Abstract
Background: Carotid endarterectomy (CEA) is the first-line surgical intervention for cases of severe carotid stenoses. Unfortunately, the restenosis rate is high after CEA. This study aims to demonstrate the predictive role of carotid plaque features and inflammatory biomarkers (monocyte-to-lymphocyte ratio (MLR), neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), systemic inflammatory index (SII), Systemic Inflammation Response Index (SIRI), and Aggregate Index of Systemic Inflammation (AISI)) in carotid restenosis and mortality at 12 months following CEA. Methods: The present study was designed as an observational, analytical, retrospective cohort study and included all patients over 18 years of age with a minimum of 70% carotid stenosis and surgical indications for CEA admitted to the Vascular Surgery Clinic, Emergency County Hospital of Targu Mures, Romania between 2018 and 2021. Results: According to our results, the high pre-operative values of inflammatory biomarkers—MLR (OR: 10.37 and OR: 6.11; p < 0.001), NLR (OR: 34.22 and OR: 37.62; p < 0.001), PLR (OR: 12.02 and OR: 16.06; p < 0.001), SII (OR: 18.11 and OR: 31.70; p < 0.001), SIRI (OR: 16.64 and OR: 9.89; p < 0.001), and AISI (OR: 16.80 and OR: 8.24; p < 0.001)—are strong independent factors predicting the risk of 12-month restenosis and mortality following CEA. Moreover, unstable plaque (OR: 2.83, p < 0.001 and OR: 2.40, p = 0.04) and MI (OR: 3.16, p < 0.001 and OR: 2.83, p = 0.005) were independent predictors of all outcomes. Furthermore, AH (OR: 2.30; p = 0.006), AF (OR: 1.74; p = 0.02), tobacco (OR: 2.25; p < 0.001), obesity (OR: 1.90; p = 0.02), and thrombotic plaques (OR: 2.77; p < 0.001) were all independent predictors of restenosis, but not for mortality in all patients. In contrast, antiplatelet (OR: 0.46; p = 0.004), statin (OR: 0.59; p = 0.04), and ezetimibe (OR:0.45; p = 0.03) therapy were protective factors against restenosis, but not for mortality. Conclusions: Our data revealed that higher preoperative inflammatory biomarker values highly predict 12-month restenosis and mortality following CEA. Furthermore, age above 70, unstable plaque, cardiovascular disease, and dyslipidemia were risk factors for all outcomes. Additionally, AH, AF, smoking, and obesity were all independent predictors of restenosis but not of mortality in all patients. Antiplatelet and statin medication, on the other hand, were protective against restenosis but not against mortality.
Keywords: MLR, NLR, PLR, SII, SIRI, AISI, carotid restenosis, carotid plaque, biomarkers
1. Introduction
Stroke is one of the medical emergencies that presents a high mortality rate, currently occupying second place worldwide as a cause of mortality [1,2,3]. The main mechanism underlying ischemic stroke is atherosclerosis, which is represented by the formation and progression of atherosclerotic plaques in the carotid arteries [4,5,6]. Often, atherosclerotic plaque does not generate specific symptoms, but vulnerable/unstable plaques present an increased risk of ischemic stroke [4,5].
Carotid endarterectomy (CEA) is the most effective, first-line surgical intervention for cases of severe carotid stenoses [7,8,9]. CEA is performed by surgical removal of atherosclerotic plaque from the level of the bifurcation of the common carotid artery and the level of the internal carotid artery, to reduce the risk of developing a stroke [10]. According to published studies, the rate of post-endarterectomy restenosis ranges from 5 to 37% depending on the definition of restenosis and the follow-up duration [11,12,13].
The presence of risk factors (smoking, obesity, dyslipidemia), as well as the local and systemic inflammatory response are involved in post-endarterectomy restenosis [14,15,16]. Regarding the characteristics of atherosclerotic plaques, these were associated with the negative evolution of the patient with ischemic stroke [17,18,19,20,21], as well as with stent restenosis [22].
Among the systemic inflammatory biomarkers, the most accessible and easy to implement in current medical practice are hematological reports based on the total number of monocytes, neutrophils, lymphocytes, and platelets, respectively: monocyte-to-lymphocyte ratio (MLR), neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), systemic inflammatory index (SII), systemic Inflammation Response Index (SIRI), and aggregate index of systemic inflammation (AISI), whose predictive role has been demonstrated in the case of cardiovascular pathologies [17,18,19,20,21,22,23], oncological pathologies [24,25,26,27], chronic kidney disease [28,29,30], and, more recently, in the case of COVID-19 patients [31,32,33,34,35,36,37].
This study aims to demonstrate the predictive role of carotid plaque features and systemic inflammatory biomarkers in carotid restenosis and mortality at 12 months following CEA.
2. Materials and Methods
2.1. Study Design
The present study was designed as an observational, analytical, retrospective cohort study and included all patients over 18 years of age with a minimum of 70% carotid stenosis and surgical indications for CEA admitted to the Vascular Surgery Clinic, Emergency County Hospital of Targu Mures, Romania between 2018 and 2021. Patients with active tumors, hematological disease, restenosis after CEA, or contralateral CEA were all excluded. Regarding restenosis at 12 months, all patients enrolled in this study were initially divided into two groups named “No Stenosis” and “Restenosis”. The ideal cut-off value for all inflammatory biomarkers was used to calculate 12-month restenosis and mortality.
2.2. Data Collection
The patient’s age, gender, and hospitalization period were extracted from the hospital’s electronic database. Regarding comorbidities, the following cardiac pathologies were recorded: arterial hypertension (AH), atrial fibrillation (AF), ischemic heart disease (IHD), history of myocardial infarction (MI), chronic heart failure (CHF), as well as other pathologies: chronic kidney disease (CKD), peripheral arterial disease (PAD), and diabetes mellitus (DM).
The following were extracted from the pre-operative laboratory analyses: hemoglobin level, hematocrit level, number of neutrophils, monocytes, platelets, lymphocytes, glucose level, total cholesterol level, triglyceride level, blood urea nitrogen (BUN), creatinine, and Glomerular filtration rate (GFR).
2.3. Inflammatory Markers
Inflammatory biomarkers were determined from the first blood test result. The ratio was calculated using the equations:
-
-
MLR = monocytes/lymphocytes
-
-
NLR = neutrophils/lymphocytes
-
-
PLR = platelets/lymphocytes
-
-
SII = (neutrophils * platelets)/lymphocytes
-
-
SIRI = (monocytes * platelets)/lymphocytes
-
-
AISI = (neutrophils * monocytes * platelets)/lymphocytes
2.4. Surgical Technique
All patients were operated on by the same surgical team, under cervical block. The surgical intervention was carried out in the conventional method, the common, internal, and external carotid artery being prepared first, followed by clamping at the level of the three carotid arteries. Before clamping, 5000 IU of intravenous heparin was administered, and in case of post-clamping neurological changes, an intravascular shunt (FlexcelTM Carotid Shunt, LeMaitre®, North Brunswick, NJ, USA) was used. Later, carotid endarterectomy was performed through longitudinal arteriotomy, with removal of the atherosclerotic plaque, and sent for histological evaluation. Finally, carotid artery reconstruction was performed using a 6:0 prolene thread and an intradermal suture (Ethicon, Norderstedt, Germany).
2.5. Study Outcomes
The primary endpoints were the occurrence of restenosis higher than 50% and mortality at 12 months. The number of days spent in the hospital was recorded as a secondary outcome. The primary outcomes were stratified for the optimal cut-off value of inflammatory biomarkers.
2.6. Follow-Up Strategy
Patients were evaluated by Doppler ultrasound, 4 weeks, 6 months, and 12 months after the intervention. Restenosis was recorded if, during the ultrasonographic examination, at the level of the internal carotid artery, a stenosis of at least 50% was detected after CEA. If patients did not show up for their control visits and did not phone ahead of time to reschedule, the family was called to find out the patient’s situation. Mortality was recorded through telephone contact with the family.
