Abstract
Because of the critical role of the CD40-CD40 ligand (CD40L) pathway in the induction and effector phases of immune responses, we investigated the effects of CD40 ligation on the control of Trypanosoma cruzi infection. First, we observed that supernatants of murine spleen cells stimulated by CD40L-transfected 3T3 fibroblasts (3T3-CD40L transfectants) prevent the infection of mouse peritoneal macrophages (MPM) by T. cruzi. This phenomenon depends on de novo production of nitric oxide (NO) as it is prevented by the addition of N-nitro-l-arginine methyl ester, a NO synthase inhibitor. NO production requires interleukin (IL)-12-mediated gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α) synthesis as demonstrated by inhibition experiments using neutralizing anti-IL-12, anti-IFN-γ, and anti-TNF-α monoclonal antibodies (MAb). We found that an activating anti-CD40 MAb also directly stimulates IFN-γ-activated MPM to produce NO and thereby to control T. cruzi infection. To determine the in vivo relevance of these in vitro findings, mice were injected with 3T3-CD40L transfectants or 3T3 control fibroblasts at the time of T. cruzi inoculation. We observed that in vivo CD40 ligation dramatically reduced both parasitemia and the mortality rate of T. cruzi-infected mice. A reduced parasitemia was still observed when the injection of 3T3-CD40L transfectants was delayed 8 days postinfection. It was abolished by injection of anti-IL-12 MAb. Taken together, these data establish that CD40 ligation facilitates the control of T. cruzi infection through a cascade involving IL-12, IFN-γ, and NO.
CD40 is a cell surface receptor expressed by various cells (B lymphocytes, dendritic cells, hematopoietic progenitors, endothelial cells, and epithelial cells) including monocytes and macrophages (56). Interaction of CD40 with its CD40 ligand (CD40L) (4, 22) triggers a pleiotropic pathway involved in both humoral and cellular immunity. By exerting potent biological activities on CD4+ T cells and antigen-presenting cells such as dendritic cells and macrophages (49), this pathway plays a major role in anti-infective host defense (21). Indeed, CD40-CD40L interactions result in the secretion of multiple cytokines such as interleukin (IL)-1, IL-6, IL-8, IL-10, IL-12, gamma interferon (IFN-γ), and tumor necrosis factor alpha (TNF-α) by immunocompetent cells. In particular, IL-12 has emerged as a potent immunoregulatory cytokine involved in the control of intracellular infections (44, 54, 55).
Trypanosoma cruzi is a hemoflagellate protozoan parasite with intracellular multiplication. It infects humans as well as domestic and wild mammals and is the etiological agent of Chagas’ disease (51). Experimental infection of BALB/c mice mimics the human disease and allows the study of host defense mechanisms. It displays an acute phase characterized by high parasitemia, followed by a chronic phase during which parasites become undetectable in peripheral blood while persisting in tissues. Various cytokines are implicated in the control of T. cruzi infection in mice including IL-12, IFN-γ, and TNF-α (1–3, 8, 25, 42, 45, 47, 53). IFN-γ and TNF-α have been shown to induce nitric oxide (NO) synthesis (13, 33), which in turn plays a crucial role in the control of T. cruzi infection in mice both in vitro and in vivo (1, 19, 24, 34, 35, 37–40, 57). The present work was undertaken to analyze the effect of CD40 ligation on cell infection in vitro and to investigate whether a CD40L stimulation was able to protect mice against T. cruzi infection.
MATERIALS AND METHODS
SC and mouse peritoneal macrophages.
Spleens were harvested from male BALB/c mice (6 to 8 weeks old) purchased from Bantin & Kingman Universal (Hull, United Kingdom) and maintained in our animal facilities on standard laboratory chow. Suspensions of erythrocyte-free spleen cells (SC) were obtained by spleen dilaceration and treatment for 30 s with distilled sterile water. SC (107 cells/ml) were then suspended in RPMI 1640 medium (GIBCO, Grand Island, N.Y.). They were plated in a 24-well cell culture plate (Nunc, Roskilde, Denmark) and incubated in a 5% CO2 and water-saturated atmosphere.
Mouse peritoneal macrophages (MPM) were harvested from male BALB/c mice by washing their peritoneal cavities with chilled Hank’s balanced salt solution without Ca2+ and Mg2+ (pH 7.4; GIBCO) (38). They were allowed to adhere (2 × 105 cells/well) on round sterile coverslips (Thermanox, 13-mm diameter; Miles Scientific, Naperville, Ill.) in 24-well microplates for 2 h at 37°C in a 5% CO2 atmosphere. Nonadherent cells were removed by washing.
SC and MPM were cultured in RPMI 1640 medium supplemented with N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES) (25 mM), glutamine (25 mM), fetal calf serum (10%), penicillin (100 IU/ml), and streptomycin (100 μg/ml; GIBCO).
Infection of MPM and mice with T. cruzi.
For in vitro experiments, T. cruzi trypomastigotes (Tehuantepec strain) were obtained from infected fibroblasts as previously described (14). Trypomastigotes (106 parasites/well) were added to MPM in a 5:1 parasite-to-cell ratio. After 24 h, cultures were washed to remove free parasites, and MPM were further incubated for 24 h. Then, cells were fixed with methanol and stained with Giemsa stain. The percentage of infected MPM and the mean number of amastigotes per infected MPM were recorded after microscopical examination of at least 200 cells per well. A parasitic index was calculated by multiplying the percentage of infected MPM times the mean number of amastigotes per infected MPM (58).
For in vivo experiments, BALB/c mice were inoculated intraperitoneally with 100 blood-form trypomastigotes in 0.2 ml of Alsever’s solution. Parasitemia was monitored by counting trypomastigotes in blood samples collected by tail incision every 2 days and every day around the peak of parasitemia. Survival rates were determined daily.
CD40L-transfected fibroblasts.
