Abstract
The gut microbiota (GM) is a complex and dynamic population of microorganisms living in the human gastrointestinal tract that play an important role in human health and diseases. Recent evidence suggests a strong direct or indirect correlation between GM and both male and female fertility: on the one hand, GM is involved in the regulation of sex hormone levels and in the preservation of the blood–testis barrier integrity; on the other hand, a dysbiotic GM is linked to the onset of pro-inflammatory conditions such as endometriosis or PCOS, which are often associated with infertility. Exposure to endocrine-disrupting chemicals (EDCs) is one of the main causes of GM dysbiosis, with important consequences to the host health and potential transgenerational effects. This perspective article aims to show that the negative effects of EDCs on reproduction are in part due to a dysbiotic GM. We will highlight (i) the link between GM and male and female fertility; (ii) the mechanisms of interaction between EDCs and GM; and (iii) the importance of the maternal–fetal GM axis for offspring growth and development.
Keywords: endocrine disruptors, gut microbiota, dysbiosis, reproduction, infertility
1. Introduction
In this perspective, we will describe mounting evidence that suggests a damaging effect of endocrine-disrupting chemicals (EDCs) on the gut microbiota (GM), and we will bring experimental, clinical, and epidemiological evidence that shows how dysregulation of the GM eubiotic condition might be the linking ring between EDCs and male and female infertility. Whilst this dysregulation of EDCs on the GM is evident during adult life, we will suggest that it might occur also during the early post-natal phases of life and fetal development, speculating, for the latter, a possible transgenerational effect.
2. Gut Microbiota: A New Player in Town
The GM is a complex and dynamic population of microorganisms living in the human gastrointestinal tract that exerts biochemical functions otherwise absent in the host. GM is considered a hidden metabolic organ of the human body influencing human health and diseases [1]. Particularly, GM regulates a range of physiological functions [2] mainly involved in (i) preserving the intestinal barrier integrity [3], (ii) protecting against pathogens [4], (iii) regulating host immunity [5,6], (iv) ensuring energy metabolism [7], and (v) modulating immune development [8].
The main GM phyla are Firmicutes, Bacteroidetes, Actinobacteria, Proteobacteria, Fusobacteria, and Verrucomicrobia, with the former two representing 90% of the whole population [9]. The biodiversity of GM is of utmost importance since it serves as a functional expansion of host genomes. Indeed, the GM’s genome, named gut microbiome, harbors different extra genes encoding enzymatic proteins, non-encoded by the host, that contribute to the regulation of the host physiology [10].
The Key Role of Gut Microbiota in Health and Disease
The GM is a potential controller of wellness and disease. As demonstrated by experimental, clinical, and epidemiological evidence, a dysbiotic microbiota (i.e., a microbiota that deviates from the “eubiotic” status in terms of diversity and functionality [11]) is implicated in a range of diseases in adults, including inflammatory bowel disease [12,13], arthritis [14], cancer [15], neurological and neuropsychiatric disorders [16], cardiometabolic [17] and cardiovascular [18] disease, obesity [19], type 2 diabetes [20,21,22], and, as detailed below, infertility.
Gut eubiosis is pivotal also for the maintenance of the intestinal barrier integrity, essential to prevent the so-called leaky-gut syndrome (LGS), a condition that causes intestinal permeability resulting in the permeation of antigens, endotoxins, and pathogens and the altered production of neurotransmitters and metabolites such as short-chain fatty acids, leading to chronic low-grade inflammation, a state linked to the development of several diseases [23].
Furthermore, increasing evidence suggests that the acquisition and development of a healthy microbiota in the infant are pivotal to exerting long-lasting beneficial effects in disease prevention [24]. The maternal microbial reservoir is crucial in the maternal-to-infant passage. Maternal vaginal, oral, gut, skin, and breast milk microbial communities contribute to establishing the infant’s own gut microbial community [25,26,27,28], thus regulating correct fetal growth, neurodevelopment, and immune programming [29,30] and providing a prophylactic potential of non-communicable diseases (NCDs), such as obesity, immunoinflammatory disorders, and neurocognitive complications [31].
Extensive microbial colonization takes place post-partum [24] through the mode of delivery, contact with the mother (such as skin-to-skin care), maternal diet, and breastfeeding [32]; however, gut colonization might occur already during the prenatal period [33]. Recent, although controversial [30], findings question the dogma that the womb is sterile, suggesting that the fetus incorporates an initial microbial inoculum already before birth (in utero colonization hypothesis) [29,30,34,35,36], followed by postnatally supplemented maternal microbes.
3. The Dangerous Effects of Endocrine-Disrupting Chemicals on the Gut Microbiota
Several factors can affect GM composition and homeostasis, and EDCs are among the most critical [37]. EDCs are highly heterogeneous environmental contaminants that include both natural and synthetic molecules [38]. Phytoestrogens (e.g., genistein and coumestrol) are natural EDCs found in several human and animal food, whereas solvents/lubricants (e.g., polychlorinated biphenyls and dioxins), plastics (e.g., bisphenol A), plasticizers (e.g., phthalates), pesticides and fungicides (e.g., methoxychlor, dichlorodiphenyltrichloroethane, and vinclozolin), and pharmaceutical agents (e.g., diethylstilbestrol) are synthetic EDCs applied to anthropic activities [39]. EDCs enter the organism through the food chain, resulting in adverse interference with hormonally controlled functions [38]. Indeed, they exert their toxicity mimicking estrogen and/or androgen hormone actions, binding to their specific endogenous receptors. They promote impaired activation, synthesis, and secretion of endogenous hormones, thus influencing several hormonal and metabolic processes [38,40]. Moreover, some EDCs show genotoxic effects [41,42] and perturb the epigenetic landscape, inducing alterations of target cells [43,44]. A number of clinical and experimental studies have shown the impact of EDCs on human GM [45]. For instance, increased urinary lead (Pb) was associated with significant changes in the human adult gut microbiota biodiversity [46], affecting both the richness of microbial taxa (α-diversity) and the variability in taxa composition (β-diversity) [47].
Post-natal exposure to di-(2-ethylhexyl) phthalate (DEHP) altered GM composition in new-born babies, with levels of Bifidobacterium longum, a key microbial species for normal gut colonization and development [48], significantly decreased compared to control [49]. Moreover, using an in vitro simulator of the human intestinal microbial ecosystem (SHIME), Wang and colleagues demonstrated that BPA exposure significantly changed the variability of the microbial community and increased the percentage of microbes shared in ascending, transverse, and descending colons, observing an upregulated expression of genes related to estrogenic effect and oxidative stress [50].
Many other experimental studies extensively reviewed by Galvez-Ontiveros and colleagues [45] have been conducted in model animals such as rodents, zebrafish, rabbits, and dogs, showing that exposure to polychlorinated biphenyls, parabens, phytoestrogens, metals, triclosan and triclocarban, phthalates, and BPA and its analogs affects GM composition and functionality and triggers metabolic disease.
