Abstract
Temporomandibular disorders (TMDs) are a multifactorial joint disease of the masticatory system. The possible etiological factors involved in the onset and progression of TMDs include oral parafunctional habits (OPFHs) and postural habits (PHs). However, little information is available on the association between OPFHs and PHs and a predisposition to TMDs. Thus, to investigate whether the presence of OPFH and PH predisposes individuals to TMDs, a survey-based cross-sectional cohort study of self-reported TMD was performed. A total of 2292 patients with TMD were recruited for the survey. Through one-to-one propensity score matching, 166 patients with and without sleep bruxism (SB) were selected. The SB group had a significantly higher risk of masticatory muscular pain or fatigue than the non-SB group (p = 0.018). Furthermore, the SB group without other OFPHs and PHs did not show a significantly higher risk of TMD symptoms than the non-SB group. Diurnal clenching and bad posture also affect the stomatognathic system, causing pain or fatigue; however, it did not result in TMDs in patients without any OPFHs and PHs. This implies that OPFHs and PHs may increase the risk of TMD symptoms in coexistence with other habits.
Keywords: temporomandibular disorders, oral parafunctional habit, cross sectional cohort study, propensity score matching analysis, postural habit
1. Introduction
Temporomandibular disorders (TMDs) are a collective term used to describe pain and functional disturbance of the masticatory system [1,2]. Masticatory system pain and disturbance affect oral health-related quality of life [3]. Furthermore, prolonged pain may affect the overall quality of life of the patient [4]. Thus, clinicians should aim for rapid elimination of TMD-related pain to allow for improvement in masticatory function.
According to the Diagnostic Criteria for TMD (DC/TMD) Axis I, TMDs can be divided into pain-related TMDs including masticatory muscle disorders and arthralgia, and intra-articular disorder including disc displacement with or without reduction and mouth opening limitation and osteoarthritis [5]. Epidemiological surveys have shown that TMD is the second most common musculoskeletal disorder that causes pain and disability [6,7] and that musculoskeletal pain occurs in 2–12% of the population [8,9,10], while 10% of the population reported severe symptoms [11]. In a large population study, tenderness of the TMJ and masticatory muscle was observed in 5% and 15% of the population, respectively [12]; however, these symptoms were self-reported by approximately 4% and 6–8% of the patients, respectively [13,14]. The reported prevalence of TMD symptoms and signs may differ widely depending on the method of examination, diagnostic criteria, age, and patient sample selection [15,16,17]. The commonly used treatments for muscular and arthrogenous TMDs are clarified into four types: non-invasive management modalities including occlusal splint, medications, orthotics, and physical therapy; minimally invasive modalities including arthrocentesis, arthroscopy, and intra-articular injections of corticosteroids or hyaluronic acid; invasive surgical modalities including arthroplasty, osteotomy, and osteodistraction; and salvage procedures including total joint replacement [18,19].
Numerous factors can be considered in the etiology of TMDs. Oral parafunctional habits (OPFHs), such as bruxism, diurnal clenching, and thumb sucking, are common and usually have negligible effects on the stomatognathic apparatus [20]. Sleep bruxism (SB) is a movement disorder characterized by repetitive masticatory muscle activity during sleep, and it could be associated with myofascial pain and arthralgia [21]. However, when the activity exceeds the individual resistance threshold or the host’s adaptive capacity decreases, it may cause damage to the dentition, muscles, and TMJ [22]. Several researchers have suggested that OPFHs are a possible etiological factor in the progression of TMDs in growing populations [22,23,24,25]. Furthermore, postural habits (PHs), such as bad posture and sleeping in face down position, are considered indicative of a potential TMD [26,27]. However, little information is available on the association between OPFHs and PHs and a predisposition to TMDs.
Thus, to investigate whether the presence of OPFH and PH predisposes to TMDs, we performed a survey-based cross-sectional cohort study of self-reported TMD among outpatients who visited the Temporomandibular Joint Clinic of Tokushima University Hospital. We aimed to assess the prevalence of self-reported subjective symptoms indicative of TMD and their correlation with OPFHs and PHs associated with the onset and progression of TMDs.
2. Materials and Methods
2.1. Participants
Outpatients with the chief complaint of TMD symptoms who visited the Temporomandibular Joint Clinic at Tokushima University Hospital between April 2010 and September 2021 were recruited. The inclusion criteria for the patients were age >12 years and at least one TMD symptom according to the DC/TMD [5]. The exclusion criteria were uncertain symptoms of TMD; orofacial pain, such as trigeminal neuralgia; toothache; and pain of dental origin.
The Ethics Committee of Tokushima University Hospital approved the study, and written informed consent was obtained from each patient after sufficient explanation of the research purposes (No. 2279-4).
2.2. Questionnaire
All participants were asked to fill out a unique self-reported questionnaire, including items on TMD symptoms, general and oral health, OPFHs and PHs, and some sociodemographic issues. The answer alternatives to all questions were “yes” or “no”, except for items 9 and 14 (Table 1). All patients answered the prepared 21 items in the self-reported questionnaire before the clinical examination. Of the 21 items, 6 items evaluated the present TMD symptoms for the patients, and the remaining included the past medical history or present/past OPFH and PH. In addition, multiple-choice questions regarding the following topics were added to the questionnaire: TMD symptoms (TMJ pain during various mandibular movements (item 1); masticatory muscle pain and fatigue (item 2); difficulty in mouth opening (item 3); TMJ sounds, such as clicking or popping (item 4); frequent headaches and stiff shoulder (item 5); and joint luxation (item 6)), and OPFH and PH (SB (item 16), diurnal grinding or clenching (DC) (item 17), chin on hand (CH) (item 18), face down (FD) (item 19), and bad posture (BP) (item 20)). Furthermore, two items in the questionnaire about previous TMJ treatment (item 9) and chewing side (item 13) with three answer choices were included. Patients were asked to grade two items on “maximum present pain related to this symptom (item 7)” and “degree of daily hindrance related to this symptom (item 8)”. These were rated on a visual analog scale (VAS) with a 100 mm straight horizontal line anchored by descriptors at each end. For “maximum present pain related to this symptom”, which measured painful experiences, the descriptor was “no pain” on the left end and “worst pain” on the right end; for the “degree of daily hindrance related to this symptom”, it was “not at all” on the left and “hardest time” on the right end.
Table 1.
