Abstract
Interleukin-1 receptor antagonist (IL-1ra) is a naturally occurring cytokine whose only known function is the inhibition of interleukin-1 (IL-1). Using a reverse genetic approach in mice, we previously showed that increasing IL-1ra gene dosage leads to reduced survival of a primary listerial infection. In this study, we characterize further the role of endogenously produced IL-1ra and, by inference, IL-1 in murine listeriosis. IL-1ra overexpression inhibits, but does not eliminate, primary immune responses, reducing survival and increasing bacterial loads in the target organs. We demonstrate that IL-1ra functions in the innate immune response to regulate the peak leukocyte levels in the blood, the accumulation of leukocytes at sites of infection, and the activation of macrophages during a primary infection. Reduced macrophage class II major histocompatibility complex expression was observed despite increased gamma interferon (IFN-γ) levels, suggesting that IL-1 activity is essential along with IFN-γ for macrophage activation in vivo. We also show that IL-1ra plays a more limited role during secondary listeriosis, blunting the strength of the delayed-type hypersensitivity response to listerial antigen while not significantly altering cellular immunity to a second infectious challenge. When these results are compared to those for other mutant mice, IL-1ra appears to be unique among the cytokines studied to date in its regulation of leukocyte migration during primary listeriosis.
Listeria monocytogenes is an intracellular bacterial pathogen which primarily infects macrophages and hepatocytes. Resolution of an L. monocytogenes infection requires a coordinated innate and cellular immune response dependent upon the regulated recruitment and activation of specific classes of leukocytes (WBC). The most rapidly responding cell type is the neutrophil, which infiltrates the infected tissue in response to as yet poorly defined chemotactic signals released by hepatocytes that concomitantly undergo apoptosis (21). Macrophages and natural killer (NK) cells are then recruited to the infectious foci to aid in the destruction of infected cells and the removal of cellular debris, as well as the engulfment and killing of Listeria organisms that may have been released extracellularly. Finally, Listeria-specific T cells are recruited and activated, resulting in the formation of granulomatous lesions and eventually sterilizing immunity (10, 14, 26). Mice which survive a primary infection with Listeria are immune due to the presence of Listeria-specific memory T cells. Such mice display two characteristic responses: a delayed-type hypersensitivity (DTH) to listerial antigen and an acquired cellular resistance to reinfection with L. monocytogenes.
Listeriosis is a good model for analyzing the reciprocal inductive activation of macrophages and lymphocytes that occurs during the immune response, as well as a valuable tool for dissecting the innate immune system. On a molecular level, the roles of many cytokines in the immune response to Listeria have been described. The infected macrophage produces cytokines, including interleukin-12 (IL-12), tumor necrosis factor alpha (TNF-α), and IL-1, which induce lymphocytes to produce gamma interferon (IFN-γ). NK cells are the primary source of IFN-γ during the early innate immune response. The macrophages are then activated by the IFN-γ, possessing high levels of bactericidal activity and upregulating surface major histocompatibility complex (MHC) molecules. Finally, sterilizing immunity is achieved by the development of a Th1-dominated cellular immune response.
The IL-1 family of cytokines has a wide spectrum of metabolic, physiologic, hematopoietic, inflammatory, and immunologic activities. IL-1α and IL-1β, the two agonists, and IL-1ra, the receptor antagonist, are related polypeptides that have approximately equal affinities for the two IL-1 receptors. It has recently been demonstrated that IL-1 signal transduction appears to occur solely via the type I IL-1 receptor (IL-1RI), since IL-1RI-deficient mice do not respond to exogenously administered IL-1 (7, 15). The complex of IL-1 bound to IL-1RI associates with the IL-1 receptor accessory protein (IL-1RAcP). Formation of this ternary complex then leads to high-affinity IL-1 binding and signal transduction (8). IL-1ra competes directly with IL-1 for receptor binding. However, the complex of IL-1ra and IL-1RI does not recruit IL-1RAcP and therefore does not lead to signal transduction.
Using transgenic mice, we have previously shown that endogenous IL-1ra modulates the host immune response to listeriosis. Deletion of the IL-1ra gene increases survival and reduces the bacterial load in the infected organs, while overexpression of IL-1ra results in the converse phenotypic alterations (12). Similarly, pretreatment with IL-1 has been shown to potentiate the immune response to many bacterial infections, including those with L. monocytogenes (1). Antibody neutralization studies have shown the same effect which we observe with IL-1ra overproduction, i.e., IL-1 neutralization results in decreased survival and increased bacterial titers in both SCID and immunocompetent mice (11, 22). In SCID mice, the resulting exacerbation of listerial infection was accompanied by decreases in leukocytosis, neutrophil recruitment, and macrophage activation (23). Because these studies were performed with SCID mice, the role of IL-1 in the T-cell response to listeriosis remains undetermined. Investigation of these detailed findings in immunocompetent mice is needed because the complex interactions that occur between innate and specific immune systems are lacking in the simplified model of innate immunity of the SCID mouse. Additionally, in vivo antibody neutralization has potential side effects that might complicate interpretation of the results. For example, rather than only neutralizing the effect of the protein under study, antibodies to membrane proteins like the IL-1 receptor or IL-1α could target cells for destruction via the antibody-dependent complement-mediated pathway. IL-1ra transgenic mice offer a direct and practical approach to studying the obligate role of the IL-1 signal transduction pathway in vivo.
In this report, we further define the role of IL-1ra in murine listeriosis with mice which overexpress the secreted form of IL-1ra under the control of its endogenous promoter. We show that IL-1ra acts to inhibit, but not eliminate, the immune response during primary listeriosis. Specifically, IL-1ra limits at least three aspects of the early innate immune response: leukocytosis, WBC recruitment to sites of infection, and macrophage activation. In contrast, IL-1ra plays a more limited role during secondary immune responses. The development and expression of cell-mediated immunity are not significantly altered by IL-1ra overexpression. However, the DTH inflammatory response to listerial antigen is significantly reduced.
MATERIALS AND METHODS
Mice.
As described in detail elsewhere, transgenic mice overexpressing the secreted form of IL-1ra under control of its endogenous promoter were created by standard methods on a C57BL6/J × CBA/J hybrid background (12). To maximize the gene dosage of IL-1ra, we created a line of mice hemizygous at two unlinked loci of integration, called T14 and T16. This overexpressing line, designated T20, produces approximately 16-fold more IL-1ra than do wild-type mice (12). Transgenic mice for these experiments were generated from mating mice homozygous for the transgene at both loci with wild-type B6CBAF1/J mice. Nontransgenic wild-type control animals were, therefore, generated by mating wild-type B6CBAF1/J mice. All of the mice used in these experiments were 8- to 15-week-old males, bred in a specific-pathogen-free facility and handled in accordance with institutional guidelines. As transgenic mice on a B6 (N = 7) background became available, they were used in similar experiments. T20 males (n = 5) and wild-type control littermates (n = 1) or pure C57BL6/J mice (n = 4) were infected with 5.2 × 105 CFU. The basic findings presented below on the influence of IL-1ra overexpression on the immune response to listeriosis were confirmed in these mice. Because the preponderance of data was gathered from mice on the mixed background, those results are presented in the text.
Bacteria and infection.
