Abstract
Continuous development of personalized treatments is undoubtedly beneficial for oncogenic patients’ comfort and survival rate. Mutant TP53 is associated with a worse prognosis due to the occurrence of metastases, increased chemoresistance, and tumor growth. Currently, numerous compounds capable of p53 reactivation or the destabilization of mutant p53 are being investigated. Several of them, APR-246, COTI-2, SAHA, and PEITC, were approved for clinical trials. This review focuses on these novel therapeutic opportunities, their mechanisms of action, and their significance for potential medical application.
Keywords: mutant p53, gain-of-function, targeted therapy, p53 reactivation, review
1. Introduction
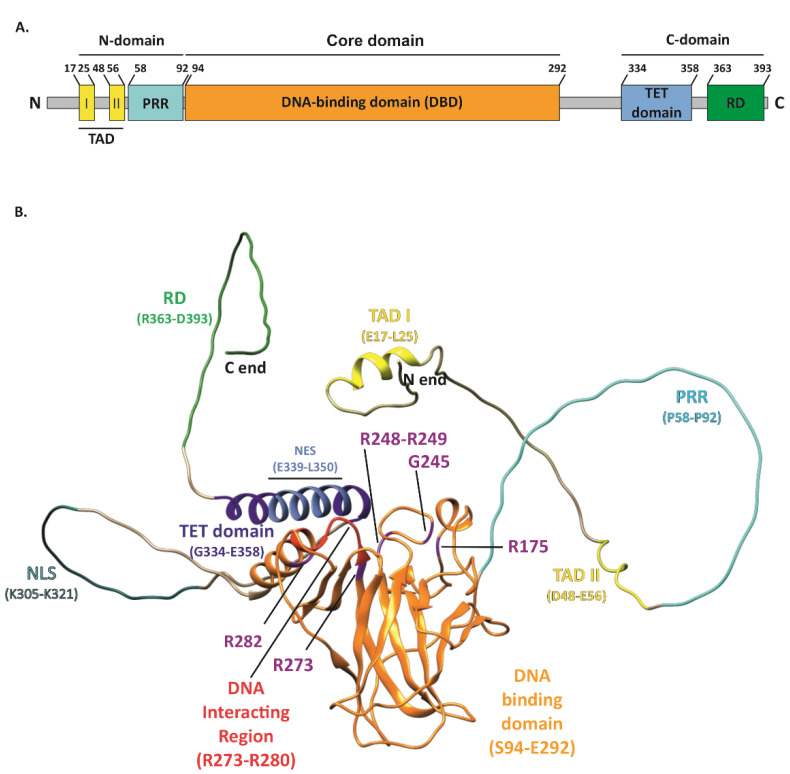
The tumor suppressor p53, encoded by the TP53 gene and known as “the guardian of the genome” [1], performs a variety of functions in cancer prevention. The basic unit of the p53 protein consists of three major functional domains such as an N-terminal transactivation domain (TAD), a core DNA-binding domain (DBD)—the main target for mutations, and a C-terminal regulatory domain (CTD) (Figure 1A) [2]. The p53 protein, to exert its function, binds in a sequence-specific manner to the DNA-binding sites by forming a tetramer, via four self-assembling p53 molecules, which are stabilized by protein–protein and base-stacking interactions [3]. As a gene-expression regulator, it mainly controls how the cell behaves under stress conditions. p53-specific responses consist of the activation of mechanisms such as cell cycle arrest, apoptosis, and senescence [4,5,6,7]. Responsible for such pivotal processes, p53 can deliver considerable damage if mutated, thus becoming “the guardian of the cancer cell” [8]. TP53 is the most frequently mutated gene in cancers. It has been recognized that 50% of cancer patients acquire certain types of TP53 gene alterations [9,10]. The potential role of p53 as a specific target in modern therapies against cancers is being widely discussed. Numerous attempts at this approach have already been made. TP53 mutations are labeled as loss of function (LOF) or gain of function (GOF). The presence of GOF TP53 mutants increases the malignancy of tumors in various ways. Occurring metastases, greater chemoresistance, invasiveness, and shorter survival are typical traits of GOF. These mutations account for 30% of all missense mutations in the TP53 gene. Widespread screening of patients allowed for the recognition of the hotspots R175, G245, R248, R249, R273, and R282, which are present in the DNA-binding domain (DBD) or near this interface of p53 [11].
Figure 1.
Structure of the p53 protein. (A) Simplified representation of the secondary structure showing domain organization of the human p53 protein (Uniprot #P04637). TAD, transactivation domain; PRR, proline-rich region; DBD, DNA-binding domain; TET, tetramerization domain; RD, regulatory domain. (B) Schematic representation of the p53 3D structure with the GOF mutation sites shown (purple). The TAD domain is shown in yellow with TAD I and II motifs indicated (yellow); PRR (cyan); DBD (orange) with direct DNA-binding region indicated (red); bipartite nuclear localization signal (NLS) (sea green); TET domain (dark blue) including nuclear export signal (NES) (light blue); and C-terminal RD (green). The structure of p53 was only partially solved by crystallography/X-ray diffraction or NMR; therefore, the 3D structure prediction of full-length protein was performed with AlphaFold DB, DeepMind Technologies Limited [12,13], and visualized with UCSF Chimera, an extensible molecular modeling system developed by the Resource for Biocomputing, Visualization, and Informatics at the University of California, San Francisco [14].
2. Pivotal Functions and Regulation Mechanisms of Wild-Type p53 (WTp53)
Non-altered p53 acts as a transcription regulator in charge of the cellular responses to stress factors, hypoxia, nutritional stress, differentiation signals, and DNA damage. Under stress conditions, an affected cell can respond in numerous ways. p53 induces cell cycle arrest, apoptosis, or senescence. Each of those processes is regulated by p53 and its target genes. Cell cycle arrest is induced by p21 and p57; apoptosis is activated via Puma, also known as BCL2 binding component 3, Bax (BCL2-associated X), and Noxa (phorbol-12-myristate-13-acetate-induced protein 1); and senescence is induced via p27Kip1 (cyclin-dependent kinase inhibitor 1B, encoded by CDKN1B gene) and Pai1 (phosphoribosylanthranilate isomerase 1), while TIGAR (TP53 induced glycolysis regulatory phosphatase) and glutaminase 2 (encoded by GLS2) are responsible for the arrangement of metabolic changes [7,15,16]. Additionally, p53 upregulates the expression of death receptor DR5 and, thus, may mediate apoptosis in part via DR5 [17]. The negative regulation of p53 activity is dependent on the Mdm2 protein, which binds to p53 and, as a result, inhibits its transcriptional functions, thus promoting proteasomal degradation. WTp53 oncogenic suppressor functions are regulated by the presence of molecular chaperones, which shape the proper tertiary and quaternary structure of the protein [18,19] However, mutant p53 (MUTp53) benefits from this mechanism, due to the chaperones that stabilize MUTp53, thus allowing it to escape proteasomal degradation [20]. It is a well-known fact that MUTp53 is abundant in cancer cells compared to WTp53 in non-tumorigenic cells, which indicates that the mutated protein is more stable [21]. Although MUTp53 undergoes the degradation mechanisms orchestrated by Mdm2 in normal tissues, the same process fails in tumors for an unknown reason [22]. Several studies were conducted to explain this phenomenon. Cancer tissues have the tendency to express HSP70 or HSP90, which stabilize MUTp53, therefore allowing its accumulation in the cell [23]. However, this explanation undermines the regulatory patterns, including those controlled by Mdm2 or other E3 ligase proteins [24].
3. Features of GOF p53 Mutants
Missense mutations occurring in the six hot spots consist of eight mutants, which account for nearly 30% of all missense mutations. They consist of R175H, G245S, R248Q, R248W, R249S, R273C, R273H, and R282W. The gain of function characteristics are designated to all of them, although the mechanisms behind their novel functionalities are different. This group can be further divided, based on the dysfunctionality that is present in the final protein. Mutation in the contact mutants, R248Q, R248W, R273H, and R273C, occurs in their DNA-binding domain, directly affecting their ability to control the transcription of targeted genes. Conformational mutants such as R175H, G245S, R249S, and R282H, on the other hand, are unable to fold properly, leading to the loss of the zinc coordinates and, thus, general DNA-binding activity [25,26,27,28,29,30]. Contact mutants and conformational (structure) mutants show decreased thermostability. Altered proteins are not capable of binding to the designated sites; however, they are capable of binding to new sequences, can regulate completely different genes, as a result, and produce new phenotypes [30]. Consequently, each of the substitutions can affect the newly formed protein differently; hence, each mutant promotes distinct GOF hallmarks caused by individual molecular mechanisms, which may require a novel approach in therapy. The clinical approach is highly complicated, because many factors must be taken into account; nevertheless, the result could notably benefit the condition of cancer patients [7,31,32]. More than 40% of cancer-related gene expression depends on the SWI/SNF signaling pathway. SWI/SNF is a subfamily of ATP-dependent chromatin remodeling complexes, which serve broad roles in the transcriptional regulation of differentiation and proliferation across many lineages. Lately, it has been shown that MUTp53 can interact with the SWI/SNF complex, resulting in chromatin being in an open state due to the histone modifications. As a consequence, changes in the expression of cancer-related genes were observed [33]. Other proteins undergo changes in their activity via MUTp53, such as p63 and p73 [34], which, in turn, inhibit apoptosis instead of inducing it. Various other transcription factors are under the influence of MUTp53 both in negative and positive regulatory activity: ETS2, NF-kB (nuclear factor kappa B), HIF-1α (hypoxia-inducible factor 1 subunit alpha), SMAD, SREBP (sterol regulatory element binding protein), and NF-Y (nuclear transcription factor Y) [29].