3T3 fibroblasts transfected with the gene encoding CD40L (3T3-CD40L transfectants) were obtained as follows. The 5′ region of the mCD40L, including the Kozack sequence, the leader sequence, and the first 136 codons, was amplified by reverse transcriptase-PCR of mRNA derived from activated EL4 T cells. The primers used for amplification were based on the published sequence of the mCD40L cDNA (4). The 460-bp downstream region of mCD40L was obtained by BamHI and HindIII digestion of the pHβAPr-1-neo-mCD40L-mCD8α plasmid (kindly provided by P. Lane, Basel Institute for Immunology, Basel, Switzerland) (31). The complete mCD40L cDNA was then cloned into the pCI-neo vector (Promega, Leiden, The Netherlands). Flow cytometry analysis of pCI-neo-mCD40L-transfected COS-7 cells, by using a biotinylated anti-mCD40L antibody (Pharmingen, San Diego, Calif.), showed that mCD40L was correctly assembled and expressed on the cell surface. Plasmid pCI-neo-mCD40L was used for the lipofection (LipoTaxi; Stratagene, Westburg, The Netherlands) of NIH 3T3 cells (American Type Culture Collection, Rockville, Md.). mCD40L-expressing cells were selected on the basis of growth in the presence of G418 sulfate (2 mg/ml, final concentration; Alexis Corporation, San Diego, Calif.) and flow cytometry. For a negative control in the different assay systems used, a 3T3 cell line transfected with empty pCI vector DNA (3T3 control fibroblasts) was selected.
3T3-CD40L transfectants and 3T3 control fibroblasts were grown in Dulbeco’s modified Eagle medium (GIBCO) supplemented with fetal calf serum (5%), penicillin (100 IU/ml), and streptomycin (100 μg/ml), and for transfected cells, with G418 sulfate (2 mg/ml). They were harvested after trypsin-EDTA treatment (GIBCO) and irradiated at 30 Gy (Mark I-68A irradiator; J.L. Shepherd and Associates, San Fernando, Calif.) to prevent further cell replication. They were used to induce in vitro CD40 triggering on SC. 3T3-CD40L transfectants (5 × 104) were seeded together with 5 × 106 SC per well (24-well culture plate) in 500 μl of culture medium.
For in vivo experiments, mice were intravenously injected with 106 3T3-CD40L transfectants in 150 μl of phosphate-buffered saline (PBS). 3T3 control fibroblasts and PBS were used as controls. To assess the kinetics of IL-12 secretion, blood samples (100 μl/mouse) were taken from uninfected mice by retroorbital puncture on days 0, 1, 4, 7, and 10 postinjection. Individual blood samples were centrifuged (10 min, 800 × g), and plasma samples were stored at −70°C until use. For in vivo neutralization of IL-12, anti-IL-12 monoclonal antibody (MAb) (see below, 1.3 mg) was injected intraperitoneally on the day of infection (day 0) and then on days 2 and 4 postinfection (p.i.).
MAbs and reagents.
Neutralizing anti-IFN-γ MAb (R4-6A2; immunoglobulin G1 [IgG1]) and anti-TNF-α MAb (MP6-XT3; IgG1) were purchased from Pharmingen. The anti-IL-12 MAb (C17.8; IgG2a) used in the in vitro experiments was purchased from Genzyme (Cambridge, Mass.). Corresponding control isotype-matched antibodies R3-34 (IgG1) and R35-95 (IgG2a) were purchased from Pharmingen. The hybridoma FGK45, producing an IgG2aκ specific for murine CD40, was kindly provided by A. Rolink (Basel Institute for Immunology, Basel, Switzerland) (43). The hybridoma cells were cultured in standard conditions in RPMI 1640 containing 1% bovine serum. The rat MAb was purified by affinity chromatography with a mouse anti-rat kappa MAb immobilized on Sepharose beads and 3.5 M MgCl2 as elution buffer. Eluted antibodies were extensively dialyzed against PBS and filter sterilized. A nonrelated rat MAb was purified similarly and used as a negative control. Another anti-CD40 MAb (3/23; IgG2a) was from Pharmingen. The in vitro working concentration for all MAbs was 10 μg/ml except for anti-CD40 MAb (20 μg/ml). For in vivo experiments, ascitic MAb anti-IL-12 (C17.8; IgG2a) was used (kindly provided by V. Flamand, Université Libre de Bruxelles, Brussels, Belgium). The ascitic IgG2a antibodies used in vivo as a control were a kind gift from H. Bazin (Université Catholique de Louvain, Brussels, Belgium). FGK45 anti-CD40 MAb was also tested in vivo for its ability to protect mice against T. cruzi infection. For this purpose, we used high doses of FGK45 compared with the one used by others (46): eight mice were injected intravenously with 200 μg of FGK45 MAb at the time of T. cruzi inoculation, and four of them received again 100 μg of MAb at days 1 and 4 p.i. Two control groups of five mice were injected with isotype-matched control MAb or PBS.
N-nitro-l-arginine methyl ester (NAME, 5 mM; Sigma Chemical Co., St. Louis, Mo.) was used as the competitive inhibitor of NO synthase.
Recombinant murine IFN-γ (rIFN-γ; 10 U/ml) was kindly supplied by A. Billiau and H. Herremans (Rega Institute, Leuven, Belgium).
The concentration of endotoxin in all the reagents and media was below 80 pg/ml according to the colorimetric limulus amoebocyte lysate assay (detection limit, 1 pg/ml) (Coatest endotoxin; Chromogenix, Mölndal, Sweden).
Cytokine determinations and nitrite assay.
Enzyme-linked immunosorbent assay (ELISA) kits were purchased from Genzyme for determination of IL-12 (p40 and p70), IFN-γ, and TNF-α. The lower limits of detection of these assays were, respectively, 15, 30, 20 and 35 pg/ml. NO production by MPM was assayed by measuring nitrite, its stable degradation product, by the Griess reaction (20). Supernatants (50 μl) from cultured MPM were harvested after 24 h and mixed with 50 μl of Griess solution (1% sulfanilamide, 0.1% naphthylethylene diamine dihydrochloride, 2% H3PO4). The absorbance was measured at 540 nm in a microplate ELISA reader (Spectracount Microplate Photometer; Packard, Meriden, Conn.). Sodium nitrite (NaNO2) diluted in culture medium was used as a standard. The detection limit of the assay was 2.5 μM.