Endocrine-Disrupting Chemicals and Gut Microbiota: Mechanisms of Interaction
Gastrointestinal microbiota and EDC interactions are multiple and interdependent. On the one hand, environmental contaminants alter gastrointestinal bacteria composition and/or the metabolic activity that shapes the host’s microbiotype; on the other hand, GM extensively metabolizes environmental chemicals, thus modulating their toxicity in the host [51]. Indeed, the microbiota is pictured as an additional organ involved in xenobiotic biotransformation [52] and influencing the pharmacokinetics of environmental chemicals [53]. Therefore, an altered symbiotic flora may differently modulate chemical toxicity [11,45].
There are three main mechanisms by which EDCs and GM interact [51]:
-
(1)
Through the direct and indirect metabolism of xenobiotics, i.e., chemical substances that are not produced by the organism. More specifically, xenobiotics enter the human body mainly through the gastrointestinal tract and reach the distal gut where they can be directly metabolized by GM performing diverse chemical transformations such as hydrolysis, removal of the succinate group, dehydroxylation, acetylation, deacetylation, proteolysis, denitration, deconjugation, or thiazole ring opening. In other circumstances, after ingestion, xenobiotics such as the non-polar ones are transported to the liver for detoxification where they are oxidized and subsequently eliminated in urine or secreted into the bile. In the latter case, they move to the small intestine where they can be absorbed, or they progress down to the large intestine where they are metabolized by the GM.
-
(2)
By altering the microbial diversity and thus inducing dysbiosis. For instance, it has been demonstrated that both BPA and ethinylestradiol exposure can elevate the amount of Bifidobacterium spp. in mice, leading to metabolic disorders [54]; instead, methylparaben, diethyl phthalate, and triclosan (or their mixture) exposure modifies, in rats, the ratio between Bacteroidetes and Firmicutes spp. [55], a relevant marker of gut dysbiosis [56]. Moreover, EDCs reduce the number of microbial species such as Lactobacillus spp., important for xenobiotic biotransformation and involved in the maintenance of a proper intestinal barrier [57], thus resulting in the enhanced absorption of contaminants and toxicity in the host.
-
(3)
EDCs interfere with the GM enzymatic activity. Indeed, GM significantly contributes to the host metabolism by providing enzymes encoded by the gut microbiome (i.e., the genome of GM) which are involved in both the xenobiotic and endobiotic metabolism. Among the most important of these enzymes, β-glycosidase catalyzes the hydrolysis of plant polyphenol glycosides, and β-glucuronidase (GUSB) catalyzes the removal of glucuronic acid from liver-produced glucuronides [58]. Consequently, EDCs, by perturbating GM, may alter host physiological processes mediated by these enzymes.
4. Gut Microbiota and (In)Fertility
Emerging evidence indicates that GM composition is key also in reproductive health for both women and men.
4.1. Female Fertility
Variations to the GM homeostasis may affect female fertility via different pathways. Primarily, GM is able to influence female fertility by affecting the level of sex hormones [59]. Indeed, as described before, the gut microbiome encodes different enzymes involved in host metabolism, and one of them, the enzyme GUSB, is responsible for the metabolism and modulation of circulating estrogen hormones [60] since it deconjugates estrogens, enabling their binding to estrogen receptors and leading to physiological downstream effects [61,62]. Therefore, changes to the microbial population encoding the enzyme GUSB, known as the estrobolome, affect the endogenous estrogen metabolism by modulating the enterohepatic circulation of these hormones, with a subsequent impact on the woman’s hormonal balance and, therefore, on her fertility [59].
Secondly, GM seems inextricably linked to female infertility due to the important relationship that exists between a healthy GM and the immune system [63]. Indeed, a dysbiotic GM is observed in several infertility-related disorders such as endometriosis [64,65], polycystic ovary syndrome (PCOS), insulin resistance (IR) [66,67,68,69,70,71], and obesity [72,73], characterized by an unbalanced immune profile and pro-inflammatory status, known to negatively affect fertility [74]. All these conditions are characterized by a reduced GM biodiversity and specific microbial imbalances in both the gut and reproductive tract leading to immune dysfunction, compromised immunosurveillance, and altered immune cell profiles. For instance, in women affected by endometriosis, diminished Lactobacillus spp. dominance, an altered Firmicutes:Bacteroidetes ratio, and an abundance of vaginosis-related bacteria and other opportunistic pathogens [64,65] determine upregulated ovarian estrogen secretion through neuro-active metabolites that stimulate GnRH neurons, in turn worsening hormonal homeostasis [64]. A further example is that of PCOS patients, in which the GM shows an abnormal Escherichia:Shigella ratio and an excess of Bacteroides compared to healthy women [71], a condition occurring also in IR and in obese women characterized by an increased Firmicutes:Bacteroidetes ratio [56,72]. Moreover, GM exerts a role in the pathogenesis of thyroid autoimmune disease, a frequent disorder in infertility patients [75,76,77], and a relationship between the gut microbiome and premature ovarian insufficiency (POI) has been suggested [78,79].
Lastly, GM eubiosis plays a key role in female fertility since it has been demonstrated that GM can influence the whole genital tract microbiota through a continuous crosstalk between uterus and vagina ecosystems [80]. Therefore, a condition of dysbiosis in the gut could possibly lead to vaginal and uterine dysbiosis, negatively affecting endometrial receptivity at the time of implantation [81,82].
Noteworthy, oral administration of probiotics influences vaginal microbiota composition and immunity [23], and different microbial species, such as the Gram-positive Lactobacillus spp. that dominates the vaginal microbiota in physiological conditions, originate from the gut [83]. Moreover, GM dysbiosis can induce the leaky-gut syndrome leading to intestinal permeability and leakage of bacteria and bacterial products from the gut into the circulation, thus affecting the female genital tract microbiota [80].
4.2. Male Fertility
Growing interest has been devoted also to the role of the microbiome in male reproduction. First, differences in GM between genders have been demonstrated, with a considerably less variety of gut microbiota in men as compared to women, with sex hormones likely responsible for these differences [84,85]. Although the underlying mechanisms by which GM contributes to the regulation of androgens and steroids are still unclear, it is known that some gut microbes can express steroid-processing enzymes and produce steroid hormones with an impact on the metabolism [86,87], and in turn, sex steroids themselves may regulate the structure and function of the GM [87,88].
The GM communicates with the distal organs of the host, including the testis, through various mechanisms, and it may affect male reproduction at several levels.
It has been suggested that a defective gut barrier function can end with the dissemination within the blood flow of microbial-associated molecular patterns (MAMPs) such as lipopolysaccharide, lipoprotein acids, peptidoglycans, and lipoproteins into the testis and epididymis. Indeed, immune system activation, induced by GM translocation, leads to testicular and epididymal inflammation and, also, triggers insulin resistance together with gastrointestinal hormones which, in turn, affect the secretion of sex hormones [luteinizing hormone (LH), follicle-stimulating hormone (FSH), and testosterone (T)] and their role in the regulation of spermatogenesis [89].