2.7. Histopathological and Morphometrical Analysis
The samples were taken from the intern carotid artery. The carotid plaque was immersed in formalin 4% in a container that was at least five times the size of the fragment (at room temperature). The routine Hematoxylin–Eosin staining protocol was followed (the pieces were fixed, placed in 70% alcohol for one hour, 96% alcohol for a maximum of 24 h, then absolute alcohol for one hour, and then two xylene baths were performed, after which the tissue was placed in two paraffin baths of 30 min each, and it was sectioned at the microtomy in three sections at different levels with dimensions of 3 microns, and then the staining process followed). The procedure for Red Oil staining was used to examine the fatty deposits. There was no decalcification for any type of lesion. The histological type of the atherosclerotic plaques was analyzed and classified using the classification proposed by Virmani et al. [38], with consideration of morphopathological features such as intimal thickness, the presence of calcifications, fatty deposits, and plaque rupture.
2.8. Statistical Analysis
SPSS for Mac OS version 28.0.1.0 was used for statistical analysis (SPSS, Inc., Chicago, IL, USA). Chi-square tests were used to assess the associations of the ratios with category factors, while Student’s t- or Mann–Whitney tests were used to assess differences in continuous variables. To analyze the predictive power and to establish the cut-off values of inflammatory biomarkers, receiver operating characteristic (ROC) curve analysis was utilized. The ROC curve analysis was used to determine the appropriate MLR, NLR, PLR, SII, SIRI, and AISI cut-off values based on the Youden index (Youden Index = Sensitivity + Specificity − 1, ranging from 0 to 1). To identify independent predictors of 12-month restenosis and mortality after CEA, a multivariate logistic regression analysis using variables with p < 0.1 was undertaken.
3. Results
During the studied period, 369 patients underwent carotid endarterectomy. Of the patients, 190 were male (51.49%), and the mean age was 71.33 ± 11.61 (39–94). At the pre-operative carotid ultrasound, at the level of the symptomatic carotid, 268 patients presented stenoses between 70 and 90%, while 101 patients had stenoses greater than 90% but with no occlusion. At the histopathological analysis of the extracted plaques, 213 (57.72%) were stable plaques, and 156 (42.28%) showed signs of instability. Pre-operative pharmacological therapy included anticoagulant medication for 95 patients (25.75%), antiplatelet medication for 178 patients (48.24%), and statins for 222 patients (60.16%). Over the 12 months, 38 patients died (10.38%). The rest of the recorded variables are presented in Table 1.
Table 1.
The baseline characteristic data of all patients and divided according to the restenosis risk at 24 months.
| Variables | All Patients n = 369 |
No Stenosis n = 294 |
Restenosis n = 75 |
p-Value (OR; CI 95%) |
|---|---|---|---|---|
| Age mean ± SD (min–max) | 71.33 ± 11.61 (39–94) |
69.91 ± 11.35 (39–91) |
76.90 ± 10.97 (44–94) |
<0.0001 |
| Male/Female sex no. (%) | 190 (51.49%) 179 (48.51%) |
157 (53.30%) 137 (46.60%) |
33 (44.00%) 42 (56.00%) |
0.14 (1.45; 0.87–2.42) |
| Comorbidities and Risk factors | ||||
| AH, no. (%) | 240 (65.04%) | 181 (61.56%) | 59 (78.67%) | 0.006 (2.30; 1.26–4.19) |
| IHD, no. (%) | 146 (39.57%) | 104 (35.37%) | 42 (56.00%) | 0.001 (2.32; 1.38–3.89) |
| AF, no. (%) | 105 (28.46%) | 68 (23.13%) | 37 (49.33%) | <0.0001 (3.23; 1.90–5.48) |
| CHF, no. (%) | 84 (22.76%) | 66 (22.45%) | 18 (24.00%) | 0.77 (1.09; 0.60–1.98) |
| MI, no. (%) | 88 (23.85%) | 56 (19.05%) | 32 (42.67%) | <0.0001 (3.16; 1.83–5.44) |
| DM, no. (%) | 108 (29.27%) | 82 (27.89%) | 26 (34.67%) | 0.25 (1.37; 0.79–2.35) |
| CKD, no. (%) | 69 (18.70%) | 54 (18.37%) | 15 (20.00%) | 0.74 (1.11; 0.58–2.10) |
| PAD, no. (%) | 86 (23.31%) | 67 (22.79%) | 19 (25.33%) | 0.64 (1.14; 0.63–2.06) |
| Tobacco, no. (%) | 80 (21.68%) | 53 (18.03%) | 27 (36.00%) | 0.001 (2.55; 1.46–4.46) |
| Obesity, no. (%) | 94 (25.47%) | 67 (22.79%) | 27 (36.00%) | 0.02 (1.90; 1.10–3.28) |
| Dyslipidemia, no. (%) | 114 (30.89%) | 68 (23.13%) | 46 (61.33%) | <0.0001 (5.27; 3.07–9.02) |
| Ipsilateral ICA Stenosis | ||||
| 70–90%, no. (%) | 268 (72.62%) | 242 (82.31%) | 26 (34.67%) | <0.0001 (8.77; 4.99–15.38) |
| 90–99%, no. (%) | 101 (27.37%) | 52 (17.68%) | 49 (65.33%) | |
| Contralateral ICA Stenosis | ||||
| <50%, no. (%) | 218 (59.07%) | 176 (59.86%) | 42 (56%) | 0.54 (0.85; 0.51–1.42) |
| 50–70%, no. (%) | 89 (24.11%) | 69 (23.46%) | 20 (26.67%) | 0.56 (1.18; 0.66–2.11) |
| >70%, no. (%) | 62 (16.80%) | 49 (16.67%) | 13 (17.33%) | 0.89 (1.04; 0.53–2.05) |
| Histological Type of Carotid Plaque | ||||
| Stable Plaques, no. (%) | 213 (57.72%) | 169 (57.48%) | 44 (58.66%) | 0.85 (1.04; 0.62–1.75) |
| Fibroatheroma, no. (%) | 88 (23.85%) | 65 (22.10%) | 23 (30.67%) | 0.12 (1.55; 0.88–2.73) |
| Fibrocalcific, no. (%) | 125 (33.88%) | 104 (35.37%) | 21 (28%) | 0.22 (0.71; 0.40–1.24) |
| Unstable Plaques, no. (%) | 156 (42.28%) | 109 (37.07%) | 47 (62.67%) | 0.0001 (2.84; 1.68–4.81) |