RESULTS
3T3-CD40L transfectants induce T. cruzi infection clearance through a NO-mediated IL-12-dependent pathway.
The involvement of CD40 ligation in T. cruzi infection was first tested in vitro by using a two-step procedure. As T. cruzi readily infects 3T3 fibroblasts (data not shown), SC were first cocultured with either 3T3-CD40L transfectants, 3T3 control fibroblasts, or medium. Supernatants of these cocultures were then added to MPM cultured at the time of T. cruzi addition. The parasitic index was calculated on MPM, 48 h later. Supernatants from CD40L-activated SC clearly improved control of T. cruzi infection by MPM, whereas supernatants of SC alone or cocultured with 3T3 control fibroblasts did not exert a significant effect (Fig. 1A).
FIG. 1.
Effect of supernatants from CD40L-activated SC cultures on T. cruzi infection of MPM. Supernatants harvested from SC (open bars), from SC incubated with 3T3 control fibroblasts (hatched bars), or from CD40L-activated SC (black bars) were added to MPM at the time of T. cruzi infection, in the presence or absence of NAME, and the parasitic index (A) and nitrite levels (B) were measured. Data are means ± standard deviations from three independent experiments performed in duplicate. ∗∗∗, P < 0.001 compared to 3T3 control fibroblasts (Student’s t test).
In light of the well-known protective role of NO in T. cruzi infection, NO levels were determined in culture supernatants of MPM. Supernatants from CD40L-activated SC induced a strong up-regulation of NO production by MPM. By contrast, the NO level was low when MPM were incubated with supernatants from SC-3T3 control fibroblast cultures or SC alone (Fig. 1B). The addition of NAME to MPM at the time of SC supernatant addition abolished the NO overproduction as well as their effect on the parasitic index (Fig. 1).
To identify the causative agents responsible for the induction of NO production by MPM, IFN-γ and TNF-α levels were measured in supernatants from CD40L-activated SC. As expected, IFN-γ and TNF-α synthesis was induced by 3T3-CD40L transfectants (data not shown). Furthermore, the addition of anti-IFN-γ and anti-TNF-α MAbs to CD40L-activated SC inhibited NO production by MPM, demonstrating that NO production was dependent upon the presence of these two cytokines (Table 1).
TABLE 1.
Nitrite production is dependent on IFN-γ and TNF-α production by CD40L-activated SCa
| MAb added to CD40L-activated SC | Nitrite production (μM) by macrophages |
|---|---|
| Isotype control | 60.7 ± 5.1 |
| Anti-IFN-γ | 11.7 ± 5.7*** |
| Anti-TNF-α | 12.9 ± 2.6*** |
SC were activated with 3T3-CD40L transfectants in the presence of anti-IFN-γ or anti-TNF-α MAb. Supernatants were harvested after 48 h and transferred to MPM cultures at the time of T. cruzi infection. Nitrite levels were measured after 24 h. Data are means ± standard deviations of three independent experiments performed in duplicate. ∗∗∗, P < 0.001 compared to isotype control (Student’s t test).
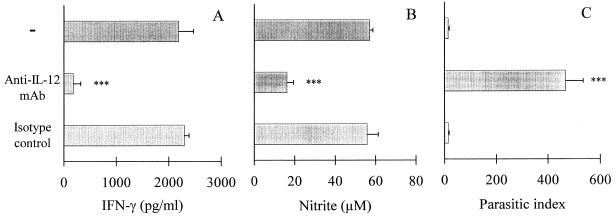
IL-12 is a major component of a complex biochemical pathway inducing IFN-γ synthesis and leading to NO production by macrophages. Accordingly, IL-12 p40 and IL-12 p70 (the bioactive heterodimer) levels were assayed in the supernatants from CD40L-activated SC collected after 48 h (Table 2). In contrast to supernatants from SC cultured in the presence of 3T3 control fibroblasts or medium alone, we found a significant increase of both IL-12 p40 and p70 when SC had been cocultured with 3T3-CD40L transfectants. The addition of neutralizing anti-IL-12 MAb to the culture of CD40L-activated SC inhibited the production of IFN-γ by SC (Fig. 2A). The supernatants also failed to induce NO production by MPM (Fig. 2B) and lost their clearing effect against T. cruzi infection (Fig. 2C).
TABLE 2.
Production of IL-12 p40 and IL-12 p70 by SC incubated with 3T3-CD40L transfectantsa
| Treatment of SC | IL-12 p40 (pg/ml) | IL-12 p70 (pg/ml) |
|---|---|---|
| None | 3,052 ± 1,079 | <15 |
| 3T3 | 5,852 ± 3,201 | <15 |
| 3T3-CD40L | 34,266 ± 10,523* | 63.8 ± 35.3* |
SC were incubated with either 3T3 control fibroblasts or 3T3-CD40L transfectants for 48 h. IL-12 p40 and IL-12 p70 levels were measured in culture supernatants. Data are means ± standard deviations from three (IL-12 p40) and five (IL-12 p70) independent experiments performed in duplicate. ∗, P < 0.05 compared to 3T3 control fibroblasts (Student’s t test).
FIG. 2.
Effect of neutralizing anti-IL-12 MAb added to CD40L-activated SC on their IFN-γ and NO production and parasitic index of T. cruzi-infected MPM. SC were activated with 3T3-CD40L transfectants in the presence of anti-IL-12 MAb, isotype-matched control, or medium for 48 h. IFN-γ levels were measured in culture supernatants after 48 h (A). Then, culture supernatants were transferred to T. cruzi-infected MPM. Nitrite levels (B) and parasitic index (C) were measured after 24 and 48 h, respectively. Data are means ± standard deviations from three independent experiments performed in duplicate. ∗∗∗, P < 0.001 compared to data obtained with culture supernatants harvested from SC incubated with isotype-matched control (Student’s t test).
