Abstract
Natural resistance of humans to the cattle pathogen Trypanosoma brucei brucei has been attributed to the presence in human serum of nonimmune factors that lyse the parasite. Normal human serum contains two trypanosome lytic factors (TLFs). TLF1 is a 500-kDa lipoprotein, which is reported to contain apolipoprotein A-I (apoA-I), haptoglobin-related protein (Hpr), hemoglobin, paraoxonase, and apoA-II, whereas TLF2 is a larger, poorly characterized particle. We report here a new immunoaffinity-based purification procedure for TLF2 and TLF1, as well as further characterization of the components of each purified TLF. Immunoaffinity-purified TLF1 has a specific activity 10-fold higher than that of TLF1 purified by previously described methods. Moreover, we find that TLF1 is a lipoprotein particle that contains mainly apoA-I and Hpr, trace amounts of paraoxonase, apoA-II, and haptoglobin, but no detectable hemoglobin. Characterization of TLF2 reveals that it is a 1,000-kDa protein complex containing mainly immunoglobulin M, apoA-I, and Hpr but less than 1% detectable lipid.
African trypanosomes are unicellular eukaryotic protozoans that infect both animals and humans. They are usually transmitted by the bite of the tsetse fly, and they live in the bloodstream of their mammalian hosts. The trypanosome continuously evades the immune system, systematically changing its surface coat by switching expression among a thousand distinct genes encoding the variant surface glycoprotein (6). Nevertheless, humans are resistant to the widespread Trypanosoma subspecies T. brucei brucei due to the presence of nonimmune serum factors that lyse the invading trypanosome (12). In contrast, two other subspecies, T. b. gambiense and T. b. rhodesiense, infect humans and cause sleeping sickness because they are resistant to lysis by human serum.
A factor from human serum that lyses T. b. brucei was characterized in 1978 (3) as a subset of high-density lipoprotein (HDL) and is now known as trypanosome lytic factor 1 (TLF1). More recently, Smith et al. (39) identified Hpr (haptoglobin [Hp]-related protein) as a unique component of TLF1 that is not found in nontrypanolytic HDL. Hpr shares over 90% amino acid sequence identity with Hp, an abundant serum protein (0.2 to 2 mg/ml) that binds free hemoglobin (Hb) and facilitates its clearance via receptors in the liver (5). The physiological role and biological properties of Hpr are unknown. The current model explaining trypanolysis requires binding of TLF1 to a receptor in the trypanosome flagellar pocket, followed by endocytosis and subsequent delivery to lysosomes (10). The low lysosomal pH is hypothesized to activate an Hpr-dependent peroxidase activity that results in lipid peroxidation, lysis of the lysosomal membrane, and autodigestion of the parasite (25, 39). However, the physiological significance of TLF1 has been questioned due to the presence of a natural inhibitor within serum that may mask TLF1 activity in normal human serum (NHS). The inhibitor of TLF1 has been identified as Hp (32, 38), which blocks TLF1-mediated lysis by an unknown mechanism.
A second distinct trypanosome lytic factor, termed TLF2, has been identified in NHS (3, 12, 20, 21, 29, 32, 41, 42). TLF2 is a ∼1,000-kDa particle that is not inhibited by Hp in vitro and accounts for most or all of the observed trypanosome lytic activity in NHS (32). We have shown previously that antibodies against the TLF1 components apolipoprotein A-I (apoA-I) and Hpr can immunodeplete TLF2 trypanolytic activity, indicating that apoA-I and Hpr are proteins common to both lytic factors (43). We report here on the characterization of both TLF1 and TLF2 from NHS following purification by an immunoaffinity method that uses a monoclonal antibody (MAb) recognizing the common and critical component Hpr.
MATERIALS AND METHODS
NHS.
Serum (100 ml) was collected from healthy fasted donors. Individual humans exhibit one of three different Hp haplotypes that can be distinguished by molecular mass. Hp type 1-1 is a dimer with a molecular mass of 86 to 100 kDa, while types 2-1 and 2-2 are polymers with molecular masses that range from 200 to 400 kDa and from 400 to 1,000 kDa, respectively. Serum from a donor with Hp type 1-1 was used for purification since its lower molecular weight more easily permits separation from the high-molecular-weight TLFs. Cofractionation of Hp with TLF is not desirable because Hp is an inhibitor of TLF1, and an anti-Hp affinity purification step is used in TLF purification (see below).
Trypanolytic assay.
A fluorescence-based assay was used to measure trypanolysis (42). The assay involves incubating the test sample with 2 × 106 trypanosomes at 37°C for 2 h in a final volume of 200 μl of Dulbecco modified Eagle medium with 1% bovine serum albumin (BSA), followed by the addition of the acetoxymethyl ester of calcein, which is cell permeant and nonfluorescent. Upon entering viable cells, the probe is cleaved by intracellular nonspecific esterases, releasing the highly fluorescent free acid. This product is retained within the cell, allowing fluorimetric quantitation of the number of unlysed cells. Trypanolytic units were determined for samples at each stage of the purification; 1 U of activity lyses 50% of the trypanosomes at 37°C in 2 h under our assay conditions. Serum-sensitive (Etat 1.9s) and serum-resistant (Etat 1.9r [35]) trypanosome subspecies were routinely examined to ensure that lysis was specific.
Purification of TLF1 and TLF2 from NHS.
NHS (100 ml) was adjusted to a density of 1.25 g/ml with KBr and ultracentrifuged at 49,000 rpm (228, 306 × g) in a near-vertical rotor (NVTi 65; Beckman) for 16 h at 10°C (31). The top 25% of the gradient (ρ = 1.0 to 1.25 g/ml) was collected and pooled to yield 24 ml of lipoproteins containing TLF1, while the bottom 50% of the gradient constituted the infranate containing TLF2. For TLF1 purification, the density of the lipoprotein fraction was adjusted to 1.3 g/ml with KBr and 4-ml aliquots were layered under 8 ml of 0.9% NaCl. The lipoproteins were then centrifuged at 49,000 rpm (228,306 × g) for 3 h at 10°C (NVTi 65 rotor; Beckman); HDL was harvested as a yellow band in the center of the tubes (ρ = 1.10 to 1.25 g/ml). The original infranates containing TLF2 (ρ = 1.27 to 1.3 g/ml) were pooled to yield 56 ml. Both TLF1 and TLF2 pools were dialyzed against three changes of Tris-buffered saline (TBS; 50 mM Tris-HCl, 150 mM NaCl [pH 7.5]) at 4°C and then concentrated by ultrafiltration (XM300 filter membrane; Amicon). TLF1 was concentrated to 2 ml (50 mg of protein/ml), and TLF2 was concentrated to 20 ml (200 mg/ml). To preserve trypanolytic activity, these concentrated protein samples were stored at −80°C until used for further purification.