This condition determines: (i) the activation of specialized receptors known as pattern recognition receptors which serve as MAMP sensors that recognize the essential microbial components and trigger an immune response; (ii) Langerhans islet inflammation; and (iii) gastrointestinal hormonal changes with insulin resistance and the alteration of LH, FSH, and free T levels [89].
Moreover, a novel role for the GM in the regulation of testicular development and function has been outlined, demonstrating the involvement of the GM in the regulation of the endocrine function of the testis and the integrity of the blood–testis barrier (BTB) [90].
Noteworthy, in addition to the GM, the testicular microenvironment is not completely sterile, containing low amounts of Actinobacteria, Bacteroidetes, Firmicutes, and Proteobacteria. Although the role of testicular bacterial species in the testis remains to be elucidated, it has been suggested that these microbes play an important role in regulating and shaping the immune response in the testis. The gut microbes and testicular microbes together can influence human reproduction, as demonstrated recently in the study of Zhang et al. [91], in which GM altered bile acid levels and affected vitamin A absorption in the gut. Thus, the abnormal vitamin A metabolism can be transferred to the testis through the blood circulation, ultimately resulting in a sharp decline in spermatogonia differentiation in the metabolic syndrome model. Alfano et al. [92] showed a dysbiotic bacterial community in idiopathic nonobstructive azoospermia (iNOA) patients, describing a decrease in Clostridium abundance in those patients with unsuccessful sperm retrieval compared to successful sperm-retrieval patients. This finding has potential clinical relevance since the presence of the class Clostridia has been linked with improved sperm motility and morphology [93,94].
In addition, these authors suggested a link between gut microbes and testicular microbes since testicular bacterial microbiome modifications observed in iNOA men were similar to those previously reported in the gut of elderly individuals [92].
Altered intestinal flora can contribute to increased serum trimethylamine-N-oxide levels, promote vascular inflammation leading to cavernous endothelial and smooth muscle cell damage and to the development of erectile dysfunction [95].
EDCs can cause dysbiosis of the GM with consequent alteration of both the function and the anatomy of the intestinal barrier, activation of the immune system, development of metabolic disorders, and IR which, in turn, affect spermatogenesis and sperm viability [89]. La Merrill et al. [44] identified ten specific key characteristics or main levels where EDCs can interfere with hormone regulation and action, resulting in reproduction disorders. On the other hand, several EDCs compromise BTB integrity and consequently sperm quantity and quality [96], and the GM can protect the germ cells from environmental noxious substances, including EDCs themselves [90]. Indeed, in mice, the GM can modulate the permeability of the blood–testis barrier and influence intratesticular testosterone as well as serum LH and FSH levels. Conversely, the exposure of germ-free mice to commensal bacteria such as Clostridium tyrobutyricum can restore the blood–testis barrier integrity [90].
5. Endocrine-Disrupting Chemicals, Gut Microbiota, and Reproductive Health: An Intricate Triad with Possible Implication for Future Generations
EDC exposure during adulthood has been clearly associated with negative effect for human health; however, EDC exposure in both pre- and post-natal periods may exert even worse consequences: Firstly, because in this phase the human gut is much less resilient and much more responsive to external and environmental factors than the adult gut. Indeed, fetuses are exposed to a greater risk than adults from food contaminants due to their higher absorption rate, poor detoxification and elimination capacity, faster cell proliferation, and the still immature DNA repair mechanism [36]. Secondly, because during the perinatal and neonatal period, the microbial ecosystem inside undergoes an unprecedented process of shaping [25]. Therefore, any disturbance in this timeframe may lead to more detrimental effects than at any other moment in life affecting both the acquisition and constitution of a healthy GM, with subsequent implications for the exposed individual, offspring health, and their reproductive capability. In light of this, it is not surprising that a growing literature is showing the negative consequences of prenatal EDC exposure on offspring reproductive health.
For instance, it has been recently highlighted that gestational EDC exposure causes serious damage to the reproductive system in male offspring along with disturbing the GM. Gestational exposure to dibutyl phthalate determines an increased abundance of Bacteroidetes, Prevotella, and P. copri and causes gut dysbacteriosis in the offspring with also an increase in seminiferous atrophy and spermatogenic cell apoptosis [97]. Moreover, phthalate exposure during the gestational period seems to cause testicular damage in the offspring and abnormal phenotypes (e.g., cryptorchidism, loss of reproductive organs, hypospadias) [97]. Lastly, prenatal EDC exposure and the consequent gut–genital microbiota damage are considered at the origin and pathogenesis of endometriosis [65] and male infertility [57,97] in the offspring. Overall, these studies suggest that environmental chemicals may impair in utero programming and, in some cases (e.g., BPA), may be associated with a trans-generationally increased risk of infertility [98].
A key point now is the understanding of the underlying mechanisms regulating the observed transgenerational effects. In this regard, some authors speculated that specific GM strains can induce epigenetic changes in the host genes relevant to immunological, metabolic, and neurological development and functions [24]. For instance, Lactobacillus and Bifidobacterium can affect DNA methylation by regulating methyl-donor availability through their production of folate [99], or, via butyrate production, they act as histone deacetylase (HDAC) inhibitors and suppressors of nuclear factor-kB (NF-kB) activation, upregulating the expression of PPARγ and decreasing interferon-γ production [100,101]. Moreover, an increased Firmicutes:Bacteroidetes ratio seems to affect DNA methylation [102]. For instance, Kumar and colleagues [103] reported distinct DNA methylation profiles in blood samples from women 6 months after delivery, depending on the predominance of either Firmicutes or Bacteroidetes and Proteobacteria phyla in their fecal microbiota during pregnancy. The differences in methylation patterns affected genes whose function is linked to obesity, metabolism, and inflammation, thus highlighting their link between metabolic disorders and gut microbiota [104].
The exact mechanism by which GM chemically modulate epigenetic marks remains to be clarified. The existence of a “microbiota–nutrient metabolism–host epigenetic” axis has been postulated [105]. According to this hypothesis, the GM acts as a regulator of DNA methylation and histone modifications by altering the levels of nutrients and metabolites: on one hand, directly inhibiting enzymes that catalyze the processes, and on the other, altering the availability of substrates necessary for the enzymatic reactions. In other words, the link between epigenetic marks and GM could be mediated by host-microbial metabolites, acting as substrates and cofactors for epigenetic enzymes. In this scenario, EDCs inducing GM dysbiosis may induce a downstream effect on epigenetic programming and regulation with critical consequences if occurring during the first 1000 days of life of a human individual, a period in which epigenetic DNA imprinting activity is most active and different factors such as nutrition, microbiome, and epigenome play a key role in developmental programming, influencing the individual susceptibility to the development of diseases later in life [24].
