Abstract
Bacteria persisting in periodontal pockets are exposed to elevated temperatures during periods of inflammation. Temperature is an environmental factor that can modulate gene expression. Consequently, in the present study we examined the effect of temperature on the expression of virulence determinants by the periodontopathogen, Porphyromonas gingivalis. P. gingivalis W50 was grown in a complex medium under hemin excess at pH 7.0 and at a constant temperature of either 37, 39, or 41°C; cultures were monitored for protease and hemagglutinin activity. P. gingivalis grew well at all three temperatures. An increase in growth temperature from 37 to 39°C resulted in a 65% reduction in both total arginine- and lysine-specific activities (P < 0.01). A further rise in growth temperature to 41°C led to even greater reductions in arginine-specific (82%; P < 0.001) and lysine-specific (73%; P < 0.01) activities. These reductions were also associated with an altered distribution of individual arginine-specific enzyme isoforms. At 41°C, there was a disproportionate reduction in the level of the heterodimeric RI protease, which also contains adhesin domains. The reduction also correlated with a markedly diminished hemagglutination activity of cells, especially in those grown at 41°C, and a reduced immunoreactivity with a monoclonal antibody which recognizes gene products involved in hemagglutination. Thus, as the environmental temperature increased, P. gingivalis adopted a less aggressive phenotype, while retaining cell population levels. The coordinate down-regulation of virulence gene expression in response to an environmental cue linked to the intensity of the host inflammatory response is consistent with the clinically observed cyclical nature of disease progression in periodontal diseases.
Periodontal diseases are a group of chronic inflammatory conditions of the supporting tissues of the teeth. The microflora associated with these diseases is diverse (13, 31), but Porphyromonas gingivalis is considered to be one of the most significant components and produces a range of putative virulence determinants including proteases, hemagglutinins, and lipopolysaccharide (8, 9, 15, 21, 26). This organism is usually absent or undetectable in health but is frequently a major component of the subgingival plaque in disease (13, 19). This suggests that under the environmental conditions which prevail during the host inflammatory response, P. gingivalis is able to outcompete other members of the subgingival microflora and reach significant levels within the microbial community. Temperature is one of the factors which is known to change as a consequence of an increased inflammatory response (32), especially in sites such as periodontal pockets (11). The aim of the present study was to determine the effect of temperature on the expression of some of the proposed virulence determinants of P. gingivalis that have received most attention recently, namely the arginine- and lysine-specific proteases and hemagglutination activity.
MATERIALS AND METHODS
Bacterial strain and growth conditions.
Porphyromonas gingivalis W50 was grown in a 2-liter capacity chemostat (FT Applikon, Schiedam, The Netherlands) operated at a working volume of 700 ml. The pH of the culture was maintained at 7.0 (± 0.1) by the automatic addition of 1 M NaOH and 0.5 M HCl, and the temperature was controlled at 37°C (± 0.1°C). The culture vessel was sparged with a gas mixture of oxygen-free nitrogen (95% [vol/vol]) and carbon dioxide (5% [vol/vol]) to maintain anaerobic conditions; once bacterial growth was initiated, the Eh of the culture fell to −350 mV, and this value was maintained throughout the cultivation process. The medium was BHI (brain heart infusion broth; Oxoid) supplemented with 5 mg of hemin (Sigma)/liter to achieve hemin excess levels. The medium flow rate was adjusted to give a dilution rate (D) of 0.1 h−1, corresponding to a mean generation time of 6.9 h. P. gingivalis W50 was grown to late-logarithmic phase in anaerobic batch culture at 37°C with the same growth medium, and 100 ml of the culture was used to inoculate the chemostat. The medium was introduced initially at a very slow rate and left overnight to reach the required working volume of 700 ml; once this value was attained, the medium flow rate was increased to give the desired D (0.1 h−1). In subsequent experiments, chemostat cultures were started at 37°C (± 0.1°C) prior to increasing the temperature to either 39 (± 0.1) or 41°C (± 0.1°C). At each temperature, the chemostat was allowed to achieve a steady state (10 culture volume changes, i.e., approximately 4 days) after inoculation, and samples were taken from steady-state cultures for analysis over 6 days.
Estimation of biomass.
The biomass of the culture was determined by daily measurements of the optical density at 540 nm (B105 UV/VIS spectrophotometer; Jenway) dry weight, and viable counts of the culture, as previously described (20).
Measurements of enzyme activity.
