Abstract
Simple Summary
We aimed to compare etiopathogenic features of 28 penile squamous cell carcinomas (PSCC) from Mozambique, an African country with a high prevalence of human papillomavirus (HPV) and HIV, with 51 PSCC from Spain, a European country with a low prevalence of HPV and HIV. All tumors underwent HPV testing and p16 and p53 immunohistochemistry. PSCC with either p16 overexpression and/or positivity for HPV were considered HPV-associated. The two sites showed striking etiopathogenic differences. Patients from Mozambique were significantly younger than those from Spain and showed mostly HPV-associated, p53-wild-type PSCC, contrary to older patients from Spain showing mostly HPV-independent, p53-altered tumors. These data may be valuable for primary prevention of PSCC worldwide.
Abstract
Penile squamous cell carcinomas (PSCC) are classified by the World Health Organization into two categories based on their relationship with the human papillomavirus (HPV): HPV-associated and HPV-independent. We compared a cohort of PSCC from Mozambique, a sub-Saharan country in southeast Africa with a high prevalence of HPV and HIV infection, and Spain, a country in southwestern Europe with a low prevalence of HPV and HIV, to study the distribution of the etiopathogenic categories of these tumors in both sites. A total of 79 PSCC were included in the study (28 from Mozambique and 51 from Spain). All cases underwent HPV-DNA polymerase chain reaction (PCR) testing, genotyping, and immunohistochemistry for p16 and p53. Any PSCC showing either p16 overexpression or HPV-DNA in PCR analysis was considered HPV-associated. Overall, 40/79 (50.6%) tumors were classified as HPV-associated and 39 (49.4%) as HPV-independent. The two sites showed marked differences: 25/28 (89.3%) tumors from Mozambique and only 15/51 (29.4%) from Spain were HPV-associated (p < 0.001). HPV16 was the most frequent HPV type identified in 64.0% (16/25) of the HPV-associated tumors from Mozambique, and 60.0% (9/15) from Spain (p = 0.8). On average, patients from Mozambique were almost two decades younger than those from Spain (mean age 50.9 ± 14.9 and 69.2 ± 13.3, respectively [p < 0.001]). In conclusion, significant etiopathogenic differences between PSCC in Mozambique and Spain were observed, with a remarkably high prevalence of HPV-associated tumors in Mozambique and a relatively low prevalence in Spain. These data may have important consequences for primary prevention of PSCC worldwide.
Keywords: penile cancer, Mozambique, Spain, HPV, p16, p53
1. Introduction
Penile cancer is an uncommon malignancy with an estimated age-standardized incidence worldwide of 0.80 per 100,000 person-year in 2018 [1] that typically affects men in their fifth to seventh decades of life. Over 95% of penile cancers are penile squamous cell carcinomas (PSCC) [2]. Several risk factors for PSCC have been identified, including sexual behavior, history of warts/condylomas/human papillomavirus (HPV) infection, lack of circumcision, phimosis, poor penile hygiene, smoking and chronic inflammatory conditions such as lichen sclerosus [3]. In its 2016 classification, the World Health Organization (WHO) recommended separating PSCC based on their HPV status into HPV-associated and HPV-independent tumors [4,5], with this categorization having been maintained in the recent WHO 2022 revision [6]. This WHO classification recognizes a clear association between histological types and their relationship with HPV, with several histological variants of PSCC that are typically HPV-associated and other histological variants known as HPV-independent [6].
HPV-associated PSCC frequently arise on an intraepithelial precursor named HPV-associated penile intraepithelial neoplasia (PeIN), a lesion with a basaloid, warty, or warty–basaloid morphology [7], also called high-grade squamous intraepithelial lesion (HSIL) according to the consensus of the Lower Anogenital Squamous Terminology standardization project [8]. HPV-associated PeIN is characterized by immature basal-appearing cells involving the whole thickness of the epithelium. Both HPV-associated PSCC and PeIN show strong, block type staining for p16 immunohistochemistry (IHC) [7,9,10,11]. Contrarily, HPV-independent PSCC frequently arise on a precursor lesion named non-HPV-related PeIN or differentiated PeIN, a lesion characterized by atypical basal cells with normal maturation in the superficial layers that usually develops in a context of inflammatory conditions of the penis, such as lichen sclerosus [7,9,10]. Both HPV-independent PSCC and its precursor lesion show no overexpression of p16 and frequently display an abnormal pattern of p53 [7,9,10].
The incidence of PSCC has marked geographical variability, with the highest incidence centered in low- and middle-income countries (LMIC), especially in southern Africa, South Asia, and South America, and a significantly lower, albeit rising, incidence in most European countries [12]. Unfortunately, while the epidemiology of PSCC in high-income areas is relatively well known, data on PSCC in LMIC, particularly in sub-Saharan Africa, are very sparse. The largest published study encompassing 1010 PSCC, involved only 19 tumors (1.8%) from sub-Saharan African countries [13]. The high prevalence of HPV infection may have a significant impact in terms of the proportion of HPV-associated and HPV-independent tumors. In addition, the high prevalence of HIV in these areas [14] may have also a significant impact in the rates of HPV-associated and -independent PSCC, as this condition multiplies the risk of persistent HPV infection and, consequently, leads to increased incidence of HPV-associated premalignant lesions and carcinomas [15].
The aim of this study was to compare the differential etiopathogenic characteristics of PSCC in Mozambique, a sub-Saharan country in South-East Africa with high prevalence of HPV and HIV and Spain, a European country with low ratesof HPV and HIV [16].