The HDL preparation (2 ml) containing TLF1 was fractionated by size on a Superose 6 column (1.5 by 60 cm; Pharmacia, Piscataway, N.J.) at a flow rate of 0.4 ml/min. Trypanolytic fractions (450 to 650 kDa) were pooled and concentrated. The TLF2 preparation was fractionated in 10 runs by loading 2 ml on a Superose 6 column (1.5 by 60 cm) at a flow rate of 0.4 ml/min. Trypanolytic fractions (700 to 1,200 kDa) were pooled and concentrated. To preserve trypanolytic activity, these concentrated TLF samples were stored at −80°C until used for further purification. The remaining purification steps, affinity, and sizing were performed in 1 day, and lytic activity was analyzed. Freezing and thawing of the final samples resulted in a 50% loss of TLF1 lytic units and a 90% loss of TLF2 lytic units per round.
An affinity column was prepared by covalently coupling a MAb raised against human Hp (H-6395, a mouse immunoglobulin G1 [IgG1] from Sigma, St. Louis, Mo.) via amino groups to a HighTrap N-hydroxysuccinimide column as instructed by the manufacturer (Pharmacia). The TLF pools were pumped onto the column in TBS at room temperature and allowed to bind for 10 min. Following washing with TBS (5 column volumes), the bound fraction was eluted with 100 mM glycine–150 mM NaCl (pH 3.0). Collected fractions (1 ml) were immediately neutralized with 20 μl of 1.5 M Tris-HCl (pH 8). Trypanolytic fractions were pooled, concentrated, and subsequently fractionated on a Superose 6 high-resolution column (HR; 1 by 30 cm; Pharmacia) at a flow rate of 0.2 ml/min to verify that the molecular mass of the TLFs remained unchanged. TLF1 and TLF2 retained their original molecular masses of ∼500 and ∼1,000 kDa, respectively.
Polyacrylamide gel electrophoresis (PAGE) and silver staining.
Gradient gels of 10 to 15% acrylamide were prepared and run according to the method of Laemmli (19). Gels were fixed in 50% methanol and then stained with silver by the method of Wray et al. (46).
Amino acid sequencing.
For N-terminal sequence analysis, TLF1 and TLF2 samples were separated by sodium dodecyl sulfate (SDS)-PAGE on 12% gels and transferred to polyvinylidene difluoride (PVDF) membranes (Immobilon P; Millipore), using 3-cyclohexylamino-1-propanesulfonic acid (pH 11) containing 10% (vol/vol) methanol. Membranes were stained with Coomassie blue, and the protein bands were excised and sequenced on a model 477A protein sequencer (Applied Biosystems) as described elsewhere (8).
Immunodepletion studies.
All antibodies—mouse MAbs against human IgM (IgG2b, I-6385; Sigma) and human Hp (IgG1, H-6395; Sigma), polyclonal sheep anti-human apoA-I (AHP213 [Serotec, Indianapolis, Ind.] or 726 478, [Boehringer Mannheim, Germany]), and goat anti-human apoA-I (AB740; Chemicon, Temecula, Calif.)—used in immunodepletion studies were prebound to protein G beads (Pharmacia) for 60 min on a rotating wheel at 4°C. Antibody-coated beads were washed three times with TBS and transferred to clean tubes prior to the addition of the TLFs. TLFs (1 to 2 lytic units) were incubated with the antibody-coated beads for 60 min on a rotating wheel at 4°C. The beads were then pelleted by centrifugation, and the supernatants were assayed for trypanosome lytic activity.
Western blotting and ECL.
Proteins separated by SDS-PAGE (15% gel) were transferred to PVDF membranes as described previously (44). Membranes were blocked with 10% BSA in TBS containing 0.1% Tween (TBST). The primary antibodies, rabbit anti-human IgM μ chain (I-0140; Sigma), sheep anti-human apoA-I (726 478; Boehringer Mannheim), sheep anti-human apoA-II (726 486; Boehringer Mannheim), and rabbit anti-human Hp (H-8636; Sigma), were diluted 1:10,000 in TBST containing 4% BSA. Mouse anti-human paraoxonase (F41F2-K; R. W. James, Geneva University Hospital, Geneva, Switzerland) was diluted 1:50 in TBST containing 4% BSA. The secondary antibodies, goat anti-rabbit IgG conjugated to horseradish peroxidase (HRP) (W401B; Promega), rabbit anti-sheep IgG conjugated to HRP (605 345; Boehringer Mannheim), and rabbit anti-mouse IgG conjugated to HRP (W402B; Promega), were diluted 1:20,000 in TBST containing 4% BSA. The immunoreactive bands were revealed by enhanced chemiluminescence (ECL) as described by the manufacturer (Amersham).
Paraoxonase/arylesterase assay.
Paraoxonase/arylesterase was measured essentially by the method of Zech et al. (47). Paraoxon (Sigma) was resuspended in TBS containing 4 mM CaCl2 to a final concentration of 8 mM. Aliquots of 100 μl were placed in a 96-well microtiter plate, and the reaction was initiated by the addition of an equal volume of TLF1 (100 lytic units, 1 μg) or TLF2 (3 lytic units, 1.8 μg). The absorbance change at 405 nm was monitored at 22°C. Arylesterase activity was tested at 270 nm in a total volume of 1.0 ml with Tris-HCl (pH 8.5)—5 mM p-nitrophenylacetate—1 mM CaCl2. Paraoxonase/arylesterase unit activity is measured as micromoles of p-nitrophenol released per minute. MAb F41F2-K, specific for paraoxonase, was kindly provided by R. W. James.
Lipid assay.
Lipid content was quantitated by the method of Marsh and Weinstein (22).
Depletion of IgM and IgG from NHS.
NHS was diluted twofold and incubated at 4°C for 90 min with 50 μl of either agarose-linked goat anti-human IgM (A-9935; Sigma), agarose-linked goat anti-human IgG (A-3543; Sigma), or TBS. The agarose beads were removed by centrifugation, and the supernatants were evaluated for trypanolytic activity by microscopic analysis. The thoroughness of IgM depletion was analyzed by immunoblotting the supernatants with an anti-IgM MAb (I-6385; Sigma).
Enzyme-linked immunosorbent assay.
Levels of IgM and apoA-I were measured by using a standard sandwich enzyme-linked immunosorbent assay technique (9). Monoclonal anti-IgM (I-6385; Sigma) and anti-apoA-I (MAB010; Chemicon) antibodies were used as capture antibodies (coating concentration, 10 μg/ml), and corresponding polyclonal rabbit anti-IgM (I-0140; Sigma) and goat anti-apoA-I (AB740; Chemicon) antibodies were used as reporting antibodies. Alkaline phosphatase-conjugated secondary antibodies (Sigma) were used for detection.
RESULTS
Purification of TLF1 and TLF2.