Activating anti-CD40 MAb directly enhances parasite control by IFN-γ-activated macrophages.
We also triggered the CD40-CD40L pathway by using agonistic anti-CD40 MAb (FGK45) to directly activate MPM in vitro. MPM were infected with T. cruzi trypomastigotes and treated with anti-CD40 MAb together with a suboptimal concentration of 10 U of rIFN-γ ml. FGK45 anti-CD40 MAb clearly augments NO production by IFN-γ-activated MPM (Table 3). Similar results were found with another anti-CD40 MAb (3/23, data not shown). This NO up-regulation correlated with an improved control of T. cruzi infection, which was blocked in the presence of NAME but still active in the presence of neutralizing anti-IL-12 MAb (Table 3).
TABLE 3.
Effect of activating anti-CD40 MAb on IFN-γ-activated MPMa
| Treatment | Parasitic index
|
Nitrite (μM)
|
||
|---|---|---|---|---|
| Isotype control | Anti-CD40 MAb | Isotype control | Anti-CD40 MAb | |
| None | 377 ± 53 | 118 ± 30** | 23.6 ± 1.3 | 43.1 ± 4.4** |
| NAME | 459 ± 81 | 428 ± 93 | 6.4 ± 3.6 | 7.7 ± 3.9 |
| Anti-IL-12 MAb | 355 ± 104 | 115 ± 11** | 23.9 ± 3.0 | 41.5 ± 7.1** |
MPM were treated at the time of T. cruzi infection with 10 U of rIFN-γml in combination with isotype-matched control MAb or FGK45 anti-CD40 MAb in the presence or absence of NAME or neutralizing anti-IL-12 MAb. Parasitic index and nitrite levels were measured. Data are from three independent experiments performed in duplicate. ∗∗, P < 0.01 compared to isotype-matched control MAb (Student’s t test).
Injection of 3T3-CD40L transfectants protects mice against T. cruzi infection.
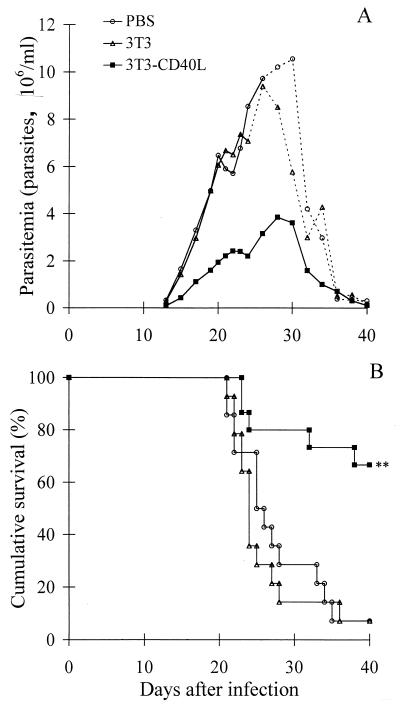
To evaluate in vivo the protective effect of CD40 stimulation on T. cruzi infection, mice were injected with 3T3-CD40L transfectants, 3T3 control fibroblasts, or PBS at the same time of T. cruzi inoculation. The injection of 3T3-CD40L transfectants reduced the peak of parasitemia (Fig. 3A) and considerably increased the survival rate of T. cruzi-infected mice (Fig. 3B). Most of the mice (10 of 15; n = 3) survived the acute phase of infection and entered the chronic phase. In contrast, only one of the infected mice survived after 3 weeks when the groups were injected either with 3T3 control fibroblasts (1 of 14; n = 3) or PBS (1 of 14; n = 3). These data indicate that a single injection of 3T3-CD40L transfectants protects most of the mice against fatal infection. However, injection of 400 μg of activating anti-CD40 MAb did not protect mice against T. cruzi infection (data not shown).
FIG. 3.
Effect of injection of 3T3-CD40L transfectants in T. cruzi-infected mice. Three groups of mice were inoculated with T. cruzi and injected with either 3T3-CD40L transfectants, 3T3 control fibroblasts, or PBS. Parasitemia (A) and cumulative survival (B) are reported. Dotted lines represent the parasitemia when less than 50% of mice were still alive. Data were pooled from three independent experiments (n = 14 or 15 mice per group). For parasitemia, the difference between the experimental and control groups was significant (P < 0.01, Mann-Whitney U test) for the period from day 15 to day 24 p.i. ∗∗, P < 0.002 (χ2 analysis).
We also tested the ability of 3T3-CD40L transfectants to modify the course of an established infection. For this purpose, mice were inoculated with T. cruzi and injected with 3T3-CD40L transfectants or 3T3 control fibroblasts 8 days later. Treatment by 3T3-CD40L transfectants reduced the peak of parasitemia to 4.0 × 106 parasite/ml versus 9.2 × 106 parasites/ml in mice injected with control transfectants (P < 0.05 between day 20 and 29 p.i., Mann-Whitney U test). In parallel, the lethality rate was slightly reduced (on day 41, 14% of control mice survived versus 40% of the treatment group); however, this difference did not reach significance (χ2 analysis).
The protective effect of CD40 ligation in T. cruzi-infected mice is related to up-regulation of IL-12.
