Abstract
Background: The aim of this study was to evaluate the impact of adverse lifestyle factors on outcomes in patients with human papillomavirus (HPV)-related oropharyngeal squamous cell carcinoma (OPSCC). Methods: From 2010 to 2019, 150 consecutive non-metastatic OPSCC patients receiving curative treatment in our institution were retrospectively enrolled. HPV positivity was defined as p16 expression ≥75%. The effects of adverse lifestyle factors on overall survival (OS) and disease-free survival (DFS) on OPSCC patients were determined. Results: The median follow-up duration was 3.6 years. Of the 150 OPSCCs, 51 (34%) patients were HPV-positive and 99 (66%) were HPV-negative. The adverse lifestyle exposure rates were 74.7% (n = 112) alcohol use, 57.3% (n = 86) betel grid chewing, and 78% (n = 117) cigarette smoking. Alcohol use strongly interacted with HPV positivity (HR, 6.00; 95% CI, 1.03–35.01), leading to an average 26.1% increased risk of disease relapse in patients with HPV-positive OPSCC. Heavy smoking age ≥30 pack-years was associated with increased risk of death (HR, 2.05; 95% CI, 1.05–4.00) and disease relapse (HR, 1.99; 95% CI, 1.06–3.75) in OPSCC patients. In stratified analyses, the 3-year absolute risk of disease relapse in HPV-positive OPSCC patients reached up to 50% when alcohol use and heavy smoking for ≥30 pack-years were combined. Conclusions: Alcohol acted as a significant treatment-effect modifier for DFS in HPV-positive OPSCC patients, diluting the favorable prognostic effect of HPV positivity. Heavy smoking age ≥30 pack-years was an independent adverse prognostic factor of OS and DFS in OPSCC patients. De-intensification treatment for HPV-related OPSCC may be avoided when these adverse lifestyle factors are present.
Keywords: alcohol, smoking, betel nut, human papillomavirus (HPV), oropharyngeal cancer, treatment-effect modifier, prognostic factor
1. Introduction
Over the past few decades, the prevalence of human papillomavirus (HPV)-related oropharyngeal squamous cell carcinoma (OPSCC) has increased rapidly—particularly in high-income countries [1,2]. Unlike other head and neck squamous cell carcinomas (HNSCCs), HPV-related OPSCCs have distinct clinical presentations: the patients tend to be younger and the cancers are less associated with smoking and more associated with primary tonsillar tumors and cystic cervical lymph node metastasis [3]. HPV-16 accounts for at least 85% of all HPV-related OPSCCs [4]. Two HPV oncogenes, E6 and E7, are key drivers of HPV-mediated carcinogenesis. E6 and E7 involve increased degradation of the tumor suppressor proteins p53 and Rb, respectively, resulting in the loss of cell-cycle checkpoint activation in response to DNA damage and uncontrolled licensing of DNA replication—which together result in genomic instability and resistance to apoptosis [5,6]. p16 is upregulated during the process of E7-directed epigenetic reprogramming [7]. Thus, p16 overexpression is a surrogate marker for HPV-related OPSCC [8]. The cutoff of p16 positivity by immunohistochemistry (IHC) staining is nuclear expression ≥+2/+3 intensity and ≥75% distribution [9].
The prognostic significance of HPV status in OPSCC has been established; patients with HPV-related OPSCC have a more favorable treatment response and longer survival time than HPV-unrelated OPSCC [10,11,12]. The American Joint Committee on Cancer (AJCC) has defined HPV-positive and HPV-negative OPSCCs as separate entities because of their distinct tumor characteristics, biological behaviors, and treatment outcomes [9,13].
Asian OPSCC patients have poorer treatment outcomes than other ethnicities [14,15]. One suspected reason for this is the lower rate of HPV positivity in OPSCC, which is about 30% to 50% in Asians but 70% to 85% in Western populations [2]. Higher rates of alcohol use, betel grid chewing, and cigarette smoking (ABC lifestyle factors) in Asia also might contribute to poorer prognosis [14]. The ABC lifestyle factors are especially common in Southeast Asia—especially in low socioeconomic and less-educated populations [16]. ABC lifestyle factors usually coexist, which may contribute to a dramatically increased risk of developing HNSCC in multi-user persons compared with that in persons who have never been exposed to ABC factors [17]. Although the role of ABC lifestyle factors has been well established in the development of HNSCC [18,19], less is known about their prognostic significance in patients with HPV-positive OPSCC. Epidemiologic studies of HPV-positive OPSCC have been conducted mostly in Western countries and are therefore not generalizable to non-Western countries [20], where factors such as cultural and behavioral differences might result in different etiologies in HPV-positive OPSCC. Wider geographically based investigations are necessary to guide region-specific clinical treatments and public health policies.
As de-intensification treatment protocols in patients with HPV-positive OPSCC are currently applied [21,22,23], it is important to identify patients where such attempts may not be safe. We hypothesized that ABC lifestyle factors moderate the effects of p16 status on survival in OPSCC patients. This study aimed to evaluate the impact of ABC lifestyle factors on treatment outcomes in patients with HPV-positive OPSCC and to optimize the selection of a subgroup of HPV-positive OPSCC patients for de-intensification treatment.
2. Materials and Methods
2.1. Patients
One hundred and fifty OPSCC patients who had completed a course of curative treatment, consisting of surgery and radiotherapy (RT)-based therapy from January 2010 to October 2019, were consecutively collected and analyzed. All patients had received a complete staging work-up before treatment and were followed to determine their treatment response and survival. The exclusion criteria were: (1) other underlying malignancy or distant metastasis at the time that OPSCC was diagnosed; (2) lack of available pretreatment primary tumor specimens to re-evaluate p16 expression by IHC staining; (3) lack of pretreatment contrast-enhanced computed tomography (CT) images of the head and neck to re-evaluate clinical staging. The Institutional Review Board approved this retrospective study.
2.2. Demographic and Clinical Data
Patient data—including age, gender, tumor subsites, history of ABC lifestyle (alcohol consumption, betel grid chewing, cigarette smoking), smoking age (number of cumulative pack-years of smoking), treatment-related profiles (surgery, radiotherapy, chemotherapy), and outcome data—were gathered by retrospective chart review. The clinical and pathological staging that had been determined previously were re-evaluated and revised based on the seventh and eighth editions of the AJCC staging system [9,13].