TLF1 and TLF2 were purified by a four-step procedure (Tables 1 and 2). The TLFs were first separated from each other by density gradient centrifugation (see Materials and Methods for details). TLF1, which is a form of HDL, had a buoyant density predominantly of 1.20 to 1.25 g/ml, whereas TLF2 equilibrated at 1.28 g/ml (results not shown).
TABLE 1.
Purification of TLF1
| Sample | Protein (mg) | Activity (U) | Sp act (U/mg) | Purification (fold)a | Recovery (%) |
|---|---|---|---|---|---|
| HDL | 100 | 100,000b | 1,000 | 50 | 100 |
| Affinity column | 0.5 | 35,000 | 70,000 | 4,900 | 35 |
| Superose 6HRc | 0.3 | 35,000 | 117,000 | 8,190 | 35 |
Adjusted to allow for the removal of Hp, an inhibitor of TLF1, at the first stage of purification.
There is a 50-fold increase in activity due to the removal of the TLF1 inhibitor Hp, which partitions into the infranate. Most or all of the observed activity in the whole serum is TLF2 (2,000 lytic units).
450- to 650-kDa region.
TABLE 2.
Purification of TLF2
| Sample | Protein (mg) | Activity (U) | Sp act (U/mg) | Purification (fold) | Recovery (%) |
|---|---|---|---|---|---|
| Whole serum, 100 ml | 7,000 | 2,000 | 0.29 | 1.0 | 100 |
| Infranate | 4,000 | 2,000 | 0.5 | 1.8 | 100 |
| Superose 6a | 75 | 3,260 | 43 | 148 | 163b |
| Affinity column eluate | 3 | 2,500 | 830 | 2,860 | 125 |
| Superose 6HRa | 0.6 | 983 | 1,600 | 5,520 | 49c |
700- to 1,200-kDa region.
The apparent increase in activity upon size fractionation is due to the presence of residual TLF1 (∼1%) in the infranate, which becomes separated from the endogenous inhibitor Hp (90 kDa). The leading edge of the TLF1 peak at 450 to 650 kDa contaminates the TLF2 peak.
This final size separation of TLF2 from TLF1 on Superose 6HR (high resolution) is more effective due to the low amount of protein (3 mg) applied to the column compared to the initial size fractionation (Superose 6 preparative), in which a large amount of protein was applied (400 mg). It also separates contaminating Hp and albumin (∼50% protein).
TLF1.
The HDL fraction containing TLF1 showed a 50-fold increase in total activity relative to serum. This is due to the removal of the TLF1 inhibitor Hp (32, 38), most of which partitions to the infranate. The HDL was then subjected to immunoaffinity chromatography on a column containing an immobilized MAb to human Hp. This antibody also recognizes Hpr. A 70-fold increase in specific activity, most likely due to the removal of nonlytic HDL, was observed in the eluate. The small amount of contaminating Hp (free and complexed with nonlytic HDL) was removed by size fractionation in the final step of purification. The final preparation had a specific activity more than 8,000-fold greater than that of serum, and the recovery was 35%.
TLF2.
The Hp content in the serum used for this purification scheme was 270 μg/ml. An Hp concentration of 200 μg/ml was sufficient to inhibit all of the TLF1 activity equivalent to that found in 1 ml of serum. Therefore, for the purpose of calculating specific activities and recoveries, we assumed that all of the trypanolytic activity in serum was TLF2 mediated (32). This assumption was corroborated by the recovery of an equivalent amount of lytic activity in the lipoprotein-free infranate relative to that observed in whole serum. The infranate was then concentrated and sized on a Superose 6 column in order to separate TLF2 from Hp (90 kDa). The pooled 700- to 1,200-kDa region had a total lytic activity about twice that of the infranate. This was due to TLF1 contamination in the infranate, which was masked due to the presence of Hp in the infranate. After passage through the Sepharose column, TLF1 (450 to 650 kDa) becomes separated from free Hp and its activity is unmasked. Thus, the leading edge of TLF1 contaminates the lagging edge of TLF2 (700 to 1,200 kDa). The pooled TLF2 was then subjected to immunoaffinity chromatography, and the contaminating TLF1 was removed by repurification on Sepharose 6HR column. The resulting fractions yielded a purification of 5,500-fold and a recovery of 49% relative to the activity of TLF2 in whole serum.
The purification and yields of TLF1 and TLF2 during various steps of the fractionation are shown in Tables 1 and 2, respectively. By this procedure, a 100-ml aliquot of serum yielded 300 μg of purified TLF1 and 600 μg of purified TLF2 within 4 to 5 days. In other experiments, affinity purification was performed with polyclonal antibodies to Hp, but the yields of TLF1 and TLF2 were more than 20-fold lower (data not shown). As is usually the case with polyclonal antibodies, the avidity of binding the antigen is much higher than that for MAbs, and even more extreme elution conditions can fail to release the immunogen. Despite complete binding of TLF to the polyclonal antibody column, we were unable to elute the TLF in an active form. Evaluation of the purified preparations in trypanolytic assays revealed that 1 lytic unit of activity is equivalent to ∼10 ng of TLF1 and ∼600 ng of TLF2. Based on the recovery of lytic units, NHS contains ∼10 μg of TLF1 per ml (1,000 lytic units) that are inhibited by Hp and ∼12 μg of TLF2 per ml (20 lytic units).
PAGE and protein sequencing of TLF1 and TLF2.
Purified TLF1 and TLF2 were analyzed by SDS-PAGE. Silver-stained gels revealed that both TLF1 and TLF2 contained proteins of 40, 36, 28, and 13.5 kDa (Fig. 1). TLF2 additionally had several unique bands: a prominent 85-kDa protein, and less prominent 50-, 45-, 31-, and 29-kDa proteins. TLF2 proteins were analyzed by N-terminal microsequencing.
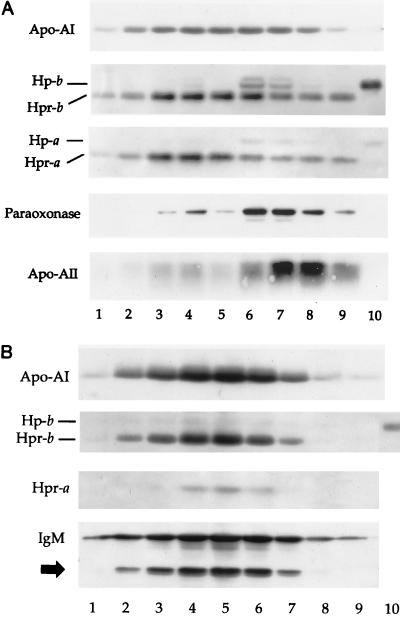
FIG. 1.