At each steady state, a small volume of the culture was removed directly from the chemostat during growth at 37, 39, and 41°C to test for arginine-specific and lysine-specific enzyme activities of P. gingivalis. Some of the removed culture was centrifuged at 11,600 × g at 4°C in a microcentrifuge (MSE Microcentaur; Sanyo), and both whole culture and supernatant were tested for enzyme activity. Activity was measured in 1 ml of 0.1 M Tris-HCl–10 mM l-cysteine–10 mM CaCl2, pH 8.0, containing 0.5 mM dl-BApNA (N-α-benzoyl-dl-arginine-p-nitroanilide; Sigma) or 0.25 mM AcLyspNA (N-α-acetyl-lysine-p-nitroanilide; Sigma) at 30°C, as described previously (28), by using a spectrophotometer supplied with an enzyme kinetic program (UV1601; Shimadzu) and a temperature-controlled cuvette chamber. One unit of enzyme activity is defined as that amount of enzyme which hydrolyzes 1 nmol of substrate under the conditions of the assay per min.
Separation of arginine-specific protease isoforms.
The extracellular arginine-specific proteases of P. gingivalis W50 exist in several isoforms, referred to as RI, RIA, and RIB, which are all derived from prpR1 (27). Therefore, the effect of temperature on the distribution of these isoforms was studied. One liter of culture was collected on ice overnight from the chemostat outflow and then centrifuged at 10,000 × g for 60 min at 4°C (Sorvall RC-5B refrigerated centrifuge). The pelleted cells were stored at −85°C, and the supernatant was brought to 85% saturation by the addition of solid ammonium sulfate with continuous mixing at 4°C overnight for complete precipitation of the enzyme. The mixture was then centrifuged as described above, and the supernatant was assayed to confirm complete precipitation of enzyme activity. The pellet was first extracted with 50 mM sodium acetate buffer, pH 5.3, containing 0.0055% (wt/vol) 3-14 Zwittergent (Calbiochem) to solubilize RI and RIA enzyme activities, which were separated from insoluble material by centrifugation. RI and RIA were then separated by an affinity chromatography step as described previously (28). RIB activity in the pellet fraction was solubilized by repeated extraction with 50 mM sodium acetate buffer containing 0.05% (wt/vol) Zwittergent.
Total RNA extraction for Northern blot analysis of prpR1 gene.
Fresh P. gingivalis culture (1.5 ml) was removed directly from the chemostat at each steady state and centrifuged at 11,600 × g in a microcentrifuge at 4°C for 5 min. The pelleted cells were mixed with 1 ml of Total Isolation Reagent (Advanced Biotechnologies Ltd, Surrey, United Kingdom), and RNA was prepared in accordance with the manufacturer’s protocol. Finally, RNA was resuspended in 50 μl of diethyl pyrocurbonate-treated water at 65°C for 5 min (27). RNA was resolved in a denaturing 1% agarose-formaldehyde gel by electrophoresis (29) and then transferred to a Hybond N+ membrane (Amersham) by vacuum blotting (Vacu-Aid; Hybaid). The membrane was then probed with a 32P-labelled DNA probe specific for the coding region of the catalytic (α) domain of the protease. Labelling of an agarose gel-purified 270-bp SphI-EcoRV fragment of the α-encoding domain of prpR1 with [α-32P]dCTP and the hybridization, washing, and autoradiography conditions have been described previously (1).
Hemagglutination of RBC with P. gingivalis.
Blood from a healthy volunteer was removed by venipuncture into 1.6 mg ml−1 of EDTA. The collected blood was centrifuged at 1,200 × g for 5 min and the supernatant was discarded. Erythrocytes (RBC) were washed once with phosphate-buffered saline (PBS)–10 mM EDTA and twice with PBS. An approximate 5% RBC solution in PBS was prepared and stored at 4°C until needed. To establish the minimum hemagglutination dose (MHD), 50 μl of doubling dilutions of supernatants of P. gingivalis cell culture grown under steady state conditions at 37, 39, and 41°C were prepared in a 96-well microtiter plate. Equal volumes of a 0.5% solution prepared from RBC stock solution and PBS were added to each well followed by incubation at room temperature for 2 h. The MHD was the highest dilution of the supernatant that caused visible hemagglutination.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis was performed on 12% polyacrylamide gels according to the method of Laemmli (18). Western blotting, with monoclonal antibody 1A1, has been described previously (7). Control blot assays were performed with rabbit antiserum raised to a P. gingivalis 47-kDa surface antigen which was purified as described previously (4).