Questions for patients with TMD at the initial visit.
| TMJ Symptoms |
|---|
| 1. Do you have pain in the jaw joint? |
| 2. Do you have masticatory muscular pain or fatigue? |
| 3. Is it hard for you to open your mouth? |
| 4. Do you have clicking or popping sound in either or both jaws? |
| 5. Do you ever have frequent headaches or neck and/or shoulder pain? |
| 6. Do you ever have difficulty closing after opening wide? (joint luxation) |
| 7. How do you experience maximum pain related to this symptom? (Could you check the bars?) |
| 8. How do you have a daily hindrance related to this symptom? (Could you check the bars?) |
| Medical history or characteristics |
| 9. Have you ever had a previous medical history of TMD? |
| If you answered “Yes”, was previous medical history same or different side for presence of TMD symptom? |
| 10. Have you ever had a previous TMD treatment? |
| 11. Have you ever had systemic bone or joint problems, such as rheumatic arthritis, gout, osteoporosis, or osteoarthritis? |
| 12. Have you ever injured your head, neck, jaw, or spine? |
| 13. Have you ever had orthodontic treatment? |
| 14. Which side do you usually or exclusively use during chewing, “Right side”, “Left side”, or “Both sides”? |
| 15. Do you consider yourself a nervous person? |
| 16. Do you frequently follow long-term desk work, long-time driving, hobbies, or sports? |
| Oral parafunctional or postural habits |
| 17. Do you grind your teeth during your sleep? (sleep bruxism) |
| 18. Do you clench or grind your teeth during the day? (awake bruxism or tooth contact habits) |
| 19. Do you rest your chin on your hand? |
| 20. Do you sleep lying face down? |
| 21. Do you have bad posture such as a hunch? |
2.3. Setting of Outcomes
The clinical outcomes were set as the present TMD symptoms, which included “TMJ pain during various mandibular movements (item 1)”, “masticatory muscle pain and fatigue (item 2)”, “difficulty in mouth opening (item 3)”, “TMJ sounds, such as clicking or popping (item 4)”, “frequent headaches and stiff shoulder (item 5)”, and “joint luxation (item 6)” from answers of alternatively self-reported questionnaires.
2.4. Statistical Analysis
In the present study, the estimated propensity score of each patient was used to perform individual matching analysis to determine whether OPFH and PH were present. Statistical analysis was performed using SPSS 27.0 (SPSS Inc., Chicago, IL, USA). To calculate the propensity score, a logistic regression model was fitted for each symptom and the patients’ constitution, including age, sex, habits, and history. To evaluate the effect of OPFH and PH on TMD symptoms, the estimated propensity score is required to calculate C-statistics, which is represented by the area under the receiver operating characteristic (ROC) curve and is an indicator for evaluating the goodness of fit [28]. Each patient who had an OPFH or PH was matched with a patient who had no habits with the closest estimated propensity on the logit scale within a specified range (≤0.2 of the pooled standard deviation of estimated logits) as a caliper using a specific OPFH or PH. The propensity score was calculated in the absence of other habits except for the targeted habit.
Fisher’s exact test was used to compare the six present TMD symptoms between the groups with and without OPFHs and PHs. Logistic regression analysis for each TMD symptom was performed in propensity-matched patients to analyze the odds ratios and 95% confidence intervals with reference to the absence of OPFHs and PHs. The two VAS scales for pain and daily hindrance were compared between the presence and absence of each habit using the Mann–Whitney U test.
In the present study, two different methods were used to analyze the effect of OPFHs and PHs: (1) all items, including other OPFHs and PHs except for targeted habits were used, and (2) patients without other OPFHs and PHs were selected to investigate the effect of targeted habits on the onset and progression of TMD symptoms.
3. Results
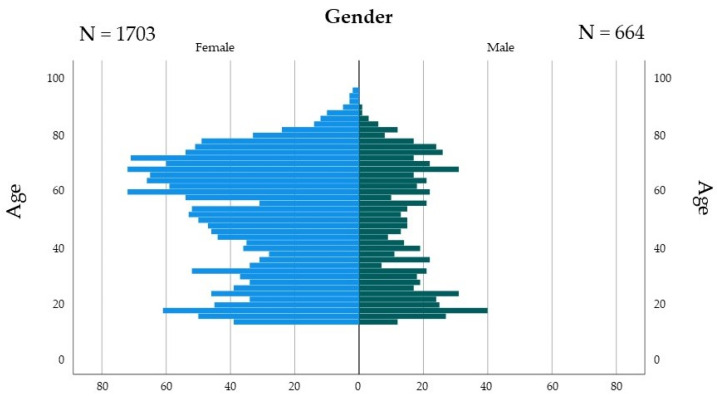
Of 2367 outpatients, 2292 patients with TMD (639 males and 1653 female) were recruited for the survey using the self-reported questionnaire (Figure 1). The remaining 75 patients who did not fill in the questionnaire items, responded to an indefinite answer, or were below 12 years of age were excluded. Because this analysis focused on each OPFH or PH, one-to-one propensity score matching was performed for each habit to patient history.
Figure 1.
Demographic data of TMD patients for age.
Table 2 shows the demographic and clinical characteristics of TMD patients with and without SB. Nine items, including age, medical history of TMD, orthodontic treatment experience, nervousness, hobby, and involvement in sports, and four OPFHs were significantly different between TMD groups with and without SB before propensity score matching (Table 2-(1)). As these items were regarded as confounding factors, we performed propensity score matching to remove these factors. A total of 904 patients with and without SB were selected, and the C-statistic for goodness of fit was calculated using the area under the ROC curve. The computed value was 0.683 in the propensity score model. After propensity score matching, patient distributions were closely balanced (Table 2-(2)). It was observed that the values for all items did not differ significantly between the TMD patients with and without SB.
Table 2.
Demographic and clinical characteristics of TMD patients with and without SB. (1) Before propensity score matching. (2) After propensity score matching.