2. Methods
2.1. Case Selection
The computer records of the Department of Pathology of the Maputo Central Hospital (MCH) and the Hospital Clinic of Barcelona (HCB)-two tertiary referral health institutions in Mozambique and Spain, respectively, were revised looking for all the cases diagnosed with PSCC. The period revised in the MCH was from January 2018 to December 2020, being from January 2000 to December 2020 in the HCB.
All PSCC identified during these periods were retrieved, and the material available was histologically reviewed. The inclusion criteria for the study were: (1) the presence of invasive PSCC and (2) the material available for HPV detection by polymerase chain reaction (PCR), and p16 and p53 IHC.
The ethics committees of both the Faculty of Medicine of the Eduardo Mondlane University & MCH and HCB approved the study (refs. CIBS FM&HCM/071/2017 and HCB/2020/1207, respectively).
2.2. Histological Review
Sections of all the tumors stained with hematoxylin and eosin were reviewed by two pathologists (CM, NR) to confirm the presence of invasive carcinoma. Following the WHO criteria, several histological types were considered as probably related to HPV (basaloid, papillary–basaloid, warty, wart–basaloid, clear cell, and lymphoepithelioma-like) and other histological variants were considered as not related to HPV infection (usual type [keratinizing], verrucous [including cuniculatum], papillary and sarcomatoid) [4,6]. After revision, the most representative and well-preserved paraffin block, containing the most obvious pathological findings of each case, was selected for HPV detection and IHC staining.
2.3. Tissue Preparation, Nucleic-Acid Isolation and HPV-DNA Detection
DNA extraction was performed on 10µm whole sections of formalin-fixed paraffin-embedded tissue from surgical specimens or penile biopsies. The microtome blade was replaced between cases. No microdissection was performed but, in all cases, the tissue analyzed included the invasive tumor. The highest safety measures were carried out while sectioning and performing sample preparation to avoid cross-contamination. DNA was extracted after overnight incubation in 20 μL of proteinase K solution at 56 °C. Subsequently, the DNA was isolated using a commercial kit (QIAamp DNA FFPE Tissue Kit; Qiagen, Hilden, Germany) according to the manufacturing instructions. Samples were quantified using the NanoDrop 2000 (Thermo Fisher Scientific, Waltham, MA, USA) to determine DNA concentrations for the genotyping test. Samples with less than 10 µL of isolated DNA were excluded from the study.
The SPF10 PCR and LiPA25 system were used for HPV-DNA detection and typing (version 1, Labo Biomedical Products, Rijswijk, The Netherlands). A volume of 10 µL of isolated DNA was PCR-amplified using the INNO-LiPA HPV Genotyping Extra II kit (Fujirebio, Gent, Belgium). The same kit was used for HPV genotyping. This system allows the genotyping of HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68, 26, 53, 66, 70, 73, 82, 6, 11, 40, 42, 43, 44, 54, 61, 62, 67, 81, 83 and 89. HPV DNA-positive samples not hybridizing with any of the 32 probes were classified as HPV type X (HPV X or undetermined type).Each run was performed with positive and negative controls to monitor the efficiency of DNA isolation, PCR amplification, hybridization, and genotyping procedures.
2.4. p16 IHC
All tumors were stained with a p16 monoclonal antibody using the CINtec Histology Kit (clone E6H4; Roche-mtm-Laboratories, Heidelberg, Germany). The tumors were considered as p16 positive if they showed strong and diffuse block-like staining, and were considered as negative when the staining was patchy or completely negative.
All IHC stains were conducted at the department of Pathology of the HCB, employing the BenchMark ULTRA platform (Roche-Ventana, Tucson, AZ, USA).
2.5. Criteria for Classifying a Tumor as HPV-Associated or HPV-Independent
In this study any PSCC showing either p16 IHC overexpression and/or HPV-DNA in the PCR analysis were considered as HPV-associated. All PSCC showing a negative result for the two techniques (p16 IHC and HPV-DNA PCR) were considered as HPV-independent.
As it has recently been proposed that a second biomarker, such as p16 or E6*I mRNA, should also be used to consider a tumor as etiologically associated with HPV [13], a second, more restrictive scenario was also calculated, including only tumors showing both HPV-DNA in the PCR analysis and p16 IHC overexpression as HPV-associated. In this restrictive scenario, tumors showing a discrepant result (HPV-DNA negative and p16 positive or HPV-DNA positive and p16 negative) were considered as HPV-independent.
2.6. p53 IHC
A monoclonal antibody was used for p53 staining (clone DO-7, Dako, Carpinteria, CA, USA). The interpretation of staining was conducted in the invasive tumor following the pattern-based framework recently developed for vulvar carcinomas [17,18]. This framework includes two normal (wild-type) and four abnormal (suggestive of mutant protein) patterns. Normal patterns included (a) scattered basal nuclear positivity (b) staining in cells in the mid-epithelial layers, with absent basal staining. Abnormal p53 patterns comprised: (1) basal overexpression (strong and continuous staining of the basal cells), (2) diffuse overexpression (strong and continuous staining of the basal and suprabasal cells (3) cytoplasmic staining (with or without nuclear positivity), and (4) null pattern (absence of staining in the tumoral cells with conserved staining in the normal skin, stromal or inflammatory cells).
2.7. Statistical Analysis
The SPSS 28.0 statistical package (SPSS, Chicago, IL, USA) was used to perform the data analyses. Categorical variables are shown as absolute numbers and percentages. And were analyzed with the chi-square or Fisher exact test. Quantitative variables are shown as mean ± standard deviation, and were compared with Student’s t test or analysis of variance test.
3. Results
3.1. General Features
Overall, 79 tumors were included in the study, 28 from the MCH (Mozambique) and 51 from the HCB (Spain). The age, HIV status, histological variant, p16 IHC, HPV-DNA PCR, and p53 IHC results of the PSCC from Mozambique and Spain are shown in Table 1 and Table 2, respectively.
