Abstract
The potential of different parasite proteinases for use as vaccine candidates against fascioliasis in sheep was studied by vaccinating animals with the cathepsin L proteinases CL1 and CL2 and with leucine aminopeptidase (LAP) purified from adult flukes. In the first trial, sheep were immunized with CL1 or CL2 and the mean protection levels obtained were 33 and 34%, respectively. Furthermore, a significant reduction in egg output was observed in sheep vaccinated either with CL1 (71%) or with CL2 (81%). The second trial was performed to determine the protective potential of the two cathepsin L proteinases assayed together, as well as in combination with LAP, and of LAP alone. The combination of CL1 and CL2 induced higher levels of protection (60%) than those produced when these enzymes were administered separately. Those sheep that received the cocktail vaccine including CL1, CL2, and LAP were significantly protected (78%) against metacercarial challenge, but vaccination with LAP alone elicited the highest level of protection (89%). All vaccine preparations induced high immunoglobulin G titers which were boosted after the challenge infection, but no correlations between antibody titers and worm burdens were found. However, the sera of those animals vaccinated with LAP contained LAP-neutralizing antibodies. Reduced liver damage, as assessed by the level of the liver enzyme gamma-glutamyl transferase, was observed in the groups vaccinated with CL1, CL2, and LAP or with LAP alone.
Proteinases produced by parasitic helminths play critical roles in maintaining the balance between parasite and host. For example, proteinases can participate in the disruption of immune defense mechanisms directed against parasite tissues, in facilitating the migration of the parasite through host tissues, and in the acquisition of nutrition from the host (17, 38). Accordingly, parasite proteinases are believed to be important candidates toward which vaccines could be directed with the view of upsetting this parasite-host relationship.
Fasciola hepatica is the causative agent of fascioliasis, or liver fluke disease; it infects a wide range of mammals, including cattle, sheep, and humans. Immature and mature flukes secrete endoproteinases, predominantly two cysteine proteinases that have been purified and characterized as cathepsin L proteinases. They have been termed CL1 and CL2 because they differ in their physicochemical properties and show unique specificities toward fluorogenic peptidic substrates (11, 35). CL1, which is secreted by all stages of the parasite that exist in the mammalian host, can cleave host immunoglobulins and prevent attachment of eosinophils to newly excysted juveniles in vitro (7, 34, 35). It has been suggested that CL2, which can cleave fibrinogen in a manner that produces a fibrin clot, prevents excessive bleeding at feeding points within the bile ducts (12). Both cysteinyl proteinases can also degrade extracellular matrix and basement membrane molecules such as collagen types I, III, and IV, fibronectin, and laminin. Thus, roles in tissue penetration and immune system evasion have been attributed to these enzymes (4).
More recently, an exoproteinase has been isolated from an F. hepatica detergent-soluble extract and characterized as a leucine aminopeptidase (LAP) because of its preferential specificity for cleaving the substrate leucine–7-amino-4-methylcoumarin (NHMec). Histochemical methods showed that the LAP activity in the liver fluke was associated with the epithelial cells that line the digestive tract of the parasite. This enzyme most likely functions in the final stages of the catabolism of peptides that are generated by the degradation of host tissue by endoproteinases, such as the cathepsin L proteinases, and are absorbed by the epithelial cells (1).
Immunoprophylactic control of liver fluke disease has been attempted by using either parasite extracts or defined functional parasite antigens, with some success (37). Vaccine preparations containing the CL1 and CL2 proteinases induced high levels (>70%) of protection against an F. hepatica challenge infection in cattle (10). However, the effects of such a vaccine have not yet been tested in sheep, a host that is very important from an epidemiological point of view and that shows little or no natural immunity to F. hepatica. In the present study, we have carried out vaccine trials in sheep, using CL1, CL2, and LAP, either alone or in combination, and have demonstrated that these molecules can induce high levels of protection against F. hepatica challenge infection in this animal.
MATERIALS AND METHODS
Parasites.
Metacercariae used in this study were obtained in our laboratory by passage through the intermediate host snail Limnea viatrix and maintained on 0.4% carboxymethyl cellulose until used. Mature F. hepatica flukes were obtained from the bile ducts of infected cattle at a local abattoir.
Purification of parasite enzymes.
Cathepsins CL1 and CL2 were purified to homogeneity from the excretion-secretion (E/S) products of mature flukes as previously described (11, 35). Briefly, mature flukes were washed six times in 0.1 M phosphate-buffered saline (PBS), pH 7.3, and incubated for 8 h at 37°C in RPMI 1640, pH 7.3, containing 2% glucose, 30 mM HEPES, and 25 mg of gentamicin per ml. The flukes were removed, and the culture medium was centrifuged at 15,000 × g for 1 h at 4°C. The supernatant (containing E/S products) was then collected, filtered, and stored at −20°C until used. E/S products, concentrated 50-fold by ultrafiltration, were applied to a Sephacryl S-200 HR column (Pharmacia). Fractions of 3 ml were collected and assayed for cathepsin L activity, using the fluorogenic substrate N-benzyloxycarbonyl (Z)-Phe-Arg-NHMec. Fractions containing enzyme activity were pooled and applied to a QAE A50 anion-exchange column. The proteins were eluted with a continuous gradient of NaCl (0 to 400 mM), and the fractions were assayed for cathepsin L activity by using Z-Phe-Arg-NHMec and tosyl-Gly-Pro-Arg-NHMec for CL1 and CL2, respectively.
LAP was purified from the detergent-soluble extracts as follows. Washed adult flukes were killed by freezing for 30 min at −20°C and washed twice with PBS at 4°C. One gram (wet weight) of tissue was incubated in a solution consisting of 10 ml of 1% deoxycholic acid (DOC) in 0.15 M glycine buffer (pH 9.0)–0.5 M NaCl for 60 min at room temperature, 30 min at 37°C, and then 30 min at 4°C. The DOC-extracted material was centrifuged at 20,000 × g for 60 min, and the supernatant was stored at −80°C until used. Detergent from the membrane extract was removed by using a Bio-Beads SM-2 column (Bio-Rad) equilibrated in 0.01 M K2HPO4, pH 7.4. Membrane extract fractions containing LAP activity (detected with the fluorogenic substrate leucine-NHMec) were applied onto a calibrated Superdex 200 HR 16/50 column (Pharmacia) equilibrated with 0.05 M Tris-HCl, pH 8.5. Samples were eluted with the same buffer at a flow rate of 3.5 ml/min. Fractions (1 ml) containing LAP activity were pooled, filtered through a 0.45-μm-pore-size nylon membrane filter (Millipore, Bedford, Mass.), and applied to a bestatin affinity column (5 by 1 cm) equilibrated with 0.05 M Tris-HCl, pH 8.5, prepared as previously described (14). The column was washed with 10 bed volumes of the equilibration buffer at a flow rate of 0.3 ml/min. Stepwise elution was carried out with 0.05 M Tris-HCl buffer, pH 8.5, containing 0.1, 0.25, 0.5, and 1.0 M NaCl. Fractions with enzyme activity were pooled, dialyzed against 0.1 M glycine (pH 8.0) containing 0.02% sodium azide, and stored at 4°C until used.
