Abstract
Neurodegenerative diseases exert an overwhelming socioeconomic burden all around the globe. They are mainly characterized by modified protein accumulation that might trigger various biological responses, including oxidative stress, inflammation, regulation of signaling pathways, and excitotoxicity. These disorders have been widely studied during the last decade in the hopes of developing symptom-oriented therapeutics. However, no definitive cure has yet been discovered. Tea is one of the world’s most popular beverages. The same plant, Camellia Sinensis (L.).O. Kuntze, is used to make green, black, and oolong teas. Green tea has been most thoroughly studied because of its anti-cancer, anti-obesity, antidiabetic, anti-inflammatory, and neuroprotective properties. The beneficial effect of consumption of tea on neurodegenerative disorders has been reported in several human interventional and observational studies. The polyphenolic compounds found in green tea, known as catechins, have been demonstrated to have many therapeutic effects. They can help in preventing and, somehow, treating neurodegenerative diseases. Catechins show anti-inflammatory as well as antioxidant effects via blocking cytokines’ excessive production and inflammatory pathways, as well as chelating metal ions and free radical scavenging. They may inhibit tau protein phosphorylation, amyloid beta aggregation, and release of apoptotic proteins. They can also lower alpha-synuclein levels and boost dopamine levels. All these factors have the potential to affect neurodegenerative disorders. This review will examine catechins’ neuroprotective effects by highlighting their biological, pharmacological, antioxidant, and metal chelation abilities, with a focus on their ability to activate diverse cellular pathways in the brain. This review also points out the mechanisms of catechins in various neurodegenerative and cognitive diseases, including Alzheimer’s, Parkinson’s, multiple sclerosis, and cognitive deficit.
Keywords: catechins, neuroprotective, neurodegenerative diseases, epigallocatechin gallate, cognitive defect
1. Introduction
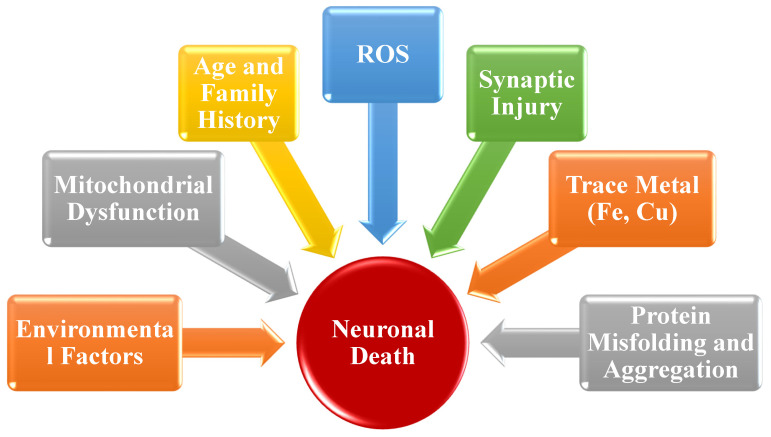
Neurodegenerative diseases (NDs) are one of the most prevalent health problems, affecting millions of people all around the world [1]. Because of the aging population, longer life, and changing environments, the worldwide burden of NDs is increasing. According to the World Health Organization (WHO), NDs account for 12% of total world mortality, 16.8% of deaths in developing countries, and 13% in developed countries. It is estimated that, by 2030, neurological disorders, such as Alzheimer’s disease (AD), Parkinson’s disease (PD), and other dementias, will constitute 38% of worldwide disability as calculated by years of life lost owing to disability [2]. According to the WHO, the global cost of dementia was estimated to be USD 818 billion in 2015, which was approximately 1.1% of the global gross domestic product (GDP) [3]. The NDs, including AD, PD, multiple sclerosis (MS), Huntington’s disease (HD), and amyotrophic lateral sclerosis (ALS), are caused by diverse etiological causative factors, such as genetic, molecular, and environmental factors. Inflammation, excessive reactive oxygen species (ROS), epigenetic instability, and aging are the primary determinants of neurodegenerative diseases. The molecular factors involved in NDs are (1) particular protein dynamics coupled to degradation and aggregation of the impaired protein, (2) formation of free radicals and oxidative stress, (3) mitochondrial dysfunction and deficient bioenergetics, and (4) exposure to pesticides and metal toxicity [4], as shown in Figure 1.
Figure 1.
Pathophysiological aspects that can cause neurodegeneration.
Neuronal deterioration accelerates with age and is particularly severe in old age, as observed with the numerous neuropathologies. To date, most neurodegenerative diseases have remained incurable. As previously stated, the current ongoing treatment just relieves symptoms but does not hinder the disease progression, focusing the need for more effective treatment strategies [5]. Development of new drugs for NDs is hampered by the lack of understanding of the biological perspective of these multifactorial disorders, the blood–brain barrier that prevents drug flow to the brain, and the scarcity of clinically relevant animal models for new drug testing [6]. Although NDs are pathologically characterized by disease-specific misfolded protein aggregation and modified cellular stress response, researchers have focused exclusively on reduction in misfolded protein load; however, the outcomes were disappointing [7,8]. Due to the late onset and asymptomatic nature of most neurodegenerative diseases, treatment begins at later disease stages and has restricted benefits to the patients. If interventions are initiated early, they might be able to largely prevent or slow the disease progression. These therapies via lowering or even removing the primary stressor may be able to restore neuronal function [9].
Flavonoids are primary molecules for establishment of a new class of clinically effective therapeutic agents for neurodegenerative disorders. Flavonoids have been linked to a decreased risk of neurodegenerative disorders when consumed regularly [10]. Besides their antioxidant properties, these polyphenolic compounds have neuroprotective characteristics due to their ability to interact with cellular signaling pathways, followed by translation and transcription, which influence cellular function in both physiological and pathological conditions [8,9]. The most popular beverage used throughout the world, tea, can be divided into six categories based on the degree of fermentation, such as non-fermented form (green tea), white tea (micro-fermented), yellow tea (slightly fermented), oolong tea (semi-fermented), black tea (fully fermented), and dark tea (post-fermented) [11,12,13]. Tea includes a wide range of bioactive compounds, including flavonoids, caffeine, polyphenols, free amino acids, theanine, methylxanthine, several lipids, volatile molecules, as well as mineral substances [14,15,16,17]. Green tea usually contains a higher content of polyphenols than black tea due to polyphenol biodegradation during processing of dark tea, which has beneficial effects on human health [15].
Catechins, also known as flavan-3-ols, constitute about 70–80% of tea polyphenols and are in abundance in the young tea plant’s buds and leaves [18,19]. Meanwhile, green tea has stronger antioxidant activity in vitro as compared to dark tea. Dark tea, on the other hand, frequently exhibits greater in vivo antioxidant activity as compared to green tea due to the bioavailability of polyphenol biodegradation products [12,20]. Numerous studies have demonstrated that tea has a variety of health benefits, including memory and cognitive improvements, cardiovascular disease prevention, anti-cancerous, anti-obesity, and sedative [21,22,23,24,25]. The effect of tea on neuron function has generated great attention over the last few decades, and several studies have proved its neuroprotective properties. For instance, tea may help in reducing the morbidity and severity of Alzheimer’s and Parkinson’s disease, as well as the risk of developing depression symptoms via reducing inflammation and oxidative stress, regulating intracellular signaling pathways, metal chelation, and modulating the hypothalamus–pituitary–adrenal (HPA) axis and monoamine neurotransmitter levels [26,27,28]. Epigallocatechin gallate (EGCG) was able to attenuate any deficiencies in nest building and Barnes maze performance [29]. Moreover, EGCG has been shown to ameliorate central memory deficit in water mazes and novel object recognition tests [30]. EGCG also protects the majority of motor deficits induced by rotenone in grip strength measurement, Rota rod, beam-crossing task, and open-field test [31]. EGCG-loaded nanoparticles show promising application potential due to their degradability and biocompatibility [32]. It was evident that EGCG-loaded nanoparticles ameliorate neurobehavioral deficits in novel object recognition, open-field, and Morris water maze test [33].
2. Catechins: Biosynthesis and Mechanism of Action
2.1. Synthesis and Structure of Catechins
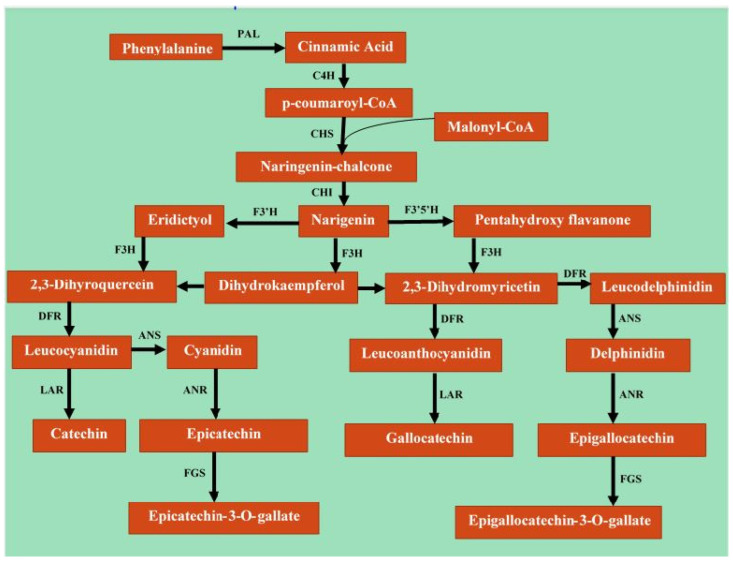
Catechins are formed of a three-carbon core coupled to two phenolic nuclei (aromatic rings) with many hydroxyl groups [34,35]. The catechins in tea are categorized into two groups known as epistructured catechins and non-epistructured catechins [36]. Epistructured catechins are the major tea catechins. Epigallocatechin gallate (EGCG) is the most abundant catechin in green tea, followed by epigallocatechin (EGC), epicatechin gallate (ECG), and epicatechin (EC). Non-epistructured catechins include gallocatechin (GC), gallocatechin gallate (GCG), catechins (C), and catechins gallate (CG) and are, on the other hand, only found in trace amounts in tea [37]. Catechins are produced in Camellia sinensis plant’s leaves via shikimic–cinnamic acid and acetic–malonic metabolic pathways (Figure 2). The shikimic acid pathways form chalcone and gallic acid, which ultimately synthesize various catechins [38]. The content of particular catechins in a fresh leaf of tea varies depending on the region of cultivation, nutrition of the plant, variety, kind of leaves (young or coarse), and time of the year. Frequently, the catechins profile in the extract of green tea leaf is comprised of EGCG 10–15%, EGC 6–10%, ECG 2–3%, and epicatechins 2% [39].
Figure 2.