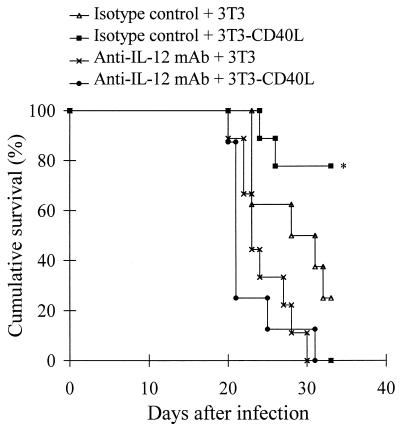
To assess the role of IL-12 in the protective effect of CD40 ligation in vivo, we first measured IL-12 p40 levels in the serum of 3T3-CD40L-injected mice. These experiments confirmed that CD40-CD40L interactions induce IL-12 synthesis in vivo (Fig. 4). We then evaluated the effect of IL-12 neutralization on the outcome of infection. For this, neutralizing anti-IL-12 MAb (or its isotype-matched control) was coinjected with 3T3-CD40L transfectants in T. cruzi-infected mice. Mortality follow-up showed that protection obtained with injection of 3T3-CD40L transfectants was prevented by IL-12 neutralization but not by injection of the isotype-matched control (Fig. 5). These data establish that CD40L-mediated protection in vivo depends on IL-12 release.
FIG. 4.
Kinetics of IL-12 synthesis in serum of 3T3-CD40L transfectant-injected mice. IL-12 p40 levels were measured in blood samples obtained from mice injected with 3T3-CD40L transfectants (n = 10) (black bars) or 3T3 control fibroblasts (n = 8) (open bars). Data are means ± standard errors of the means. ∗, P < 0.05; ∗∗∗, P < 0.01 compared to day 0 (Student’s t test).
FIG. 5.
Effect of injection of neutralizing anti-IL-12 MAb on T. cruzi-infected and 3T3-CD40L transfectant-treated mice. Mice (n = 10) were inoculated with T. cruzi trypomastigotes and injected with 3T3-CD40L transfectants or 3T3 control fibroblasts. Neutralizing anti-IL-12 MAb or isotype-matched control was injected intraperitoneally on the day of infection (day 0) and then on days 2 and 4 p.i. Cumulative survival was reported. ∗, P < 0.05 (χ2 analysis).
DISCUSSION
In the present study, we demonstrate that CD40 ligation leads to the control of T. cruzi infection through the induction of NO. Several studies have shown that CD40-CD40L interactions could result in NO production (32, 48, 50, 52). Our results indicate that this is achieved by inducing IL-12 production as well as by direct stimulation of IFN-γ-activated macrophages. In vitro, high levels of IL-12, IFN-γ, and TNF-α are produced by CD40L-activated SC. Supernatants from these SC cultures stimulate MPM, which become able to control T. cruzi infection through NO production. This NO production is inhibited when neutralizing anti-IL-12, anti-IFN-γ, or anti-TNF-α MAbs are added to the SC cultures with 3T3-CD40L transfectants. These data confirm that CD40-CD40L interactions among SC promote the synthesis of IL-12 (9, 27, 31), which in turn, induces IFN-γ secretion (10, 41, 44, 55). IFN-γ acts in synergy with TNF-α to stimulate the production of NO (17), resulting in parasite clearing. Finally, CD40 ligation also directly stimulates IFN-γ-activated MPM to produce NO and thereby to control T. cruzi infection. This cascade is probably operative in vivo as a single injection of 3T3-CD40L transfectants to mice at the time of T. cruzi inoculation exerts a clear-cut protective effect which is mediated by IL-12. We show also that a single injection of 3T3-CD40L transfectants even 8 days p.i. is still able to reduce parasitemia. This is in line with previous studies showing that treatment of T. cruzi-infected mice with anti-IL-12 MAb has an exacerbating effect on both parasitemia and mortality (3) while an exogenous supply of IL-12 protects mice (25).
Our results are also consistent with a recent study showing an IL-12-dependent protection in mice infected with Leishmania (a closely-related parasitic protozoa) and injected with anti-CD40-stimulating MAb (15). Furthermore, exacerbated Leishmania infection is observed when CD40-CD40L interactions are disrupted (6, 23, 26, 48). Likewise, a CD40-CD40L-mediated protective effect is also observed with other pathogens such as Cryptosporidium parvum (12) and Pneumocystis carinii (59). In contrast, this is not the case with other pathogens such as Borrelia burgdorferi (16), Listeria monocytogenes (21), Mycobacterium tuberculosis (7), and Histoplasma capsulatum (60). This discrepancy could be explained by differential abilities of infectious agents to induce IL-12 production by the host (7, 60). CD40-CD40L interaction would be determinant only when IL-12 production induced by pathogens is insufficient, as has been shown in the course of Leishmania infection (5).
3T3-CD40L transfectants are found to be more efficient than activating anti-CD40 MAb in improving parasite control, and this data is in agreement with previous observations obtained with B cells (30). This is most likely due to a more efficient cross-linking of CD40 molecules by CD40L expressed at high density at the fibroblast membrane compared with the agonistic anti-CD40 IgG MAb.
CD40L stimulating properties have already been used in the treatment of tumors, tumor regression being linked to restoration of major histocompatibility complex class I expression by tumor cells (18, 28, 36), IL-12 overproduction, or potentiation of host antigen-presenting cell functions. Moreover, CD40 engagement restores, at least in vitro, production of IL-12 by cells from human immunodeficiency virus (HIV)-infected patients and it stimulates macrophages to produce HIV-1-suppressive chemokines (11, 29). According to our results, activation of the immune system through CD40 ligation could also be considered a potent strategy for immunotherapy of parasitic diseases.
ACKNOWLEDGMENTS
We thank H. Herremans and A. Billiau (Rega Institute, Leuven, Belgium) for providing rIFN-γ; A. Scheich, who edited the English text; V. Vercruysse for valuable technical assistance; and I. Mazza for help in preparing the manuscript.
This work was supported by grants from Action de Recherche Concertée de la Communauté Française de Belgique, the Fonds Emile Defay, the Sportvereniging tegen Kanker (M.d.V.), and the Fund for Scientific Research-Flanders.