| Thrombotic Plaque, no. (%) | 73 (19.78%) | 47 (15.98%) | 26 (34.67%) | 0.0004 (2.78; 1.57–4.92) |
| With A Thrombus in Organization, no. (%) | 41 (11.11%) | 30 (10.20%) | 11 (14.67%) | 0.27 (1.51; 0.71–3.17) |
| Thin-Cap Fibro-Atheroma, no. (%) | 26 (7.05%) | 20 (6.80%) | 6 (8%) | 0.71 (1.19; 0.46–3.07) |
| Calcified Nodule, no. (%) | 16 (4.34%) | 12 (4.08%) | 4 (5.33%) | 0.63 (1.32; 0.41–4.22) |
| Pre-Operative Drug Therapy | ||||
| Anticoagulant, no. (%) | 95 (25.75%) | 78 (26.53%) | 17 (22.67%) | 0.49 (0.81; 0.44–1.47) |
| Antiplatelet, no. (%) | 178 (48.24%) | 153 (52.04%) | 25 (33.33%) | 0.004 (0.46; 0.27–0.78) |
| Statins, no. (%) | 222 (60.16%) | 184 (62.59%) | 38 (50.67%) | 0.06 (0.61; 0.36–1.02) |
| Ezetimibe, no. (%) | 77 (20.86%) | 68 (23.12%) | 9 (12%) | 0.03 (0.45; 0.21–0.95) |
| PCSK9I, no. (%) | 21 (5.69%) | 18 (6.12%) | 3 (4%) | 0.48 (0.63; 0.18–2.22) |
| Laboratory data | ||||
| Hemoglobin g/dL median (Q1–Q3) |
13.7 (12.5–14.86) | 13.81 (12.75–14.99) | 13.2 (10.96–14.4) | 0.0004 |
| Hematocrit % median (Q1–Q3) |
41.9 (38.2–45) | 42.0 (39.02–45.01) | 41.23 (34.27–44.5) | 0.003 |
| Glucose mg/dL median (Q1–Q3) |
115 (95–145.8) | 110 (94–133.7) | 146 (120.75–173.75) | <0.0001 |
| Cholesterol mg/dL median (Q1–Q3) |
178.2 (146.4–215.2) | 177.75 (148.02–214.07) | 180.1 (146–239.25) | 0.09 |
| Triglyceride mg/dL median (Q1–Q3) |
119.1 (90.7–165.3) | 117.35 (93.55–163.67) | 123.1 (81.4–170.15) | 0.22 |
| GFR (mL/min/1.73 m2) median (Q1–Q3) |
76.06 (57.47–92.24) | 77.93 (63.28–93.46) | 62.44 (37.42–86.08) | <0.0001 |
| BUN mg/dL median (Q1–Q3) |
41.1 (31.2–54.8) | 39.15 (30.05–50.6) | 51.2 (37.75–97.2) | <0.0001 |
| Creatinine mg/dL median (Q1–Q3) |
0.90 (0.76–1.12) | 0.89 (0.75–1.08) | 1.10 (0.78–1.52) | 0.0003 |
| Neutrophils ×10³/µL median (Q1–Q3) |
4.78 (3.47–7.12) | 4.33 (3.33–6.04) | 7.79 (5.43–9.85) | <0.0001 |
| Lymphocytes ×10³/µL median (Q1–Q3) |
2.10 (1.48–2.87) | 2.35 (1.70–3.19) | 1.36 (0.79–1.72) | <0.0001 |
| Monocyte ×10³/µL median (Q1–Q3) |
0.53 (0.4–0.72) | 0.51 (0.4–0.68) | 0.60 (0.4–0.92) | 0.003 |
| PLT ×10³/µL median (Q1–Q3) |
234 (189.4–280) | 234 (188.62–277.57) | 240.9 (199–323.85) | 0.03 |
| MLR, median (Q1–Q3) | 0.24 (0.16–0.41) | 0.22 (0.15–0.31) | 0.50 (0.32–0.73) | <0.0001 |
| NLR, median (Q1–Q3) | 2.37 (1.25–4.68) | 1.82 (1.15–3.19) | 6.38 (4.39–9.06) | <0.0001 |
| PLR, median (Q1–Q3) | 106.49 (79.95–160.84) | 97.01 (74.88–134.3) | 206.77 (128.17–307.96) | <0.0001 |
| SII, median (Q1–Q3) | 528.57 (302.95–1089.97) | 433.84 (265.83–744.45) | 1635.22 (1039.23–2795.66) | <0.0001 |
| SIRI, median (Q1–Q3) | 1.06 (0.62–2.80) | 0.92 (0.56–1.74) | 4.31 (2.69–5.66) | <0.0001 |
| AISI, median (Q1–Q3) | 265.39 (136.85–666.86) | 209.2 (125.01–419.96) | 1095.2 (593.47–1721.68) | <0.0001 |
| Outcomes | ||||
| Mortality, no. (%) | 38 (10.30%) | 18 (6.12%) | 20 (26.67%) | <0.0001 (5.57; 2.77–11.22) |
| Length of hospital stay, mean ± SD |
3.93 ± 0.85 | 3.93 ± 0.79 | 4.07 ± 1.06 | 0.054 |
AH = arterial hypertension; IHD = ischemic heart disease; AF = atrial fibrillation; CHF = chronic heart failure; MI = myocardial infarction; DM = diabetes mellitus; CKD = chronic kidney disease; PAD = peripheral arterial disease; ICA = internal carotid artery; PCSK9i = proprotein convertase subtilisin/kexin type-9 inhibitors; GFR = glomerular filtration rate; PLT = total platelet count; BUN = blood urea nitrogen; MLR = monocyte-to-lymphocyte ratio; NLR = neutrophil-to-lymphocyte ratio; PLR = platelet-to-lymphocyte ratio; SII = systemic inflammatory index; SIRI = systemic inflammation response index; AISI = aggregate index of systemic inflammation.
Patients whose restenosis occurred in the 12 months following CEA were older (p < 0.0001), had higher incidences of cardio-vascular comorbidities (AH (p = 0.006), IHD (p = 0.001), AF (p < 0.0001), and MI (p < 0.0001)), and higher incidences of all risk factors enrolled, as seen in Table 1. In terms of ICA stenosis and carotid plaque features, in the restenosis group was a higher incidence of severe ipsilateral stenosis (90–99%; p < 0.0001), as well a higher incidence of unstable plaques (p = 0.0001) and especially thrombotic plaque (p = 0.0004). As pre-operative drug therapy, patients with no stenosis for 12-month follow-up had a higher incidence of antiplatelet use (p = 0.004) and ezetimibe (p = 0.03).
Regarding the laboratory findings, patients in the restenosis group had higher bun (p < 0.0001), creatinine (p = 0.0003), neutrophil (p < 0.0001), monocyte (p = 0.003), platelet (p = 0.03), and all systemic inflammatory biomarker (p < 0.0001) values as well as lower hemoglobin (p = 0.0004), hematocrit (p = 0.003), GFR (p < 0.0001), and lymphocyte (p < 0.0001). Moreover, there were higher incidences of mortality (p < 0.0001) Table 1.
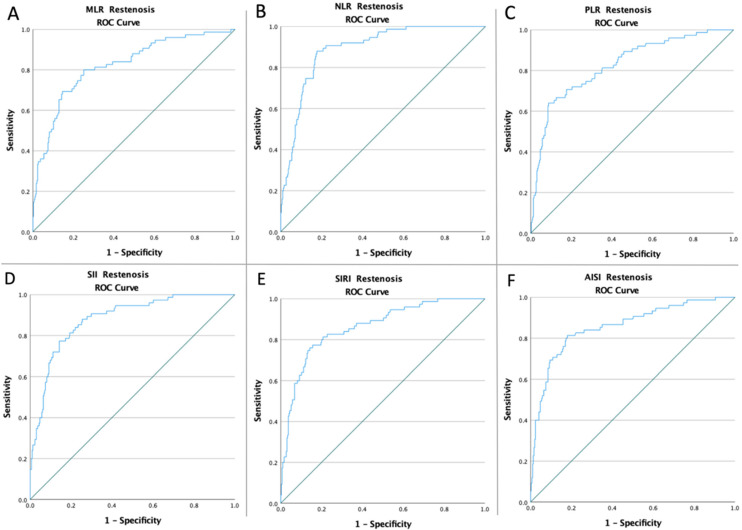
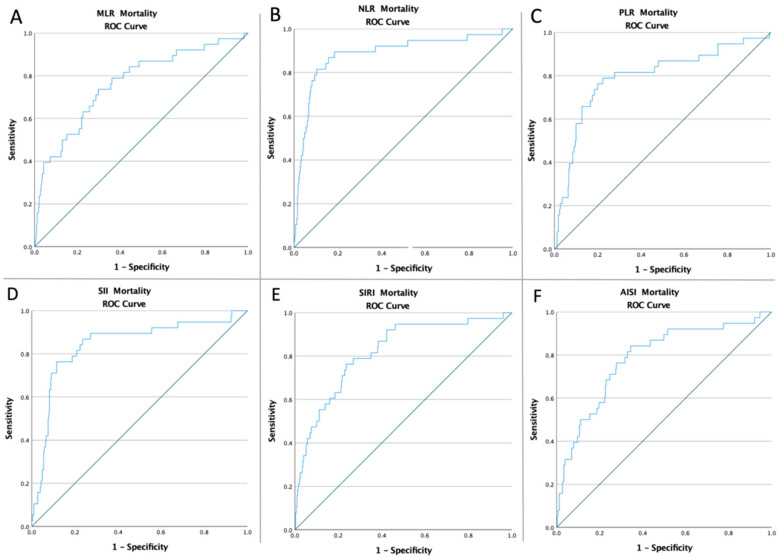
The ROC curves of all inflammatory biomarkers were created to determine whether the baseline of these markers was predictive of 12-month restenosis and mortality following CEA (Figure 1 and Figure 2). The optimal cut-off value obtained from Youden’s index, areas under the curve (AUC), and the predictive accuracy of the markers are listed in Table 2.