6. Conclusions and Future Perspectives
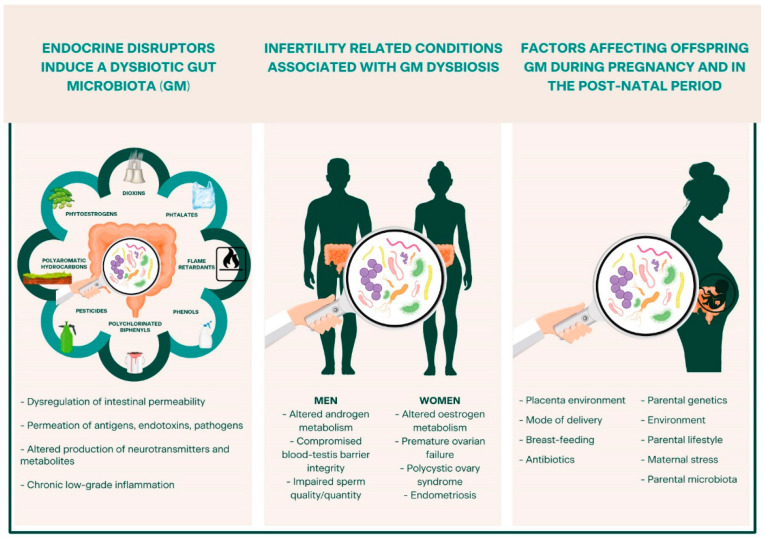
Figure 1 highlights the main aspects described in this review: emerging concepts provide evidence of the correlation between EDC exposure, GM dysbiosis, and the occurrence of a range of infertility-related diseases in the exposed individual and in the offspring, shedding light on an intriguing triad (EDCs–GM–(in)fertility) and on its possible involvement in the (dis)regulation of reproductive health in both men and women.
Figure 1.
Overview of the intricate relationship among endocrine-disrupting chemicals (EDCs), gut microbiota (GM), and reproductive health. EDCs induce GM damage through several mechanisms of action, leading to GM dysbiosis, intestinal permeability, and chronic low-grade inflammation, conditions linked to several diseases, including those related to infertility. When a dysbiotic condition occurs during pregnancy or in the early post-natal period, it may lead to even worse detrimental effects, affecting both the acquisition and constitution of a healthy GM, with subsequent implications on the exposed individual, the offspring’s health, and reproductive capability.
In the era of precision medicine, a better understanding of the role of GM in reproduction opens the possibility to develop novel strategies to prevent or treat infertility and the diseases associated with it, such as maternal dietary modification [106], probiotic and prebiotic supplementation, and fecal microbiota transplantation [107]. Moreover, the recent correlation between microbiota composition and host epigenome suggests that the enrichment for certain microbial species could modulate unique gene expression signatures [8]. The epigenome is a dynamic player in host-microbiota crosstalk functional in precision medicine [108]. Should this fascinating hypothesis be confirmed, the control of epigenetic substrate levels produced by microbial species could represent a new avenue for clinical approaches with pre, pro or post-biotic supplementation to regulate epigenetic enzymes in the gut.
Author Contributions
G.F., P.R., D.C., S.G., M.Z., F.M.U. and L.R. conceived the manuscript. G.F., M.A. and M.F. conducted the literature search. G.F., P.R., D.C., M.F., L.S. and M.Z. drafted the manuscript. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
M.F. was supported by Fondazione Umberto Veronesi. P.R., M.Z. and S.G were supported by the Italian Ministry of Education, University and Research (MUR): Dipartimenti di Eccellenza Program (2018–2022)—Department of Biology and Biotechnology “L. Spallanzani”, University of Pavia.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Thursby E., Juge N. Introduction to the human gut microbiota. Biochem. J. 2017;474:1823–1836. doi: 10.1042/BCJ20160510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kho Z.Y., Lal S.K. The Human Gut Microbiome—A Potential Controller of Wellness and Disease. Front. Microbiol. 2018;9:1835. doi: 10.3389/fmicb.2018.01835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takiishi T., Fenero C.I.M., Camara N.O.S. Intestinal barrier and gut microbiota: Shaping our immune responses throughout life. Tissue Barriers. 2017;5:e1373208. doi: 10.1080/21688370.2017.1373208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pickard J.M., Zeng M.Y., Caruso R., Nunez G. Gut microbiota: Role in pathogen colonization, immune responses, and inflammatory disease. Immunol. Rev. 2017;279:70–89. doi: 10.1111/imr.12567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu H.J., Wu E. The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes. 2012;3:4–14. doi: 10.4161/gmic.19320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zheng D., Liwinski T., Elinav E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020;30:492–506. doi: 10.1038/s41422-020-0332-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heiss C.N., Olofsson L.E. Gut Microbiota-Dependent Modulation of Energy Metabolism. J. Innate Immun. 2018;10:163–171. doi: 10.1159/000481519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woo V., Alenghat T. Epigenetic regulation by gut microbiota. Gut Microbes. 2022;14:2022407. doi: 10.1080/19490976.2021.2022407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rinninella E., Raoul P., Cintoni M., Franceschi F., Miggiano G.A.D., Gasbarrini A., Mele M.C. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms. 2019;7:14. doi: 10.3390/microorganisms7010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hooper L.V., Gordon J.I. Commensal host-bacterial relationships in the gut. Science. 2001;292:1115–1118. doi: 10.1126/science.1058709. [DOI] [PubMed] [Google Scholar]
- 11.Velmurugan G., Ramprasath T., Gilles M., Swaminathan K., Ramasamy S. Gut Microbiota, Endocrine-Disrupting Chemicals, and the Diabetes Epidemic. Trends Endocrinol. Metab. 2017;28:612–625. doi: 10.1016/j.tem.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 12.Pu D., Zhang Z., Feng B. Alterations and Potential Applications of Gut Microbiota in Biological Therapy for Inflammatory Bowel Diseases. Front. Pharmacol. 2022;13:906419. doi: 10.3389/fphar.2022.906419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gill P.A., Inniss S., Kumagai T., Rahman F.Z., Smith A.M. The Role of Diet and Gut Microbiota in Regulating Gastrointestinal and Inflammatory Disease. Front. Immunol. 2022;13:866059. doi: 10.3389/fimmu.2022.866059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guan Z., Luo L., Liu S., Guan Z., Zhang Q., Li X., Tao K. The Role of Depletion of Gut Microbiota in Osteoporosis and Osteoarthritis: A Narrative Review. Front. Endocrinol. 2022;13:847401. doi: 10.3389/fendo.2022.847401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sadrekarimi H., Gardanova Z.R., Bakhshesh M., Ebrahimzadeh F., Yaseri A.F., Thangavelu L., Hasanpoor Z., Zadeh F.A., Kahrizi M.S. Emerging role of human microbiome in cancer development and response to therapy: Special focus on intestinal microflora. J. Transl. Med. 2022;20:301. doi: 10.1186/s12967-022-03492-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mitrea L., Nemes S.A., Szabo K., Teleky B.E., Vodnar D.C. Guts Imbalance Imbalances the Brain: A Review of Gut Microbiota Association With Neurological and Psychiatric Disorders. Front. Med. 2022;9:813204. doi: 10.3389/fmed.2022.813204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Callender C., Attaye I., Nieuwdorp M. The Interaction between the Gut Microbiome and Bile Acids in Cardiometabolic Diseases. Metabolites. 2022;12:65. doi: 10.3390/metabo12010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rajendiran E., Ramadass B., Ramprasath V. Understanding connections and roles of gut microbiome in cardiovascular diseases. Can. J. Microbiol. 2021;67:101–111. doi: 10.1139/cjm-2020-0043. [DOI] [PubMed] [Google Scholar]
