Abstract
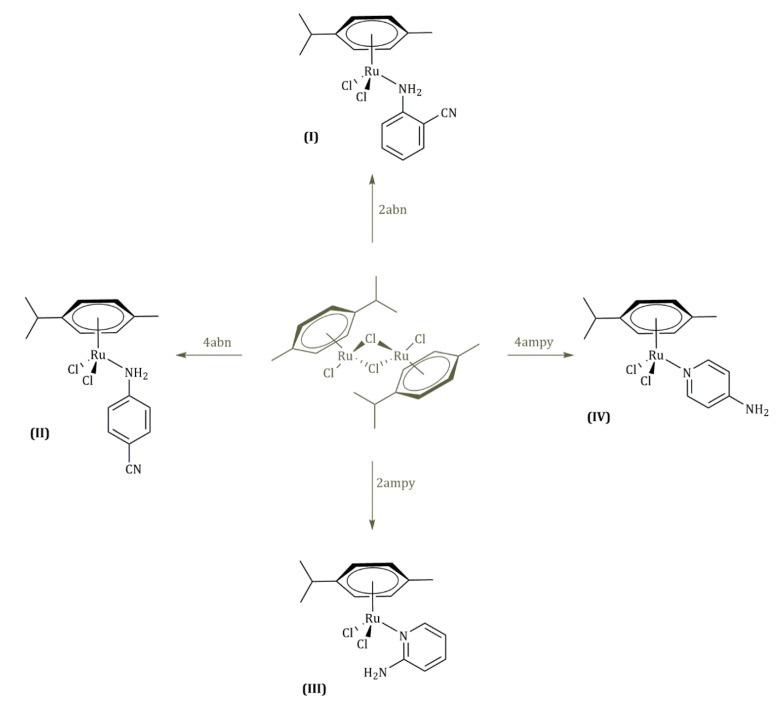
When the [Ru(p-cymene)(μ-Cl)Cl]2 complex is made to react, in dichloromethane, with the following ligands: 2-aminobenzonitrile (2abn), 4-aminobenzonitrile (4abn), 2-aminopyridine (2ampy) and 4-aminopyridine (4ampy), after addition of hexane, the following compounds are obtained: [Ru(p-cymene)Cl2(2abn)] (I), [Ru(p-cymene)Cl2(4abn)] (II), [Ru(p-cymene)Cl2(2ampy] (III) and [Ru(p-cymene)Cl2(μ-(4ampy)] (IV). All the compounds are characterized by elemental analysis of carbon, hydrogen and nitrogen, proton nuclear magnetic resonance, COSY 1H-1H, high-resolution mass spectrometry (ESI), thermogravimetry and single-crystal X-ray diffraction (the crystal structure of III is reported and compared with the closely related literature of II). The cytotoxicity effects of complexes were described for cervical cancer HeLa cells via 3-(4.5-dimethylthiazol-2-yl)-2.5-diphenyltetrazolium bromide (MTT) assay. The results demonstrate a low in vitro anticancer potential of the complexes.
Keywords: ruthenium, complexes, cytotoxicity, anticancer activity, HeLa, MTT assay
1. Introduction
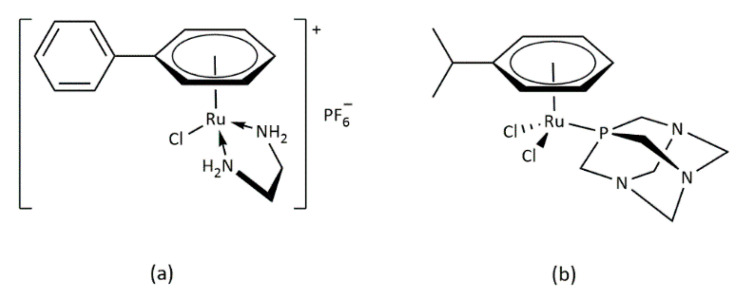
In addition to cis-platinum [1] and other similar compounds such as carbo-platin [2,3,4] or allyl-platinum [5,6,7], it has also been shown that numerous ruthenium (II) compounds are able to exhibit similar properties for cancer therapy [8]. Figure 1 presents some of those that lately have received special attention [9,10,11,12,13].
Figure 1.
Structures of some antitumor active ruthenium complexes. (a) [(η6-biphenil)Ru(en)Cl]PF6 and (b) [(η6-p-cymene)Ru(pta)Cl2].
Recently, the use of platinum-based compounds has been limited because of their lack of cellular selectivity and drug resistance. Ruthenium is attracting the interest of researchers as a promising alternative to platinum-based complexes due to their several oxidation states, Ru (II), Ru (III) and Ru (IV), and improved cellular selectivity [14]. In this way, ruthenium (II) complexes display excellent structural, photophysical and biological properties, which makes them promising anticancer compounds [15]. The mechanism of action for many ruthenium complexes differs from the DNA-binding mechanism typically associated with platinum compounds, having a wider range of intracellular targets [16]. In addition, ruthenium-based drugs have demonstrated superior therapeutic efficiency in robust metastatic cancers or cisplatin resistant tumors [17].