Statistical analysis.
Data were expressed as means ± standard deviations. Differences among means were analyzed for statistical significance by Student’s t test.
RESULTS
Growth of P. gingivalis at different temperatures.
P. gingivalis W50 was able to grow well at each of the temperatures tested. Optimal growth was at 37°C as judged by dry weight and viable counts (Table 1). Increases in temperature to 39 and 41°C resulted in significant reductions in culture dry weight (P < 0.05 and P = 0.001, respectively), although viable counts were not significantly affected. Growth was stable at each temperature as indicated by the low standard deviations in viable count and dry weight at each steady state (Table 1).
TABLE 1.
Effect of temperature on the growth characteristics of P. gingivalis W50
| Growth temp (°C) | Mean (± SD) dry wt (mg ml−1[n = 6])a | Mean (± SD) viable counts (CFU ml−1[n = 6]) |
|---|---|---|
| 37 | 2.8 ± 0.4 | 1.5 × 109 ± 0.6 × 109 |
| 39 | 2.2 ± 0.5* | 1.4 × 109 ± 0.4 × 109 |
| 41 | 1.8 ± 0.4** | 1.3 × 109 ± 0.5 × 109 |
*, P < 0.05 for results at 37°C versus results at 39°C; **, P = 0.001 for results at 37°C versus results at 41°C.
Effect of growth temperature on protease activity.
Increasing the growth temperature led to a decrease in the total arginine- and lysine-specific protease activities of whole cultures and cell-free supernatant of P. gingivalis W50 (Table 2). Maximum activities were obtained during steady-state growth at 37°C. When the temperature was increased to 39°C the total arginine- and lysine-specific enzyme activities were reduced significantly (P < 0.01) to 33 and 35%, respectively, of their levels at 37°C. There was also a significant reduction in both enzyme activities in cell-free supernatants (P < 0.01). The ratio of total arginine-specific to lysine-specific activities remained the same, at 37 and 39°C, as did the ratio of whole culture to supernatant lysine-specific activity at both temperatures. However, the ratio of arginine-specific activity of whole culture to that of the supernatant increased from 4:1 at 37°C to 5:1 at 39°C (Table 2).
TABLE 2.
Effect of growth temperature on protease activity of P. gingivalis W50a
| Growth temp (°C) | Mean (± SD) arginine-specific activity (nmol mg−1 min−1[n = 12])
|
Mean (± SD) lysine-specific activity (nmol mg−1 min−1[n = 12])
|
||
|---|---|---|---|---|
| W | S | W | S | |
| 37 | 325 ± 74 | 79 ± 42 | 62 ± 18 | 15 ± 8 |
| 39 | 109 ± 59* | 21 ± 9* | 22 ± 10* | 6 ± 2* |
| 41 | 58 ± 16** | 15 ± 5** | 17 ± 3** | 7 ± 1** |
W, whole culture; S, supernatant; *, P < 0.01 for results at 37°C versus results at 39°C; **, P < 0.001 for results at 37°C versus results at 41°C.
An increase in growth temperature to 41°C caused a further decrease in protease activity (Table 2). Compared with the data obtained at 37°C, the total arginine-specific and lysine-specific activities were reduced by 82 and 73%, respectively (P < 0.001). The ratio of arginine-specific to lysine-specific activities shifted from approximately 5:1 at 37°C to 3:1 at 41°C.
Distribution of arginine-specific protease isoforms.
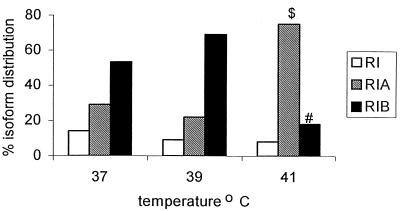
In addition to an effect on total arginine specific protease activity, temperature also influenced the relative proportions of the different isoforms. The effect of growth temperature on the distribution of the three isoforms of PrpRI (RI, RIA, and RIB) (28) is presented in Fig. 1. At growth temperatures of 37 and 39°C the three isoforms of the arginine-specific protease exhibited a similar distribution. However, at 41°C the proportion of the monomer, RIA, was significantly increased (P < 0.01) and there was a corresponding significant decrease (P < 0.05) in the proportion of the vesicle and membrane-associated RIB enzyme at this temperature compared to those at 37 and 39°C.