HPV status was determined by re-examination of p16 nuclear expression in the pretreatment primary tumor by IHC staining. After tissue specimens from our human biobank were collected, all the slides were re-evaluated by a head and neck pathologist with 30-years’ experience to determine the HPV status. HPV positivity was defined as the presence of p16 expression in ≥75% of carcinoma cells, with nuclear reactivity on IHC staining [9].
2.3. Treatments
The standard primary treatments for OPSCC were surgery and RT-based therapy. Definitive concurrent chemoradiotherapy (CCRT) with a platinum-based chemotherapeutic regimen was most often used in locally advanced OPSCC. The curative-intent radiation dose to the primary tumor and grossly involved lymph nodes was 60–74 Gy in 1.8–2.2 Gy per fraction, delivered daily with intensity-modulated radiotherapy or volumetric-modulated arc therapy techniques. Induction chemotherapy was allowed before primary treatments. Adjuvant treatments after primary surgery were indicated when patients with adverse pathological features—including positive/close surgical margin, extranodal extension, pT3–pT4 disease, positive lymph node metastasis, perineural invasion, lymphovascular space invasion, or any other concern—when determined to be appropriate by multidisciplinary discussion.
2.4. Statistical Analysis
Baseline characteristics were presented as mean (standard deviation) for continuous variables and number (frequency) for categorical variables. The Kaplan–Meier survival method was used to depict the curves for the distribution of time to death or relapse and log-rank tests were carried out to evaluate differences between HPV-positive and HPV-negative OPSCC patients. Overall survival (OS) was defined as the date of initial treatment to the date of death or last follow-up. Disease-free survival (DFS) was defined as the date of initial treatment to the date of disease relapse (locoregional recurrence and/or distant metastasis) or death. The p-value of continuous variables was calculated by the two-sample t-test whereas the p-value of categorical variables was calculated by the chi-square test and Fisher’s exact test.
Among patients with OPSCC, univariable Cox proportional hazard models were applied to identify significant patient characteristics associated with OS and DFS—including p16 status, gender, age, clinical stage, tumor subsites, initial treatment, and ABC lifestyle factors. We hypothesized that ABC lifestyle factors could modify the effects of p16 status for OS and DFS. We used multivariable Cox proportional hazard models with patient characteristics and p16 status and lifestyle factors as interaction terms by using the stepwise variable selection method to select relevant variables for OS and DFS. The criteria for the model fitting were based on the Akaike information criterion. Furthermore, multivariate models were constructed with interaction terms that were selected by the stepwise method and significant and clinically relevant variables from univariate analyses. The multicollinearity and proportional hazard assumption of the models were checked; none of the models showed high multicollinearity and the proportional hazard assumption was met.
The stratified analyses were made according to alcohol use and smoking age to estimate 3-year and 5-year cumulative risks of disease relapse in HPV-positive patients using multivariate models. The interaction plot was depicted by HPV status and alcohol use to show changes in the cumulative risk of disease relapse, which was estimated using multivariate models, in different situations. A two-tailed p value < 0.05 was considered statistically significant. All statistical results were carried out with R (version 4.1.0) software and Quanta for Medical Care AI: AI Medical Platform (QOCA AIM) 2.0 version (Quanta Computer Inc., Taoyuan, Taiwan).
3. Results
3.1. Patient Characteristics
One hundred and fifty patients were analyzed in this study; 99 (66%) had HPV-negative OPSCC and 51 (34%) were HPV-positive OPSCC patients. The mean age at diagnosis of OPSCC was 54.4 years; most were locally advanced OPSCCs. The ABC lifestyle exposure rates were: 112/150 (74.7%) patients showed alcohol consumption, 86/150 (57.3%) betel grid chewing, and 117/150 (78%) cigarette smoking. More than half of all patients (79/150; 52.7%) had concomitant ABC lifestyle exposure. Among the 150 patients, 39 (26%) were treated with primary surgery and 111 (74%) were treated with primary RT-based therapy (106 CCRT and 5 RT only). In the primary surgery group, 10 patients underwent induction chemotherapy before surgery and 35 underwent adjuvant RT/CCRT after surgery. In the primary RT-based therapy group, 22 patients underwent induction chemotherapy before definitive RT/CCRT. The baseline characteristics of the study population are summarized in Table 1.
Table 1.
Baseline characteristics of the study population.
| Variable | p16 (−), n = 99 | p16 (+), n = 51 | p-Value | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Male, n, % | 93 | 93.94 | 37 | 72.55 | <0.001 |
| Female, n, % | 6 | 6.06 | 14 | 27.45 | <0.001 |
| Age, mean, SD | 53.3 | 9.22 | 56.7 | 9.71 | 0.036 |
| Age, n, % | 0.085 | ||||
| <50 | 36 | 36.36 | 10 | 19.61 | |
| 50–59 | 35 | 35.35 | 20 | 39.22 | |
| ≥60 | 28 | 28.28 | 21 | 41.18 | |
| Cigarette smoking, n, % | 91 | 91.92 | 26 | 50.98 | <0.001 |
| Smoking age (pack-years), mean, SD | 31.8 | 26.8 | 14.4 | 17.9 | <0.001 |
| Smoking age (pack-years), n, % | <0.001 | ||||
| 0 | 8 | 8.08 | 25 | 49.02 | |
| 1–9 | 7 | 7.07 | 3 | 5.88 | |
| 10–19 | 14 | 14.14 | 0 | 0.00 | |
| 20–29 | 18 | 18.18 | 10 | 19.61 | |
| ≥30 | 52 | 52.53 | 13 | 25.49 | |
| Alcohol use, n, % | 86 | 86.87 | 26 | 50.98 | <0.001 |