Silver-stained SDS–12% polyacrylamide gel of reduced TLF1 and TLF2. One lytic unit of TLF2 and 7.5 lytic units of TLF1 were reduced in sample buffer, and the polypeptides were separated by PAGE. Bands identified by protein sequencing of their N termini (heavy chain [H-chain] and light chain [L-chain]) are indicated with white arrows. We could not obtain the sequences of the two bands indicated with the black arrow but they may be proteolytic products of the IgM μ chain due to their immunoreactivity with anti-μ-chain polyclonal antibody (see Fig. 2). Asterisks indicate positions of molecular weight markers migrating at 94, 67, 43, 30, 20.1, and 14 kDa (top to bottom).
The major component at 85 kDa yielded the amino acid sequence EVQLVEVESGGGLVQPG, which corresponds to an immunoglobulin heavy chain (subgroup VHIII). By immunoblot analysis, both the 85-kDa protein and the minor components at 50 to 45 kDa (indicated by black arrows in Fig. 1 and 2B) reacted with polyclonal antibodies to the μ chain (Fig. 2B). In addition, the sequences retrieved from the proteins migrating at 29 to 31 kDa (YVLTQSPXLS and DIVMTQSPLS) revealed the presence of immunoglobulin κ and λ light chains.
FIG. 2.
Western blot of fractions from final step of purification of TLF1 (A) and TLF2 (B). Superose 6HR fractions encompassing molecular mass ranges of 650 to 200 kDa for TLF1 (A, lanes 1 to 9) and 1,200 to 700 kDa for TLF2 (B, lanes 1 to 9) were reduced in sample buffer. The polypeptides were separated by SDS-PAGE prior to transfer to PVDF membranes. The blots were sequentially immunoblotted with antibodies raised against apoA-I, apoA-II, Hp and Hpr, paraoxonase, and IgM as indicated on LHS. The blots were stripped between each immunoblotting step by incubation in 0.2 M glycine (pH 2.8). The black arrow corresponds to that in Fig. 1. Lanes 10, haptoglobin standard.
Amino acid sequencing revealed the presence of Hpr as well as a lesser amount of Hp both in TLF1 and TLF2. Hpr was identified by the sequence of the 13.5-kDa component SDLGAVISLLLWGRQLFALYSG. As previously described for TLF1 (38), the Hpr α chain in TLF2 contains an uncleaved signal sequence. The Hpr β chain corresponds to the 36-kDa protein with the N-terminal sequence ILGGHLDAKGSF. While the protein migrating at 40 kDa yielded an identical sequence, its larger molecular mass corresponds to that of the Hp β chain. The Hp α chain was not sequenced but is accounted for by the faintly stained band that migrates just above it. Finally, the 28-kDa band yielded the sequence DEPPQSPWDRVK, which corresponds to apoA-I (Fig. 1).
Western blot analysis of the final purification profile.
We analyzed the peak distribution of the identified TLF proteins with respect to each other. The fractions from the final purification through the Superose 6HR column were separated by SDS-PAGE, transferred to PVDF membranes, and probed for each protein with antibodies specifically directed against apoA-I, apoA-II, Hp and Hpr, IgM μ chain, and paraoxonase (Fig. 2). Figure 2A represents a composite Western blot analysis of purified TLF1 using Abs to individual proteins (indicated on the LHS). Lanes 3 to 5 have peak lytic activity, and correspond to the TLF1 analyzed by the silver-stained gel in Fig. 1. The paraoxonase comigrates with Hp because they have the same molecular weight (see clear zone in center of Hp bands in lanes 4, 6, and 7) and would therefore appear as one band on the silver-stained gel. We sometimes detect paraoxonase in TLF1 by Western blot analysis, although paraoxonase does not correlate with TLF1 lytic activity. The role, if any, of paraoxonase in TLF1 lytic activity remains unclear. We detect a trace of apoA-II in highly purified TLF1 by Western analysis (Fig. 2A), but anti-apoA-II antibodies failed to immunodeplete trypanolytic activity. However, the same anti-apoA-II antibodies are able to immunodeplete lytic activity from less pure TLF1 preparations (data not shown). Together these data suggest that TLF1 (like HDL) is heterogeneous in nature, and although some TLF1 particles may contain apoA-II, the lipoprotein is not required for lytic activity. Lanes 6 to 9 represent the Hp-containing HDL that copurifies on the affinity column. This has negligible lytic activity despite the presence of Hpr; this most likely results from inhibition by the coeluting Hp. There is substantially more (∼10-fold) paraoxonase and apoA-II in the HDL peak than in the TLF1 peak.
Figure 2B represents the final purification profile of TLF2 through Superose 6HR, revealing the presence of Hpr, apoA-I, and IgM comigrating as a single peak; there was no trace of paraoxonase or apoA-II. Lanes 4 to 6 have peak lytic activity. Chemical analysis of TLF2 did not reveal the presence of lipid components, in contrast to TLF1, which is composed of 40% lipid (11). This is consistent with the buoyant density of each particle: 1.28 g/ml for TLF2 and 1.21 to 1.25 g/ml for TLF1. In a previous study, Hb was detected in a preparation of TLF1 (4). We did not detect Hb in purified TLF2, and Hb in TLF1 is considered a contaminant (see Table 3 and Discussion). Recent analysis also indicates that Hb is not a component of TLF1 (25).
TABLE 3.
Characteristics of TLF1 and TLF2
| Characteristic | TLF1 | TLF2 |
|---|---|---|
| Mol wt | 500,000 | 1,000,000 |
| % Lipid | 40 | <1 |
| Hpr | + | + |
| ApoA-I | + | + |
| IgM | − | + |
| Paraoxonase/arylesterasea | ∼20 ng/2 μg | <20 ng/4 μg |
| ApoA-IIb | Trace detected | Not detected |
| Inhibited by Hp | + | − |
| Hbc | 22 ng/μg | <1 ng/1.8 μg |
Paraoxonase, 0.000012 U/μg of HDL; arylesterase, 0.00013 U/μg of HDL; no activity was detected in 1 μg of TLF1 or 1.8 μg of TLF2. Paraoxonase protein was not detected in TLF2 (4 μg) by antiparaoxonase MAb F41F2-K, which has a limit of detection of 20 ng. Paraoxonase protein was detected in TLF1 at a level is estimated to be 10-fold less than that detected in HDL; 1 μg of HDL contains ∼70 ng of paraoxonase.
Not detected by silver stain or Coomassie blue stain; trace amounts were detected by ECL Western blotting.
Limit of detection of 1 ng by immunoblot with polyclonal goat anti-human Hb (Sigma). Hb detected in TLF1 cofractionates with Hp but not Hpr or lytic activity (∼500 kDa) in the final size fraction of purification, which may represent contaminating Hp-Hb complexes of ∼150 kDa.
Immunodepletion of TLF1 and TLF2 trypanolytic activity.