L. monocytogenes EGD was kept virulent by continuous passage through mice. All inocula were prepared and verified by titering post hoc (12). Control mice were either sham infected with vehicle only or uninfected. Sham infection did not alter any parameters compared to uninfected mice (data not shown). For studying survival during a primary infection, doses ranged from 500 CFU to 1.3 × 106 CFU in 100 μl administered intravenously (i.v.). For investigation of innate immune responses, inocula in the range of 2.3 × 103 to 4.2 × 103 CFU were administered intraperitoneally. To study secondary immune responses, mice were immunized with a sublethal dose of 103 CFU i.v. and allowed fully to resolve the primary infection (shown to occur by day 14). Seventeen days later, immune mice were challenged i.v. with high doses of Listeria ranging from 6.7 × 104 to 2.7 × 106 CFU which were lethal to control naive mice. Some groups of infected mice were monitored for survival while others were sacrificed by CO2 inhalation for analysis of peripheral blood and peritoneal cells, histological examination of the liver and spleen, and in situ hybridization as detailed below. Bacteria were enumerated by homogenization of organs in sterile phosphate-buffered saline (PBS) and plating serial log dilutions (12).
Blood and serum collection and analysis.
Blood was collected by cardiac puncture at the time of sacrifice. An aliquot of approximately 150 μl was stored in an EDTA-coated collection tube (Microtainer; Becton Dickinson, Rutherford, N.J.), and total WBC were counted in a hemacytometer after dilution in 3% acetic acid to lyse erythrocytes. Standard blood smears were prepared and stained with May-Grunwald and Giemsa stains (Sigma, St. Louis, Mo.). Differentials were determined by counting at least 200 WBC per sample in duplicate in randomly selected fields by an investigator who was blinded to the genotypes of the mice. Absolute lymphocyte, monocyte, and neutrophil numbers were calculated by multiplying the total WBC by the percentages obtained for each cell type. The remaining blood (200 μl to 1 ml) was collected into a serum separation tube (Vacutainer; Becton Dickinson), and serum was isolated according to the manufacturer’s protocol. Serum samples were stored at −80°C until used for IL-1ra protein determinations with a sandwich enzyme-linked immunosorbent assay (ELISA) as previously described (12). IFN-γ levels were also measured by ELISA with the antibody pair of R46A2 and XMG1.2 (PharMingen, San Diego, Calif.) according to the manufacturer’s protocol.
Peritoneal exudate cell (PEC) collection and analysis.
PECs were harvested from the mice by peritoneal lavage. Five milliliters of PBS was injected into the peritoneal cavity, the abdomen was vigorously massaged, the fluid was withdrawn, and PECs were stored on ice. Total viable cell number was determined by counting the trypan blue-excluding cells. For differential cell counting, an aliquot of PECs was centrifuged, resuspended in a minimal volume, and applied to slides with a fine paintbrush. After air drying, differential counts were determined as described for the blood smears above.
I-A surface expression on peritoneal macrophages.
PECs were resuspended in medium (RPMI 1460, 10% fetal calf serum [Sigma], 100 U of penicillin per ml, 100 μg of streptomycin sulfate per ml) at a density of 5 × 105 cells/ml. A 0.5-ml portion of cells was allowed to adhere to ethanol-washed 12-mm glass coverslips for 2 h at 37°C with 5% CO2. Nonadherent cells were removed by washing three times with PBS. Adherent cells were fixed for 10 min in 1% paraformaldehyde at 4°C and processed for immunofluorescence with a monoclonal antibody against Ia antigen (Chemicon International, Temecula, Calif.) and a fluorescein-conjugated Fab′ goat anti-mouse immunoglobulin G as the secondary antibody. When tested on resident peritoneal macrophages from pure C57BL6/J (I-Ab) and CBA/J (I-Ak) mice, this Ia antibody recognized both isotypes present in the mixed strain background with equivalent immunofluorescent staining. At least 200 adherent macrophages were counted by using a standard fluorescence microscope to determine the percentage of I-A+ macrophages, and the total numbers were calculated by multiplying this percentage by the number of macrophages present in the PECs. The adherent cell populations were determined to be ≥96% macrophages by both morphology and Mac-1 or F4/80 staining.
Histology and in situ hybridization.
At the time of sacrifice, portions of the liver and spleen were fixed in 10% buffered formalin and then paraffin embedded. Serial, or near-serial, 5- to 6-μm sections from each sample were stained with hematoxylin-eosin (Sigma) or tissue Gram stain (Sigma) or processed for in situ hybridization for detection of IL-1ra message. Histomorphometric analysis was performed by an investigator blinded to the genotypes of the mice. Hematoxylin-eosin-stained sections were examined in a standard light microscope, and at least five randomly chosen low-power fields (total magnification, ×63) were analyzed per organ. The results of three to five mice per genotype were combined to determine the average number of infectious lesions and percentage of necrotic lesions on a given day postinfection. Infectious lesions were defined as any focal accumulation of WBC or infected cells within the hepatic parenchyma. Any lesions that had obvious signs of hepatocellular necrosis associated with the infectious microabscesses were defined as necrotic. The in situ hybridization protocol was a modification of that of Winter et al. (28). For detection of IL-1ra mRNA, we used digoxigenin-labeled cRNA antisense and sense probes transcribed from a template containing a 348-bp EcoRI-EcoRV fragment from the 5′ end of the IL-1ra cDNA (29) subcloned into pBluescript SK(+) (Stratagene, La Jolla, Calif.). This probe contains the 5′ untranslated region and exon 1, which are unique to secreted IL-1ra, as well as exons 2 and 3, which are common to the secreted and intracellular forms.
DTH measurements.
To elicit a DTH response, the left hindpaw of each mouse was injected with listerial antigen in the form of 107 CFU of heat-killed L. monocytogenes (HKLM) in 25 μl of PBS. The contralateral paw was injected with vehicle only to control for nonspecific inflammatory reactions. HKLM was prepared by incubation of a known quantity of bacteria at 72°C for 2 h. Lack of viability was confirmed by attempting to grow both plate and liquid cultures at 37°C. The extent of footpad swelling was measured with a dial microcaliper (Bel-Art Products, Pequannock, N.J.) before challenge and at 24, 48, and 72 h postchallenge. Each measurement was performed in duplicate or triplicate and averaged for each footpad of every mouse. The systematic error was determined to be ±0.03 mm for these measurements. The percent footpad swelling was calculated by dividing the thickness of the HKLM-injected footpad by the thickness of the contralateral vehicle-injected footpad. By 72 h, the DTH response was no longer significantly detected in either wild-type or IL-1ra-overexpressing mice.
Statistics.
We used the following statistical tests: for bacterial titers, Mann-Whitney U test; for WBC, PECs, and DTH response, Student’s t test; for survival, χ2 analysis with Fisher’s exact correction on endpoint data. Significance was assigned to P values of ≤0.05. Statistical analyses were performed with GB-Stat software (Dynamic Microsystems, Silver Spring, Md.).
RESULTS
Primary listeriosis. (i) IL-1ra overexpression converts sublethal doses of Listeria to lethal ones and retards the rate of clearance of bacteria from infected organs.