| Betel quid chewing, n, % | 76 | 76.77 | 10 | 19.61 | <0.001 |
| ABC concomitant use, n, % | <0.001 | ||||
| 3 | 70 | 70.7 | 9 | 17.65 | |
| 2 of 3 | 20 | 20.2 | 12 | 23.53 | |
| Tumor subsite, n, % | 0.002 | ||||
| Tonsil | 47 | 47.47 | 40 | 78.43 | |
| Soft palate | 22 | 22.22 | 6 | 11.76 | |
| Tongue base | 23 | 23.23 | 5 | 9.80 | |
| Posterior pharyngeal wall | 7 | 7.07 | 0 | 0.00 | |
| Clinical stage (AJCC 7th ed.), n, % | 0.041 | ||||
| Stage I | 2 | 2.02 | 1 | 1.96 | |
| Stage II | 7 | 7.07 | 0 | 0.00 | |
| Stage III | 6 | 6.06 | 9 | 17.65 | |
| Stage IVA | 68 | 68.69 | 37 | 72.55 | |
| Stage IVB | 16 | 16.16 | 4 | 7.84 | |
| Clinical stage (AJCC 8th ed.), n, % | <0.001 | ||||
| Stage I | 2 | 2.02 | 26 | 50.98 | |
| Stage II | 7 | 7.07 | 15 | 29.41 | |
| Stage III | 5 | 5.05 | 10 | 19.61 | |
| Stage IVA | 55 | 55.56 | 0 * | 0.00 * | |
| Stage IVB | 30 | 30.3 | 0 * | 0.00 * | |
| Initial treatment, n, % | 0.024 | ||||
| Surgery | 32 | 32.32 | 7 | 13.73 | |
| RT-based therapy | 67 | 67.68 | 44 | 86.27 | |
| CCRT | 66 | 66.67 | 40 | 78.43 | |
| RT only | 1 | 1.01 | 4 | 7.84 | |
| Disease relapse, n, % | 58 | 58.59 | 13 | 25.49 | <0.001 |
| LRR | 25 | 25.25 | 6 | 11.76 | |
| DM | 18 | 18.18 | 5 | 9.80 | |
| LRR + DM | 15 | 15.15 | 2 | 3.92 | |
| Mortality, n, % | 61 | 61.62 | 9 | 17.65 | <0.001 |
| DOD | 47 | 47.47 | 5 | 9.80 | |
| Dead, other reason | 14 | 14.14 | 4 | 7.84 | |
* The clinical stage of nonmetastatic HPV-positive OPSCC was downstaged to stage III or less in the eighth edition of the American Joint Committee on Cancer (AJCC) staging system. Abbreviations: n, number of patients; SD, standard deviation; ABC, alcohol/betel nut/cigarette; AJCC, American Joint Committee on Cancer; ed., edition; RT, radiotherapy; CCRT, concurrent chemoradiotherapy; LRR, locoregional recurrence; DM, distant metastasis; DOD, died of disease.
Between the two groups of HPV-positive and HPV-negative OPSCC patients, there was no significant difference in the age distribution and clinical stage. In patients with HPV-positive OPSCC, the dominant tumor subsite was the tonsil and the majority received primary RT-based therapy. Patients with HPV-negative OPSCC had a significantly higher proportion of male gender, a higher exposure rate to ABC lifestyle factors, and a higher smoking age (Table 1).
3.2. Treatment Outcomes
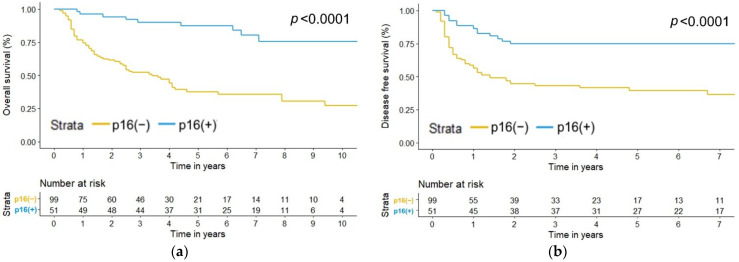
The median follow-up time was 3.6 years. The recurrence rate was 47.3%, with 71 of the 150 patients developing disease relapse (locoregional recurrence and/or distant metastasis). The mortality rate was 46.7%, which meant that 70 of the 150 patients had expired by the time of analysis. Patients with HPV-positive OPSCC had significantly lower disease relapse and mortality rates than those with HPV-negative OPSCC (p < 0.001; Table 1). The 3-year overall survival (OS) and disease-free survival (DFS) rates for HPV-positive versus HPV-negative OPSCC patients were 90% versus 52% and 74.5% versus 42.9%, respectively (both p values < 0.0001; Figure 1).
Figure 1.
Kaplan–Meier estimate of (a) overall survival (p < 0.0001) and (b) disease-free survival (p < 0.0001) by p16 status.
3.3. Factors Affecting Overall Survival (OS)
In the multivariate analysis, the HPV status (positive: hazard ratio, 0.09; 95% CI, 0.02–0.44), clinical stage (stage IVA: hazard ratio, 2.72; 95% CI, 1.05–7.00; stage IVB: hazard ratio, 15.62; 95% CI, 5.29–46.13), and smoking age (≥30 pack-years: hazard ratio, 2.05; 95% CI, 1.05–4.00) were significant prognostic factors for OS in OPSCC patients (Table 2). There was no significant interaction between HPV positivity and ABC lifestyle factors; that is, ABC lifestyle factors were not significant treatment-effect modifiers for OS in HPV-positive OPSCC (Table 2).
Table 2.
Univariate and multivariate analyses for overall survival.
| Variable | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| Stepwise Selection * | Selected Predictors # | |||||
| HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| Covariate | ||||||
| p16 (ref. = negative) | 0.18 | (0.09, 0.37) | 0.08 | (0.02, 0.35) | 0.09 | (0.02, 0.44) |
| Gender (ref. = male) | 0.15 | (0.04, 0.60) | 0.52 | (0.12, 2.33) | ||
| Age (5-year increments) | 0.89 | (0.78, 1.02) | ||||
| Clinical stage (ref. = stage I–III) & | ||||||
| Stage IVA | 2.69 | (1.07, 6.76) | 2.55 | (1.00, 6.49) | 2.72 | (1.05, 7.00) |
| Stage IVB | 12.11 | (4.41, 33.26) | 16.26 | (5.76, 45.86) | 15.62 | (5.29, 46.13) |
| Tumor subsite (ref. = other sites than tonsil) | 0.46 | (0.28, 0.73) | 0.77 | (0.45, 1.33) | ||
| Initial treatment (ref. = surgery) | 0.88 | (0.52, 1.48) | 1.02 | (0.58, 1.81) | ||
| Smoking age (ref. = <20 pack-years) | ||||||
| 20–29 | 1.78 | (0.85, 3.70) | 0.96 | (0.41, 2.23) | 1.15 | (0.46, 2.90) |
| ≥30 | 2.98 | (1.67, 5.32) | 1.90 | (1.02, 3.54) | 2.05 | (1.05, 4.00) |
| Alcohol use (ref. = none) | 3.19 | (1.58, 6.44) | 0.90 | (0.39, 2.08) | ||
| Betel quid chewing (ref. = none) | 2.35 | (1.40, 3.96) | 0.85 | (0.45, 1.61) | ||
| Interaction term | ||||||
| p16: Smoking age (20–29 pack-years) | 8.00 | (1.16, 55.01) | 5.74 | (0.79, 41.53) | ||
| p16: Smoking age (≥30 pack-years) | 2.94 | (0.43, 19.93) | 2.53 | (0.36, 17.85) | ||
| p16: Alcohol use | ||||||
| p16: Betel quid chewing | ||||||
* Variable selection employed the stepwise method by the Akaike information criterion. # The Cox proportional hazard model was constructed with interaction terms that were selected by the stepwise method and significant and clinically relevant variables from univariate analyses. & The clinical stage was defined by the 7th edition of the American Joint Committee on Cancer (AJCC) staging system. Significant values of HR and 95% CI are in bold. Abbreviations: HR, hazard ratio; CI, confidence interval; ref., reference.
