes | 0.000012 | 0.0024 | 0.028 | |
| Veillonella | 0.0172 | 0.0006 | 0.039 | |||
| Ishaq et al., 2018 [32] | GD | Prevotella_9 | 0.4970 | 0.1952 | 0.034 | |
| Haemophilus | 0.1358 | 0.0099 | 0.049 | |||
| Dialister | 0.0110 | 0.0445 | 0.047 | |||
| Alistipes | 0.0180 | 0.0474 | 0.025 | |||
| Faecalibacterium | 0.0289 | 0.0562 | 0.014 | |||
| Yang et al., 2022 [38] | GD | Actinobacteria | 0.1229 | 0.0175 | 0.003 | |
| TM7 | 0.0001 | 0.00001 | 0.011 | |||
| Firmicutes | 0.6088 | 0.7522 | 0.044 | |||
| Cyanobacteria | 0.0002 | 0.00003 | 0.050 | |||
| El-Zawawy et al., 2021 [41] | GD and HT | Bacteroidetes | 0.738 | 0.326 | <0.001 | |
| Firmicutes | 0.248 | 0.543 | <0.001 | |||
| Prevotella | 0.4000 | 0.0161 | 0.006 | |||
| Ishaq et al., 2017 [43] | HT | Dialister | 0.0091 | 0.0446 | 0.029 | |
| Zhao et al., 2018 [45] | HT | Firmicutes | 0.826 | 0.691 | <0.001 | |
| Bacteroidetes | 0.099 | 0.227 | <0.001 | |||
| Faecalibacterium | 0.0987 | 0.1510 | 0.004 | |||
| Bacteroides | 0.0613 | 0.1330 | <0.001 | |||
| Prevotella_9 | 0.0183 | 0.0601 | <0.001 | |||
| Blautia | 0.0977 | 0.0586 | <0.001 | |||
| Roseburia | 0.0398 | 0.0312 | 0.010 | |||
| Lachnoclostridium | 0.0241 | 0.0285 | 0.013 | |||
| Ruminococcus_torques_group | 0.0306 | 0.0200 | 0.002 | |||
| Romboutsia | 0.0235 | 0.0146 | 0.006 | |||
| Dorea | 0.0200 | 0.0138 | 0.006 | |||
| Fusicatenibacter | 0.0186 | 0.0100 | <0.001 | |||
| Eubacterium_hallii_group | 0.0258 | 0.0110 | <0.001 | |||
Legend: AITD, autoimmune thyroid disease; GD, Graves’ disease; GO, Graves’ orbitopathy; HT, Hashimoto’s thyroiditis.
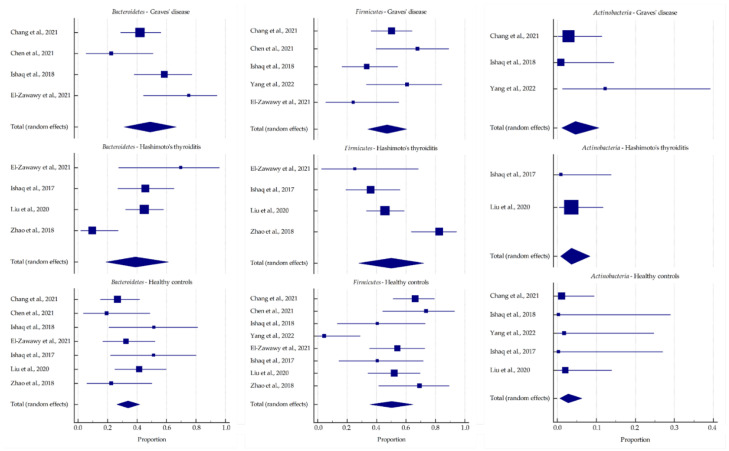
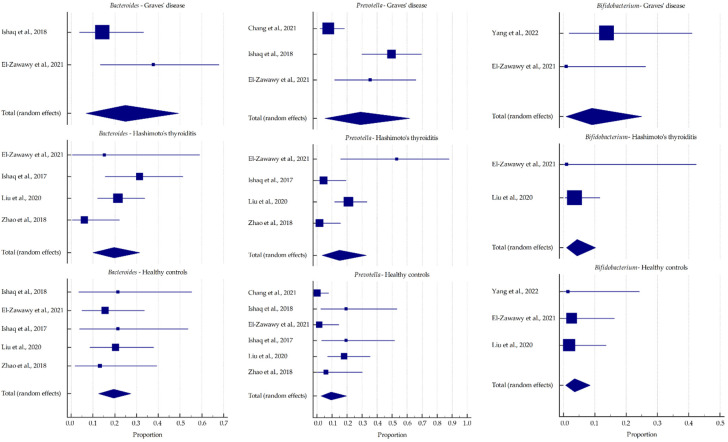
The pooled relative abundance calculated for the most common phyla and genera from the included studies is represented in Figure 5 and Figure 6, respectively, and their values are presented in Table 4. In GD patients, a trend toward an increased abundance of Bacteroidetes and Actinobacteria was observed at the phylum level. This was reflected in a higher abundance of Prevotella and Bifidobacterium at the genus level. In summary, HT patients showed an abundance of the selected microbiota similar to those of healthy subjects.
Figure 5.
Forest plots representing the pooled relative abundance for the most common phyla in the included studies [30,31,32,38,41,43,44,45].
Figure 6.
Forest plots representing the pooled relative abundance for the most common genera in the included studies [30,32,38,41,43,44,45].
Table 4.
Pooled relative abundance (%) for the most common microbiota in autoimmune thyroid disease patients.
| Microbial Phylum/Genus |
Graves’ Disease (A) |
Hashimoto’s Thyroiditis (B) |
Healthy Controls (C) |
p-Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean, % | −95% CI | +95% CI | Mean, % | −95% CI | +95% CI | Mean, % | −95% CI | +95% CI | A vs. B | A vs. C | B vs. C | |
| Bacteroidetes | 48.964 | 31.446 | 66.615 | 39.038 | 19.009 | 61.224 | 33.948 | 26.366 | 41.968 | 0.487 | 0.089 | 0.628 |
| Bacteroides | 24.806 | 6.786 | 49.378 | 19.629 | 10.027 | 31.489 | 19.498 | 12.611 | 27.466 | 0.653 | 0.560 | 0.985 |
| Prevotella | 28.911 | 5.242 | 61.787 | 14.965 | 3.338 | 32.884 | 9.451 | 2.638 | 19.853 | 0.360 | 0.127 | 0.513 |
| Firmicutes | 47.207 | 34.033 | 60.582 | 49.955 | 27.895 | 72.025 | 50.185 | 35.499 | 64.854 | 0.835 | 0.779 | 0.986 |
| Actinobacteria | 4.692 | 1.087 | 10.638 | 3.641 | 0.824 | 8.347 | 2.77 | 0.632 | 6.356 | 0.737 | 0.482 | 0.713 |
| Bifidobacterium | 9.027 | 0.759 | 24.955 | 4.354 | 0.894 | 10.238 | 3.54 | 0.656 | 8.593 | 0.390 | 0.274 | 0.794 |
Legend: CI, confidence interval.