REFERENCES
- 1.Abrahamson I A, Coffman R L. Cytokine and nitric oxide regulation of the immunosuppression in Trypanosoma cruzi infection. J Immunol. 1995;155:3955–3963. [PubMed] [Google Scholar]
- 2.Abrahamson I A, Coffman R L. Trypanosoma cruzi: IL-10, TNF, IFN-gamma, and IL-12 regulate innate and acquired immunity to infection. Exp Parasitol. 1996;84:231–244. doi: 10.1006/expr.1996.0109. [DOI] [PubMed] [Google Scholar]
- 3.Aliberti J C S, Cardoso M A G, Martins G A, Gazzinelli R T, Vieira L Q, Silva J S. Interleukin-12 mediates resistance to Trypanosoma cruzi in mice and is produced by murine macrophages in response to live trypomastigotes. Infect Immun. 1996;64:1961–1967. doi: 10.1128/iai.64.6.1961-1967.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armitage R J, Fanslow W C, Strockbine L, Sato T A, Clifford K N, Macduff B M, Anderson D M, Gimpel S D, Davis-Smith T, Maliszewski C R, Clark E A, Smith C A, Grabstein K H, Cosman D, Spriggs M K. Molecular and biological characterization of a murine ligand for CD40. Nature. 1992;357:80–82. doi: 10.1038/357080a0. [DOI] [PubMed] [Google Scholar]
- 5.Belkaid Y, Butcher B, Sacks D L. Analysis of cytokine production by inflammatory mouse macrophages at the single-cell level: selective impairment of IL-12 induction in Leishmania-infected cells. Eur J Immunol. 1998;28:1389–1400. doi: 10.1002/(SICI)1521-4141(199804)28:04<1389::AID-IMMU1389>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 6.Campbell K A, Ovendale P J, Kennedy M K, Fanslow W C, Reed S G, Maliszewski C R. CD40 ligand is required for protective cell-mediated immunity to Leishmania major. Immunity. 1996;4:283–289. doi: 10.1016/s1074-7613(00)80436-7. [DOI] [PubMed] [Google Scholar]
- 7.Campos-Neto A, Ovendale P, Bement T, Koppi T A, Fanslow W C, Rossi M A, Alderson M R. CD40 ligand is not essential for the development of cell-mediated immunity and resistance to Mycobacterium tuberculosis. J Immunol. 1998;160:2037–2041. [PubMed] [Google Scholar]
- 8.Cardillo F, Voltarelli J C, Reed S G, Silva J S. Regulation of Trypanosoma cruzi infection in mice by gamma interferon and interleukin 10: role of NK cells. Infect Immun. 1996;64:128–134. doi: 10.1128/iai.64.1.128-134.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cella M, Scheidegger D, Palmer-Lehman K, Lane P, Lanzavecchia A, Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J Exp Med. 1996;184:747–752. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan S H, Perussia B, Gupta J W, Kobayashi M, Pospisil M, Young H A, Wolf S F, Young D, Clark S C, Trinchieri G. Induction of IFN-γ production by NK cell stimulatory factor (NKSF): characterization of the responder cells and synergy with other inducers. J Exp Med. 1991;173:869–879. doi: 10.1084/jem.173.4.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chougnet C, Thomas E, Landay A L, Kessler H A, Buchbinder S, Scheer S, Shearer G M. CD40 ligand and IFN-γ synergistically restore IL-12 production in HIV-infected patients. Eur J Immunol. 1998;28:646–656. doi: 10.1002/(SICI)1521-4141(199802)28:02<646::AID-IMMU646>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 12.Cosyns M, Tsirkin S, Jones M, Flavell R A, Kikutani H, Hayward A R. Requirement for CD40-CD40 ligand interaction for elimination of Cryptosporidium parvum from mice. Infect Immun. 1998;66:603–607. doi: 10.1128/iai.66.2.603-607.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deng W, Thiel B, Tannenbaum C S, Hamilton T A, Stuehr D J. Synergistic cooperation between T cell lymphokines for induction of the nitric oxide synthase gene in murine peritoneal macrophages. J Immunol. 1993;151:322–329. [PubMed] [Google Scholar]
- 14.El Bouhdidi A, Truyens C, Rivera M-T, Bazin H, Carlier Y. Trypanosoma cruzi infection in mice induces a polyisotypic hyper-gammaglobulinaemia and parasite-specific response involving high IgG2a concentrations and highly avid IgG1 antibodies. Parasite Immunol. 1994;16:69–76. doi: 10.1111/j.1365-3024.1994.tb00325.x. [DOI] [PubMed] [Google Scholar]
- 15.Ferlin W G, von der Weld T, Cottrez F, Ferrick D A, Coffman R L, Howard M C. The induction of a protective response in Leishmania major-infected BALB/c mice with anti-CD40 mAb. Eur J Immunol. 1998;28:525–531. doi: 10.1002/(SICI)1521-4141(199802)28:02<525::AID-IMMU525>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 16.Fikrig E, Barthold S W, Chen M, Grewal I S, Craft J, Flavell R A. Protective antibodies in murine Lyme disease arise independently of CD40 ligand. J Immunol. 1996;157:1–3. [PubMed] [Google Scholar]
- 17.Frankova D, Zidek Z. IFN-γ-induced TNF-α is a prerequisite for in vitro production of nitric oxide generated in murine peritoneal macrophages by IFN-γ. Eur J Immunol. 1998;28:838–843. doi: 10.1002/(SICI)1521-4141(199803)28:03<838::AID-IMMU838>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 18.Funakoshi S, Taub D D, Asai O, Hirano A, Ruscetti F W, Longo D L, Murphy W J. Effects of CD40 stimulation in the prevention of human EBV-lymphomagenesis. Leuk Lymphoma. 1997;24:187–199. doi: 10.3109/10428199709039007. [DOI] [PubMed] [Google Scholar]