4. Chemoresistance Mechanisms Established by MUTp53
Cancer cells’ ability to manage oxidative stress is possible due to the presence of the xCT (also known as SLC7A11), a functional subunit of the cystine/glutamate antiporter system xc-. xCT is coded by the SLC7A11 (solute carrier family 7, member 11) gene, and its overexpression is observed in various types of cancer cells, especially in cancer stem cells (CSC). xCT alters metabolic pathways via participation in glutathione biosynthesis and, in this way, protects cancer cells from oxidative stress conditions and ferroptosis [35]. Generally, the presence of xCT in cells is associated with the promotion of tumor progression and induction of chemoresistance through the detoxification activity of GSH-mediated reactive oxygen species (ROS). It was shown that WTp53- as well as p53-carrying GOF missense mutations can inhibit the expression of SLC7A11 and sensitize cells to ferroptosis. It was also shown that cells with non-functional p53 are highly resistant to chemotherapeutics and radiotherapy; however, the knockout of the SLC7A11 gene results in the restoration of sensitivity to applied therapy. These observations are related to GSH depletion and, consequently, to the reduced protection from oxidative stress upon xCT inhibition. This suggests that, in the resistance to oxidative stress, the regulation of the xCT-glutathione axis plays an important role, which allows the tumor to survive in unsuitable conditions. Therefore, the inhibition of the xCT-glutathione axis may represent a promising approach to overcoming resistance associated with MUTp53 [36,37]. This significantly increases the resistance to therapeutic drugs, such as cisplatin, adriamycin, and etoposide, even when compared to cancer cell lines with TP53 knockdown [38]. Etoposide resistance was also observed in the study conducted by Scian et al., where it was linked to the increased expression of NF-κB2 induced by MUTp53R273H and R175H. Interestingly, the mutant D281G did not cause such effects [39]. Cisplatin resistance can be overcome in the mutant R273H via depletion of ataxia-telangiectasia (ATM) and the Rad3-related protein (ATR) activator DNA2 [40]. The understanding of these mechanisms is crucial for the successful treatment of patients. Overall, studies show that MUTp53 is involved in the increased expression of MDR1 (multidrug resistance gene 1) [41]. Each type of GOF MUTp53 can manifest several chemoresistance mechanisms due to different proteins and genes being affected. For instance, R273H is resistant to doxorubicin and methotrexate via the inhibition of apoptosis through procaspase-3 downregulation [42]. The resistance to gemcitabine occurs due to MUTp53 phosphorylation, which induces CDK1 (cyclin-dependent kinase 1) and CCNB1 (cyclin B1) expression [43], while R273H mutant is resistant to cisplatin via YAP/β-arrestin1 pathway [44]. Long non-coding RNAs (lncRNAs) are associated with chemoresistance and the proliferation of tumors. Cells carrying the R273H mutation were established to have more in common with CSC than other mutants. Moreover, lnc273-31 and lnc273-34 were required for CSC to establish the self-renewal feature. Generally, epithelial–mesenchymal transition (EMT), migration, invasion, and chemoresistance were established as the characteristics of R273H mutant cells, which demonstrate the high expression of lnc273-31 and lnc273-34. However, this effect was not manifested in R175H or R248W p53 mutants [45]. Another noteworthy chemoresistance mechanism involves the Mdm2-mediated ubiquitination and degradation of the mutant p53. It was observed that the p53R248Q mutant’s resistance to cisplatin could be modulated by fibroblast growth factor-inducible 14 (Fn14). High-grade serous ovarian cancer cells became sensitive to cisplatin, due to p53R248Q degradation, which was possible when expression of Fn14 was restored [46].
5. Possible Therapeutic Approaches
Novel strategies in the therapies targeting MUTp53 have been presented over the past decade. These strategies include the elimination of mutant p53 and restoration or reactivation of WTp53, destabilization of MUTp53, or inhibition of the downstream signaling resulting from mutant p53 gain of function and, thus, initiation of synthetic lethality in the cells expressing mutant p53. Numerous compounds targeting MUTp53 have already been discovered, and various brilliant reviews have already described them in a comprehensive manner [26,47,48,49,50,51]. However, only a few of these compounds have reached the clinical stage of research. This review focuses on the current therapeutic opportunities for oncologic patients. The molecules taken into consideration for this article are APR-246 (PRIMA-1MET, eprenetapopt), COTI-2, vorinostat (SAHA), and PEITC (phenethyl isothiocyanate). All of the reviewed molecules are summed up in Table 1.
Table 1.
Therapeutic compounds currently being investigated in clinical trials related to the treatment of cancer.
| Drug | Type of Drug |
No. of Registered Clinical Trials Related to Cancer Treatment |
Mechanism | Targeted p53 Mutants |
Phases of Clinical Trials |
Discovery |
|---|---|---|---|---|---|---|
|
APR-246
(Eprenetapopt) |
Small molecule-cysteine thiol group targeting compound | 13 | Restoration of the native conformation by binding to thiol groups in the core domain | R175H, R273H | I–III | 2002 [52] |
| COTI-2 | Zn2+ chelator | 1 | Inhibition of MUTp53 misfolding | R175H, R273H, R273C, R282W | I | 2016 [53] |
|
SAHA
(Vorinostat) |
HDAC inhibitor | 283 | Inhibition of the Hsp90 complex- induction of degradation of the mutant p53 | R249S, R273H | I–III | 2011 [54] |
| PEITC | Phytochemical | 7 | Restoration of the native conformation Oxidative stress |
R175H | I–III | 2016 [55] |
6. APR-246 (PRIMA-1MET, Eprenetapopt)
APR-246 was discovered by cell-based screening in 2002 by Bykov and collaborators [52]. This molecule is a prodrug that, after proper conversion, targets the conformational p53 mutants and restores their native form [56], therefore restoring their transcriptive functions and allowing them to regulate the expression of targeted genes, such as PUMA, NOXA, and BAX, which, in consequence, promotes apoptosis [52,57].
APR-246 is transformed into a reactive compound methylene quinuclidinone (MQ), which is capable of covalent binding to the thiol groups in MUTp53 and WTp53 [58]. This process restores the MUTp53 DNA-binding ability. Depending on the residue, where the MQ molecule binds, it displays different mechanisms. For instance, the MQ binding to the 277-cysteine residue stabilizes the p53-DNA interface. MQ-C124 (cysteine residue 124) and MQ-C229 (cysteine residue 229) support the interface between p53 dimers. Overall, these processes function as support for the p53-DNA complexes [59]. APR-246 cytotoxicity is induced due to the accumulation of ROS. MUTp53 itself suppresses the expression of SLC7A11 by targeting NRF2 (nuclear factor erythroid 2-related factor 2), which prevents the formation of the antioxidant-glutathione (GSH). MQ, additionally, leads to ROS abundance by binding to the thiol groups of GSH [57,60,61]. This topic was further investigated by Milne and co-workers using a human cell line of non-small cell lung cancer H1299 with mutant p53R175H or R273H. The authors revealed that this mechanism disrupts the functioning of the R175H mutant but not of R273H. APR-246 demonstrates higher toxicity, when SLC7A11 is downregulated, but only for the R175H mutant and not for R273H [62]. This indicates that some mutants are more vulnerable to APR-246, while others are not. Behind these processes, a different mechanism is in charge, which results in distinct sensitivity to the applied drug. However, some studies claim that the effectiveness of APR-246 is not dependent on the p53 status [63,64,65]. Despite former research, TP53 mutation status may not be the best predictive factor for APR-246 sensitivity. Recently it was concluded that the SLC7A11 expression is a significantly more precise factor. Additionally, the SLC7A11 genetic regulators, such as ATF4, Mdm2, WTp53, and c-Myc, modulate the cancer resistance to APR-246 [65]. Intriguingly, some results show that cell line CCRF-SB with WTp53 is particularly resistant to APR-246 [66]. Nevertheless, APR-246 is a promising therapeutic in combination with other clinically used chemotherapeutics. APR-246 affects cells with TP53 mutations, such as OVCAR-3R248Q (human ovarian cancer), NSCLCR248W, and R273H (non-small cell lung carcinoma), to become sensitive to doxorubicin and cisplatin [57]. Different types of mutation significantly impact the response to the applied therapy. In the pancreatic ductal adenocarcinoma cell line with GOF TP53 (R248W cell line), it was discovered that introducing WTp53 increased the sensitivity to APR-246 [67].