In addition, significant correlations between microbiota alterations (at the phylum and genus levels) and the thyroid functional parameters determined, such as thyroid peroxidase antibody (TPOAb), TSH-receptor antibody (TRAb), thyroglobulin antibody (TGAb), and thyroid-stimulating hormone (TSH), are presented in Table 5.
Table 5.
Significant correlations between microbiota alterations with the thyroid functional parameters determined.
| TPOAb | TRAb | TGAb | TSH | |||||
|---|---|---|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | Positive | Negative | Positive | Negative | |
| Phyla | Firmicutes [41], Bacteroidetes [30,36,41], Actinobacteria [30] | Firmicutes [30], Proteobacteria [36], Synergistetes [31,36] | Firmicutes [41], Bacteroidetes [41] | Proteobacteria [36], Synergistetes [31,36] | Actinobacteria [36] | Synergistetes [31] | Firmicutes [30,36], Proteobacteria [36], Synergistetes [31,36] | Bacteroidetes [30,36] |
| Genera | Blautia [33,45], Lactobacillus [31], Streptococcus [36,45], Veillonella [36], Alistipes [40], Prevotella [30,36,41], Bifidobacterium [38] | Blautia [36], Faecalibacterium [30,40,45], Phascolarctobacterium [36,45], Alistipes [36], Bacteroides [33,36,45], Prevotella [45] | Lactobacillus [31], Streptococcus [36], Veillonella [36], Prevotella [36], Bifidobacterium [38] | Blautia [36], Lactobacillus [36], Phascolarctobacterium [31,36], Alistipes [36], Bacteroides [36] | Blautia [45], Streptococcus [36,45], Prevotella [36], Bifidobacterium [38] |
Phascolarctobacterium [36,45], Bacteroides [45], Prevotella [45] |
Blautia [36], Faecalibacterium [30], Lactobacillus [36], Phascolarctobacterium [31,36], Alistipes [36], Bacteroides [36] | Lactobacillus [31], Streptococcus [36], Veillonella [36], Prevotella [30,36] |
Legend: TPOAb, thyroid peroxidase antibody; TRAb, TSH-receptor antibody; TGAb, thyroglobulin antibody; TSH, thyroid-stimulating hormone.
3. Discussion
3.1. Microbiota Alpha-Diversity in AITD Patients
The diversity of the bacterial microbiota and the abundance of microbial species are characterized using specific alpha-diversity indices. Chao1 and ACE indices reflect the richness, whereas the Simpson index and the Shannon index reflect the community diversity (both richness and evenness) [30].
In all the studies included in this review, lower levels of richness indices (ACE and Chao1) were reported in GD patients than in healthy subjects. Statistically significant differences were reported in three studies [32,33,36]. For the Shannon index, significantly lower scores (including GO patients) were reported in six studies [31,33,34,35,36,37], and for the Simpson index, significantly lower scores were reported in three studies [31,34,36] and significantly higher scores were reported in one study [33]. One study reported strikingly contrasting results, reporting higher values (but not statistically significant) for all alpha-diversity indices in GD patients [30]. Despite this study, the meta-analysis showed a clear trend toward decreasing values of all indices in GD patients compared with healthy controls. It can be hypothesized that the lower diversity may be associated with the inflammatory response to the altered host immune function [33]. In addition, the decrease in microbial diversity may lead to a reduction in its functional capabilities, making it more susceptible to deleterious effects due to external disturbances [46]. Furthermore, this state is associated with several pathological conditions such as obesity, diabetes, inflammatory bowel diseases, polycystic ovary syndrome, and colorectal cancer [47,48,49,50,51]. Interestingly, Chen et al. [31] reported that microbiota diversity was significantly improved after 3–5 months of methimazole treatment.
However, in two studies, higher values of richness indices were reported in HT patients than in healthy subjects [43,45]. Similar findings were reported for the Simpson and the Shannon indices in these studies. The Shannon index was lower in HT patients than in healthy subjects in two studies [41,44]. The present meta-analysis confirmed increases in both richness indices and the Shannon index (as opposed to the Simpson index) in HT patients. This may be related to intestinal dysmotility and thus longer gastrointestinal transit time, predisposing to bacterial overgrowth in patients with hypothyroidism [52]. Although high microbiota diversity is generally associated with better health outcomes, it can also cause damaging effects such as increased protein breakdown and decreased polyphenol conversion, mucus secretion, and epithelial turnover [18].
Moreover, a Good’s coverage index of more than 99% was reported in the majority of the included studies, indicating that the current sequencing depth represented the true situation of fecal samples for gut microbiota. It should also be noted that the type of diet, eating habits, and geographical provenance can strongly influence gut microbiota diversity [53,54]. However, the effect of dietary factors was not considered in most of the studies, and only five studies excluded pure vegetarians [30,31,34,35,41]. Losasso et al. [55] reported a significantly higher richness in vegetarians than in omnivorous participants based on the Chao1 indices. However, these differences between omnivores and vegans or vegetarians were not confirmed in other studies [56,57]. Another potential confounding factor was cigarette smoking, one of the most important risk factors for GD and GO [58], since it was found to affect the composition of the gut microbiota [59,60,61].
3.2. Microbiota Relative Abundance in GD and HT Patients
In recent years, a possible role of the gut microbiome in the development and progression of diversity has been suggested, and its characteristics have been evaluated. Similar to the alpha-diversity analysis results, the intestinal microbiota composition at the phylum and genus levels was markedly different between GD patients and healthy controls. Firmicutes and Bacteroidetes phyla are the predominant components of the human gut microbiota, together comprising 90% of the total community. The relationship between these two phyla, expressed as the Firmicutes/Bacteroidetes ratio (F/B ratio), is a relevant marker of gut dysbiosis and is associated with various pathological conditions [62,63]. In most of the included studies, the F/B ratio was lower in GD patients than in healthy individuals, suggesting that this index could contribute to the pathogenesis of GD. A similar relationship was observed in GO patients [34,35]. However, Yang et al. [39] reported a significantly higher proportion of Firmicutes and a significantly lower proportion of Bacteroidetes in GD patients than in controls. Interestingly, an increased F/B ratio is usually observed in obesity [47]. Therefore, the effect of thyroid hormones on the composition and functioning of the intestinal microbiota, in addition to the increased basal metabolic rate, may result in weight changes [33]. This meta-analysis reported that the abundance of Bacteroidetes was higher in AITD patients, but that of Firmicutes did not differ compared with healthy subjects.