Figure 1.
ROC curve analysis concerning Restenosis (A) for the MLR (AUC: 0.822; p < 0.0001), (B) for the NLR (AUC: 0.890; p < 0.0001), (C) for the PLR (AUC: 0.825; p < 0.0001), (D) for the SII (AUC: 0.880; p < 0.0001), (E) for the SIRI (AUC: 0.861; p < 0.0001), and (F) for the AISI (AUC: 0.857; p < 0.0001).
Figure 2.
ROC curve analysis concerning Mortality (A) for the MLR (AUC: 0.765; p < 0.0001), (B) for the NLR (AUC: 0.885; p < 0.0001), (C) for the PLR (AUC: 0.794; p < 0.0001), (D) for the SII (AUC: 0.844; p < 0.0001), (E) for the SIRI (AUC: 0.819; p < 0.0001), and (F) for the AISI (AUC: 0.784; p < 0.0001).
Table 2.
The AUC of the ROC curve, 95% confidence interval, sensitivity, and specificity of the preoperative inflammatory markers.
| Variables | Cut-Off | AUC | Std. Error | 95% CI | Sensitivity | Specificity | p-Value |
|---|---|---|---|---|---|---|---|
| Restenosis | |||||||
| MLR | 0.30 | 0.822 | 0.028 | 0.767–0.878 | 80% | 74.8% | <0.0001 |
| NLR | 3.47 | 0.890 | 0.019 | 0.854–0.927 | 90.7% | 77.9% | <0.0001 |
| PLR | 143.05 | 0.825 | 0.028 | 0.771–0.879 | 72% | 79.6% | <0.0001 |
| SII | 881.55 | 0.880 | 0.021 | 0.839–0.921 | 81.3% | 80.6% | <0.0001 |
| SIRI | 2.01 | 0.861 | 0.024 | 0.814–0.908 | 81.3% | 79.3% | <0.0001 |
| AISI | 465.42 | 0.857 | 0.026 | 0.806–0.908 | 82.7% | 77.9% | <0.0001 |
| Mortality | |||||||
| MLR | 0.31 | 0.765 | 0.044 | 0.679–0.850 | 73.7% | 70.1% | <0.0001 |
| NLR | 4.41 | 0.885 | 0.034 | 0.818–0.953 | 89.5% | 81.6% | <0.0001 |
| PLR | 155.07 | 0.794 | 0.044 | 0.708–0.880 | 78.9% | 77.6% | <0.0001 |
| SII | 921.47 | 0.844 | 0.038 | 0.769–0.919 | 86.8% | 76.4% | <0.0001 |
| SIRI | 2.17 | 0.819 | 0.037 | 0.747–0.891 | 78.9% | 73.1% | <0.0001 |
| AISI | 504.97 | 0.784 | 0.041 | 0.704–0.864 | 76.3% | 72.2% | <0.0001 |
AUC = area under curve; Std = standard; CI = confidence interval; MLR = monocyte-to-lymphocyte ratio; NLR = neutrophil-to-lymphocyte ratio; PLR = platelet-to-lymphocyte ratio; SII = systemic inflammatory index; SIRI = systemic inflammation response index; AISI = aggregate index of systemic inflammation.
The restenosis risk, mortality, and length of hospital stay were further analyzed after dividing the patients into paired groups according to the optimal cut-off value of inflammatory biomarkers. Moreover, there was a higher incidence of restenosis risk and mortality rate for all the inflammatory biomarkers, as seen in Table 3.
Table 3.
Univariate analysis of inflammatory biomarkers and restenosis risk and mortality.
| Restenosis | Mortality | |
|---|---|---|
| Low-MLR vs. high-MLR | 15/225 (6.67%) vs. 60/144 (41.67%) p < 0.0001 |
10/237 (4.22%) vs. 28/132 (21.21%) p < 0.0001 |
| Low-NLR vs. high-NLR | 7/236 (2.97%) vs. 68/133 (51.13%) p < 0.0001 |
4/274 (1.46%) vs. 34/95 (57.36%) p < 0.0001 |
| Low-PLR vs. high-PLR | 21/255 (8.24%) vs. 54/114 (47.37%) p < 0.0001 |
8/265 (3.02%) vs. 30/104 (28.85%) p < 0.0001 |
| Low-SII vs. high-SII | 14/251 (5.58%) vs. 61/118 (51.69%) p < 0.0001 |
5/258 (1.94%) vs. 33/111 (29.73%) p < 0.0001 |
| Low-SIRI vs. high-SIRI | 14/247 (5.67%) vs. 61/122 (50%) p < 0.0001 |
8/248 (3.23%) vs. 30/121 (24.79%) p < 0.0001 |
| Low-AISI vs. high-AISI | 13/242 (5.37%) vs. 62/127 (48.82%) p < 0.0001 |
9/247 (3.64%) vs. 29/122 (23.77%) p < 0.0001 |
MLR = monocyte-to-lymphocyte ratio; NLR = neutrophil-to-lymphocyte ratio; PLR = platelet-to-lymphocyte ratio; SII = systemic inflammatory index; SIRI = systemic inflammation response index; AISI = aggregate index of systemic inflammation.
A multivariate analysis was used to determine the association between the inflammatory biomarkers, underlying risk factors, restenosis, and mortality risk at 12 months following CEA. A high baseline value of all systemic inflammatory markers was a strong independent predictor of restenosis and mortality (for all p < 0.0001). Moreover, as shown in Table 4, age above 70 (OR: 3.26; p < 0.001 and OR: 5.44; p = 0.007), MI (OR: 3.16; p < 0.001 and OR: 2.83; p = 0.005), dyslipidemia (OR: 5.27; p < 0.001 and OR: 2.80; p = 0.005), and unstable plaques (OR: 2.83; p < 0.001 and OR: 2.40; p = 0.04) were all independent predictors of restenosis and mortality. Furthermore, AH (OR: 2.30; p = 0.006), AF (OR: 1.74; p = 0.02), tobacco (OR: 2.25; p < 0.001), obesity (OR: 1.90; p = 0.02), and thrombotic plaques (OR: 2.77; p < 0.001) were all independent predictors of restenosis, but not for mortality in all patients. In contrast, antiplatelet (OR: 0.46; p = 0.004), statin (OR: 0.59; p = 0.04), and ezetimibe (OR:0.45; p = 0.03) therapy were protective factors against restenosis, but not for mortality (Table 4).
Table 4.
Multivariate analysis for predictors of restenosis and mortality at 12 months following CEA.
| Restenosis | Mortality | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-Value | OR | 95% CI | p-Value | |
| Age > 70 | 3.26 | 1.79–5.93 | <0.001 | 5.44 | 1.59–18.60 | 0.007 |
| AH | 2.30 | 1.26–4.19 | 0.006 | 2.13 | 0.77–5.85 | 0.14 |
| IHD | 1.42 | 0.95–2.10 | 0.08 | 1.11 | 0.71–1.73 | 0.64 |
| AF | 1.74 | 1.06–2.87 | 0.02 | 1.26 | 0.85–1.87 | 0.24 |
| MI | 3.16 | 1.83–5.44 | <0.001 | 2.83 | 1.37–5.85 | 0.005 |
| Tobacco | 2.55 | 1.46–4.46 | <0.001 | 2.31 | 0.97–5.50 | 0.058 |
| Obesity | 1.90 | 1.10–3.28 | 0.02 | 1.94 | 0.93–4.05 | 0.07 |
| Dyslipidemia | 5.27 | 3.07–9.02 | <0.001 | 2.80 | 1.37–5.71 | 0.005 |
| Unstable Plaques | 2.83 | 1.67–4.78 | <0.001 | 2.40 | 1.02–5.63 | 0.04 |
| Thrombotic Plaques | 2.77 | 1.57–4.90 | <0.001 | 1.73 | 0.69–4.35 | 0.24 |
| Antiplatelet | 0.46 | 0.27–0.78 | 0.004 | 1.07 | 0.47–2.46 | 0.85 |
| Statins | 0.59 | 0.35–0.78 | 0.04 | 0.75 | 0.32–1.72 | 0.50 |
| Ezetimibe | 0.45 | 0.21–0.95 | 0.03 | 0.74 | 0.24–2.24 | 0.74 |
| high-MLR | 10.37 | 5.38–18.58 | <0.001 | 6.11 | 2.86–13.04 | <0.001 |
| high-NLR | 34.22 | 14.99–78.12 | <0.001 | 37.62 | 12.87–109.97 | <0.001 |
| high-PLR | 12.02 | 6.62–17.88 | <0.001 | 16.06 | 5.78–44.61 | <0.001 |
| high-SII | 18.11 | 9.46–34.66 | <0.001 | 31.70 | 10.37–96.84 | <0.001 |
| high-SIRI | 16.64 | 8.72–31.74 | <0.001 | 9.89 | 4.37–22.37 | <0.001 |
| high-AISI | 16.80 | 8.69–32.45 | <0.001 | 8.24 | 3.76–18.08 | <0.001 |
AH = arterial hypertension; IHD = ischemic heart disease; AF = atrial fibrillation; MI = myocardial infarction; MLR = monocyte-to-lymphocyte ratio; NLR = neutrophil-to-lymphocyte ratio; PLR = platelet-to-lymphocyte ratio; SII = systemic inflammatory index; SIRI = systemic inflammation response index; AISI = aggregate index of systemic inflammation.