Biosynthesis of catechins in Camellia sinensis plant’s leaves via shikimic–cinnamic acid and acetic–malonic metabolic pathways. PAL, phenylalanine ammonia-lyase; C4H, cinnamate 4-hydroxylase; CHS, chalcone synthase; CHI, chalcone isomerase; F3H, flavanone 3-hydroxylase; F3′H, flavonoid 3′-hydroxylase; F3′5′H, flavonoid 3′,5′-hydroxylase; DFR, dihydroflavonol 4-Reductase; LAR, leucocyanidin reductase; ANR, anthocyanidin reductase; ANS, anthocyanidin synthase; FGS, flavan-3-ol gallate synthase.
2.2. Physical and Chemical Properties of Catechins
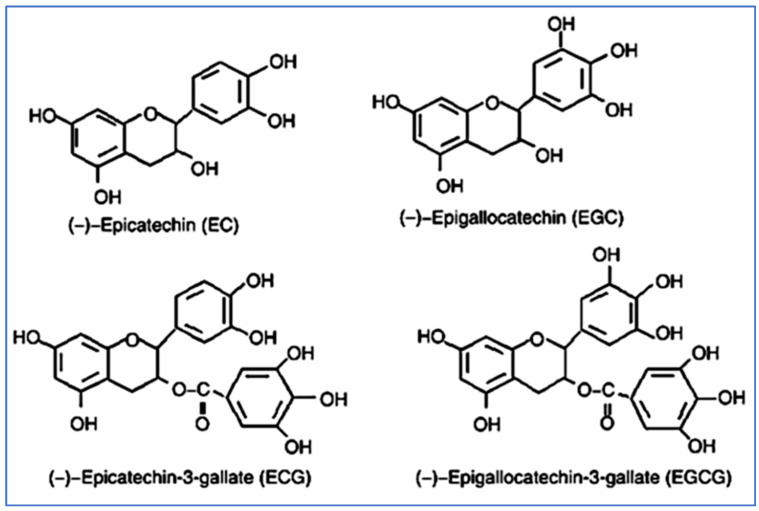
Catechins are colorless, but they have bitter and astringent flavors [40]. A variety of catechins have distinct flavors, such as ECG and EGCG (Figure 3), which are bitter and astringent in taste, while EC and EGC have a bitter but sweet aftertaste [41,42]. Catechins can combine with proteins and caffeine to form precipitates, making the solution cloudy, known as cream formation [43]. Moreover, catechins precipitate when interacting with enzymes, including lipoxygenase, pepsin, amylase, lipase, and trypsin, thereby inhibiting their activity. Catechins with ester bonds, such as ECG and EGCG, have a higher ability to produce precipitates with enzyme interaction as compared to catechins containing non-ester bonds.
Figure 3.
Different types of catechins.
Isolation of catechins for their use in food can be achieved by precipitating catechins with protein or caffeine. Catechins have a strong iron-binging ability as the galloyl group in their structure binds to iron in food, preventing its absorption in the body [44]. Tea catechins are highly reactive and unstable in the presence of heat, oxidizing enzymes, and alkaline conditions. The oxidized catechins produce thearubegins and theaflavins in the presence of peroxidase (POD) and polyphenol oxidase (PPO) enzymes [45]. At 40 °C and a pH of 5.5, POD and PPO activity are optimal [46]. As a result, adjusting the temperature and pH can slow the activity of both these enzymes [47]. Temperature adjustment requires caution because epistructured catechins could epimerize to non-epistructured catechins at a high temperature above 95 °C. Furthermore, even though catechins are extremely stable in acidic solution (pH 4), their stability decreases with the rise in pH from 4 to 8, and catechins become highly unstable above pH 8 in alkaline solution. These qualities can be used to stabilize the catechins when added to foods. Due to their strong antioxidative properties because of hydroxyl groups in their structure, the catechins can scavenge reactive oxygen species (ROS), including superoxide radicals, nitric acid, hydroxyl radicals, peroxynitrite, singlet oxygen, and nitrogen dioxide, all contributing important roles in carcinogenesis [48]. The scavenging ability of hydroxyl radical (HO) declines in order of ECG, EC, EGCG, and EGC [49]. Furthermore, catechins have the ability to capture peroxyl radicals, hindering free radical chain reactions and halting lipid peroxidation [50]. The efficiency of four primary catechins in reducing lipid peroxidation decreases in the following order: EGCG, ECG, EGC, and EC [49]. This characteristic is crucial while adding catechins to foods, particularly those rich in oil or fat.
2.3. Bioavailability of Catechin
Depending on adsorption, distribution, metabolism, and excretion processes (ADME), the disposition of EGCG is at its peak at 90 min and is nearly undetectable after 24 h of its oral intake [51]. After oral administration, the EGCG reaches the stomach. The acidic environment of the stomach favors its structural stability [52]. Then, the portion of EGCG becomes absorbed in the small intestines. A minute EGCG concentration seems to be observed in peripheral blood because of the large fraction of EGCG in small intestines transmitted to large intestines, which causes the transformation of this molecule via enterocytes, forming approximately eleven catechin ring fission products. These ringed fission products are present conjugated as well as free form in plasma. The EGCG forms could cross the blood–brain barrier and reach brain parenchyma, promoting neuritogenesis and ultimately reducing neurodegeneration [53]. The remaining EGCG is metabolized via liver cells and converted into sulfated, glucuronide, and methylated intermediates, which are excreted in urine [54]. Several factors are involved in maintenance of polyphenols’ stability [51,55]. The alkaline environment and high temperature affect structural stability, enhancing chemical EGCG degradation [56]. Many studies hypothesized that, in humans, the oral bioavailability of EGCG is low and decreases along with food [57]. Some studies have reported selectivity of EGCG with different vitamins and minerals, suggesting that vitamins and fish oil reduce EGCG oxidation, while minerals, such as chrome and selenium, improve bioavailability and antioxidant activities [58,59,60]. Andreu-Fernandez et al. [61] suggested that the EGCG concentration in plasma is highest after Teavigo intake following overnight fasting, while the EGCG, when taken along with food supplements, improves the stability inside the body.
2.4. Catechins and Neurodegeneration
As low-molecular-weight (MW) secondary metabolites, flavonoids are present in vascular plants and are grouped into flavonols, flavones, flavan-3-ol, flavanones, anthocyanins, and isoflavones. The three-ring structure of all phenolic compounds with a 15-carbon skeleton includes two six-carbon benzene rings connected by a heterocyclic ring of pyran or pyrone. Further, 2-Phenyl-3,4-dihydro-2H-chromen-3-ol serves as the backbone of flavan-3-ols, the catechins. The catechins groups include epicatechins, catechins, epicatechins gallate (ECG), epigallocatechin gallate (EGCG), and gallocatechin (GC) [62]. About 60% of catechins in green tea are made up of EGCG, comprising a galloyl group, a tetrahydropyran moiety, a pyrogallol ring, and a benzenediol ring [63]. The chemical structure of the molecule will determine its biological function. The interface of the molecule in the biological matrix is influenced by the quantity of hydroxyl groups and their position [64]. It is demonstrated that EGCG has a greater antioxidative effect than EC or EGC due to its higher hydroxyl content [65]. Moreover, EGCG possesses two structures, 4-keto,3-hydroxyl or 4-keto, 5-hydroxyl moiety, and ortho-3′,4′-dihydroxy moiety, enabling chelation and neutralization of the metal ions [66]. Therefore, the use of catechins, especially EGCG, may be effective in treatment of certain neurodegenerative diseases.
2.4.1. Anti-Inflammatory and Antioxidant Activity
Catechins’ most well-known biological actions are their free radical scavenging and antioxidant characteristics. The cellular components, such as protein, nucleic acids, DNA, and lipids, due to excessive ROS production and accumulation of defective mitochondria in brain cells, have been linked to neurodegenerative diseases, such as AD [67]. Catechins diminish lipid peroxidation [68]. As free scavengers, the phenolic hydroxyl groups have antioxidant characteristics, preventing new free radical production and regulation of protein synthesis associated with redox balance, such as maintenance of superoxide dismutase, catalase, and Nicotinamide dioxide phosphate (NADPH) [69]. By phosphorylating the p38 MAPK kinase and ERK 1/2 signaling pathways, the catechins increase the nuclear factor erythroid 2-related factor-2 (NRF2) activity [70]. Epigallocatechin gallate boosts the Nrf2 levels that are decreased in the hippocampus of patients suffering from Alzheimer’s disease and enhances expression of HO-1, which modulates cellular response towards oxidative stress, thereby protecting against cellular death [71,72]. Catechins modulate neuro-inflammation, which is characterized by microglial activation, through immunomodulatory characteristics. They also have a significant impact on neurodegenerative disease progression, including AD, HD, PD, and amyotrophic lateral sclerosis [73]. When compared with controls, co-cultures of the main catechins in green tea and lipopolysaccharide (LPS) significantly decreased the levels of interleukin-6 (IL-6) and tumor necrosis factor (TNF-α) in human neutrophils. Catechins increase levels of Nrf2 mRNA and anti-oxidative activities in activated B cells and decrease the expression of nuclear factor kappa light chain enhancer of activated B cell (NFB) protein and toll-like receptor 4 (TLR4) [74]. In in vitro LPS-induced neuro-inflammatory models, EGCG decreases nitric oxide (NO) and TNF-α production and reverses motor impairment [75]. Four months of EGCG consumption in APP/PS1 transgenic mice mitigated amyloid (A) plaques in the hippocampus and prevented activation of microglia. Along with higher levels of anti-inflammatory cytokines IL-10 and IL-13, lower levels of IL-1 were also observed [76]. These results suggested that catechins protect neurons via diminishing inflammatory mediators production through NFB, Nrf2, and TLR4/NFB pathways, as well as altering microglial activation. Interestingly, due to the iron-chelating characteristics of catechins, the iron deposition in neurons and microglia is inhibited, which might be due to ferroprotein 1 (FPN1) and divalent metal transporter 1 (DMT1) [77]. People who have neurodegenerative diseases have toxic aggregates of AB due to iron, which accumulate in their brains [78]. As a result, EGCG may affect AB levels by destabilising AB plaques, preventing aggregation of hyperphosphorylated tau or inhibiting translation of the amyloid precursor protein (APP) due to a decrease in labile fe2+ [79].