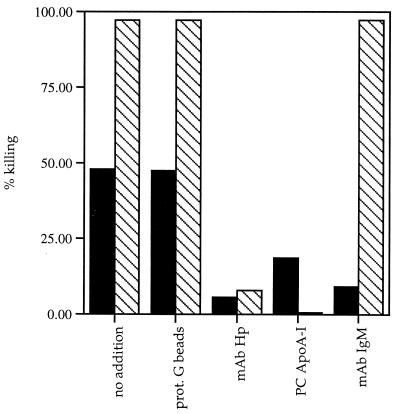
To ascertain whether the identified proteins that purify with TLF2 represent integral components of the lytic factor, antibodies against each TLF2-associated protein were tested for the ability to immunodeplete lytic activity. Control immunodepletion experiments were performed with TLF1. Although protein G beads alone do not immunodeplete activity, a MAb to human Hp, which cross-reacts with Hpr (43), immunodepleted lytic activity from both purified TLF2 and TLF1 (Fig. 3). A polyclonal antiserum to human apoA-I totally depleted TLF1 but only partially depleted TLF2. Although four different antisera to human apoA-I have been tested in these immunodepletion studies, none of them completely remove TLF2 activity; this may reflect inaccessibility of apoA-I in a fraction of the TLF2 particles. In contrast, MAbs to human IgM μ chain immunodepleted lytic activity from TLF2 but not from TLF1 (Fig. 3).
FIG. 3.
Immunodepletion of TLF activity. Protein G beads (50 μl) were preincubated with 100 μg of the various antibodies. Following washing, 1 to 2 lytic units of TLF was added to the beads and incubated for 60 min at 4°C. The supernatants were then tested for trypanolytic activity. Shown are lytic activities of TLF2 (solid bars) and TLF1 (hatched bars) remaining after no addition (control) and after immunoprecipitation with protein (prot.) G beads only, anti-Hp MAb, goat anti-apoA-I polyclonal (PC) antibody, and anti-IgM MAb.
Coimmunoprecipitation of TLF components.
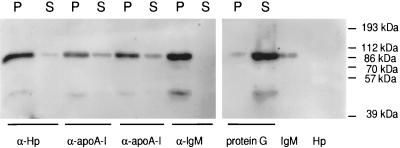
To substantiate that IgM, apoA-I, and Hpr proteins were associated in a single TLF2 protein complex, we investigated whether IgM was coimmunoprecipitated by antibodies to apoA-I and Hpr. As shown in Fig. 4, antibodies recognizing either Hp (and therefore Hpr) or apoA-I coprecipitated the vast majority of the IgM. The IgM remaining in the supernatant after incubation with anti-Hp may be a contaminant, as there was no detectable Hpr in the supernatant (not shown). IgM that does not coprecipitate with anti-apoA-I may reflect TLF2 in which the apoA-I epitopes are masked, because both Hpr and apoA-I (not shown) as well as residual lytic activity (Fig. 3, column 5) are found in the supernatant. The 45-kDa band recognized by the polyclonal antiserum to the IgM μ chain most likely corresponds to a proteolytic fragment of IgM shown in Fig. 1 and 2B (black arrows); this polyclonal antiserum does not cross-react with Hp, which migrates at about 40 kDa (Fig. 4, last lane). This finding confirms that TLF2 is a protein complex containing IgM, Hpr, and apoA-I.
FIG. 4.
TLF2 is a protein complex. One microgram of TLF2 was immunoprecipitated by antibodies to human Hp (MAb; α-Hp), human apoA-I (polyclonal antibodies from sheep and goat; α-apoA-I), and human IgM (MAb; α-IgM). Following immunoprecipitation, the pellet (P) and supernatant (S) were separated by reducing SDS-PAGE (12% gel) and transferred to PVDF membranes. The IgM μ chain was detected with rabbit anti-IgM μ-chain antibody followed by goat anti-rabbit IgG-HRP and exposed for 30 s by ECL.
Analysis of TLF2 proteins in serum fractions.
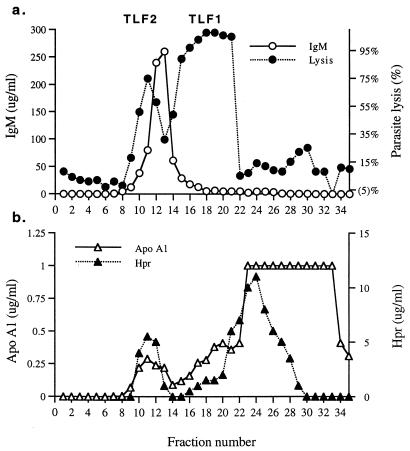
To directly examine the cofractionation of the identified components with lytic activity, fractions of NHS separated by gel filtration were analyzed for IgM, Hpr, apoA-I, and parasite lysis (Fig. 5). Serum IgM fractionated as a single peak corresponding to the expected molecular weight of IgM pentamers. Although lytic TLF2 fractions and IgM-containing fractions overlapped, the TLF2 peak did not coincide with the IgM peak (Fig. 5a), consistent with the notion that only a subfraction of IgM is trypanolytic. The larger molecular weight of TLF2 than of the bulk of IgM is consistent with our findings that TLF2 is a complex composed of a single pentameric IgM unit bound to apoA-I and Hpr. Further analysis of serum fractions revealed the coincidence of TLF2, apoA-I, and Hpr peaks (Fig. 5). The correlation of Hpr concentration with TLF1 and TLF2 lytic activity has been reported previously (16). The sharp drop in TLF1 lytic activity at fraction 22, despite the presence of Hpr and apoA-I in subsequent fractions, is due to cofractionation of inhibitory Hp (32, 38, 43).
FIG. 5.
Analysis of TLF2 component proteins in serum fractions. NHS was fractionated by size exclusion chromatography, and collected fractions were assayed for trypanolytic activity (a) and indicated proteins (a and b).
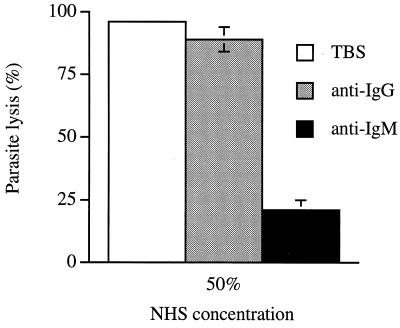
Immunodepletion of IgM and TLF2 trypanolytic activity from serum.
We have previously hypothesized that the primary trypanolytic activity in unfractionated NHS is due to TLF2, because TLF1 activity is largely or completely masked by inhibition with endogenous Hp (9). The identification of IgM as a component that is unique to TLF2 relative to TLF1 provides an independent means to evaluate this observation through immunodepletion of NHS with anti-IgM. Figure 6 shows that immunodepletion with agarose-linked anti-IgM removes ∼80% of the trypanolytic activity from human serum, while depletion with agarose-linked anti-IgG had a minimal effect. Western blot analysis revealed that a small residual fraction of IgM was not removed from NHS by immunodepletion, even upon repeated incubations of serum with anti-IgM agarose (data not shown). Although the explanation for this is unknown, other experiments showed that the residual lytic activity in the IgM-depleted serum was not inhibited by further addition of 250 μg of Hp per ml (data not shown). These data show that TLF2 is the main trypanolytic factor in human serum.