APR-246 is currently registered for 13 clinical trials in patients suffering from various cancers (Table 2). Within those clinical trials, some results are promising. The use of eprenetapopt in patients diagnosed with hematologic malignancies and solid tumors has been proven safe [68,69,70,71], while, in another study, it was confirmed to give better results when combined with azacitidine [72], even greater than azacitidine alone [73], in patients with leukemias.
Table 2.
Currently registered clinical trials for the use of APR-246 in oncology patients.
| ClinicalTrials.gov Identifier |
Status | Conditions | Last Update |
Available Results |
Phase |
|---|---|---|---|---|---|
| NCT04214860 | Completed | Myeloid Malignancy | 19 January 2022 | No | I |
| NCT03931291 | Completed | Acute Myeloid Leukemia (AML), Myelodysplastic Syndromes (MDS) |
19 January 2022 | Yes [68] | II |
| NCT04383938 | Completed | Bladder Cancer, Gastric Cancer, Non-Small Cell Lung Cancer (NSCLC), Urothelial Carcinoma, Advanced Solid Tumor |
3 June 2022 | Yes [69] | I–II |
| NCT03588078 | Unknown | MDS with gene mutations, AML with gene mutations, Myeloproliferative Neoplasm (MPNs), Chronic Myelomonocytic Leukemia (CMML) |
30 January 2020 | Yes [72] | I–II |
| NCT04419389 | Suspended | Non-Hodgkin Lymphoma (NHL), Chronic Lymphocytic Leukemia (CLL), Mantle Cell Lymphoma (MCL) |
3 June 2022 | No | I–II |
| NCT03745716 | Completed | MDS with MUTp53 | 12 July 2022 | Yes [https://clinicaltrials.gov/ct2/show/NCT03745716 accessed on 2 October 2022] | III |
| NCT03268382 | Completed | High-grade Serous Ovarian Cancer (HGSC) with MUTp53 | 21 July 2022 | No | II |
| NCT03072043 | Completed | MDS, AML, MPNs; CMML with MUTp53 |
24 January 2022 | Yes [73] | I–II |
| NCT00900614 | Completed | Hematologic Neoplasms, Prostatic Neoplasms |
31 July 2019 | No | I |
| NCT02098343 | Completed | HGSC with MUTp53 | 13 October 2022 | Yes [71] | I–II |
| NCT03391050 | Terminated | Melanoma | 31 July 2019 | No | I–II |
| NCT04990778 | Withdrawn | MCL | 10 March 2022 | No | II |
7. COTI-2
COTI-2 is a third generation of thiosemicarbazone that targets p53 mutants and restores their native conformation. The molecule was registered for clinical trials and further studied in 2016 by Salim and co-workers via machine learning in silico screening (computational platform known as CHEMSA) [53]. COTI-2 is a Zn2+ chelator that binds to the misfolded mutant p53 and restores its proper folding [53,74]. COTI-2 is effective independently of p53, and its mechanism involves the activation of AMPK and inhibition of the oncogenic mTOR pathways, which suggests the presence of other targets. Its actions result in cell senescence rather than apoptosis, probably due to its effect on p21 [75]. It was reported that COTI-2 is safe in animal models [53].
The efficacy of this therapeutic agent has been tested on multiple cell lines and mice xenografts. Mostly, it demonstrated antiproliferative activity, greater than the one observed with the use of cetuximab or erlotinib, both of which were already approved for cancer treatments. COTI-2 was tested on human cell lines with different kinds of TP53 mutations, HT-29 (colon cancer, p53R273H), HCT-15 (colorectal adenocarcinoma, p53S241F), OVCAR-3 (ovarian carcinoma, p53R248Q), K562 (chronic myelogenous leukemia, p53Q136fs*13), SF-268 (glioblastoma, p53R273H), SNB-19 (glioblastoma, p53R273H), T47D (breast cancer, p53L194F), MDA-MB-231 (breast cancer, p53R280K), KRAS mutations (MDA-MB-231, colorectal cancer cell line SW620 with p53R273H), PIK3CA mutations (breast cancer cell line MCF7 with WTp53, HT-29, T47D), APC mutations (colon cancer cell line COLO-205 with p53Y103F, HCT-15), and PTEN mutations (glioblastoma cell line SF-295 with p53R248Q, SNB-19), which showed its therapeutic potential by inhibiting the growth of all the above mentioned cell lines. The results of the study conducted by Salim and co-workers offered several promising discoveries. COTI-2 was also compared to approved therapeutics such as cetuximab, erlotinib, cisplatin, and carmustine on various colon cancer and glioblastoma cell lines, in which it was proven to be more effective [53]. COTI-2 was also evaluated as an additional therapeutic component. Lindemann and co-workers demonstrated how additional usage of COTI-2 resulted in a higher sensitivity to cisplatin and radiation in head and neck squamous cell carcinoma (HNSCC), regardless of TP53 status [76]. Another study showed that cells with MUTp53 were more sensitive to COTI-2 than cells with WTp53. However, COTI-2’s effectiveness was present in all types of p53 mutations, regardless of whether they were a conformational or a contact mutant. Additionally, in this study, COTI-2 was also effective independently of p53. Cells, upon receiving the treatment, demonstrated greater p63 levels and p63’s enhanced binding to the promoters of p21 and Puma [77].
There is one registered clinical trial for COTI-2. This is a phase I study of COTI-2, as a monotherapy or in combination with cisplatin (Table 3). The patients with HNSCC must have confirmed TP53 mutations; however, the recruitment status is currently unknown.
Table 3.
Clinical trial for the use of COTI-2 in oncology patients.
| ClinicalTrials.gov Identifier | Status | Conditions | Last Update | Available Results | Phase |
|---|---|---|---|---|---|
| NCT02433626 | Unknown | Ovarian Cancer, Fallopian Tube Cancer, Endometrial Cancer, Cervical Cancer, Peritoneal Cancer, Head and Neck Cancer (HNSCC), Colorectal Cancer, Lung Cancer, Pancreatic Cancer |
1 February 2019 | No | I |
8. Vorinostat (SAHA)
Suberoylanilide hydroxamic acid (SAHA, Vorinostat) is a histone deacetylase inhibitor (HDACi). Originally it was used for cutaneous T-cell lymphoma treatment. SAHA was already approved by the FDA; however, more possibilities for its use in cancer therapy are continuously under development. Recently, it was shown to target MUTp53 specifically and induce its degradation [54]. Primarily, it regulates the acetylation of proteins including nucleosomal histones. SAHA induces apoptosis, as a result of cytochrome c release and ROS accumulation via the mitochondria-mediated pathway. In consequence, SAHA does not require functional p53 [78]. Nevertheless, p53-mediated apoptosis after treatment with SAHA has also been reported in another study [78,79].
SAHA induces cell senescence, independently of MUTp53 status. The mechanism is still under examination. Some studies suggest that it promotes MUTp53 degradation selectively, in this case in MDA-MB-231R280K and DLD1S241F (colorectal adenocarcinoma); however, SAHA-induced cell death was only present in the MDA-MB-231 cells and not in DLD1 [80].
Additionally, SAHA is capable of inducing apoptosis in cells that lack p53 mutations in a mechanism completely independent from p53. The mechanism consists of p21WAF1/CIP1 elevation via the inhibition of Mdm2, but only in the LNCaP (human prostate adenocarcinoma) cell line and not in MCF-7, despite elevated levels of p53 and p27Kip1 [81,82]. A different study conducted by Drozdkova and co-workers also suggested the p53-independent mechanism of SAHA in cancers. This is due to the apoptosis occurring in the tested cell lines with a mutation in the TP53 gene (U266A161T and RPMI8226E285K), although it had a greater impact on RPMI226 cells [83].
The study conducted by Huang and co-workers suggests a direct reaction between SAHA and p53, in cases where apoptosis was a result of p53 activation via phosphorylation. This research was carried out on an in vitro model, where nasopharyngeal carcinoma cells showed that SAHA activates tumor suppressors such as p53 and Rb1 (retinoblastoma protein), while, at the same time, inactivating AMPK (5′ AMP-activated protein kinase) signaling, which leads to apoptosis [84]. SAHA is highly effective in cell lines with p53 mutations, breast cancer, MDA-MB-231R280K, BT-474E285K, and prostate adenocarcinoma PC3p.K139fs*31, where the antiproliferative effect was present due to the increased expression of CDKN1A (cyclin-dependent kinase inhibitor 1A encoding p21), while, at the same time, CCND1 (cyclin D1) and TP53 expression levels decreased [85].
Successful in a variety of cancers in in vitro research (lymphoma, myeloma, leukemia, mesothelioma, colon carcinoma, NSSCLC, bladder, breast, prostate, ovarian, renal cell, thyroid, pancreatic, endometrial cancer, melanoma, glioblastoma) and well-tolerated by patients diagnosed with cutaneous T-cell lymphoma, SAHA has established its multipurpose use [86]. In the clinicaltrials.gov database, there appears to be 354 registered studies when using the phrase “SAHA” or “vorinostat” in the research table. The majority of those studies investigate the opportunities of cancer therapy; however, other conditions such as HIV infections (NCT01365065, NCT02707900, NCT03803605), Cushing’s disease (NCT04339751), sickle cell diseases, anemias (NCT01000155), Niemann–Pick disease (NCT02124083), and Alzheimer’s disease (NCT03056495) can also be found.