FIG. 1.
Effect of temperature on the distribution of RI, RIA, and RIB protease isoforms in the supernatant of P. gingivalis W50 at different growth temperatures. $, P < 0.01 for results at 41°C versus results at 37 and 39°C; #, P < 0.05 for results at 41°C versus results at 37 and 39°C.
Northern blot analysis of prpR1 gene.
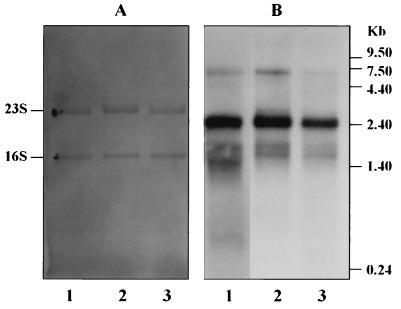
Northern analysis of mRNA from the three growth temperatures is shown in Fig. 2. Prior to hybridization, membranes were briefly stained with 0.04% methylene blue in 0.5 M Na-acetate, pH 6.0 (27), to confirm equal loading and transfer. The probe used in these studies hybridizes to transcripts derived from both prpR1 (approximately 6 kb) and a second arginine-specific protease gene, prR2 (approximately 2.5 kb), the products of which are cell-associated enzymes in this strain (28). Transcripts from both loci were detected in the continuously grown P. gingivalis cell extracts, with full-length prpR1 forming a relatively small percentage of the total arginine-specific protease message; a similar profile occurs in batch-grown cells (27). However, an increase in temperature to 41°C caused a greater reduction in prpR1 message compared to that from prR2. This may reflect the effect of increasing temperature on either the production or the rate of decay of prpR1 message.
FIG. 2.
Analysis of prpR1 and prR2 mRNA in steady-state cells of P. gingivalis W50 at different growth temperatures. (A) Methylene blue stain of total RNA to confirm equivalent sample loadings. (B) Northern blot of membrane in panel A following hybridization with 32P-labelled DNA probe derived from the α-coding domain of prpR1. Lanes 1, cells grown at 37°C; lanes 2, cells grown at 39°C; lanes 3, cells grown at 41°C. Full-length prpR1 transcript is approximately 6 kb and prR2 transcript is 2.5 kb.
Hemagglutination of RBCs with P. gingivalis.
The ability of P. gingivalis culture supernatants from the three different growth temperatures to hemagglutinate RBCs is presented in Table 3. The highest hemagglutination titers were observed when P. gingivalis was grown at 37°C (1:256). Growth at 39°C resulted in a onefold reduction in hemagglutination titer of the culture supernatant (1:128). At a growth temperature of 41°C, however, a threefold reduction of hemagglutination end point (1:32 dilution) was observed.
TABLE 3.
Effect of growth temperature on the hemagglutinin activity of culture supernatants of P. gingivalis W50
| Growth temp (°C) | Hemagglutination end point |
|---|---|
| 37 | 1:256 |
| 39 | 1:128 |
| 41 | 1:32 |
Western blot analysis.
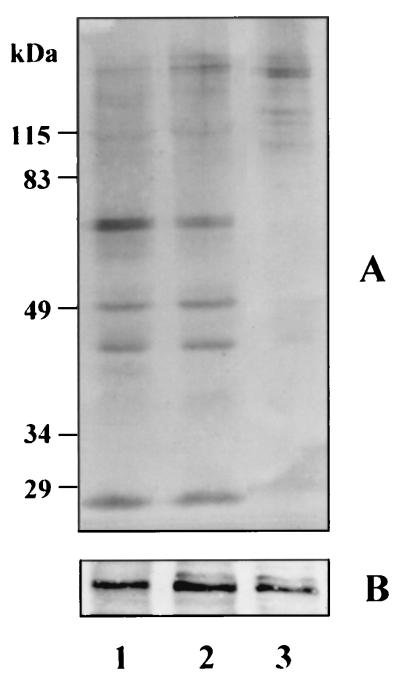
The monoclonal antibody, 1A1, reacted with multiple bands on Western blots of P. gingivalis cells grown at 37°C (Fig. 3A), which is consistent with our previous observations that this antibody recognizes a determinant present on multiple gene products of this organism (7). Because of proteolytic processing of the products of these genes it is not possible to assign an identity unequivocally to each protein band. However, from previous studies (7) the band at approximately 50 kDa most likely represents the adhesin chain of the RI protease heterodimer. Growth at higher temperatures resulted in a progressive decrease in immunoreactive components, and this was particularly evident in cells grown at 41°C, suggesting that increasing temperature may coordinately down-regulate this family of sequence and antigenically related gene products.