FIG. 6.
Trypanolytic activity of IgM- and IgG-depleted NHS. Error bars show means ± standard deviation (n = 3).
DISCUSSION
Characterization of highly purified TLF2 revealed that it is a protein complex containing mainly IgM, apoA-I, Hpr, and a small amount of Hp (Fig. 1 and Table 3). Our analysis of TLF1 revealed apoA-I and Hpr (Fig. 1) as the main proteins as well as the presence of a small amount of paraoxonase, apoA-II, and Hp (Fig. 2). It is possible that Hp is a contaminant that copurifies with TLF by the anti-Hp affinity purification method.
The IgM associated with TLF2 (12 μg/ml) represents a small fraction of total IgM in serum (500 to 1,900 μg/ml). We have made several attempts to remove all of the lytic activity from human serum by immunodepletion of the IgM. As shown in Fig. 6, immunodepletion of serum with anti-IgM removes ∼80% of the activity. However, immunoblotting the depleted supernatant revealed that we are unable to completely deplete the sample of IgM. The nature of the remaining 20% lytic activity is uncertain, although the residual activity is not inhibited by added Hp and therefore may correspond to TLF2. In any case, these results confirm that the main trypanolytic activity in serum is due to TLF2.
Although IgM was previously observed in partially purified preparations of TLF2, it was considered a contaminant (15). This notion was fostered from work by Rifkin (34), who used an anti-IgM serum to deplete human IgM with no decrease in trypanocidal activity. In contrast, Aaronovitch and Terry (1) concluded that the active trypanolytic factor in NHS was IgM because a rabbit antiserum to human IgM strongly inhibited the trypanocidal activity. The discrepancy in their results could be explained if the serum used by Rifkin was from an individual with low endogenous Hp levels such that TLF1 was active (see fractionation of LDL-free serum in Fig. 6A of Rifkin’s report [34]).
The presence of apoA-I and Hpr in both TLF1 and TLF2 suggests that both components play a role in trypanolysis or in the assembly of the lytic particles. Although lytic activity in fractionated serum does not correlate with the concentration of apoA-I (Fig. 5), serum from an individual with familial apoA-I deficiency was not trypanolytic (29), suggesting that apoA-I is required. However, TLF activity could have been masked. In addition, the levels of Hpr or Hp were not assessed in this patient. In contrast, sera from patients with Tangiers disease, in which levels of apoA-I are dramatically lowered, were found to be trypanolytic (42), which raised questions about the role of apoA-I in trypanolysis. As sera of patients with Tangiers disease have low levels of HDL (2 to 3%) (45), immunoprecipitation of all of the HDL with antisera against apoA-I would better address the role of apoA-I in TLF.
Evidence that Hpr may confer lytic activity comes from the observation that sera of only some apes and Old World monkeys have trypanolytic activity (37); these primates possess an Hpr gene, whereas all other animals studied to date do not (24). The exception is chimpanzee serum, which is not trypanolytic, probably due to a frameshift mutation in the Hpr sequence resulting in premature termination of translation (24). Additionally, the levels of both Hpr (32) and trypanocidal activity (13) are elevated in plasma from full-term pregnant women. We find that the lytic activity of TLF1 and TLF2 correlates with Hpr concentration (Fig. 2 and 4 and reference 43).
Purified TLF1 and TLF2 have specific activities of ∼10 and ∼600 ng/lytic units, respectively. Compared on a molar basis, 1 lytic unit contains 18-fold more TLF2 (1,000 kDa) than TLF1 (300-kDa protein in 500-kDa particle). The immunoaffinity-based purification of TLF1 yielded a 13- to 25-fold increase in specific activity relative to preparations obtained by other purification methods (10, 39). TLF1 is labile at 4°C, and the discrepancy between specific activities may partially reflect the more rapid purification procedure used here (4 versus 8 days). In contrast to previous reports (10, 11, 39), immunoaffinity-purified TLF1 contained no detectable paraoxonase and apoA-II by silver staining or sequencing, although low levels are observed in TLF1 fractions by a more sensitive immunoblotting method. This finding, coupled with the higher specific activity of immunoaffinity-purified TLF1, suggests that TLF1 purified by previous methods is contaminated with nonlytic HDL. The Hpr-based immunoaffinity purification may provide the best means to discriminate lytic TLF from nonlytic HDL, because Hpr appears to be required for lytic activity. A caveat of this immunoaffinity purification method is that any contaminating plasma Hp will be concentrated and will purify with TLF, although most Hp can be removed subsequently by a further round of size fractionation. HDL isolated by an immunoaffinity technique using an antibody to apoA-I (17) contained Hp (sequence of the Hp β chain) in a subset of HDL particles (ρ > 1.21 g/ml). Similar to the case for TLF1, these HDL particles lie outside the traditional HDL density range (ρ = 1.063 to 1.21 g/ml), and it is possible that Hpr was misidentified as Hp, as the β-chain N termini are identical. Although it is unlikely, we cannot rule out the possibility that Hp is a minor component of TLF1 and/or TLF2.
It is unclear how the components are arranged and held together in the TLF2 complex. Hydrophobic interactions may play a prominent role because apoA-I is an amphipathic protein, and Hpr contains an uncleaved hydrophobic signal peptide. The mechanism by which they bind to IgM is unknown. Our data indicate that TLF2-associated IgM is not clonal, since smears of both κ and λ chains were observed by SDS-PAGE, and different TLF2 preparations contain different proportions of each chain (not shown). Although not rigorously shown, it is likely that TLF2-associated IgM represents a secreted polymeric IgM and not a complexed form of monomeric IgM containing a transmembrane domain. The latter migrates with an apparent molecular weight higher than that of secreted μ chains by SDS-PAGE (9), and we do not detect any difference in the mobility of the μ chain between TLF2-associated IgM and the bulk of serum IgM.
In addition to apolipoproteins, a variety of other plasma proteins combine with HDL particles (14, 17, 30), and it is conceivable that TLF1 is formed in a similar manner by binding to Hpr. Over 95% of apoA-I in plasma is associated with HDL, while the remainder is found in the plasma fraction with a density of >1.21 g/ml. This high-density apoA-I can exist as lipid-poor or lipid-free protein (2, 18) or, in the presence of free apoA-II, can form an apoA-I/apoA-II adduct (28). Analogously, it is conceivable that it instead forms an apoA-I/Hpr adduct, which then interacts with IgM in plasma to form TLF2.