3.4. Factors Affecting Disease-Free Survival (DFS)
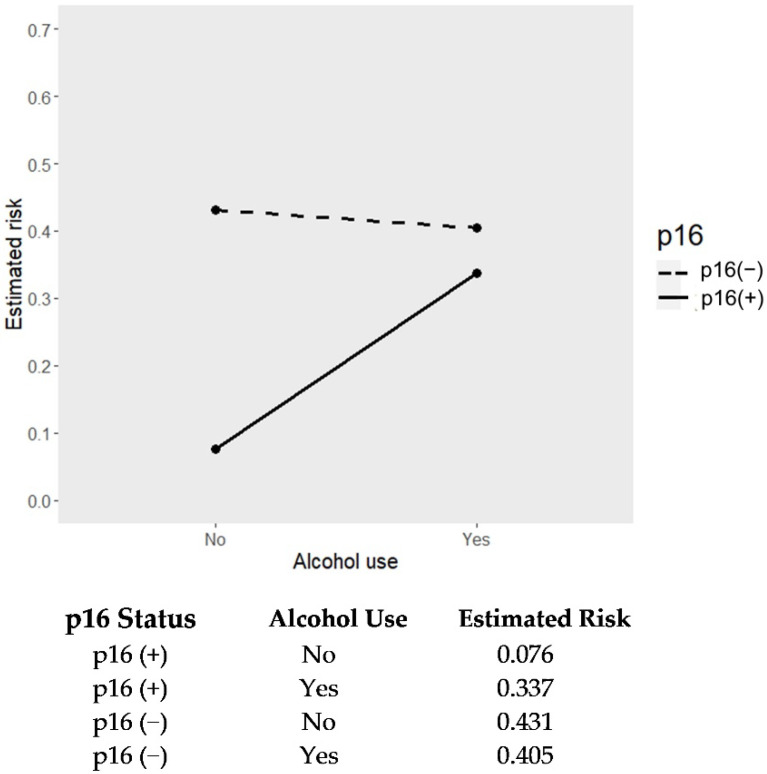
In the multivariate analysis, the HPV status (positive: hazard ratio, 0.10; 95% CI, 0.02–0.49), clinical stage (stage IVA: hazard ratio, 2.87; 95% CI, 1.11–7.41; stage IVB: hazard ratio, 8.43; 95% CI, 2.83–25.08), tumor subsite (tonsil: hazard ratio, 0.46; 95% CI, 0.27–0.80), and smoking age (≥30 pack-years: hazard ratio, 1.99; 95% CI, 1.06–3.75) were significant prognostic factors for DFS in OPSCC patients (Table 3). Moreover, there was a strong interaction between HPV positivity and alcohol use (alcohol use: hazard ratio, 6.00; 95% CI, 1.03–35.01), which meant that alcohol was a significant treatment-effect modifier for DFS in HPV-positive OPSCC patients (Table 3). The presence of alcohol exposure diluted the favorable prognostic effect of HPV positivity in OPSCC patients. In a median follow-up duration of 3.6 years, alcohol use contributed to an average 26.1% increased risk of disease relapse in patients with HPV-positive OPSCC, whereas there was no risk increment in those with HPV-negative OPSCC (Figure 2). By stratification of smoking age among HPV-positive OPSCC patients with alcohol use, the 3-year absolute risk of disease relapse was 33% in those with smoking age <20 pack-years and up to 50% in those ≥30 pack-years (Table 4).
Table 3.
Univariate and multivariate analyses for disease-free survival.
| Variable | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| Stepwise Selection * | Selected Predictors # | |||||
| HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| Covariate | ||||||
| p16 (ref. = negative) | 0.31 | (0.17, 0.56) | 0.12 | (0.03–0.58) | 0.10 | (0.02, 0.49) |
| Gender (ref. = male) | 0.32 | (0.12, 0.88) | 1.12 | (0.36, 3.42) | ||
| Age (5-year increments) | 0.98 | (0.87, 1.11) | 1.12 | (0.97–1.28) | ||
| Clinical stage (ref. = stage I–III) & | ||||||
| Stage IVA | 2.75 | (1.09, 6.90) | 3.6 | (1.37–9.45) | 2.87 | (1.11, 7.41) |
| Stage IVB | 10.68 | (3.88, 29.41) | 12.35 | (4.09–37.26) | 8.43 | (2.83, 25.08) |
| Tumor subsite (ref. = other sites than tonsil) | 0.34 | (0.21, 0.55) | 0.49 | (0.26–0.83) | 0.46 | (0.27, 0.80) |
| Initial treatment (ref. = surgery) | 1.05 | (0.62, 1.78) | 1.21 | (0.68, 2.16) | ||
| Smoking age (ref. = <20 pack-years) | ||||||
| 20–29 | 1.43 | (0.71, 2.88) | 1.69 | (0.77–3.70) | 1.64 | (0.74, 3.63) |
| ≥30 | 2.07 | (1.20, 3.55) | 1.88 | (1.01–3.49) | 1.99 | (1.06, 3.75) |
| Alcohol use (ref. = none) | 2.68 | (1.37, 5.23) | 0.66 | (0.27–1.62) | 0.57 | (0.23, 1.39) |
| Betel quid chewing (ref. = none) | 1.77 | (1.08, 2.92) | 0.91 | (0.48, 1.72) | ||
| Interaction term | ||||||
| p16: Smoking age (20–29 pack-years) | ||||||
| p16: Smoking age (≥30 pack-years) | ||||||
| p16: Alcohol use | 4.4 | (0.78–24.7) | 6.00 | (1.03, 35.01) | ||
| p16: Betel quid chewing | ||||||
* Variable selection employed the stepwise method by the Akaike information criterion. # The Cox proportional hazard model was constructed with interaction terms that were selected by the stepwise method and significant and clinically relevant variables from univariate analyses. & The clinical stage was defined by the 7th edition of the American Joint Committee on Cancer (AJCC) staging system. Significant values of HR and 95% CI are in bold. Abbreviations: HR, hazard ratio; CI, confidence interval; ref., reference.