Mice which overexpress IL-1ra consistently showed a survival difference after being inoculated with 5,000 CFU of Listeria organisms (25 versus 88% survival for wild-type control mice) (also see reference 12). However, IL-1ra overexpression does not completely block the immune response since mice overproducing IL-1ra were able to survive listerial inocula below a threshold of approximately 1,500 CFU. In contrast with other transgenic mice (e.g., IFN-γ receptor knockout mice [2]), the effect of IL-1ra overexpression on survival was confined to a relatively narrow dose range. While there are many possible reasons for this result, the most likely explanation lies in the unique biological function of this cytokine which possesses no known agonist function. Increasing the endogenous production of IL-1ra would attenuate, but not ablate, IL-1 signaling in vivo. Such an alteration, compared to receptor- or agonist-null mutations, would predictably be more subtle and dose sensitive yet nonetheless important for understanding the delicate balance normally maintained between IL-1 and IL-1ra and how critical disruption of that balance can be in determining the outcome of infection. The intermediate sensitivity of heterozygously disrupted IL-1ra mutant mice in comparison with wild-type mice and homozygous null mutants underscores this point (12), as many heterozygous null cytokine mutants reportedly have a wild-type phenotype.
To demonstrate that this increased lethality was directly due to an impaired immune response, we quantified the bacterial burden in the target organs of both IL-1ra-overexpressing and wild-type control mice that were inoculated with sublethal doses of Listeria. At 4 h after i.v. inoculation, the bacterial titers in both liver and spleen were the same in wild type and IL-1ra overexpressors (data not shown), demonstrating that the initial rate of uptake of bacteria from the bloodstream was unaltered. On all subsequent days of infection, higher bacterial titers were recovered from the livers and spleens of mice overexpressing IL-1ra (Fig. 1). The maximal difference was detected on day 4 of infection, a time which typically marks the peak of bacterial burden and the period of transition between the innate and cellular immune responses. By day 12, wild-type mice had apparently cleared the bacterial infection, while IL-1ra overexpressors still had low, but detectable, levels of bacteria in both the liver and the spleen. Only on day 14 was the listerial infection eradicated in the IL-1ra-overexpressing mice (data not shown).
FIG. 1.
Bacterial titers throughout the course of a primary infection. Data from a representative experiment of four is shown. Wild-type (WT) and IL-1ra-overexpressing (T20) mice were infected i.v. with 1,150 CFU of Listeria, and the bacterial burdens in the organs from individual mice were determined on various days postinfection. The titers present in the livers and spleens are shown as CFU per gram of organ to correct for differences in sample size. The mean values are indicated by horizontal lines. Note that the scales differ in the various panels of the figure. The differences in bacterial titer were significant (P ≤ 0.05 [Mann-Whitney U test]) only on days 4 and 12 postinfection, although the trend toward higher mean titers in IL-1ra overexpressors is consistently observed at other time points.
These results suggested that IL-1ra overexpression increased mortality by lowering the rate of bacterial elimination from the target organs during both the earlier, innate immune phase and the later, cellular immune phase of the host response. However, IL-1ra does not prevent the eventual development of sterilizing immunity because the IL-1ra-overexpressing mutants can eradicate the infection. To investigate further the role of IL-1ra in innate immunity, we infected wild-type and IL-1ra-overexpressing mice intraperitoneally with a sublethal dose of Listeria, sacrificed them on days 1 through 4 postinfection, and analyzed their immune responses at a cellular and molecular level.
(ii) IL-1ra partially inhibits leukocytosis.
Because of the postulated role of IL-1 in the hematopoietic changes that occur during disease states, we determined the effect of IL-1ra on circulating WBC during the course of a primary listerial infection. The total number and differentials of WBC in the bloodstream were determined by standard methods. There was no statistically significant difference between wild-type mice and IL-1ra overexpressors in the absence of stimulus (data not shown), indicating that normal hematopoiesis was not significantly altered in IL-1ra overexpressors.
During a primary listerial infection, wild-type mice displayed a prominent leukocytosis that peaked at 24 h postinfection and then rapidly declined toward baseline levels. The leukocytosis was primarily due to a dramatic increase in the absolute numbers of neutrophils and monocytes. Overexpression of IL-1ra significantly reduced the peak level of leukocytosis. We consistently observed an IL-1ra-mediated reduction in leukocytosis of 23 to 36% in experiments with a range of sublethal inocula and a greater than 50% reduction with lethal doses. The differential cell counts were unaltered (data not shown), leading to an absolute decrease in the numbers of circulating neutrophils, monocytes, and lymphocytes at 24 h postinfection.
(iii) WBC extravasation to the infected peritoneum is suppressed by IL-1ra overexpression.
We next determined how this reduction in leukocytosis might be related to the targeting of WBC to sites of infection. We analyzed peritoneal cells from the same mice that were sublethally infected for the above-described studies. In response to the listerial infection, there is a rapid cellular influx into the peritoneal cavity (Fig. 2). In wild-type mice, the rapid initial increase on day 1 is primarily due to a rise in the number of neutrophils (from [2.33 ± 2.14] × 104 to [1.51 ± 0.34] × 106 in a representative experiment). The number of macrophages remained fairly constant, with only a slight increase on day 1 (from [2.28 ± 0.33] × 106 to [3.42 ± 0.74] × 106). In contrast, the number of lymphocytes increased more slowly, with the number beginning to rise on day 2 and continuing an upward trend through day 4 (from [2.71 ± 0.37] × 106 to [3.34 ± 0.62] × 106), during the same period in which the numbers of neutrophils and macrophages were returning to baseline levels.
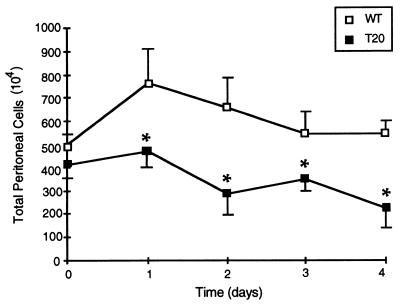
FIG. 2.
Peritoneal exudate cells. The total number of PECs is shown for both wild-type (WT) and IL-1ra-overexpressing (T20) mice during a primary listerial infection. All mice were infected intraperitoneally on day 0, and individual mice were sacrificed on each of four subsequent days. Sham-infected mice had no alterations in WBC numbers compared to uninfected controls (data not shown). The day 0 data shown here is derived from uninfected mice. The data at each time point represents the mean ± the SEM of three to five individual mice. For clarity, positive error bars are shown for wild-type mice and negative error bars are shown for the IL-1ra-overexpressing mice; where not shown, the error bar is smaller than the size of the data point itself. Data from a representative experiment of three is shown; only those differences which were statistically significant in all experiments are indicated by an asterisk (P ≤ 0.05).
In mice which overexpress IL-1ra, the cellular influx in response to listerial infection was significantly inhibited (Fig. 2). In fact, the total number of cells in the infected peritoneum never increased and actually declined below baseline levels by day 4 postinfection (from [4.10 ± 0.67] × 106 to [1.63 ± 0.55] × 106). As was the case for the circulating WBC, the differential cell counts were not altered by IL-1ra (data not shown). Therefore, the overexpression of IL-1ra blocks the accumulation of all types of WBC at sites of extravascular infection. A simple interpretation of these results is that this inhibition of WBC accumulation within the infected peritoneal cavity is caused by obstruction of the proper migration of the WBC.
(iv) IL-1ra also reduces WBC migration to other sites of infection.