Some years ago, our research group described the synthesis of the compound [Ru(p-cymene)Cl(o-phen)]PF6, (o-phen = o-phenylenediamine) [18], which has shown to be an excellent antitumor agent [19,20]. Moreover, complexes [Ru(p-cymene)Cl2L], L being one of the following substituted pyridines: 2-fluoro-5-aminopyridine, 5-amino-2-chloropyridine and 2-bromo-5-aminopyridine, have been isolated and characterized and have shown moderate cytotoxicity against lung carcinoma A549 and breast cancer MCF-7 cell lines [21]. Recently, J. Ruiz et al. [22] have described a set of ruthenium (II) complexes containing p-cymene, and a C-N ligand with a non-coordinated CHO group, capable of exhibiting anticancer properties towards several human cancer cell lines, including cells of the epithelial ovarian carcinoma A2780, CDDP-resistant ovarian cancer A2780cisR and breast cancer MCF-7. They have also tested the compounds in the non-tumorigenic BGM and CHO cells, finding high selectivity towards cancer cells over normal cells. Ismail et al. [23] have synthesized two new hybrid half-sandwiched Ru(II) arene complexes of a general formula [η6-(p-cymene)Ru(L)Cl] (where L = 1-(Benzazol-2-yl)-3-(thiophen-2-yl) propane-1,3-dione), which have exhibited significant inhibitory activity against human breast and lung cancer cells (MCF-7 and A549), finding lower IC50 values than those of clinical cisplatin drugs. Recently, our group has prepared new compounds of ruthenium (II), neutral and ionic that behave as anticancer agents, especially one of them: [Ru(p-cymene)(2amfol)Cl2];, (2amfol = 2-aminephenol) against HeLa and MCF-7 cells [24].
Nowadays, scientists worldwide have expanded the use of specific features of organometallic ruthenium compounds (e.g., structural diversity, ligand exchangeability, redox and catalytic properties) for medicinal purposes with surprising results. The aim of this work is the synthesis and characterization of organometallic ruthenium compounds, obtained by reacting [Ru(p-cymene) Cl(μ-Cl)]2 against potentially bidentate ligands with two donor nitrogen atoms, obtaining the new complexes [Ru(p-cymene) Cl2L]; L = 2abn, 4abn, 2ampy and 4ampy. In addition, a study of their properties as antitumor agents was carried out by MTT assay on HeLa cells.
2. Results and Discussion
Although the ligands used are potentially bidentate, they always act as monodentate across the amine nitrogen (abn) or pyridine nitrogen (ampy). This behavior has been previously observed on ruthenium (II) complexes [18,24]. Only D. S. Pandey et al. have described a related compound in which the CN group acts as a bridge [25].
The four ruthenium (II) complexes shown in Figure 2 were obtained with good yields, and they are air-stable solids that present colors in the yellow–orange range. All of them gave satisfactory analyses of carbon, hydrogen and nitrogen (see Table 1). Moreover, all the isolated complexes are neutral in acetone solutions 5 × 10−4 M [25].
Figure 2.
Complexes I, II, III and IV.
Table 1.
Color, yield, Elemental Analysis, Exact Mass and Decomposition temperatures from complexes I, II, III and IV.
| Comp. | Color | Yield (%) | Analysis a (%) | Exact Mass a (g/mol) | M.P. b (°C) | ||||
|---|---|---|---|---|---|---|---|---|---|
| C | H | N | [M-H]+ | [M-Cl]+ | [M-2Cl]+ | ||||
| I | Yellow–orange | 70 | 48.09 (48.12) | 4.73 (4.75) | 6.40 (6.60) | 388.8792 (388.8812) | - | 69 | |
| II | Yellow–orange | 90 | 48.25 (48.12) | 4.77 (4.75) | 6.53 (6.60) | 425.3591 (425.3418) | - | - | 90 |
| III | Orange | 85 | 44.86 (45.01) | 5.06 (5.04) | 6.83 (7.00) | - | - | 329.0595 (329.4365) | 78 |
| IV | Light Brown | 90 | 44.70 (45.01) | 4.87 (5.04) | 6.95 (7.00) | - | 329.0599 (329.4365) | 72 | |
a Calculates values in parenthesis. b Decomposition temperatures from the thermogravimetric curves.
The X-ray diffraction structures of complexes II [26] and III [27] have been previously described, however, in the case of II, no additional data have been presented and, in the case of III, in our hands, another polymorph with a different space group is obtained.
2.1. Molecular Structure
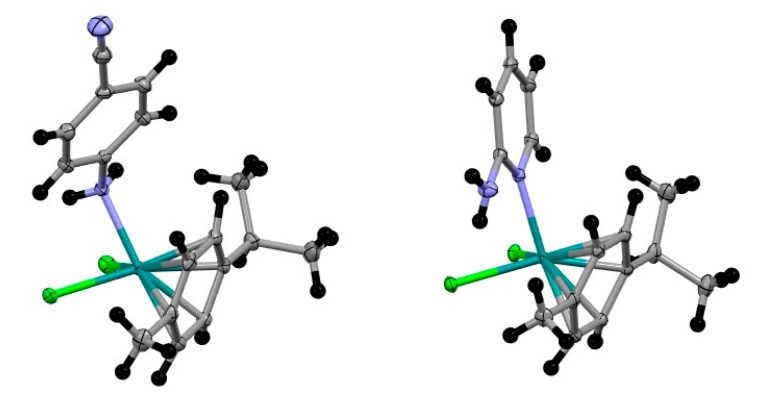
Complexes exhibit distorted octahedral geometry: three positions occupied by chlorine and N atoms and the three remaining by p-cymene ligand. The highest deviation from the ideal 90 degree bond angle is N-RuCl2, 80.17o (II) and N-Ru-Cl2, 86.22° (III). Most relevant molecular parameters are shown in Table 2. The difference in the hybridization of coordinated nitrogen explains the difference in Ru-N distances: coordinated N in II is sp3 and in III is sp (see Figure 3). Both Cl-Ru distances are similar in RuCl2(p-cymene) (4-aminobenzonitrile), but in RhCl2(p-cymene) (2-aminopyridine), Cl-Rh distances are different (Table 2); the reason is an intramolecular hydrogen bond N-H···Cl that lengthens Rh-Cl(1) distance.