2.4.2. Autophagic and Neuritogenic Activity
The neuritogenic activity of EGCG and its metabolites was observed in human neuroblastoma SH-SY5Y cells [80]. In PCl2 (TrkB) cells, a very minute concentration of EGCG (0.5M) and unfractionated green tea polyphenols enhance the neurotrophic potential of brain-derived neurotrophic factor (BDNF) [81,82], whereas the in vivo findings are yet to be confirmed. Autophagy is linked to aging, which protects neural cells by removing protein aggregates and degrading obsolete cell structures [83,84]. Protein aggregates play a significant role in neurodegenerative diseases. Catechin via different mechanisms, including transcription factor EB (TFEB), mTOR (molecular target of rapamycin), and 5′ AMP-activated protein kinase (AMPK), may affect autophagy [85,86]. By suppressing the expression of the DNA methyltransferase 2 (DNMT2) gene, Khali et al. [87] have reported upregulated expression of autophagal genes (Atg5 and LC3) in response to EGCG, both in in vivo and in vitro studies. Interestingly, continuous EGCG treatment reduces neuronal apoptosis in the hippocampal CA1 region, thereby reducing the learning and memory deficit in rats subjected to chronic unexpected mild stress. The decrease in apoptotic cells, soluble and insoluble A1, and restoration of autophagic flux in the hippocampus CA1 region of the stressed rats may be the cause of this improvement [88]. Catechins also increase beclin-1, which regulates autophagy and endocytosis. Catechins also have neuroprotective effects via protein kinase C signaling through reducing Bax, caspase-3, Bad, and poly(ADP-ribose) polymerase (PARP) [89,90,91].
2.4.3. Dual-Specific Tyrosine Phosphorylation Regulated Kinase 1a Inhibition
EGCG has demonstrated an antagonist of a serine/threonine kinase known as dual-specificity tyrosine phosphorylation-regulated kinase (DYRK1A). DYRK1A is found on 21q22.2 loci of chromosome 21. DYRK1A is considered a substantial contributor to cognitive dysfunction because of its role in neuron differentiation, neurogenesis, synaptic plasticity, and cell death [92]. In mice overexpressing DYRK1A, EGCG-enriched supplements neutralize plasma and neuronal markers (NFkB and BDNF). Moreover, EGCG is safer for heart and liver function and also crosses the blood–brain barrier (BBB) [93].
2.5. The Role of Catechins in Various Neurodegenerative Disorders
Over the years, research on the molecular mechanism of catechins indicates their noteworthy potential in prevention and management of neurodegenerative diseases. The neuroprotective effect of catechins in various neurodegenerative diseases has been listed in Table 1.
Table 1.
Neuroprotective effect of catechins in various neurodegenerative diseases.
| Objective | Experimental Model | Disease | Outcome | References |
|---|---|---|---|---|
| EGCG | In vitro | Alzheimer’s | Inhibition of Tau aggregation and oxidation | [94] |
| EGCG | In vitro | Huntington disease | Inhibitory effect on htt aggregation | [95] |
| CAT | Rat model | Parkinson disease | Improve rotational behavior, locomotion, and memory | [96,97] |
| EGCG | Rat model | Alzheimer’s | Decrease oxidative stress and improve cholinergic synaptic and mitochondrial functions | [98] |
| EGCG | Mice model | Multiple sclerosis | Decrease onset of disease and clinical severity. Reduce inflammatory infiltrates. Increase Olig 1 expression | [99,100] |
| Green tea | Drosophila model | Huntington disease | Green tea consumption may modulate symptoms | [101] |
| EGCG | Human studies | Multiple Sclerosis | Improve muscle metabolism, counteract NOX overactivation, decrease plasma NAA levels | [102,103] |
| EGCG | Human studies | Down syndrome | Improve visual recognition memory | [104] |
| EGCG | Human studies | Alzheimer’s | Low prevalence of cognitive impairment, decrease oxidative stress, and lipid peroxidation | [105,106] |
2.5.1. Alzheimer’s Disease (AD)
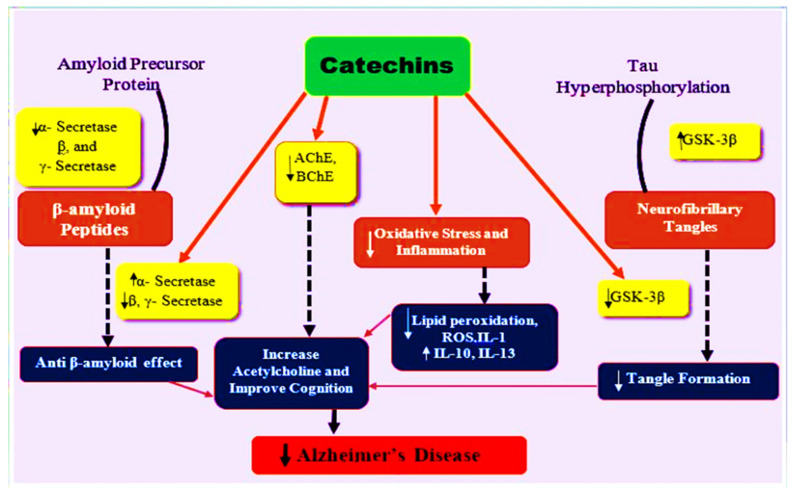
Numerous in vivo, in vitro, and in silico studies have been conducted on the molecular mechanism of catechins [91,107,108,109]. Antioxidant properties of catechins may help protect against neurodegeneration. Increased oxidative stress is linked to late-onset neurodegenerative diseases, as previously stated [110,111]. Peroxidized lipids, proteins, and oxidized DNA have all been found to be higher in Alzheimer’s disease patients [112]. Green tea catechins (0.5 percent green tea catechins in water) were given to rats for 26 weeks and were found to prevent amyloid-induced cognitive impairment. Both hippocampal and plasma levels of lipid peroxide and ROS were decreased by 20% as compared to controls [113]. EGCG was also shown to have a similar effect by Biasibetti et al. [114]. The researchers evaluated the effect of EGCG in a streptozotocin-induced dementia rat model. Cognitive deficits determined via the Morris water maze were reversed after one-month oral administration of EGCG (10 mg/kg/day). Moreover, the ROS and NO levels were dramatically reduced [114]. These antioxidative boosts may be due to the radical scavenging activity and iron-chelating abilities of catechins [115,116,117]. Catechins can chelate metal ions, such as copper and iron, by blocking the Fenton reaction [118] as these metal ions have been found to accumulate in people suffering from Alzheimer’s’ disease [119]. These outcomes demonstrate that catechins can attenuate the oxidative stress in the brain and peripheral tissues as well as prevent cognitive-deficit-related behavioral changes. Pro-inflammatory components, such as cytokines and cytotoxic compounds, are secreted from injured and damaged neurons, which could lead to cell death [120]. Lee et al. [121] demonstrated that EGCG pretreatment (1.5/3 mg/kg for 3 weeks) inhibits LPS-induced cognitive deficit and quashed cytokines and inflammatory proteins. Another in vivo study reported that EGCG reduced responses associated with LPS-induced inflammation in BV-2 microglia [122]. In Alzheimer’s’ disease, catechins affect the PKC-related pathways that are involved in cell survival and production of soluble non-toxic amyloid β [123,124]. Levtes et al. [125] found that low EGCG concentration (1–5 µM) induces production of sAPP from PC12 cells and human neuroblastoma, and 2 weeks of oral EGCG administration (2 mg/kg/day) enhances PKC α and ε in the hippocampus of mice as compared to controlled mice. Kaur et al. [126] and Kim et al. [127] investigated AchE inhibition with tea polyphenols. Kaur et al. [126] conducted research on aged Wistar rats fed green tea extract (0.5%) for 8 weeks and have shown significantly better learning and memory performance (measured through a passive avoidance test). Cerebrum’s AchE activity seemed reduced in older rats as compared to young rats. Kim et al. [127] reported the reversal of scopolamine-induced amnesia by (0.2% tea polyphenol) diet-based treatment. Tea polyphenols decrease AchE activity in addition to behavioral changes. Besides in vitro and in vivo studies, many in silico studies have also been carried out on tea polyphenol and acetylcholine esterase enzymes [109,128]. Hyperphosphorylation of tau protein and excessive neuronal β-amyloid deposition, inflammation, and oxidative stress are considered to be key contributors to development of AD. Green tea catechins possess a neuroprotective effect against Alzheimer’s disease by increasing anti-oxidant activity and inhibiting inflammation, oxidative stress, and hampering the action of beta-amyloid aggregation and anticholinesterase and other mechanisms, as shown in Figure 4.
Figure 4.
The neuroprotective mechanism of catechins in Alzheimer’s disease. A, β, and γ-secretase produce β-amyloid from amyloid precursor proteins, while GSK-3β catalyzes tau hyperphosphorylation that leads to neurofibrillary tangles, which ultimately results in oxidative stress and inflammation. The catechins inhibit β, γ-secretase, and GSK-3β activity, thereby preventing amyloid plaques and neurofibrillary tangles formation, as well as possessing anti-inflammatory and anti-oxidative properties and increasing acetylcholine levels in the synaptic cleft, thereby improving the decline in cognitive function in AD.
2.5.2. Parkinson’s Disease
Parkinson’s disease is a slowly progressive neurological condition of the motor system that typically affects people above the age of 50. However, people under 50 can also be affected. A-synuclein is a protein containing 140 amino acids, discovered in the brain, mostly expressed in the pre-synaptic cleft of nerve cells, involved in neuronal differentiation, regulation of dopamine synthesis, and suppression of neuronal apoptosis. In normal physiological conditions, α-synuclein cannot form a fibrillary structure due to the balance between its monomeric and oligomeric form. Furthermore, ubiquitin-proteasome machinery and lysosomal autophagic pathways remove the excess α-synuclein formed [129]. However, if α-synuclein levels are increased, there occurs impaired mitochondrial function, and its disruption with the membrane increases the predisposition of this non-toxic structure to aggregate and cause disruption in the normal neuronal mechanism, leading to neuron death [130,131,132]. Preventing aggregation formation is thus a vital step in preventing PD pathogenesis. The green tea polyphenol epigallocatechin-3-gallate (EGCG) aids in decreasing α-synuclein aggregation and toxicity in in vitro PCl2 cells. It also becomes attached to natively unfolded synuclein polypeptide chains to prevent formation of synuclein by forming an unstructured oligomer. It also impedes monomeric synuclein in addition to fibrillary intermediates [133].