Table 1.
Age, HIV status, histological variant, Human Papillomavirus (HPV)-DNA result, p16 and p53 immunohistochemical features of the tumors from Mozambique.
| Case | Age | HIV Status | Histological Variant | p16 IHC | HPV-DNA Detection and Type | p53 IHC |
|---|---|---|---|---|---|---|
| HPV-associated carcinomas | ||||||
| M2 | 40 | NA | Warty/basaloid | + | 70 | Normal |
| M3 | 40 | + | Usual type | + | 45, 51, 70 | Normal |
| M5 | 98 | NA | Warty/basaloid | + | 16 | Normal |
| M7 | 44 | NA | Basaloid | + | 16 | Normal |
| M8 | 38 | NA | Warty/basaloid | + | 18, 39 | Normal |
| M10 | 62 | − | Warty | + | 16 | Normal |
| M11 | 38 | NA | Warty/basaloid | + | 16 | Normal |
| M12 | 39 | NA | Warty/basaloid | + | 16 | Normal |
| M14 | 51 | NA | Warty | + | 18, 31, 44, 6 | Normal |
| M15 | 41 | NA | Warty/basaloid | + | 16 | Normal |
| M16 | 34 | NA | Warty/basaloid | + | 16 | Normal |
| M17 | 30 | NA | Basaloid | + | 16 | Normal |
| M18 | 39 | NA | Warty/basaloid | + | 16 | Normal |
| M20 | 43 | + | Basaloid | + | 16 | Abnormal |
| M22 | 66 | NA | Basaloid | + | 68 | Normal |
| M23 | 45 | + | Warty/basaloid | + | 16, 68 | Normal |
| M24 | 51 | + | Basaloid | + | 16 | Normal |
| M25 | 43 | + | Warty | + | 16 | Normal |
| M27 | 68 | + | Basaloid | + | 16, 52, 82, 11 | Normal |
| M28 | 52 | − | Basaloid | + | 16 | Normal |
| M6 | 56 | NA | Warty | + | Negative | Normal |
| M26 | 59 | NA | Basaloid | + | Negative | Normal |
| M1 | 68 | NA | Warty/basaloid | − | 16, 11 | Abnormal |
| M9 | 51 | NA | Usual type | − | X | Normal |
| M13 | 53 | NA | Basaloid | − | 18, 31, 52 | Normal |
| HPV-independent carcinomas | ||||||
| M4 | 65 | NA | Warty | − | Negative | Abnormal |
| M19 | 74 | NA | Warty | − | Negative | Abnormal |
| M21 | 38 | NA | Usual type | − | Negative | Abnormal |
NA: not available: + positive; − negative; X: undetermined HPV genotype.
Table 2.
Age, HIV status, histological variant, Human Papillomavirus (HPV)-DNA result, p16 and p53 immunohistochemical features of the tumors from Spain.
| Case | Age | HIV Status | Histological Variant | p16 | HPV-DNA Detection and Type | p53 |
|---|---|---|---|---|---|---|
| HPV-associated carcinomas | ||||||
| S3 | 51 | − | Basaloid | + | 16 | Abnormal |
| S9 | 61 | − | Warty | + | 16 | Normal |
| S14 | 58 | − | Basaloid | + | 16 | Normal |
| S21 | 52 | − | Usual type | + | 16, 18 | Normal |
| S22 | 81 | − | Lymphoepithelioma-like | + | 16 | Normal |
| S38 | 45 | + | Warty-basaloid | + | 16 | Normal |
| S45 | 67 | − | Warty | + | 6 | Normal |
| S46 | 87 | − | Warty-basaloid | + | 16 | Normal |
| S51 | 59 | − | Basaloid | + | 16 | Normal |
| S12 | 60 | − | Warty | + | − | Abnormal |
| S29 | 94 | − | Basaloid | + | − | Normal |
| S30 | 63 | − | Basaloid | + | − | Normal |
| S31 | 64 | − | Basaloid | + | − | Normal |
| S13 | 60 | − | Usual type | − | 16 | Abnormal |
| S49 | 76 | − | Basaloid | − | X | Normal |
| HPV-independent carcinomas | ||||||
| S1 | 62 | − | Verrucous | − | − | Normal |
| S2 | 65 | − | Usual type | − | − | Normal |
| S4 | 59 | − | Usual type | − | − | Abnormal |
| S5 | 53 | − | Usual type | − | − | Abnormal |
| S6 | 53 | − | Usual type | − | − | Abnormal |
| S7 | 78 | − | Usual type | − | − | Abnormal |
| S8 | 54 | − | Usual type | − | − | Abnormal |
| S10 | 81 | − | Usual type | − | − | Abnormal |
| S11 | 83 | − | Usual type | − | − | Normal |
| S15 | 74 | − | Basaloid | − | − | Abnormal |
| S16 | 70 | − | Usual type | − | − | Abnormal |
| S17 | 81 | − | Usual type | − | − | Abnormal |
| S18 | 85 | − | Warty-basaloid | − | − | Normal |
| S19 | 63 | − | Usual type | − | − | Normal |
| S20 | 59 | − | Usual type | − | − | Abnormal |
| S23 | 80 | − | Usual type | − | − | Normal |
| S24 | 72 | − | Basaloid | − | − | Abnormal |
| S25 | 74 | − | Usual type | − | − | Normal |
| S26 | 73 | − | Usual type | − | − | Normal |
| S27 | 77 | − | Usual type | − | − | Abnormal |
| S28 | 65 | − | Usual type | − | − | Abnormal |
| S32 | 85 | − | Verrucous | − | − | Normal |
| S33 | 79 | − | Usual type | − | − | Abnormal |
| S34 | 75 | − | Usual type | − | − | Normal |
| S35 | 86 | − | Usual type | − | − | Abnormal |
| S36 | 70 | − | Verrucous (cuniculatum) | − | − | Normal |
| S37 | 56 | − | Usual type | − | − | Abnormal |
| S39 | 59 | − | Usual type | − | − | Abnormal |
| S40 | 40 | − | Usual type | − | − | Abnormal |
| S41 | 83 | − | Verrucous | − | − | Normal |
| S42 | 75 | − | Usual type | − | − | Abnormal |
| S43 | 44 | − | Usual type | − | − | Abnormal |
| S44 | 67 | − | Warty-basaloid | − | − | Abnormal |
| S47 | 96 | − | Usual type | − | − | Normal |
| S48 | 85 | − | Usual type | − | − | Normal |
| S50 | 89 | − | Usual type | − | − | Normal |
NA: not available: + positive; − negative; X: undetermined HPV genotype.