At lower taxonomic levels, the abundance of the genera was much more variable in both GD patients and healthy controls. Compared with the healthy controls, a significantly higher abundance of Bacteroides was reported in GD patients in two studies [30,33]. However, Shi et al. [35] reported that Bacteroides was significantly less abundant in the intestinal microbiota of GD patients than in healthy controls. Similar findings were reported in a murine model of GD/GO, where the INDIGO European consortium identified several disease-associated taxa, including reduced Bacteroides [64]. The gut microbiota of the murine model of GD/GO was also manipulated by antibiotics, probiotics, and human fecal material transfer, which resulted in the onset and modulation of the disease, thus confirming the effect of gut microbiota on GO [65]. The same international research group later confirmed a lower abundance of Bacteroides and a higher abundance of Actinobacteria in GD and GO patients compared with healthy controls (data unpublished). Bacteroides ferments glucose and lactate to SCFAs other than butyrate, such as acetate, succinate, and propionate, resulting in the reduction in mucin synthesis, tight junctions, and increased intestinal permeability, also known as the leaky gut syndrome (LGS). Furthermore, this leads to the disruption of gut homeostasis and may be involved in the pathogenesis and exacerbation of autoimmune disorders [66]. Moreover, in GD patients, a significant increase in Prevotella was reported in five studies [30,32,33,37,41]. In chronic inflammatory diseases, Prevotella mediate mucosal inflammation, which leads to the systemic dissemination of inflammatory mediators and bacterial products. Species of this genus predominantly activate Toll-like receptor 2, thus inducing the secretion of Th17-polarizing cytokines such as interleukin-1β, interleukin-6, and interleukin-23, and promote neutrophil recruitment by stimulating interleukin-17 production [67]. Yan et al. [37] reported that Prevotella might also affect the therapeutic efficacy of drugs for GD. Similarly, a higher abundance of Prevotella was reported in GO patients [34].
In addition, a higher abundance of the phylum Actinobacteria was reported, compared with healthy subjects, especially for two genera. Interestingly, the role of Bifidobacterium in immunopathogenesis is not clear since they may be protective or progressive in autoimmune diseases depending on the species [68]. For example, Bifidobacterium bifidum induces interleukin-17 secretion, promoting Th17 polarization, which is associated with autoimmune diseases [69]. Furthermore, the increased abundance of Collinsella is associated with an excessive interleukin-17 release and the altered permeability of the intestinal mucosa [70].
However, the relative abundance of the gut microbiota in HT patients was similar to that of healthy subjects, showing the reverse trend of alterations compared with GD patients. Blautia, the genus representative of the Firmicutes phylum, is an example of inverse dependencies between GD and HT. It is hypothesized that these commensal bacteria can mediate beneficial anti-inflammatory effects [71]. In addition, the abundance of Blautia was found to be significantly negatively correlated with visceral fat accumulation regardless of gender [72].
It is important to emphasize that similar to diversity, the relative abundance of the gut microbiota is also influenced by the type of diet. Previous studies reported that the Mediterranean diet was associated with the abundance of fiber-degrading bacterial genera such as Bifidobacterium, Prevotella, and Roseburia, and the suppression of Streptococcus and Ruminococcus [57,73]. However, the Western diet with higher fast-food consumption was characterized by decreased levels of Lactobacillus and Faecalibacterium [73,74].
3.3. Correlations between Microbiota Alterations and Thyroid Functional Parameters
The role of microbiota in the development of AITDs could be more clearly understood by investigating the relationships between changes (both functional and immunological) in its composition and thyroid functional parameters. Among the thyroid functional parameters determined, the most significant correlations were reported for TPOAb. Similarly, TSH and TRAb levels were often correlated with microbiota alterations. Only a few bacteria were correlated with the TGAb level.
However, the exact directions of these correlations are difficult to determine. At the phylum level, Bacteroidetes correlated negatively with the TSH level and positively with TPOAb and TRAb levels [30,36,41]. On the other hand, Proteobacteria and Synergistetes showed strikingly contrasting relationships [31,36].
At the genus level, especially in the phylum Firmicutes, a significant discrepancy in results was observed. Veillonella and Streptococcus correlated negatively with the TSH level and positively with TPOAb and TRAb levels, the latter genus correlated also with TGAb [36,45]. Moreover, Bifidobacterium showed the same findings [38]. However, Faecalibacterium and Phascolarctobacterium strains, as well as Bacteroides strains, showed contrasting correlations [30,31,33,36,40,45].
These findings confirm the significant correlations between some of the gut bacteria and thyroid parameters, indicating that microbiota alterations could be closely related to the development and progression of AITDs. Veillonella and Streptococcus are responsible for the development of oral diseases such as periodontitis and caries [75]. Both these genera interact metabolically and induce cytokine secretion by dendritic cells, resulting in an excessive immune response that may disrupt thyroid autoimmunity [76]. Moreover, Lactobacillus and Bifidobacterium strains have amino acid sequences familiar with TG and TPO, which can selectively bind with autoantibodies, triggering AITDs via molecular simulation mechanisms [77].
In contrast, Faecalibacterium is considered a protective factor in autoimmune processes, and its lower abundance stimulates the development of gastrointestinal disorders such as inflammatory bowel diseases and colorectal cancer [78]. Moreover, it is associated with a significant decrease in thyroid-stimulating immunoglobulin antibodies [40]. Similarly, a lower abundance of Phascolarctobacterium may lead to the altered production of SCFAs and thus an imbalance in immune homeostasis, increasing the host’s susceptibility to digestive and metabolic diseases [79]. At the phylum level, a lower abundance of Synergistetes may be involved in the balance of Th17/Treg differentiation, affecting the synthesis and secretion of autoantibodies in patients with autoimmune diseases [80].
Furthermore, the use of antithyroid drugs (ATD) might affect the gut microbiota composition. Little is known in this regard, except for in vitro studies that reported minor effects of ATD on 40 selected bacterial strains, even though other types of drugs were also able to inhibit the growth of one or more bacterial strains [81].
Moreover, gender, as well as the levels of sex hormones, could also affect the composition of intestinal microbes [82]. In women, subclinical thyroid abnormalities are more common and challenging to resolve [83]. The occurrence of subclinical hypothyroidism (SCH) may be associated with small intestinal bacterial overgrowth (SIBO). Wang et al. [84] reported a higher TPOAb-positive rate in SIBO-positive patients compared with SIBO-negative patients. In another study on pregnant women with SCH, differences in the gut microbiota composition and metabolic function were observed between TPOAb-positive and TPOAb-negative patients [85].
3.4. Study Limitations
The limitations of this systematic review include, in particular, the limitations of the selected studies. Sample sizes of these studies were relatively small, sometimes unmatched by age or gender in the case of control subjects. The majority of the selected studies were conducted in Asia, and one study each was conducted in Europe, Africa, and South America. Unfortunately, it was not possible to include all studies in the meta-analysis as complete data on diversity indices and relative abundance of the selected phyla and genera were not available (but only in diagrams with inaccurate scales). Only one study explicitly described GO patients, even though data from other independent groups are becoming available. It should be noted that all these studies used fecal samples to determine microbiota alterations, but the oral microbiome was not investigated, which may be of interest for further studies.