| (1) Before Propensity Score Matching | ||||
|---|---|---|---|---|
| SB | ||||
| Yes (=Presence) | No (=Absence) | p–Value | ||
| Age (years) | 43.6 (28–57.8) (N = 484) |
53.4 (29.9–67.9) (N = 1804) |
<0.001 | |
| Gender | Female | 352 (21.3%) | 1301 (78.7%) | 0.995 |
| (N = 2292) | Male | 136 (21.3%) | 503 (78.7%) | |
| Medical history of TMJ (N = 2211) |
Yes and Same | 121 (24%) | 383 (76%) | <0.001 |
| Yes and Difference | 146 (25.7%) | 421 (74.3%) | ||
| No | 205 (18%) | 935 (82%) | ||
| TMD treatment | Yes | 128 (24.1%) | 403 (75.9%) | 0.079 |
| (N = 2269) | No | 357 (20.5%) | 1381 (79.5%) | |
| Systemic history | Yes | 95 (19%) | 405 (81%) | 0.153 |
| (N = 2276) | No | 390 (22%) | 1386 (78%) | |
| Head and neck injury | Yes | 89 (23.8%) | 285 (76.2%) | 0.205 |
| (N = 2277) | No | 397 (20.9%) | 1506 (79.1%) | |
| Orthodontic treatment | Yes | 70 (29.4%) | 168 (70.6%) | 0.001 |
| (N = 2285) | No | 418 (20.4%) | 1629 (79.6%) | |
| Chewing side (N = 2257) |
Both side | 174 (20.1%) | 691 (79.9%) | 0.147 |
| Right side | 164 (20.3%) | 642 (79.7%) | ||
| Left side | 141 (24.1%) | 445 (75.9%) | ||
| Nervous person | Yes | 336 (23.3%) | 1106 (76.7%) | 0.003 |
| (N = 2262) | No | 147 (17.9%) | 673 (82.1%) | |
| Desk work and recreation | Yes | 294 (23.9%) | 936 (76.1%) | 0.002 |
| (N = 2217) | No | 183 (18.5%) | 804 (81.5%) | |
| SB | Yes | 278 (34.4%) | 530 (65.6%) | <0.001 |
| (N = 2292) | No | 210 (14.2%) | 1274 (85.8%) | |
| CH | Yes | 141 (25.8%) | 405 (74.2%) | 0.003 |
| (N = 2292) | No | 347 (19.9%) | 1399 (80.1%) | |
| FD | Yes | 93 (25.1%) | 277 (74.9%) | 0.049 |
| (N = 2292) | No | 395 (20.6%) | 1527 (79.4%) | |
| BP | Yes | 243 (24.4%) | 752 (75.6%) | 0.001 |
| (N = 2292) | No | 245 (18.9%) | 1052 (81.1%) | |
| (2) After Propensity Score Matching | ||||
| SB | ||||
| Yes (=Presence) | No (=Absence) | p –Value | ||
| Age (years) | 43.4 (27.4–58.4) (N = 452) |
43.6 (24.1–59.8) (N = 452) |
0.873 | |
| Gender | Female | 330 (50.5%) | 323 (49.5%) | 0.603 |
| (N = 904) | Male | 122 (48.6%) | 129 (51.4%) | |
| Medical history of TMJ (N = 904) |
Yes and Same | 116 (48.9%) | 121 (51.1%) | 0.920 |
| Yes and Difference | 137 (50.7%) | 133 (49.3%) | ||
| No | 199 (50.1%) | 198 (49.9%) | ||
| TMD treatment | Yes | 121 (48%) | 131 (52%) | 0.458 |
| (N = 904) | No | 331 (50.8%) | 321 (49.2%) | |
| Systemic history | Yes | 91 (50%) | 91 (50%) | 1.000 |
| (N = 904) | No | 361 (50%) | 361 (50%) | |
| Head and neck injury | Yes | 88 (49.7%) | 89 (50.3%) | 0.933 |
| (N = 904) | No | 364 (50.1%) | 363 (49.9%) | |
| Orthodontic treatment | Yes | 63 (52.1%) | 58 (47.9%) | 0.625 |
| (N = 904) | No | 389 (49.7%) | 394 (50.3%) | |
| Chewing side (N = 940) |
Both side | 164 (47.4%) | 182 (52.6%) | 0.465 |
| Right side | 150 (51.4%) | 142 (48.6%) | ||
| Left side | 138 (51.9%) | 128 (48.1%) | ||
| Nervous person | Yes | 311 (49.8%) | 313 (50.2%) | 0.886 |
| (N = 904) | No | 141 (50.4%) | 139 (49.6%) | |
| Desk work and recreation | Yes | 278 (50.5%) | 273 (49.5%) | 0.733 |
| (N = 904) | No | 174 (49.3%) | 179 (50.7%) | |
| DC | Yes | 255 (49.7%) | 258 (50.3%) | 0.840 |
| (N = 904) | No | 197 (50.4%) | 194 (49.6%) | |
| CH | Yes | 132 (49.8%) | 133 (50.2%) | 0.942 |
| (N = 904) | No | 320 (50.1%) | 319 (49.9%) | |
| FD | Yes | 86 (47.8%) | 94 (52.2%) | 0.505 |
| (N = 904) | No | 366 (50.6%) | 358 (49.4%) | |
| BP | Yes | 221 (49.1%) | 229 (50.9%) | 0.595 |
| (N = 904) | No | 231 (50.9%) | 223 (49.1%) | |
To examine whether SB affects TMD symptoms, patients without other OPFHs and PHs were included (Table 3). A total of 675 patients had no OPFHs or PHs regardless of SB presence. Significant differences in age and habitual chewing side were found between the patients with and without SB (Table 3). Through one-to-one propensity score matching, 166 patients with and without SB were selected. The C-statistic for goodness of fit in the propensity score model was calculated as 0.620.
Table 3.
Demographic and clinical characteristics of TMD patients with and without SB, in case the other OPFHs and PHs are absent.