The clinical presentation of PSCC was markedly different in the two sites, with the patients from Mozambique being on average almost two decades younger than patients from Spain (mean age of the patients from Mozambique 50.9 ± 14.9 years and mean age in Spain 69.2 ± 13.3 years [p < 0.001]). HIV status was available in 8 cases from Mozambique and in all cases from Spain. Six out of 8 (75.0%) of the Mozambican patients were HIV positive in contrast with 1/51 (1.9%) of the Spanish patients.
3.2. Association with HPV
Overall 40/79 (50.6%) tumors were classified as HPV-associated and 39 (49.4%) as HPV-independent. However, marked differences were observed between the two sites: 25/28 (89.3%) tumors from Mozambique vs. only 15/51 (29.4%) from Spain were HPV-associated. Using stricter criteria (restrictive scenario, i.e., HPV-DNA identified by PCR and p16 IHC overexpression) 20/28 PSCC (71.4%) from Mozambique and 9/51 (17.6%) from Spain were considered as HPV-associated. In both scenarios the differences were statistically significant (p < 0.001).
HPV16 was the most frequent HPV type identified in 64.0% (16/25) of the HPV-associated tumors from Mozambique and in 60.0% (9/15) of the tumors from Spain (p = 0.8). No differences in the distribution of HPV type were identified between the two sites in any of the scenarios (p = 1). The prevalence of infection by multiple HPV types was slightly higher in Mozambique than in Spain, although the differences did not reach statistical significance (28.0% [7/25] vs. 6.7% [1/15], respectively; p = 0.219).
3.3. Histological Subtypes
Of the 40 tumors associated with infection by HPV, 36 (90.0%) were of the histological variants defined as related to HPV according to the WHO (16 [40.0%] basaloid, 7 [17.5%] warty, 12 [30.0%] mixed basaloid/warty, and 1 [2.5%] lymphoepithelioma-like), but four (10.0%) were of the usual type, one of the variants considered as not related to HPV by the WHO. Contrarily, 33/39 (84.6%) of the HPV-independent PSCC were of the histological variants defined as not related to HPV according to the WHO (29 [74.4%] usual type, 3 [7.6%] verrucous, 1 [2.4%] cuniculatum), but 6 (15.4%) were variants classified as HPV-related by the WHO (2 [5.1%] basaloid, 2 [5.1%] warty, and 2 [5.1%] mixed warty/basaloid).
3.4. p53 IHC Results
p53 IHC showed a normal staining pattern suggestive of wild-type protein in 35/40 (87.5%) of the HPV-associated tumors, and only 5/40 (12.5%) showed an abnormal pattern suggestive of a mutated p53. No differences were observed between Mozambique and Spain (p = 0.38). Contrarily, the HPV-independent PSCC showed an abnormal pattern of p53 IHC staining in 24/39 (61.5%) and a normal pattern in 15/39 (38.5%) (p < 0.0001).
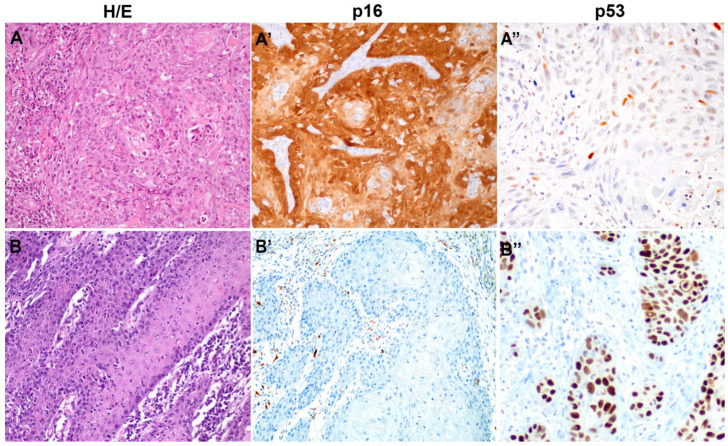
Figure 1 shows an example of the histological and IHC features of the HPV-associated and HPV-independent types of PSCC.
Figure 1.
Histological and immunohistochemical features of a representative example of Human Papillomavirus (HPV)-associated (A,A′,A″) and HPV-independent (B,B′,B″) penile squamous cell carcinoma (PSCC). (A,B, hematoxylin and eosin, 200×), p16 (A′,B′) and p53 (A″,B″), HPV-associated PSCC, the most frequent type in Mozambique, is characterized by diffuse positive p16 staining and scattered irregular nuclear positivity for p53. HPV-independent PSCC, the most frequent type in Spain, is characterized by keratinizing histology, negative p16 staining and marked overexpression of p53.