The purification processes were assessed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) under nonreducing and reducing (10 mM dithiothreitol [final concentration]) conditions (16). After electrophoresis, the gels were stained with Coomassie brilliant blue R250 or by using a silver stain kit (Bio-Rad). Phosphorylase B (97 kDa), albumin (66 kDa), ovalbumin (45 kDa), carbonic anhydrase (30 kDa), trypsin inhibitor (20 kDa), and lactalbumin (14 kDa) were used as molecular mass markers.
Immunization protocol.
Male Corriedale sheep (2 years old) were housed in a paddock with an artificial water supply. The animals were purchased from a fluke-free area and shown to be free of infection by fecal analysis and enzyme-linked immunosorbent assay (ELISA). All vaccines were prepared by mixing 100 μg of purified proteinase in Freund’s complete adjuvant for the first immunization (priming; week 0) or Freund’s incomplete adjuvant for the second immunization (booster; week 4) and administered subcutaneously.
In the first trial, groups of 10 animals were immunized with CL1 (group 1), CL2 (group 2), or PBS (control; group 3). In the second trial, groups of 10 animals were immunized with a combination (100 μg of each proteinase/dose) of CL1 and CL2 (group 1) or CL1, CL2, and LAP (group 2), with LAP alone (group 3), or with PBS (control; group 4). Six weeks after the immunization (week 6), each of the first-trial group members was orally challenged with a gelatin capsule containing 500 metacercariae. Animals in the second trial were orally challenged with 300 metacercariae. During the trials, a number of animals (14 each in trials 1 and 2) died as the result of a drought associated with a very hot summer. Animal death was not associated with any particular group in the trials. Sheep were humanely slaughtered 18 (first trial) or 20 (second trial) weeks after the first immunization. Flukes in the main bile ducts and gall bladder were removed. The liver was then cut into 1-cm-long pieces, which were subsequently soaked in water at 37°C for 30 min, squeezed, and forced through a 300-μm-mesh sieve; the retained material was analyzed for immature or damaged flukes. Blood was collected from all sheep prior to the first immunization and weekly until the time of slaughter. The serum was obtained and then stored at −80°C.
Fecal egg counts (FEC).
In the first vaccination trial, feces were removed from the rectum after slaughter. Three grams of dried feces was diluted in 40 ml of a water-detergent solution and resuspended by vortexing. The sample was then washed through sieves of different pore sizes (300, 150, and 32 μm), and the filtrate was left at room temperature for 3 min. The number of F. hepatica eggs in the sediment was estimated as previously described (28).
Liver enzyme analysis.
Liver damage was assessed in all immunized and control animals of both trials by measuring the levels of the liver enzymes aspartate transaminase (AST; EC 2.6.1.1) and gamma-glutamyl transferase (GGT; EC 2.3.2.2) in sera from blood samples taken weekly from the time of priming (week 0) to the day of slaughter, using commercial kits according to the manufacturer’s instructions (Boehringer).
Analysis of antibody responses by ELISA.
Antibody responses were analyzed by ELISA. The wells of polystyrene microplates (Nunc, Roskilde, Denmark) were coated with a 3-μg/ml solution of purified CL1, CL2, or LAP in PBS and incubated overnight at 4°C. The remaining binding sites were blocked with a solution consisting of 5% dry milk in PBS for 1 h at 37°C, and the plate wells were washed three times, for 3 min each, in washing solution (0.05% Tween 20 in PBS). Sera were diluted 1:200 in dilution buffer (2.5% dry milk and 0.05% Tween 20 in PBS) and added to the wells (100 μl per well). After a 1-h incubation at 37°C, the plates were washed as described above, and bound antibody was detected with peroxidase-conjugated rabbit anti-sheep immunoglobulin G (IgG) (1:7,000 in dilution buffer) and the substrates o-phenylenediamine and H2O2 (Sigma Chemical Co., St. Louis, Mo.). The enzyme reaction was stopped after 15 min by addition of 10% HCl (50 μl/well) and the optical densities at 492 nm (OD492) were determined.
For data analysis of antiproteinase antibody concentrations, a pool of serum samples from infected sheep was used as the reference serum. Antibody concentrations, expressed in terms of arbitrary units and OD492 values, corresponding to dilutions of the reference serum were correlated by linear-regression analysis. ELISA data (OD492 values) for every sample were converted to antibody concentrations equivalent to this reference for analytical consistency.
Immunoblot analysis of antibody responses in sheep.
Mature F. hepatica E/S products and purified LAP were electrophoretically separated by SDS-PAGE (12% gels) under reducing conditions. Separated proteins were electrophoresed onto nitrocellulose paper in accordance with a standard method (39). Following protein transfer, the nitrocellulose filters were incubated in blocking buffer (5% dry milk and 1.5% bovine serum albumin [BSA] in PBS) for 1 h at room temperature. After three washes of 5 min each with a solution consisting of 0.1% BSA in PBS–0.05% Tween 20, the membranes were incubated in sera from the vaccinated and control sheep (diluted 1:200 in dilution solution [0.1% BSA in PBS-Tween 20]) for 1 h at room temperature. Following the incubation period, the membranes were washed as described above and then incubated with peroxidase-conjugated anti-sheep IgG (Sigma, Poole, Dorset, United Kingdom) diluted 1:1,000 in dilution solution. After three washes, the blots were developed by using the substrate 4-chloro-1-naphthol and H2O2.
Assay of inhibition of LAP by antibodies.