Table 2.
Relevant molecular parameters for II and III.
| RuCl2(p-Cymene)(4-Aminobenzonitrile) | RhCl2(p-Cymene)(2-Aminopyridine) | ||
|---|---|---|---|
| Ru-N | 2.1756(18) Å | Ru-N | 2.1693(14) Å |
| Ru-Cl(1) | 2.4260(5) Å | Ru-Cl(1) | 2.4545(8) Å |
| Ru-Cl(2) | 2.4219(5) Å | Ru-Cl(2) | 2.4153(8) Å |
| N-Ru-Cl(1) | 82.57(5)o | N-Ru-Cl(1) | 88.14(3)o |
| N-Ru-Cl(2) | 80.17(5)o | N-Ru-Cl(2) | 88.86(3)o |
| Cl(1)-Ru-Cl(2) | 89.261(17)o | Cl(1)-Ru-Cl(2) | 86.22(3)o |
| Ru-Centroide(p-cymene) | 1.421 Å | Ru-Centroide(p-cymene) | 1.432 Å |
| Hydrogen bond N(2)-H(01)···Cl(1) [Å and o] d(N-H): 0.851(16); d(H···Cl): 2.418(17); d(N···Cl): 3.1917(16); <(NHCl): 151(2). |
|||
| Molecular surface | 537.2 Å2 | Molecular surface | 491.6 Å2 |
| Molecular volume | 299.9 Å3 | Molecular volume | 280.8 Å3 |
| Ovality | 1.730 | Ovality | 1.669 |
Figure 3.
Molecular structure of II (left) and III (right).
Difference in molecule surface and volume (Table 2) matches differences in these parameters for ligands 4-aminobenzonitrile and 2-aminopyridine. These ligands also originally differ in ovality.
2.2. Supramolecular Structure
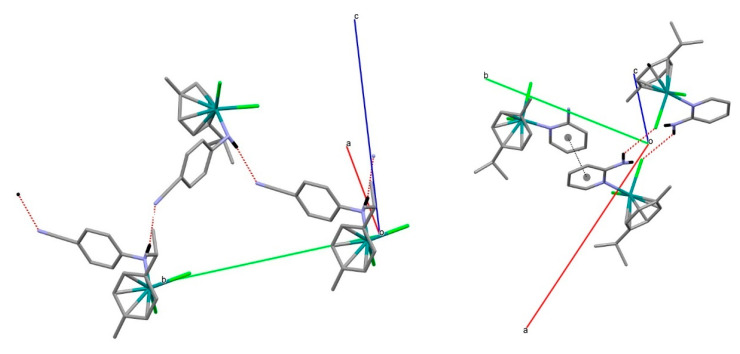
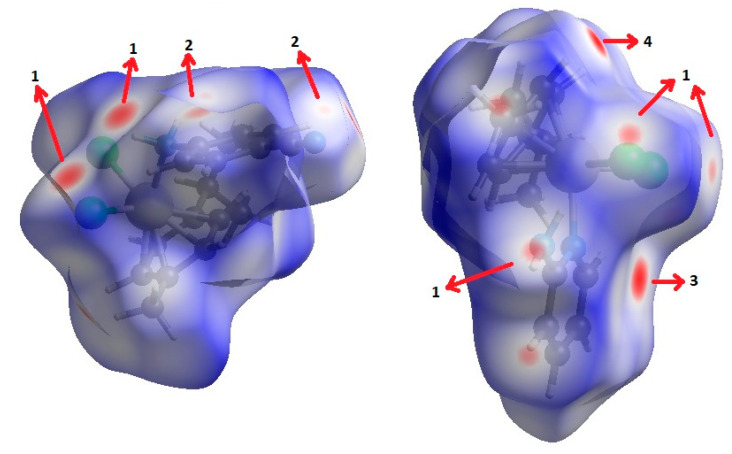
The most relevant features of crystal packing in II are shown in Figure 4. The structure consists of infinite chains of molecules linked by N-H···N≡C contacts. In addition, there are C-H···Cl and N-H···Cl contacts. This is confirmed by the Hirshfeld surface (HS) analysis [28,29,30,31]. Figure 5 shows the dnorm Hirshfeld surface; its associated 2D fingerprint can be decomposed into contributions from N···H/H···N interactions (these make up 13.8% of the surface area of the HS) and H···Cl/Cl···H interactions (21.1%). The Supplementary Materials contains fingerprint plots.
Figure 4.
Supramolecular packing in II (left) and III (right).
Figure 5.
View of the Hirshfeld surfaces mapped over dnorm property of II (left) and III (right). The labels 1, 2, 3 and 4 represent Cl···H/H···Cl, N···H/H···N, π···π and C-H···π interactions, respectively.