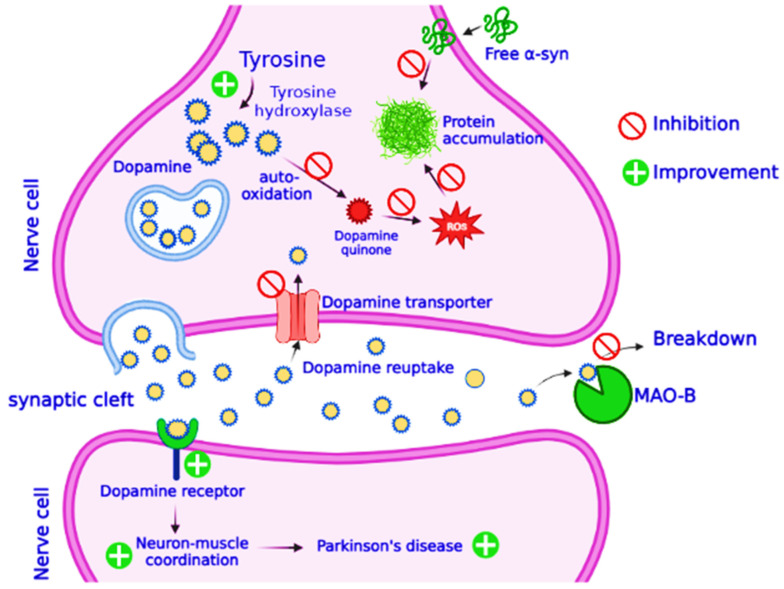
PD affects dopaminergic neurons in the Substantia Nigra pars compacta (SNpc) region of the brain [134]. According to an estimate, 80% of dopaminergic neurons die during PD, reducing the levels of dopamine in the brain [135]. Dopamine deficiency produces aberrant nerve firing and motor control loss [136]. Neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydopyridine (MPTP) causes dopaminergic neuronal death in substantia nigra and is widely used in discovering the molecular mechanism involved in pathogenesis of PD [137]. In cynomolgus monkeys given MPTP, catechins-rich polyphenol extract alleviated motor deficit, recovered tyrosine hydroxylase and dopamine levels, and reduced α-synuclein oligomers and their aggregation [138]. Pre-treatments with green tea extracts and EGCG reduced the loss of dopamine in male C57/BL mice by significantly altering striatal antioxidants, such as superoxide dismutase (SOD) and catalase. They also inhibit TH reduction, the enzyme that catalyzes L-DOPA (L-dihydrophenylalanine) synthesis from tyrosine in the dopamine biosynthetic pathway [139]. In MPTP C57/BL mice, pretreatment of EGCG decreases α-synuclein expression and hinders neuronal death by increasing Bcl-2 and lowering Bax expression. It also provided neuroprotection by boosting striatal protein kinase C-α (PKC-α) [140]. In human NB SH-SY5Y cells, EGCG increases the expression of PKC, resulting in Bad degradation [141]. Activated PKC promotes neuronal survival by stimulating ERK and JNK [142]. EGCG also prevents neurodegeneration in CHO cells expressing dopamine transporters (DAT) by inhibiting MPP+ absorption and transfer to presynaptic dopaminergic neurons [143]. COMT inhibitors block conversion of L-DOPA to 3-O-methyl dopa and are used in junctions to treat PD. EGCG has been demonstrated to inhibit COMT, both in vivo and in vitro, preventing further methylation of L-DOPA, suggested to be given in combination with other medication to improve their availability and efficiency in the brain (Figure 5) [144]. In addition to upregulating dopamine conversion, EGCG inhibits MAO-B in aged rat brains, indicating its multi-potential function in the prevention of PD [145]. Iron accumulation in the substantia nigra is regarded to be a pathogenic feature of PD. When the BBB is disrupted and iron transport and storage mechanisms fail, the iron gathers in brain, which causes oxidative stress, aggregation of α-synuclein, neuro-inflammation, and ultimately cell death [146]. Iron chelation therapy is being studied as a therapeutic approach for treatment of PD. Green tea polyphenols’ potential as iron chelators, as well as other neuro-rescue characteristics, may aid to alleviate Parkinson’s disease (Figure 5).
Figure 5.
Role of green tea catechins in prevention of Parkinson’s disease. The protective mechanism of catechins has been elaborated. Parkinson’s disease is characterized by dopamine deficiency, and all treatment measures are aimed at introduction of dopamine as a medicine and its preservation. The figure illustrates conversion of tyrosine to dopamine by the action of an enzyme tyrosine hydroxylase; newly synthesized dopamine is packed into vesicles and released into the synaptic cleft, where it binds with the dopamine-specific receptors and contributes its role in signaling between two nerve cells. Normally, dopamine is reabsorbed to the nerve cells and reused, or it is broken down by the action of an enzyme monoamine oxidase B (MAO-B). Presence of green tea catechins inhibits MAO-B activity and promotes dopamine reabsorption. Catechins also inhibit plaque formation by the proteins and autoxidation of dopamine.
2.5.3. Huntington’s Disease (HD)
Huntington’s disease is initiated by repeated enlargement of unstable polyglutamine (poly Q) within the first exon of the IT-15 gene, producing Huntington (htt) protein [147]. The presence of htt fibril aggregates causes progressive damage to striatal and cortical neurons and also produces neuronal inclusions with aggregated htt protein [148]. Chorea, marked by fidgety movements, is a common symptom of HD. Tetrabenazine (TBZ) has been proven beneficial for chorea treatment as it reduces dopamine levels by suppressing the vesicular monoamine transporter 2 (VMAT-2) in the central nervous system. Few antipsychotic medications have also been used for such purposes in the past [149]. Choline esterase inhibitors (Rivastigmine) have been demonstrated to improve cognitive functions in progressive HD-related dementia patients; however, more placebo-controlled trials are required [150]. In the last few years, most research has focused on compounds preventing mutant htt aggregation. EGC and other green compounds are powerful inhibitors of htt1 aggregation. In vitro HD models were used to demonstrate that green tea polyphenols can regulate the early phase of polyQ growth, therefore impeding development of amyloid fibril processes. The potential benefits of EGCG and its derivatives against HD have been reported in many animal studies [151]. EGCG inhibits polyQ aggregation and protects neuronal cells expressing mutant htt protein [152]. Alterations in the lipid content of cellular and subcellular membranes also cause amyloid protein accumulation. It also had a better affinity for phospholipids than non-mutated HTT, causing changes in phospholipid double-layer stability. Beasley et al. [95] investigated the effect of lipid vesicles on EGCG or curcumin to modulate htt, lipid linkage, and aggregated htt. Because EGCG inhibits amyloid formation in the presence of lipid vesicles regardless of how it interacts with the membrane environment, it is suggested that EGCG could be used to treat HD and other disorders where protein aggregation is altered and coupled to amyloid deposition. A clinical trial examining the effect of the maximum routine dose of EGCG (1200 mg) on cognitive functioning of HD patients indicates intriguing preliminary findings; however, more human clinical trials are required to determine EGCG effects as a therapeutic candidate for HD treatment.
2.5.4. Multiple Sclerosis (MS)
Multiple sclerosis is a chronic neurodegenerative auto-immune disease characterized by local lymphocytic infiltration, causing inflammation and demyelination of neurons as well as astroglial proliferation with neuronal injury [153]. It can produce a wide range of neurologic symptoms, including sensory impairments, movement issues, and impaired vision. The most prevalent clinical form of MS is RRMS (relapsing–remitting multiple sclerosis), distinguished by relapse with clinical manifestation along with partial or complete recovery [154]. Yet, there is no definite cure for MS. However, many disease-modifying treatments (DMT) for reducing relapses and disease progression have been reported [155]. Many studies have studied DMT and EGCG in the experimental autoimmune encephalomyelitis (EAE) model, specifically used for MS research. Inflammatory infiltrates and the onset of disease were significantly delayed [99]. Other research examined EGCG’s effects in cuprizone-induced MS animals. EGCG enhanced oligodendrocyte transcription factor 1 and remyelination-related proteolipid protein in the cerebral cortex [100,156]. In both animal models, EGCG therapy improved the mice’s clinical symptoms or molecular pathways. Few studies have investigated catechins’ influence on human populations, chiefly on RRMS. Bellman-Strobl et al. [157] conducted an analysis of EGCG + GA in 122 RRMS patients. When compared to the placebo, the 800 md/day oral EGCG + GA dose for an 18-month duration revealed no significant improved in MRI and clinical activities. A double-blind clinical experiment was conducted to investigate the antioxidant therapy mechanism in MS patients and showed that RRMS patients had lower NOX levels in CD11b+monocytes when given GA + 600 mg EGCG. The daily dose of 600 mg of EGCG in RRMS patients for 12 months has been reported to increase muscle metabolism during exercise [158]. Lovera et al. [103] conducted a study to assess the efficacy of polyphenon E (green tea extract containing 50% EGCG) in patients suffering from MS. They discovered that 800 mg EGCG enhances levels of N-acetyl aspartate in the brain, indicating a neuroprotective effect. However, due to abnormal liver function tests, the dose had to be stopped in five out of seven patients, concluding that polyphenon E may have an increased risk of hepatotoxicity. Other researchers assessed the EGCG treatment safety and determined that it was a safe drug, with comparable side effects in placebo as well as treatment groups [157,159]. In addition to EGCG, polyphenon E contains other polyphenols containing low quantities of caffeine that are metabolized in the liver. Overall, catechins’ effectivity in MS patients is still unclear.
2.5.5. Amyotrophic Lateral Sclerosis and Frontotemporal Dementia
Amyotrophic lateral sclerosis (ALS) is a less common neurodegenerative disease, characterized by progressive loss of upper and lower motor neurons, which leads to weakness in limbs and swallowing and speech difficulties [160]. The progression of the disease ultimately results in paralysis and death from respiratory failure [161]. Frontotemporal dementia (FTD) is also associated with changes in the temporal and frontal lobes that manifest changes in personality, language, behavior, and motor skills [162,163]. EGCG has been reported to have a protective role in ALS in protecting motor neuron cells from mitochondrial damage and oxidative stress [164]. Oral EGCG supplements significantly slow the onset of symptoms, improve motor function, and expand lifespan [165,166]. EGCG upregulates the PI3K/Akt signaling pathway, which mediates GSK-3 activity, increasing neurofibrillary tangles and neuronal death [164]. An increase in PI3K/Akt and a reduction in death signals, including caspase-3, cleaved PARP, and cytosolic cytochrome c, are observed in ALS animals, which supports these findings [165]. On microglia and astrocytes, EGCG also possesses anti-inflammatory and anti-oxidant properties [166]. Furthermore, despite its presumed chelating properties, it has little effect on iron metabolism [167]. In FTD, inhibition of tau filament formation was observed for ECG but not for EC [168].