Figure 2.
Interaction plot for estimated risk of disease relapse according to p16 status and alcohol use.
Table 4.
The cumulative risks of disease relapse in HPV-related oropharyngeal cancer by alcohol and smoking age.
| Smoking Age | Follow-Up | p16 (+) | |
|---|---|---|---|
| Absolute Risk (95% CI) | Absolute Risk (95% CI) | ||
| Alcohol (−) | Alcohol (+) | ||
| <20 pack-years | 3 years | 0.12 (0.11, 0.14) | 0.33 (0.24, 0.39) |
| 5 years | 0.14 (0.12, 0.15) | 0.35 (0.26, 0.42) | |
| 20–29 pack-years | 3 years | 0.19 (0.18, 0.20) | 0.45 (0.35, 0.51) |
| 5 years | 0.21 (0.20, 0.21) | 0.47 (0.37, 0.54) | |
| ≥30 pack-years | 3 years | 0.22 (0.21, 0.24) | 0.50 (0.38, 0.57) |
| 5 years | 0.24 (0.22, 0.25) | 0.52 (0.41, 0.59) | |
Abbreviations: HPV, human papilloma virus; CI, confidence interval.
4. Discussion
In this study, we demonstrated that alcohol was a significant treatment-effect modifier for DFS in HPV-positive OPSCC patients. The presence of alcohol use diluted the favorable prognostic effect of HPV positivity in OPSCC patients, leading to an average 26.1% increased risk of disease relapse. A heavy smoking age of ≥30 pack-years was a poor prognostic factor of all-cause and disease-specific mortality among OPSCC patients, regardless of HPV status. On the other hand, betel grid chewing made no contribution to effect modification or the prediction of treatment outcomes in patients with OPSCC.
Confusion between treatment-effect modifiers and prognostic factors is common. Effect modifiers, also called effect moderators, are factors that influence how well an intervention affects the outcome. Prognostic factors are factors that predict the outcome of a disease [24]. Scientifically, effect modifiers must be differentiated from prognostic factors, but it is more challenging to claim that a factor is an effect modifier rather than a prognostic factor. Prognostic factors are familiar to oncologists and are used to provide patients with a more accurate prognosis, but they do not help identify which patients will respond best to a specific intervention. Effect modification has recently become of particular interest in oncology in the era of targeted therapy and immunotherapy, where the effectiveness of a treatment might largely depend on host or tumor factors [25,26]—as does HPV-related OPSCC. Due to the heterogeneous tumor behavior present in HPV-positive and HPV-negative OPSCCs, the variety of variables affecting treatment outcomes may play different roles as effect modifiers or prognostic factors. Determining the treatment-effect modifiers in HPV-positive OPSCC helps to identify subgroups of patients who respond better or worse to de-escalation treatments.
Our data indicate that alcohol is a significant treatment-effect modifier for DFS in HPV-positive OPSCC patients. Exposure to alcohol is well-known as a dominant etiologic factor of HNSCC. A large case-control study conducted by Lee et al. involved 740 HNSCC patients in Taiwan [25]; although the patients enrolled in this study were heterogeneous, the results showed a significant positive dose–response relationship between pre-diagnosis alcohol use and worse OS in HNSCC. This association was more significant for non-oral cavity HNSCC than for oral HNSCC. A possible mechanism for this is the polymorphism of the ethanol-metabolizing genes ADH1B and ALDH2, which modify the relationship between pre-diagnosis alcohol use and the OS of HNSCC patients—providing a possible biological explanation [27]. However, unlike our study, this analysis did not adjust for HPV status (due to a lack of access to the tumor tissues to test for HPV)—thus prohibiting its generalization to HPV-positive OPSCC. A recent study in Belgium demonstrated that alcohol use was a poor prognostic factor for OS in OPSCC patients and established a simplified scoring system composed of p16 status, smoking, and alcohol [28]. Another study showed that alcohol consumption was an independent factor for survival among patients with HPV-negative OPSCC rather than for those with HPV-positive OPSCC [29]. So far, relevant studies on the impact of alcohol use on HPV-positive OPSCC are scanty and contradictory. To the best of our knowledge, our study was the first to highlight the role of alcohol use as a treatment-effect modifier for HPV-positive OPSCC, which had not been previously evaluated and reported. The findings of our provided supporting evidence that p16 expression is not the only key factor for survival in OPSCC patients and demonstrated that the favorable prognostic effect of HPV positivity in OPSCC patients can be diluted by alcohol use.
Our study also demonstrated that smoking was not a treatment-effect modifier for HPV-positive OPSCC, but heavy smoking age ≥30 pack-years was a significant prognostic indicator of worse OS and DFS in OPSCC patients. A considerable amount of literature has explored the association between smoking and HPV-positive OPSCC. First, the association between smoking and the pathogenesis of HPV-positive OPSCC was suggested. The potential pathways of smoking-related carcinogenesis were likely attributed to cellular alterations and DNA damage, promoting infection by and the persistence of HPV [30]. By pooling two large head and neck cancer studies with HPV serology data, Anantharaman et al. demonstrated that smoking was consistently associated with increased risks of both HPV-positive and HPV-negative OPSCC [31]. Second, the association between smoking and treatment-related outcomes in HPV-positive OPSCC has been widely explored. The results remained somewhat controversial, with some studies reporting smoking as a poor prognostic factor independent of HPV status, which is consistent with our findings [32,33], and others reporting smoking exposure as a poor prognostic factor within the context of HPV-positive OPSCC [34,35]. Though there are some conflicting results, most studies agree that smoking is associated with worse OS and a trend towards worse DFS in HPV-positive OPSCC [36]. Third, the association between the amount of smoking and worse survival outcomes in HPV-positive OPSCC has been investigated. There is no consensus on the cutoff for high-risk smokers. Several previous studies have reported smoking age >10 pack-years as a cutoff delineating higher risk HPV-positive OPSCC patients [10,37]. Other smoking metrics reported in the literature have included smoking age >20 pack-years, >20 cigarettes daily, total pack-years, current smoking, and ever smoking—with variable prognostic effects on survival outcomes [38,39,40]. The divergence in outcomes determined by these smoking metrics might be due to heterogeneous patient populations, various sample sizes, and different lifestyle factors, with exposure influenced by different cultures. However, most studies have agreed that the heavier the smoking, the worse the survival outcomes [36]. Our study recommended a cutoff of ≥30 pack-years smoking age for risk stratification in Asian OPSCC populations. As more than three-quarters of our study population had a smoking history—with the majority being heavy smokers—and more than half also had ABC lifestyle exposure, our study populations were more reflective of current conditions among OPSCC patients in Southeast Asia [14,16,41].