In spite of complex III possessing smaller molecular weight, it shows larger density than II. This more compact crystal packing for III can be explained in part by the π···π stacking interaction between 2ampy ligands (Figure 4) with centroids at 3.695 Å distance. In addition, there are C-H···π and N-H···Cl contacts. Figure 5 shows the dnorm Hirshfeld surface; its associated 2D fingerprint can be decomposed into contributions from C···H/H···C interactions (these make up 10.5% of the surface area of the HS), H···Cl/Cl···H interactions (21.8%) and C···C interactions (3.9%). The Supplementary Materials contains fingerprint plots.
Complexes I and II show absorptions due to ν(CN) in the 2230 cm−1 environment that agree that the ligand does not behave as a bridge through nitrile nitrogen. However, the three bands assignable to NH2 appear between 3200 and 3100 cm−1. The coordination through the pyridine nitrogen atom in III and IV is evidenced by the displacement of two bands at 604 cm−1 (in-plane ring deformation) and 405 cm−1 (out-of-plane ring deformation) to higher frequencies (631 and 424 cm−1, respectively). Complexes I, II, III and IV exhibit bands assignable to ν(NH) in the 3400–3100 range, about 100 cm−1 below the free ligand [32]. In I and II, the decrease in NH2 frequencies is due to the coordination of amine group to the ruthenium atom. However, in the case of III and IV, where the neutral ligand is bound to the ruthenium via the pyridinic nitrogen, the decrease in NH2 frequencies may be due to intra-(III) or inter-(IV) molecular NH2-Cl interactions. All the isolated compounds show two absorptions assigned to ν(RuCl) at about 290–270 cm−1.
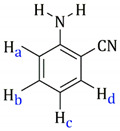
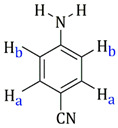
Table 1 also includes the data obtained from their high-resolution mass spectra (ESI), and Table 3 show relevant data from Proton Nuclear Magnetic Resonance. The assignments of the aromatic ring signals of the ligands (2abn and 2ampy) in I and III were made through COSY 1H-1H experiments.
Table 3.
1H-NMR data of I, II, III and IV.
| Complex | 1H δ(SiMe4) (in CD2CCl2) | Ligand Structure |
|---|---|---|
| I | 7.37–7.28 (m, Hb + Hd) 6.82 (d, Ha, Jab = 8.1Hz) 6.67 (t, Hc, Jbc = 8.1Hz) 5.88–5.72 (dd, –C6H4, J = 6.2 Hz) 5.47 (s, br, –NH2) 2.78 (spt, 1H, –CH(CH3)2, J = 6.9 Hz) 1.61 (s, –CH3) 1.30 (d, –CH(CH3)2, J = 6.9 Hz) |
|
| II | 7.23 (m, Ha) 6.54 (m, Hb) 5.75–5.53 (dd, –C6H4, J = 6.2 Hz) 4,72 (s, br, –NH2) 2.65 (spt, –CH(CH3)2) 2.07 (s, –CH3) 1.18 (d, 6H, –CH(CH3)2, J = 6.8 Hz) |
|
| III | 8.54 (d, Hd, J = 4.8 Hz) 7.42 (pst, Hc) 6.62 (pst, Hb) 6.58 (d, Ha, J = 8.0 Hz) 6.12 (s, br, –NH2) 5.53–5.31 (dd, –C6H4, J = 6.0 Hz) 2.93 (spt, –CH(CH3)2, J = 7.2 Hz) 1.96 (s, –CH3) 1.28 (d, 6H, –CH(CH3)2, J = 6.8 Hz) |
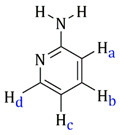
|
| IV | 8.33 (d, Ha, J = 6.9 Hz) 6.43 (d, Hb, J = 6.9 Hz) 4.55 (s, br, –NH2) 5.37–5.137 (dd, –C6H4, J = 6.0 Hz) 2.90 (spt, –CH(CH3)2, J = 6.9 Hz) 2.12 (s, –CH3) 1.27 (d, –CH(CH3)2, J = 6.9 Hz) |
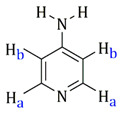
|
In relation to the mass spectra, under the working conditions used, the molecular peak corresponding to the exact mass is not observed, but others due to [M-Cl]+ are observed. This type of behavior has been previously observed [22,24].