FIG. 3.
Western blot analysis of P. gingivalis W50 grown at different temperatures with monoclonal antibody 1A1 (A) and rabbit antiserum to a P. gingivalis 47-kDa surface antigen (B). Lane 1, cells grown at 37°C; lane 2, cells grown at 39°C; lane 3, cells grown at 41°C.
In order to examine whether this response represents a general phenomenon which affects all cell surface and/or extracellular proteins, we also examined the level of expression of a 47-kDa antigen, which is a novel surface-associated glutamate dehydrogenase of P. gingivalis (17). There was no detectable difference in the level of this protein in P. gingivalis cells grown at different temperatures on the basis of reactivity with rabbit antiserum on Western blots (Fig. 3B). Hence, there appears to be selective effects on surface protein expression in response to increases in the growth temperature of this organism.
DISCUSSION
Periodontal disease is a result of a dynamic and multifactorial interaction between the subgingival microbial community and the host, the outcome of which is a destructive inflammatory response (25). The role of individual organisms is complex. For example, P. gingivalis produces a number of potential virulence factors which may contribute to tissue damage by both direct and indirect mechanisms. However, the proteolytic activities of this organism appear to be of particular relevance to perturbation of tissue homeostasis (15, 21, 26). In particular, proteases with specificity for arginyl and lysyl peptide bonds are able to proteolytically inactivate a number of key host proteins involved in the control of the inflammatory response and the innate and specific host defences.
Recent studies have emphasized the potential importance of arginine-specific proteases of P. gingivalis. In vitro studies have suggested a role in the subversion of the opsono-phagocytic capacity of complement through the fluid phase proteolytic inactivation of C3 and C5 and the concomitant release of the chemoattractant C5a (33). Furthermore, proteolytic cleavage at arginyl peptide bonds by this organism leads to the degradation of kininogen and the production of the vasoactive peptide, bradykinin (16). In addition, proteolysis may lead to the inactivation of host protease inhibitors (6, 23) that are central to the control of plasma cascades involved in clotting and fibrinolysis. Finally, the lysine-specific proteases of P. gingivalis are able to prevent clot formation by the proteolysis of fibrinogen (30), and proteases of P. gingivalis are also able to degrade immunoglobulins, iron-binding proteins (15), and antimicrobial peptides (10). The likely net effect of these activities is a deregulation of the local inflammatory response and a disruption of the host defenses to this and other subgingival organisms. Thus, P. gingivalis could play a pivotal role in determining the effectiveness of the host response to the microbial challenge and the inflammatory status at subgingival sites.
It is well established that expression of bacterial virulence determinants is modulated by changes in environmental conditions. The transition from health to disease at subgingival sites is accompanied by marked changes in key environmental parameters including nutrient profile, pH, Eh, and temperature. Although it has been shown previously (20, 22) that pH and nutrients regulate protease production by P. gingivalis, this is the first study to examine the effect of temperature. An increase in temperature had little impact on the growth of P. gingivalis W50 but had significant effects on arginine- and lysine-specific protease activities and hemagglutination. Increasing the growth temperature from 37 to 39°C resulted in approximately a 65% reduction (P < 0.01) in total activity of both proteases, and a further increase in growth temperature to 41°C led to reductions of 82 and 73% in the arginine-specific and lysine-specific activities, respectively (P < 0.001).
The change in total arginine protease activity was associated with an altered distribution of the individual enzyme isoforms. Previous investigations have demonstrated that the extracellular arginine specific protease activity of P. gingivalis W50 is composed of three forms (RI, RIA, and RIB) which are all derived from prpR1 (27). RI is a heterodimeric enzyme comprising the catalytic (α) polypeptide in noncovalent association with a second (β) adhesin polypeptide which is derived from the C-terminal extension of the PrpRI initial translation product. RIA is the free catalytic α polypeptide and RIB is a highly post-translationally modified form of the α polypeptide which appears to contain approximately 30% (wt/wt) carbohydrate. While RI and RIA are both soluble, RIB is exclusively located in the extracellular vesicles and membrane fragments of the culture supernatant. The factors which govern the formation of these three isoforms from the same gene are unknown, although Northern blot analysis of P. gingivalis mRNA suggests that the monomeric enzymes may result from translation of a 3′ truncated transcript of prpR1 (27). In the present study there was a disproportionate reduction in the level of the heterodimeric RI protease during growth at 41°C. This enzyme has a number of properties in addition to proteolysis which are mediated through the adhesin chain of the dimer. For example, monoclonal antibodies to a determinant within this adhesin domain block hemagglutination by P. gingivalis whole cells in vitro (7) and are able to retard recolonization of deep periodontal pockets in patients following passive immunization (5).