- 19.Asadi A., Shadab Mehr N., Mohamadi M.H., Shokri F., Heidary M., Sadeghifard N., Khoshnood S. Obesity and gut-microbiota-brain axis: A narrative review. J. Clin. Lab. Anal. 2022;36:e24420. doi: 10.1002/jcla.24420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bouter K.E., van Raalte D.H., Groen A.K., Nieuwdorp M. Role of the Gut Microbiome in the Pathogenesis of Obesity and Obesity-Related Metabolic Dysfunction. Gastroenterology. 2017;152:1671–1678. doi: 10.1053/j.gastro.2016.12.048. [DOI] [PubMed] [Google Scholar]
- 21.Ogunrinola G.A., Oyewale J.O., Oshamika O.O., Olasehinde G.I. The Human Microbiome and Its Impacts on Health. Int. J. Microbiol. 2020;2020:8045646. doi: 10.1155/2020/8045646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cho I., Blaser M.J. The human microbiome: At the interface of health and disease. Nat. Rev. Genet. 2012;13:260–270. doi: 10.1038/nrg3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kinashi Y., Hase K. Partners in Leaky Gut Syndrome: Intestinal Dysbiosis and Autoimmunity. Front. Immunol. 2021;12:673708. doi: 10.3389/fimmu.2021.673708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Indrio F., Martini S., Francavilla R., Corvaglia L., Cristofori F., Mastrolia S.A., Neu J., Rautava S., Russo Spena G., Raimondi F., et al. Epigenetic Matters: The Link between Early Nutrition, Microbiome, and Long-term Health Development. Front. Pediatr. 2017;5:178. doi: 10.3389/fped.2017.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laursen M.F., Bahl M.I., Licht T.R. Settlers of our inner surface—Factors shaping the gut microbiota from birth to toddlerhood. FEMS Microbiol. Rev. 2021;45:fuab001. doi: 10.1093/femsre/fuab001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chu D.M., Ma J., Prince A.L., Antony K.M., Seferovic M.D., Aagaard K.M. Maturation of the infant microbiome community structure and function across multiple body sites and in relation to mode of delivery. Nat. Med. 2017;23:314–326. doi: 10.1038/nm.4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pannaraj P.S., Li F., Cerini C., Bender J.M., Yang S., Rollie A., Adisetiyo H., Zabih S., Lincez P.J., Bittinger K., et al. Association Between Breast Milk Bacterial Communities and Establishment and Development of the Infant Gut Microbiome. JAMA Pediatr. 2017;171:647–654. doi: 10.1001/jamapediatrics.2017.0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferretti P., Pasolli E., Tett A., Asnicar F., Gorfer V., Fedi S., Armanini F., Truong D.T., Manara S., Zolfo M., et al. Mother-to-Infant Microbial Transmission from Different Body Sites Shapes the Developing Infant Gut Microbiome. Cell Host Microbe. 2018;24:133–145.e5. doi: 10.1016/j.chom.2018.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gao Y., Nanan R., Macia L., Tan J., Sominsky L., Quinn T.P., O’Hely M., Ponsonby A.L., Tang M.L.K., Collier F., et al. The maternal gut microbiome during pregnancy and offspring allergy and asthma. J. Allergy Clin. Immunol. 2021;148:669–678. doi: 10.1016/j.jaci.2021.07.011. [DOI] [PubMed] [Google Scholar]
- 30.Koleva P.T., Kim J.S., Scott J.A., Kozyrskyj A.L. Microbial programming of health and disease starts during fetal life. Birth Defects Res. C Embryo Today. 2015;105:265–277. doi: 10.1002/bdrc.21117. [DOI] [PubMed] [Google Scholar]
- 31.Hosseinkhani F., Heinken A., Thiele I., Lindenburg P.W., Harms A.C., Hankemeier T. The contribution of gut bacterial metabolites in the human immune signaling pathway of non-communicable diseases. Gut Microbes. 2021;13:1–22. doi: 10.1080/19490976.2021.1882927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neu J. Developmental aspects of maternal-fetal, and infant gut microbiota and implications for long-term health. Matern. Health Neonatol. Perinatol. 2015;1:6. doi: 10.1186/s40748-015-0007-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller W.B., Jr. The Eukaryotic Microbiome: Origins and Implications for Fetal and Neonatal Life. Front. Pediatr. 2016;4:96. doi: 10.3389/fped.2016.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Funkhouser L.J., Bordenstein S.R. Mom knows best: The universality of maternal microbial transmission. PLoS Biol. 2013;11:e1001631. doi: 10.1371/journal.pbio.1001631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perez-Cano F.J. What Does Influence the Neonatal Microbiome? Nutrients. 2020;12:2472. doi: 10.3390/nu12082472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sarron E., Perot M., Barbezier N., Delayre-Orthez C., Gay-Queheillard J., Anton P.M. Early exposure to food contaminants reshapes maturation of the human brain-gut-microbiota axis. World J. Gastroenterol. 2020;26:3145–3169. doi: 10.3748/wjg.v26.i23.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hasan N., Yang H. Factors affecting the composition of the gut microbiota, and its modulation. PeerJ. 2019;7:e7502. doi: 10.7717/peerj.7502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gore A.C., Chappell V.A., Fenton S.E., Flaws J.A., Nadal A., Prins G.S., Toppari J., Zoeller R.T. Executive Summary to EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr. Rev. 2015;36:593–602. doi: 10.1210/er.2015-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Diamanti-Kandarakis E., Bourguignon J.P., Giudice L.C., Hauser R., Prins G.S., Soto A.M., Zoeller R.T., Gore A.C. Endocrine-disrupting chemicals: An Endocrine Society scientific statement. Endocr. Rev. 2009;30:293–342. doi: 10.1210/er.2009-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kabir E.R., Rahman M.S., Rahman I. A review on endocrine disruptors and their possible impacts on human health. Environ. Toxicol. Pharmacol. 2015;40:241–258. doi: 10.1016/j.etap.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 41.Claxton L.D., Houk V.S., Hughes T.J. Genotoxicity of industrial wastes and effluents. Mutat. Res. 1998;410:237–243. doi: 10.1016/S1383-5742(98)00008-8. [DOI] [PubMed] [Google Scholar]