It was also possible that the reduced levels of WBC in the circulation and the peritoneal cavity might have been due to their accumulation at other sites of infection, namely, the liver and spleen. To test this possibility, we histologically examined these organs from the same infected mice. We consistently observed that the IL-1ra transgenic mice had a greater number of infectious lesions and that a greater percentage of those lesions appeared to be necrotic and poorly resolved. These histopathological differences were most apparent following a lethal infection since higher listerial doses induce greater numbers of individual foci of infection for quantification and comparison. For lethally infected mice on day 2 postinfection, the average number of infectious lesions in the liver was 0.71 ± 0.19 for wild type and 1.56 ± 0.32 for IL-1ra overexpressors (P = 0.019 [Student’s t test]), and the percentage of the lesions which were necrotic was (10 ± 4)% for wild-type mice compared to (36 ± 6)% for IL-1ra overexpressors (P = 0.004 [Student’s t test]). These necrotic liver lesions always had reduced levels of WBC infiltration, as judged by hematoxylin-eosin staining, and more gram-positive organisms, primarily within the hepatocytes associated with necrotic foci (typical liver histopathology is shown in Fig. 3). Both the greater number and necrotic appearance of liver lesions and greater bacterial burden in the liver and spleen support the idea that WBC migration to sites of infection in these target organs was reduced by IL-1ra overexpression.
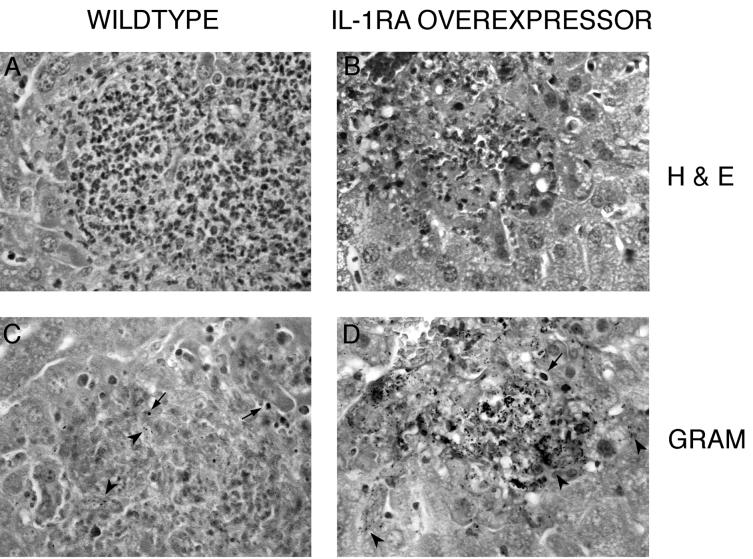
FIG. 3.
Histology of the livers of Listeria-infected mice. Representative sections of infectious foci and surrounding hepatocytic parenchyma from IL-1ra overexpressors and wild-type controls on day 2 postinfection are shown (original magnification, ×400). Hematoxylin-eosin (H & E) staining shows coagulative necrosis of infected hepatocytes and a marked reduction in WBC accumulation at the infectious focus of mice overexpressing IL-1ra (B) compared to that seen in wild-type mice (A). Gram staining of serial sections shows the localization of bacteria within both phagosomes (arrows) and necrotizing hepatocytes (arrowheads). While most of the bacteria are efficiently contained within the granuloma of wild-type mice (C), large numbers of bacteria appear to be free in the cytoplasm of infected hepatocytes within and beyond the initial infectious lesion in the IL-1ra overexpressors (D).
(v) Macrophage activation is inhibited by IL-1ra despite increased IFN-γ levels.
During a listerial infection, macrophages become activated to possess bactericidal activity and to present antigens to T cells. We determined whether IL-1ra overexpression altered macrophage activation, as well as recruitment. The work of others has shown that expression of class II MHC molecules is an accurate marker for macrophage activation in response to listerial infection (cf. reference 22). We therefore measured macrophage activation by determining the level of surface expression of class II MHC by immunofluorescence. As has been previously reported, we found that in uninfected mice 10 to 14% of the peritoneal macrophages are I-A+. This baseline percentage was not changed in our transgenic mice. In wild-type mice, we observed a rapid increase in I-A expression beginning on day 2 postinfection (Fig. 4A). IL-1ra-overexpressing mice had a significantly lower percentage of I-A+ macrophages in their peritoneal cavity (Fig. 4A) from days 2 through 4. In fact, the absolute number of activated macrophages was almost constant throughout the course of the infection (Fig. 4B). On day 4 postinfection, the IL-1ra overexpressors have approximately half the wild-type number of peritoneal macrophages (Fig. 2) yet less than one-fifth as many I-A+ macrophages (Fig. 4B). Therefore, the reduced influx of macrophages cannot completely account for the diminution in activated macrophages. Thus, it appears that IL-1ra prevents both the recruitment of macrophages into the infected peritoneum and the activation of resident macrophages.
FIG. 4.
Activation of peritoneal macrophages during listeriosis. The percentage (A) and total numbers (B) of peritoneal macrophages expressing class II MHC, as judged by antibody staining for I-A antigen, are shown for wild type (WT) and IL-1ra overexpressors (T20). The data at each time point represents the mean ± SEM of three to five individual mice. Day 0 data in the experiment shown is derived from uninfected mice. Data from a representative experiment of three is shown; only those differences which were statistically significant in all experiments are indicated by an asterisk (P ≤ 0.05).
Because IL-1 does not directly induce MHC class II expression in macrophages, IL-1ra overexpression might be altering I-A expression indirectly, by causing a reduction in the levels of IFN-γ, a direct inducer of macrophage activation. Therefore, we measured the levels of IFN-γ present in the serum of the sublethally infected mice used for the above-described studies. In mice overexpressing IL-1ra, the levels of circulating IFN-γ were elevated three- to fourfold compared to wild-type controls (8.9 ± 3.1 ng/ml for wild type and 33.3 ± 1.4 ng/ml for overexpressors on day 4 postinfection [mean ± standard deviation; n = 4 mice/genotype; P < 0.05 by Student’s t test]). Similar qualitative results were obtained by measuring the levels of mRNA for IFN-γ by Northern blotting in the livers and spleens of the infected mice (data not shown).
Since this result was unexpected, we wondered whether the observed increase in IFN-γ might simply be caused by the higher bacterial load present in the IL-1ra transgenic mice. In order to obtain genotype-matched mice with comparable levels of infection, we inoculated individual mice with a range of listerial doses. In mice of the same genotype, there was a correlation between the level of infection and the amount of IFN-γ (Table 1). However, in mice harboring the same bacterial burden, serum IFN-γ levels were consistently two- to threefold higher in IL-1ra overexpressors. A doubling of IFN-γ levels in mice of the same genotype required an increase in bacterial titers of several orders of magnitude, well below the two- to sixfold increase in mean bacterial titers seen for IL-1ra overexpressors compared to wild-type mice administered the same listerial inoculum (Fig. 1). From this data, we conclude that IL-1ra overexpression in vivo causes an overproduction of IFN-γ during listeriosis while simultaneously inhibiting macrophage activation.
TABLE 1.