The coding sequence for the β adhesin chain of RI has been identified in several other genes of P. gingivalis including kgp, tla, and hagA, which code for a lysine-specific protease precursor, an outer membrane receptor involved in hemin utilization, and a high-molecular-weight hemagglutinin, respectively (2, 14, 24). The epitope for the monoclonal antibody, 1A1, used in the current investigation is present in the products of all of these genes. Growth at elevated temperature led to a significant decrease in the immunoreactivity of whole cells with monoclonal antibody 1A1, and this was particularly evident at 41°C. This loss of expression of the monoclonal antibody 1A1-reactive proteins at higher growth temperatures is consistent with the lowered hemagglutination activity of these cultures and the decreased arginine- and lysine-specific protease activities (derived from prpR1 and kgp, respectively) and may reflect a coordinate down-regulation of the other members of this family of sequence-related genes.
Temperature has been shown to down-regulate the expression of another binding determinant in P. gingivalis W50. Amano et al. (3) showed that a shift in temperature from 37 to 39°C resulted in a 54% reduction in the amount of fimbriae as well as in decreased expression of mRNA for fimA. Likewise, a decrease in growth temperature from 39 to 34°C resulted in an 11-fold increase in activity of the fimA promoter of P. gingivalis and an increased ability of P. gingivalis to adhere to Streptococcus gordonii and to invade primary cultures of gingival epithelial cells (34). These findings suggest that P. gingivalis responds to a rise in temperature by down-regulating the expression of determinants involved in surface binding.
At first sight it might appear surprising that P. gingivalis down-regulates the expression of determinants that are associated with its pathogenicity under conditions that are present during active disease. However, this may represent a key strategy by which this organism is able to maintain itself under the hostile conditions of an inflamed periodontal pocket. Even at a temperature of 41°C there was no reduction in the viable count of P. gingivalis, suggesting that it is well adapted for growth at these elevated temperatures. Since the proteases have been shown to deregulate the inflammatory response, decreased expression of the proteases during growth at elevated temperatures may enable the organism to lessen the intensity of this host response by preserving the integrity of the host controlling mechanisms.
Collectively, the data suggest P. gingivalis responds to an increased temperature by adopting a less inflammatory and aggressive phenotype while retaining its population levels. This phenotypic response also includes other survival strategies, such as reduced adhesion to host substrates (3, 34), thereby enhancing the potential for cells to spread to other, more favorable sites, and an increase in the expression of superoxide dismutase (3), which may protect against the oxidative burst of neutrophils. The coordinate down-regulation of virulence gene expression in response to environmental cues linked to the intensity of inflammation is consistent with the clinically observed cyclical nature of disease progression (12).
ACKNOWLEDGMENT
This work was supported in part by the Medical Research Council of Great Britain (PG9318173).