2.5.6. Fetal Alcohol Spectrum Disorders (FASD)
Alcohol has many adverse effects on CNS, including neuro-immune dysregulation, neurotransmitter problems, epigenetic modification, as well as oxidative stress [169]. Chronic intake of alcohol leads to cognitive impairment, with loss of axons, atrophy of white matter, and brain cell demyelination [170,171]. The rat brain analysis revealed an association between alcohol consumed and degree of white matter atrophy [172]. FASD referred to the group of disabilities as the result of prenatal exposure to alcohol, marked by neurobehavioral dysfunction, dysmorphology of the face, and stunted growth [173,174]. To date, FASD and other alcohol-related changes have no treatment, and the traits remain throughout life; however, early intervention, including developmental therapy or behavioral interventions, may resist certain disorders’ and disabilities’ development and progression [175,176]. The potential of catechins to permeate various organs and their anti-oxidative effects bring them to the table for FASD treatment options [177]. In the murine model, 50 to 100 mg/kg EGCG treatment has been observed to increase glutathione and SOD levels, along with lipid peroxide and NO levels reduction in neonates [178]. Pregnant mice administered 400 mg/kg EGCG reported a decline in production of H2O2 and malondialdehyde (MDA) [179]. On the other hand, 30 mg/kg EGCG treatment for prenatal alcoholic exposure (PAE) resulted in N rf2 reduction [180]. As EGCG can cross the BBB, it has been shown that it can improve the brain development of a fetus impaired by ethanol [177]. In an in vitro rhombencephalic-neuron-cultured model from the rat fetus, EGCG prevents ethanol-induced neuronal death [181]. It also decreases the expression of NFB and caspase-3 in a rat model [178]. Microcephaly and PAE-related cognitive deficits may be prevented by reducing apoptosis. In addition to its antioxidant and anti-apoptotic properties, EGCG has been shown to reduce the ethanol-induced inflammatory response (decreased interleukin-1 and tumor necrosis factor-α levels) [148]. A study conducted on PAE mice showed that a daily 30 mg/kg EGCG dose increased expression of neuronal nuclei (NeuN) and doublecortin (DCX), which could help in preventing the loss of mature neurons and the delays in maturation due to ethanol. EGCG upregulates BDNF and glial fibrillary acidic protein (GFAP), thus compensating for the early astrocyte differentiation and neural plasticity abnormalities caused due to maternal alcohol consumption [180]. The tea catechins, including EGCG, EC, and catechins, have been observed to inhibit DNMT1-mediated DNA methylation in in vitro models [182]. EGCG effectivity against FASD DNA methylation was not found in the literature, so it would be informative to observe the effect of catechins on the DNA methylation pattern of fetal cells. Finally, the aforementioned disruptions in embryonic neurodevelopment result in behavioral and cognitive abnormalities, including impaired learning and memory having been observed to be ameliorated via EGCG treatment [104]. It is interesting to note that DS and FASD have many similar traits, including craniofacial dysmorphology, cognitive and behavioral disorders, and growth deficiencies. DYRK1A inhibition has been shown to aid neuronal plasticity in patients suffering from Down syndrome, suggesting that this molecular pathway should be investigated in FASD disease.
2.5.7. Down Syndrome (DS)
Down syndrome is a genetic disease related to intellectual disabilities, caused by chromosomal 21 third copy, characterized by typical facial traits, including epicanthus, flattened face, up-slanted eyes, and hypotonia. The cognitive abnormalities in Down syndrome range in severity owing to the imbalance between huge synaptic suppression in the hippocampus and excessive cerebral cortex activation [183]. DS may cause a lack of flexibility in reaction to the environment, resulting in failure to adapt to a new situation. The major outcomes are changes in synaptic structure, which affects the information processing and storage capacity of brain networks. The neuropsychological traits include prominent hippocampal-dependent deficits impacting spatial memory, and reduced cognitive abilities [184]. EGCG, because of its pro-cognitive effects, has been recommended for Down syndrome [185]. EGCG also suppresses metalloproteinase 9 (MMP9), which is dysregulated in DS [186]. DYRK1A is over-expressed in Down syndrome and is considered to be the major source of cognitive dysfunction in DS patients. Researchers found that different doses of EGCG are associated with inhibition of DYRK1A in the brain. Thus, DS treatment can target the active DYRK1A concentration to improve behavioral issues via the GABAergic and glutamatergic pathways [187,188]. Environmentally enriched EGCG treatment in Ts65Dn mice has shown to improve memory and learning. A 42 mg/kg/day EGCG dose has also shown to retrieve deregulation of phosphoprotein in the hippocampus, reverse the kinome deregulation process, and restore the epigenetic profile. Because of all these potential pathways, green tea derivatives may enhance cognition [189,190,191,192]. EGCG therapy restores mitochondrial bioenergetics and biogenesis, which were significantly reduced in Down syndrome. It enhances proliferation of neuronal progenitor cells and neuronal plasticity and inhibits ROS production [193,194]. It is possible that neonatal EGCG therapy is more effective in reducing DS impairments. However, long-term EGCG effects on hippocampus physiology are still not proven [195].
3. Conclusions
To date, few therapeutic strategies for treating neurodegenerative diseases are available, so it is critical to discover novel preventive and therapeutic neuroprotective agents. Catechins appeared to have neuroprotective effects, as evident from both in vivo and in vitro studies against Alzheimer’s, Parkinson’s, multiple sclerosis, Huntington’s disease, and other neurodegenerative diseases. Catechins, especially EGCG, the most commonly used catechins, possess antioxidant, anti-inflammatory, anti-apoptotic and neuritogenic, and metal chelation characteristics, along with the ability to trigger diverse molecular mechanisms, such as lipid peroxidation inhibition, induce metal-chelating effects on amyloid-B, and α-synuclein fibrillation, inhibiting amyloid-β fibrillation. They produce anti-inflammatory effects by regulating the levels of inflammatory markers, including TNF-α, NF-kB, IL-6, IL-1 NFB, and NO, increasing antioxidants, such as GSH, SOD, and CAT, and decreasing lipid peroxidation by increasing Nrf2 protein expression. Moreover, catechins have the ability to cross the blood–brain barrier, which makes them feasible neuroprotective candidates against neurodegenerative diseases. However, more research, particularly clinical trials in humans, is required to understand catechins’ involvement in various biochemical pathways associated with neurological diseases. Future studies should examine regulation of neurological mechanisms and targeted brain regions where neuronal failure occurs, as well as bioavailability and optimal dosage for the best outcomes with no side effects and toxicity aspects regarding catechins consumption. Cognitive training along with pharmacological therapies would open up a world of possibilities for reversing the neuronal degeneration observed in such disorders.
Acknowledgments
The authors are thankful to Umm Al-Qura University, Makkah, Saudi Arabia for supporting this project (Project number 224UQU4310387DSR37).
Author Contributions
Conceptualization, O.A. and M.H.D.; methodology, A.S.A.A.; software, R.R.; validation, R.R. and S.I.A.; formal analysis, W.H.A.; investigation, B.N.M.; resources, S.I.; data curation, S.I.; writing—original draft preparation, I.K.; writing—review and editing, M.S.N.; visualization, S.N.; supervision, M.S.N.; project administration, R.R.; funding acquisition, O.A.; All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
The Project was funded by Deanship of Scientific Research at Umm Al-Qura University, and this work was supported by Grant Code (Project Code: 22 UQU4310387DSR37).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gonçalves P.B., Sodero A.C.R., Cordeiro Y. Green Tea Epigallocatechin-3-gallate (EGCG) Targeting Protein Misfolding in Drug Discovery for Neurodegenerative Diseases. Biomolecules. 2021;11:767. doi: 10.3390/biom11050767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feigin V.L., Vos T., Nichols E., Owolabi M.O., Carroll W.M., Dichgans M., Deuschl G., Parmar P., Brainin M., Murray C. The global burden of neurological disorders: Translating evidence into policy. Lancet Neurol. 2020;19:255–265. doi: 10.1016/S1474-4422(19)30411-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feigin V.L., Nichols E., Alam T., Bannick M.S., Beghi E., Blake N., Culpepper W.J., Dorsey E.R., Elbaz A., Ellenbogen R.G., et al. Global, regional, and national burden of neurological disorders, 1990-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:459–480. doi: 10.1016/S1474-4422(18)30499-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sheikh S., Haque E., Mir S.S. Neurodegenerative diseases: Multifactorial conformational diseases and their therapeutic interventions. J. Neurodegener. Dis. 2013;2013:563481. doi: 10.1155/2013/563481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gribkoff V.K., Kaczmarek L.K. The need for new approaches in CNS drug discovery: Why drugs have failed, and what can be done to improve outcomes. Neuropharmacology. 2017;120:11–19. doi: 10.1016/j.neuropharm.2016.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Danon J.J., Reekie T.A., Kassiou M.J.T.i.C. Challenges and opportunities in central nervous system drug discovery. Trends Chem. 2019;1:612–624. doi: 10.1016/j.trechm.2019.04.009. [DOI] [Google Scholar]