4. Materials and Methods
4.1. Search Strategy and Data Extraction
A systematic review was conducted up to 23 May 2022, according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement guidelines [86], using the databases PubMed, Scopus, and Web of Science. The search queries included the following:
For PubMed: (((thyroid OR Graves) AND (orbitopathy OR ophthalmopathy)) OR ((Graves OR Hashimoto) AND (disease OR thyroiditis))) AND (microbiome OR microbiota OR microflora)
For Scopus: TITLE-ABS-KEY((((thyroid OR Graves) AND (orbitopathy OR ophthalmopathy)) OR ((Graves OR Hashimoto) AND (disease OR thyroiditis))) AND (microbiome OR microbiota OR microflora))
For Web of Science: TS = ((((thyroid OR Graves) AND (orbitopathy OR ophthalmopathy)) OR ((Graves OR Hashimoto) AND (disease OR thyroiditis))) AND (microbiome OR microbiota OR microflora)).
The results were filtered by the publication date (i.e., studies published after 2000). The title, abstract, and full text of the results were screened by two independent investigators. Studies included in this review matched all the predefined criteria according to PICOS (“Population”, “Intervention”, “Comparison”, “Outcomes”, and “Study design”), as shown in Table 6. A detailed search flowchart is presented in the Section 2. The study protocol was registered in the international prospective register of systematic reviews PROSPERO (CRD42022335984).
Table 6.
Inclusion and exclusion criteria according to the PICOS.
| Parameter | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| Population | Patients with autoimmune thyroid diseases, including Graves’ disease and Hashimoto’s thyroiditis, aged 0–99 years, both sexes | Patients with other autoimmune diseases |
| Intervention | Not applicable | |
| Comparison | Not applicable | |
| Outcomes | Alterations in microbiota composition, including richness, diversity, and abundance indices | Alterations in microbiota composition without determined diversity indices |
| Study design | Case–control, cohort, and cross-sectional studies | Literature reviews, case reports, expert opinions, letters to the editor, conference reports |
| Published after 2000 | Not published in English |
The results of the meta-analysis were presented in forest plots using MedCalc Statistical Software, version 19.5.3 (MedCalc Software Ltd., Ostend, Belgium). The meta-analysis was performed using the subgroups GD and HT. Pooled standardized mean differences for diversity indices were calculated as continuous variables and pooled proportions for the relative abundance at the phylum and genus levels.
4.2. Quality Assessment and Critical Appraisal for the Systematic Review of the Included Studies
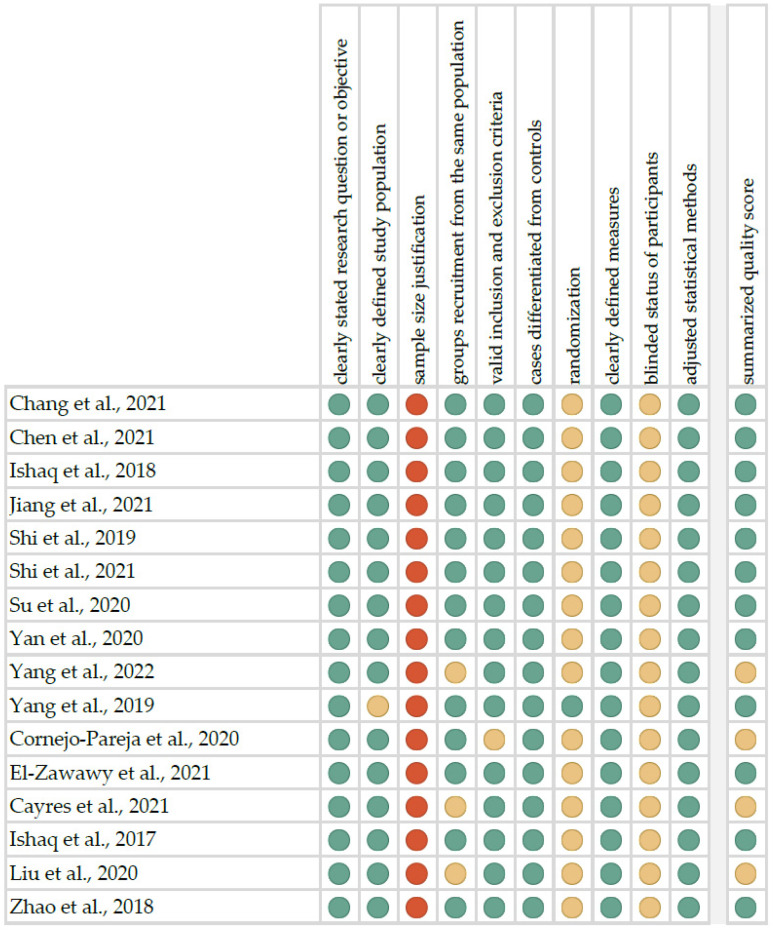
The risk of bias in each of the selected studies was assessed using the “Study Quality Assessment Tool” issued by the National Heart, Lung, and Blood Institute within the National Institute of Health [87]. This questionnaire was answered by the two independent investigators, and any disagreements were resolved by discussion between them. The summarized quality assessment for the individual studies is represented in Figure 7. Critical appraisal was summarized by adding the points for each criterion of potential risk (points: 1—low, 0.5—unspecified, 0—high). Twelve studies (75.0%) were classified as having “good” quality (≥80% total score) and four (25.0%) as having “intermediate” quality (≥60% total score). The level of evidence was evaluated using the classification of the Oxford Centre for Evidence-Based Medicine levels for diagnosis [88]. All of the included studies showed the third or fourth level of evidence (in this 5-grade scale).
Figure 7.
Quality assessment, including the main potential risk of bias (risk level: green—low, yellow—unspecified, red—high; quality score: green—good, yellow—intermediate, red—poor) [30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45].
5. Conclusions
The findings of this systematic review showed that significant alterations in the diversity and composition of the intestinal microbiota can be observed in both GD and HT patients. Compared with healthy subjects, higher diversity indices were observed in HT patients, whereas lower values were observed in GD patients. Moreover, a higher relative abundance of Bacteroidetes and Actinobacteria was observed in GD patients. Changes in the composition of microbiota are most commonly correlated with TPOAb levels. Further studies are required to confirm these findings.