4. Discussion
The role of inflammation in the progression of atherosclerosis is well-acknowledged [39,40,41]. Gibson et al. [42], Tamhane et al. [43], and Duffy et al. [44] demonstrated the role of neutrophils and lymphocytes in the negative evolution of patients with coronary disease through the progression of coronary atherosclerotic plaques. Furthermore, the applicability of some of the inflammatory biomarkers analyzed in this research (NLR, PLR, and MLR) has been demonstrated in the negative evolution of patients with acute ischemic stroke [45,46,47,48,49], as well as restenosis and neurological complications following CEA and carotid stenting [50,51,52,53,54,55].
According to our results, the high pre-operative values of inflammatory biomarkers—MLR (OR: 10.37 and OR: 6.11; p < 0.001), NLR (OR: 34.22 and OR: 37.62; p < 0.001), PLR (OR: 12.02 and OR: 16.06; p < 0.001), SII (OR: 18.11 and OR: 31.70; p < 0.001), SIRI (OR: 16.64 and OR: 9.89; p < 0.001), and AISI (OR: 16.80 and OR: 8.24; p < 0.001)—are strong independent factors predicting the risk of 12-month restenosis and mortality following CEA. Moreover, unstable plaque (OR: 2.83, p < 0.001 and OR: 2.40, p = 0.04) and MI (OR: 3.16, p < 0.001 and OR: 2.83, p = 0.005) were independent predictors of all outcomes, as seen in Table 4.
Similar to our findings, older patients, cardiovascular diseases, smoking, obesity, and dyslipidemia have all been identified as risk factors for restenosis following CEA [16,56,57]. Furthermore, Zhou et al. [58] and Hellings et al. [59] demonstrated that the histopathological characteristics of carotid plaques are associated with carotid restenosis after CEA.
In studies recently reported in the literature, the NLR and PLR have been assessed regarding restenosis and poor outcome following ECA. Thus, Halazun et al. [51] found that high values of NLR > 5 (OR, 3.38; 95% CI, 1.81–6.27; p < 0.001) were associated with cognitive dysfunction in 432 CEA patients. Furthermore, King et al. [52] reported a correlation between pre-operative values of NLR > 3 and an increased risk of stroke and death following CEA for asymptomatic carotid artery stenosis. According to Deşer et al. [53], PLR > 145,304 (83.3% sensitivity, 73.8% specificity; p = 0.002) is an independent predictor for stroke (p = 0.047).
In the case of 285 patients with acute ischemic stroke, Sadeghi et al. found that high values of NLR > 5.73, 24 h after thrombolysis, are associated with a poor functional prognosis [45]. Similarly, Lee et al. recently published research in which they proved that high NLR > 6.2 and PLR > 103.6 levels are predictive factors for failed reperfusion following endovascular treatment in 282 patients with acute ischemic stroke [46]. Furthermore, Lattanzi et al. demonstrated, in two articles, that NLR > 6.4 (OR: 1.11; 95% CI: 1.04–1.18; p = 0.001) predicts early neurological deterioration [47,52] and SIRI > 3.8 was an independent predictor of futile recanalization (OR: 2.88; 95% CI: 1.56–5.30; p < 0.001) [60,61] for ischemic stroke patients undergoing endovascular treatment.
Scicchitano et al. [61] and Marzullo et al. [62] studied and established that the soluble suppressor of tumorigenicity (sST-2) works as an independent predictor of mortality and carotid plaque in individuals with carotid atherosclerotic plaque following CEA. The relevance of nutraceutical and dietary carotenoid supplementation in the daily diet in terms of dyslipidemia and lipidic management was established by two systematic reviews published by Scicchitano et al. [63] and Ciccone et al. [64].
The primary outcome of this research is that pre-operative systemic inflammatory biomarkers have a high predictive role in the risk of restenosis occurrence and mortality following CEA. As seen in Table 4, cardiovascular diseases (AH, AF, and MI), unstable plaques, elderly patients, and other risk factors (tobacco, obesity, and dyslipidemia) all predict 12-month restenosis. Moreover, antiplatelet, statin, and ezetimibe medication are protective factors for 12-month restenosis after CEA. To the best of our knowledge, this is the first research to evaluate the predictive role of carotid plaque morphology, pre-operative drug therapy, red blood cell biomarkers, and risk of restenosis and mortality at 12 months following CEA.
Although this study included 369 patients who underwent CEA for four years and had significant results with the high level of sensitivity and specificity of the analyzed inflammatory biomarkers in the prediction of restenosis and mortality at 12 months after CEA, it has certain limitations.
Limitations
Our study has some limitations. Firstly, we must consider the monocentric and retrospective design of the study. Secondly, we excluded patients who benefited from carotid stenting, or patients with contralateral CEA. Moreover, regarding the exclusion criteria (malignancy and hematological disease), the results cannot be extrapolated to the general population. In the future, we recommend a multicentric prospective study, following the inflammatory status and poor outcomes of patients after CEA. Furthermore, additional research is necessary to support our findings.
5. Conclusions
Our data revealed that higher preoperative inflammatory biomarker values (MLR, NLR, PLR, SII, SIRI, and AISI) highly predict 12-month restenosis and mortality following CEA. Furthermore, during the study period, age above 70, unstable plaque, cardiovascular disease, and dyslipidemia were risk factors for 12-month restenosis and mortality. Additionally, AH, AF, smoking, and obesity were all independent predictors of restenosis but not of mortality in all patients. Antiplatelet, statin, and ezetimibe medication, on the other hand, were protective against restenosis but not against mortality. Given their ease of use and inexpensive cost, these ratios can be used for preoperative-risk group stratification and improved patient management of restenosis following CEA.
Acknowledgments
This paper was published with the support of George Emil Palade University of Medicine, Pharmacy, Sciences and Technology of Targu Mures and is part of a Ph.D. thesis from the Doctoral School of Medicine and Pharmacy within the George Emil Palade University of Medicine, Pharmacy, Science, and Technology of Targu Mures with the title “The modulatory role of inflammation and perivascular adipose tissue in the occurrence and progression of atherosclerosis”, which will be presented by Raluca Niculescu, having the approval of all authors.