An in vitro inhibitory assay was developed to investigate the effect of antibodies induced in the vaccinated sheep on the LAP activity. Protein A affinity column-purified IgG preparations from infected and control animals were assayed as follows. A 100-μl volume of LAP (60 μg) was preincubated with 100 μl of purified IgG (50 μg) at 37°C for 1 h. A 100-μl volume of this reaction mixture was then added, in duplicate, to wells of polystyrene microplates preloaded with 100 μl of 0.1 M glycine buffer, pH 8.3. The plate was then incubated for 10 min at 37°C prior to the addition of 50 μl of 4 mM l-leucine p-nitroanilide (pNA) to each well. LAP incubated without purified IgG served as a control. The OD405 was determined at time zero and following a 2-h incubation at 37°C, and the amount (in nanomoles) of pNA released per minute per milligram of protein was estimated as previously described (19). Protein concentrations were determined by using a Micro-BCA protein assay kit (Pierce, Rockford, Ill.) (22).
Statistical methods.
Arithmetic means were calculated for the worm burdens, FEC, liver enzymes, and ELISA values of the vaccinated and control groups. Percentage protection was calculated as [u − (v/u)] × 100, where u is the mean value for the negative-control group and v is the mean value for the experimental group. The statistical significance of values determined for worm burdens and FEC was analyzed by using the Mann-Whitney U test. The relationship between ELISA values and worm burden was studied by using the Spearman correlation test.
RESULTS
Purification of parasite enzymes.
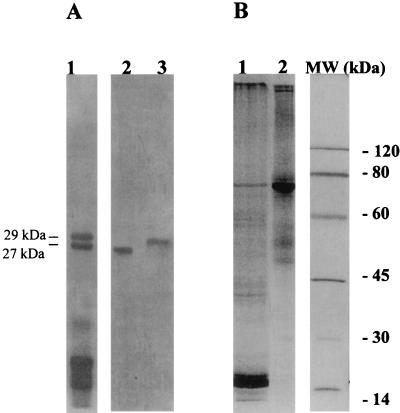
F. hepatica cathepsin L proteinases were purified to homogeneity from E/S products by a combination of gel filtration and ion-exchange chromatography. Analysis by SDS-PAGE (under reducing conditions) revealed that the purified preparations contained single proteins of 27.5 kDa, corresponding to CL1 (34), and of 29 kDa, corresponding to CL2 (11) (Fig. 1A). The F. hepatica 65-kDa LAP was isolated to homogeneity from DOC extracts of adult flukes by gel filtration and Bestatin affinity chromatography (Fig. 1B) (1).
FIG. 1.
Purification of F. hepatica proteinases used in vaccination trials. (A) Reducing SDS-PAGE analysis, using a 15% gel, of cathepsin L-containing fractions eluted from a Sephacryl S-200 HR column (lane 1), purified 27-kDa CL1 (lane 2), and 29-kDa CL2 (lane 3). (B) Nonreducing SDS-PAGE analysis, using a 10% gel, of a detergent-soluble extract (DOC) from adult flukes (lane 1) and Bestatin affinity-purified LAP (lane 2). Shown on the right are molecular mass standards.
ELISA analysis of sera obtained from sheep immunized with CL1 or CL2 in trial 1.
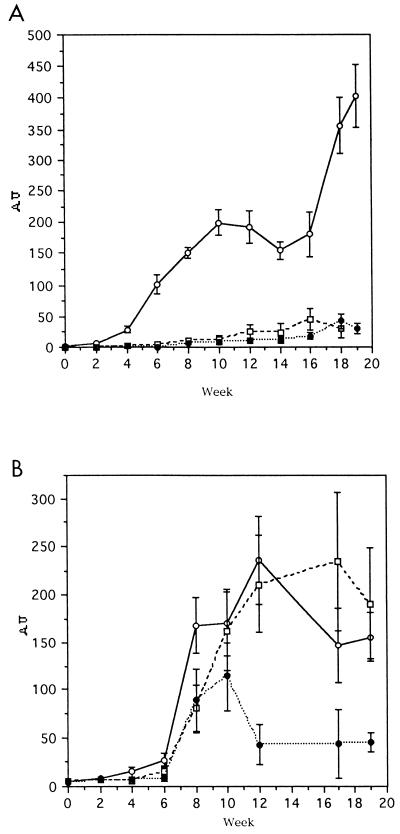
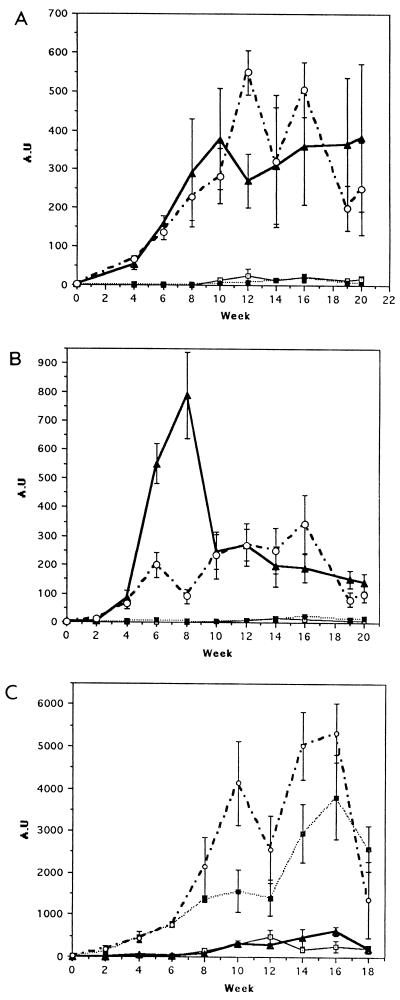
Antibodies reactive with the vaccine molecules appeared in the sera of sheep within 4 weeks after the initial immunization and showed a sharp increase following the challenge infection at week 6. The level of antibody response elicited by CL1 was higher than that produced by CL2 (Fig. 2A). In addition, antibodies induced by immunizations with CL1 cross-reacted with the CL2 antigen, and vice versa. Specific antibodies to CL1 and CL2 appeared in the sera of nonvaccinated control animals at week 8, i.e., 2 weeks after the challenge infection. Throughout the course of infection, these animals produced a higher level of anti-CL2 antibodies, which peaked at week 10, than anti-CL1 antibodies (Fig. 2).
FIG. 2.
ELISA analysis of antibody responses against CL1 and CL2 in sheep from trial 1. Titers of anti-CL1 (A) and anti-CL2 (B) specific IgGs in serum samples taken every 2 weeks during the course of the experiment were determined by ELISA. CL1 and CL2 were probed with serum from each animal, and each titer represents the mean response in animals immunized with CL1 (group 1) (open circles), CL2 (group 2) (open squares), or PBS (group 3) (closed circles). AU, arbitrary units.