- 42.Choudhuri S., Kaur T., Jain S., Sharma C., Asthana S. A review on genotoxicity in connection to infertility and cancer. Chem. Biol. Interact. 2021;345:109531. doi: 10.1016/j.cbi.2021.109531. [DOI] [PubMed] [Google Scholar]
- 43.Rattan S., Flaws J.A. The epigenetic impacts of endocrine disruptors on female reproduction across generationsdagger. Biol. Reprod. 2019;101:635–644. doi: 10.1093/biolre/ioz081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.La Merrill M.A., Vandenberg L.N., Smith M.T., Goodson W., Browne P., Patisaul H.B., Guyton K.Z., Kortenkamp A., Cogliano V.J., Woodruff T.J., et al. Consensus on the key characteristics of endocrine-disrupting chemicals as a basis for hazard identification. Nat. Rev. Endocrinol. 2020;16:45–57. doi: 10.1038/s41574-019-0273-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Galvez-Ontiveros Y., Paez S., Monteagudo C., Rivas A. Endocrine Disruptors in Food: Impact on Gut Microbiota and Metabolic Diseases. Nutrients. 2020;12:1158. doi: 10.3390/nu12041158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eggers S., Safdar N., Sethi A.K., Suen G., Peppard P.E., Kates A.E., Skarlupka J.H., Kanarek M., Malecki K.M.C. Urinary lead concentration and composition of the adult gut microbiota in a cross-sectional population-based sample. Environ. Int. 2019;133:105122. doi: 10.1016/j.envint.2019.105122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walters K.E., Martiny J.B.H. Alpha-, beta-, and gamma-diversity of bacteria varies across habitats. PLoS ONE. 2020;15:e0233872. doi: 10.1371/journal.pone.0233872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matamoros S., Gras-Leguen C., Le Vacon F., Potel G., de La Cochetiere M.F. Development of intestinal microbiota in infants and its impact on health. Trends Microbiol. 2013;21:167–173. doi: 10.1016/j.tim.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 49.Yang Y.N., Yang Y.S.H., Lin I.H., Chen Y.Y., Lin H.Y., Wu C.Y., Su Y.T., Yang Y.J., Yang S.N., Suen J.L. Phthalate exposure alters gut microbiota composition and IgM vaccine response in human newborns. Food Chem. Toxicol. 2019;132:110700. doi: 10.1016/j.fct.2019.110700. [DOI] [PubMed] [Google Scholar]
- 50.Wang Y., Rui M., Nie Y., Lu G. Influence of gastrointestinal tract on metabolism of bisphenol A as determined by in vitro simulated system. J. Hazard. Mater. 2018;355:111–118. doi: 10.1016/j.jhazmat.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 51.Claus S.P., Guillou H., Ellero-Simatos S. The gut microbiota: A major player in the toxicity of environmental pollutants? NPJ Biofilms Microbiomes. 2016;2:16003. doi: 10.1038/npjbiofilms.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li N., Li J., Zhang Q., Gao S., Quan X., Liu P., Xu C. Effects of endocrine disrupting chemicals in host health: Three-way interactions between environmental exposure, host phenotypic responses, and gut microbiota. Environ. Pollut. 2021;271:116387. doi: 10.1016/j.envpol.2020.116387. [DOI] [PubMed] [Google Scholar]
- 53.Rosenfeld C.S. Gut Dysbiosis in Animals Due to Environmental Chemical Exposures. Front. Cell Infect. Microbiol. 2017;7:396. doi: 10.3389/fcimb.2017.00396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Javurek A.B., Spollen W.G., Johnson S.A., Bivens N.J., Bromert K.H., Givan S.A., Rosenfeld C.S. Effects of exposure to bisphenol A and ethinyl estradiol on the gut microbiota of parents and their offspring in a rodent model. Gut Microbes. 2016;7:471–485. doi: 10.1080/19490976.2016.1234657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hu J., Raikhel V., Gopalakrishnan K., Fernandez-Hernandez H., Lambertini L., Manservisi F., Falcioni L., Bua L., Belpoggi F., Teitelbaum L.S., et al. Effect of postnatal low-dose exposure to environmental chemicals on the gut microbiome in a rodent model. Microbiome. 2016;4:26. doi: 10.1186/s40168-016-0173-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Magne F., Gotteland M., Gauthier L., Zazueta A., Pesoa S., Navarrete P., Balamurugan R. The Firmicutes/Bacteroidetes Ratio: A Relevant Marker of Gut Dysbiosis in Obese Patients? Nutrients. 2020;12:1474. doi: 10.3390/nu12051474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hampl R., Starka L. Endocrine disruptors and gut microbiome interactions. Physiol. Res. 2020;69:S211–S223. doi: 10.33549/physiolres.934513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McIntosh F.M., Maison N., Holtrop G., Young P., Stevens V.J., Ince J., Johnstone A.M., Lobley G.E., Flint H.J., Louis P. Phylogenetic distribution of genes encoding beta-glucuronidase activity in human colonic bacteria and the impact of diet on faecal glycosidase activities. Environ. Microbiol. 2012;14:1876–1887. doi: 10.1111/j.1462-2920.2012.02711.x. [DOI] [PubMed] [Google Scholar]
- 59.He S., Li H., Yu Z., Zhang F., Liang S., Liu H., Chen H., Lu M. The Gut Microbiome and Sex Hormone-Related Diseases. Front. Microbiol. 2021;12:711137. doi: 10.3389/fmicb.2021.711137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Baker J.M., Al-Nakkash L., Herbst-Kralovetz M.M. Estrogen-gut microbiome axis: Physiological and clinical implications. Maturitas. 2017;103:45–53. doi: 10.1016/j.maturitas.2017.06.025. [DOI] [PubMed] [Google Scholar]