Serum levels of IFN-γ and bacterial titers in wild-type and IL-1ra-overexpressing micea
| Genotype | Bacterial titer | IFN-γ level |
|---|---|---|
| WT | 3.87 | 9.15 |
| T20 | 3.86 | 18.20 |
| WT | 4.70 | 12.60 |
| T20 | 4.69 | 24.20 |
| WT | 5.97 | 13.35 |
| T20 | 5.90 | 31.80 |
| T20 | 5.95 | 34.60 |
| WT | 8.83 | 31.54 |
| T20 | 8.99 | 102.80 |
Mice were infected with various inocula of Listeria and sacrificed for quantification of the bacterial load in target organs (log CFU per gram of liver) as described in the text and for measurement of serum IFN-γ levels (nanograms per milliliter) by ELISA with a limit of detection of 0.286 ng/ml. Genotype-matched individual mice harboring roughly equivalent levels of infection were chosen. WT, wild type; T20, IL-1ra overexpressor.
(vi) Listeriosis induces IL-1ra expression in WBC and hepatocytes.
Since the expression of IL-1ra in murine listeriosis is not described in the literature, we examined the temporal and spatial expression pattern of IL-1ra during a primary listerial infection. In wild-type mice, the levels of mRNA detected by Northern blotting correlate positively with bacterial load as determined by titering (data not shown). We also measured the level of IL-1ra protein in the serum of infected mice administered a range of inocula and collected at various stages of infection. We detected circulating IL-1ra only in lethally infected mice on the day before death, similar to the results reported for IL-1 (11).
Using in situ hybridization, we have observed that the infiltrating WBC express high levels of IL-1ra in the livers of infected wild-type mice (Fig. 5). While it was often difficult to discriminate individual cells within the cellular influx at infectious lesions, hybridization signal has been clearly seen within neutrophils. Occasionally, IL-1ra hybridization signal is visible in individual scattered cells within the hepatic sinuses and blood vessels in the liver (data not shown), consistent with the idea of migrating WBC being recruited from the circulation and being the primary source of IL-1ra, as has been reported for other inflammatory cytokines (27). We also detected lower levels of IL-1ra signal in hepatocytes directly adjacent to infectious foci. These hepatocytes did not appear to be infected, as judged by Gram staining of serial sections.
FIG. 5.
Distribution of cells expressing IL-1ra mRNA in the livers of infected mice detected by in situ hybridization. Shown are representative serial sections from the liver of a wild-type mouse 3 days after infection with a sublethal dose of Listeria (original magnification, ×160). (A) In situ hybridization with the antisense probe shows very strong IL-1ra mRNA expression in the WBC throughout the hepatic granuloma. A weaker but clear signal is also detected in the hepatocytes surrounding the infectious focus. (B) In situ hybridization with the negative control sense probe shows the specificity of the IL-1ra signal. (C) Hematoxylin-eosin-stained near-serial section shows the infectious lesion marked by a strong WBC influx as well as the surrounding apparently uninfected hepatic parenchyma.
This wild-type expression pattern was mimicked by the IL-1ra transgene both temporally and spatially except that there were greater levels of message in the overexpressing mice in response to similar infectious stimuli, as expected (data not shown). We conclude that there is no ectopic expression of IL-1ra in our transgenic mice and that it is overexpression of the secreted form of IL-1ra in its normal induction pattern that is responsible for the dominant phenotypes which we have observed in our mice.
Secondary listeriosis.
The results above demonstrate a role for IL-1ra in the innate immune response to Listeria infection. However, it remained possible that this could have obscured any subsequent role of IL-1ra in the later adaptive immune response. Bacterial titers remained elevated late in infection (days 7 to 12 [Fig. 1]) in IL-1ra overexpressors compared to wild-type mice. Additionally, the decreased number of MHC class II-positive, activated macrophages might be expected to reduce the level of CD4+ helper T-cell activation and ultimately the strength of the CD8+ cellular immune response. Therefore, to address the role of IL-1ra in specific T-cell immunity in vivo, we compared the secondary immune responses of IL-1ra overexpressors and wild-type controls.
(i) IL-1ra blunts the DTH response to listerial antigen.
To determine whether IL-1ra overexpression regulates the DTH response, we challenged immunized mice by injecting HKLM into one hind footpad and vehicle only into the contralateral control footpad. At 48 h postchallenge, wild-type mice have an average DTH swelling of (141.6 ± 1.9)% versus only (128.6 ± 1.6)% for IL-1ra overexpressors (mean ± standard error of the mean [SEM], n = 40 mice/genotype, P < 0.01, Student’s t test). There is less footpad swelling in IL-1ra overexpressors after administration of listerial antigen throughout the duration of the response. These differences were not due to nonspecific inflammatory reactions in the mice because naive wild-type and IL-1ra-overexpressing mice had no significant footpad swelling in response to Listeria antigen ([99.9 ± 0.4]% for wild type, [100.4 ± 0.4]% for IL-1ra overexpressors [mean ± SEM, n = 9 mice/genotype, P = 0.89, Student’s t test]). In addition, vehicle-injected footpads of immune mice of both genotypes showed no difference compared to prechallenge measurements. Since IL-1ra overexpression partially suppresses the DTH response to listerial antigen, we conclude that IL-1 signaling is involved in T-cell-mediated inflammatory reactions.
(ii) Cellular immunity to secondary infection is unaltered in IL-1ra overexpressors.
We were also interested in establishing the effect of IL-1ra on the function of the secondary immune response to reinfection. Immune mice were challenged with high doses of Listeria, lethal to naive mice of both genotypes. Over a large dose range, we could detect no significant alteration in the acquired cellular immunity of the mice overexpressing IL-1ra (Table 2), as judged by either survival or bacterial titers following secondary bacterial challenge. In order to investigate further the effect of IL-1ra during a secondary listerial infection, we measured the same parameters that were altered during primary infection. There was no difference in the number or composition of WBC in either the circulation or the peritoneal cavity on days 1 and 2 postinfection (data not shown). Histopathological examination of the liver and spleen also failed to reveal any obvious differences in lesion number or quality. All of these results are further evidence against an obligate role for IL-1 and IL-1ra in secondary as opposed to primary listeriosis.
TABLE 2.
Survival and bacterial titers in mice following a secondary Listeria infectiona
| Genotype | Inoculum | Survivalb
|
Titer (log CFU/g)c
|
||||
|---|---|---|---|---|---|---|---|
| % (no. surviving/total no.) | Pd | n | Liver | Spleen | Pd | ||
| WT | 6.7 × 104 | 100 (10/10) | 10 | 4.05 ± 0.54 | 3.75 ± 0.39 | ||
| T20 | 6.7 × 104 | 100 (10/10) | 10 | 4.28 ± 0.25 | 4.00 ± 0.43 | 0.23 (l), 0.11 (s) | |
| WT | 5.1 × 105 | 100 (10/10) | NDe | ND | |||
| T20 | 5.1 × 105 | 100 (10/10) | ND | ND | |||
| WT | 1.3 × 106 | 87 (13/15) | 10 | 5.10 ± 0.08 | 4.20 ± 0.14 | ||
| T20 | 1.3 × 106 | 73 (11/15) | 0.32 | 10 | 5.18 ± 0.10 | 4.29 ± 0.15 | 0.08 (l), 0.17 (s) |
| WT | 2.7 × 106 | 32 (8/25) | ND | ND | |||
| T20 | 2.7 × 106 | 20 (5/25) | 0.26 | ND | ND | ||
Immunized wild-type (WT) and IL-1ra transgenic (T20) mice were infected i.v. with the various inocula of Listeria given in the table.