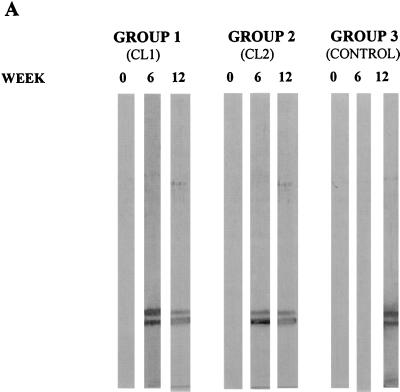
Immunoblot analysis using serum from animals in trial 1.
Adult F. hepatica E/S products were separated by SDS-PAGE (12% gel) under reducing conditions, electrotransferred to nitrocellulose membranes, and probed with sera obtained from vaccinated and control animals at weeks 0, 6, and 12 (Fig. 3A). Antibodies reactive with both CL1 and CL2 components of liver fluke E/S products were present in the sera of vaccinated animals in groups 1 and 2 on the day of challenge (week 6). Antibodies reactive with these antigens were not present in the sera of the nonvaccinated control animals at this time but were present by week 12, i.e., 6 weeks after the challenge infection. Serum samples taken from all groups at week zero showed no immunoreactivity to E/S product proteins (Fig. 3A).
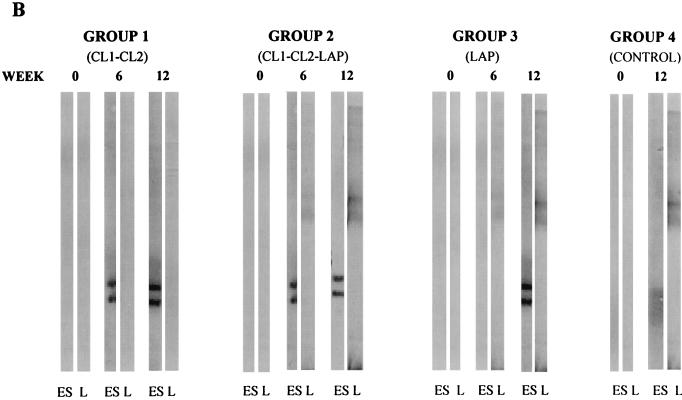
FIG. 3.
Western blot analysis of IgG response against F. hepatica proteinases in sheep during the course of vaccination trials. (A) For the first vaccination trial, E/S products from mature flukes, separated by SDS-PAGE (12% gel) under reducing conditions and electrotransferred onto nitrocellulose filters, were probed with pools of serum obtained from animals in each group at weeks 0 (primary immunization), 6 (time of infection), and 12 (6 weeks postinfection). Sheep were immunized with 100 μg of either CL1 (group 1) or CL2 (group 2) or with PBS (group 3; control) per immunization. (B) The development of specific IgG antibodies against F. hepatica cathepsin L proteinases and LAP at various time points during trial 2 is shown. Pooled sera obtained from animals in each sheep group at weeks 0 (primary immunization), 6 (time of infection), and 12 (6 weeks postinfection) were probed against E/S products (ES) or LAP (L). Animals were immunized with 100 μg each of CL1 and CL2 (group 1), 100 μg each of CL1, CL2, and LAP (group 2), 100 μg of LAP (group 3), or PBS (group 4; control) per immunization.
Parasite burdens and FEC in the animals in trial 1.
Twelve weeks after the infection with metacercariae of F. hepatica, the animals were slaughtered and the parasites in the bile ducts and liver tissue were counted (Table 1). The vaccinated animals in groups 1 and 2 showed mean reductions in fluke burden of 34% (P < 0.001) and 33% (P > 0.05), respectively. The FEC analysis showed a significant reduction of eggs in the vaccinated groups compared to the control group. For group 1, the mean reduction was 71%, and for group 2 it was 81%.
TABLE 1.
Worm recovery and FEC in sheep vaccinated against F. hepatica infection with CL1 and CL2 in trial 1
| Group (antigen) | No. of flukes recovered
|
% Reduction in no. of flukes vs control | FEC (mean ± SD) | % Reduction in FEC vs control | |
|---|---|---|---|---|---|
| Mean ± SD | Flukes/animal | ||||
| 1 (CL1) | 109 ± 24.6 | 126, 87, 124, 149, 116, 85, 77 | 34 | 125.4 ± 80 | 71 |
| 2 (CL2) | 107 ± 40.1 | 74, 162, 65, 127 | 33 | 82.3 ± 90 | 81 |
| 3 (none [control]) | 161.4 ± 21.4 | 150, 135, 194, 150, 178 | 426 ± 120.2 | ||
Antibody responses of sheep vaccinated with CL1, CL2, and LAP in trial 2.
In the second trial, combinations of CL1 and CL2 (group 1) and of CL1, CL2, and LAP (group 2) were assessed as cocktail vaccines. F. hepatica LAP was also tested alone (group 3). The mean antibody titers against CL1 in these groups are shown in Fig. 4A. Groups 1 and 2 showed antibody responses to the vaccine after the second week postpriming (Fig. 4A). This response increased up to 6 weeks postinfection (week 12) and remained relatively constant up to the end of the experiment, at week 20. The antibody response elicited in animals of group 3 (which were given LAP alone) was similar to that of the control group, with only very low titers being exhibited after week 12.
FIG. 4.
ELISA analysis of antibody responses against CL1, CL2, and LAP in sheep from trial 2. Titers of anti-CL1 (A), anti-CL2 (B), and anti-LAP (C) specific IgGs in serum samples taken every 2 weeks during the course of the experiment were determined by ELISA. Antigens were probed with serum from each animal, and each titer represents the mean response in animals immunized with a combination of CL1 and CL2 (group 1) (closed triangles), a combination of CL1, CL2, and LAP (group 2) (open circles), LAP alone (group 3) (closed squares), or PBS (group 4) (open squares). AU, arbitrary units.
The dynamics of the antibody response against CL2 are shown in Fig. 4B. The antibody response elicited in the animals of group 1 showed a sharp peak at week 8. The levels of specific antibodies in this group decreased at week 10 and then remained relatively constant until the end of the experiment. The antibody response of group 3 was similar to that of group 2 with the absence of the peak at week 8.
When the specific antibody response against F. hepatica LAP was examined, animals in groups 2 and 3 showed the strongest response, with minimal levels being elicited in the animals of groups 1 and 4 (Fig. 4C).