Current knowledge on the sites of synthesis of the individual protein components of the TLFs leads us to suggest that the particles are formed in the serum. Polymeric IgM is synthesized by plasma cells. Hp is primarily synthesized in the liver and to a lesser extent in adipocytes and lung epithelia (shown in mice) (7). ApoA-I is synthesized primarily in the intestine and the liver before secretion into the lymph and plasma, where it binds lipids to form HDL. The sites of Hpr synthesis are unclear (4, 26); although the promoter is active in liver cells, Hpr transcripts have not been detected. It has been noted that plasma samples from individuals suffering from liver diseases have diminished trypanocidal activities (27). Additionally, a unique form of Hpr is expressed by a variety of human tumor cells and is elevated in sera from cancer patients (40). Low levels of IgM, Hp, and lipid-free apoA-I are also found in lymph, urine, and cerebrospinal fluid; these sources have not yet been tested for trypanolytic activity (15, 23, 36).
ACKNOWLEDGMENTS
This work was supported in part by National Institutes of Health grants to J.R. (A141233) and S.T. (A140206) and by the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases.
We thank Photini Sinnis, Soren Gantt, and Amy Shore for reviewing the manuscript. We thank R. W. James for the gift of MAb F41F2-K, specific for paraoxonase.
REFERENCES
- 1.Aaronovitch S, Terry R J. The trypanolytic factor in human serum. Trans R Soc Trop Med Hyg. 1972;66:344. doi: 10.1016/0035-9203(72)90227-1. [DOI] [PubMed] [Google Scholar]
- 2.Aszalos B F, Sloop C H, Wong L, Roheim P S. Two-dimensional electrophoresis of plasma lipoproteins: recognition of new apo A-I-containing subpopulations. Biochim Biophys Acta. 1993;1169:291–300. doi: 10.1016/0005-2760(93)90253-6. [DOI] [PubMed] [Google Scholar]
- 3.Barth P. A new method for the isolation of the trypanocidal factor from normal human serum. Acta Trop. 1989;46:71–73. doi: 10.1016/0001-706x(89)90019-3. [DOI] [PubMed] [Google Scholar]
- 4.Bensi G, Raugei G, Klefenz H, Cortese R. Structure and expression of the human haptoglobin locus. EMBO J. 1985;4:119–126. doi: 10.1002/j.1460-2075.1985.tb02325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bowman B H. Hepatic plasma proteins: mechanism of function and regulation. San Diego, Calif: Academic Press, Inc.; 1993. pp. 159–167. [Google Scholar]
- 6.Cross G A M. Cellular and genetic aspects of antigenic variation in trypanosomes. Annu Rev Immunol. 1990;8:83–110. doi: 10.1146/annurev.iy.08.040190.000503. [DOI] [PubMed] [Google Scholar]
- 7.Friedrichs W E, Navarijo-Ashbaugh A L, Bowman B H, Yang F. Expression of inflammatory regulation of haptoglobin gene in adipocytes. Biochem Biophys Res Commun. 1995;209:250–256. doi: 10.1006/bbrc.1995.1496. [DOI] [PubMed] [Google Scholar]
- 8.Ghiso J, Matsubara E, Koudinov A, Choi-Miura N H, Tomita M, Wisniewski T, Frangione B. The cerebrospinal fluid soluble form of Alzheimer’s amyloid betas is complexes to SP-40,40 (apolipoprotein J), an inhibitor of the complement membrane-attack complex. Biochem J. 1993;233:27–30. doi: 10.1042/bj2930027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grant J. In: Immunological methods in bacteriology. Weir D M, editor. Oxford, England: Blackwell; 1978. [Google Scholar]
- 10.Hagar K M, Pierce M A, Moore D R, Tytler E M, Esko J D, Hajduk S L. Endocytosis of a cytotoxic human high density lipoprotein results in disruption of acidic intracellular vesicles and subsequent killing of African trypanosomes. J Cell Biol. 1994;126:155–167. doi: 10.1083/jcb.126.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hajduk S L, Moore D R, Vasudevacharya J, Siqueria H, Torri A F, Tytler E M, Esko J D. Lysis of Trypanosoma brucei by a toxic subspecies of human high density lipoprotein. J Biol Chem. 1989;264:5210–5217. [PubMed] [Google Scholar]
- 12.Hawking F. The differentiation of Trypanosoma rhodesiense from T. brucei by means of human serum. Trans R Soc Trop Med Hyg. 1973;67:517–527. doi: 10.1016/0035-9203(73)90082-5. [DOI] [PubMed] [Google Scholar]
- 13.Hawking F, Ramsden D B, Whytock S. The trypanocidal action of human serum and of baboon plasma. Trans R Soc Trop Med Hyg. 1973;67:501–516. doi: 10.1016/0035-9203(73)90081-3. [DOI] [PubMed] [Google Scholar]
- 14.James R W, Hochstrasser D, Tissot J D, Funk M, Appel R, Barja F, Pelligrini C, Muller A F, Pometta D. Protein heterogeneity of lipoprotein particles containing apolipoprotein A-I without apolipoprotein A-II and apolipoprotein A-I with apolipoprotein A-II isolated from human plasma. J Lipid Res. 1988;29:1557–1571. [PubMed] [Google Scholar]
- 15.Katnik I, Dobryszycka W. Enzyme immunoassay to measure low levels of haptoglobin in biological fluids. J Immunoassay. 1990;11:503–517. doi: 10.1080/01971529008055047. [DOI] [PubMed] [Google Scholar]
- 16.Kuhajda F P, Katumuluwa A I, Pasternack G R. Expression of haptoglobin-related protein and its potential as a tumor antigen. Proc Natl Acad Sci USA. 1989;86:1188–1192. doi: 10.1073/pnas.86.4.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kunitake S T, Carilli C T, Lau K, Protter A A, Naya-Vigne J, Kane J P. Identification of proteins associated with apolipoprotein A-I containing lipoproteins purified by selected affinity immunosorption. Biochemistry. 1994;33:1988–1993. doi: 10.1021/bi00174a003. [DOI] [PubMed] [Google Scholar]
- 18.Kunitake S T, La Sala K J, Kane J P. Apolipoprotein A-I-containing lipoproteins with pre-beta electrophoretic mobility. J Lipid Res. 1985;26:549–555. [PubMed] [Google Scholar]
- 19.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:630–634. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 20.Lorenz P, Betschart B, Owen J S. Trypanosoma brucei brucei and high-density lipoproteins: old and new thoughts on the identity and mechanism of the trypanocidal factor in human serum. Parasitol Today. 1995;11:348–352. doi: 10.1016/0169-4758(95)80191-x. [DOI] [PubMed] [Google Scholar]