Compared to previous studies, the strength of our study was the clear definition of HPV positivity as the presence of p16 expression in ≥75% of carcinoma cells, showing nuclear reactivity on IHC staining [9]. We repeated p16 immunostaining tests in all the pretreatment primary tumor tissues, which were reviewed by a 30-year-experienced head and neck pathologist to accurately discriminate between HPV positivity and negativity. Furthermore, all the pretreatment CT images of the head and neck region were reviewed by a 15-year-experienced radiologist to revise clinical staging based on the seventh and eighth editions of the AJCC staging system to accurately display disease status. Detailed ABC lifestyle exposure histories and an adequate follow-up duration (median 3.6 years) made our results more convincing. Despite the retrospective study design, the consecutive enrollment of qualified OPSCC participants made the internal validity of patient selection solid and reliable. Finally, and most importantly, this was the first study providing the new concept, with convincing evidence, that alcohol is a treatment-effect modifier for HPV positivity.
The limitation of this study was the lack of quantification of alcohol consumption. Detailed quantification of alcohol consumption can include information on drinking status, frequency, the level of drinking, and drink-years [27]. Due to the retrospective nature of this study, our information on alcohol use relied on medical record reviews. There might be some inaccurate reporting due to recall bias when taking histories, or potential falsification of alcohol history due to guilt or shame. Further studies would benefit from including more objective measures of alcohol quantification such as questionnaires or prospective study designs.
5. Conclusions
Alcohol acted as a significant treatment-effect modifier for DFS in HPV-positive OPSCC patients, diluting the favorable prognostic effect of HPV positivity. A heavy smoking age of ≥30 pack-years was an independent adverse prognostic factor for OS and DFS in OPSCC patients. The 3-year absolute risk of disease relapse reached up to 50% in HPV-positive OPSCC patients when alcohol use and a heavy smoking age of ≥30 pack-years were combined. The presence of alcohol use and a history of heavy smoking should be considered critical factors when making treatment decisions between standard and de-intensification protocols among HPV-positive OPSCC patients. Further large-scale studies are warranted to confirm these findings.
Acknowledgments
We are grateful for the technical services provided by the Bioinformatics Group of the Smart Healthcare Solution in National Cheng Kung University Hospital. We also give thanks for the support from the Human Biobank, Research Center of Clinical Medicine, National Cheng Kung University Hospital.
Author Contributions
Conceptualization, Y.-H.L. and Y.-S.T.; methodology, Y.-H.L. and C.-C.S.; software, C.-C.S.; formal analysis, C.-C.S.; writing—original draft preparation, Y.-H.L., C.-C.S., S.-Y.W., W.-T.H., Y.-H.W. and Y.-S.T.; writing—review and editing, Y.-S.T.; supervision, H.H.W.C., J.-R.H. and C.-H.L.; funding acquisition, Y.-H.L. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was approved by the Institutional Review Board of National Cheng Kung University Hospital (A-ER-108-477) for studies involving humans.
Informed Consent Statement
Patient consent was waived due to the retrospective nature of the study.
Data Availability Statement
The data presented in this study are available on reasonable request from the corresponding author. The data are not publicly available due to privacy.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan (grant number NCKUH-11005005).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lechner M., Liu J., Masterson L., Fenton T.R. HPV-associated oropharyngeal cancer: Epidemiology, molecular biology and clinical management. Nat. Rev. Clin. Oncol. 2022;19:306–327. doi: 10.1038/s41571-022-00603-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carlander A.F., Jakobsen K.K., Bendtsen S.K., Garset-Zamani M., Lynggaard C.D., Jensen J.S., Grønhøj C., Buchwald C.V. A Contemporary Systematic Review on Repartition of HPV-Positivity in Oropharyngeal Cancer Worldwide. Viruses. 2021;13:1326. doi: 10.3390/v13071326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldenberg D., Begum S., Westra W.H., Khan Z., Sciubba J., Pai S.I., Califano J.A., Tufano R.P., Koch W.M. Cystic lymph node metastasis in patients with head and neck cancer: An HPV-associated phenomenon. Head Neck. 2008;30:898–903. doi: 10.1002/hed.20796. [DOI] [PubMed] [Google Scholar]
- 4.Kreimer A.R., Clifford G.M., Boyle P., Franceschi S. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: A systematic review. Cancer Epidemiol. Biomark. Prev. 2005;14:467–475. doi: 10.1158/1055-9965.EPI-04-0551. [DOI] [PubMed] [Google Scholar]
- 5.Scheffner M., Werness B.A., Huibregtse J.M., Levine A.J., Howley P.M. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990;63:1129–1136. doi: 10.1016/0092-8674(90)90409-8. [DOI] [PubMed] [Google Scholar]