4. Discussion
In this study we provide relevant information in relation to the epidemiology of PSCC in sub-Saharan Africa, a geographical area in which these data are particularly scant. Our series of PSCC from sub-Saharan Africa and Europe shows relevant etiopathogenic differences between the two sites and highlights the extremely high prevalence of HPV-associated PSCC in Mozambique compared with the low prevalence in Spain. Over 89% of the PSCC in Mozambique but only 29% of the PSCC from Spain were HPV-associated (p < 0.001). Using the restrictive scenario (i.e., p16 IHC overexpression and HPV-DNA identified by PCR), the proportion of HPV-associated tumors from Mozambique was still extremely high (71.4%), whereas the percentage of HPV-associated tumors in Spain dropped to 17.6% (p < 0.001). HPV16 was the most frequent HPV type identified in both sites (64.0% and 60.0% HPV-associated tumors from Mozambique and Spain, respectively), similar to what has been reported in other studies [13,19].
The results of our Spanish cohort are in keeping with most studies from high-income countries. In these sites, PSCC is typically a disease of old men, and a significant proportion arise independently from HPV, frequently within the background of inflammatory conditions of the penile skin, such as lichen sclerosus and lichen simplex chronicus [13,14,20]. Contrarily, information from LMIC sites is very scant [21], but existing data suggest that PSCC may have a distinctive epidemiological background, with most tumors arising via an HPV-associated pathway [22]. A high prevalence of HPV infections has been reported in many LMIC, with Mozambique showing an incidence as high as 63% [23]. As HPV-associated tumors tend to affect younger individuals, patients from Spain were almost two decades older in average than patients from Mozambique, a pattern also observed in vulvar squamous cell carcinomas [24].
Although the high prevalence of HPV infection should be considered the main factor the high prevalence of HPV-associated PSCC in the Mozambican cohort, the high prevalence of HIV in Mozambique [25] has been suggested as a relevant contributor to the rise in HPV-associated carcinomas in Mozambique and also in other sub-Saharan countries [26]. A community-based study in a rural district of Maputo Province (Mozambique) showed a 37% prevalence of HIV in men 28–47 years old [25]. A high risk of developing HPV-related cancers [27] has been observed in people living with HIV, and the two viruses (HPV and HIV type 1) have been categorized as carcinogens by the International Agency for Research on Cancer [28]. Nevertheless, whereas the association between HIV and cervical cancer has been well established [29], the link between HPV-associated PSCC and HIV has not been evaluated. Interestingly, 75% of the Mozambican patients for whom clinical data were available were HIV positive. In contrast, the prevalence of HIV-positive patients in the Spanish series was 1.9%. Finally, the limited access to the health system might also contribute to this high incidence, as a large part of population in Mozambique lives in rural areas where access to health care is poor.
Interestingly, although 90% of the HPV-associated tumors were basaloid, warty, mixed basaloid/warty, or lymphoepithelioma-like, the types of PSCC considered, following the WHO classification, as tumors related to HPV [4,6], 10% of the HPV-associated PSCC in our series were of the usual type. Similarly, whereas 85% of the HPV-independent tumors were of the usual type or verrucous, 15% were basaloid, warty, or mixed warty/basaloid, in other words, the histological types considered in this classification as related to HPV. Similar results have been reported in the largest study on penile tumors [13]. These findings suggest that diagnosis based on pure morphological criteria may be misleading in a small but significant proportion of tumors and indicate that p16 IHC and/or HPV-DNA testing should be required to properly classify PSCC as HPV-associated or HPV-independent. In this regard, we recently reported that a small proportion of HPV-independent intraepithelial precursors of penile cancer may be indistinguishable from the HPV-associated precursors [30]. This overlap between HPV-associated and -independent tumors is a well-known phenomenon in vulvar tumors [31] and head and neck tumors, and the WHO classification of both types of neoplasms stresses the need for p16 IHC and/or HPV-DNA testing to adequately classify these tumors because of the better prognosis of HPV-associated compared with HPV-independent tumors in all these sites [32,33]. Growing evidence suggesting that this better behavior of HPV-associated tumors also applies to PSCC [34,35] stresses the need for these ancillary studies to properly classify PSCC. Nevertheless, ancillary studies to detect HPV in the tumor are probably not necessary in sub-Saharan countries, as most PSCC are HPV-associated given that HPV-independent tumors are extremely infrequent and laboratory capacities are low [36].
Interestingly, abnormal p53 IHC-mutant patterns were identified in a high percentage of HPV-independent PSCC and in only a small percentage of HPV-associated tumors, a phenomenon already reported in studies based on IHC, in addition to studies analyzing TP53 mutations [37,38,39].
A subset of tumors showed discrepant p16 IHC and HPV-DNA results. It is difficult to draw conclusions on the significance of these findings and on whether these cases are or are not etiologically related to HPV with the data of the present study. It has recently been proposed that in addition to the identification of the DNA of the HPV, a second biomarker, such as p16 or E6 mRNA, should also be required to consider a tumor as etiologically associated with HPV [13]. On the other hand, studies conducted in vulvar squamous cell carcinoma suggest that p16 IHC is more reliable than HPV-DNA detection tests using formalin-fixed, paraffin-embedded material [31].
5. Conclusions
Most PSCC in Mozambique, a country in southeastern Africa, which probably reflects the epidemiological picture of many sub-Saharan African countries, are HPV-associated and arise in young men. In contrast, most PSCC diagnosed in Europe are HPV-independent and affect old men. These data suggest that extending HPV vaccination programs to males in LMIC could, in addition to the benefits of tackling the cervical cancer burden, reduce a significant proportion of penile cancer.