| (1) Before Propensity Score Matching | ||||
|---|---|---|---|---|
| SB | ||||
| Yes (=Presence) | No (=Absence) | p–Value | ||
| Age (years) | 54.8 (39.5–67.7) (N = 89) |
63.6 (47.1–73.2) (N = 586) |
0.001 | |
| Gender | Female | 59 (12.7%) | 404 (87.3%) | 0.616 |
| (N = 675) | Male | 30 (14.2%) | 182 (85.8%) | |
| Medical history of TMJ (N = 647) |
Yes and Same | 15 (12.5%) | 105 (87.5%) | 0.278 |
| Yes and Difference | 23 (17.3%) | 110 (82.7%) | ||
| No | 47 (11.9%) | 347 (88.1%) | ||
| TMD treatment | Yes | 21 (14.8%) | 121 (85.2%) | 0.527 |
| (N = 667) | No | 67 (12.8%) | 458 (87.2%) | |
| Systemic history | Yes | 22 (11.6%) | 167 (88.4%) | 0.479 |
| (N = 671) | No | 66 (13.7%) | 416 (86.3%) | |
| Head and neck injury | Yes | 14 (14.7%) | 81 (85.3%) | 0.643 |
| (N = 672) | No | 75 (13%) | 502 (87%) | |
| Orthodontic treatment | Yes | 7 (17.1%) | 34 (82.9%) | 0.453 |
| (N = 673) | No | 82 (13%) | 550 (87%) | |
| Chewing side (N = 669) |
Both side | 33 (11.5%) | 255 (88.5%) | 0.021 |
| Right side | 24 (11%) | 195 (89%) | ||
| Left side | 32 (19.8%) | 130 (80.2%) | ||
| Nervous person | Yes | 53 (15.3%) | 294 (84.7%) | 0.131 |
| (N = 666) | No | 36 (11.3%) | 283 (88.7%) | |
| Desk work and recreation | Yes | 45 (15.6%) | 243 (84.4%) | 0.105 |
| (N = 659) | No | 42 (11.3%) | 329 (88.7%) | |
| (2) After Propensity Score Matching | ||||
| SB | ||||
| Yes (=Presence) | No (=Absence) | p –Value | ||
| Age (years) | 55.9 (39.6–67.7) (N = 83) |
52.9 (28.4–72.9) (N = 83) |
0.626 | |
| Gender | Female | 55 (50.9%) | 53 (49.1%) | 0.745 |
| (N = 166) | Male | 28 (48.3%) | 30 (51.7%) | |
| Medical history of TMJ (N = 166) |
Yes and Same | 15 (51.7%) | 14 (48.3%) | 0.978 |
| Yes and Difference | 22 (50%) | 22 (50%) | ||
| No | 46 (49.5%) | 47 (50.5%) | ||
| TMD treatment | Yes | 19 (51.4%) | 18 (48.6%) | 0.852 |
| (N = 166) | No | 64 (49.6%) | 65 (50.4%) | |
| Systemic history | Yes | 21 (48.8%) | 22 (51.2%) | 0.859 |
| (N = 166) | No | 62 (50.4%) | 61 (49.6%) | |
| Head and neck injury | Yes | 14 (42.4%) | 19 (57.6%) | 0.331 |
| (N = 166) | No | 69 (51.9%) | 64 (48.1%) | |
| Orthodontic treatment | Yes | 6 (40%) | 9 (60%) | 0.417 |
| (N = 166) | No | 77 (51%) | 74 (49%) | |
| Chewing side (N = 166) |
Both side | 33 (47.8%) | 36 (52.2%) | 0.594 |
| Right side | 22 (46.8%) | 25 (53.2%) | ||
| Left side | 28 (56%) | 22 (44%) | ||
| Nervous person | Yes | 48 (50%) | 48 (50%) | 1.000 |
| (N = 166) | No | 35 (50%) | 35 (50%) | |
| Desk work and recreation | Yes | 42 (51.9%) | 39 (48.1%) | 0.641 |
| (N = 166) | No | 41 (48.2%) | 44 (51.8%) | |
Table 4 shows the results of the logistic regression analysis of TMD symptoms involving SB presence in the propensity-matched group in comparison with the non-SB group. The SB group had a significantly higher risk of masticatory muscular pain or fatigue compared with the non-SB group (odds ratio = 1.523; 95% confidence interval, 1.074–2.159; p = 0.018), including medical history, OPFHs, and PHs. However, the degree of maximum pain of daily hindrance did not differ significantly between the SB and non-SB groups, regardless of masticatory muscle pain or fatigue. Furthermore, the SB group without other OFPHs and PHs showed no significantly higher risk of TMD symptoms than the non-SB group (Table 4).
Table 4.
Logistic regression analysis of TMD symptoms with the presence of targeted habit with reference to the absence of SB in the propensity-matched group. (1) Regardless of the presence or absence of other OPFHs and PHs. (2) In cases where other OPFHs and PHs are absent, except for the targeted habits.
| (1) | Habit | N | Odds Ratio | 95% Confidence Interval | p–Value |
|---|---|---|---|---|---|
| Pain in jaw joint | SB | 841 | 1.051 | 0.776–1.425 | 0.746 |
| DC | 1228 | 1.372 | 1.063–1.770 | 0.015 | |
| CH | 935 | 0.954 | 0.715–1.272 | 0.749 | |
| FD | 623 | 1.186 | 0.836–1.683 | 0.339 | |
| BP | 1269 | 0.890 | 0.695–1.139 | 0.354 | |
| Masticatory muscular pain/fatigue | SB | 829 | 1.523 | 1.074–2.159 | 0.018 |
| DC | 1209 | 1.477 | 1.100–1.982 | 0.009 | |
| CH | 908 | 1.127 | 0.782–1.623 | 0.521 | |
| FD | 608 | 0.824 | 0.535–1.269 | 0.378 | |
| BP | 1240 | 1.306 | 0.972–1.754 | 0.076 | |
| Hard to open mouth | SB | 829 | 0.781 | 0.594–1.027 | 0.077 |
| DC | 1214 | 1.023 | 0.816–1.282 | 0.847 | |
| CH | 913 | 1.045 | 0.804–1.357 | 0.742 | |
| FD | 610 | 1.238 | 0.900–1.703 | 0.189 | |
| BP | 1248 | 0.880 | 0.704–1.100 | 0.261 | |
| Clicking/popping in jaw | SB | 852 | 0.823 | 0.620–1.092 | 0.176 |
| DC | 1244 | 0.877 | 0.696–1.104 | 0.176 | |
| CH | 938 | 0.902 | 0.691–1.178 | 0.448 | |
| FD | 638 | 1.357 | 0.978–1.883 | 0.068 | |
| BP | 1291 | 1.169 | 0.930–1.469 | 0.180 | |
| Headache/shoulder stiffness | SB | 824 | 1.218 | 0.926–1.602 | 0.159 |
| DC | 1206 | 1.509 | 1.203–1.894 | <0.001 | |
| CH | 908 | 0.948 | 0.730–1.229 | 0.685 | |
| FD | 606 | 1.169 | 0.850–1.610 | 0.337 | |
| BP | 1238 | 1.599 | 1.278–2.002 | <0.001 | |
| Joint luxation | SB | 827 | 0.748 | 0.400–1.400 | 0.363 |
| DC | 1206 | 0.739 | 0.424–1.288 | 0.285 | |
| CH | 909 | 0.752 | 0.425–1.329 | 0.325 | |
| FD | 606 | 1.386 | 0.696–2.761 | 0.352 | |
| BP | 1237 | 0.870 | 0.520–1.457 | 0.596 | |
| (1) | Targeted Habit | ||||
| Habit | N | Yes (=Presence) | No (=Absence) | p –Value | |
| Maximum Pain (VAS) | SB | 888 | 40.00 (11.50–56.50) | 40.00 (12.00–61.00) | 0.548 |
| DC | 1291 | 41.50 (18.00–60.00) | 36.00 (9.00–55.00) | 0.003 | |
| CH | 976 | 35.00 (13.00–55.00) | 42.00 (13.25–60.00) | 0.151 | |