- 61.Ervin S.M., Li H., Lim L., Roberts L.R., Liang X., Mani S., Redinbo M.R. Gut microbial beta-glucuronidases reactivate estrogens as components of the estrobolome that reactivate estrogens. J. Biol. Chem. 2019;294:18586–18599. doi: 10.1074/jbc.RA119.010950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Plottel C.S., Blaser M.J. Microbiome and malignancy. Cell Host Microbe. 2011;10:324–335. doi: 10.1016/j.chom.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rooks M.G., Garrett W.S. Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 2016;16:341–352. doi: 10.1038/nri.2016.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jiang I., Yong P.J., Allaire C., Bedaiwy M.A. Intricate Connections between the Microbiota and Endometriosis. Int. J. Mol. Sci. 2021;22:5644. doi: 10.3390/ijms22115644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Garcia-Penarrubia P., Ruiz-Alcaraz A.J., Martinez-Esparza M., Marin P., Machado-Linde F. Hypothetical roadmap towards endometriosis: Prenatal endocrine-disrupting chemical pollutant exposure, anogenital distance, gut-genital microbiota and subclinical infections. Hum. Reprod. Update. 2020;26:214–246. doi: 10.1093/humupd/dmz044. [DOI] [PubMed] [Google Scholar]
- 66.Qi X., Yun C., Sun L., Xia J., Wu Q., Wang Y., Wang L., Zhang Y., Liang X., Wang L., et al. Publisher Correction: Gut microbiota-bile acid-interleukin-22 axis orchestrates polycystic ovary syndrome. Nat. Med. 2019;25:1459. doi: 10.1038/s41591-019-0562-8. [DOI] [PubMed] [Google Scholar]
- 67.Giampaolino P., Foreste V., Di Filippo C., Gallo A., Mercorio A., Serafino P., Improda F.P., Verrazzo P., Zara G., Buonfantino C., et al. Microbiome and PCOS: State-of-Art and Future Aspects. Int. J. Mol. Sci. 2021;22:2048. doi: 10.3390/ijms22042048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.He F.F., Li Y.M. Role of gut microbiota in the development of insulin resistance and the mechanism underlying polycystic ovary syndrome: A review. J. Ovarian Res. 2020;13:73. doi: 10.1186/s13048-020-00670-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kriebs A. IL-22 links gut microbiota to PCOS. Nat. Rev. Endocrinol. 2019;15:565. doi: 10.1038/s41574-019-0255-x. [DOI] [PubMed] [Google Scholar]
- 70.Yurtdas G., Akdevelioglu Y. A New Approach to Polycystic Ovary Syndrome: The Gut Microbiota. J. Am. Coll. Nutr. 2020;39:371–382. doi: 10.1080/07315724.2019.1657515. [DOI] [PubMed] [Google Scholar]
- 71.Guo J., Shao J., Yang Y., Niu X., Liao J., Zhao Q., Wang D., Li S., Hu J. Gut Microbiota in Patients with Polycystic Ovary Syndrome: A Systematic Review. Reprod. Sci. 2022;29:69–83. doi: 10.1007/s43032-020-00430-0. [DOI] [PubMed] [Google Scholar]
- 72.Saad M.J., Santos A., Prada P.O. Linking Gut Microbiota and Inflammation to Obesity and Insulin Resistance. Physiology. 2016;31:283–293. doi: 10.1152/physiol.00041.2015. [DOI] [PubMed] [Google Scholar]
- 73.Scheithauer T.P.M., Rampanelli E., Nieuwdorp M., Vallance B.A., Verchere C.B., van Raalte D.H., Herrema H. Gut Microbiota as a Trigger for Metabolic Inflammation in Obesity and Type 2 Diabetes. Front. Immunol. 2020;11:571731. doi: 10.3389/fimmu.2020.571731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.de Rivero Vaccari J.P. The Inflammasome in Reproductive Biology: A Promising Target for Novel Therapies. Front. Endocrinol. 2020;11:8. doi: 10.3389/fendo.2020.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang J.W., Liao X.X., Li T. Thyroid Autoimmunity in Adverse Fertility and Pregnancy Outcomes: Timing of Assisted Reproductive Technology in AITD Women. J. Transl. Int. Med. 2021;9:76–83. doi: 10.2478/jtim-2021-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Poppe K. Management of Endocrine Disease: Thyroid and female infertility: More questions than answers?! Eur. J. Endocrinol. 2021;184:R123–R135. doi: 10.1530/EJE-20-1284. [DOI] [PubMed] [Google Scholar]
- 77.Twig G., Shina A., Amital H., Shoenfeld Y. Pathogenesis of infertility and recurrent pregnancy loss in thyroid autoimmunity. J. Autoimmun. 2012;38:J275–J281. doi: 10.1016/j.jaut.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 78.Wu J., Zhuo Y., Liu Y., Chen Y., Ning Y., Yao J. Association between premature ovarian insufficiency and gut microbiota. BMC Pregnancy Childbirth. 2021;21:418. doi: 10.1186/s12884-021-03855-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jiang L., Fei H., Tong J., Zhou J., Zhu J., Jin X., Shi Z., Zhou Y., Ma X., Yu H., et al. Hormone Replacement Therapy Reverses Gut Microbiome and Serum Metabolome Alterations in Premature Ovarian Insufficiency. Front. Endocrinol. 2021;12:794496. doi: 10.3389/fendo.2021.794496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Amabebe E., Anumba D.O.C. Female Gut and Genital Tract Microbiota-Induced Crosstalk and Differential Effects of Short-Chain Fatty Acids on Immune Sequelae. Front. Immunol. 2020;11:2184. doi: 10.3389/fimmu.2020.02184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Benner M., Ferwerda G., Joosten I., van der Molen R.G. How uterine microbiota might be responsible for a receptive, fertile endometrium. Hum. Reprod. Update. 2018;24:393–415. doi: 10.1093/humupd/dmy012. [DOI] [PubMed] [Google Scholar]
- 82.Wang J., Li Z., Ma X., Du L., Jia Z., Cui X., Yu L., Yang J., Xiao L., Zhang B., et al. Translocation of vaginal microbiota is involved in impairment and protection of uterine health. Nat. Commun. 2021;12:4191. doi: 10.1038/s41467-021-24516-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Amabebe E., Anumba D.O.C. The Vaginal Microenvironment: The Physiologic Role of Lactobacilli. Front. Med. 2018;5:181. doi: 10.3389/fmed.2018.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dominianni C., Sinha R., Goedert J.J., Pei Z., Yang L., Hayes R.B., Ahn J. Sex, body mass index, and dietary fiber intake influence the human gut microbiome. PLoS ONE. 2015;10:e0124599. doi: 10.1371/journal.pone.0124599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Haro C., Rangel-Zuniga O.A., Alcala-Diaz J.F., Gomez-Delgado F., Perez-Martinez P., Delgado-Lista J., Quintana-Navarro G.M., Landa B.B., Navas-Cortes J.A., Tena-Sempere M., et al. Intestinal Microbiota Is Influenced by Gender and Body Mass Index. PLoS ONE. 2016;11:e0154090. doi: 10.1371/journal.pone.0154090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Diviccaro S., Giatti S., Borgo F., Falvo E., Caruso D., Garcia-Segura L.M., Melcangi R.C. Steroidogenic machinery in the adult rat colon. J. Steroid. Biochem. Mol. Biol. 2020;203:105732. doi: 10.1016/j.jsbmb.2020.105732. [DOI] [PubMed] [Google Scholar]