- 6.Huh K., Zhou X., Hayakawa H., Cho J.Y., Libermann T.A., Jin J., Harper J.W., Munger K. Human papillomavirus type 16 E7 oncoprotein associates with the cullin 2 ubiquitin ligase complex, which contributes to degradation of the retinoblastoma tumor suppressor. J. Virol. 2007;81:9737–9747. doi: 10.1128/JVI.00881-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McLaughlin-Drubin M.E., Crum C.P., Münger K. Human papillomavirus E7 oncoprotein induces KDM6A and KDM6B histone demethylase expression and causes epigenetic reprogramming. Proc. Natl. Acad. Sci. USA. 2011;108:2130–2135. doi: 10.1073/pnas.1009933108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Larsen C.G., Gyldenløve M., Jensen D.H., Therkildsen M.H., Kiss K., Norrild B., Konge L., von Buchwald C. Correlation between human papillomavirus and p16 overexpression in oropharyngeal tumours: A systematic review. Br. J. Cancer. 2014;110:1587–1594. doi: 10.1038/bjc.2014.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lydiatt W.M., Patel S.G., O’Sullivan B., Brandwein M.S., Ridge J.A., Migliacci J.C., Loomis A.M., Shah J.P. Head and Neck cancers-major changes in the American Joint Committee on cancer eighth edition cancer staging manual. CA Cancer J. Clin. 2017;67:122–137. doi: 10.3322/caac.21389. [DOI] [PubMed] [Google Scholar]
- 10.Ang K.K., Harris J., Wheeler R., Weber R., Rosenthal D.I., Nguyen-Tân P.F., Westra W.H., Chung C.H., Jordan R.C., Lu C., et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N. Engl. J. Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fakhry C., Westra W.H., Li S., Cmelak A., Ridge J.A., Pinto H., Forastiere A., Gillison M.L. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J. Natl. Cancer Inst. 2008;100:261–269. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen-Tan P.F., Zhang Q., Ang K.K., Weber R.S., Rosenthal D.I., Soulieres D., Kim H., Silverman C., Raben A., Galloway T.J., et al. Randomized phase III trial to test accelerated versus standard fractionation in combination with concurrent cisplatin for head and neck carcinomas in the Radiation Therapy Oncology Group 0129 trial: Long-term report of efficacy and toxicity. J. Clin. Oncol. 2014;32:3858–3866. doi: 10.1200/JCO.2014.55.3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Sullivan B., Huang S.H., Su J., Garden A.S., Sturgis E.M., Dahlstrom K., Lee N., Riaz N., Pei X., Koyfman S.A., et al. Development and validation of a staging system for HPV-related oropharyngeal cancer by the International Collaboration on Oropharyngeal cancer Network for Staging (ICON-S): A multicentre cohort study. Lancet Oncol. 2016;17:440–451. doi: 10.1016/S1470-2045(15)00560-4. [DOI] [PubMed] [Google Scholar]
- 14.Chen T.C., Wu C.T., Ko J.Y., Yang T.L., Lou P.J., Wang C.P., Chang Y.L. Clinical characteristics and treatment outcome of oropharyngeal squamous cell carcinoma in an endemic betel quid region. Sci. Rep. 2020;10:526. doi: 10.1038/s41598-019-57177-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsai M.H., Cheng Y.J., Pao T.H., Hsueh W.T., Chen H.H.W., Wu Y.H. Association of Primary Treatment Modality for Advanced-Stage Oropharyngeal Squamous Cell Carcinoma With Survival Outcomes. JAMA Netw. Open. 2021;4:e2112067. doi: 10.1001/jamanetworkopen.2021.12067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ko Y.C., Chiang T.A., Chang S.J., Hsieh S.F. Prevalence of betel quid chewing habit in Taiwan and related sociodemographic factors. J. Oral Pathol. Med. 1992;21:261–264. doi: 10.1111/j.1600-0714.1992.tb01007.x. [DOI] [PubMed] [Google Scholar]
- 17.Hsu W.L., Chien Y.C., Chiang C.J., Yang H.I., Lou P.J., Wang C.P., Yu K.J., You S.L., Wang L.Y., Chen S.Y., et al. Lifetime risk of distinct upper aerodigestive tract cancers and consumption of alcohol, betel and cigarette. Int. J. Cancer. 2014;135:1480–1486. doi: 10.1002/ijc.28791. [DOI] [PubMed] [Google Scholar]
- 18.Auguste A., Deloumeaux J., Joachim C., Gaete S., Michineau L., Herrmann-Storck C., Duflo S., Luce D. Joint effect of tobacco, alcohol, and oral HPV infection on head and neck cancer risk in the French West Indies. Cancer Med. 2020;9:6854–6863. doi: 10.1002/cam4.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar R., Rai A.K., Das D., Das R., Kumar R.S., Sarma A., Sharma S., Kataki A.C., Ramteke A. Alcohol and Tobacco Increases Risk of High Risk HPV Infection in Head and Neck Cancer Patients: Study from North-East Region of India. PLoS ONE. 2015;10:e0140700. doi: 10.1371/journal.pone.0140700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fakhry C., Westra W.H., Wang S.J., van Zante A., Zhang Y., Rettig E., Yin L.X., Ryan W.R., Ha P.K., Wentz A., et al. The prognostic role of sex, race, and human papillomavirus in oropharyngeal and nonoropharyngeal head and neck squamous cell cancer. Cancer. 2017;123:1566–1575. doi: 10.1002/cncr.30353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marur S., Li S., Cmelak A.J., Gillison M.L., Zhao W.J., Ferris R.L., Westra W.H., Gilbert J., Bauman J.E., Wagner L.I., et al. E1308: Phase II Trial of Induction Chemotherapy Followed by Reduced-Dose Radiation and Weekly Cetuximab in Patients With HPV-Associated Resectable Squamous Cell Carcinoma of the Oropharynx- ECOG-ACRIN Cancer Research Group. J. Clin. Oncol. 2017;35:490–497. doi: 10.1200/JCO.2016.68.3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hegde J.V., Shaverdian N., Felix C., Wang P.C., Veruttipong D., Hsu S., Riess J.W., Rao S.D., Daly M.E., Chen A.M. Functional Outcomes After De-escalated Chemoradiation Therapy for Human Papillomavirus-Positive Oropharyngeal Cancer: Secondary Analysis of a Phase 2 Trial. Int. J. Radiat. Oncol. Biol. Phys. 2018;100:647–651. doi: 10.1016/j.ijrobp.2017.10.045. [DOI] [PubMed] [Google Scholar]