2.3. In Vitro Cytotoxicity
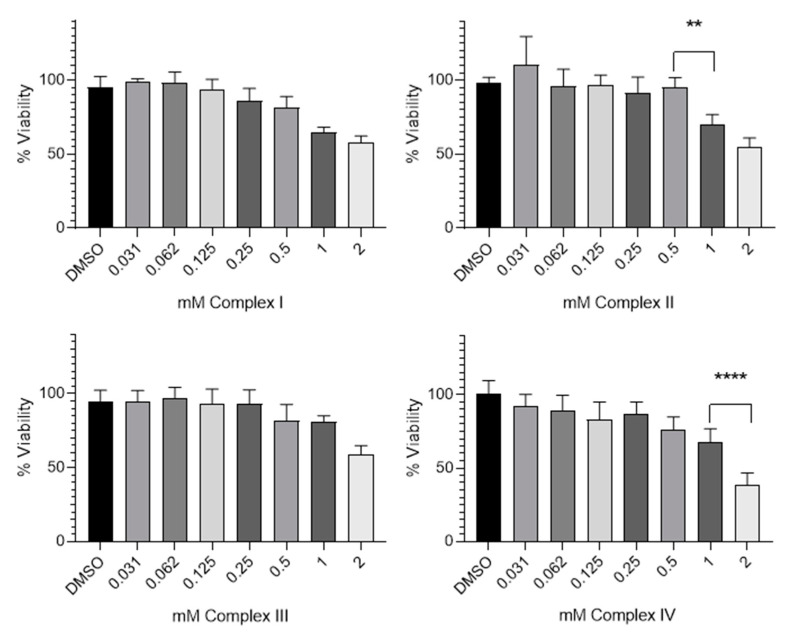
The in vitro cytotoxic effects of Complex I, Complex II, Complex III and Complex IV were assessed with the MTT assay after 48 h of exposure in the HeLa cell line. This cell line was selected due to its origin to determine if any of the complexes have anticancer activity. HeLa cells are derived from a cervical carcinoma and have been widely used in cytotoxicity studies [33]. To study the effects of the complexes, concentrations between 0.031–2 mM were evaluated. As complexes were dissolved in DMSO (0.5% v/v), this solvent without complex was used at the same concentration as the control. Cells without treatment were used as the control (data not revealed). After 48 h of incubation with several concentrations of the complexes, the cell viability decreased very slowly or remained almost constant as the concentration increased (Figure 6). However, Complex IV was more cytotoxic than the others, revealing an IC50 value, defined as the minimal concentration of a drug that is required for 50% inhibition in vitro of 1.6 ± 0.004 mM.
Figure 6.
Cytotoxicity effect of Complex I, Complex II, Complex III and Complex IV on HeLa cell line. Data are expressed as percentage of cell viability ± SD versus concentration. ** indicates p < 0.01 and **** indicates p < 0.0001, compared with the adjacent column.
Table 4 shows the cytotoxicity expressed as IC50 mean values (mM) of the complexes synthesized and cisplatin exposed to HeLa cells for 48 h. As can be seen, the cytotoxic potency is negligible on the HeLa cell line studied.
Table 4.
Cytotoxicity expressed as IC50 mean values (mM) of the complexes synthesized and cisplatin exposed to HeLa cells for 48 h.
| Compound | IC50, mM |
|---|---|
| Complex I | >2 |
| Complex II | >2 |
| Complex III | >2 |
| Complex IV | 1.60 ± 0.004 |
| cisplatin | 0.06 ± 0.002 |
3. Materials and Methods
The solvents were dried by conventional methods. The ligands were commercial-grade chemicals and [Ru(p-cymene)Cl2]2 were prepared by the method published in [34]. 1H NMR spectra were recorder on a Bruker Advance 200, 300 or 400 MHz instrument. IR spectra were recorded on a 100 FT-IR Spectrometer as Nujol mulls. The C, H and N analyses were obtained with a LECO CNHS-932 elemental microanalyzer. Thermal decomposition studies were carried out on a simultaneous analyzer TGA-DTA of TA Instruments. High-resolution (HR)-ESI-MS spectrometry was realized by HPLC Spectrometer/MS TOF Agilent Model 6220.
3.1. X-ray Data Collection, Structure Solution and Refinement for II and III
Diffraction data were collected on a Bruker D8 QUEST diffractometer with monochromated Mo-Kα radiation (Kα = 0.71073 Å), performing ω and φ scans at 100(2) K. SADABS 2016/2 absorption correction was performed (Bruker (2016). APEX3, SADABS and SAINT. Bruker AXS Inc., Madison, Wisconsin, USA)
The structures were solved by direct methods [35] and refined anisotropically on F2 using the program SHELXL-2018 [36]. CIF files were validated using checkCIF [37]. Numerical details are presented in Table 5. Hydrogen atoms bound to C were located at geometrically idealized positions and were allowed to ride on the parent atoms; hydrogen atoms bound to N atoms were discernible from difference-Fourier maps and were subsequently refined with N-H distance restraints. Crystal data for II were reported previously [26]; we collected data at low temperature in order to achieve a more precise structural study.
Table 5.
X-ray Data Collection Parameters for II and III.
| II | III | |
|---|---|---|
| formula | C17H20Cl2N2Ru | C15H20Cl2N2Ru |
| fw | 424.32 | 400.30 |
| cryst color, habit | orange, prism | orange, prism |
| cryst size (mm) | 0.090 × 0.050 × 0.030 | 0.18 × 0.07 × 0.04 |
| cryst syst | monoclinic | monoclinic |
| space group | P21/n (#14) | P21/c (#14) |
| a (Å) | 8.9362(9) | 16.509(7) |
| b (Å) | 12.8438(12) | 13.112(5) |
| c (Å) | 14.9730(16) | 7.167(2) |
| α (°) | 90 | 90 |
| β (°) | 91.063(4) | 93.90(2) |
| γ (°) | 90 | 90 |
| V (Å3) | 1718.2(3) | 1547.8(10) |
| Z value | 4 | 4 |
| Dcalcd (g/cm3) | 1.640 | 1.718 |
| F 000 | 856 | 808 |
| no. of reflns measd | 37,748 | 69,142 |
| no. of observations | 5265 | 4719 |
| no. of variables | 208 | 192 |
| R1 | 0.0271 | 0.0180 |
| wR2 | 0.0563 | 0.0434 |
| goodness of fit | 1.050 | 0.903 |
The generated Hirshfeld surfaces and the associated 2D fingerprint plots are extracted using the CrystalExplorer17 software [38].