Selected clinical trials of SAHA, whether used alone or in combination with other drugs, cover a variety of cancers, and the range is summarized in Table 4. The effectiveness of vorinostat treatment in neoplasms should be carefully examined due to the quantity of conducted clinical trials.
Table 4.
Clinical trial for the use of SAHA in oncology patients.
| ClinicalTrials.gov Identifier | Status | Conditions | Last Update |
Available Results | Phase |
|---|---|---|---|---|---|
| NCT00735826 | Completed | Aerodigestive Tract Cancer, Lung Cancer, Esophageal Cancer, Head and Neck Cancer (HNSCC) |
12 October 2018 | No | NA |
| NCT02538510 | Completed | HNSCC, Squamous Cell Carcinoma, Nasopharynx Carcinoma, Salivary Gland Carcinoma |
13 September 2022 | Yes [87] | I–II |
| NCT00616967 | Active, Not Recruiting |
Breast Cancer | 3 February 2022 | Yes [88] | II |
| NCT01153672 | Completed | Breast Cancer | 6 September 2019 | Yes [https://clinicaltrials.gov/ct2/show/NCT01153672 accessed on 2 October 2022] | NA |
| NCT00967057 | Completed | Leukemia | 12 August 2013 | Yes [89,90] | III |
| NCT00121225 | Completed | Melanoma | 29 January 2019 | Yes [91] | II |
| NCT00948688 | Terminated | Pancreatic Cancer | 10 May 2017 | No | I–II |
| NCT01075113 | Completed | Liver Cancer | 20 August 2019 | Yes [92] | I |
| NCT02042989 | Completed | Advanced Cancers with MUTp53 | 11 July 2022 | No | I |
| NCT01738646 | Completed | Glioblastoma | 6 March 2017 | Yes [93] | II |
NA—not applicable.
9. PEITC
β-phenylethyl isothiocyanate (PEITC) is a phytochemical that can be found in cruciferous vegetables. This compound acts as a Zn2+ chelator, which inhibits the misfolding of MUTp53. Its therapeutic properties targeting cancer diseases were proposed by Aggarwal et al., who found this compound through cell-based screening [55]. Various studies revealed that PEITC caused oxidative stress, which inhibited the growth of cancer cells [94,95,96,97].
Studies conducted in the mouse breast cancer xenograft model (the SK-BR-3 xenograft mouse) have shown that PEITC selectively targets p53 mutants. This compound acted preferentially towards the p53R175H hotspot mutant and successfully restored its native conformation. In consequence, this mutant was exposed to proteasome-mediated degradation [55]. Moreover, it was shown that PEITC efficiently inhibited tumor growth [55]. In vitro studies have shown the effectiveness of PEITC in treating oral cancer cells with MUTp53 [95]. PEITC is active towards structural (R175H) and contact (R248W) mutants; however, it targets structural mutants favorably, as proven in xenograft prostate cancer mouse models, which resulted in tumor growth inhibition [98]. PEITC affects not only p53 but also other cell-cycle-associated proteins such as CDC25C (M-phase inducer phosphatase 3) and cyclin A2, which cause cell cycle arrest. It was shown that PEITC induces apoptosis in IPEC-J2 cells (intestinal porcine epithelial cell line) by lowering the mitochondrial membrane potential by releasing cytochrome c to the cytoplasm. This cascade of events involves the activation of caspase-9, caspase-3, and PARP 1 (Poly [ADP-ribose] polymerase 1) [97]; therefore, it is still effective in cells lacking the p53 activity. As demonstrated in a study conducted by Liu et al., PEITC lengthened the survival of mice with leukemia. In this experimental model, apoptosis was induced via the decrease in the Mcl-1 (Induced Myeloid Leukemia Cell Differentiation protein) survival molecule, as a result of glutathione depletion and ROS accumulation [99]. Generally, the oxidative stress induced by PEITC and, thus, its effectiveness in eliminating cancer cells, was reported in various studies [96]. The mechanism behind cell cycle arrest in the G2/M phase caused by PEITC application involves oxidative stress in the DNA-damage-induced ATM–Chk2-p53-related pathway [94]. PEITC induces G2/M cell cycle arrest and inhibits the growth of oral cancer cells via the decrease in the Mcl-1 survival molecule, as a result of glutathione depletion and ROS accumulation [99]. Currently, 10 studies with the use of PEITC are registered, and 7 of them include cancer treatment. All of the studies are summarized in Table 5. However, without the published data, it is difficult to determine the legitimacy of the treatment involving PEITC. Nevertheless, one study claims this molecule is potentially effective in inhibiting cancerogenesis in smokers [100].
Table 5.
Clinical trial for the use of PEITC.
| ClinicalTrials.gov Identifier | Status | Conditions | Last Update | Available Results | Phase |
|---|---|---|---|---|---|
| NCT03700983 | Completed | Head and Neck Cancer |
9 October 2018 | No | NA |
| NCT03034603 | Active, Not Recruiting |
Head and Neck Neoplasms |
10 October 2022 | No | NA |
| NCT00691132 | Completed | Lung Cancer | 12 May 2017 | Yes [100] | II |
| NCT00968461 | Withdrawn | Leukemia | 15 April 2013 | No | I |
| NCT01790204 | Completed | Oral Cancer with MUTp53 | 23 March 2015 | No | I–II |
| NCT00005883 | Completed | Lung Cancer | 28 March 2011 | No | I |
| NCT02468882 | Unknown | Long-term Effects Secondary to Cancer |
11 June 2015 | No | III |
| NCT03978117 | Recruiting | Healthy | 5 April 2022 | No | II |
| NCT05354453 | Recruiting | Healthy | 19 October 2022 | No | I |
| NCT05070585 | Recruiting | Metabolic Disturbance |
9 June 2022 | No | I–II |
NA—not applicable.
10. Conclusions
Targeted, personalized therapy still has a long way to go, but, with each study, that goal becomes closer. A key question to ask at this stage, of any cutting-edge therapeutic work, is what benefit it may bring to the patient.
The results of the in vitro and in vivo studies of new potential drugs often show the benefits of their use. Unfortunately, at the stage of clinical trials, the expected benefits of treatment often turn out to be far from ideal, especially in oncological patients. When cancer occurs, the changes in p53 are most notably related to its antitumor functions. Not only do mutations of p53 appear but also other abnormalities; thus, specific approaches for such a broad group of patients might be almost impossible. Taking into account variables such as interactions with other genes and metabolites and the dominant negative effects, regardless of whether the mutation is germinal or somatic, the application of personalized therapy in the clinic seems almost impossible [32]. Nevertheless, progress in personalized treatment is still being achieved; for instance, a recent study conducted by Klimovich et al. states that even partial p53 reactivation can induce cancer regression in mice, when the MUTp53E177R variant is considered. However, other mutants have not been studied yet. Researchers point out that when even a partial loss in activity of p53 is introduced, the cancer risk is increased. However, when there is minimal to no activity at all, the phenomena known as p53 addiction [38,101,102] occurs, which means that the cancer cell becomes addicted to the presence of MUTp53 or to the loss of WTp53 activity. The potential medical treatment involves triggering just a small activity of WTp53 through the applied drug, which, in turn, is enough to reduce the tumor [103]. The use of combination treatment when one drug reactivates MUTp53 and the other targets a specific cancer is very promising. As observed in the clinical trial NCT03072043, treatment with eprenetapopt and azacytidine was beneficial to patients with myelodysplastic syndromes and with oligoblastic acute myeloid leukemia, which carries p53 mutants [73]. Other therapeutic approaches such as, for instance, indirect therapy via Mdm2 [31] should also be taken into consideration. Many therapeutics, which are inhibitors of Mdm2 or Mdm4, have entered clinical trials but have yet to be proven to be safe and effective in cancer treatment, such as milademetan, RG7112, RG7388, CGM097, HDM201, and ALRN-6924. Indirect therapy against MUTp53 may prove to be more beneficial and easier to introduce to the clinic, regardless of the specific type of TP53 mutation. Some recent reviews argue that targeting MUTp53 will provide effective treatment in the future. The development of machine learning technology may come with an easier answer to the future of cancer treatment. Recently a study using such technology reported new genes (GPSM2, OR4N2, CTSL2, SPERT, RPE65) that may be associated with p53 functions, which seem to be a better fit for the platinum-based therapies for patients than their TP53 status [104]. The ongoing discussion between researchers on whether personal therapy, which considers the investigation of molecules targeting the exact type of mutation of TP53, should be pursued or not has yet to be unraveled [105,106,107,108,109].
Acknowledgments
The authors would like to thank Iwona Gajór and Krzysztof Flis for assisting with the manuscript correction.