Author Contributions
Conceptualization, N.S.-G., D.G. and N.Z.; methodology, N.S.-G., D.G., N.Z. and K.N.; formal analysis, D.G. and K.N.; investigation and data curation, D.G., N.Z. and K.N.; writing—original draft preparation, N.S.-G., D.G., N.Z. and K.N.; writing—review and editing, N.S.-G., I.M., T.K., M.S. and M.R.; visualization, D.G., N.Z. and K.N.; supervision, M.S. and M.R. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bogusławska J., Godlewska M., Gajda E., Piekiełko-Witkowska A. Cellular and Molecular Basis of Thyroid Autoimmunity. Eur. Thyroid J. 2022;11:e210024. doi: 10.1530/ETJ-21-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luty J., Ruckemann-Dziurdzińska K., Witkowski J.M., Bryl E. Immunological Aspects of Autoimmune Thyroid Disease—Complex Interplay between Cells and Cytokines. Cytokine. 2019;116:128–133. doi: 10.1016/j.cyto.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 3.Mikoś H., Mikoś M., Obara-Moszyńska M., Niedziela M. The Role of the Immune System and Cytokines Involved in the Pathogenesis of Autoimmune Thyroid Disease (AITD) Endokrynol. Pol. 2014;65:150–155. doi: 10.5603/EP.2014.0021. [DOI] [PubMed] [Google Scholar]
- 4.Mikulska A.A., Karaźniewicz-Łada M., Filipowicz D., Ruchała M., Główka F.K. Metabolic Characteristics of Hashimoto’s Thyroiditis Patients and the Role of Microelements and Diet in the Disease Management—An Overview. Int. J. Mol. Sci. 2022;23:6580. doi: 10.3390/ijms23126580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Szczepanek-Parulska E., Adamska M., Korda O., Kosicka W., Skowrońska D., Świejkowska A., Tuzimek D., Dadej D., Krygier A., Ruchała M. Changes in Complete Blood Count Parameters Influenced by Endocrine Disorders. Endokrynol. Pol. 2021;72:261–270. doi: 10.5603/EP.a2021.0059. [DOI] [PubMed] [Google Scholar]
- 6.Mohammad M.Y.H., Bushulaybi N.A., AlHumam A.S., AlGhamdi A.Y., Aldakhil H.A., Alumair N.A., Shafey M.M. Prevalence of Depression among Hypothyroid Patients Attending the Primary Healthcare and Endocrine Clinics of King Fahad Hospital of the University (KFHU) J. Fam. Med. Prim. Care. 2019;8:2708–2713. doi: 10.4103/jfmpc.jfmpc_456_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Antonelli A., Ferrari S.M., Ragusa F., Elia G., Paparo S.R., Ruffilli I., Patrizio A., Giusti C., Gonnella D., Cristaudo A., et al. Graves’ Disease: Epidemiology, Genetic and Environmental Risk Factors and Viruses. Best Pract. Res. Clin. Endocrinol. Metab. 2020;34:101387. doi: 10.1016/j.beem.2020.101387. [DOI] [PubMed] [Google Scholar]
- 8.Sawicka-Gutaj N., Ziółkowska P., Wojciechowska K., Shawkat S., Czarnywojtek A., Warchoł W., Sowiński J., Szczepanek-Parulska E., Ruchała M. Eye Symptoms in Patients with Benign Thyroid Diseases. Sci. Rep. 2021;11:18706. doi: 10.1038/s41598-021-98232-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gontarz-Nowak K., Szychlińska M., Matuszewski W., Stefanowicz-Rutkowska M., Bandurska-Stankiewicz E. Current Knowledge on Graves’ Orbitopathy. J. Clin. Med. 2021;10:16. doi: 10.3390/jcm10010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rinninella E., Raoul P., Cintoni M., Franceschi F., Miggiano G.A.D., Gasbarrini A., Mele M.C. What Is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms. 2019;7:14. doi: 10.3390/microorganisms7010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campaniello D., Corbo M.R., Sinigaglia M., Speranza B., Racioppo A., Altieri C., Bevilacqua A. How Diet and Physical Activity Modulate Gut Microbiota: Evidence, and Perspectives. Nutrients. 2022;14:2456. doi: 10.3390/nu14122456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Docimo G., Cangiano A., Romano R.M., Pignatelli M.F., Offi C., Paglionico V.A., Galdiero M., Donnarumma G., Nigro V., Esposito D., et al. The Human Microbiota in Endocrinology: Implications for Pathophysiology, Treatment, and Prognosis in Thyroid Diseases. Front. Endocrinol. 2020;11:586529. doi: 10.3389/fendo.2020.586529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knezevic J., Starchl C., Tmava Berisha A., Amrein K. Thyroid-Gut-Axis: How Does the Microbiota Influence Thyroid Function? Nutrients. 2020;12:1769. doi: 10.3390/nu12061769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fasano A. Zonulin and Its Regulation of Intestinal Barrier Function: The Biological Door to Inflammation, Autoimmunity, and Cancer. Physiol. Rev. 2011;91:151–175. doi: 10.1152/physrev.00003.2008. [DOI] [PubMed] [Google Scholar]
- 15.Zhang L., Masetti G., Colucci G., Salvi M., Covelli D., Eckstein A., Kaiser U., Draman M.S., Muller I., Ludgate M., et al. Combining Micro-RNA and Protein Sequencing to Detect Robust Biomarkers for Graves’ Disease and Orbitopathy. Sci. Rep. 2018;8:8386. doi: 10.1038/s41598-018-26700-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benvenga S., Guarneri F. Molecular Mimicry and Autoimmune Thyroid Disease. Rev. Endocr. Metab. Disord. 2016;17:485–498. doi: 10.1007/s11154-016-9363-2. [DOI] [PubMed] [Google Scholar]
- 17.Hou J., Tang Y., Chen Y., Chen D. The Role of the Microbiota in Graves’ Disease and Graves’ Orbitopathy. Front. Cell. Infect. Microbiol. 2021;11:739707. doi: 10.3389/fcimb.2021.739707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fröhlich E., Wahl R. Microbiota and Thyroid Interaction in Health and Disease. Trends Endocrinol. Metab. TEM. 2019;30:479–490. doi: 10.1016/j.tem.2019.05.008. [DOI] [PubMed] [Google Scholar]
- 19.Mohammad Hosseini A., Majidi J., Baradaran B., Yousefi M. Toll-Like Receptors in the Pathogenesis of Autoimmune Diseases. Adv. Pharm. Bull. 2015;5:605–614. doi: 10.15171/apb.2015.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bargiel P., Szczuko M., Stachowska L., Prowans P., Czapla N., Markowska M., Petriczko J., Kledzik J., Jędrzejczyk-Kledzik A., Palma J., et al. Microbiome Metabolites and Thyroid Dysfunction. J. Clin. Med. 2021;10:3609. doi: 10.3390/jcm10163609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamamoto E.A., Jørgensen T.N. Relationships Between Vitamin D, Gut Microbiome, and Systemic Autoimmunity. Front. Immunol. 2020;10:3141. doi: 10.3389/fimmu.2019.03141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu H., Liu H., Liu C., Shang M., Wei T., Yin P. Gut Microbiome and the Role of Metabolites in the Study of Graves’ Disease. Front. Mol. Biosci. 2022;9:841223. doi: 10.3389/fmolb.2022.841223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Virili C., Centanni M. Does Microbiota Composition Affect Thyroid Homeostasis? Endocrine. 2015;49:583–587. doi: 10.1007/s12020-014-0509-2. [DOI] [PubMed] [Google Scholar]