- 7.Mallucci G.R., Klenerman D., Rubinsztein D.C.J.A.R.o.C., Biology D. Developing therapies for neurodegenerative disorders: Insights from protein aggregation and cellular stress responses. Annu. Rev. Cell Dev. Biol. 2020;36:165–189. doi: 10.1146/annurev-cellbio-040320-120625. [DOI] [PubMed] [Google Scholar]
- 8.Cummings J., Lee G., Ritter A., Sabbagh M., Zhong K., Research D.T., Interventions C. Alzheimer’s disease drug development pipeline: 2020. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2020;6:e12050. doi: 10.1002/trc2.12050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Solanki I., Parihar P., Mansuri M.L., Parihar M.S.J.A.i.n. Flavonoid-based therapies in the early management of neurodegenerative diseases. Adv. Nutr. Int. Rev. J. 2015;6:64–72. doi: 10.3945/an.114.007500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cummings J., Aisen P.S., DuBois B., Frölich L., Jack C.R., Jones R.W., Morris J.C., Raskin J., Dowsett S.A., Scheltens P.J.A.s.r., et al. Drug development in Alzheimer’s disease: The path to 2025. Alzheimers Res. Ther. 2016;8:39. doi: 10.1186/s13195-016-0207-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bokuchava M.A., Skobeleva N.I., Sanderson G.W. Nutrition. The biochemistry and technology of tea manufacture. Crit. Rev. Food Sci. Nutr. 1980;12:303–370. doi: 10.1080/10408398009527280. [DOI] [PubMed] [Google Scholar]
- 12.Zhao C.-N., Tang G.-Y., Cao S.-Y., Xu X.-Y., Gan R.-Y., Liu Q., Mao Q.-Q., Shang A., Li H.-B. Phenolic profiles and antioxidant activities of 30 tea infusions from green, black, oolong, white, yellow and dark teas. Antioxidants. 2019;8:215. doi: 10.3390/antiox8070215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang G.-Y., Meng X., Gan R.-Y., Zhao C.-N., Liu Q., Feng Y.-B., Li S., Wei X.-L., Atanasov A.G., Corke H., et al. Health functions and related molecular mechanisms of tea components: An update review. Int. J. Mol. Sci. 2019;20:6196. doi: 10.3390/ijms20246196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Venkata S.P.C., Indra P. The aroma, taste, color and bioactive constituents of tea. J. Med. Plants Res. 2011;5:2110–2124. [Google Scholar]
- 15.Samanta S. Potential bioactive components and health promotional benefits of tea (Camellia sinensis) J. Am. Nutr. Assoc. 2022;41:65–93. doi: 10.1080/07315724.2020.1827082. [DOI] [PubMed] [Google Scholar]
- 16.Khan N., Mukhtar H. Tea and health: Studies in humans. Curr. Pharm. Des. 2013;19:6141–6147. doi: 10.2174/1381612811319340008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang G.-Y., Zhao C.-N., Xu X.-Y., Gan R.-Y., Cao S.-Y., Liu Q., Shang A., Mao Q.-Q., Li H.-B.J.A. Phytochemical composition and antioxidant capacity of 30 Chinese teas. Antioxidants. 2019;8:180. doi: 10.3390/antiox8060180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Graham H.N. Green tea composition, consumption, and polyphenol chemistry. Prev. Med. 1992;21:334–350. doi: 10.1016/0091-7435(92)90041-F. [DOI] [PubMed] [Google Scholar]
- 19.Liu M., Tian H.-l., Wu J.-H., Cang R.-R., Wang R.-X., Qi X.-H., Xu Q., Chen X.-H.J.H.r. Relationship between gene expression and the accumulation of catechin during spring and autumn in tea plants (Camellia sinensis L.) Hortic. Res. 2015;2:15011. doi: 10.1038/hortres.2015.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cao S.-Y., Li B.-Y., Gan R.-Y., Mao Q.-Q., Wang Y.-F., Shang A., Meng J.-M., Xu X.-Y., Wei X.-L., Li H.-B.J.F. The in vivo antioxidant and hepatoprotective actions of selected Chinese teas. Foods. 2020;9:262. doi: 10.3390/foods9030262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yokogoshi H. Health Benefits of Green Tea: An Evidence-Based Approach. Nova Science Publishers, Inc. Hauppauge; New York, NY, USA: 2017. 22 Green Tea in the Protection against Neurodegeneration; p. 185. [Google Scholar]
- 22.Suzuki T., Miyoshi N., Hayakawa S., Imai S., Isemura M., Nakamura Y. Beverage Impacts on Health and Nutrition. Springer; Berlin/Heidelberg, Germany: 2016. Health benefits of tea consumption; pp. 49–67. [Google Scholar]
- 23.Miyoshi N., Pervin M., Suzuki T., Unno K., Isemura M., Nakamura Y.J.B.T. Therapy. Green tea catechins for well-being and therapy: Prospects and opportunities. Bot. Targets Ther. 2015;5:85–96. [Google Scholar]
- 24.Cao S.-Y., Zhao C.-N., Gan R.-Y., Xu X.-Y., Wei X.-L., Corke H., Atanasov A.G., Li H.-B. Effects and mechanisms of tea and its bioactive compounds for the prevention and treatment of cardiovascular diseases: An updated review. Antioxidants. 2019;8:166. doi: 10.3390/antiox8060166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu X.-Y., Zhao C.-N., Cao S.-Y., Tang G.-Y., Gan R.-Y., Li H.-B. Effects and mechanisms of tea for the prevention and management of cancers: An updated review. Crit. Rev. Food Sci. Nutr. 2020;60:1693–1705. doi: 10.1080/10408398.2019.1588223. [DOI] [PubMed] [Google Scholar]
- 26.Dong X., Yang C., Cao S., Gan Y., Sun H., Gong Y., Yang H., Yin X., Lu Z. Tea consumption and the risk of depression: A meta-analysis of observational studies. Aust. N. Z. J. Psychiatry. 2015;49:334–345. doi: 10.1177/0004867414567759. [DOI] [PubMed] [Google Scholar]
- 27.Zhu W.-L., Shi H.-S., Wei Y.-M., Wang S.-J., Sun C.-Y., Ding Z.-B., Lu L. Green tea polyphenols produce antidepressant-like effects in adult mice. Pharmacol. Res. 2012;65:74–80. doi: 10.1016/j.phrs.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 28.Chen S.-Q., Wang Z.-S., Ma Y.-X., Zhang W., Lu J.-L., Liang Y.-R., Zheng X.-Q. Neuroprotective effects and mechanisms of tea bioactive components in neurodegenerative diseases. Molecules. 2018;23:512. doi: 10.3390/molecules23030512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walker J.M., Klakotskaia D., Ajit D., Weisman G.A., Wood W.G., Sun G.Y., Serfozo P., Simonyi A., Schachtman T.R. Beneficial effects of dietary EGCG and voluntary exercise on behavior in an Alzheimer’s disease mouse model. J. Alzheimer’s Dis. 2015;44:561–572. doi: 10.3233/JAD-140981. [DOI] [PubMed] [Google Scholar]
- 30.Ettcheto M., Cano A., Manzine P.R., Busquets O., Verdaguer E., Castro-Torres R.D., García M.L., Beas-Zarate C., Olloquequi J., Auladell C. Epigallocatechin-3-Gallate (EGCG) improves cognitive deficits aggravated by an obesogenic diet through modulation of unfolded protein response in APPswe/PS1dE9 mice. Mol. Neurobiol. 2020;57:1814–1827. doi: 10.1007/s12035-019-01849-6. [DOI] [PubMed] [Google Scholar]
- 31.Tseng H.-C., Wang M.-H., Chang K.-C., Soung H.-S., Fang C.-H., Lin Y.-W., Li K.-Y., Yang C.-C., Tsai C.-C. Protective effect of (−) epigallocatechin-3-gallate on rotenone-induced parkinsonism-like symptoms in rats. Neurotox. Res. 2020;37:669–682. doi: 10.1007/s12640-019-00143-6. [DOI] [PubMed] [Google Scholar]
- 32.Liu Z., Li X., Wu X., Zhu C. A dual-inhibitor system for the effective antifibrillation of Aβ40 peptides by biodegradable EGCG–Fe (iii)/PVP nanoparticles. J. Mater. Chem. B. 2019;7:1292–1299. doi: 10.1039/C8TB03266A. [DOI] [PubMed] [Google Scholar]
- 33.Singh N.A., Bhardwaj V., Ravi C., Ramesh N., Mandal A.K.A., Khan Z.A. EGCG nanoparticles attenuate aluminum chloride induced neurobehavioral deficits, beta amyloid and tau pathology in a rat model of Alzheimer’s disease. Front. Aging Neurosci. 2018;10:244. doi: 10.3389/fnagi.2018.00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hara Y. Green Tea: Health Benefits and Applications. CRC press; Boca Raton, FL, USA: 2001. [Google Scholar]
- 35.Ninomiya M. Chemical and physicochemical properties of green tea polyphenols. In: Yamamoto T., Juneja L.R., Chu D.C., Kim M., editors. Chemistry and Applications of Green Tea. ORC; New York, NY, USA: 1997. pp. 23–35. [Google Scholar]
- 36.Masukawa Y., Matsui Y., Shimizu N., Kondou N., Endou H., Kuzukawa M., Hase T. Determination of green tea catechins in human plasma using liquid chromatography–electrospray ionization mass spectrometry. J. Chromatogr. B. 2006;834:26–34. doi: 10.1016/j.jchromb.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 37.Chu K.O., Pang C.C. Pharmacokinetics and Adverse Effects of Drugs: Mechanisms and Risks Factors. Vol. 17 IntechOpen; London, UK: 2018. Pharmacokinetics and disposition of green tea catechins. [Google Scholar]
- 38.Vuong Q.V., Golding J.B., Nguyen M., Roach P.D. Extraction and isolation of catechins from tea. J. Sep. Sci. 2010;33:3415–3428. doi: 10.1002/jssc.201000438. [DOI] [PubMed] [Google Scholar]
- 39.Shi J., Xue S.J., Kakuda Y. Green tea induced thermogenesis controlling body weight. In: Ho C.T., Lin J.-K., Shahidi F., editors. Tea and Tea Products: Chemistry and Health-Promoting Properties. CRC Press; Boca Raton, FL, USA: 2009. pp. 221–232. [Google Scholar]
- 40.Balentine D.A., Harbowy M.E., Graham H.N. Caffeine. CRC Press; Boca Raton, FL, USA: 2019. Tea: The Plant and its Manufacture; Chemistry and Consumpatin of the Beverage; pp. 35–72. [Google Scholar]
- 41.Vuong Q.V., Stathopoulos C.E., Nguyen M.H., Golding J.B., Roach P.D. Isolation of green tea catechins and their utilization in the food industry. Food Rev. Int. 2011;27:227–247. doi: 10.1080/87559129.2011.563397. [DOI] [Google Scholar]
- 42.Khanongnuch C., Unban K., Kanpiengjai A., Saenjum C. Recent research advances and ethno-botanical history of miang, a traditional fermented tea (Camellia sinensis var. assamica) of northern Thailand. J. Ethn. Foods. 2017;4:135–144. doi: 10.1016/j.jef.2017.08.006. [DOI] [Google Scholar]
- 43.Penders M.H., Jones D.P., Needham D., Pelan E.G. Mechanistic study of equilibrium and kinetic behaviour of tea cream formation. Food Hydrocoll. 1998;12:9–15. doi: 10.1016/S0268-005X(98)00040-X. [DOI] [Google Scholar]
- 44.Zijp I.M., Korver O., Tijburg L.B. Effect of tea and other dietary factors on iron absorption. Crit. Rev. Food Sci. Nutr. 2000;40:371–398. doi: 10.1080/10408690091189194. [DOI] [PubMed] [Google Scholar]