Immunoblot analysis using serum obtained from the animals in trial 2.
Immunoblot analysis was carried out with serum obtained from the animals in the second trial, using E/S products and purified LAP electrophoretically separated by nonreducing SDS-PAGE on 10 and 12.5% gels, respectively, and electroblotted as described above (Fig. 3B). At week zero, none of the sera contained antibodies reactive with the E/S products. Sera obtained from animals of group 3 (vaccinated with LAP) at weeks 6 and 12 contained antibodies reactive with purified LAP but not with E/S products. Animals in group 1 (immunized with CL1 and CL2) produced antibodies reactive with CL1 and CL2 but not with LAP, whereas the antibodies in the sera of animals in group 2 (immunized with CL1, CL2, and LAP) reacted against the CL1 and CL2 in the E/S products, as well as with LAP. Sera obtained from the control animals were reactive with CL1, CL2, and LAP at week 12 (6 weeks postchallenge) only.
Parasite burdens in animals of trial 2.
In the second trial, fluke burdens were assessed 13 weeks after the metacercarial challenge (Table 2). The mean recovery of flukes in the control group ± the standard deviation was 72 ± 13, which represents 24% of the infection dose. Vaccination with a combination of CL1 and CL2 (group 1) induced a protection level of 60% (P > 0.05). The levels of protection observed in group 3 (LAP alone) and group 2 (CL1-CL2-LAP combination) were 89.6 and 79%, respectively (P < 0.001 and P < 0.01, respectively). Notably, four animals of the six in group 2 and four animals of the six in group 3 did not harbor any flukes in their livers.
TABLE 2.
Worm recovery in sheep vaccinated against F. hepatica infection with CL1, CL2, and LAP in trial 2
| Group (antigen[s]) | No. of flukes recovered
|
% Reduction vs control | |
|---|---|---|---|
| Mean ± SD | Flukes/animal | ||
| 1 (CL1-CL2) | 23.11 ± 34.5 | 56, 89, 0, 0, 0, 0, 1, 5, 57 | 60 |
| 2 (CL1-CL2-LAP) | 12.1 ± 17.6 | 43, 0, 0, 30, 0, 0 | 79 |
| 3 (LAP) | 6.5 ± 15.4 | 1, 0, 0, 38, 0, 0 | 89.6 |
| 4 (none [control]) | 72 ± 13 | 126, 59, 54, 56, 65 | |
Analysis of liver enzymes in sera of vaccinees and controls.
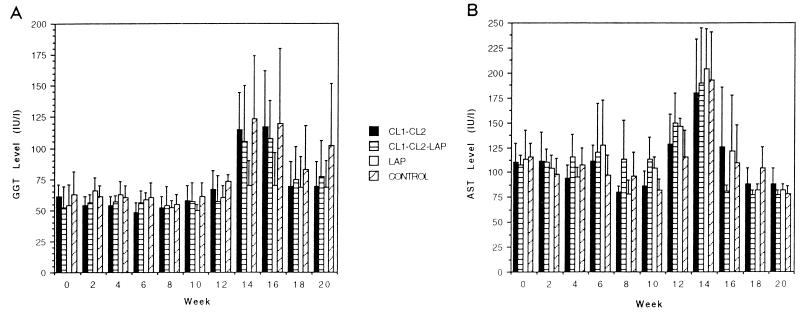
The levels of GGT began to rise at week 14 (8 weeks after infection) in nonvaccinated control animals (group 4). Animals immunized with CL1 and CL2 (group 1) exhibited an enzyme profile similar to that of the controls. The levels of GGT observed in the animals vaccinated with a combination of CL1, CL2, and LAP (group 2) were significantly lower than the levels reached in the animals immunized with CL1 and CL2 (group 1) and in the control group (group 4). Finally, the group immunized with LAP alone (group 3) showed no increase in the level of GGT throughout the course of the experiment (Fig. 5A). No differences in the AST levels in the sera of vaccinated and control animals were observed throughout the course of the experiment (Fig. 5B).
FIG. 5.
Kinetic analysis of GGT (A) and AST (B) levels in sera of sheep of trial 2. Sera were obtained every 2 weeks, until the end of the experiment, from all animals in group 1 (vaccinated with CL1 plus CL2), group 2 (vaccinated with CL1, CL2, and LAP), group 3 (vaccinated with LAP), and group 4 (PBS control). Bars represent the mean levels of enzymatic activity within each group ± the standard deviations.
Inhibitory assays.
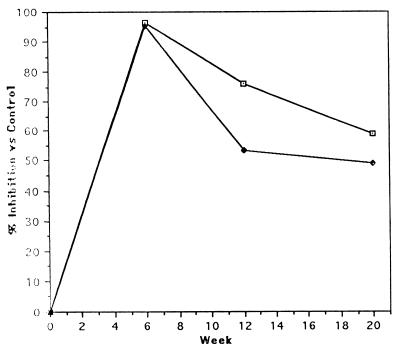
Results obtained after incubation of LAP with purified IgG are relative to those of the sheep from the control group (Fig. 6). At the time of the challenge infection (week 6), sera of the sheep vaccinated with LAP (group 3) or the CL1-CL2-LAP combination (group 2) contained antibodies that inactivated LAP activity. The mean inhibitory values were 92 and 90%, respectively, of the control animal values. Six weeks later, the sera of the sheep immunized with LAP continued to contain inhibitory antibodies (44% inhibition of LAP) (Fig. 6). After this time point, no differences between vaccinated animals and controls were observed.
FIG. 6.
Neutralization of F. hepatica LAP activity following incubation with anti-LAP IgG. Affinity-purified IgG from LAP-immunized and control animals from the second trial (50 μg) was preincubated with F. hepatica LAP (60 μg) prior to incubation in microtiter plates with the substrate l-leucine pNA. OD405 readings were determined at 0 min and 2 h, and the change in OD was calculated and converted to nanomoles of pNA released per minute per milligram of protein. In the graph is shown the percentage of inhibition produced by IgG from animals in group 2 (immunized with CL1, CL2, and LAP) (⧫) and group 3 (immunized with LAP) (⊡) at different time points, relative to the inhibition observed with IgG for the control group.