3.2. Synthesis of the Complexes
These were prepared according to the following procedure, very similarly to reference [18]. To a dichloromethane (15 mL) solution of [(p-cymene)RuCl2]2 (0.4902 mmol), the appropriate ligand (0.9804 mmol for I, II and III; and 0.4902 mmol for IV) was added. The resulting suspension was stirred for 1 h and then was concentrated. The solid obtained was separated by filtration, crystallized from dichloromethane/ether and repeatedly washed with diethyl ether.
The crystals used for X-ray diffraction were obtained by slow diffusion of hexane over solutions of II and III in dichloromethane.
3.3. Cytotoxicity Assays
3.3.1. Cell Culture
Human cervical cancer cells (HeLa) were acquired from the American Type Tissue Culture Collection (ATCC, USA). Cell lines were maintained in the Dulbecco’s Modified Eagle Medium (DMEM) with a low content of glucose (1 g/L) supplemented with 10% (v/v) fetal bovine serum (FBS), 1 mM glutamax, 1% antibiotics (penicillin-streptomycin) and 1 mM pyruvate. Cells were subcultured, and medium was changed once a week. A total of 0.25% trypsine-0.25 mM EDTA was used.
3.3.2. MTT Assay
A total of 3000 cells/well of HeLa carcinoma cell line were seeded onto 96-well plates and incubated at 37 °C in 5% CO2. After 24 h, the culture medium of each well was replaced with fresh medium, and cells were treated with different concentrations of Complex I, Complex II, Complex III and Complex IV; these solutions were diluted in DMSO to obtain a maximum concentration per well of 2 mM. In each experiment, growth medium without nanoparticles was used as a control. Cells were incubated at 37 °C for 48 h. Then, the media were removed and 200 µL of 1% MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) was added. After 4 h of incubation, the MTT was removed and 100 µL of dimethyl sulfoxide DMSO was added. The absorbance was measured at 560 nm in a microplate reader Fluostar Omega spectrophotometer. Each sample was tested in three independent sets with triplicate points.
4. Conclusions
Four ruthenium(II) complexes were designed and synthesized, namely [Ru(p-cymene)Cl2(2abn)] (I), [Ru(p-cymene)Cl2(4abn)] (II), [Ru(p-cymene)Cl2(2ampy] (III) and [Ru(p-cymene)Cl2(μ-(4ampy)] (IV). Good yields were obtained, and all are air-stable solids exhibited colors in the yellow–orange range. All the compounds were fully characterized by elemental analysis of carbon, hydrogen and nitrogen, proton nuclear magnetic resonance, COSY 1H-1H, high-resolution mass spectrometry (ESI), thermogravimetry and single-crystal X-ray diffraction. Furthermore, the cytotoxic effect of the complexes was evaluated against HeLa cells, and cell viability was found to decrease very slowly in all cases. Only complex IV was more cytotoxic than the others, but it presented a much higher IC50 than cisplatin. However, although the results of the synthesized complexes do not show high cytotoxicity values against HeLa cells compared with cisplatin, further studies on the synthesis and characterization of ruthenium complexes are certainly very useful to be able to relate the structures to their unique and versatile biochemical properties.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27217264/s1, NMR spectra; Hirshfeld Surface analysis for Complexe II and for Complexe III.
Author Contributions
Methodology, G.V., J.P., M.G.F. and M.G.M.; investigation, M.G.F., I.M. and M.G.M.; Supervision, J.P., G.V. and G.G. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained in the article and in Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are available from the authors.
Funding Statement
This work has been partially supported by the grant ref. PID2020-113081RB-I00 funded by MCIN/AEI/10.13039/501100011033 and part of the grant ref. 20977/PI/18 funded by the research support program of the Seneca Foundation of Science and Technology of Murcia, Spain. Marta G. Fuster acknowledges support from FPI grant (Ref. PRE2018-086441) funded by MCIN/AEI/10.13039/501100011033 and by “ESF Investing in your future” Imane Moulefera acknowledges support from the Spanish Ministry of Universities under “Margarita Salas program”, financed by Next Generation EU.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rosenberg B., Vancamp L., Trosko J.E., Mansour V.H. Platinum Compounds: A New Class of Potent Antitumour Agents. Nature. 1969;222:385–386. doi: 10.1038/222385a0. [DOI] [PubMed] [Google Scholar]