Author Contributions
Conceptualization, K.A.R.; writing—original draft preparation, K.A.R., A.P., M.S., and Z.G.; writing—review and editing, K.A.R. and S.F.; supervision, K.A.R. and S.F.; manuscript revision, figures, and tables preparation, K.A.R. and S.F. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the National Science Centre, Poland, grant number 2019/33/N/NZ4/02773 to K.A.R., and by the Center for Translational Medicine, Warsaw University of Life Sciences, Poland.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lane D.P. Cancer. p53, guardian of the genome. Nature. 1992;358:15–16. doi: 10.1038/358015a0. [DOI] [PubMed] [Google Scholar]
- 2.Tan Y.S., Mhoumadi Y., Verma C.S. Roles of computational modelling in understanding p53 structure, biology, and its therapeutic targeting. J. Mol. Cell Biol. 2019;11:306–316. doi: 10.1093/jmcb/mjz009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kitayner M., Rozenberg H., Kessler N., Rabinovich D., Shaulov L., Haran T.E., Shakked Z. Structural basis of DNA recognition by p53 tetramers. Mol. Cell. 2006;22:741–753. doi: 10.1016/j.molcel.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 4.Kruiswijk F., Labuschagne C.F., Vousden K.H. p53 in survival, death and metabolic health: A lifeguard with a licence to kill. Nat. Rev. Mol. Cell Biol. 2015;16:393. doi: 10.1038/nrm4007. [DOI] [PubMed] [Google Scholar]
- 5.Zilfou J.T., Lowe S.W. Tumor suppressive functions of p53. Cold Spring Harb. Perspect. Biol. 2009;1:a001883. doi: 10.1101/cshperspect.a001883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bieging K.T., Mello S.S., Attardi L.D. Unravelling mechanisms of p53-mediated tumour suppression. Nat. Rev. Cancer. 2014;14:359–370. doi: 10.1038/nrc3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alvarado-Ortiz E., de la Cruz-Lopez K.G., Becerril-Rico J., Sarabia-Sanchez M.A., Ortiz-Sanchez E., Garcia-Carranca A. Mutant p53 Gain-of-Function: Role in Cancer Development, Progression, and Therapeutic Approaches. Front. Cell Dev. Biol. 2020;8:607670. doi: 10.3389/fcell.2020.607670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mantovani F., Collavin L., Del Sal G. Mutant p53 as a guardian of the cancer cell. Cell Death Differ. 2019;26:199–212. doi: 10.1038/s41418-018-0246-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lawrence M.S., Stojanov P., Mermel C.H., Robinson J.T., Garraway L.A., Golub T.R., Meyerson M., Gabriel S.B., Lander E.S., Getz G. Discovery and saturation analysis of cancer genes across 21 tumour types. Nature. 2014;505:495–501. doi: 10.1038/nature12912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bouaoun L., Sonkin D., Ardin M., Hollstein M., Byrnes G., Zavadil J., Olivier M. TP53 Variations in Human Cancers: New Lessons from the IARC TP53 Database and Genomics Data. Hum. Mutat. 2016;37:865–876. doi: 10.1002/humu.23035. [DOI] [PubMed] [Google Scholar]
- 11.Baugh E.H., Ke H., Levine A.J., Bonneau R.A., Chan C.S. Why are there hotspot mutations in the TP53 gene in human cancers? Cell Death Differ. 2018;25:154–160. doi: 10.1038/cdd.2017.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jumper J., Evans R., Pritzel A., Green T., Figurnov M., Ronneberger O., Tunyasuvunakool K., Bates R., Žídek A., Potapenko A., et al. Highly accurate protein structure prediction with AlphaFold. Nature. 2021;596:583–589. doi: 10.1038/s41586-021-03819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Varadi M., Anyango S., Deshpande M., Nair S., Natassia C., Yordanova G., Yuan D., Stroe O., Wood G., Laydon A., et al. AlphaFold Protein Structure Database: Massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Res. 2021;50:D439–D444. doi: 10.1093/nar/gkab1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pettersen E.F., Goddard T.D., Huang C.C., Couch G.S., Greenblatt D.M., Meng E.C., Ferrin T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 15.Kastenhuber E.R., Lowe S.W. Putting p53 in Context. Cell. 2017;170:1062–1078. doi: 10.1016/j.cell.2017.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simabuco F.M., Morale M.G., Pavan I.C.B., Morelli A.P., Silva F.R., Tamura R.E. p53 and metabolism: From mechanism to therapeutics. Oncotarget. 2018;9:23780–23823. doi: 10.18632/oncotarget.25267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sheikh M.S., Fornace A.J., Jr. Death and decoy receptors and p53-mediated apoptosis. Leukemia. 2000;14:1509–1513. doi: 10.1038/sj.leu.2401865. [DOI] [PubMed] [Google Scholar]
- 18.Stindt M.H., Muller P.A., Ludwig R.L., Kehrloesser S., Dotsch V., Vousden K.H. Functional interplay between MDM2, p63/p73 and mutant p53. Oncogene. 2015;34:4300–4310. doi: 10.1038/onc.2014.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sherman M.Y., Gabai V., O’Callaghan C., Yaglom J. Molecular chaperones regulate p53 and suppress senescence programs. FEBS Lett. 2007;581:3711–3715. doi: 10.1016/j.febslet.2007.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wawrzynow B., Zylicz A., Zylicz M. Chaperoning the guardian of the genome. The two-faced role of molecular chaperones in p53 tumor suppressor action. Biochim. Biophys. Acta Rev. Cancer. 2018;1869:161–174. doi: 10.1016/j.bbcan.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 21.Prives C., White E. Does control of mutant p53 by Mdm2 complicate cancer therapy? Genes Dev. 2008;22:1259–1264. doi: 10.1101/gad.1680508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lang G.A., Iwakuma T., Suh Y.A., Liu G., Rao V.A., Parant J.M., Valentin-Vega Y.A., Terzian T., Caldwell L.C., Strong L.C., et al. Gain of function of a p53 hot spot mutation in a mouse model of Li-Fraumeni syndrome. Cell. 2004;119:861–872. doi: 10.1016/j.cell.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 23.Yue X., Zhao Y., Xu Y., Zheng M., Feng Z., Hu W. Mutant p53 in Cancer: Accumulation, Gain-of-Function, and Therapy. J. Mol. Biol. 2017;429:1595–1606. doi: 10.1016/j.jmb.2017.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chipuk J.E., Maurer U., Green D.R., Schuler M. Pharmacologic activation of p53 elicits Bax-dependent apoptosis in the absence of transcription. Cancer Cell. 2003;4:371–381. doi: 10.1016/S1535-6108(03)00272-1. [DOI] [PubMed] [Google Scholar]
- 25.Stein Y., Rotter V., Aloni-Grinstein R. Gain-of-Function Mutant p53: All the Roads Lead to Tumorigenesis. Int. J. Mol. Sci. 2019;20:6197. doi: 10.3390/ijms20246197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bykov V.J.N., Eriksson S.E., Bianchi J., Wiman K.G. Targeting mutant p53 for efficient cancer therapy. Nat. Rev. Cancer. 2018;18:89–102. doi: 10.1038/nrc.2017.109. [DOI] [PubMed] [Google Scholar]
- 27.Bullock A.N., Fersht A.R. Rescuing the function of mutant p53. Nat. Rev. Cancer. 2001;1:68–76. doi: 10.1038/35094077. [DOI] [PubMed] [Google Scholar]
- 28.Bullock A.N., Henckel J., Fersht A.R. Quantitative analysis of residual folding and DNA binding in mutant p53 core domain: Definition of mutant states for rescue in cancer therapy. Oncogene. 2000;19:1245–1256. doi: 10.1038/sj.onc.1203434. [DOI] [PubMed] [Google Scholar]
- 29.Kim M.P., Lozano G. Mutant p53 partners in crime. Cell Death Differ. 2018;25:161–168. doi: 10.1038/cdd.2017.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim E., Deppert W. Interactions of mutant p53 with DNA: Guilt by association. Oncogene. 2007;26:2185–2190. doi: 10.1038/sj.onc.1210312. [DOI] [PubMed] [Google Scholar]
- 31.Hu J., Cao J., Topatana W., Juengpanich S., Li S., Zhang B., Shen J., Cai L., Cai X., Chen M. Targeting mutant p53 for cancer therapy: Direct and indirect strategies. J. Hematol. Oncol. 2021;14:157. doi: 10.1186/s13045-021-01169-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sabapathy K., Lane D.P. Therapeutic targeting of p53: All mutants are equal, but some mutants are more equal than others. Nat. Reviews. Clin. Oncol. 2018;15:13–30. doi: 10.1038/nrclinonc.2017.151. [DOI] [PubMed] [Google Scholar]