- 45.Zhao T., Li C., Wang S., Song X. Green tea (Camellia sinensis): A review of its phytochemistry, pharmacology, and toxicology. Molecules. 2022;27:3909. doi: 10.3390/molecules27123909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Subramanian N., Venkatesh P., Ganguli S., Sinkar V.P. Role of polyphenol oxidase and peroxidase in the generation of black tea theaflavins. J. Agric. Food Chem. 1999;47:2571–2578. doi: 10.1021/jf981042y. [DOI] [PubMed] [Google Scholar]
- 47.Gulati A., Rawat R., Singh B., Ravindranath S.D. Application of microwave energy in the manufacture of enhanced-quality green tea. J. Agric. Food Chem. 2003;51:4764–4768. doi: 10.1021/jf026227q. [DOI] [PubMed] [Google Scholar]
- 48.Yeasmen N., Orsat V. Maximization of the recovery of phenolic compounds from sugar maple leaves. Biomass Convers. Biorefinery. 2022:1–16. doi: 10.1007/s13399-022-02904-4. [DOI] [Google Scholar]
- 49.Guo Q., Zhao B., Li M., Shen S., Xin W. Studies on protective mechanisms of four components of green tea polyphenols against lipid peroxidation in synaptosomes. Biochim. Biophys. Acta. 1996;1304:210–222. doi: 10.1016/S0005-2760(96)00122-1. [DOI] [PubMed] [Google Scholar]
- 50.Terao J., Piskula M., Yao Q. Protective effect of epicatechin, epicatechin gallate, and quercetin on lipid peroxidation in phospholipid bilayers. Arch. Biochem. Biophys. 1994;308:278–284. doi: 10.1006/abbi.1994.1039. [DOI] [PubMed] [Google Scholar]
- 51.Lee M.-J., Maliakal P., Chen L., Meng X., Bondoc F.Y., Prabhu S., Lambert G., Mohr S., Yang C.S. Pharmacokinetics of tea catechins after ingestion of green tea and (−)-epigallocatechin-3-gallate by humans: Formation of different metabolites and individual variability. Cancer Epidemiol. Biomark. Prev. 2002;11:1025–1032. [PubMed] [Google Scholar]
- 52.Zeng L., Ma M., Li C., Luo L. Stability of tea polyphenols solution with different pH at different temperatures. Int. J. Food Prop. 2017;20:1–18. doi: 10.1080/10942912.2014.983605. [DOI] [Google Scholar]
- 53.Pervin M., Unno K., Takagaki A., Isemura M., Nakamura Y. Function of Green Tea Catechins in the Brain: Epigallocatechin Gallate and its Metabolites. Int. J. Mol. Sci. 2019;20:3630. doi: 10.3390/ijms20153630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lu H., Meng X., Yang C.S. Enzymology of methylation of tea catechins and inhibition of catechol-O-methyltransferase by (-)-epigallocatechin gallate. Drug Metab. Dispos. Biol. Fate Chem. 2003;31:572–579. doi: 10.1124/dmd.31.5.572. [DOI] [PubMed] [Google Scholar]
- 55.Scholl C., Lepper A., Lehr T., Hanke N., Schneider K.L., Brockmöller J., Seufferlein T., Stingl J.C. Population nutrikinetics of green tea extract. PLoS ONE. 2018;13:e0193074. doi: 10.1371/journal.pone.0193074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li N., Taylor L.S., Mauer L.J. Degradation kinetics of catechins in green tea powder: Effects of temperature and relative humidity. J. Agric. Food Chem. 2011;59:6082–6090. doi: 10.1021/jf200203n. [DOI] [PubMed] [Google Scholar]
- 57.Naumovski N., Blades B.L., Roach P.D. Food Inhibits the Oral Bioavailability of the Major Green Tea Antioxidant Epigallocatechin Gallate in Humans. Antioxidants. 2015;4:373–393. doi: 10.3390/antiox4020373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shirai N., Suzuki H. Effects of simultaneous intakes of fish oil and green tea extracts on plasma, glucose, insulin, C-peptide, and adiponectin and on liver lipid concentrations in mice fed low- and high-fat diets. Ann. Nutr. Metab. 2008;52:241–249. doi: 10.1159/000140516. [DOI] [PubMed] [Google Scholar]
- 59.Peters C.M., Green R.J., Janle E.M., Ferruzzi M.G. Formulation with ascorbic acid and sucrose modulates catechin bioavailability from green tea. Food Res. Int. 2010;43:95–102. doi: 10.1016/j.foodres.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Giunta B., Hou H., Zhu Y., Salemi J., Ruscin A., Shytle R.D., Tan J. Fish oil enhances anti-amyloidogenic properties of green tea EGCG in Tg2576 mice. Neurosci. Lett. 2010;471:134–138. doi: 10.1016/j.neulet.2010.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Andreu-Fernández V., Almeida Toledano L., Pizarro N., Navarro-Tapia E., Gómez-Roig M.D., de la Torre R., García-Algar Ó. Bioavailability of Epigallocatechin Gallate Administered With Different Nutritional Strategies in Healthy Volunteers. Antioxidants. 2020;9:440. doi: 10.3390/antiox9050440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fraga C.G., Croft K.D., Kennedy D.O., Tomás-Barberán F.A. The effects of polyphenols and other bioactives on human health. Food Funct. 2019;10:514–528. doi: 10.1039/C8FO01997E. [DOI] [PubMed] [Google Scholar]
- 63.Reygaert W.C. Green Tea Catechins: Their Use in Treating and Preventing Infectious Diseases. BioMed Res. Int. 2018;2018:9105261. doi: 10.1155/2018/9105261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Botten D., Fugallo G., Fraternali F., Molteni C. Structural Properties of Green Tea Catechins. J. Phys. Chem. B. 2015;119:12860–12867. doi: 10.1021/acs.jpcb.5b08737. [DOI] [PubMed] [Google Scholar]
- 65.Weinreb O., Mandel S., Amit T., Youdim M.B.H. Neurological mechanisms of green tea polyphenols in Alzheimer’s and Parkinson’s diseases. J. Nutr. Biochem. 2004;15:506–516. doi: 10.1016/j.jnutbio.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 66.Van Acker S.A.B.E., Van Den Berg D.-j., Tromp M.N.J.L., Griffioen D.H., Van Bennekom W.P., Van Der Vijgh W.J.F., Bast A. Structural aspects of antioxidant activity of flavonoids. Free Radic. Biol. Med. 1996;20:331–342. doi: 10.1016/0891-5849(95)02047-0. [DOI] [PubMed] [Google Scholar]
- 67.Fang E.F., Hou Y., Palikaras K., Adriaanse B.A., Kerr J.S., Yang B., Lautrup S., Hasan-Olive M.M., Caponio D., Dan X., et al. Mitophagy inhibits amyloid-β and tau pathology and reverses cognitive deficits in models of Alzheimer’s disease. Nat. Neurosci. 2019;22:401–412. doi: 10.1038/s41593-018-0332-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Andrade S., Loureiro J.A., Pereira M.C. Green tea extract-biomembrane interaction study: The role of its two major components, (−)-epigallocatechin gallate and (−)-epigallocatechin. Biochim. Biophys. Acta (BBA)—Biomembr. 2021;1863:183476. doi: 10.1016/j.bbamem.2020.183476. [DOI] [PubMed] [Google Scholar]
- 69.Bernatoniene J., Kopustinskiene D.M. The Role of Catechins in Cellular Responses to Oxidative Stress. Molecules. 2018;23:6626. doi: 10.3390/molecules23040965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yang G.-Z., Wang Z.-J., Bai F., Qin X.-J., Cao J., Lv J.-Y., Zhang M.-S. Epigallocatechin-3-Gallate Protects HUVECs from PM2.5-Induced Oxidative Stress Injury by Activating Critical Antioxidant Pathways. Molecules. 2015;20:6626–6639. doi: 10.3390/molecules20046626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ramsey C.P., Glass C.A., Montgomery M.B., Lindl K.A., Ritson G.P., Chia L.A., Hamilton R.L., Chu C.T., Jordan-Sciutto K.L. Expression of Nrf2 in Neurodegenerative Diseases. J. Neuropathol. Exp. Neurol. 2007;66:75–85. doi: 10.1097/nen.0b013e31802d6da9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Romeo L., Intrieri M., D’Agata V., Mangano N.G., Oriani G., Ontario M.L., Scapagnini G. The Major Green Tea Polyphenol, (-)-Epigallocatechin-3-Gallate, Induces Heme Oxygenase in Rat Neurons and Acts as an Effective Neuroprotective Agent against Oxidative Stress. J. Am. Coll. Nutr. 2009;28:492S–499S. doi: 10.1080/07315724.2009.10718116. [DOI] [PubMed] [Google Scholar]
- 73.Degan D., Ornello R., Tiseo C., Carolei A., Sacco S., Pistoia F. The Role of Inflammation in Neurological Disorders. Curr. Pharm. Des. 2018;24:1485–1501. doi: 10.2174/1381612824666180327170632. [DOI] [PubMed] [Google Scholar]
- 74.Marinovic M.P., Morandi A.C., Otton R. Green tea catechins alone or in combination alter functional parameters of human neutrophils via suppressing the activation of TLR-4/NFκB p65 signal pathway. Toxicol. Vitr. 2015;29:1766–1778. doi: 10.1016/j.tiv.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 75.Cheng C.-Y., Barro L., Tsai S.-T., Feng T.-W., Wu X.-Y., Chao C.-W., Yu R.-S., Chin T.-Y., Hsieh M.F. Epigallocatechin-3-Gallate-Loaded Liposomes Favor Anti-Inflammation of Microglia Cells and Promote Neuroprotection. Int. J. Mol. Sci. 2021;22:3037. doi: 10.3390/ijms22063037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bao J., Liu W., Zhou H.-Y., Gui Y.-R., Yang Y.-H., Wu M.-J., Xiao Y.-F., Shang J.-T., Long G.-F., Shu X.-J. Epigallocatechin-3-gallate Alleviates Cognitive Deficits in APP/PS1 Mice. Curr. Med. Sci. 2020;40:18–27. doi: 10.1007/s11596-020-2142-z. [DOI] [PubMed] [Google Scholar]
- 77.Urrutia P., Aguirre P., Esparza A., Tapia V., Mena N.P., Arredondo M., González-Billault C., Núñez M.T. Inflammation alters the expression of DMT1, FPN1 and hepcidin, and it causes iron accumulation in central nervous system cells. J. Neurochem. 2013;126:541–549. doi: 10.1111/jnc.12244. [DOI] [PubMed] [Google Scholar]
- 78.Sheelakumari R., Kesavadas C., Varghese T., Sreedharan R.M., Thomas B., Verghese J., Mathuranath P.S. Assessment of Iron Deposition in the Brain in Frontotemporal Dementia and Its Correlation with Behavioral Traits. Am. J. Neuroradiol. 2017;38:1953. doi: 10.3174/ajnr.A5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mandel S.A., Avramovich-Tirosh Y., Reznichenko L., Zheng H., Weinreb O., Amit T., Youdim M.B.H. Multifunctional Activities of Green Tea Catechins in Neuroprotection. Neurosignals. 2005;14:46–60. doi: 10.1159/000085385. [DOI] [PubMed] [Google Scholar]
- 80.Unno K., Pervin M., Nakagawa A., Iguchi K., Hara A., Takagaki A., Nanjo F., Minami A., Nakamura Y. Blood–Brain Barrier Permeability of Green Tea Catechin Metabolites and their Neuritogenic Activity in Human Neuroblastoma SH-SY5Y Cells. Mol. Nutr. Food Res. 2017;61:1700294. doi: 10.1002/mnfr.201700294. [DOI] [PubMed] [Google Scholar]