| FD | 671 | 35.00 (12.00–56.00) | 35.00 (10.00–55.00) | 0.769 | |
| BP | 1346 | 40.00 (14.00–56.00) | 40.00 (14.00–58.00) | 0.616 | |
| Daily Hindrance (VAS) | SB | 877 | 47.00 (20.00–62.00) | 45.00 (20.00–60.00) | 0.828 |
| DC | 1282 | 49.00 (22.00–64.00) | 45.50 (19.00–56.00) | 0.010 | |
| CH | 969 | 40.00 (18.00–55.25) | 47.00 (20.00–60.00) | 0.062 | |
| FD | 670 | 42.00 (19.00–56.00) | 40.00 (18.50–58.00) | 0.887 | |
| BP | 1336 | 49.00 (21.25–61.75) | 45.00 (20.00–57.00) | 0.078 | |
| (2) | Habit | N | Odds Ratio | 95% Confidence Interval | p–Value |
| Pain in jaw joint | SB | 149 | 0.844 | 0.375–1.901 | 0.683 |
| DC | 355 | 1.281 | 0.792–2.073 | 0.312 | |
| CH | 136 | 1.040 | 0.479–2.260 | 0.921 | |
| FD | 99 | 1.531 | 0.661–3.547 | 0.319 | |
| BP | 430 | 1.017 | 0.672–1.540 | 0.935 | |
| Masticatory muscular pain/fatigue | SB | 142 | 1.810 | 0.727–4.504 | 0.198 |
| DC | 342 | 1.466 | 0.850–2.526 | 0.167 | |
| CH | 131 | 0.395 | 0.097–1.598 | 0.180 | |
| FD | 96 | 1.955 | 0.459–8.318 | 0.358 | |
| BP | 416 | 1.618 | 0.913–2.866 | 0.097 | |
| Hard to open mouth | SB | 145 | 0.698 | 0.363–1.342 | 0.281 |
| DC | 344 | 0.901 | 0.589–1.379 | 0.632 | |
| CH | 133 | 1.092 | 0.551–2.165 | 0.801 | |
| FD | 96 | 1.635 | 0.711–3.758 | 0.246 | |
| BP | 420 | 0.975 | 0.664–1.432 | 0.897 | |
| Clicking/popping in jaw | SB | 152 | 0.820 | 0.429–1.568 | 0.549 |
| DC | 356 | 0.732 | 0.479–1.119 | 0.149 | |
| CH | 140 | 0.530 | 0.270–1.041 | 0.064 | |
| FD | 106 | 0.659 | 0.295–1.473 | 0.308 | |
| BP | 444 | 1.052 | 0.714–1.551 | 0.798 | |
| Headache/shoulder stiffness | SB | 142 | 1.474 | 0.758–2.867 | 0.252 |
| DC | 342 | 1.060 | 0.692–1.624 | 0.790 | |
| CH | 131 | 0.914 | 0.450–1.856 | 0.804 | |
| FD | 96 | 1.497 | 0.650–3.446 | 0.342 | |
| BP | 418 | 1.713 | 1.160–2.532 | 0.007 | |
| Joint luxation | SB | 143 | 0.250 | 0.027–2.294 | 0.187 |
| DC | 342 | 0.347 | 0.090–1.330 | 0.107 | |
| CH | 131 | 0.774 | 0.198–3.022 | 0.712 | |
| FD | 97 | 0.285 | 0.054–1.488 | 0.117 | |
| BP | 416 | 0.532 | 0.218–1.297 | 0.159 | |
| (2) | Targeted Habit | ||||
| Habit | N | Yes (=Presence) | No (=Absence) | p –Value | |
| Maximum Pain (VAS) | SB | 162 | 29.50 (8.00–53.50) | 34.00 (5.00–54.75) | 0.916 |
| DC | 376 | 42.00 (17.50–61.00) | 35.00 (10.00–55.00) | 0.068 | |
| CH | 144 | 30.00 (12.50–51.50) | 35.00 (3.00–53.00) | 0.997 | |
| FD | 105 | 35.00 (12.25–58.00) | 23.00 (0.00–53.00) | 0.110 | |
| BP | 461 | 46.00 (13.50–60.75) | 35.00 (5.00–58.50) | 0.073 | |
| Daily Hindrance (VAS) | SB | 158 | 45.00 (19.00–52.00) | 48.50 (19.75–58.00) | 0.382 |
| DC | 372 | 45.50 (20.00–63.00) | 46.00 (15.50–60.00) | 0.313 | |
| CH | 145 | 37.00 (12.50–50.00) | 39.00 (14.00–51.50) | 0.698 | |
| FD | 107 | 45.00 (25.00–53.00) | 39.00 (14.25–53.75) | 0.352 | |
| BP | 459 | 50.00 (23.50–59.50) | 38.50 (13.00–56.25) | 0.007 | |
SB: sleep bruxism, DC: diurnal grinding or clenching, CH: chin on hand, FD: face down, BP: bad posture. In bold: Variables with significant difference.
For other OFPHs or PHs except SB, the demographic and clinical characteristics of TMD patients with and without DC, CH, FD, or BP were evaluated (Tables S1–S4). Before propensity score matching, the results indicated significant differences in items 5 to 7 between TMD patients with and without DC, CH, FD, or BP. After propensity score matching, patient distributions were closely balanced between TMD patients with and without each OPFH or PH. Furthermore, to identify the single habit as the causative factor for TMD, single-targeted habits, except the other OFPHs and PHs, were analyzed (Tables S5–S8). For each single targeted habit (DC, CH, FD, or BP), the demographic and clinical characteristics of TMD patients without other OFPHs or PHs, except for each single targeted habit, before and after propensity score matching. Before propensity score matching, items 3 to 7 showed significant differences between the presence and absence of each single targeted habit. However, there were no significant differences between the presence and absence of each single targeted habit after propensity score matching. All C-statistics for goodness of fit were calculated around 0.700 in the propensity score model. Notably, the DC group had a significantly higher risk of jaw pain (odds ratio = 1.372; 95% confidence interval, 1.063–1.770; p = 0.015), masticatory pain or fatigue (odds ratio = 1.477; 95% confidence interval, 1.100–1.982; p = 0.009), and headache or shoulder stiffness (odds ratio = 1.509; 95% confidence interval, 1.203–1.894; p < 0.001) than the non-DC group (Table 4). Furthermore, the DC group had significantly higher maximum pain and daily hindrance than the non-DC group (p = 0.003 and p = 0.010, respectively). However, patients with TMD without other OPFHs and PHs, except for DC, showed no significantly higher risk of TMD symptoms (Table 4). The BP group had a significantly higher risk of headache or shoulder stiffness (odds ratio = 1.599; 95% confidence interval, 1.278–2.002; p < 0.001) than the non-BP group (Table 4). Furthermore, TMD patients without other OPFHs and PHs, except for BP, showed a significantly higher risk of headache or shoulder stiffness (odds ratio = 1.713; 95% confidence interval, 1.160–2.532; p = 0.007) than the non-BP group (Table 4). However, as CH and FD did not have significant differences in all items between TMD patients with and without CH or FD, OPFHs and PHs did not affect TMD symptoms (Table 4).