- 33.Pfister N.T., Fomin V., Regunath K., Zhou J.Y., Zhou W., Silwal-Pandit L., Freed-Pastor W.A., Laptenko O., Neo S.P., Bargonetti J., et al. Mutant p53 cooperates with the SWI/SNF chromatin remodeling complex to regulate VEGFR2 in breast cancer cells. Genes Dev. 2015;29:1298–1315. doi: 10.1101/gad.263202.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferraiuolo M., Di Agostino S., Blandino G., Strano S. Oncogenic Intra-p53 Family Member Interactions in Human Cancers. Front. Oncol. 2016;6:77. doi: 10.3389/fonc.2016.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ruiu R., Rolih V., Bolli E., Barutello G., Riccardo F., Quaglino E., Merighi I.F., Pericle F., Donofrio G., Cavallo F., et al. Fighting breast cancer stem cells through the immune-targeting of the xCT cystine-glutamate antiporter. Cancer Immunol. Immunother. CII. 2019;68:131–141. doi: 10.1007/s00262-018-2185-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McCubrey J.A., Meher A.K., Akula S.M., Abrams S.L., Steelman L.S., LaHair M.M., Franklin R.A., Martelli A.M., Ratti S., Cocco L., et al. Wild type and gain of function mutant TP53 can regulate the sensitivity of pancreatic cancer cells to chemotherapeutic drugs, EGFR/Ras/Raf/MEK, and PI3K/mTORC1/GSK-3 pathway inhibitors, nutraceuticals and alter metabolic properties. Aging. 2022;14:3365–3386. doi: 10.18632/aging.204038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.He C., Li L., Guan X., Xiong L., Miao X. Mutant p53 Gain of Function and Chemoresistance: The Role of Mutant p53 in Response to Clinical Chemotherapy. Chemotherapy. 2017;62:43–53. doi: 10.1159/000446361. [DOI] [PubMed] [Google Scholar]
- 38.Bossi G., Lapi E., Strano S., Rinaldo C., Blandino G., Sacchi A. Mutant p53 gain of function: Reduction of tumor malignancy of human cancer cell lines through abrogation of mutant p53 expression. Oncogene. 2006;25:304–309. doi: 10.1038/sj.onc.1209026. [DOI] [PubMed] [Google Scholar]
- 39.Scian M.J., Stagliano K.E., Anderson M.A., Hassan S., Bowman M., Miles M.F., Deb S.P., Deb S. Tumor-derived p53 mutants induce NF-kappaB2 gene expression. Mol. Cell. Biol. 2005;25:10097–10110. doi: 10.1128/MCB.25.22.10097-10110.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu K., Lin F.T., Graves J.D., Lee Y.J., Lin W.C. Mutant p53 perturbs DNA replication checkpoint control through TopBP1 and Treslin. Proc. Natl. Acad. Sci. USA. 2017;114:E3766–E3775. doi: 10.1073/pnas.1619832114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sampath J., Sun D., Kidd V.J., Grenet J., Gandhi A., Shapiro L.H., Wang Q., Zambetti G.P., Schuetz J.D. Mutant p53 cooperates with ETS and selectively up-regulates human MDR1 not MRP1. J. Biol. Chem. 2001;276:39359–39367. doi: 10.1074/jbc.M103429200. [DOI] [PubMed] [Google Scholar]
- 42.Wong R.P., Tsang W.P., Chau P.Y., Co N.N., Tsang T.Y., Kwok T.T. p53-R273H gains new function in induction of drug resistance through down-regulation of procaspase-3. Mol. Cancer Ther. 2007;6:1054–1061. doi: 10.1158/1535-7163.MCT-06-0336. [DOI] [PubMed] [Google Scholar]
- 43.Fiorini C., Cordani M., Padroni C., Blandino G., Di Agostino S., Donadelli M. Mutant p53 stimulates chemoresistance of pancreatic adenocarcinoma cells to gemcitabine. Biochim. Et Biophys. Acta. 2015;1853:89–100. doi: 10.1016/j.bbamcr.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 44.Tocci P., Cianfrocca R., Di Castro V., Rosano L., Sacconi A., Donzelli S., Bonfiglio S., Bucci G., Vizza E., Ferrandina G., et al. beta-arrestin1/YAP/mutant p53 complexes orchestrate the endothelin A receptor signaling in high-grade serous ovarian cancer. Nat. Commun. 2019;10:3196. doi: 10.1038/s41467-019-11045-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao Y., Li Y., Sheng J., Wu F., Li K., Huang R., Wang X., Jiao T., Guan X., Lu Y., et al. P53-R273H mutation enhances colorectal cancer stemness through regulating specific lncRNAs. J. Exp. Clin. Cancer Res. CR. 2019;38:379. doi: 10.1186/s13046-019-1375-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu A.Y., Gu L.Y., Cang W., Cheng M.X., Wang W.J., Di W., Huang L., Qiu L.H. Fn14 overcomes cisplatin resistance of high-grade serous ovarian cancer by promoting Mdm2-mediated p53-R248Q ubiquitination and degradation. J. Exp. Clin. Cancer Res. CR. 2019;38:176. doi: 10.1186/s13046-019-1171-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Binayke A., Mishra S., Suman P., Das S., Chander H. Awakening the “guardian of genome”: Reactivation of mutant p53. Cancer Chemother. Pharmacol. 2019;83:1–15. doi: 10.1007/s00280-018-3701-x. [DOI] [PubMed] [Google Scholar]
- 48.Gomes A.S., Ramos H., Inga A., Sousa E., Saraiva L. Structural and Drug Targeting Insights on Mutant p53. Cancers. 2021;13:3344. doi: 10.3390/cancers13133344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Parrales A., Iwakuma T. Targeting Oncogenic Mutant p53 for Cancer Therapy. Front. Oncol. 2015;5:288. doi: 10.3389/fonc.2015.00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schulz-Heddergott R., Moll U.M. Gain-of-Function (GOF) Mutant p53 as Actionable Therapeutic Target. Cancers. 2018;10:188. doi: 10.3390/cancers10060188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li Y., Wang Z., Chen Y., Petersen R.B., Zheng L., Huang K. Salvation of the fallen angel: Reactivating mutant p53. Br. J. Pharmacol. 2019;176:817–831. doi: 10.1111/bph.14572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bykov V.J., Issaeva N., Shilov A., Hultcrantz M., Pugacheva E., Chumakov P., Bergman J., Wiman K.G., Selivanova G. Restoration of the tumor suppressor function to mutant p53 by a low-molecular-weight compound. Nat. Med. 2002;8:282–288. doi: 10.1038/nm0302-282. [DOI] [PubMed] [Google Scholar]
- 53.Salim K.Y., Maleki Vareki S., Danter W.R., Koropatnick J. COTI-2, a novel small molecule that is active against multiple human cancer cell lines in vitro and in vivo. Oncotarget. 2016;7:41363–41379. doi: 10.18632/oncotarget.9133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li D., Marchenko N.D., Moll U.M. SAHA shows preferential cytotoxicity in mutant p53 cancer cells by destabilizing mutant p53 through inhibition of the HDAC6-Hsp90 chaperone axis. Cell Death Differ. 2011;18:1904–1913. doi: 10.1038/cdd.2011.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aggarwal M., Saxena R., Sinclair E., Fu Y., Jacobs A., Dyba M., Wang X., Cruz I., Berry D., Kallakury B., et al. Reactivation of mutant p53 by a dietary-related compound phenethyl isothiocyanate inhibits tumor growth. Cell Death Differ. 2016;23:1615–1627. doi: 10.1038/cdd.2016.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bykov V.J., Issaeva N., Zache N., Shilov A., Hultcrantz M., Bergman J., Selivanova G., Wiman K.G. Reactivation of mutant p53 and induction of apoptosis in human tumor cells by maleimide analogs. J. Biol. Chem. 2005;280:30384–30391. doi: 10.1074/jbc.M501664200. [DOI] [PubMed] [Google Scholar]
- 57.Mohell N., Alfredsson J., Fransson A., Uustalu M., Bystrom S., Gullbo J., Hallberg A., Bykov V.J., Bjorklund U., Wiman K.G. APR-246 overcomes resistance to cisplatin and doxorubicin in ovarian cancer cells. Cell Death Dis. 2015;6:e1794. doi: 10.1038/cddis.2015.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lambert J.M., Gorzov P., Veprintsev D.B., Soderqvist M., Segerback D., Bergman J., Fersht A.R., Hainaut P., Wiman K.G., Bykov V.J. PRIMA-1 reactivates mutant p53 by covalent binding to the core domain. Cancer Cell. 2009;15:376–388. doi: 10.1016/j.ccr.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 59.Degtjarik O., Golovenko D., Diskin-Posner Y., Abrahmsen L., Rozenberg H., Shakked Z. Structural basis of reactivation of oncogenic p53 mutants by a small molecule: Methylene quinuclidinone (MQ) Nat. Commun. 2021;12:7057. doi: 10.1038/s41467-021-27142-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Menichini P., Monti P., Speciale A., Cutrona G., Matis S., Fais F., Taiana E., Neri A., Bomben R., Gentile M., et al. Antitumor Effects of PRIMA-1 and PRIMA-1(Met) (APR246) in Hematological Malignancies: Still a Mutant P53-Dependent Affair? Cells. 2021;10:98. doi: 10.3390/cells10010098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Omar S.I., Tuszynski J. The molecular mechanism of action of methylene quinuclidinone and its effects on the structure of p53 mutants. Oncotarget. 2018;9:37137–37156. doi: 10.18632/oncotarget.26440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Milne J.V., Zhang B.Z., Fujihara K.M., Dawar S., Phillips W.A., Clemons N.J. Transketolase regulates sensitivity to APR-246 in p53-null cells independently of oxidative stress modulation. Sci. Rep. 2021;11:4480. doi: 10.1038/s41598-021-83979-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tessoulin B., Descamps G., Moreau P., Maiga S., Lode L., Godon C., Marionneau-Lambot S., Oullier T., Le Gouill S., Amiot M., et al. PRIMA-1Met induces myeloma cell death independent of p53 by impairing the GSH/ROS balance. Blood. 2014;124:1626–1636. doi: 10.1182/blood-2014-01-548800. [DOI] [PubMed] [Google Scholar]