Acknowledgments
The authors are thankful to the Pathology and MCH Cancer Registry databases for helping to obtain the data and the Calouste Gulbenkian Foundation, Portugal, and partners (Camões—Institute of Cooperation and Language, Portugal; Millennium BCP Foundation, Portugal; and Millennium BIM, Mozambique), which allowed the implementation of the MCH Cancer Registry under the project ‘Improving the diagnosis and treatment of oncological diseases in Mozambique’. ISGlobal receives support from the Spanish Ministry of Science and Innovation through the “Centro de Excelencia Severo Ochoa 2019–2023” Program (CEX2018-000806-S), and support from the Generalitat de Catalunya through the CERCA Program.
Author Contributions
Conceptualization, J.O., C.C., C.M., N.R. and M.d.P.; Methodology, L.C., C.M., A.S., I.T., L.M., R.L.d.C., N.T., O.S. and L.L.; Software, M.d.P. and C.M.; Validation, N.T., O.S., F.F., I.B., L.L, C.L., M.T.R.-C. and I.R.-C.; Formal Analysis, M.d.P., L.C., I.T., N.T., O.S. and M.R.I.; Investigation, C.C., F.F., L.C., L.L., I.T., I.R.-C., I.B., O.S., C.L., M.T.R.-C., M.R.I., N.T., L.M. and A.S.; J.O. and C.M. Resources, J.O. and C.C.; Data Curation, L.C., I.B., O.S. and C.C.; Writing—Original Draft Preparation, C.M., C.C., L.C., A.S., J.O., N.R., R.L.d.C., F.F. and M.R.I.; Writing—Review & Editing, A.S., C.C., C.M., F.F., L.L., I.T., I.R.-C., I.B., O.S., C.L., M.T.R.-C., M.R.I., M.d.P., N.T. and L.M.; L.C., I.R.-C., N.R. and J.O. Visualization, C.M. and J.O.; Supervision, I.R.-C., A.S., R.L.d.C. and L.M.; Project Administration, I.R.-C. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of the Eduardo Mondlane University/Maputo Central Hospital and Hospital Clínic of Barcelona (Protocol codes FM&HCM/071/2017 and HCB/2020/1207, respectively).
Informed Consent Statement
Informed consent was obtained from the individuals involved in the study.
Data Availability Statement
All the data is shown in the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
No official funding was received to carry out this work.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Barnholtz-Sloan J.S., Maldonado J.L., Pow-sang J., Guiliano A.R. Incidence trends in primary malignant penile cancer. Urol. Oncol. 2007;25:361–367. doi: 10.1016/j.urolonc.2006.08.029. [DOI] [PubMed] [Google Scholar]
- 3.Daling J.R., Madeleine M.M., Johnson L.G., Schwartz S.M., Shera K.A., Wurscher M.A., Carter J.J., Porter P.L., Galloway D.A., McDougall J.K., et al. Penile cancer: Importance of circumcision, human papillomavirus and smoking in in situ and invasive disease. Int. J. Cancer. 2005;116:606–616. doi: 10.1002/ijc.21009. [DOI] [PubMed] [Google Scholar]
- 4.Moch H., Cubilla A.L., Humphrey P.A., Reuter V.E., Ulbright T.M. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part A: Renal, Penile, and Testicular Tumours. Eur. Urol. 2016;70:93–105. doi: 10.1016/j.eururo.2016.02.029. [DOI] [PubMed] [Google Scholar]
- 5.Cubilla A.L., Velazquez E.F., Amin M.B., Epstein J., Berney D.M., Corbishley C.M. Members of the ISUP Penile Tumor Panel The World Health Organisation 2016 classification of penile carcinomas: A review and update from the International Society of Urological Pathology expert-driven recommendations. Histopathology. 2018;72:893–904. doi: 10.1111/his.13429. [DOI] [PubMed] [Google Scholar]
- 6.Moch H., Amin M.B., Berney D.M., Compérat E.M., Gill A.J., Hartmann A., Menon S., Raspollini M.R., Rubin M.A., Srigley J.R., et al. The 2022 World Health Organization Classification of Tumours of the Urinary System and Male Genital Organs—Part A: Renal, Penile, and Testicular Tumours. Eur. Urol. 2022;82:458–468. doi: 10.1016/j.eururo.2022.06.016. [DOI] [PubMed] [Google Scholar]
- 7.Chaux A., Pfannl R., Lloveras B., Alejo M., Clavero O., Lezcano C., Muñoz N., de Sanjosé S., Bosch X., Hernández-Pérez M., et al. Distinctive association of p16INK4a overexpression with penile intraepithelial neoplasia depicting warty and/or basaloid features: A study of 141 cases evaluating a new nomenclature. Am. J. Surg. Pathol. 2010;34:385–392. doi: 10.1097/PAS.0b013e3181cdad23. [DOI] [PubMed] [Google Scholar]
- 8.Darragh T.M., Colgan T.J., Thomas Cox J., Heller D.S., Henry M.R., Luff R.D., McCalmont T., Nayar R., Palefsky J.M., Stoler M.H., et al. The Lower Anogenital Squamous Terminology Standardization project for HPV-associated lesions: Background and consensus recommendations from the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology. Int. J. Gynecol. Pathol. 2013;32:76–115. doi: 10.1097/PGP.0b013e31826916c7. [DOI] [PubMed] [Google Scholar]
- 9.Chaux A., Velazquez E.F., Amin A., Soskin A., Pfannl R., Rodríguez I.M., Barreto J.E., Lezcano C., Ayala G., Netto G.J., et al. Distribution and characterization of subtypes of penile intraepithelial neoplasia and their association with invasive carcinomas: A pathological study of 139 lesions in 121 patients. Hum. Pathol. 2012;43:1020–1027. doi: 10.1016/j.humpath.2011.07.025. [DOI] [PubMed] [Google Scholar]
- 10.Chaux A., Pfannl R., Rodríguez I.M., Barreto J.E., Velazquez E.F., Lezcano C., Piris A., Netto G.J., Cubilla A.L. Distinctive immunohistochemical profile of penile intraepithelial lesions: A study of 74 cases. Am. J. Surg. Pathol. 2011;35:553–562. doi: 10.1097/PAS.0b013e3182113402. [DOI] [PubMed] [Google Scholar]