Some groups of mice were monitored for survival for at least 7 days. No deaths were observed beyond day 5.
Other groups of mice were sacrificed for bacterial titer determination. The data presented here is from mice sacrificed on day 2. Similar results were obtained on day 1 (data not shown). Naive mice of both genotypes were infected at the same time as the immune mice. They were unable to survive any of the inocula used and had titers approximately 3 logs higher than those for immune mice (data not shown).
The P values were calculated for comparisons of data between wild-type and IL-1ra-overexpressing mice separately for each dose of Listeria. Statistical comparisons used were the Mann-Whitney U test for bacterial titers and χ2 analysis with Fisher’s exact test correction for endpoint survival data. l, liver; s, spleen.
ND, not determined.
One trivial explanation for these results could have been that IL-1ra is not expressed, or not properly overexpressed by our transgene, during secondary immune responses. However, by in situ hybridization studies, we observed that the IL-1ra expression pattern in the liver is comparable during primary and secondary listeriosis (Fig. 5 and data not shown). The only major difference is that, since granuloma development is accelerated in secondary infection, detection of IL-1ra mRNA was correspondingly accelerated. As in primary infection, the temporal and spatial expression pattern detected in IL-1ra overexpressors was unaltered.
DISCUSSION
We present evidence that IL-1ra is a normal part of the cytokine network induced during primary murine listeriosis and that overexpression of IL-1ra reduces survival and retards the rate of eradication of bacteria from infected tissues. IL-1ra acts to limit at least three aspects of the host innate immune response: leukocytosis, WBC accumulation at sites of infection, and macrophage activation. Taken together, these results indicate that IL-1ra overexpression causes a global defect in WBC migration during murine listeriosis. The WBC appear to be partially blocked early in recruitment, perhaps reflecting an inability to move from the bone marrow into the bloodstream, which then leads to the subsequent reduction in the numbers of WBC that migrate to the actual sites of infection in the peritoneal cavity, liver, and spleen. The ability of exogenously administered IL-1 to elicit the release of inflammatory cells from the bone marrow and the specific blockade by recombinant IL-1ra and antibodies to the IL-1RI support this interpretation of our data (17). Additionally, IL-1 can increase the expression of cell adhesion molecules and chemokines that directly regulate the trafficking of WBC during infection (5). However, we cannot currently exclude the possibility that IL-1ra might also inhibit the division of resident peritoneal macrophages that can also occur during listeriosis (20) or that the neutrophils are undergoing more rapid apoptosis, as has been recently suggested by studies with IL-6-deficient mice (19). It is also noteworthy that, while earlier studies implicated IL-1 in the recruitment of neutrophils alone (21), we have observed a more generalized effect on all classes of WBC. It is not known whether this is due to an inherent difference between immunocompetent and SCID mice (or between different strains of mice) or differences in methodology (antibody neutralization of receptor and agonists versus overproduction of an antagonist from its endogenous promoter). Alternatively, it might indicate some novel function for IL-1ra that is distinct from the IL-1 signaling pathway.
We also found that overproduction of IL-1ra during primary listeriosis caused a decrease in macrophage MHC class II expression and yet an increase in IFN-γ levels in the serum. Taken at face value, the in vivo results indicate that IL-1ra is a negative regulator of MHC expression and further indicate that IFN-γ alone is not the sole determinant of MHC class II upregulation. As no agonist activities have been found for IL-1ra, the effect is likely to be mediated via antagonism of IL-1 that is essential for MHC expression. These results also support the findings of Rogers et al. (23) but contrast with those of Hunter et al. (13) indicating that IL-1 is not a direct inducer of IFN-γ in vivo. In contrast to our results, antibody neutralization of IL-1 in SCID mice resulted in a complete absence of MHC class II induction and normal levels of IFN-γ production. This difference might be due to an incomplete blockade of the IL-1 signal in our mice that overexpress IL-1ra. However, we still have no direct explanation for why MHC class II expression is inhibited by IL-1ra overexpression while IFN-γ levels are upregulated. It is possible that IL-1ra is inhibiting the expression of IFN-γ receptors on the macrophages or is increasing the expression of IL-10, which has been shown to inhibit IFN-γ-induced I-A expression in vitro (25).
We also conclude from this data that IL-1ra overproduction from its endogenous promoter causes a dysregulation of the cytokine network that ultimately results in increased production of IFN-γ. Presumably, this reflects an in vivo regulatory mechanism that compensates for reduced levels of IL-1 signaling by elevating the production of other inflammatory cytokines. Our previous results, which revealed an unexpected parallel regulation of IL-1 and IL-1ra following lipopolysaccharide administration (12), support a model wherein overproduction of the anti-inflammatory cytokine IL-1ra leads to an increase in proinflammatory cytokine production in vivo. Our preliminary data with IL-1ra transgenic mice indicates that IFN-γ is also regulated in parallel with IL-1ra following lipopolysaccharide administration (13a). Alternatively, both the decrease in MHC class II expression and the increase in IFN-γ levels may occur simply because IL-1ra overexpression causes an increase in bacterial burden, since these phenotypes have been shown to result from extremely high levels of Listeria infection in wild-type mice (24). However, this explanation is unlikely because IL-1ra overexpressors harboring the same level of infection as wild-type controls have two- to threefold-increased serum IFN-γ concentrations. Additionally, we rarely detected Listeria-infected peritoneal macrophages isolated from these mice (data not shown).
By in situ hybridization, we investigated the wild-type expression pattern of IL-1ra in the liver during murine listeriosis. IL-1ra message was expressed strongly by the infiltrating WBC throughout the liver lesions, in agreement with evidence that neutrophils and macrophages are the primary cellular sources of IL-1ra (5). This result corresponds well with the expression patterns described by Wagner and Czuprynski (27) for the proinflammatory cytokines TNF-α and IL-1α. Interestingly, IL-1ra expression was also detected at lower levels in apparently uninfected hepatocytes directly surrounding the granulomatous lesions. This expression pattern suggests the following model of resolution of an active listerial infection in the liver: WBC, expressing both IL-1 and IL-1ra, are encased within a ring of IL-1ra produced by the uninfected hepatocytes directly adjacent to the lesion which mark the boundary between the infected cells to be killed and the healthy cells to be spared. This hepatocyte-derived IL-1ra would act to limit the range of action of WBC-derived IL-1, confining it exclusively to local action within the infected tissue. In this model, the host tissue would be protected from any potentially destructive action of IL-1 without inhibition of the beneficial functions of IL-1 within the infectious lesion itself, i.e., avoiding immunopathology but not causing immunosuppression. IL-1ra overexpression in the hepatocytes would be predicted to increase the concentration of IL-1ra extracellularly and therefore extend the zone of IL-1ra by simple diffusion. This increased level of IL-1ra would then reduce the effective concentration of IL-1 and shift the balance toward immunosuppression rather than immunoprotection. The predicted outcome is seen in the hematoxylin-eosin- and Gram-stained liver sections of IL-1ra overexpressors in comparison to wild-type mice: a lack of clear delineation of the boundary between infected and uninfected tissue reflected in a reduction of WBC accumulation at the infection focus and the spread of viable bacteria into hepatocytes beyond the initial lesion site (Fig. 5).