- 19.Gazzinelli R T, Oswald I P, Hieny S, James S L, Sher A. The microbicidal activity of interferon-γ-treated macrophages against Trypanosoma cruzi involves an l-arginine-dependent, nitrogen oxide-mediated mechanism inhibitable by interleukin-10 and transforming growth factor-β. Eur J Immunol. 1992;22:2501–2506. doi: 10.1002/eji.1830221006. [DOI] [PubMed] [Google Scholar]
- 20.Green L C, Wagner D A, Golowski J, Skipper P L, Wishnock J S, Tannenbaum S R. Analysis of nitrate, nitrite and (15N) nitrate in biological fluids. Anal Biochem. 1982;126:136–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 21.Grewal I S, Borow P, Pamer E G, Oldstone M B A, Flavell R A. The CD40-CD154 system in anti-infective host defense. Curr Opin Immunol. 1997;9:491–497. doi: 10.1016/s0952-7915(97)80100-8. [DOI] [PubMed] [Google Scholar]
- 22.Grewal I S, Flavell R A. CD40 and CD154 in cell-mediated immunity. Annu Rev Immunol. 1998;16:111–135. doi: 10.1146/annurev.immunol.16.1.111. [DOI] [PubMed] [Google Scholar]
- 23.Heinzel F P, Rerko R M, Hujer A M. Underproduction of interleukin-12 in susceptible mice during progressive leishmaniasis is due to decreased CD40 activity. Cell Immunol. 1998;184:129–142. doi: 10.1006/cimm.1998.1267. [DOI] [PubMed] [Google Scholar]
- 24.Hölscher C, Köhler G, Müller U, Mossmann H, Schaub G A, Brombacher F. Defective nitric oxide effector functions lead to extreme susceptibility of Trypanosoma cruzi-infected mice deficient in gamma interferon receptor or inducible nitric oxide synthase. Infect Immun. 1998;66:1208–1215. doi: 10.1128/iai.66.3.1208-1215.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hunter C A, Slifer T, Araujo F. Interleukin-12-mediated resistance to Trypanosoma cruzi is dependent on tumor necrosis factor alpha and gamma interferon. Infect Immun. 1996;64:2381–2386. doi: 10.1128/iai.64.7.2381-2386.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kamanaka M, Yu P, Yasui T, Yoshida K, Kawabe T, Horii T, Kishimoto T, Kikutani H. Protective role of CD40 in Leishmania major infection at two distinct phases of cell-mediated immunity. Immunity. 1996;4:275–281. doi: 10.1016/s1074-7613(00)80435-5. [DOI] [PubMed] [Google Scholar]
- 27.Kato T, Yamane H, Nariuchi H. Differential effects of LPS and CD40 ligand stimulations on the induction of IL-12 production by dendritic cells and macrophages. Cell Immunol. 1997;181:59–67. doi: 10.1006/cimm.1997.1196. [DOI] [PubMed] [Google Scholar]
- 28.Khanna R, Cooper L, Kienzle N, Moss D J, Burrows S R, Khanna K K. Engagement of CD40 antigen with soluble CD40 ligand up-regulates peptide transporter expression and restores endogenous processing function in Burkitt’s lymphoma cells. J Immunol. 1997;159:5782–5785. [PubMed] [Google Scholar]
- 29.Kornbluth R S, Kee K, Richman D D. CD40 ligand (CD154) stimulation of macrophages to produce HIV-1-suppressive beta-chemokines. Proc Natl Acad Sci USA. 1998;95:5205–5210. doi: 10.1073/pnas.95.9.5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lamman J D, Claassen E, Noelle R J. Functions of CD40 and its ligand, gp39 (CD40L) Crit Rev Immunol. 1996;16:59–108. doi: 10.1615/critrevimmunol.v16.i1.40. [DOI] [PubMed] [Google Scholar]
- 31.Lane P, Brocker T, Hubele S, Padovan E, Lanzavecchia A, McConnel F. Soluble CD40 ligand can replace the normal T cell-derived CD40 ligand signal to B cells in T cell-dependent activation. J Exp Med. 1993;177:1209–1213. doi: 10.1084/jem.177.4.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu L, Bonham C A, Chambers F G, Watkins S C, Hoffman R A, Simmons R L, Thomson A W. Induction of nitric oxide synthase in mouse dendritic cells by IFN-γ, endotoxin, and interaction with allogeneic T cells. J Immunol. 1996;157:3577–3586. [PubMed] [Google Scholar]
- 33.MacMicking J, Xie Q-w, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol. 1997;15:323–350. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- 34.Metz G, Carlier Y, Vray B. Trypanosoma cruzi upregulates nitric oxide release by IFN-γ-preactivated macrophages, limiting cell infection independently of the respiratory burst. Parasite Immunol. 1993;15:693–699. doi: 10.1111/j.1365-3024.1993.tb00584.x. [DOI] [PubMed] [Google Scholar]
- 35.Munoz-Fernandez M A, Fernandez M A, Fresno M. Synergism between tumor necrosis factor-α and interferon-γ on macrophage activation for the killing of intracellular Trypanosoma cruzi through a nitric oxide-dependent mechanisms. Eur J Immunol. 1992;22:301–307. doi: 10.1002/eji.1830220203. [DOI] [PubMed] [Google Scholar]
- 36.Nakajima A, Kodama T, Morimoto S, Azuma M, Taked K, Oshima H, Yoshino S-I, Yagata H, Okumura K. Antitumor effect of CD40 ligand: elicitation of local and systemic antitumor responses by IL-12 and B7. J Immunol. 1998;161:1901–1907. [PubMed] [Google Scholar]
- 37.Norris K A, Schrimpf J E, Flynn J L, Morris S M., Jr Enhancement of macrophage microbicidal activity: supplemental arginine and citrulline augment nitric oxide production in murine peritoneal macrophages and promote intracellular killing of Trypanosoma cruzi. Infect Immun. 1995;63:2793–2796. doi: 10.1128/iai.63.7.2793-2796.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Olivares Fontt E, Vray B. Relationship between granulocyte macrophage-colony stimulating factor, tumor necrosis factor-α and Trypanosoma cruzi infection in murine macrophages. Parasite Immunol. 1995;17:135–141. doi: 10.1111/j.1365-3024.1995.tb01015.x. [DOI] [PubMed] [Google Scholar]