- 11.Canete-Portillo S., Velazquez E.F., Kristiansen G., Egevad L., Grignon D., Chaux A., Cubilla A.L. Report From the International Society of Urological Pathology (ISUP) Consultation Conference on Molecular Pathology of Urogenital Cancers V: Recommendations on the Use of Immunohistochemical and Molecular Biomarkers in Penile Cancer. Am. J. Surg. Pathol. 2020;44:E80–E86. doi: 10.1097/PAS.0000000000001477. [DOI] [PubMed] [Google Scholar]
- 12.Fu L., Tian T., Yao K., Chen X.-F., Luo G., Gao Y., Lin Y.-F., Wang B., Sun Y., Zheng W., et al. Global Pattern and Trends in Penile Cancer Incidence: Population-Based Study. JMIR Public Health Surveill. 2022;8:e34874. doi: 10.2196/34874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alemany L., Cubilla A., Halec G., Kasamatsu E., Quirós B., Masferrer E., Tous S., Lloveras B., Hernández-Suarez G., Lonsdale R., et al. Role of Human Papillomavirus in Penile Carcinomas Worldwide. Eur. Urol. 2016;69:953–961. doi: 10.1016/j.eururo.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 14.Dwyer-Lindgren L., Cork M.A., Sligar A., Steuben K.M., Wilson K.F., Provost N.R., Mayala B.K., VanderHeide J.D., Collison M.L., Hall J.B., et al. Mapping HIV prevalence in sub-Saharan Africa between 2000 and 2017. Nature. 2019;570:189–193. doi: 10.1038/s41586-019-1200-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Looker K.J., Rönn M.M., Brock P.M., Brisson M., Drolet M., Mayaud P., Boily M.C. Evidence of synergistic relationships between HIV and Human Papillomavirus (HPV): Systematic reviews and meta-analyses of longitudinal studies of HPV acquisition and clearance by HIV status, and of HIV acquisition by HPV status. J. Int. AIDS Soc. 2018;21:e25110. doi: 10.1002/jia2.25110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakagawa F., Phillips A.N., Lundgren J.D. Update on HIV in Western Europe. Curr. HIV/AIDS Rep. 2014;11:177–185. doi: 10.1007/s11904-014-0198-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rakislova N., Alemany L., Clavero O., Saco A., Torné A., Del Pino M., Munmany M., Rodrigo-calvo M.T., Guerrero J., Marimon L., et al. P53 immunohistochemical patterns in HPV-independent squamous cell carcinomas of the vulva and the associated skin lesions: A study of 779 cases. Int. J. Mol. Sci. 2020;21:8091. doi: 10.3390/ijms21218091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tessier-Cloutier B., Kortekaas K.E., Thompson E., Pors J., Chen J., Ho J., Prentice L.M., McConechy M.K., Chow C., Proctor L., et al. Major p53 immunohistochemical patterns in in situ and invasive squamous cell carcinomas of the vulva and correlation with TP53 mutation status. Mod. Pathol. 2020;33:1595–1605. doi: 10.1038/s41379-020-0524-1. [DOI] [PubMed] [Google Scholar]
- 19.Ferrándiz-Pulido C., Masferrer E., De Torres I., Lloveras B., Hernandez-Losa J., Mojal S., Salvador C., Morote J., Ramon Y., Cajal S., et al. Identification and genotyping of human papillomavirus in a Spanish cohort of penile squamous cell carcinomas: Correlation with pathologic subtypes, p16(INK4a) expression, and prognosis. J. Am. Acad. Dermatol. 2013;68:73–82. doi: 10.1016/j.jaad.2012.05.029. [DOI] [PubMed] [Google Scholar]
- 20.Kirrander P., Sherif A., Friedrich B., Lambe M., Hakansson U. Swedish National Penile Cancer Register: Incidence, tumour characteristics, management and survival. BJU Int. 2016;117:287–292. doi: 10.1111/bju.12993. [DOI] [PubMed] [Google Scholar]
- 21.Bandini M., Ahmed M., Basile G., Watkin N., Master V., Zhu Y., Prakash G., Rodriguez A., Ssebakumba M.K., Leni R., et al. A global approach to improving penile cancer care. Nat. Rev. Urol. 2022;19:231–239. doi: 10.1038/S41585-021-00557-Y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buonaguro F.M., Tornesello M.L., Salatiello I., Okong P., Buonaguro L., Beth-Giraldo E., Biryahwaho B., Sempala S.D.K., Giraldo G. The uganda study on HPV variants and genital cancers. J. Clin. Virol. 2000;19:31–41. doi: 10.1016/S1386-6532(00)00131-1. [DOI] [PubMed] [Google Scholar]
- 23.Maueia C., Murahwa A., Manjate A., Andersson S., Sacarlal J., Kenga D., Mussá T., Williamson A.L. Identification of the Human Papillomavirus Genotypes, According to the Human Immunodeficiency Virus Status in a Cohort of Women from Maputo, Mozambique. Viruses. 2021;14:24. doi: 10.3390/v14010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rakislova N., Manzotti C., Saude O. Differential etiopathogenic features of vulvar squamous cell carcinomas in sub-Saharan Africa and Europe. Int. J. Cancer. 2022 doi: 10.1002/ijc.34314. online ahead of print . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.González R., Munguambe K., Aponte J.J., Bavo C., Nhalungo D., Macete E., Alonso P.L., Menéndez C., Naniche D. High HIV prevalence in a southern semi-rural area of Mozambique: A community-based survey. HIV Med. 2012;13:581–588. doi: 10.1111/J.1468-1293.2012.01018.X. [DOI] [PubMed] [Google Scholar]
- 26.Lorenzoni C., Vilajeliu A., Carrilho C., Ismail M.R., Castillo P., Augusto O., García-Basteiro A.L., Sidat M., de Sanjosé S., Menéndez C., et al. Trends in cancer incidence in Maputo, Mozambique, 1991–2008. PLoS ONE. 2015;10:e0130469. doi: 10.1371/journal.pone.0130469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frisch M., Biggar R.J., Goedert J.J. Human papillomavirus-associated cancers in patients with human immunodeficiency virus infection and acquired immunodeficiency syndrome. J. Natl. Cancer Inst. 2000;92:1500–1510. doi: 10.1093/jnci/92.18.1500. [DOI] [PubMed] [Google Scholar]
- 28.Known and Probable Human Carcinogens. [(accessed on 20 July 2022)]. Available online: https://www.cancer.org/healthy/cancer-causes/general-info/known-and-probable-human-carcinogens.html.