Author Contributions
Conceptualization, methodology, writing—original draft preparation, R.N. and E.R.; software, E.M.A. (Emil Marian Arbănași), R.K. and R.M.M.; formal analysis, investigation, C.M.C. and I.G.C.; resources, A.H.S.; data curation, A.H.S. and A.C.T.; writing—review and editing, E.M.A. (Eliza Mihaela Arbănași), A.S. and V.V.; project administration, visualization, supervision, A.V.M. and O.S.C.; validation, all authors. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Targu Mures Emergency County Hospital, Romania (protocol code 30418, on 7 December 2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tsao C.W., Aday A.W., Almarzooq Z.I., Alonso A., Beaton A.Z., Bittencourt M.S., Boehme A.K., Buxton A.E., Carson A.P., Commodore-Mensah Y., et al. Heart Disease and Stroke Statistics—2022 Update: A Report From the American Heart Association. Circulation. 2022;145:e153–e639. doi: 10.1161/CIR.0000000000001052. [DOI] [PubMed] [Google Scholar]
- 2.Feigin V.L., Stark B.A., Johnson C.O., Roth G.A., Bisignano C., Abady G.G., Abbasifard M., Abbasi-Kangevari M., Abd-Allah F., Abedi V., et al. Global, Regional, and National Burden of Stroke and Its Risk Factors, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021;20:795–820. doi: 10.1016/S1474-4422(21)00252-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yi X., Zhu L., Sui G., Li J., Luo H., Yu M., Wang C., Chen X., Wei W., Bao S. Inflammation and Endothelial Function Relevant Genetic Polymorphisms and Carotid Plaque in Chinese Population. J. Atheroscler. Thromb. 2020;27:978–994. doi: 10.5551/jat.53074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peng M., Wang L., Xia Y., Tao L., Liu Y., Huang F., Li S., Gong X., Liu X., Xu G. High Dietary Inflammatory Index Is Associated with Increased Plaque Vulnerability of Carotid in Patients with Ischemic Stroke. Stroke. 2020;51:2983–2989. doi: 10.1161/STROKEAHA.120.029035. [DOI] [PubMed] [Google Scholar]
- 5.Fabiani I., Palombo C., Caramella D., Nilsson J., De Caterina R. Imaging of the Vulnerable Carotid Plaque: Role of Imaging Techniques and a Research Agenda. Neurology. 2020;94:922–932. doi: 10.1212/WNL.0000000000009480. [DOI] [PubMed] [Google Scholar]
- 6.Willey J.Z., Pasterkamp G. The Role of the Vulnerable Carotid Plaque in Embolic Stroke of Unknown Source. J. Am. Coll. Cardiol. 2022;79:2200–2202. doi: 10.1016/j.jacc.2022.04.004. [DOI] [PubMed] [Google Scholar]
- 7.Whooley J.L., David B.C., Woo H.H., Hoh B.L., Raftery K.B., Hussain Siddiqui A., Westerveld M., Amin-Hanjani S., Ghogawala Z. Carotid Revascularization and Its Effect on Cognitive Function: A Prospective Nonrandomized Multicenter Clinical Study. J. Stroke Cerebrovasc. Dis. 2020;29:104702. doi: 10.1016/j.jstrokecerebrovasdis.2020.104702. [DOI] [PubMed] [Google Scholar]
- 8.Kleindorfer D.O., Towfighi A., Chaturvedi S., Cockroft K.M., Gutierrez J., Lombardi-Hill D., Kamel H., Kernan W.N., Kittner S.J., Leira E.C., et al. 2021 Guideline for the Prevention of Stroke in Patients with Stroke and Transient Ischemic Attack: A Guideline from the American Heart Association/American Stroke Association. Stroke. 2021;52:e364–e467. doi: 10.1161/STR.0000000000000375. [DOI] [PubMed] [Google Scholar]
- 9.Brott T.G., Halperin J.L., Abbara S., Bacharach J.M., Barr J.D., Bush R.L., Cates C.U., Creager M.A., Fowler S.B., Friday G., et al. 2011 ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS Guideline on the Management of Patients with Extracranial Carotid and Vertebral Artery Disease: Executive Summary: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American Stroke Association, American Association of Neuroscience Nurses, American Association of Neurological Surgeons, American College of Radiology, American Society of Neuroradiology, Congress of Neurological Surgeons, Society of Atherosclerosis Imaging and Prevention, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of NeuroInterventional Surgery, Society for Vascular Medicine, and Society for Vascular Surgery. J. Am. Coll. Cardiol. 2011;57:1002–1044. doi: 10.1016/j.jacc.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 10.Howell S.J. Carotid Endarterectomy. Br. J. Anaesth. 2007;99:119–131. doi: 10.1093/bja/aem137. [DOI] [PubMed] [Google Scholar]
- 11.DeGroote R.D., Lynch T.G., Jamil Z., Hobson R.W. Carotid Restenosis: Long-Term Noninvasive Follow-up after Carotid Endarterectomy. Stroke. 1987;18:1031–1036. doi: 10.1161/01.STR.18.6.1031. [DOI] [PubMed] [Google Scholar]
- 12.Lattimer C.R., Burnand K.G. Recurrent Carotid Stenosis after Carotid Endarterectomy. Br. J. Surg. 1997;84:1206–1219. [PubMed] [Google Scholar]
- 13.Keagy B.A., Edrington R.D., Poole M.A., Johnson G. Incidence of Recurrent or Residual Stenosis after Carotid Endarterectomy. Am. J. Surg. 1985;149:722–725. doi: 10.1016/S0002-9610(85)80173-2. [DOI] [PubMed] [Google Scholar]
- 14.Herder M., Johnsen S.H., Arntzen K.A., Mathiesen E.B. Risk Factors for Progression of Carotid Intima-Media Thickness and Total Plaque Area: A 13-Year Follow-up Study: The Tromsø Study. Stroke. 2012;43:1818–1823. doi: 10.1161/STROKEAHA.111.646596. [DOI] [PubMed] [Google Scholar]
- 15.Brunelli N., Altamura C., Costa C.M., Altavilla R., Palazzo P., Maggio P., Marcosano M., Vernieri F. Carotid Artery Plaque Progression: Proposal of a New Predictive Score and Role of Carotid Intima-Media Thickness. Int. J. Environ. Res. Public Health. 2022;19:758. doi: 10.3390/ijerph19020758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nguyen D.T., Bérczi Á., Nyárády B.B., Szőnyi Á., Philippovich M., Dósa E. Short- and Mid-Term Outcomes of Stenting in Patients with Isolated Distal Internal Carotid Artery Stenosis or Post-Surgical Restenosis. J. Clin. Med. 2022;11:5640. doi: 10.3390/jcm11195640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arbănași E.M., Mureșan A.V., Coșarcă C.M., Kaller R., Bud T.I., Hosu I., Voidăzan S.T., Arbănași E.M., Russu E. Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio Impact on Predicting Outcomes in Patients with Acute Limb Ischemia. Life. 2022;12:822. doi: 10.3390/life12060822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Russu E., Mureșan A.V., Arbănași E.M., Kaller R., Hosu I., Voidăzan S., Arbănași E.M., Coșarcă C.M. The Predictive Role of NLR and PLR in Outcome and Patency of Lower Limb Revascularization in Patients with Femoropopliteal Disease. J. Clin. Med. 2022;11:2620. doi: 10.3390/jcm11092620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taurino M., Aloisi F., Del Porto F., Nespola M., Dezi T., Pranteda C., Rizzo L., Sirignano P. Neutrophil-to-Lymphocyte Ratio Could Predict Outcome in Patients Presenting with Acute Limb Ischemia. J. Clin. Med. 2021;10:4343. doi: 10.3390/jcm10194343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Drugescu A., Roca M., Zota I.M., Costache A.-D., Gavril O.I., Gavril R.S., Vasilcu T.F., Mitu O., Esanu I.M., Roca I.-C., et al. Value of the Neutrophil to Lymphocyte Ratio and Platelet to Lymphocyte Ratio in Predicting CPET Performance in Patients with Stable CAD and Recent Elective PCI. Med. Kaunas Lith. 2022;58:814. doi: 10.3390/medicina58060814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Efros O., Beit Halevi T., Meisel E., Soffer S., Barda N., Cohen O., Kenet G., Lubetsky A. The Prognostic Role of Neutrophil-to-Lymphocyte Ratio in Patients Hospitalized with Acute Pulmonary Embolism. J. Clin. Med. 2021;10:4058. doi: 10.3390/jcm10184058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strazzulla A., Abroug Ben Halima S., Chouchane I., Rezek M., Pinto Stiebler M., Hamrouni S., Maalaoui M., Ghriss N., Guedec-Ghelfi R., Moini C., et al. The Predictive Value of Cell Blood Count Parameters to Diagnose Pulmonary Embolism in Patients with SARS-CoV-2 Infection: A Case Control Study. Antibiotics. 2022;11:60. doi: 10.3390/antibiotics11010060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arbănași E.M., Mureșan A.V., Arbănași E.M., Kaller R., Cojocaru I.I., Coșarcă C.M., Russu E. The Neutrophil-to-Lymphocyte Ratio’s Predictive Utility in Acute Pulmonary Embolism: Systematic Review. J. Cardiovasc. Emergencies. 2022;8:25–30. doi: 10.2478/jce-2022-0005. [DOI] [Google Scholar]