- 24.Virili C., Fallahi P., Antonelli A., Benvenga S., Centanni M. Gut Microbiota and Hashimoto’s Thyroiditis. Rev. Endocr. Metab. Disord. 2018;19:293–300. doi: 10.1007/s11154-018-9467-y. [DOI] [PubMed] [Google Scholar]
- 25.Fernández-García V., González-Ramos S., Martín-Sanz P., Laparra J.M., Boscá L. Beyond Classic Concepts in Thyroid Homeostasis: Immune System and Microbiota. Mol. Cell. Endocrinol. 2021;533:111333. doi: 10.1016/j.mce.2021.111333. [DOI] [PubMed] [Google Scholar]
- 26.Virili C., Centanni M. “With a Little Help from My Friends”—The Role of Microbiota in Thyroid Hormone Metabolism and Enterohepatic Recycling. Mol. Cell. Endocrinol. 2017;458:39–43. doi: 10.1016/j.mce.2017.01.053. [DOI] [PubMed] [Google Scholar]
- 27.Vought R.L., Brown F.A., Sibinovic K.H., Daniel E.G.M. Effect of Changing Intestinal Bacterial Flora on Thyroid Function in the Rat. Horm. Metab. Res. 1972;4:43–47. doi: 10.1055/s-0028-1094095. [DOI] [PubMed] [Google Scholar]
- 28.Ferreira R.L.U., Sena-Evangelista K.C.M., de Azevedo E.P., Pinheiro F.I., Cobucci R.N., Pedrosa L.F.C. Selenium in Human Health and Gut Microflora: Bioavailability of Selenocompounds and Relationship with Diseases. Front. Nutr. 2021;8:685317. doi: 10.3389/fnut.2021.685317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gorini F., Vassalle C. Selenium and Selenoproteins at the Intersection of Type 2 Diabetes and Thyroid Pathophysiology. Antioxidants. 2022;11:1188. doi: 10.3390/antiox11061188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang S.-C., Lin S.-F., Chen S.-T., Chang P.-Y., Yeh Y.-M., Lo F.-S., Lu J.-J. Alterations of Gut Microbiota in Patients with Graves’ Disease. Front. Cell. Infect. Microbiol. 2021;11:663131. doi: 10.3389/fcimb.2021.663131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen J., Wang W., Guo Z., Huang S., Lei H., Zang P., Lu B., Shao J., Gu P. Associations between Gut Microbiota and Thyroidal Function Status in Chinese Patients with Graves’ Disease. J. Endocrinol. Investig. 2021;44:1913–1926. doi: 10.1007/s40618-021-01507-6. [DOI] [PubMed] [Google Scholar]
- 32.Ishaq H.M., Mohammad I.S., Shahzad M., Ma C., Raza M.A., Wu X., Guo H., Shi P., Xu J. Molecular Alteration Analysis of Human Gut Microbial Composition in Graves’ Disease Patients. Int. J. Biol. Sci. 2018;14:1558–1570. doi: 10.7150/ijbs.24151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiang W., Yu X., Kosik R.O., Song Y., Qiao T., Tong J., Liu S., Fan S., Luo Q., Chai L., et al. Gut Microbiota May Play a Significant Role in the Pathogenesis of Graves’ Disease. Thyroid Off. J. Am. Thyroid Assoc. 2021;31:810–820. doi: 10.1089/thy.2020.0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shi T.-T., Xin Z., Hua L., Zhao R.-X., Yang Y.-L., Wang H., Zhang S., Liu W., Xie R.-R. Alterations in the Intestinal Microbiota of Patients with Severe and Active Graves’ Orbitopathy: A Cross-Sectional Study. J. Endocrinol. Investig. 2019;42:967–978. doi: 10.1007/s40618-019-1010-9. [DOI] [PubMed] [Google Scholar]
- 35.Shi T.-T., Xin Z., Hua L., Wang H., Zhao R.-X., Yang Y.-L., Xie R.-R., Liu H.-Y., Yang J.-K. Comparative Assessment of Gut Microbial Composition and Function in Patients with Graves’ Disease and Graves’ Orbitopathy. J. Endocrinol. Investig. 2021;44:297–310. doi: 10.1007/s40618-020-01298-2. [DOI] [PubMed] [Google Scholar]
- 36.Su X., Yin X., Liu Y., Yan X., Zhang S., Wang X., Lin Z., Zhou X., Gao J., Wang Z., et al. Gut Dysbiosis Contributes to the Imbalance of Treg and Th17 Cells in Graves’ Disease Patients by Propionic Acid. J. Clin. Endocrinol. Metab. 2020;105:dgaa511. doi: 10.1210/clinem/dgaa511. [DOI] [PubMed] [Google Scholar]
- 37.Yan H.-X., An W.-C., Chen F., An B., Pan Y., Jin J., Xia X.-P., Cui Z.-J., Jiang L., Zhou S.-J., et al. Intestinal Microbiota Changes in Graves’ Disease: A Prospective Clinical Study. Biosci. Rep. 2020;40:BSR20191242. doi: 10.1042/BSR20191242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang M., Li F., Zhang R., Wu Y., Yang Q., Wang F., Yu Z., Liu J., Cha B., Gong Q., et al. Alteration of the Intestinal Microbial Flora and the Serum IL-17 Level in Patients with Graves’ Disease Complicated with Vitamin D Deficiency. Int. Arch. Allergy Immunol. 2022;183:225–234. doi: 10.1159/000518949. [DOI] [PubMed] [Google Scholar]
- 39.Yang M., Sun B., Li J., Yang B., Xu J., Zhou X., Yu J., Zhang X., Zhang Q., Zhou S., et al. Alteration of the Intestinal Flora May Participate in the Development of Graves’ Disease: A Study Conducted among the Han Population in Southwest China. Endocr. Connect. 2019;8:822–828. doi: 10.1530/EC-19-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cornejo-Pareja I., Ruiz-Limón P., Gómez-Pérez A.M., Molina-Vega M., Moreno-Indias I., Tinahones F.J. Differential Microbial Pattern Description in Subjects with Autoimmune-Based Thyroid Diseases: A Pilot Study. J. Pers. Med. 2020;10:192. doi: 10.3390/jpm10040192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.El-Zawawy H.T., Ahmed S.M., El-Attar E.A., Ahmed A.A., Roshdy Y.S., Header D.A. Study of Gut Microbiome in Egyptian Patients with Autoimmune Thyroid Diseases. Int. J. Clin. Pract. 2021;75:e14038. doi: 10.1111/ijcp.14038. [DOI] [PubMed] [Google Scholar]
- 42.Cayres L.C.d.F., de Salis L.V.V., Rodrigues G.S.P., van Helvoort Lengert A., Biondi A.P.C., Sargentini L.D.B., Brisotti J.L., Gomes E., de Oliveira G.L.V. Detection of Alterations in the Gut Microbiota and Intestinal Permeability in Patients With Hashimoto Thyroiditis. Front. Immunol. 2021;12:579140. doi: 10.3389/fimmu.2021.579140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ishaq H.M., Mohammad I.S., Guo H., Shahzad M., Hou Y.J., Ma C., Naseem Z., Wu X., Shi P., Xu J. Molecular Estimation of Alteration in Intestinal Microbial Composition in Hashimoto’s Thyroiditis Patients. Biomed. Pharmacother. Biomed. Pharmacother. 2017;95:865–874. doi: 10.1016/j.biopha.2017.08.101. [DOI] [PubMed] [Google Scholar]