- 87.Ly L.K., Rowles J.L., 3rd, Paul H.M., Alves J.M.P., Yemm C., Wolf P.M., Devendran S., Hudson M.E., Morris D.J., Erdman J.W., Jr., et al. Bacterial steroid-17,20-desmolase is a taxonomically rare enzymatic pathway that converts prednisone to 1,4-androstanediene-3,11,17-trione, a metabolite that causes proliferation of prostate cancer cells. J. Steroid. Biochem. Mol. Biol. 2020;199:105567. doi: 10.1016/j.jsbmb.2019.105567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Arroyo P., Ho B.S., Sau L., Kelley S.T., Thackray V.G. Letrozole treatment of pubertal female mice results in activational effects on reproduction, metabolism and the gut microbiome. PLoS ONE. 2019;14:e0223274. doi: 10.1371/journal.pone.0223274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang Y., Xie Z. Exploring the role of gut microbiome in male reproduction. Andrology. 2022;10:441–450. doi: 10.1111/andr.13143. [DOI] [PubMed] [Google Scholar]
- 90.Al-Asmakh M., Stukenborg J.B., Reda A., Anuar F., Strand M.L., Hedin L., Pettersson S., Soder O. The gut microbiota and developmental programming of the testis in mice. PLoS ONE. 2014;9:e103809. doi: 10.1371/journal.pone.0103809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang T., Sun P., Geng Q., Fan H., Gong Y., Hu Y., Shan L., Sun Y., Shen W., Zhou Y. Disrupted spermatogenesis in a metabolic syndrome model: The role of vitamin A metabolism in the gut-testis axis. Gut. 2022;71:78–87. doi: 10.1136/gutjnl-2020-323347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Alfano M., Ferrarese R., Locatelli I., Ventimiglia E., Ippolito S., Gallina P., Cesana D., Canducci F., Pagliardini L., Vigano P., et al. Testicular microbiome in azoospermic men-first evidence of the impact of an altered microenvironment. Hum. Reprod. 2018;33:1212–1217. doi: 10.1093/humrep/dey116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hou D., Zhou X., Zhong X., Settles M.L., Herring J., Wang L., Abdo Z., Forney L.J., Xu C. Microbiota of the seminal fluid from healthy and infertile men. Fertil. Steril. 2013;100:1261–1269. doi: 10.1016/j.fertnstert.2013.07.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Weng S.L., Chiu C.M., Lin F.M., Huang W.C., Liang C., Yang T., Yang T.L., Liu C.Y., Wu W.Y., Chang Y.A., et al. Bacterial communities in semen from men of infertile couples: Metagenomic sequencing reveals relationships of seminal microbiota to semen quality. PLoS ONE. 2014;9:e110152. doi: 10.1371/journal.pone.0110152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li H., Qi T., Huang Z.S., Ying Y., Zhang Y., Wang B., Ye L., Zhang B., Chen D.L., Chen J. Relationship between gut microbiota and type 2 diabetic erectile dysfunction in Sprague-Dawley rats. J. Huazhong Univ. Sci. Technol. Med. Sci. 2017;37:523–530. doi: 10.1007/s11596-017-1767-z. [DOI] [PubMed] [Google Scholar]
- 96.Wu D., Huang C.J., Jiao X.F., Ding Z.M., Zhang S.X., Miao Y.L., Huo L.J. Bisphenol AF compromises blood-testis barrier integrity and sperm quality in mice. Chemosphere. 2019;237:124410. doi: 10.1016/j.chemosphere.2019.124410. [DOI] [PubMed] [Google Scholar]
- 97.Zhang T., Zhou X., Zhang X., Ren X., Wu J., Wang Z., Wang S., Wang Z. Gut microbiota may contribute to the postnatal male reproductive abnormalities induced by prenatal dibutyl phthalate exposure. Chemosphere. 2022;287:132046. doi: 10.1016/j.chemosphere.2021.132046. [DOI] [PubMed] [Google Scholar]
- 98.Rodriguez-Caro H., Williams S.A. Strategies to reduce non-communicable diseases in the offspring: Negative and positive in utero programming. J. Dev. Orig. Health Dis. 2018;9:642–652. doi: 10.1017/S2040174418000569. [DOI] [PubMed] [Google Scholar]
- 99.Rossi M., Amaretti A., Raimondi S. Folate production by probiotic bacteria. Nutrients. 2011;3:118–134. doi: 10.3390/nu3010118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Berni Canani R., Di Costanzo M., Leone L. The epigenetic effects of butyrate: Potential therapeutic implications for clinical practice. Clin. Epigenetics. 2012;4:4. doi: 10.1186/1868-7083-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fofanova T.Y., Petrosino J.F., Kellermayer R. Microbiome-Epigenome Interactions and the Environmental Origins of Inflammatory Bowel Diseases. J. Pediatr. Gastroenterol. Nutr. 2016;62:208–219. doi: 10.1097/MPG.0000000000000950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ramos-Molina B., Sanchez-Alcoholado L., Cabrera-Mulero A., Lopez-Dominguez R., Carmona-Saez P., Garcia-Fuentes E., Moreno-Indias I., Tinahones F.J. Gut Microbiota Composition Is Associated With the Global DNA Methylation Pattern in Obesity. Front. Genet. 2019;10:613. doi: 10.3389/fgene.2019.00613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kumar H., Lund R., Laiho A., Lundelin K., Ley R.E., Isolauri E., Salminen S. Gut microbiota as an epigenetic regulator: Pilot study based on whole-genome methylation analysis. mBio. 2014;5:e02113–e02114. doi: 10.1128/mBio.02113-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yang J.Y., Kweon M.N. The gut microbiota: A key regulator of metabolic diseases. BMB Rep. 2016;49:536–541. doi: 10.5483/BMBRep.2016.49.10.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Miro-Blanch J., Yanes O. Epigenetic Regulation at the Interplay Between Gut Microbiota and Host Metabolism. Front. Genet. 2019;10:638. doi: 10.3389/fgene.2019.00638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Druart C., Alligier M., Salazar N., Neyrinck A.M., Delzenne N.M. Modulation of the gut microbiota by nutrients with prebiotic and probiotic properties. Adv. Nutr. 2014;5:624S–633S. doi: 10.3945/an.114.005835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gulliver E.L., Young R.B., Chonwerawong M., D’Adamo G.L., Thomason T., Widdop J.T., Rutten E.L., Rossetto Marcelino V., Bryant R.V., Costello S.P., et al. Review article: The future of microbiome-based therapeutics. Aliment. Pharmacol. Ther. 2022;56:192–208. doi: 10.1111/apt.17049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Peery R.C., Pammi M., Claud E., Shen L. Epigenome—A mediator for host-microbiome crosstalk. Semin. Perinatol. 2021;45:151455. doi: 10.1016/j.semperi.2021.151455. [DOI] [PubMed] [Google Scholar]