In contrast to its role as a survival determinant in primary infection, IL-1ra does not significantly affect the acquired cellular resistance to rechallenge with viable Listeria. Bacterial titers in target organs and survival after a secondary infection are comparable in wild-type and IL-1ra-overexpressing mice. Also, while we observed the same basic expression pattern of IL-1ra in secondary and primary listeriosis, there is no alteration in WBC migration and recruitment in either the bloodstream or to sites of infection during secondary infection. However, the DTH response to listerial antigen is reduced by IL-1ra overproduction, indicating that IL-1 signaling participates in T-cell-mediated inflammation. In agreement with this conclusion, we have found that lethality from superantigen-induced septic shock is reduced in mice overexpressing IL-1ra (unpublished data). Other recent studies have also shown that the DTH response to methylated bovine serum albumin is reduced in IL-1RI-deficient mice (15) and that the development of collagen-induced arthritis is inhibited in IL-1ra overexpressors (16). Thus, the dependency of various T-cell-mediated inflammatory responses on proper levels of IL-1 signaling in vivo seems clearly established.
The dissociation of DTH inflammatory responses from immune protection in listeriosis has been well characterized on a cellular level by adoptive cell transfer studies and by selective T-cell subset depletion with monoclonal antibodies. These studies have established that it is primarily the CD4+ T cells which mediate the inflammatory responses while the CD8+ T cells are primarily responsible for the expression of cell-mediated immunity (18). Therefore, our data suggests that IL-1ra overexpression partially inhibits CD4+ helper cells but not CD8+ cytotoxic effector cells in vivo, although the exact mechanism is not clear. We do not currently know whether the overexpression of IL-1ra is inhibiting the sensitization or elicitation phase of the DTH response, or both. However, the ability of IL-1ra to reduce inflammation without altering the expression of cellular immunity could have important clinical implications in allowing for the treatment of cell-mediated inflammatory diseases without the risk of significantly suppressing adaptive immune responses. This would be limited, however, by the fact that IL-1ra treatment would be predicted to inhibit primary immune responses to novel infectious pathogens.
Taken together, we conclude that the role of IL-1ra in vivo is to limit the inflammatory and innate immune responses during a listerial infection, presumably via its antagonism of IL-1 binding to its receptor. Overexpression of IL-1ra causes a shift in the IL-1/IL-1ra ratio, reducing the net in vivo biological activity of IL-1. Decreased IL-1 signaling results in a reduced level of immune system activation in response to a primary listerial infection (and presumably to other infectious processes). Once this initial block is overcome, i.e., by previous infection in immune mice, there is no longer a critical dependence upon IL-1 signaling in the immune response, but the inflammatory response remains partially dependent upon the IL-1/IL-1ra ratio.
Recently, several studies investigating listeriosis in strains of transgenic mice lacking either the TNF receptor p55 (6), the IFN-γ receptor (2), IL-10 (3), or IL-6 (4) have been published. A comparison of the phenotypes of these mice to that presented for the IL-1ra overexpressors reveals certain unique as well as overlapping functions of these cytokines. In general, all proinflammatory cytokines (TNF-α, IFN-γ, and IL-6) enhance, and anti-inflammatory cytokines (IL-1ra and IL-10) antagonize, the innate immune response, resulting in altered susceptibilities, bacterial titers, and liver pathologies during a primary listerial infection. Leukocytosis is partially dependent upon IL-1 signaling. The generalized effect on all classes of WBC appears unique to IL-1, although neutrophilia requires IL-6 as well. Whether IL-1 and IL-6 both serve critical and nonredundant roles in neutrophil mobilization or whether IL-1 acts via induction of IL-6 in vivo remains undetermined. Extravasation of WBC to sites of infection appears to have an absolute requirement for IL-1, while TNF-α, IFN-γ, and IL-6 are all dispensable. The upregulation of MHC class II molecules on macrophages is critically dependent upon both IL-1 and IFN-γ, which seem to have nonredundant roles, as discussed above. An unexpected result is that an apparently normal cellular immunity can develop and function independently of both IFN-γ (9) and IL-1 signaling. To date, only IL-10 has been shown to regulate the efficacy of both the innate and the secondary immune responses during listeriosis.
The role of the IL-1 receptor and IL-1ra in listerial resistance is important to separate from other genetic modifiers that may exist in mouse strains of mixed background. It is crucially important because the IL-1RI knockout mice of Labow et al. (15) which were of B6, 129 mixed background resembled the IL-1ra overexpressors in listerial sensitivity, as did those IL-1 receptor knockout mice of Glaccum et al. (7). However, the latter mice did not maintain their increased sensitivity after backcrossing to C57BL/6 five generations. Our experiments have used mice of a B6, CBA background carrying the IL-1ra transgene compared to similar mice without the transgene. As several independent experiments gave similar results, the altered sensitivity and cellular responses were attributed to the presence of the IL-1ra transgene. Furthermore, as transgenic mice on a B6 (N = 7) background have become available, we have observed the same increased sensitivity to Listeria. The studies presented here provide the baseline data for future work to resolve the relative contributions of IL-1ra, IL-1 receptor, and other genetic loci in the responses to listerial infection. This work will ultimately determine whether IL-1ra functions independently of the IL-1 receptor. Until now, we have assumed that the only activity of IL-1ra is to complete with IL-1 for the IL-1 receptor. If, when compared on similar genetic backgrounds, the receptor knockout mice lack the phenotypic responses of the IL-1ra transgenic overexpressors, then IL-1ra may function independently of the IL-1RI and alternative mechanisms of action of IL-1ra would have to be considered.
ACKNOWLEDGMENTS
This work was supported by NIH grant 2T32 DK07328 (to V.M.I.), by NIH grant K11 AI01116 (to E.H.), and by NIH grant AI 42861 (to D.H.).
We thank Sarah M. Paul for technical assistance with these experiments. We are also grateful to members of our laboratory for helpful suggestions and comments on the manuscript and to Paul B. Rothman for criticisms of the manuscript and reagents for the IFN-γ ELISA.