DISCUSSION
Because sheep show little or no natural resistance to infection with the liver fluke, many attempts have been made to stimulate protective immune responses in these animals by the use of primary homologous infections (15, 25, 27, 29–32), infections with irradiated, attenuated metacercariae (2, 5, 6), or vaccination with either somatic (24, 26) or secreted (27) antigens. The consistent failure of these attempts has led researchers to search for defined F. hepatica functional antigens as potential vaccine targets (37). Accordingly, vaccination with purified liver fluke glutathione S-transferase provided the first example of the induction of high levels of protection (57%) in sheep (28).
Due to the participation of cathepsin L proteinases in mechanisms crucial to parasite survival, such as immune evasion and tissue penetration, these enzymes have been considered candidates for vaccines against fascioliasis (4, 8, 9, 11, 35). Wijffels et al. reported that vaccination of sheep with a mixture of cysteine proteinases (which most likely contained both CL1 and CL2) did not significantly reduce worm burdens following a challenge infection but did reduce the parasite egg output by 70% (40). Recent experiments, performed in cattle, using purified cathepsins CL1 and CL2 in combination with a parasite hemoglobin (Hb) demonstrated their protective properties. CL1 alone induced a mean level of protection of 43%. When CL1 was used in combination with Hb, the level of protection achieved was 51.9%. While the vaccine efficacy of CL2 alone was not assessed, a combination of CL2 and Hb elicited a protection level of 72.4% compared with the nonvaccinated controls. In addition, this vaccine combination reduced the viability of eggs produced by remaining parasites by >98% (10).
In the present study, we demonstrated the achievement of significant levels of protection against fascioliasis in sheep by vaccination with cocktails of parasite proteolytic enzymes. In the first trial, despite the differences in the antibody responses elicited when CL1 and CL2 were administrated separately, the levels of protection achieved were 34 and 33% and the reductions in egg output were 70 and 81%, respectively. Although statistical analysis did not reveal any correlation between anti-cathepsin L IgG titers and worm burdens recovered, further dissection of the humoral response in the immunized animals—for example, through the study of the IgG subclass- or IgE-specific response—could help to clarify the issue. Mulcahy et al. recently demonstrated a negative correlation between the burden of flukes recovered from cattle vaccinated with cathepsin L proteinases and the level of IgG2 antibodies elicited (18).
Although no comparative trial for CL1 and CL2 was performed in cattle, the results presented here showed that these two enzymes had nearly equal protective potentials in sheep. Although differences in synthetic-substrate specificity suggest that the cathepsin L proteinases have different roles in digestion, migration, and immune evasion, they have shown very similar cleaving activities on protein substrates such as the extracellular matrix components fibronectin and laminin (4) and human IgG subclass molecules (3a). Moreover, the amino acid sequences of these enzymes are 79.4% similar, which would also account for the observed cross-reactivity of the induced antibody responses in the vaccinated sheep (13, 23).
The antifecundity effects induced by vaccination with the purified CL1 and CL2 are consistent with the similar findings of Wijffels et al. (40), who vaccinated sheep with a mixture of liver fluke cathepsin L proteinases, and with those of Dalton et al., who showed an almost complete antiembryonation effect in cattle by using CL2 in combination with Hb (10). This effect may be mediated by the direct binding of antibody to parasite enzymes, since we have shown that the binding of rabbit anti-cathepsin L antibodies from immunized or experimentally infected animals was capable of neutralizing the enzymatic activity (21, 33). This remarkable effect on egg output could have a profound impact on disease transmission by reducing the level of snail infection.
The second trial was performed to determine the protective potential of the cathepsin L proteinases in combination and to examine the effects of the addition of LAP, an exopeptidase recently isolated in our laboratory (1). The level of protection achieved in the second trial when CL1 and CL2 were mixed in equal amounts was 60% of that of the control group. These results suggest an additive effect of CLs in eliciting protection against parasite challenge. However, the protective effects in the two trials are not entirely comparable, since smaller numbers of metacercariae were administered in the second trial. The maximum levels of protection were achieved in group 3 (immunized with LAP) and group 2 (immunized with CL1-CL2-LAP), with protection reaching 89.6 and 79%, respectively. In addition, several animals in both groups harbored no worms in their livers. It is assumed that the observed increment in protection for group 2, compared to group 1, is due to the presence of LAP in the vaccination mixture.
F. hepatica LAP alone was able to induce a very high level of protection—in fact, the highest yet reported in sheep. Aminopeptidases are a family of zinc-dependent exopeptidases that hydrolyze amino acid residues at the amino termini of peptide substrates. Among other functions, they participate in terminal degradation of proteins, protein maturation, and regulatory processes of cellular metabolism (3). The F. hepatica LAP, isolated from adult fluke DOC-soluble extracts, shows a preferential cleavage of Leu-NHMec but also exhibits activity on other substrates, like Arg-NHMec and Phe-NHMec. Since LAP activity was histochemically localized to the epithelial cells lining the gut of the adult fluke, it was postulated that this enzyme participates in the final breakdown of small polypeptides and dipeptides previously generated by cathepsin L proteinases and a dipeptidylpeptidase (8). It is plausible, therefore, that vaccination with LAP induces a humoral immune response capable of neutralizing this enzyme at the apical membrane of the gut cells. In support of this hypothesis is the finding that affinity-purified IgG antibodies from LAP-vaccinated animals in groups 2 and 3 could inhibit LAP activity. However, there was not a statistically significant correlation between antibody titers against LAP and worm burdens in any of the groups. Low anti-LAP IgG levels were produced following infection in the nonvaccinated animals, indicating that the enzyme must be somehow exposed to the host immune system early in the migratory phase. We have also detected LAP activity in parasite extracts from newly excysted juveniles and 3-week-old immature flukes (unpublished data).
This is not the first report of aminopeptidase-elicited protective immunity against parasites. The Haemonchus contortus H11 antigen, a membrane glycoprotein derived from the intestinal microvilli of the parasite, is an LAP (36). Sheep vaccinated with H11 are significantly protected against challenge infection with H. contortus; reductions of 60% in male worms and 84% in female worms and an antireproductive effect consisting of a 90% reduction in FEC were determined by Newton et al. (20).
Since extensive liver damage is a pathological consequence of liver fluke infection, the extent of the damage in immunized and control animals for both trials was examined by measuring the levels of the liver enzymes AST and GGT in their sera. Increased AST is indicative of liver damage, particularly injury to the liver parenchyma cells. In both trials, the levels of AST in the immunized animals throughout the course of the infection did not show significant differences from the control group levels, indicating the success of the infection. Increased levels of GGT are indicative of bile duct hyperplasia and in liver fluke infections are associated with the arrival and residence of flukes in the bile ducts, which begin approximately 9 weeks postinfection. The levels of GGT elicited in groups 3 (vaccinated with LAP) and 2 (vaccinated with CL1, CL2, and LAP) in the second trial were significantly lower than those detected in the control group. These results are in accordance with the very small numbers of parasites recovered from these groups at necropsy and suggest that the protection achieved in these groups is directed against the immature migrating flukes at the liver parenchyma.