- 44.Liu S., An Y., Cao B., Sun R., Ke J., Zhao D. The Composition of Gut Microbiota in Patients Bearing Hashimoto’s Thyroiditis with Euthyroidism and Hypothyroidism. Int. J. Endocrinol. 2020;2020:5036959. doi: 10.1155/2020/5036959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao F., Feng J., Li J., Zhao L., Liu Y., Chen H., Jin Y., Zhu B., Wei Y. Alterations of the Gut Microbiota in Hashimoto’s Thyroiditis Patients. Thyroid Off. J. Am. Thyroid Assoc. 2018;28:175–186. doi: 10.1089/thy.2017.0395. [DOI] [PubMed] [Google Scholar]
- 46.Sommer F., Anderson J.M., Bharti R., Raes J., Rosenstiel P. The Resilience of the Intestinal Microbiota Influences Health and Disease. Nat. Rev. Microbiol. 2017;15:630–638. doi: 10.1038/nrmicro.2017.58. [DOI] [PubMed] [Google Scholar]
- 47.Pinart M., Dötsch A., Schlicht K., Laudes M., Bouwman J., Forslund S.K., Pischon T., Nimptsch K. Gut Microbiome Composition in Obese and Non-Obese Persons: A Systematic Review and Meta-Analysis. Nutrients. 2021;14:12. doi: 10.3390/nu14010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brennan C.A., Garrett W.S. Gut Microbiota, Inflammation, and Colorectal Cancer. Annu. Rev. Microbiol. 2016;70:395–411. doi: 10.1146/annurev-micro-102215-095513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sultan S., El-Mowafy M., Elgaml A., Ahmed T.A.E., Hassan H., Mottawea W. Metabolic Influences of Gut Microbiota Dysbiosis on Inflammatory Bowel Disease. Front. Physiol. 2021;12:715506. doi: 10.3389/fphys.2021.715506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gaike A.H., Paul D., Bhute S., Dhotre D.P., Pande P., Upadhyaya S., Reddy Y., Sampath R., Ghosh D., Chandraprabha D., et al. The Gut Microbial Diversity of Newly Diagnosed Diabetics but Not of Prediabetics Is Significantly Different from That of Healthy Nondiabetics. mSystems. 2020;5:e00578-19. doi: 10.1128/mSystems.00578-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Torres P.J., Siakowska M., Banaszewska B., Pawelczyk L., Duleba A.J., Kelley S.T., Thackray V.G. Gut Microbial Diversity in Women with Polycystic Ovary Syndrome Correlates With Hyperandrogenism. J. Clin. Endocrinol. Metab. 2018;103:1502–1511. doi: 10.1210/jc.2017-02153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lauritano E.C., Bilotta A.L., Gabrielli M., Scarpellini E., Lupascu A., Laginestra A., Novi M., Sottili S., Serricchio M., Cammarota G., et al. Association between Hypothyroidism and Small Intestinal Bacterial Overgrowth. J. Clin. Endocrinol. Metab. 2007;92:4180–4184. doi: 10.1210/jc.2007-0606. [DOI] [PubMed] [Google Scholar]
- 53.Rinninella E., Cintoni M., Raoul P., Lopetuso L.R., Scaldaferri F., Pulcini G., Miggiano G.A.D., Gasbarrini A., Mele M.C. Food Components and Dietary Habits: Keys for a Healthy Gut Microbiota Composition. Nutrients. 2019;11:2393. doi: 10.3390/nu11102393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Senghor B., Sokhna C., Ruimy R., Lagier J.-C. Gut Microbiota Diversity According to Dietary Habits and Geographical Provenance. Hum. Microbiome J. 2018;7–8:1–9. doi: 10.1016/j.humic.2018.01.001. [DOI] [Google Scholar]
- 55.Losasso C., Eckert E.M., Mastrorilli E., Villiger J., Mancin M., Patuzzi I., Di Cesare A., Cibin V., Barrucci F., Pernthaler J., et al. Assessing the Influence of Vegan, Vegetarian and Omnivore Oriented Westernized Dietary Styles on Human Gut Microbiota: A Cross Sectional Study. Front. Microbiol. 2018;9:317. doi: 10.3389/fmicb.2018.00317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu G.D., Compher C., Chen E.Z., Smith S.A., Shah R.D., Bittinger K., Chehoud C., Albenberg L.G., Nessel L., Gilroy E., et al. Comparative Metabolomics in Vegans and Omnivores Reveal Constraints on Diet-Dependent Gut Microbiota Metabolite Production. Gut. 2016;65:63–72. doi: 10.1136/gutjnl-2014-308209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.De Filippis F., Pellegrini N., Vannini L., Jeffery I.B., La Storia A., Laghi L., Serrazanetti D.I., Di Cagno R., Ferrocino I., Lazzi C., et al. High-Level Adherence to a Mediterranean Diet Beneficially Impacts the Gut Microbiota and Associated Metabolome. Gut. 2016;65:1812–1821. doi: 10.1136/gutjnl-2015-309957. [DOI] [PubMed] [Google Scholar]
- 58.Wiersinga W.M. Smoking and Thyroid. Clin. Endocrinol. 2013;79:145–151. doi: 10.1111/cen.12222. [DOI] [PubMed] [Google Scholar]
- 59.Lee S.H., Yun Y., Kim S.J., Lee E.-J., Chang Y., Ryu S., Shin H., Kim H.-L., Kim H.-N., Lee J.H. Association between Cigarette Smoking Status and Composition of Gut Microbiota: Population-Based Cross-Sectional Study. J. Clin. Med. 2018;7:282. doi: 10.3390/jcm7090282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Biedermann L., Zeitz J., Mwinyi J., Sutter-Minder E., Rehman A., Ott S.J., Steurer-Stey C., Frei A., Frei P., Scharl M., et al. Smoking Cessation Induces Profound Changes in the Composition of the Intestinal Microbiota in Humans. PLoS ONE. 2013;8:e59260. doi: 10.1371/journal.pone.0059260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gui X., Yang Z., Li M.D. Effect of Cigarette Smoke on Gut Microbiota: State of Knowledge. Front. Physiol. 2021;12:673341. doi: 10.3389/fphys.2021.673341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stojanov S., Berlec A., Štrukelj B. The Influence of Probiotics on the Firmicutes/Bacteroidetes Ratio in the Treatment of Obesity and Inflammatory Bowel Disease. Microorganisms. 2020;8:1715. doi: 10.3390/microorganisms8111715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Magne F., Gotteland M., Gauthier L., Zazueta A., Pesoa S., Navarrete P., Balamurugan R. The Firmicutes/Bacteroidetes Ratio: A Relevant Marker of Gut Dysbiosis in Obese Patients? Nutrients. 2020;12:1474. doi: 10.3390/nu12051474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Masetti G., Moshkelgosha S., Köhling H.-L., Covelli D., Banga J.P., Berchner-Pfannschmidt U., Horstmann M., Diaz-Cano S., Goertz G.-E., Plummer S., et al. Gut Microbiota in Experimental Murine Model of Graves’ Orbitopathy Established in Different Environments May Modulate Clinical Presentation of Disease. Microbiome. 2018;6:97. doi: 10.1186/s40168-018-0478-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moshkelgosha S., Verhasselt H.L., Masetti G., Covelli D., Biscarini F., Horstmann M., Daser A., Westendorf A.M., Jesenek C., Philipp S., et al. Modulating Gut Microbiota in a Mouse Model of Graves’ Orbitopathy and Its Impact on Induced Disease. Microbiome. 