REFERENCES
- 1.Czuprynski C J, Brown J F, Young K M, Cooley A J, Kurtz R S. Effects of murine recombinant interleukin 1α on the host response to bacterial infection. J Immunol. 1988;140:962–968. [PubMed] [Google Scholar]
- 2.Dai W J, Bartens W, Köhler G, Hufnagel M, Kopf M, Brombacher F. Impaired macrophage listericidal and cytokine activities are responsible for the rapid death of Listeria monocytogenes-infected IFN-γ receptor-deficient mice. J Immunol. 1997;158:5297–5304. [PubMed] [Google Scholar]
- 3.Dai W J, Köhler G, Brombacher F. Both innate and acquired immunity to Listeria monocytogenes infection are increased in IL-10-deficient mice. J Immunol. 1997;158:2259–2267. [PubMed] [Google Scholar]
- 4.Dalrymple S A, Lucian L A, Slattery R, McNeil T, Aud D M, Fuchino S, Lee F, Murray R. Interleukin-6-deficient mice are highly susceptible to Listeria monocytogenes infection: correlation with inefficient neutrophilia. Infect Immun. 1995;63:2262–2268. doi: 10.1128/iai.63.6.2262-2268.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dinarello C A. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–2147. [PubMed] [Google Scholar]
- 6.Endres R, Luz A, Schulze H, Neubauer H, Fütterer A, Holland S M, Wagner H, Pfeffer K. Listeriosis in p47phox−/− and TRp55−/− mice: protection despite absence of ROI and susceptibility despite presence of RNI. Immunity. 1997;7:419–432. doi: 10.1016/s1074-7613(00)80363-5. [DOI] [PubMed] [Google Scholar]
- 7.Glaccum M B, Stocking K L, Charrier K, Smith J L, Willis C R, Maliszewski C, Livingston D J, Peschon J J, Morrissey P J. Phenotypic and functional characterization of mice that lack the type I receptor for IL-1. J Immunol. 1997;159:3364–3371. [PubMed] [Google Scholar]
- 8.Greenfeder S A, Nunes P, Kwee L, Labow M, Chizzonite R A, Ju G. Molecular cloning and characterization of a second subunit of the interleukin 1 receptor complex. J Biol Chem. 1995;270:13757–13765. doi: 10.1074/jbc.270.23.13757. [DOI] [PubMed] [Google Scholar]
- 9.Harty J T, Bevan M J. Specific immunity to Listeria monocytogenes in the absence of IFN gamma. Immunity. 1995;3:109–117. doi: 10.1016/1074-7613(95)90163-9. [DOI] [PubMed] [Google Scholar]
- 10.Harty J T, Lenz L L, Bevan M J. Primary and secondary immune responses to Listeria monocytogenes. Curr Opin Immunol. 1996;8:526–530. doi: 10.1016/s0952-7915(96)80041-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Havell E A, Moldawer L L, Helfgott D, Kilian P L, Sehgal P B. Type I IL-1 receptor blockade exacerbates murine listeriosis. J Immunol. 1992;148:1486–1492. [PubMed] [Google Scholar]
- 12.Hirsch E, Irikura V M, Paul S M, Hirsh D. Functions of interleukin 1 receptor antagonist in gene knockout and overproducing mice. Proc Natl Acad Sci USA. 1996;93:11008–11013. doi: 10.1073/pnas.93.20.11008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hunter C A, Chizzonite R, Remington J S. IL-1 beta is required for IL-12 to induce production of IFN-gamma by NK cells. A role for IL-1 beta in the T cell-independent mechanism of resistance against intracellular pathogens. J Immunol. 1995;155:4347–4354. [PubMed] [Google Scholar]
- 13a.Irikura, V. M., and D. Hirsh. Unpublished results.
- 14.Kaufmann S H E. Immunity to intracellular bacteria. Annu Rev Immunol. 1993;11:129–163. doi: 10.1146/annurev.iy.11.040193.001021. [DOI] [PubMed] [Google Scholar]
- 15.Labow M, Shuster D, Zetterstrom M, Nunes P, Terry R, Cullinan E B, Bartfai T, Solorzano C, Moldawer L L, Chizzonite R, McIntyre K W. Absence of IL-1 signaling and reduced inflammatory response in IL-1 type I receptor-deficient mice. J Immunol. 1997;159:2452–2461. [PubMed] [Google Scholar]
- 16.Ma Y, Thornton S, Boivin G P, Hirsh D, Hirsch R, Hirsch E. Altered susceptibility to collagen-induced arthritis in transgenic mice with aberrant expression of interleukin-1 receptor antagonist. Arthritis Rheum. 1998;41:1798–1805. doi: 10.1002/1529-0131(199810)41:10<1798::AID-ART11>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 17.McIntyre K W, Stepan G J, Kolinsky K D, Benjamin W R, Plocinski J M, Kaffka K L, Campen C A, Chizzonite R A, Kilian P L. Inhibition of interleukin 1 (IL-1) binding and bioactivity in vitro and modulation of acute inflammation in vivo by IL-1 receptor antagonist and anti-IL-1 receptor monoclonal antibody. J Exp Med. 1991;173:931–939. doi: 10.1084/jem.173.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mielke M E A, Peters C, Hahn H. Cytokines in the induction and expression of T-cell-mediated granuloma formation and protection in the murine model of listeriosis. Immunol Rev. 1997;158:79–93. doi: 10.1111/j.1600-065x.1997.tb00994.x. [DOI] [PubMed] [Google Scholar]
- 19.Mocci S, Dalrymple S A, Nishinakamura R, Murray R. The cytokine stew and innate resistance to L. monocytogenes. Immunol Rev. 1997;158:107–114. doi: 10.1111/j.1600-065x.1997.tb00996.x. [DOI] [PubMed] [Google Scholar]
- 20.North R J, Dunn P L, Conlan J W. Murine listeriosis as a model of antimicrobial defense. Immunol Rev. 1997;158:27–36. doi: 10.1111/j.1600-065x.1997.tb00989.x. [DOI] [PubMed] [Google Scholar]
- 21.Rogers H W, Callery M P, Deck B, Unanue E R. Listeria monocytogenes induces apoptosis of infected hepatocytes. J Immunol. 1996;156:679–684. [PubMed] [Google Scholar]
- 22.Rogers H W, Sheehan K C F, Brunt L M, Dower S K, Unanue E R, Schreiber R D. Interleukin 1 participates in the development of anti-Listeria responses in normal and SCID mice. Proc Natl Acad Sci USA. 1992;89:1011–1015. doi: 10.1073/pnas.89.3.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rogers H W, Tripp C S, Schreiber R D, Unanue E R. Endogenous IL-1 is required for neutrophil recruitment and macrophage activation during murine listeriosis. J Immunol. 1994;153:2093–2101. [PubMed] [Google Scholar]
- 24.Tripp C S, Needleman P, Unanue E R. Indomethacin in vivo increases sensitivity to Listeria infection in mice. J Clin Investig. 1987;79:399–403. doi: 10.1172/JCI112825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tripp C S, Wolf S F, Unanue E R. Interleukin 12 and tumor necrosis factor alpha are costimulators of interferon gamma production by natural killer cells in severe combined immunodeficiency mice with listeriosis, and interleukin 10 is a physiologic antagonist. Proc Natl Acad Sci USA. 1993;90:3725–3729. doi: 10.1073/pnas.90.8.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Unanue E R. Inter-relationship among macrophages, natural killer cells and neutrophils in early stages of Listeria resistance. Curr Opin Immunol. 1997;9:35–43. doi: 10.1016/s0952-7915(97)80156-2. [DOI] [PubMed] [Google Scholar]
- 27.Wagner R D, Czuprynski C J. Cytokine mRNA expression in livers of mice infected with Listeria monocytogenes. J Leukocyte Biol. 1993;53:525–531. doi: 10.1002/jlb.53.5.525. [DOI] [PubMed] [Google Scholar]
- 28.Winter C G, Saotome Y, Saotome I, Hirsh D. CNTF overproduction hastens onset of symptoms in motor neuron degeneration (mnd) mice. J Neurobiol. 1996;31:370–378. doi: 10.1002/(SICI)1097-4695(199611)31:3<370::AID-NEU9>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 29.Zahedi K, Seldin M F, Rits M, Ezekowitz R A B, Whitehead A S. Mouse IL-1 receptor antagonist protein: molecular characterization, gene mapping, and expression of mRNA in vitro and in vivo. J Immunol. 1991;146:4228–4233. [PubMed] [Google Scholar]