- 2.Cleare M.J., Hoeschele J.D. Anti-Tumour Platinum Compounds. Relationship Between Structure and Activity. Platin. Met. Rev. 1973;17:2–13. [Google Scholar]
- 3.Cleare M.J., Hoeschele J.D. Studies on the antitumor activity of group VIII transition metal complexes. Part I. Platinum (II) complexes. Bioinorg. Chem. 1973;2:187–210. doi: 10.1016/S0006-3061(00)80249-5. [DOI] [Google Scholar]
- 4.Boulikas T., Vougiouka M. Cisplatin and platinum drugs at the molecular level (Review) Oncol. Rep. 2003;10:1663–1682. doi: 10.3892/or.10.6.1663. [DOI] [PubMed] [Google Scholar]
- 5.Kelland L. The resurgence of platinum-based cancer chemotherapy. Nat. Rev. Cancer. 2007;7:573–584. doi: 10.1038/nrc2167. [DOI] [PubMed] [Google Scholar]
- 6.Graham J., Mushin M., Kirkpatrick P. Oxaliplatin. Nat. Rev. Drug Discov. 2004;3:11–12. doi: 10.1038/nrd1287. [DOI] [PubMed] [Google Scholar]
- 7.Wang D., Lippard S.J. Cellular processing of platinum anticancer drug. Nat. Rev. Drug Discov. 2005;4:307–320. doi: 10.1038/nrd1691. [DOI] [PubMed] [Google Scholar]
- 8.Dabrowiak J.C. Metals in Medicine. 2nd ed. John Wiley & Sons; Hoboken, NJ, USA: 2017. [Google Scholar]
- 9.Morris R.E., Aird R.E., del Socorro Murdoch P., Chen H., Cummings J., Hughes N.D., Parsons S., Parkin A., Boyd G., Jodrell D.I., et al. Inhibition of cancer cell growth by ruthenium (II) arene complexes. J. Med. Chem. 2001;44:3616–3621. doi: 10.1021/jm010051m. [DOI] [PubMed] [Google Scholar]
- 10.Bugarcic T., Habtemariam A., Stepankova J., Heringova P., Kasparkova J., Deeth R.J., Johnstone R.D.L., Prescimone A., Parkin A., Parsons S., et al. The Contrasting Chemistry and Cancer Cell Cytotoxicity of Bipyridine and Bipyridinediol Ruthenium(II) Arene Complexes. Inorg. Chem. 2008;47:11470–11486. doi: 10.1021/ic801361m. [DOI] [PubMed] [Google Scholar]
- 11.Bugarcic T., Nováková O., Halámiková A., Zerzánková L., Vrána O., Kašpárková J., Habtemariam A., Parsons S., Sadler P.J., Brabec V. Cytotoxicity, cellular uptake, and DNA interactions of new monodentate ruthenium(II) complexes containing terphenyl arenes. J. Med. Chem. 2008;51:5310–5319. doi: 10.1021/jm8003043. [DOI] [PubMed] [Google Scholar]
- 12.Scolaro C., Chaplin A.B., Hartinger C.G., Bergamo A., Cocchietto M., Keppler B.K., Sava G., Dyson P.J. Tuning the hydrophobicity of ruthenium(II)-arene (RAPTA) drugs to modify uptake, biomolecular interactions and efficacy. Dalt. Trans. 2007;2:5065–5072. doi: 10.1039/b705449a. [DOI] [PubMed] [Google Scholar]
- 13.Wee H.A., Dyson P.J. Classical and non-classical ruthenium-based anticancer drugs: Towards targeted chemotherapy. Eur. J. Inorg. Chem. 2006;2006:4003–4018. doi: 10.1002/ejic.200600723. [DOI] [Google Scholar]
- 14.Zeng J., Zhao Y., Li K., Long D., Li W., Liang L. A coordinated ruthenium-rifampicin complex reprogramming the colon carcinoma micro-environment mediated by modulation of p53/AkT/mTOR/VEGF pathway. Toxicol. Appl. Pharmacol. 2021;426:115618. doi: 10.1016/j.taap.2021.115618. [DOI] [PubMed] [Google Scholar]
- 15.Shen J., Rees T.W., Ji L., Chao H. Recent advances in ruthenium(II) and iridium(III) complexes containing nanosystems for cancer treatment and bioimaging. Coord. Chem. Rev. 2021;443:214016. doi: 10.1016/j.ccr.2021.214016. [DOI] [Google Scholar]
- 16.Coverdale J.P.C., Laroiya-McCarron T., Romero-Canelón I. Designing ruthenium anticancer drugs: What have we learnt from the key drug candidates? Inorganics. 2019;7:31. doi: 10.3390/inorganics7030031. [DOI] [Google Scholar]
- 17.Lakshmi B.A., Reddy A.S., Sangubotla R., Hong J.W., Kim S. Ruthenium(II)-curcumin liposome nanoparticles: Synthesis, characterization and their effects against cervical cancer. Colloids Surf. B Biointerfaces. 2021;204:111773. doi: 10.1016/j.colsurfb.2021.111773. [DOI] [PubMed] [Google Scholar]
- 18.García G., Solano I., Sánchez G., Santana M.D., López G., Casabó J., Molins E., Miravitlles C. Reactivity of [(η6-arene)RuCl(μ-Cl)2] towards some potentially bidentate ligands. Molecular structure of [(η6-p-cymene)RuCl(taz)]PF6 (p-cymene = p-MeC6H4CH-Me2; taz = 2,6-dimethy. J. Organomet. Chem. 1994;467:119–126. doi: 10.1016/0022-328X(94)88016-6. [DOI] [Google Scholar]
- 19.Habtemariam A., Sadler P.J. Ruthenium Compounds. Application No. US7241913B2. U.S. Patent. 2007 July 10;
- 20.Iida J., Bell-Loncella E.T., Purazo M.L., Lu Y., Dorchak J., Clancy R., Slavik J., Cutler M.L., Shriver C.D. Inhibition of cancer cell growth by ruthenium complexes. J. Transl. Med. 2016;14:48. doi: 10.1186/s12967-016-0797-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yan X.-W., Xie Y.-R., Jin Z.-M., Hu M.-L., Zhou L.-P. Three Arene-Ru(II) compounds of 2-halogen-5-aminopyridine: Synthesis, characterization and cytotoxicity. Appl. Organomet. Chem. 2017;32:e3923. doi: 10.1002/aoc.3923. [DOI] [Google Scholar]