It is notable that in the sheep that were completely protected by vaccination an increase in serum AST was detected, indicating that at least some immature flukes had penetrated the liver parenchyma of these animals. This observation suggests that the vaccine may operate on the hepatic stages of flukes and not on the prehepatic stages. Therefore, animals exposed to repeated infection, as is experienced in the field, would exhibit cumulative damage to the liver. An alternative interpretation of this observation, however, is that the vaccine requires boosting by the challenge infection to induce the protective response. In this case, animals vaccinated and then challenged could be more resistant to further infection. It will be important to address these questions by assessing the efficacy of our vaccine cocktails against a trickle challenge infection administered experimentally or naturally.
In summary, in this study we have demonstrated a very high level of protection against challenge infection in ovine fascioliasis, using a vaccine preparation based on LAP, either alone or in combination with CL1 and CL2. Current efforts focus on the molecular characterization of LAP and the production of a recombinant enzyme for use in the next series of vaccination trials.
ACKNOWLEDGMENTS
This work was supported by grants from the Consejo Nacional de Investigación, Ciencia y Tecnología, Uruguay, and the Swedish Agency for Research and Cooperation.
REFERENCES
- 1.Acosta D, Goñi F, Carmona C. Characterization and partial purification of a leucine aminopeptidase from Fasciola hepatica. J Parasitol. 1998;84:1–17. [PubMed] [Google Scholar]
- 2.Armour J, Dargie J D, Doyle J J, Murray M, Robinson P, Rushton B. Proceedings of the 3rd International Congress on Parasitology. 1974. Immunization against fasciolasis; p. 494. [Google Scholar]
- 3.Bachmair A, Finley D, Varshavky G. In vivo half-life of a protein is a function of its amino-terminal residue. Science. 1986;234:179–186. doi: 10.1126/science.3018930. [DOI] [PubMed] [Google Scholar]
- 3a.Berasaín, P. Unpublished data.
- 4.Berasaín P, Goñi F, McGonigle S, Dowd A J, Dalton J P, Frangione B, Carmona C. Proteinases secreted by Fasciola hepatica degrade extracellular matrix and basement membrane components. J Parasitol. 1997;83:1–5. [PubMed] [Google Scholar]
- 5.Boray J C. The effect of host reaction to experimental Fasciola hepatica infections in sheep and cattle. In: Soulsby E J L, editor. The reaction of the host to parasitism. Marburg, Germany: Elvert; 1967. pp. 119–122. [Google Scholar]
- 6.Campbell N J, Greeg P, Kelly J D, Dincen J K. Failure to induce homologous immunity to Fasciola hepatica in sheep vaccinated with irradiated metacercariae. Vet Parasitol. 1978;4:143–152. [Google Scholar]
- 7.Carmona C, Dowd A J, Smith A M, Dalton J P. Cathepsin L proteinase secreted by Fasciola hepatica in vitro prevents antibody-mediated eosinophil attachment to newly excysted juveniles. Mol Biochem Parasitol. 1993;62:9–18. doi: 10.1016/0166-6851(93)90172-t. [DOI] [PubMed] [Google Scholar]
- 8.Carmona C, McGonigle S, Dalton J P. A novel dipeptidyl peptidase is secreted by Fasciola hepatica. Parasitology. 1994;109:113–116. doi: 10.1017/s0031182000077817. [DOI] [PubMed] [Google Scholar]
- 9.Dalton J P, Heffernan M. Thiol proteases released in vitro by Fasciola hepatica. Mol Biochem Parasitol. 1989;35:161–166. doi: 10.1016/0166-6851(89)90118-7. [DOI] [PubMed] [Google Scholar]
- 10.Dalton J P, McGonigle S, Rolph T P, Andrews S J. Induction of protective immunity in cattle against infection with Fasciola hepatica by vaccination with cathepsin L proteinases and with hemoglobin. Infect Immun. 1996;64:5066–5074. doi: 10.1128/iai.64.12.5066-5074.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dowd A J, Smith A M, McGonigle S, Dalton J P. Purification and characterization of a second cathepsin L proteinase secreted by the parasitic trematode Fasciola hepatica. Eur J Biochem. 1994;223:91–98. doi: 10.1111/j.1432-1033.1994.tb18969.x. [DOI] [PubMed] [Google Scholar]
- 12.Dowd A J, McGonigle S, Dalton J P. Fasciola hepatica cathepsin L proteinase cleaves fibrinogen and produce a novel type of fibrin clot. Eur J Biochem. 1995;232:241–246. doi: 10.1111/j.1432-1033.1995.tb20805.x. [DOI] [PubMed] [Google Scholar]
- 13.Dowd A J, Tort J, Roche L, Ryan T, Dalton J P. Isolation of a cDNA encoding Fasciola hepatica cathepsin L2 and functional expression in Saccharomyces cerevisiae. Mol Biochem Parasitol. 1997;88:163–174. doi: 10.1016/s0166-6851(97)00090-x. [DOI] [PubMed] [Google Scholar]
- 14.Karauchi O S, Mizutani S, Okako K, Narita O, Tamoda U. Purification and characterization of human placental microsomal aminopeptidase and pregnancy serum cystyl-aminopeptidase. Enzyme. 1986;35:197–205. doi: 10.1159/000469343. [DOI] [PubMed] [Google Scholar]
- 15.Knight R A. Response of lambs to challenge infections after repeated inoculations with Fasciola hepatica cysts. Proc Helminthol Soc Wash. 1980;47:186–191. [Google Scholar]
- 16.Laemmli U K, Favre M. Maturation of the head of bacteriophage T4: DNA packaging events. J Mol Biol. 1973;80:575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- 17.McKerrow J H. Parasite proteases. Exp Parasitol. 1989;68:111–115. doi: 10.1016/0014-4894(89)90016-7. [DOI] [PubMed] [Google Scholar]