4. Discussion
Numerous epidemiological surveys of TMD have been designed and reported previously [5,28,29,30,31]. In these studies, the prevalence of TMD was examined in general subjects or patient populations, and varying incidence rates were reported, ranging from 20% to 50% [29]. These studies also indicated that TMDs have a multifactorial etiology and that OPFHs (e.g., nocturnal bruxism and clenching), DC (teeth contact habit), anatomical factors, trauma or general hypermobility of the joints, psychosocial issues (e.g., anxiety and depressive syndrome), and cervical posture are potential risk factors [29,32,33]. However, considering the presence of confounding factors, these findings imply that participants with OPFHs are subject to TMD symptoms and signs. Thus, to avoid confounds in the study design, a propensity score matching analysis was adopted in this study. The estimated propensity score is commonly applied in clinical or epidemiological studies [34]. It allows for dealing with various selection biases in retrospective studies, such as the pseudo-randomization of data [35]. Thus, this analysis can organize confounds by indication [36]. This can be applied to the comparison of observed prognostic factors, such as the effect, cost, or treatment duration between different clinical treatment groups. The estimated propensity score involves the extraneous factor that each patient had and is suitable to match the resembling patients using a fixed caliper, which is the nearest neighbor matching method. When using the propensity score-matched analysis in this study, demographic factors, individual medical or dental history, and the presence of jobs and hobbies were unified to remove confounding factors. To our knowledge, this is the first study in which a matched case–control analysis was conducted to identify the causes of TMD.
The present study showed that SB affected pain or fatigue of the masticatory muscles, and DC affected jaw pain, pain or fatigue of the masticatory muscles [21], and headache or shoulder stiffness in matched patients with a medical history and various habits. However, in patients without OPFHs and PHs, SB and DC were not considered potential risk factors for TMD symptoms. Thus, although SB or DC alone did not affect TMD symptoms, these habits might produce a risk for TMD symptoms when combined with other habits. Previous studies reported that various oral parafunctional factors can affect TMD symptoms [22,37,38,39,40]. Our results showed that masticatory muscle pain, fatigue, headache, or shoulder stiffness might develop combined OPHFs and PHs, and these TMD symptoms are recognized as a part of myofascial pain or fatigue [5]. Although management of myofascial pain or fatigue is not sufficient to resolve pathophysiological mechanisms, excessive extension or contraction might stimulate nociceptive neurons in the skeletal muscle. Furthermore, nociceptors on the terminals of nociceptive neurons develop pain or fatigue [41]. By continuing excessive activation in the peripheral nervous system, pain sensitivity might increase, and peripheral or central nervous plasticity may develop [42].
The following considerations and limitations of the present cohort survey should be addressed. First, as this cohort survey was performed using a propensity score-matched analysis, the results obtained were generalizable to the paired data by the range of propensity scores [34]. Therefore, the propensity score-matched method can reduce or remove the biases in demographic factors or medical history but not the effect of other biases from unobserved factors, such as information on internal medication, academic history, work history/current job, and annual income may persist [43]. Our score-matched analysis should be organized using the high propensity score-matched analysis because of the lack of unobserved confounding factors. Second, although SB was regarded as an OPFH in the self-report questionnaire, previous reports provided detailed information about the patients [44], for example, whether the patient is self-aware about SB and how frequently SB occurs in a week. However, each item for OPFH or PH in this self-report questionnaire was not included in detail, and this questionnaire was not applied in a pilot study with a smaller population. Finally, the questionnaire used in this survey did not include questions about occlusal status and masticatory performance [12]. The occlusal condition is an important factor in diagnosing TMDs; therefore, further surveys and investigations involving occlusal conditions are needed to identify the specific causative factors in patients with TMD.
5. Conclusions
In conclusion, we performed a propensity score-matched analysis using a large cohort of TMD patients with OFPHs and/or PHs. According to the results of our matched case–control study, SB, DC, and BP affected the stomatognathic system in terms of pain or fatigue while SB and DC did not affect TMD symptoms in patients without OPFHs and PHs. This implies that these habits might produce a risk for TMD symptoms owing to the coexistence of other habits.
Acknowledgments
The authors thank Fumihiro Matsumoto, Yoshitaka Suzuki, Emi Takegawa, Hinayo Umeda, Daisuke Ikutame, Mariko Tanaka, Shigeki Hanawa, and Sooha Kim for their clinical assistance in this study.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm11216396/s1, Table S1. Demographic and clinical characteristics of TMD patients with and without DC; Table S2. Demographic and clinical characteristics of TMD patients with and without DC if other OPFHs and PHs were absent; Table S3. Demographic and clinical characteristics of TMD patients with and without CH; Table S4. Demographic and clinical characteristics of TMD patients with and without CH if other OPFHs and PHs were absent; Table S5 Demographic and clinical characteristics of TMD patients with and without FD; Table S6. Demographic and clinical characteristics of TMD patients with and without FD if other OPFHs and PHs were absent; Table S7. Demographic and Clinical Characteristics of TMJ Patients with and without BP; Table S8. Demographic and clinical characteristics of TMD patients with and without BP if other OPFHs and PHs were absent.