REFERENCES
- 1.Aduse-Opoku J, Muir J, Slaney J M, Rangarajan M, Curtis M A. Characterization, genetic analysis, and expression studies of a protease antigen (PrpRI) of Porphyromonas gingivalis. Infect Immun. 1995;63:4744–4754. doi: 10.1128/iai.63.12.4744-4754.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aduse-Opoku J, Slaney J M, Rangarajan M, Muir J, Young K A, Curtis M A. The Tla protein of Porphyromonas gingivalis W50: a homolog of the RI protease precursor (PrpRI) is an outer membrane receptor required for growth on low levels of hemin. J Bacteriol. 1997;179:4778–4788. doi: 10.1128/jb.179.15.4778-4788.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amano A, Sharma A, Sojar H T, Kuramitsu H K, Genco R J. Effects of temperature stress and expression of fimbriae and superoxide dismutase by Porphyromonas gingivalis. Infect Immun. 1994;62:4682–4685. doi: 10.1128/iai.62.10.4682-4685.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benjamin P A, Rogers P A, U S, Johnson N W, Cole M F, Curtis M A. Increased titre and avidity of IgG antibodies to Porphyromonas gingivalis whole cells and a cell surface protein in subjects with adult periodontitis. J Periodont Res. 1997;32:31–39. doi: 10.1111/j.1600-0765.1997.tb01379.x. [DOI] [PubMed] [Google Scholar]
- 5.Booth V, Ashley F P, Lehner T. Passive immunization with monoclonal antibodies against Porphyromonas gingivalis in patients with periodontitis. Infect Immun. 1996;64:422–427. doi: 10.1128/iai.64.2.422-427.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carlsson J, Herrman B F, Hoflung J F, Sundqvist G K. Degradation of the human proteinase inhibitors alpha-1 antitrypsin and alpha-2 macroglobulin by black-pigmented Bacteroides species. J Med Microbiol. 1984;18:39–46. doi: 10.1099/00222615-18-1-39. [DOI] [PubMed] [Google Scholar]
- 7.Curtis M A, Aduse-Opoku J, Slaney J M, Rangarajan M, Booth V, Cridland J, Shepherd P. Characterization of an adherence and antigenic determinant of the RI protease of Porphyromonas gingivalis which is present on multiple gene products. Infect Immun. 1996;64:2532–2539. doi: 10.1128/iai.64.7.2532-2539.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Curtis M A. Analysis of the protease and adhesin domains of the PrpRI of Porphyromonas gingivalis. J Periodont Res. 1997;32:133–139. doi: 10.1111/j.1600-0765.1997.tb01394.x. [DOI] [PubMed] [Google Scholar]
- 9.Darveau R P, Cunningham M D, Bailey T, Seachord C, Ratcliffe K, Bainbridge B, Dietsch M, Page R C, Aruffo A. Ability of bacteria associated with chronic inflammatory disease to stimulate E-selectin expression and promote neutrophil adhesion. Infect Immun. 1995;63:1311–1317. doi: 10.1128/iai.63.4.1311-1317.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Devine, D., P. D. Marsh, R. S. Percival, M. Rangarajan, and M. A. Curtis. Modulation of antimicrobial peptide activity by products of Porphyromonas gingivalis and Prevotella spp. Microbiology, in press. [DOI] [PubMed]
- 11.Fedi P F, Jr, Killoy W J. Temperature differences at periodontal sites in health and disease. J Periodontol. 1992;63:24–27. doi: 10.1902/jop.1992.63.1.24. [DOI] [PubMed] [Google Scholar]
- 12.Goodson J M, Tanner A C R, Haffajee A D, Sornberger G C, Socransky S S. Patterns of progression and regression of advanced destructive periodontal disease. J Clin Periodontol. 1982;9:472–481. doi: 10.1111/j.1600-051x.1982.tb02108.x. [DOI] [PubMed] [Google Scholar]
- 13.Haffajee A D, Socransky S S. Microbial etiological agents of destructive periodontal diseases. Periodontology 2000. 1994;5:78–111. doi: 10.1111/j.1600-0757.1994.tb00020.x. [DOI] [PubMed] [Google Scholar]
- 14.Han N M, Whitlock J, Progulske-Fox A. The hemagglutinin gene (hagA) of Porphyromonas gingivalis 381 contains four large, contiguous direct repeats. Infect Immun. 1996;64:4000–4007. doi: 10.1128/iai.64.10.4000-4007.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holt S C, Bramanti T E. Factors in virulence expression and their role in periodontal disease pathogenesis. Crit Rev Oral Biol Med. 1991;2:177–281. doi: 10.1177/10454411910020020301. [DOI] [PubMed] [Google Scholar]