- 21.Lorenz P, James R W, Owen J S, Betschart B. Heterogeneity in the properties of the trypanolytic factor in normal human serum. Mol Biochem Parasitol. 1994;64:153–164. doi: 10.1016/0166-6851(94)90143-0. [DOI] [PubMed] [Google Scholar]
- 22.Marsh J B, Weinstein D B. Simple charring method for determination of lipids. J Lipid Res. 1966;7:574–576. [PubMed] [Google Scholar]
- 23.Mashige F, Shimizu T, Iijima S, Ohkubo A. Analysis for cerebrospinal fluid proteins by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Clin Chem. 1992;38:2008–2012. [PubMed] [Google Scholar]
- 24.McEvoy S M, Maeda N. Complex events in the evolution of the haptoglobin gene cluster in primates. J Biol Chem. 1988;263:15740–15747. [PubMed] [Google Scholar]
- 25.Muranjan M, Nussenzweig V, Tomlinson S. Characterization of the human serum trypanosome toxin, haptoglobin related protein. J Biol Chem. 1998;273:3884–3887. doi: 10.1074/jbc.273.7.3884. [DOI] [PubMed] [Google Scholar]
- 26.Oliviero S, Morrone G, Cortese R. The human haptoglobin gene: transcriptional regulation during development and acute phase induction. EMBO J. 1987;6:1905–1912. doi: 10.1002/j.1460-2075.1987.tb02450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ormerod W E, Venkatesan S. Similarities of lipid metabolism in mammalian and protozoan cells: an evolutionary hypothesis for the prevalence of atheroma. Microbiol Rev. 1982;46:296–307. doi: 10.1128/mr.46.3.296-307.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Osborne J C, Brewer J H B. Solution properties of the plasma lipoproteins. Ann N Y Acad Sci. 1980;348:104–121. doi: 10.1111/j.1749-6632.1980.tb21294.x. [DOI] [PubMed] [Google Scholar]
- 29.Owen J S, Lorenz P, Betschart B. HDL particles as the trypanosome-killing factor in human serum: an exclusive or inconclusive role? Parasitol Today. 1996;12:250–251. doi: 10.1016/0169-4758(96)80814-3. [DOI] [PubMed] [Google Scholar]
- 30.Park C T, Wright S D. Plasma lipopolysaccharide-binding protein is found associated with a particle containing apolipoprotein A-I, phospholipid, and factor H-related proteins. J Biol Chem. 1996;271:18054–18080. doi: 10.1074/jbc.271.30.18054. [DOI] [PubMed] [Google Scholar]
- 31.Poumay Y, Ronveaux-Dupal M-F. Rapid preparative isolation of concentrated low density lipoproteins and of lipoprotein-deficient serum using vertical rotor gradient ultracentrifugation. J Lipid Res. 1985;26:1476–1480. [PubMed] [Google Scholar]
- 32.Raper J, Nussenzweig V, Tomlinson S. The main lytic factor of Trypanosoma b. brucei in normal human serum is not high density lipoprotein. J Exp Med. 1996;183:1023–1029. doi: 10.1084/jem.183.3.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rifkin M R. Identification of the trypanocidal factor in normal human serum: high density lipoprotein. Proc Natl Acad Sci USA. 1978;75:3450–3454. doi: 10.1073/pnas.75.7.3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rifkin M R. Trypanosoma brucei: some properties of the cytotoxic reaction induced by normal human serum. Exp Parasitol. 1978;46:189–206. doi: 10.1016/0014-4894(78)90131-5. [DOI] [PubMed] [Google Scholar]
- 35.Rifkin M R, De Greef C, Jiwa A, Landsberger F R, Shapiro S Z. Human serum-sensitive Trypanosoma brucei rhodesiense: a comparison with serologically identical human serum-resistant clones. Mol Biochem Parasitol. 1994;66:211–220. doi: 10.1016/0166-6851(94)90148-1. [DOI] [PubMed] [Google Scholar]
- 36.Roheim P S, Edelstein D, Pinter G G. Apolipoproteins in rat serum and renal lymph. Proc Natl Acad Sci USA. 1976;73:1757–1760. doi: 10.1073/pnas.73.5.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seed J R, Sechelski J B, Loomis M R. A survey for a trypanocidal factor in primate sera. J Protozool. 1990;37:393–400. doi: 10.1111/j.1550-7408.1990.tb01163.x. [DOI] [PubMed] [Google Scholar]
- 38.Smith A B, Hajduk S L. Identification of haptoglobin as a natural inhibitor of trypanocidal activity in human serum. Proc Natl Acad Sci USA. 1995;92:10262–10266. doi: 10.1073/pnas.92.22.10262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith A B, Esko J D, Hajduk S L. Killing of trypanosomes by human haptoglobin-related protein. Science. 1995;268:284–286. doi: 10.1126/science.7716520. [DOI] [PubMed] [Google Scholar]
- 40.Taback S, Lev C, Valansi C, Aker O, Shalitin C. Transcriptionally active haptoglobin-related (Hpr) gene in hepatoma G2 and leukemia Molt-4 cells. Mol Cell Biol. 1996;15:1001–1007. doi: 10.1089/dna.1996.15.1001. [DOI] [PubMed] [Google Scholar]
- 41.Tomlinson S, Raper J. The lysis of Trypanosoma brucei brucei by human serum. Nat Biotechnol. 1996;14:717–721. doi: 10.1038/nbt0696-717. [DOI] [PubMed] [Google Scholar]
- 42.Tomlinson S, Jansen A M, Koudinov A, Ghiso J A, Choi-Miura N H, Rifkin M R, Ohtaki S, Nussenzweig V. High-density-lipoprotein-independent killing of Trypanosoma brucei brucei by human serum. Mol Biochem Parasitol. 1995;70:131–138. doi: 10.1016/0166-6851(95)00019-w. [DOI] [PubMed] [Google Scholar]
- 43.Tomlinson S, Muranjan M, Nussenzweig V, Raper J. Haptoglobin-related protein and apolipoprotein AI are components of the two trypanolytic factors in human serum. Mol Biochem Parasitol. 1997;86:117–120. [PubMed] [Google Scholar]
- 44.Towbin H, Staehelin T, Gordon M. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1978;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.von Eckardstein A, Chirazi A, Schuler-Luttmann S, Walter M, Kastelein J J P, Geisel J, Real J T, Miccoli R, Noseda G, Hobbel G, Assmann G. Plasma and fibroblasts of Tangier disease patients are disturbed in transferring phospholipids onto apolipoprotein A-I. J Lipid Res. 1998;39:987–998. [PubMed] [Google Scholar]
- 46.Wray W, Boulikas T, Wray V P, Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981;188:197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]
- 47.Zech R, Rockseisen M, Kluge K, Dewald K, Armstrong V W, Chemnitius J M. Lipoproteins and hydrolysis of organophosphorus compounds. Chem Biochem Int. 1987;87:85–94. doi: 10.1016/0009-2797(93)90028-w. [DOI] [PubMed] [Google Scholar]