- 39.Petray P, Rottenberg M, Grinstein S, Orn A. Release of nitric oxide during the experimental infection with Trypanosoma cruzi. Parasite Immunol. 1994;16:193–199. doi: 10.1111/j.1365-3024.1994.tb00340.x. [DOI] [PubMed] [Google Scholar]
- 40.Plasman N, Metz G, Vray B. Interferon-γ-activated immature macrophages exhibit a high Trypanosoma cruzi infection rate associated with a low production of both nitric oxide and tumor necrosis factor-α. Parasitol Res. 1994;80:554–558. doi: 10.1007/BF00933002. [DOI] [PubMed] [Google Scholar]
- 41.Puddu P, Fantuzzi L, Borghi P, Varano B, Rainaldi G, Guillemard E, Malorni W, Nicaise P, Wolf S F, Belardelli F, Gessani S. IL-12 induces IFN-γ expression and secretion in mouse peritoneal macrophages. J Immunol. 1997;159:3490–3497. [PubMed] [Google Scholar]
- 42.Reed S G. In vivo administration of recombinant IFN-γ induces macrophage activation, and prevents acute disease, immune suppression, and death in experimental Trypanosoma cruzi infections. 1988. J Immunol. 1988;140:4342–4347. [PubMed] [Google Scholar]
- 43.Rolink A, Melchers F, Andersson J. The SCID but not the RAG-2 gene product is required for Sμ-Sɛ heavy chain class switching. Immunity. 1996;5:319–330. doi: 10.1016/s1074-7613(00)80258-7. [DOI] [PubMed] [Google Scholar]
- 44.Romani L, Puccetti P, Bistoni F. Interleukin-12 in infectious diseases. Clin Microbiol Rev. 1997;10:611–636. doi: 10.1128/cmr.10.4.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Santos Lima E C, Garcia I, Vicentelli M-H, Vassalli P, Minoprio P. Evidence for a protective role of tumor necrosis factor in the acute phase of Trypanosoma cruzi infection in mice. Infect Immun. 1997;65:457–465. doi: 10.1128/iai.65.2.457-465.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schoenberger S P, Toes R E M, Van der Voort E I H, Offringa R, Melief C J M. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interaction. Nature. 1998;393:480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 47.Silva J P, Morrissey K, Grabstein K, Mohler D, Anderson S, Reed S G. Interleukin 10, and IFN-γ regulation of experimental Trypanosoma cruzi infection. J Exp Med. 1992;175:169–174. doi: 10.1084/jem.175.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Soong L, Xu J-C, Grewal I S, Kima P, Sun J, Longley B J, Jr, Ruddle N H, McMahon-Pratt D, Flavell R A. Disruption of CD40-CD40 ligand interactions results in an enhanced susceptibility to Leishmania amazonensis infection. Immunity. 1996;4:263–273. doi: 10.1016/s1074-7613(00)80434-3. [DOI] [PubMed] [Google Scholar]
- 49.Stout R D, Suttles J. The many roles of CD40 in cell-mediated inflammatory responses. Immunol Today. 1996;17:487–492. doi: 10.1016/0167-5699(96)10060-i. [DOI] [PubMed] [Google Scholar]
- 50.Stout R D, Suttles J, Xu J, Grewal I S, Flavell R A. Impaired T cell-mediated macrophages activation in CD40 ligand-deficient mice. J Immunol. 1996;156:8–11. [PubMed] [Google Scholar]
- 51.Tanowitz H B, Simon D, Morris S A, Weiss L M, Wittner M. Chagas’ disease. Clin Microbiol Rev. 1992;5:404–419. doi: 10.1128/cmr.5.4.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tian L, Noelle R J, Lawrence D A. Activated T cells enhance nitric oxide production by murine splenic macrophages through gp39 and LFA-1. Eur J Immunol. 1995;25:306–309. doi: 10.1002/eji.1830250152. [DOI] [PubMed] [Google Scholar]
- 53.Torrico F, Heremans H, Rivera M T, Van Marck E, Billiau A, Carlier Y. Endogenous IFN-γ is required for resistance to acute Trypanosoma cruzi infection in mice. J Immunol. 1991;146:3626–3632. [PubMed] [Google Scholar]
- 54.Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:251–276. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 55.Trinchieri G. Cytokines acting on or secreted by macrophages during intracellular infection (IL-10, IL-12, IFN-γ) Curr Opin Immunol. 1997;9:17–23. doi: 10.1016/s0952-7915(97)80154-9. [DOI] [PubMed] [Google Scholar]
- 56.Van Kooten C, Banchereau J. Functions of CD40 on B cells, dendritic cells and other cells. Curr Opin Immunol. 1997;9:330–337. doi: 10.1016/s0952-7915(97)80078-7. [DOI] [PubMed] [Google Scholar]
- 57.Vespa G N R, Cunha F Q, Silva J S. Nitric oxide is involved in control of Trypanosoma cruzi-induced parasitemia and directly kills the parasite in vitro. Infect Immun. 1994;62:5177–5182. doi: 10.1128/iai.62.11.5177-5182.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vray B, de Baetselier P, Ouaissi A, Carlier Y. Trypanosoma cruzi but not Trypanosoma brucei fails to induce a chemiluminescent signal in a macrophage hybridoma cell line. Infect Immun. 1991;59:3303–3308. doi: 10.1128/iai.59.9.3303-3308.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wiley J A, Harmsen A G. CD40 ligand is required for resolution of Pneumocystis carinii pneumonia in mice. J Immunol. 1995;155:3525–3529. [PubMed] [Google Scholar]
- 60.Zhou P, Seder R A. CD40 ligand is not essential for induction of type 1 cytokine responses or protective immunity after primary or secondary infection with Histoplasma capsulatum. J Exp Med. 1998;187:1315–1324. doi: 10.1084/jem.187.8.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]