- 29.Stelzle D., Tanaka L.F., Lee K.K., Ibrahim Khalil A., Baussano I., Shah A.S.V., McAllister D.A., Gottlieb S.L., Klug S.J., Winkler A.S., et al. Estimates of the global burden of cervical cancer associated with HIV. Lancet Glob. Health. 2021;9:e161–e169. doi: 10.1016/S2214-109X(20)30459-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ordi J., Alejo M., Fusté V., Lloveras B., Del Pino M., Alonso I., Torné A. HPV-negative vulvar intraepithelial neoplasia (VIN) with basaloid histologic pattern: An unrecognized variant of simplex (differentiated) VIN. Am. J. Surg. Pathol. 2009;33:1659–1665. doi: 10.1097/PAS.0b013e3181b40081. [DOI] [PubMed] [Google Scholar]
- 31.Rakislova N., Clavero O., Alemany L., Saco A., Quirós B., Lloveras B., Alejo M., Pawlita M., Quint W., del Pino M., et al. Histological characteristics of HPV-associated and -independent squamous cell carcinomas of the vulva: A study of 1594 cases. Int. J. Cancer. 2017;141:2517–2527. doi: 10.1002/ijc.31006. [DOI] [PubMed] [Google Scholar]
- 32.WHO Classification of Tumours of Female Reproductive Organs. Fourth Edition—WHO—OMS. [(accessed on 29 December 2019)]. Available online: https://apps.who.int/bookorders/anglais/detart1.jsp?codlan=1&codcol=70&codcch=4006.
- 33.EI-Naggar A.K., Chan J.K.C., Grandis J.R., Takata T.S.P. WHO Classification of Head and Neck Tumours. WHO; Geneva, Switzerland: 2017. [Google Scholar]
- 34.Wang B., Gu W., Wan F., Wei Y., Xiao W., Lu X., Zhang G., Zhou J., Wang Q., Ding X., et al. Prognosis of the 8th TNM Staging System for Penile Cancer and Refinement of Prognostication by Incorporating High Risk Human Papillomavirus Status. J. Urol. 2020;203:562–569. doi: 10.1097/JU.0000000000000584. [DOI] [PubMed] [Google Scholar]
- 35.Sand F.L., Rasmussen C.L., Frederiksen M.H., Andersen K.K., Kjaer S.K. Prognostic Significance of HPV and p16 Status in Men Diagnosed with Penile Cancer: A Systematic Review and Meta-analysis. Cancer Epidemiol. Biomark. Prev. 2018;27:1123–1132. doi: 10.1158/1055-9965.EPI-18-0322. [DOI] [PubMed] [Google Scholar]
- 36.Wilson M.L., Fleming K.A., Kuti M.A., Looi L.M., Lago N., Ru K. Access to pathology and laboratory medicine services: A crucial gap. Lancet. 2018;391:1927–1938. doi: 10.1016/S0140-6736(18)30458-6. [DOI] [PubMed] [Google Scholar]
- 37.McDaniel A.S., Hovelson D.H., Cani A.K., Liu C.-J., Zhai Y., Zhang Y., Weizer A.Z., Mehra R., Feng F.Y., Alva A.S., et al. Genomic Profiling of Penile Squamous Cell Carcinoma Reveals New Opportunities for Targeted Therapy. Cancer Res. 2015;75:5219–5227. doi: 10.1158/0008-5472.CAN-15-1004. [DOI] [PubMed] [Google Scholar]
- 38.Feber A., Worth D.C., Chakravarthy A., de Winter P., Shah K., Arya M., Saqib M., Nigam R., Malone P.R., Tan W.S., et al. CSN1 Somatic Mutations in Penile Squamous Cell Carcinoma. Cancer Res. 2016;76:4720–4727. doi: 10.1158/0008-5472.CAN-15-3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Y., Wang K., Chen Y., Zhou J., Liang Y., Yang X., Li X., Cao Y., Wang D., Luo L., et al. Mutational landscape of penile squamous cell carcinoma in a Chinese population. Int. J. Cancer. 2019;145:1280–1289. doi: 10.1002/ijc.32373. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data is shown in the manuscript.