- 64.Grellety T., Laroche-Clary A., Chaire V., Lagarde P., Chibon F., Neuville A., Italiano A. PRIMA-1(MET) induces death in soft-tissue sarcomas cell independent of p53. BMC Cancer. 2015;15:684. doi: 10.1186/s12885-015-1667-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fujihara K.M., Corrales Benitez M., Cabalag C.S., Zhang B.Z., Ko H.S., Liu D.S., Simpson K.J., Haupt Y., Lipton L., Haupt S., et al. SLC7A11 is a superior determinant of APR-246 (Eprenetapopt) response than TP53 mutation status. Mol. Cancer Ther. 2021;20:1858–1867. doi: 10.1158/1535-7163.MCT-21-0067. [DOI] [PubMed] [Google Scholar]
- 66.Ceder S., Eriksson S.E., Liang Y.Y., Cheteh E.H., Zhang S.M., Fujihara K.M., Bianchi J., Bykov V.J.N., Abrahmsen L., Clemons N.J., et al. Mutant p53-reactivating compound APR-246 synergizes with asparaginase in inducing growth suppression in acute lymphoblastic leukemia cells. Cell Death Dis. 2021;12:709. doi: 10.1038/s41419-021-03988-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Abrams S.L., Duda P., Akula S.M., Steelman L.S., Follo M.L., Cocco L., Ratti S., Martelli A.M., Montalto G., Emma M.R., et al. Effects of the Mutant TP53 Reactivator APR-246 on Therapeutic Sensitivity of Pancreatic Cancer Cells in the Presence and Absence of WT-TP53. Cells. 2022;11:794. doi: 10.3390/cells11050794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mishra A., Tamari R., DeZern A.E., Byrne M.T., Gooptu M., Chen Y.B., Deeg H.J., Sallman D., Gallacher P., Wennborg A., et al. Eprenetapopt Plus Azacitidine After Allogeneic Hematopoietic Stem-Cell Transplantation for TP53-Mutant Acute Myeloid Leukemia and Myelodysplastic Syndromes. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2022:JCO2200181. doi: 10.1200/JCO.22.00181. [DOI] [PubMed] [Google Scholar]
- 69.Park H., Shapiro G.I., Gao X., Mahipal A., Starr J., Furqan M., Singh P., Ahrorov A., Gandhi L., Ghosh A., et al. Phase Ib study of eprenetapopt (APR-246) in combination with pembrolizumab in patients with advanced or metastatic solid tumors. ESMO Open. 2022;7:100573. doi: 10.1016/j.esmoop.2022.100573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lehmann S., Bykov V.J., Ali D., Andren O., Cherif H., Tidefelt U., Uggla B., Yachnin J., Juliusson G., Moshfegh A., et al. Targeting p53 in vivo: A first-in-human study with p53-targeting compound APR-246 in refractory hematologic malignancies and prostate cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2012;30:3633–3639. doi: 10.1200/JCO.2011.40.7783. [DOI] [PubMed] [Google Scholar]
- 71.Deneberg S., Cherif H., Lazarevic V., Andersson P.O., von Euler M., Juliusson G., Lehmann S. An open-label phase I dose-finding study of APR-246 in hematological malignancies. Blood Cancer J. 2016;6:e447. doi: 10.1038/bcj.2016.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cluzeau T., Sebert M., Rahme R., Cuzzubbo S., Lehmann-Che J., Madelaine I., Peterlin P., Beve B., Attalah H., Chermat F., et al. Eprenetapopt Plus Azacitidine in TP53-Mutated Myelodysplastic Syndromes and Acute Myeloid Leukemia: A Phase II Study by the Groupe Francophone des Myelodysplasies (GFM) J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2021;39:1575–1583. doi: 10.1200/JCO.20.02342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sallman D.A., DeZern A.E., Garcia-Manero G., Steensma D.P., Roboz G.J., Sekeres M.A., Cluzeau T., Sweet K.L., McLemore A., McGraw K.L., et al. Eprenetapopt (APR-246) and Azacitidine in TP53-Mutant Myelodysplastic Syndromes. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2021;39:1584–1594. doi: 10.1200/JCO.20.02341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yu X., Blanden A.R., Narayanan S., Jayakumar L., Lubin D., Augeri D., Kimball S.D., Loh S.N., Carpizo D.R. Small molecule restoration of wildtype structure and function of mutant p53 using a novel zinc-metallochaperone based mechanism. Oncotarget. 2014;5:8879–8892. doi: 10.18632/oncotarget.2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fitzgerald A.L., Osman A.A., Xie T.X., Patel A., Skinner H., Sandulache V., Myers J.N. Reactive oxygen species and p21Waf1/Cip1 are both essential for p53-mediated senescence of head and neck cancer cells. Cell Death Dis. 2015;6:e1678. doi: 10.1038/cddis.2015.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lindemann A., Patel A.A., Silver N.L., Tang L., Liu Z., Wang L., Tanaka N., Rao X., Takahashi H., Maduka N.K., et al. COTI-2, A Novel Thiosemicarbazone Derivative, Exhibits Antitumor Activity in HNSCC through p53-dependent and -independent Mechanisms. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2019;25:5650–5662. doi: 10.1158/1078-0432.CCR-19-0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Synnott N.C., O’Connell D., Crown J., Duffy M.J. COTI-2 reactivates mutant p53 and inhibits growth of triple-negative breast cancer cells. Breast Cancer Res. Treat. 2020;179:47–56. doi: 10.1007/s10549-019-05435-1. [DOI] [PubMed] [Google Scholar]
- 78.Ruefli A.A., Ausserlechner M.J., Bernhard D., Sutton V.R., Tainton K.M., Kofler R., Smyth M.J., Johnstone R.W. The histone deacetylase inhibitor and chemotherapeutic agent suberoylanilide hydroxamic acid (SAHA) induces a cell-death pathway characterized by cleavage of Bid and production of reactive oxygen species. Proc. Natl. Acad. Sci. USA. 2001;98:10833–10838. doi: 10.1073/pnas.191208598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Henderson C., Mizzau M., Paroni G., Maestro R., Schneider C., Brancolini C. Role of caspases, Bid, and p53 in the apoptotic response triggered by histone deacetylase inhibitors trichostatin-A (TSA) and suberoylanilide hydroxamic acid (SAHA) J. Biol. Chem. 2003;278:12579–12589. doi: 10.1074/jbc.M213093200. [DOI] [PubMed] [Google Scholar]
- 80.Foggetti G., Ottaggio L., Russo D., Mazzitelli C., Monti P., Degan P., Miele M., Fronza G., Menichini P. Autophagy induced by SAHA affects mutant P53 degradation and cancer cell survival. Biosci. Rep. 2019;39 doi: 10.1042/BSR20181345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Natarajan U., Venkatesan T., Radhakrishnan V., Samuel S., Rasappan P., Rathinavelu A. Cell Cycle Arrest and Cytotoxic Effects of SAHA and RG7388 Mediated through p21(WAF1/CIP1) and p27(KIP1) in Cancer Cells. Medicina. 2019;55:30. doi: 10.3390/medicina55020030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Natarajan U., Venkatesan T., Rathinavelu A. Effect of the HDAC Inhibitor on Histone Acetylation and Methyltransferases in A2780 Ovarian Cancer Cells. Medicina. 2021;57:456. doi: 10.3390/medicina57050456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Drozdkova D.H., Gursky J., Minarik J., Uberall I., Kolar Z., Trtkova K.S. CDKN1A Gene Expression in Two Multiple Myeloma Cell Lines With Different P53 Functionality. Anticancer Res. 2020;40:4979–4987. doi: 10.21873/anticanres.14501. [DOI] [PubMed] [Google Scholar]