- 18.Mulcahy G, O’Connor F, McGonigle S, Dowd A J, Clery D G, Andrews S J, Dalton J P. Correlation of specific antibody titre and avidity with protection in cattle immunized against Fasciola hepatica. Vaccine. 1998;16:932–939. doi: 10.1016/s0264-410x(97)00289-2. [DOI] [PubMed] [Google Scholar]
- 19.Munn E A, Smith T, Smith H, James F, Smith F, Andrews S. Vaccination against Haemonchus contortus with denatured forms of the protective antigen H11. Parasite Immunol. 1997;19:243–248. doi: 10.1046/j.1365-3024.1997.d01-205.x. [DOI] [PubMed] [Google Scholar]
- 20.Newton S E, Morrish L E, Martin P J, Montague P E, Rolph T P. Protection against multiply drug-resistant and geographically distant strains of Haemonchus contortus by vaccination with H11, a gut membrane-derived protective antigen. Int J Parasitol. 1995;25:511–521. doi: 10.1016/0020-7519(94)00143-c. [DOI] [PubMed] [Google Scholar]
- 21.Piacenza L, Acosta D, Dowd A J, McGonigle S, Dalton J P, Carmona C. Proteinases secreted by Fasciola hepatica: time course of the inhibitory effect of serum from experimentally infected rabbits demonstrated by gelatin-substrate polyacrylamide gel electrophoresis. J Helminthol. 1997;71:333–338. doi: 10.1017/s0022149x00016151. [DOI] [PubMed] [Google Scholar]
- 22.Redinbaugh M G, Turley T B. Adaptation of the bicinchoninic acid protein assay for use with microtiter plates and sucrose gradient fractions. Anal Biochem. 1986;153:267–271. doi: 10.1016/0003-2697(86)90091-6. [DOI] [PubMed] [Google Scholar]
- 23.Roche L, Dowd A J, Tort J, McGonigle S, McSweeney A, Curley G P, Ryan T, Dalton J P. Functional expression of Fasciola hepatica cathepsin L1 in Saccharomyces cerevisiae. Eur J Biochem. 1997;245:373–380. doi: 10.1111/j.1432-1033.1997.t01-1-00373.x. [DOI] [PubMed] [Google Scholar]
- 24.Ross J G. Studies of immunity to Fasciola hepatica: acquired immunity in cattle, sheep and rabbits following natural infections and vaccine procedures. J Helminthol. 1967;41:393–399. doi: 10.1017/s0022149x0002191x. [DOI] [PubMed] [Google Scholar]
- 25.Rushton B. Ovine fasciolasis following reinfection. Res Vet Sci. 1977;22:133–134. [PubMed] [Google Scholar]
- 26.Sandeman R M, Howell M J, Campbell N. An attempt to vaccinate sheep against Fasciola hepatica using a juvenile fluke antigen sheep antibody complex. Res Vet Sci. 1980;29:255–259. [PubMed] [Google Scholar]
- 27.Sandeman R M, Howell M J. Response of sheep to challenge infection with Fasciola hepatica. Res Vet Sci. 1981;30:294–297. [PubMed] [Google Scholar]
- 28.Sexton J L, Milner A R, Panaccio M, Waddington J, Wijffels G, Chandler D, Thompson C, Wilson L, Spithill T W, Mitchell G F, Campbell N. Glutathione S-transferase. Novel vaccine against Fasciola hepatica infection in sheep. J Immunol. 1990;145:3905–3910. [PubMed] [Google Scholar]
- 29.Sinclair K B. Observations on the clinical pathology of ovine fasciolasis. Br Vet J. 1962;118:37–53. [Google Scholar]
- 30.Sinclair K B. The pathogenicity of Fasciola hepatica in previously infected corticosteroid-treated lambs. Res Vet Sci. 1970;11:205–216. [PubMed] [Google Scholar]
- 31.Sinclair K B. Acquired resistance to Fasciola hepatica in sheep. Br Vet J. 1971;127:125–136. doi: 10.1016/s0007-1935(17)37685-6. [DOI] [PubMed] [Google Scholar]
- 32.Sinclair K B. The resistance of sheep to Fasciola hepatica: studies on the development and pathogenicity of challenge infections. Br Vet J. 1973;129:236–250. doi: 10.1016/s0007-1935(17)36487-4. [DOI] [PubMed] [Google Scholar]
- 33.Smith A M, Carmona C, Dowd A J, McGonigle S, Acosta D, Dalton J P. Neutralization of the activity of a Fasciola hepatica cathepsin L proteinase by anti-cathepsin L antibodies. Parasite Immunol. 1994;16:325–328. doi: 10.1111/j.1365-3024.1994.tb00356.x. [DOI] [PubMed] [Google Scholar]
- 34.Smith A M, Dowd A J, Heffernan M, Robertson C D, Dalton J P. Fasciola hepatica. A secreted cathepsin L-like proteinase cleaves host immunoglobulin. Int J Parasitol. 1993;23:977–983. doi: 10.1016/0020-7519(93)90117-h. [DOI] [PubMed] [Google Scholar]
- 35.Smith A M, Dowd A J, McGonigle S, Keegan P S, Brenna G, Trudgett A, Dalton J P. Purification of cathepsin L-like proteinase secreted by adult Fasciola hepatica. Mol Biochem Parasitol. 1993;62:1–8. doi: 10.1016/0166-6851(93)90171-s. [DOI] [PubMed] [Google Scholar]
- 36.Smith S K, Smith W D. Immunization of sheep with an integral membrane glycoprotein complex of Haemonchus contortus and with its major polypeptide components. Res Vet Sci. 1996;60:1–6. doi: 10.1016/s0034-5288(96)90121-6. [DOI] [PubMed] [Google Scholar]
- 37.Spithill T W, Dalton J P. Progress in development of liver fluke vaccines. Parasitol Today. 1988;14:220–224. doi: 10.1016/s0169-4758(98)01245-9. [DOI] [PubMed] [Google Scholar]
- 38.Tort, J., P. J. Brindley, D. Knox, K. Wolfe, and J. P. Dalton. Proteinases of helminth parasites. Adv. Parasitol., in press. [DOI] [PubMed]
- 39.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wijffels G L, Salvatore L, Dosen M, Waddington J, Wilson L, Thompson C, Campbell N, Sexton J, Vicker J, Bowen F, Friedel T, Spithill T W. Vaccination of sheep with purified cysteine proteinases of Fasciola hepatica decreases worm fecundity. Exp Parasitol. 1994;78:132–148. doi: 10.1006/expr.1994.1014. [DOI] [PubMed] [Google Scholar]