Author Contributions
S.A., F.K., Y.M., T.M., T.O. and E.T. conceived and designed the research. S.A., Y.M., and E.T. acquired data. S.A., F.K., T.M., T.O. and E.T. analyzed data. S.A., Y.M., T.O. and E.T. wrote the manuscript. All authors gave their final approval and agreed to be accountable for all aspects of this work. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This study was approved by the Ethics Committee of Tokushima University Hospital (No. 2279-4).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by Grants-in-Aid 19K11323 (S.A.) for Science Research from the Ministry of Education, Culture, Sports, Science, and Technology, Japan.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.List T., Jensen R.H. Temporomandibular disorders: Old ideas and new concepts. Cephalalgia. 2017;37:692–704. doi: 10.1177/0333102416686302. [DOI] [PubMed] [Google Scholar]
- 2.Lövgren A., Häggman-Henrikson B., Visscher C., Lobbezoo F., Marklund S., Wänman A. Temporomandibular pain and jaw dysfunction at different ages covering the lifespan–a population based study. Eur. J. Pain. 2016;20:532–540. doi: 10.1002/ejp.755. [DOI] [PubMed] [Google Scholar]
- 3.Almoznino G., Zini A., Zakuto A., Sharav Y., Haviv Y., Hadad A., Chweidan H., Yarom N., Benoliel R. Oral health-related quality of life in patients with temporomandibular disorders. J. Oral. Facial Pain Headache. 2015;29:231–241. doi: 10.11607/ofph.1413. [DOI] [PubMed] [Google Scholar]
- 4.Tjakkes G.-H.E., Reinders J.-J., Tenvergert E.M., Stegenga B. Tmd pain: The effect on health related quality of life and the influence of pain duration. Health Qual. Life Outcomes. 2010;8:1–8. doi: 10.1186/1477-7525-8-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schiffman E., Ohrbach R., Truelove E., Look J., Anderson G., Goulet J.P., List T., Svensson P., Gonzalez Y., Lobbezoo F., et al. Diagnostic criteria for temporomandibular disorders (dc/tmd) for clinical and research applications: Recommendations of the international rdc/tmd consortium network and orofacial pain special interest groupdagger. J. Oral Facial Pain Headache. 2014;28:6–27. doi: 10.11607/jop.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gonçalves D.A., Dal Fabbro A.L., Campos J.A., Bigal M.E., Speciali J.G. Symptoms of temporomandibular disorders in the population: An epidemiological study. J. Orofac. Pain. 2010;24:270–278. [PubMed] [Google Scholar]
- 7.Jin L.J., Lamster I.B., Greenspan J.S., Pitts N.B., Scully C., Warnakulasuriya S. Global burden of oral diseases: Emerging concepts, management and interplay with systemic health. Oral Dis. 2016;22:609–619. doi: 10.1111/odi.12428. [DOI] [PubMed] [Google Scholar]
- 8.Lövgren A., Visscher C.M., Häggman-Henrikson B., Lobbezoo F., Marklund S., Wänman A. Validity of three screening questions (3q/tmd) in relation to the dc/tmd. J. Oral Rehabil. 2016;43:729–736. doi: 10.1111/joor.12428. [DOI] [PubMed] [Google Scholar]
- 9.Macfarlane T.V., Blinkhorn A.S., Davies R.M., Kincey J., Worthington H.V. Oro-facial pain in the community: Prevalence and associated impact. Community Dent. Oral Epidemiol. 2002;30:52–60. doi: 10.1034/j.1600-0528.2002.300108.x. [DOI] [PubMed] [Google Scholar]
- 10.Nilsson I.-M., List T., Drangsholt M. Prevalence of temporomandibular pain and subsequent dental treatment in swedish adolescents. J. Orofac. Pain. 2005;19:144–150. [PubMed] [Google Scholar]
- 11.LeResche L. Epidemiology of temporomandibular disorders: Implications for the investigation of etiologic factors. Crit. Rev. Oral Biol. Med. 1997;8:291–305. doi: 10.1177/10454411970080030401. [DOI] [PubMed] [Google Scholar]
- 12.Gesch D., Bernhardt O., Alte D., Kocher T., John U., Hensel E. Malocclusions and clinical signs or subjective symptoms of temporomandibular disorders (tmd) in adults. J. Orofac. Orthop. 2004;65:88–103. doi: 10.1007/s00056-004-0338-7. [DOI] [PubMed] [Google Scholar]
- 13.Kamisaka M., Yatani H., Kuboki T., Matsuka Y., Minakuchi H. Four-year longitudinal course of tmd symptoms in an adult population and the estimation of risk factors in relation to symptoms. J. Orofac. Pain. 2000;14:224–232. [PubMed] [Google Scholar]
- 14.Katz J. The epidemiology of self-reported tmj sounds and pain in young adults in israel. J. Public Health Dent. 2002;62:177–179. doi: 10.1111/j.1752-7325.2002.tb03441.x. [DOI] [PubMed] [Google Scholar]
- 15.Ohrbach R., Dworkin S.F. The evolution of tmd diagnosis: Past, present, future. J. Dent. Res. 2016;95:1093–1101. doi: 10.1177/0022034516653922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Valesan L.F., Da-Cas C.D., Réus J.C., Denardin A.C.S., Garanhani R.R., Bonotto D., Januzzi E., de Souza B.D.M. Prevalence of temporomandibular joint disorders: A systematic review and meta-analysis. Clin. Oral Investig. 2021;25:441–453. doi: 10.1007/s00784-020-03710-w. [DOI] [PubMed] [Google Scholar]
- 17.Zarb G.A., Carlsson G.E. Temporomandibular disorders: Osteoarthritis. J. Orofac. Pain. 1999;13:295–306. [PubMed] [Google Scholar]
- 18.Ferrillo M., Nucci L., Giudice A., Calafiore D., Marotta N., Minervini G., d’Apuzzo F., Ammendolia A., Perillo L., de Sire A. Efficacy of conservative approaches on pain relief in patients with temporomandibular joint disorders: A systematic review with network meta-analysis. Cranio. 2022;23:1–17. doi: 10.1080/08869634.2022.2126079. [DOI] [PubMed] [Google Scholar]
- 19.Tanaka E., Detamore M.S., Mercuri L.G. Degenerative disorders of the temporomandibular joint: Etiology, diagnosis, and treatment. J. Dent. Res. 2008;87:296–307. doi: 10.1177/154405910808700406. [DOI] [PubMed] [Google Scholar]
- 20.Mejersjö C., Ovesson D., Mossberg B. Oral parafunctions, piercing and signs and symptoms of temporomandibular disorders in high school students. Acta Odontol. Scand. 2016;74:279–284. doi: 10.3109/00016357.2015.1114668. [DOI] [PubMed] [Google Scholar]
- 21.Jiménez-Silva A., Peña-Durán C., Tobar-Reyes J., Frugone-Zambra R. Sleep and awake bruxism in adults and its relationship with temporomandibular disorders: A systematic review from 2003 to 2014. Acta Odontol. Scand. 2017;75:36–58. doi: 10.1080/00016357.2016.1247465. [DOI] [PubMed] [Google Scholar]