- 22.Ballester F.J., Ortega E., Porto V., Kostrhunova H., Davila-Ferreira N., Bautista D., Brabec V., Domínguez F., Santana M.D., Ruiz J. New half-sandwich ruthenium(ii) complexes as proteosynthesis inhibitors in cancer cells. Chem. Commun. 2019;55:1140–1143. doi: 10.1039/C8CC09211G. [DOI] [PubMed] [Google Scholar]
- 23.Ismail L.A., Alfaifi M.Y., Elbehairi S.E.I., Elshaarawy R.F.M., Gad E.M., El-Sayed W.N. Hybrid organoruthenium(II) complexes with thiophene-β-diketo-benzazole ligands: Synthesis, optical properties, CT-DNA interactions and anticancer activity. J. Organomet. Chem. 2021;949:121960. doi: 10.1016/j.jorganchem.2021.121960. [DOI] [Google Scholar]
- 24.Pujante-Galian M.A., Pérez S.A., Montalbán M.G., Carissimi G., Fuster M.G., Víllora G., García G. p-Cymene Complexes of Ruthenium (II) as Antitumor Agents. Molecules. 2020;55:5063. doi: 10.3390/molecules25215063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gupta D.K., Sahay A.N., Pandey D.S., Narendra K.J., Sharma P., Espinosa G., Cabrera A., Puerta M.C., Valerga P. Synthesis, characterization, reactivity and structure of some mono and binuclear (η6-p-cymene)ruthenium(II) complexes. J. Organomet. Chem. 1998;568:13–20. doi: 10.1016/S0022-328X(98)00571-3. [DOI] [Google Scholar]
- 26.Cheng B., Tehrani A., Hu M.-L., Morsali A. Supramolecular assemblies of Ru(II) organometallic half-sandwich complexes. CrystEngComm. 2014;16:9125–9134. doi: 10.1039/C4CE01214C. [DOI] [Google Scholar]
- 27.Aronson R., Elsegood M.R.J., Steed J.W., Tocher D.A. The reactions of [Ru(η6-arene)Cl2]2 compounds with a series of aminopyridine ligands: X-ray crystal structures of [Ru(η6-1,4-MeC6H4CHMe2)Cl2(NC5H4NH2)] and [Ru(η6-C16H16)Cl2(NC5H4NH2)] Polyhedron. 1991;10:1727–1732. doi: 10.1016/S0277-5387(00)83792-4. [DOI] [Google Scholar]
- 28.McKinnon J.J., Spackman M.A., Mitchell A.S. Novel tools for visualizing and exploring intermolecular interactions in molecular crystals. Acta Cryst. 2004;60:627–668. doi: 10.1107/S0108768104020300. [DOI] [PubMed] [Google Scholar]
- 29.Spackman M.A., Jayatilaka D. Hirshfeld surface analysis. CrystEngComm. 2009;11:19–32. doi: 10.1039/B818330A. [DOI] [Google Scholar]
- 30.Spackman M.A., McKinnon J.J. Fingerprinting intermolecular interactions in molecular crystals. CrystEngComm. 2002;4:378–392. doi: 10.1039/B203191B. [DOI] [Google Scholar]
- 31.Khan B.A., Hamdani S.S., Ahmed M.N., Hameed S., Ashfaq M., Ahmed M.S., Ibrahim M.A.A., Sidhom P.A. Synthesis, X-ray diffraction analysis, quantum chemical studies and α-amylase inhibition of probenecid derived S-alkylphthalimide-oxadiazole-benzenesulfonamide hybrids. J. Enzym. Inhib. Med. Chem. 2022;37:1464–1478. doi: 10.1080/14756366.2022.2078969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakamoto K. Infrared and Raman Spectra of Inorganic and Coordination Compounds: Part B: Applications in Coordination, Organometallic and Bioinorganic Chemistry. 6th ed. John Wiley & Sons; Hoboken, NJ, USA: 2008. [Google Scholar]
- 33.Fuster M.G., Montalbán M.G., Carissimi G., Víllora G. Improving Anticancer Therapy with Naringenin-Loaded Silk Fibroin Nanoparticles. Nanomaterials. 2020;10:718. doi: 10.3390/nano10040718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bennett M.A., Huang T.N., Matheson T.W., Smith K. Inorganic Synthesis. Volume 21. John Wiley & Sons; Hoboken, NJ, USA: 1982. pp. 74–77. [Google Scholar]
- 35.Sheldrick G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015;71:3–8. doi: 10.1107/S2053229614024218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sheldrick G.M. A short history of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2007;64:112–122. doi: 10.1107/S0108767307043930. [DOI] [PubMed] [Google Scholar]
- 37.International Union of Crystallography checkCIF. [(accessed on 13 September 2022)]. Available online: https://checkcif.iucr.org/
- 38.Spackman P.R., Turner M.J., McKinnon J.J., Wolff S.K., Grimwood D.J., Jayatilaka D., Spackman M.A. A program for Hirshfeld surface analysis, visualization and quantitative analysis of molecular crystals. J. Appl. Crystallogr. 2021;54:1006–1011. doi: 10.1107/S1600576721002910. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are contained in the article and in Supplementary Materials.