- 24.Tomioka-Inagawa R., Nakane K., Enomoto T., Tomioka M., Taniguchi T., Ishida T., Ozawa K., Takagi K., Ito H., Takeuchi S., et al. The Impact of Neutrophil-to-Lymphocyte Ratio after Two Courses of Pembrolizumab for Oncological Outcomes in Patients with Metastatic Urothelial Carcinoma. Biomedicines. 2022;10:1609. doi: 10.3390/biomedicines10071609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ginesu G.C., Paliogiannis P., Feo C.F., Cossu M.L., Scanu A.M., Fancellu A., Fois A.G., Zinellu A., Perra T., Veneroni S., et al. Inflammatory Indexes as Predictive Biomarkers of Postoperative Complications in Oncological Thoracic Surgery. Curr. Oncol. 2022;29:3425–3432. doi: 10.3390/curroncol29050276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iinuma K., Enomoto T., Kawada K., Fujimoto S., Ishida T., Takagi K., Nagai S., Ito H., Kawase M., Nakai C., et al. Utility of Neutrophil-to-Lymphocyte Ratio, Platelet-to-Lymphocyte Ratio, and Systemic Immune Inflammation Index as Prognostic, Predictive Biomarkers in Patients with Metastatic Renal Cell Carcinoma Treated with Nivolumab and Ipilimumab. J. Clin. Med. 2021;10:5325. doi: 10.3390/jcm10225325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gawiński C., Michalski W., Mróz A., Wyrwicz L. Correlation between Lymphocyte-to-Monocyte Ratio (LMR), Neutrophil-to-Lymphocyte Ratio (NLR), Platelet-to-Lymphocyte Ratio (PLR) and Tumor-Infiltrating Lymphocytes (TILs) in Left-Sided Colorectal Cancer Patients. Biology. 2022;11:385. doi: 10.3390/biology11030385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pasqui E., de Donato G., Lazzeri E., Molino C., Galzerano G., Giubbolini M., Palasciano G. High Neutrophil-to-Lymphocyte and Platelet-to-Lymphocyte Ratios Are Associated with a Higher Risk of Hemodialysis Vascular Access Failure. Biomedicines. 2022;10:2218. doi: 10.3390/biomedicines10092218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaller R., Arbănași E.M., Mureșan A.V., Voidăzan S., Arbănași E.M., Horváth E., Suciu B.A., Hosu I., Halmaciu I., Brinzaniuc K., et al. The Predictive Value of Systemic Inflammatory Markers, the Prognostic Nutritional Index, and Measured Vessels’ Diameters in Arteriovenous Fistula Maturation Failure. Life. 2022;12:1447. doi: 10.3390/life12091447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mureșan A.V., Russu E., Arbănași E.M., Kaller R., Hosu I., Arbănași E.M., Voidăzan S.T. The Predictive Value of NLR, MLR, and PLR in the Outcome of End-Stage Kidney Disease Patients. Biomedicines. 2022;10:1272. doi: 10.3390/biomedicines10061272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Halmaciu I., Arbănași E.M., Kaller R., Mureșan A.V., Arbănași E.M., Bacalbasa N., Suciu B.A., Cojocaru I.I., Runcan A.I., Grosu F., et al. Chest CT Severity Score and Systemic Inflammatory Biomarkers as Predictors of the Need for Invasive Mechanical Ventilation and of COVID-19 Patients’ Mortality. Diagnostics. 2022;12:2089. doi: 10.3390/diagnostics12092089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arbănași E.M., Halmaciu I., Kaller R., Mureșan A.V., Arbănași E.M., Suciu B.A., Coșarcă C.M., Cojocaru I.I., Melinte R.M., Russu E. Systemic Inflammatory Biomarkers and Chest CT Findings as Predictors of Acute Limb Ischemia Risk, Intensive Care Unit Admission, and Mortality in COVID-19 Patients. Diagnostics. 2022;12:2379. doi: 10.3390/diagnostics12102379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parthasarathi A., Padukudru S., Arunachal S., Basavaraj C.K., Krishna M.T., Ganguly K., Upadhyay S., Anand M.P. The Role of Neutrophil-to-Lymphocyte Ratio in Risk Stratification and Prognostication of COVID-19: A Systematic Review and Meta-Analysis. Vaccines. 2022;10:1233. doi: 10.3390/vaccines10081233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Citu C., Gorun O.M., Motoc A., Citu I.M., Gorun F., Malita D. Correlation of Lung Damage on CT Scan with Laboratory Inflammatory Markers in COVID-19 Patients: A Single-Center Study from Romania. J. Clin. Med. 2022;11:4299. doi: 10.3390/jcm11154299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cocoş R., Mahler B., Turcu-Stiolica A., Stoichiță A., Ghinet A., Shelby E.-S., Bohîlțea L.C. Risk of Death in Comorbidity Subgroups of Hospitalized COVID-19 Patients Inferred by Routine Laboratory Markers of Systemic Inflammation on Admission: A Retrospective Study. Viruses. 2022;14:1201. doi: 10.3390/v14061201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Regolo M., Vaccaro M., Sorce A., Stancanelli B., Colaci M., Natoli G., Russo M., Alessandria I., Motta M., Santangelo N., et al. Neutrophil-to-Lymphocyte Ratio (NLR) Is a Promising Predictor of Mortality and Admission to Intensive Care Unit of COVID-19 Patients. J. Clin. Med. 2022;11:2235. doi: 10.3390/jcm11082235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kudlinski B., Zgoła D., Stolińska M., Murkos M., Kania J., Nowak P., Noga A., Wojciech M., Zaborniak G., Zembron-Lacny A. Systemic Inflammatory Predictors of In-Hospital Mortality in COVID-19 Patients: A Retrospective Study. Diagnostics. 2022;12:859. doi: 10.3390/diagnostics12040859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Virmani R., Kolodgie F.D., Burke A.P., Farb A., Schwartz S.M. Lessons from Sudden Coronary Death: A Comprehensive Morphological Classification Scheme for Atherosclerotic Lesions. Arterioscler. Thromb. Vasc. Biol. 2000;20:1262–1275. doi: 10.1161/01.ATV.20.5.1262. [DOI] [PubMed] [Google Scholar]
- 39.Libby P., Ridker P.M., Maseri A. Inflammation and Atherosclerosis. Circulation. 2002;105:1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 40.Santo Signorelli S., Anzaldi M., Fiore V., Catanzaro S., Simili M., Torrisi B., Neri S. Study on Unrecognized Peripheral Arterial Disease (PAD) by Ankle/Brachial Index and Arterial Comorbidity in Catania, Sicily, Italy. Angiology. 2010;61:524–529. doi: 10.1177/0003319710371614. [DOI] [PubMed] [Google Scholar]
- 41.Ross R. Atherosclerosis—An Inflammatory Disease. N. Engl. J. Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 42.Gibson P.H., Croal B.L., Cuthbertson B.H., Small G.R., Ifezulike A.I., Gibson G., Jeffrey R.R., Buchan K.G., El-Shafei H., Hillis G.S. Preoperative Neutrophil-Lymphocyte Ratio and Outcome from Coronary Artery Bypass Grafting. Am. Heart J. 2007;154:995–1002. doi: 10.1016/j.ahj.2007.06.043. [DOI] [PubMed] [Google Scholar]