- 84.Huang H., Fu Y., Zhang Y., Peng F., Lu M., Feng Y., Chen L., Chen Z., Li M., Chen Y. Dissection of Anti-tumor Activity of Histone Deacetylase Inhibitor SAHA in Nasopharyngeal Carcinoma Cells via Quantitative Phosphoproteomics. Front. Cell Dev. Biol. 2020;8:577784. doi: 10.3389/fcell.2020.577784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hernandez-Borja F., Mercado-Sanchez I., Alcaraz Y., Garcia-Revilla M.A., Villegas Gomez C., Ordaz-Rosado D., Santos-Martinez N., Garcia-Becerra R., Vazquez M.A. Exploring novel capping framework: High substituent pyridine-hydroxamic acid derivatives as potential antiproliferative agents. Daru J. Fac. Pharm. Tehran Univ. Med. Sci. 2021;29:291–310. doi: 10.1007/s40199-021-00406-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Richon V.M. Cancer biology: Mechanism of antitumour action of vorinostat (suberoylanilide hydroxamic acid), a novel histone deacetylase inhibitor. Br. J. Cancer. 2006;95:S2–S6. doi: 10.1038/sj.bjc.6603463. [DOI] [Google Scholar]
- 87.Rodriguez C.P., Wu Q.V., Voutsinas J., Fromm J.R., Jiang X., Pillarisetty V.G., Lee S.M., Santana-Davila R., Goulart B., Baik C.S., et al. A Phase II Trial of Pembrolizumab and Vorinostat in Recurrent Metastatic Head and Neck Squamous Cell Carcinomas and Salivary Gland Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2020;26:837–845. doi: 10.1158/1078-0432.CCR-19-2214. [DOI] [PubMed] [Google Scholar]
- 88.Connolly R.M., Fackler M.J., Zhang Z., Zhou X.C., Goetz M.P., Boughey J.C., Walsh B., Carpenter J.T., Storniolo A.M., Watkins S.P., et al. Tumor and serum DNA methylation in women receiving preoperative chemotherapy with or without vorinostat in TBCRC008. Breast Cancer Res. Treat. 2018;167:107–116. doi: 10.1007/s10549-017-4503-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Eckert C., Parker C., Moorman A.V., Irving J.A., Kirschner-Schwabe R., Groeneveld-Krentz S., Revesz T., Hoogerbrugge P., Hancock J., Sutton R., et al. Risk factors and outcomes in children with high-risk B-cell precursor and T-cell relapsed acute lymphoblastic leukaemia: Combined analysis of ALLR3 and ALL-REZ BFM 2002 clinical trials. Eur. J. Cancer. 2021;151:175–189. doi: 10.1016/j.ejca.2021.03.034. [DOI] [PubMed] [Google Scholar]
- 90.Parker C., Waters R., Leighton C., Hancock J., Sutton R., Moorman A.V., Ancliff P., Morgan M., Masurekar A., Goulden N., et al. Effect of mitoxantrone on outcome of children with first relapse of acute lymphoblastic leukaemia (ALL R3): An open-label randomised trial. Lancet. 2010;376:2009–2017. doi: 10.1016/S0140-6736(10)62002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Haas N.B., Quirt I., Hotte S., McWhirter E., Polintan R., Litwin S., Adams P.D., McBryan T., Wang L., Martin L.P., et al. Phase II trial of vorinostat in advanced melanoma. Investig. New Drugs. 2014;32:526–534. doi: 10.1007/s10637-014-0066-9. [DOI] [PubMed] [Google Scholar]
- 92.Gordon S.W., McGuire W.P., 3rd, Shafer D.A., Sterling R.K., Lee H.M., Matherly S.C., Roberts J.D., Bose P., Tombes M.B., Shrader E.E., et al. Phase I Study of Sorafenib and Vorinostat in Advanced Hepatocellular Carcinoma. Am. J. Clin. Oncol. 2019;42:649–654. doi: 10.1097/COC.0000000000000567. [DOI] [PubMed] [Google Scholar]
- 93.Ghiaseddin A., Reardon D., Massey W., Mannerino A., Lipp E.S., Herndon J.E., 2nd, McSherry F., Desjardins A., Randazzo D., Friedman H.S., et al. Phase II Study of Bevacizumab and Vorinostat for Patients with Recurrent World Health Organization Grade 4 Malignant Glioma. Oncologist. 2018;23:e157–e178. doi: 10.1634/theoncologist.2017-0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yeh Y.T., Yeh H., Su S.H., Lin J.S., Lee K.J., Shyu H.W., Chen Z.F., Huang S.Y., Su S.J. Phenethyl isothiocyanate induces DNA damage-associated G2/M arrest and subsequent apoptosis in oral cancer cells with varying p53 mutations. Free Radic. Biol. Med. 2014;74:1–13. doi: 10.1016/j.freeradbiomed.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 95.Wang X., Di Pasqua A.J., Govind S., McCracken E., Hong C., Mi L., Mao Y., Wu J.Y., Tomita Y., Woodrick J.C., et al. Selective depletion of mutant p53 by cancer chemopreventive isothiocyanates and their structure-activity relationships. J. Med. Chem. 2011;54:809–816. doi: 10.1021/jm101199t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hong Y.H., Uddin M.H., Jo U., Kim B., Song J., Suh D.H., Kim H.S., Song Y.S. ROS Accumulation by PEITC Selectively Kills Ovarian Cancer Cells via UPR-Mediated Apoptosis. Front. Oncol. 2015;5:167. doi: 10.3389/fonc.2015.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Liu S., Zhu Y., Yan S., Xiao H., Yi J., Li R., Wu J., Wen L. Phenethyl isothiocyanate induces IPEC-J2 cells cytotoxicity and apoptosis via S-G2/M phase arrest and mitochondria-mediated Bax/Bcl-2 pathway. Comp. Biochem. Physiology. Toxicol. Pharmacol. CBP. 2019;226:108574. doi: 10.1016/j.cbpc.2019.108574. [DOI] [PubMed] [Google Scholar]
- 98.Aggarwal M., Saxena R., Asif N., Sinclair E., Tan J., Cruz I., Berry D., Kallakury B., Pham Q., Wang T.T.Y., et al. p53 mutant-type in human prostate cancer cells determines the sensitivity to phenethyl isothiocyanate induced growth inhibition. J. Exp. Clin. Cancer Res. CR. 2019;38:307. doi: 10.1186/s13046-019-1267-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu J., Chen G., Pelicano H., Liao J., Huang J., Feng L., Keating M.J., Huang P. Targeting p53-deficient chronic lymphocytic leukemia cells in vitro and in vivo by ROS-mediated mechanism. Oncotarget. 2016;7:71378–71389. doi: 10.18632/oncotarget.12110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yuan J.M., Stepanov I., Murphy S.E., Wang R., Allen S., Jensen J., Strayer L., Adams-Haduch J., Upadhyaya P., Le C., et al. Clinical Trial of 2-Phenethyl Isothiocyanate as an Inhibitor of Metabolic Activation of a Tobacco-Specific Lung Carcinogen in Cigarette Smokers. Cancer Prev. Res. 2016;9:396–405. doi: 10.1158/1940-6207.CAPR-15-0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vikhanskaya F., Lee M.K., Mazzoletti M., Broggini M., Sabapathy K. Cancer-derived p53 mutants suppress p53-target gene expression--potential mechanism for gain of function of mutant p53. Nucleic Acids Res. 2007;35:2093–2104. doi: 10.1093/nar/gkm099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tan B.S., Tiong K.H., Choo H.L., Chung F.F., Hii L.W., Tan S.H., Yap I.K., Pani S., Khor N.T., Wong S.F., et al. Mutant p53-R273H mediates cancer cell survival and anoikis resistance through AKT-dependent suppression of BCL2-modifying factor (BMF) Cell Death Dis. 2015;6:e1826. doi: 10.1038/cddis.2015.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Klimovich B., Meyer L., Merle N., Neumann M., Konig A.M., Ananikidis N., Keber C.U., Elmshauser S., Timofeev O., Stiewe T. Partial p53 reactivation is sufficient to induce cancer regression. J. Exp. Clin. Cancer Res. CR. 2022;41:80. doi: 10.1186/s13046-022-02269-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Keshavarz-Rahaghi F., Pleasance E., Kolisnik T., Jones S.J.M. A p53 transcriptional signature in primary and metastatic cancers derived using machine learning. Front. Genet. 2022;13:987238. doi: 10.3389/fgene.2022.987238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kennedy M.C., Lowe S.W. Mutant p53: It’s not all one and the same. Cell Death Differ. 2022;29:983–987. doi: 10.1038/s41418-022-00989-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Duffy M.J., Synnott N.C., O’Grady S., Crown J. Targeting p53 for the treatment of cancer. Semin Cancer Biol. 2022;79:58–67. doi: 10.1016/j.semcancer.2020.07.005. [DOI] [PubMed] [Google Scholar]
- 107.Wang Z., Strasser A., Kelly G.L. Should mutant TP53 be targeted for cancer therapy? Cell Death Differ. 2022;29:911–920. doi: 10.1038/s41418-022-00962-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dolma L., Muller P.A.J. GOF Mutant p53 in Cancers: A Therapeutic Challenge. Cancers. 2022;14:5091. doi: 10.3390/cancers14205091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Duffy M.J., Tang M., Rajaram S., O’Grady S., Crown J. Targeting Mutant p53 for Cancer Treatment: Moving Closer to Clinical Use? Cancers. 2022;14:4499. doi: 10.3390/cancers14184499. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.