- 16.Imamura T, Pike R N, Potempa J, Travis J. Pathogenesis of periodontitis: a major arginine-specific cysteine proteinase from Porphyromonas gingivalis induces vascular permeability enhancement through activation of kallikrein/kinin pathway. J Clin Investig. 1994;94:361–367. doi: 10.1172/JCI117330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joe A, Murray C S, McBride B C. Nucleotide sequence of a Porphyromonas gingivalis gene encoding a surface-associated glutamate dehydrogenase and construction of a glutamate dehydrogenase-deficient isogenic mutant. Infect Immun. 1994;62:1358–1368. doi: 10.1128/iai.62.4.1358-1368.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 19.Maiden M J F, Carman R J, Curtis M A, Gillett I R, Griffiths G S, Sterne J A C, Wilton J M A, Johnson N W. Detection of high-risk groups and individuals for periodontal diseases: laboratory markers based on the microbiological analysis of subgingival plaque. J Clin Periodontol. 1990;17:1–13. doi: 10.1111/j.1600-051x.1990.tb01040.x. [DOI] [PubMed] [Google Scholar]
- 20.Marsh P D, McDermid A S, McKee A S, Baskerville A. The effect of growth rate and haemin on the virulence and proteolytic activity of Porphyromonas gingivalis W50. Microbiology. 1994;140:861–865. doi: 10.1099/00221287-140-4-861. [DOI] [PubMed] [Google Scholar]
- 21.Mayrand D, Holt S C. Biology of asaccharolytic black pigmenting Bacteroides species. Microbiol Rev. 1988;52:134–152. doi: 10.1128/mr.52.1.134-152.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McDermid A S, McKee A S, Marsh P D. Effect of environmental pH on enzyme activity and growth of Bacteroides gingivalis W50. Infect Immun. 1988;56:1096–1100. doi: 10.1128/iai.56.5.1096-1100.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nilsson T, Carlsson J, Sunqvist G. Inactivation of key factors of the plasma proteinase cascade systems by Bacteroides gingivalis. Infect Immun. 1985;50:467–471. doi: 10.1128/iai.50.2.467-471.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okamoto K, Kadowaki T, Nakayama K, Yamamoto K. Cloning and sequencing of the gene encoding a novel lysine-specific cysteine protease (Lys-gingipain) in Porphyromonas gingivalis: structural relationship with the arginine specific cysteine protease (Arg-gingipain) J Biochem. 1996;120:398–406. doi: 10.1093/oxfordjournals.jbchem.a021426. [DOI] [PubMed] [Google Scholar]
- 25.Page R C, Kornman K S. The pathogenesis of periodontitis: an introduction. Periodontology 2000. 1997;14:9–11. doi: 10.1111/j.1600-0757.1997.tb00189.x. [DOI] [PubMed] [Google Scholar]
- 26.Potempa J, Travis J. Porphyromonas gingivalis proteinases in periodontitis, a review. Acta Biochim Pol. 1996;43:455–466. [PubMed] [Google Scholar]
- 27.Rangarajan M, Aduse-Opoku J, Slaney J M, Young K A, Curtis M A. The prpR1 and prR2 arginine-specific protease genes of Porphyromonas gingivalis W50 produce five biochemically distinct enzymes. Mol Microbiol. 1997;23:955–965. doi: 10.1046/j.1365-2958.1997.2831647.x. [DOI] [PubMed] [Google Scholar]
- 28.Rangarajan M, Smith S J M, U S, Curtis M A. Biochemical characterisation of the arginine-specific proteases of Porphyromonas gingivalis W50 suggests a common precursor. Biochem J. 1997;323:701–709. doi: 10.1042/bj3230701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 30.Scott C F, Whitaker E J, Hammond B F, Colman R W. Purification and characterization of a potent 70-kDa thiol lysyl-proteinase (Lys-gingivain) from Porphyromonas gingivalis that cleaves kininogens and fibrinogen. J Biol Chem. 1993;268:7935–7942. [PubMed] [Google Scholar]
- 31.Slots J, Bragd L, Wikström M, Dahlén G. The occurrence of Actinobacillus actinomycetemcomitans, Bacteroides gingivalis and Bacteroides intermedius in destructive periodontal disease in adults. J Periodontol. 1986;13:576–577. doi: 10.1111/j.1600-051x.1986.tb00849.x. [DOI] [PubMed] [Google Scholar]
- 32.Wang W C, Goldman L M, Schleider D M, Appenheimer M M, Subjeck J R, Repasky E A, Evans S S. Fever-range hyperthermia enhances l-selectin-dependent adhesion of lymphocytes to vascular endothelium. J Immunol. 1998;160:961–969. [PubMed] [Google Scholar]
- 33.Wingrove J A, DiScipio R G, Chen Z, Potempa J, Travis J, Hugli T E. Activation of complement components C3 and C5 by cysteine proteinases (gingipain-1) from Porphyromonas (Bacteroides) gingivalis. J Biol Chem. 1992;26:18902–18907. [PubMed] [Google Scholar]
- 34.Xie H, Cai S, Lamont R J. Environmental regulation of fimbrial gene expression in Porphyromonas gingivalis. Infect Immun. 1997;65:2265–2271. doi: 10.1128/iai.65.6.2265-2271.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]