2021;9:45. doi: 10.1186/s40168-020-00952-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brown C.T., Davis-Richardson A.G., Giongo A., Gano K.A., Crabb D.B., Mukherjee N., Casella G., Drew J.C., Ilonen J., Knip M., et al. Gut Microbiome Metagenomics Analysis Suggests a Functional Model for the Development of Autoimmunity for Type 1 Diabetes. PLoS ONE. 2011;6:e25792. doi: 10.1371/journal.pone.0025792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Larsen J.M. The Immune Response to Prevotella Bacteria in Chronic Inflammatory Disease. Immunology. 2017;151:363–374. doi: 10.1111/imm.12760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xu Q., Ni J.-J., Han B.-X., Yan S.-S., Wei X.-T., Feng G.-J., Zhang H., Zhang L., Li B., Pei Y.-F. Causal Relationship Between Gut Microbiota and Autoimmune Diseases: A Two-Sample Mendelian Randomization Study. Front. Immunol. 2021;12:746998. doi: 10.3389/fimmu.2021.746998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.López P., Gueimonde M., Margolles A., Suárez A. Distinct Bifidobacterium Strains Drive Different Immune Responses in Vitro. Int. J. Food Microbiol. 2010;138:157–165. doi: 10.1016/j.ijfoodmicro.2009.12.023. [DOI] [PubMed] [Google Scholar]
- 70.Astbury S., Atallah E., Vijay A., Aithal G.P., Grove J.I., Valdes A.M. Lower Gut Microbiome Diversity and Higher Abundance of Proinflammatory Genus Collinsella Are Associated with Biopsy-Proven Nonalcoholic Steatohepatitis. Gut Microbes. 2020;11:569–580. doi: 10.1080/19490976.2019.1681861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jenq R.R., Taur Y., Devlin S.M., Ponce D.M., Goldberg J.D., Ahr K.F., Littmann E.R., Ling L., Gobourne A.C., Miller L.C., et al. Intestinal Blautia Is Associated with Reduced Death from Graft-versus-Host Disease. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2015;21:1373–1383. doi: 10.1016/j.bbmt.2015.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ozato N., Saito S., Yamaguchi T., Katashima M., Tokuda I., Sawada K., Katsuragi Y., Kakuta M., Imoto S., Ihara K., et al. Blautia Genus Associated with Visceral Fat Accumulation in Adults 20-76 Years of Age. NPJ Biofilms Microbiomes. 2019;5:28. doi: 10.1038/s41522-019-0101-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Garcia-Mantrana I., Selma-Royo M., Alcantara C., Collado M.C. Shifts on Gut Microbiota Associated to Mediterranean Diet Adherence and Specific Dietary Intakes on General Adult Population. Front. Microbiol. 2018;9:890. doi: 10.3389/fmicb.2018.00890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mitsou E.K., Kakali A., Antonopoulou S., Mountzouris K.C., Yannakoulia M., Panagiotakos D.B., Kyriacou A. Adherence to the Mediterranean Diet Is Associated with the Gut Microbiota Pattern and Gastrointestinal Characteristics in an Adult Population. Br. J. Nutr. 2017;117:1645–1655. doi: 10.1017/S0007114517001593. [DOI] [PubMed] [Google Scholar]
- 75.Lenkowski M., Nijakowski K., Kaczmarek M., Surdacka A. The Loop-Mediated Isothermal Amplification Technique in Periodontal Diagnostics: A Systematic Review. J. Clin. Med. 2021;10:1189. doi: 10.3390/jcm10061189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Van den Bogert B., Meijerink M., Zoetendal E.G., Wells J.M., Kleerebezem M. Immunomodulatory Properties of Streptococcus and Veillonella Isolates from the Human Small Intestine Microbiota. PLoS ONE. 2014;9:e114277. doi: 10.1371/journal.pone.0114277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kiseleva E.P., Mikhailopulo K.I., Sviridov O.V., Novik G.I., Knirel Y.A., Szwajcer Dey E. The Role of Components of Bifidobacterium and Lactobacillus in Pathogenesis and Serologic Diagnosis of Autoimmune Thyroid Diseases. Benef. Microbes. 2011;2:139–154. doi: 10.3920/BM2010.0011. [DOI] [PubMed] [Google Scholar]
- 78.Ferreira-Halder C.V., Faria A.V.d.S., Andrade S.S. Action and Function of Faecalibacterium Prausnitzii in Health and Disease. Best Pract. Res. Clin. Gastroenterol. 2017;31:643–648. doi: 10.1016/j.bpg.2017.09.011. [DOI] [PubMed] [Google Scholar]
- 79.Wu F., Guo X., Zhang J., Zhang M., Ou Z., Peng Y. Phascolarctobacterium Faecium Abundant Colonization in Human Gastrointestinal Tract. Exp. Ther. Med. 2017;14:3122–3126. doi: 10.3892/etm.2017.4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Omenetti S., Pizarro T.T. The Treg/Th17 Axis: A Dynamic Balance Regulated by the Gut Microbiome. Front. Immunol. 2015;6:639. doi: 10.3389/fimmu.2015.00639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Maier L., Pruteanu M., Kuhn M., Zeller G., Telzerow A., Anderson E.E., Brochado A.R., Fernandez K.C., Dose H., Mori H., et al. Extensive Impact of Non-Antibiotic Drugs on Human Gut Bacteria. Nature. 2018;555:623–628. doi: 10.1038/nature25979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yang M., Wen S., Zhang J., Peng J., Shen X., Xu L. Systematic Review and Meta-Analysis: Changes of Gut Microbiota before and after Menopause. Dis. Markers. 2022;2022:3767373. doi: 10.1155/2022/3767373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Capozzi A., Scambia G., Lello S. Subclinical Hypothyroidism in Women’s Health: From Pre- to Post-Menopause. Gynecol. Endocrinol. Off. J. Int. Soc. Gynecol. Endocrinol. 2022;38:357–367. doi: 10.1080/09513590.2022.2046728. [DOI] [PubMed] [Google Scholar]
- 84.Wang B., Xu Y., Hou X., Li J., Cai Y., Hao Y., Ouyang Q., Wu B., Sun Z., Zhang M., et al. Small Intestinal Bacterial Overgrowth in Subclinical Hypothyroidism of Pregnant Women. Front. Endocrinol. 2021;12:604070. doi: 10.3389/fendo.2021.604070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wu M., Yang Y., Fan Y., Guo S., Li T., Gu M., Zhang T., Gao H., Liu R., Yin C. Characteristics of the Intestinal Flora of TPOAb-Positive Women with Subclinical Hypothyroidism in the Second Trimester of Pregnancy: A Single-Center Prospective Cohort Study. Front. Cell. Infect. Microbiol. 2022;12:794170. doi: 10.3389/fcimb.2022.794170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Study Quality Assessment Tools | NHLBI, NIH. [(accessed on 22 August 2020)]; Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools.
- 88.OCEBM Levels of Evidence. [(accessed on 22 August 2020)]. Available online: https://www.cebm.net/2016/05/ocebm-levels-of-evidence/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.