- 43.Tamhane U.U., Aneja S., Montgomery D., Rogers E.-K., Eagle K.A., Gurm H.S. Association between Admission Neutrophil to Lymphocyte Ratio and Outcomes in Patients with Acute Coronary Syndrome. Am. J. Cardiol. 2008;102:653–657. doi: 10.1016/j.amjcard.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 44.Duffy B.K., Gurm H.S., Rajagopal V., Gupta R., Ellis S.G., Bhatt D.L. Usefulness of an Elevated Neutrophil to Lymphocyte Ratio in Predicting Long-Term Mortality after Percutaneous Coronary Intervention. Am. J. Cardiol. 2006;97:993–996. doi: 10.1016/j.amjcard.2005.10.034. [DOI] [PubMed] [Google Scholar]
- 45.Sadeghi F., Sarkady F., Zsóri K.S., Szegedi I., Orbán-Kálmándi R., Székely E.G., Vasas N., Berényi E., Csiba L., Bagoly Z., et al. High Neutrophil–Lymphocyte Ratio and Low Lymphocyte–Monocyte Ratio Combination after Thrombolysis Is a Potential Predictor of Poor Functional Outcome of Acute Ischemic Stroke. J. Pers. Med. 2022;12:1221. doi: 10.3390/jpm12081221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee S.-H., Jang M.U., Kim Y., Park S.Y., Kim C., Kim Y.J., Sohn J.-H. The Neutrophil-to-Lymphocyte and Platelet-to-Lymphocyte Ratios Predict Reperfusion and Prognosis after Endovascular Treatment of Acute Ischemic Stroke. J. Pers. Med. 2021;11:696. doi: 10.3390/jpm11080696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lattanzi S., Norata D., Broggi S., Meletti S., Świtońska M., Słomka A., Silvestrini M. Neutrophil-to-Lymphocyte Ratio Predicts Early Neurological Deterioration after Endovascular Treatment in Patients with Ischemic Stroke. Life. 2022;12:1415. doi: 10.3390/life12091415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Świtońska M., Piekuś-Słomka N., Słomka A., Sokal P., Żekanowska E., Lattanzi S. Neutrophil-to-Lymphocyte Ratio and Symptomatic Hemorrhagic Transformation in Ischemic Stroke Patients Undergoing Revascularization. Brain Sci. 2020;10:E771. doi: 10.3390/brainsci10110771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kotfis K., Bott-Olejnik M., Szylińska A., Rotter I. Could Neutrophil-to-Lymphocyte Ratio (NLR) Serve as a Potential Marker for Delirium Prediction in Patients with Acute Ischemic Stroke? A Prospective Observational Study. J. Clin. Med. 2019;8:1075. doi: 10.3390/jcm8071075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dai Z., Li R., Zhao N., Han Y., Wang M., Zhang S., Bai Y., Li Z., Liang M., Xiao L., et al. Neutrophil to Lymphocyte Ratio as a Predictor of Restenosis after Angioplasty and Stenting for Asymptomatic Carotid Stenosis. Angiology. 2019;70:160–165. doi: 10.1177/0003319718784805. [DOI] [PubMed] [Google Scholar]
- 51.Halazun H.J., Mergeche J.L., Mallon K.A., Connolly E.S., Heyer E.J. Neutrophil-Lymphocyte Ratio as a Predictor of Cognitive Dysfunction in Carotid Endarterectomy Patients. J. Vasc. Surg. 2014;59:768–773. doi: 10.1016/j.jvs.2013.08.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.King A.H., Kim A.H., Kwan S., Lee J., Schmaier A.H., Kumins N.H., Harth K.C., Wong V.L., Colvard B.D., Kashyap V.S., et al. Elevated Neutrophil to Lymphocyte Ratio Is Associated with Worse Outcomes after Carotid Endarterectomy in Asymptomatic Patients. J. Stroke Cerebrovasc. Dis. 2021;30:106120. doi: 10.1016/j.jstrokecerebrovasdis.2021.106120. [DOI] [PubMed] [Google Scholar]
- 53.Deşer S.B., Yucel S.M., Demirag M.K., Guclu M.M., Kolbakir F., Keceligil H.T. The Association between Platelet/Lymphocyte Ratio, Neutrophil/Lymphocyte Ratio, and Carotid Artery Stenosis and Stroke Following Carotid Endarterectomy. Vascular. 2019;27:604–611. doi: 10.1177/1708538119847390. [DOI] [PubMed] [Google Scholar]
- 54.Casanova N., Diaz-Duran C., Nieto L., Llort C., Elosua R., Clara A. Predictive Value of Complete Blood Count-Derived Inflammatory Markers for 5-Year Survival After Carotid Endarterectomy: Implications for Practice. Angiology. 2022;73:675–681. doi: 10.1177/00033197211067581. [DOI] [PubMed] [Google Scholar]
- 55.Bao X., Zhou G., Xu W., Liu X., Ye Z., Jiang F. Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio: Novel Markers for the Diagnosis and Prognosis in Patients with Restenosis Following CAS. Biomark. Med. 2020;14:271–282. doi: 10.2217/bmm-2019-0155. [DOI] [PubMed] [Google Scholar]
- 56.Fluri F., Hatz F., Voss B., Lyrer P.A., Engelter S.T. Restenosis after Carotid Endarterectomy: Significance of Newly Acquired Risk Factors. Eur. J. Neurol. 2010;17:493–498. doi: 10.1111/j.1468-1331.2009.02858.x. [DOI] [PubMed] [Google Scholar]
- 57.Texakalidis P., Tzoumas A., Giannopoulos S., Jonnalagadda A.K., Jabbour P., Rangel-Castilla L., Machinis T., Rivet D.J., Reavey-Cantwell J. Risk Factors for Restenosis After Carotid Revascularization: A Meta-Analysis of Hazard Ratios. World Neurosurg. 2019;125:414–424. doi: 10.1016/j.wneu.2019.02.065. [DOI] [PubMed] [Google Scholar]
- 58.Zhou F., Hua Y., Ji X., Jia L. A Systemic Review into Carotid Plaque Features as Predictors of Restenosis after Carotid Endarterectomy. J. Vasc. Surg. 2021;73:2179–2188.e4. doi: 10.1016/j.jvs.2020.10.084. [DOI] [PubMed] [Google Scholar]
- 59.Hellings W.E., Moll F.L., De Vries J.-P.P.M., Ackerstaff R.G.A., Seldenrijk K.A., Met R., Velema E., Derksen W.J.M., De Kleijn D.P.V., Pasterkamp G. Atherosclerotic Plaque Composition and Occurrence of Restenosis After Carotid Endarterectomy. JAMA. 2008;299:547–554. doi: 10.1001/jama.299.5.547. [DOI] [PubMed] [Google Scholar]
- 60.Lattanzi S., Norata D., Divani A.A., Di Napoli M., Broggi S., Rocchi C., Ortega-Gutierrez S., Mansueto G., Silvestrini M. Systemic Inflammatory Response Index and Futile Recanalization in Patients with Ischemic Stroke Undergoing Endovascular Treatment. Brain Sci. 2021;11:1164. doi: 10.3390/brainsci11091164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Scicchitano P., Marzullo A., Santoro A., Zito A., Cortese F., Galeandro C., Ciccone A.S., Angiletta D., Manca F., Pulli R., et al. The Prognostic Role of ST2L and SST2 in Patients Who Underwent Carotid Plaque Endarterectomy: A Five-Year Follow-Up Study. J. Clin. Med. 2022;11:3142. doi: 10.3390/jcm11113142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Marzullo A., Ambrosi F., Inchingolo M., Manca F., Devito F., Angiletta D., Zito A., Scicchitano P., Ciccone M.M. ST2L Transmembrane Receptor Expression: An Immunochemical Study on Endarterectomy Samples. PLoS ONE. 2016;11:e0156315. doi: 10.1371/journal.pone.0156315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Scicchitano P., Cameli M., Maiello M., Modesti P.A., Muiesan M.L., Novo S., Palmiero P., Saba P.S., Pedrinelli R., Ciccone M.M. Nutraceuticals and Dyslipidaemia: Beyond the Common Therapeutics. J. Funct. Foods. 2014;6:11–32. doi: 10.1016/j.jff.2013.12.006. [DOI] [Google Scholar]
- 64.Ciccone M.M., Cortese F., Gesualdo M., Carbonara S., Zito A., Ricci G., De Pascalis F., Scicchitano P., Riccioni G. Dietary Intake of Carotenoids and Their Antioxidant and Anti-Inflammatory Effects in Cardiovascular Care. Mediat. Inflamm. 2013;2013:782137. doi: 10.1155/2013/782137. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.