- 22.Karibe H., Shimazu K., Okamoto A., Kawakami T., Kato Y., Warita-Naoi S. Prevalence and association of self-reported anxiety, pain, and oral parafunctional habits with temporomandibular disorders in japanese children and adolescents: A cross-sectional survey. BMC Oral Health. 2015;15:1–7. doi: 10.1186/1472-6831-15-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bertoli F.M.P., Bruzamolin C.D., Pizzatto E., Losso E.M., Brancher J.A., de Souza J.F. Prevalence of diagnosed temporomandibular disorders: A cross-sectional study in brazilian adolescents. PLoS ONE. 2018;13:e0192254. doi: 10.1371/journal.pone.0192254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Winocur E., Littner D., Adams I., Gavish A. Oral habits and their association with signs and symptoms of temporomandibular disorders in adolescents: A gender comparison. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006;102:482–487. doi: 10.1016/j.tripleo.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 25.Ferrillo M., Giudice A., Marotta N., Fortunato F., Di Venere D., Ammendolia A., Fiore P., de Sire A. Pain Management and Rehabilitation for Central Sensitization in Temporomandibular Disorders: A Comprehensive Review. Int. J. Mol. Sci. 2022;23:12164. doi: 10.3390/ijms232012164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao D., Zhang S., Kan H., Zhang Q. Relationship between cervical angle and temporomandibular disorders in young and middle-aged population. Cranio. 2022. pp. 1–7. Online ahead of print . [DOI] [PubMed]
- 27.Yalçınkaya E., Cingi C., Bayar Muluk N., Ulusoy S., Hanci D. Are temporomandibular disorders associated with habitual sleeping body posture or nasal septal deviation? Eur. Arch. Otorhinolaryngol. 2016;273:177–181. doi: 10.1007/s00405-014-3476-6. [DOI] [PubMed] [Google Scholar]
- 28.Westreich D., Cole S.R., Funk M.J., Brookhart M.A., Stürmer T. The role of the c-statistic in variable selection for propensity score models. Pharmacoepidemiol. Drug Saf. 2011;20:317–320. doi: 10.1002/pds.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manfredini D., Guarda-Nardini L., Winocur E., Piccotti F., Ahlberg J., Lobbezoo F. Research diagnostic criteria for temporomandibular disorders: A systematic review of axis i epidemiologic findings. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2011;112:453–462. doi: 10.1016/j.tripleo.2011.04.021. [DOI] [PubMed] [Google Scholar]
- 30.Paduano S., Bucci R., Rongo R., Silva R., Michelotti A. Prevalence of temporomandibular disorders and oral parafunctions in adolescents from public schools in southern italy. Cranio. 2020;38:370–375. doi: 10.1080/08869634.2018.1556893. [DOI] [PubMed] [Google Scholar]
- 31.Progiante P.S., Pattussi M.P., Lawrence H.P., Goya S., Grossi P.K., Grossi M.L. Prevalence of temporomandibular disorders in an adult brazilian community population using the research diagnostic criteria (axes i and ii) for temporomandibular disorders (the maringá study) Int. J. Prosthodont. 2015;28:600–609. doi: 10.11607/ijp.4026. [DOI] [PubMed] [Google Scholar]
- 32.Dıraçoǧlu D., Yıldırım N.K., Saral İ., Özkan M., Karan A., Özkan S., Aksoy C. Temporomandibular dysfunction and risk factors for anxiety and depression. J. Back Musculoskelet. Rehabil. 2016;29:487–491. doi: 10.3233/BMR-150644. [DOI] [PubMed] [Google Scholar]
- 33.Iodice G., Danzi G., Cimino R., Paduano S., Michelotti A. Association between posterior crossbite, masticatory muscle pain, and disc displacement: A systematic review. Eur. J. Orthod. 2013;35:737–744. doi: 10.1093/ejo/cjt024. [DOI] [PubMed] [Google Scholar]
- 34.Yasunaga H., Horiguchi H., Kuwabara K., Matsuda S., Fushimi K., Hashimoto H., Ayanian J.Z. Outcomes after laparoscopic or open distal gastrectomy for early-stage gastric cancer: A propensity-matched analysis. Ann. Surg. 2013;257:640–646. doi: 10.1097/SLA.0b013e31826fd541. [DOI] [PubMed] [Google Scholar]
- 35.Heinze G., Jüni P. An overview of the objectives of and the approaches to propensity score analyses. Eur. Heart J. 2011;32:1704–1708. doi: 10.1093/eurheartj/ehr031. [DOI] [PubMed] [Google Scholar]
- 36.Freemantle N., Marston L., Walters K., Wood J., Reynolds M.R., Petersen I. Making inferences on treatment effects from real world data: Propensity scores, confounding by indication, and other perils for the unwary in observational research. BMJ. 2013;347:f6409. doi: 10.1136/bmj.f6409. [DOI] [PubMed] [Google Scholar]
- 37.Glaros A., Urban D., Locke J. Headache and temporomandibular disorders: Evidence for diagnostic and behavioural overlap. Cephalalgia. 2007;27:542–549. doi: 10.1111/j.1468-2982.2007.01325.x. [DOI] [PubMed] [Google Scholar]
- 38.Michiels S., van der Wal A.C., Nieste E., Van de Heyning P., Braem M., Visscher C., Topsakal V., Gilles A., Jacquemin L., Hesters M. Conservative therapy for the treatment of patients with somatic tinnitus attributed to temporomandibular dysfunction: Study protocol of a randomised controlled trial. Trials. 2018;19:1–10. doi: 10.1186/s13063-018-2903-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paulino M.R., Moreira V.G., Lemos G.A., Silva P.L., Bonan P.R.F., Batista A.U.D. Prevalence of signs and symptoms of temporomandibular disorders in college preparatory students: Associations with emotional factors, parafunctional habits, and impact on quality of life. Cien. Saude Colet. 2018;23:173–186. doi: 10.1590/1413-81232018231.18952015. [DOI] [PubMed] [Google Scholar]
- 40.Slade G., Ohrbach R., Greenspan J., Fillingim R., Bair E., Sanders A., Dubner R., Diatchenko L., Meloto C., Smith S. Painful temporomandibular disorder: Decade of discovery from oppera studies. J. Dent. Res. 2016;95:1084–1092. doi: 10.1177/0022034516653743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Woolf C.J., Ma Q. Nociceptors—Noxious stimulus detectors. Neuron. 2007;55:353–364. doi: 10.1016/j.neuron.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 42.Iida T., Komiyama O., Obara R., Baad-Hansen L., Kawara M., Svensson P. Repeated clenching causes plasticity in corticomotor control of jaw muscles. Eur. J. Oral Sci. 2014;122:42–48. doi: 10.1111/eos.12101. [DOI] [PubMed] [Google Scholar]
- 43.Lee K.S., Jha N., Kim Y.J. Risk factor assessments of temporomandibular disorders via machine learning. Sci. Rep. 2021;11:19802. doi: 10.1038/s41598-021-98837-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carra M.C., Huynh N., Lavigne G. Sleep bruxism: A comprehensive overview for the dental clinician interested in sleep medicine. Dent. Clin. North Am. 2012;56:387–413. doi: 10.1016/j.cden.2012.01.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.