- 23.Chen A.M., Felix C., Wang P.C., Hsu S., Basehart V., Garst J., Beron P., Wong D., Rosove M.H., Rao S., et al. Reduced-dose radiotherapy for human papillomavirus-associated squamous-cell carcinoma of the oropharynx: A single-arm, phase 2 study. Lancet Oncol. 2017;18:803–811. doi: 10.1016/S1470-2045(17)30246-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vickers A.J., Steineck G. Prognosis, Effect Modification, and Mediation. Eur. Urol. 2018;74:243–245. doi: 10.1016/j.eururo.2018.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Principe D.R., Kamath S.D., Korc M., Munshi H.G. The immune modifying effects of chemotherapy and advances in chemo-immunotherapy. Pharmacol. Ther. 2022;236:108111. doi: 10.1016/j.pharmthera.2022.108111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clark G.M. Prognostic factors versus predictive factors: Examples from a clinical trial of erlotinib. Mol. Oncol. 2008;1:406–412. doi: 10.1016/j.molonc.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee W.T., Hsiao J.R., Ou C.Y., Huang C.C., Chang C.C., Tsai S.T., Chen K.C., Huang J.S., Wong T.Y., Lai Y.H., et al. The Influence of Prediagnosis Alcohol Consumption and the Polymorphisms of Ethanol-Metabolizing Genes on the Survival of Head and Neck Cancer Patients. Cancer Epidemiol. Biomark. Prev. 2019;28:248–257. doi: 10.1158/1055-9965.EPI-18-0425. [DOI] [PubMed] [Google Scholar]
- 28.Bouland C., Dequanter D., Lechien J.R., Hanssens C., De Saint Aubain N., Digonnet A., Javadian R., Yanni A., Rodriguez A., Loeb I., et al. Prognostic Significance of a Scoring System Combining p16, Smoking, and Drinking Status in a Series of 131 Patients with Oropharyngeal Cancers. Int. J. Otolaryngol. 2021;2021:8020826. doi: 10.1155/2021/8020826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saito Y., Yoshida M., Ushiku T., Omura G., Ebihara Y., Shimono T., Fukayama M., Yamasoba T., Asakage T. Prognostic value of p16 expression and alcohol consumption in Japanese patients with oropharyngeal squamous cell carcinoma. Cancer. 2013;119:2005–2011. doi: 10.1002/cncr.28015. [DOI] [PubMed] [Google Scholar]
- 30.Sinha P., Logan H.L., Mendenhall W.M. Human papillomavirus, smoking, and head and neck cancer. Am. J. Otolaryngol. 2012;33:130–136. doi: 10.1016/j.amjoto.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anantharaman D., Muller D.C., Lagiou P., Ahrens W., Holcátová I., Merletti F., Kjærheim K., Polesel J., Simonato L., Canova C., et al. Combined effects of smoking and HPV16 in oropharyngeal cancer. Int. J. Epidemiol. 2016;45:752–761. doi: 10.1093/ije/dyw069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gillison M.L., Zhang Q., Jordan R., Xiao W., Westra W.H., Trotti A., Spencer S., Harris J., Chung C.H., Ang K.K. Tobacco smoking and increased risk of death and progression for patients with p16-positive and p16-negative oropharyngeal cancer. J. Clin. Oncol. 2012;30:2102–2111. doi: 10.1200/JCO.2011.38.4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hong A.M., Martin A., Chatfield M., Jones D., Zhang M., Armstrong B., Lee C.S., Harnett G., Milross C., Clark J., et al. Human papillomavirus, smoking status and outcomes in tonsillar squamous cell carcinoma. Int. J. Cancer. 2013;132:2748–2754. doi: 10.1002/ijc.27956. [DOI] [PubMed] [Google Scholar]
- 34.Hafkamp H.C., Manni J.J., Haesevoets A., Voogd A.C., Schepers M., Bot F.J., Hopman A.H., Ramaekers F.C., Speel E.J. Marked differences in survival rate between smokers and nonsmokers with HPV 16-associated tonsillar carcinomas. Int. J. Cancer. 2008;122:2656–2664. doi: 10.1002/ijc.23458. [DOI] [PubMed] [Google Scholar]
- 35.Lassen P., Lacas B., Pignon J.P., Trotti A., Zackrisson B., Zhang Q., Overgaard J., Blanchard P. Prognostic impact of HPV-associated p16-expression and smoking status on outcomes following radiotherapy for oropharyngeal cancer: The MARCH-HPV project. Radiother. Oncol. 2018;126:107–115. doi: 10.1016/j.radonc.2017.10.018. [DOI] [PubMed] [Google Scholar]
- 36.Chen S.Y., Massa S., Mazul A.L., Kallogjeri D., Yaeger L., Jackson R.S., Zevallos J., Pipkorn P. The association of smoking and outcomes in HPV-positive oropharyngeal cancer: A systematic review. Am. J. Otolaryngol. 2020;41:102592. doi: 10.1016/j.amjoto.2020.102592. [DOI] [PubMed] [Google Scholar]
- 37.Chidambaram S., Nakken E.R., Kennedy W., Thorstad W.L., Chen S.Y., Pipkorn P., Zevallos J.P., Mazul A.L. Prognostic Significance of Smoking in Human Papillomavirus-Positive Oropharyngeal Cancer Under American Joint Committee on Cancer Eighth Edition Stage. Laryngoscope. 2020;130:1961–1966. doi: 10.1002/lary.28659. [DOI] [PubMed] [Google Scholar]
- 38.Chen S.Y., Last A., Ettyreddy A., Kallogjeri D., Wahle B., Chidambaram S., Mazul A., Thorstad W., Jackson R.S., Zevallos J.P., et al. 20 pack-year smoking history as strongest smoking metric predictive of HPV-positive oropharyngeal cancer outcomes. Am. J. Otolaryngol. 2021;42:102915. doi: 10.1016/j.amjoto.2021.102915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang S.H., Xu W., Waldron J., Siu L., Shen X., Tong L., Ringash J., Bayley A., Kim J., Hope A., et al. Refining American Joint Committee on Cancer/Union for International Cancer Control TNM stage and prognostic groups for human papillomavirus-related oropharyngeal carcinomas. J. Clin. Oncol. 2015;33:836–845. doi: 10.1200/JCO.2014.58.6412. [DOI] [PubMed] [Google Scholar]
- 40.Vawda N., Banerjee R.N., Debenham B.J. Impact of Smoking on Outcomes of HPV-related Oropharyngeal Cancer Treated with Primary Radiation or Surgery. Int. J. Radiat. Oncol. Biol. Phys. 2019;103:1125–1131. doi: 10.1016/j.ijrobp.2018.11.046. [DOI] [PubMed] [Google Scholar]
- 41.Hsiao J.R., Huang C.C., Ou C.Y., Chang C.C., Lee W.T., Tsai S.T., Huang J.S., Chen K.C., Lai Y.H., Wu Y.H., et al. Investigating the health disparities in the association between lifestyle behaviors and the risk of head and neck cancer. Cancer Sci. 2020;111:2974–2986. doi: 10.1111/cas.14530. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on reasonable request from the corresponding author. The data are not publicly available due to privacy.