- 81.Gundimeda U., McNeill T.H., Fan T.K., Deng R., Rayudu D., Chen Z., Cadenas E., Gopalakrishna R. Green tea catechins potentiate the neuritogenic action of brain-derived neurotrophic factor: Role of 67-kDa laminin receptor and hydrogen peroxide. Biochem. Biophys. Res. Commun. 2014;445:218–224. doi: 10.1016/j.bbrc.2014.01.166. [DOI] [PubMed] [Google Scholar]
- 82.Andrade V., Cortés N., Pastor G., Gonzalez A., Ramos-Escobar N., Pastene E., Rojo L.E., Maccioni R.B. N-Acetyl Cysteine and Catechin-Derived Polyphenols: A Path Toward Multi-Target Compounds Against Alzheimer’s Disease. J. Alzheimer’s Dis. 2020;75:1219–1227. doi: 10.3233/JAD-200067. [DOI] [PubMed] [Google Scholar]
- 83.Wang Y., Li C., Zhang X., Kang X., Li Y., Zhang W., Chen Y., Liu Y., Wang W., Ge M., et al. Exposure to PM2.5 aggravates Parkinson’s disease via inhibition of autophagy and mitophagy pathway. Toxicology. 2021;456:152770. doi: 10.1016/j.tox.2021.152770. [DOI] [PubMed] [Google Scholar]
- 84.Sato S., Uchihara T., Fukuda T., Noda S., Kondo H., Saiki S., Komatsu M., Uchiyama Y., Tanaka K., Hattori N. Loss of autophagy in dopaminergic neurons causes Lewy pathology and motor dysfunction in aged mice. Sci. Rep. 2018;8:2813. doi: 10.1038/s41598-018-21325-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Prasanth M.I., Sivamaruthi B.S., Chaiyasut C., Tencomnao T. A Review of the Role of Green Tea (Camellia sinensis) in Antiphotoaging, Stress Resistance, Neuroprotection, and Autophagy. Nutrients. 2019;11:474. doi: 10.3390/nu11020474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Holczer M., Besze B., Zámbó V., Csala M., Bánhegyi G., Kapuy O. Epigallocatechin-3-Gallate (EGCG) Promotes Autophagy-Dependent Survival via Influencing the Balance of mTOR-AMPK Pathways upon Endoplasmic Reticulum Stress. Oxidative Med. Cell. Longev. 2018;2018:6721530. doi: 10.1155/2018/6721530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Khalil H., Tazi M., Caution K., Ahmed A., Kanneganti A., Assani K., Kopp B., Marsh C., Dakhlallah D., Amer A.O. Aging is associated with hypermethylation of autophagy genes in macrophages. Epigenetics. 2016;11:381–388. doi: 10.1080/15592294.2016.1144007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gu H.-F., Nie Y.-X., Tong Q.-Z., Tang Y.-L., Zeng Y., Jing K.-Q., Zheng X.-L., Liao D.-F. Epigallocatechin-3-Gallate Attenuates Impairment of Learning and Memory in Chronic Unpredictable Mild Stress-Treated Rats by Restoring Hippocampal Autophagic Flux. PLoS ONE. 2014;9:e112683. doi: 10.1371/journal.pone.0112683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Choi C., Song H.-D., Son Y., Cho Y.K., Ahn S.-Y., Jung Y.-S., Yoon Y.C., Kwon S.W., Lee Y.-H. Epigallocatechin-3-Gallate Reduces Visceral Adiposity Partly through the Regulation of Beclin1-Dependent Autophagy in White Adipose Tissues. Nutrients. 2020;12:3072. doi: 10.3390/nu12103072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Reznichenko L., Amit T., Youdim M.B.H., Mandel S. Green tea polyphenol (–)-epigallocatechin-3-gallate induces neurorescue of long-term serum-deprived PC12 cells and promotes neurite outgrowth. J. Neurochem. 2005;93:1157–1167. doi: 10.1111/j.1471-4159.2005.03085.x. [DOI] [PubMed] [Google Scholar]
- 91.Mandel S.A., Amit T., Weinreb O., Reznichenko L., Youdim M.B. Simultaneous manipulation of multiple brain targets by green tea catechins: A potential neuroprotective strategy for Alzheimer and Parkinson diseases. CNS Neurosci. Ther. 2008;14:352–365. doi: 10.1111/j.1755-5949.2008.00060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tejedor F.J., Hämmerle B. MNB/DYRK1A as a multiple regulator of neuronal development. FEBS J. 2011;278:223–235. doi: 10.1111/j.1742-4658.2010.07954.x. [DOI] [PubMed] [Google Scholar]
- 93.Gu Y., Moroy G., Paul J.-L., Rebillat A.-S., Dierssen M., de la Torre R., Cieuta-Walti C., Dairou J., Janel N. Molecular rescue of Dyrk1A overexpression alterations in mice with Fontup® dietary supplement: Role of green tea catechins. Int. J. Mol. Sci. 2020;21:1404. doi: 10.3390/ijms21041404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.George R.C., Lew J., Graves D.J. Interaction of cinnamaldehyde and epicatechin with tau: Implications of beneficial effects in modulating Alzheimer’s disease pathogenesis. J. Alzheimer’s Dis. JAD. 2013;36:21–40. doi: 10.3233/JAD-122113. [DOI] [PubMed] [Google Scholar]
- 95.Beasley M., Stonebraker A.R., Hasan I., Kapp K.L., Liang B.J., Agarwal G., Groover S., Sedighi F., Legleiter J. Lipid Membranes Influence the Ability of Small Molecules To Inhibit Huntingtin Fibrillization. Biochemistry. 2019;58:4361–4373. doi: 10.1021/acs.biochem.9b00739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Teixeira M.D., Souza C.M., Menezes A.P., Carmo M.R., Fonteles A.A., Gurgel J.P., Lima F.A., Viana G.S., Andrade G.M. Catechin attenuates behavioral neurotoxicity induced by 6-OHDA in rats. Pharmacol. Biochem. Behav. 2013;110:1–7. doi: 10.1016/j.pbb.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 97.Al-Amri J.S., Hagras M.M., Mohamed I.M. Effect of epigallocatechin-3-gallate on inflammatory mediators release in LPS-induced Parkinson’s disease in rats. Indian J. Exp. Biol. 2013;51:357–362. [PubMed] [Google Scholar]
- 98.Jelenković A., Jovanović M.D., Stevanović I., Petronijević N., Bokonjić D., Zivković J., Igić R. Influence of the green tea leaf extract on neurotoxicity of aluminium chloride in rats. Phytother. Res. PTR. 2014;28:82–87. doi: 10.1002/ptr.4962. [DOI] [PubMed] [Google Scholar]
- 99.Herges K., Millward J.M., Hentschel N., Infante-Duarte C., Aktas O., Zipp F. Neuroprotective effect of combination therapy of glatiramer acetate and epigallocatechin-3-gallate in neuroinflammation. PLoS ONE. 2011;6:e25456. doi: 10.1371/journal.pone.0025456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Semnani M., Mashayekhi F., Azarnia M., Salehi Z. Effects of green tea epigallocatechin-3-gallate on the proteolipid protein and oligodendrocyte transcription factor 1 messenger RNA gene expression in a mouse model of multiple sclerosis. Folia Neuropathol. 2017;55:199–205. doi: 10.5114/fn.2017.70484. [DOI] [PubMed] [Google Scholar]
- 101.Varga J., Dér N.P., Zsindely N., Bodai L. Green tea infusion alleviates neurodegeneration induced by mutant Huntingtin in Drosophila. Nutr. Neurosci. 2020;23:183–189. doi: 10.1080/1028415X.2018.1484021. [DOI] [PubMed] [Google Scholar]
- 102.Mähler A., Steiniger J., Bock M., Klug L., Parreidt N., Lorenz M., Zimmermann B.F., Krannich A., Paul F., Boschmann M. Metabolic response to epigallocatechin-3-gallate in relapsing-remitting multiple sclerosis: A randomized clinical trial. Am. J. Clin. Nutr. 2015;101:487–495. doi: 10.3945/ajcn.113.075309. [DOI] [PubMed] [Google Scholar]
- 103.Lovera J., Ramos A., Devier D., Garrison V., Kovner B., Reza T., Koop D., Rooney W., Foundas A., Bourdette D. Polyphenon E, non-futile at neuroprotection in multiple sclerosis but unpredictably hepatotoxic: Phase I single group and phase II randomized placebo-controlled studies. J. Neurol. Sci. 2015;358:46–52. doi: 10.1016/j.jns.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.de la Torre R., de Sola S., Hernandez G., Farré M., Pujol J., Rodriguez J., Espadaler J.M., Langohr K., Cuenca-Royo A., Principe A., et al. Safety and efficacy of cognitive training plus epigallocatechin-3-gallate in young adults with Down’s syndrome (TESDAD): A double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Neurol. 2016;15:801–810. doi: 10.1016/S1474-4422(16)30034-5. [DOI] [PubMed] [Google Scholar]
- 105.Ide K., Yamada H., Takuma N., Kawasaki Y., Harada S., Nakase J., Ukawa Y., Sagesaka Y.M. Effects of green tea consumption on cognitive dysfunction in an elderly population: A randomized placebo-controlled study. Nutr. J. 2016;15:49. doi: 10.1186/s12937-016-0168-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kuriyama S., Hozawa A., Ohmori K., Shimazu T., Matsui T., Ebihara S., Awata S., Nagatomi R., Arai H., Tsuji I. Green tea consumption and cognitive function: A cross-sectional study from the Tsurugaya Project. Am. J. Clin. Nutr. 2006;83:355–361. doi: 10.1093/ajcn/83.2.355. [DOI] [PubMed] [Google Scholar]
- 107.Hong M., Yu J., Wang X., Zhan S., Wu Z., Zhang X. Tea Polyphenols as Prospective Natural Attenuators of Brain Aging. Nutrients. 2022;14:3012. doi: 10.3390/nu14153012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ide K., Yamada H. Clinical benefits of green tea consumption for cognitive dysfunction. PharmaNutrition. 2015;3:136–145. doi: 10.1016/j.phanu.2015.07.001. [DOI] [Google Scholar]
- 109.Ali B., Jamal Q.M., Shams S., Al-Wabel N.A., Siddiqui M.U., Alzohairy M.A., Al Karaawi M.A., Kesari K.K., Mushtaq G., Kamal M.A. In Silico Analysis of Green Tea Polyphenols as Inhibitors of AChE and BChE Enzymes in Alzheimer’s Disease Treatment. CNS Neurol. Disord. Drug Targets. 2016;15:624–628. doi: 10.2174/1871527315666160321110607. [DOI] [PubMed] [Google Scholar]
- 110.Bennett S., Grant M.M., Aldred S. Oxidative stress in vascular dementia and Alzheimer’s disease: A common pathology. J. Alzheimer’s Dis. JAD. 2009;17:245–257. doi: 10.3233/JAD-2009-1041. [DOI] [PubMed] [Google Scholar]
- 111.Kim G.H., Kim J.E., Rhie S.J., Yoon S. The Role of Oxidative Stress in Neurodegenerative Diseases. Exp. Neurobiol. 2015;24:325–340. doi: 10.5607/en.2015.24.4.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Praticò D. Evidence of oxidative stress in Alzheimer’s disease brain and antioxidant therapy: Lights and shadows. Ann. New York Acad. Sci. 2008;1147:70–78. doi: 10.1196/annals.1427.010. [DOI] [PubMed] [Google Scholar]
- 113.Haque A.M., Hashimoto M., Katakura M., Hara Y., Shido O. Green tea catechins prevent cognitive deficits caused by Abeta1-40 in rats. J. Nutr. Biochem. 2008;19:619–626. doi: 10.1016/j.jnutbio.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 114.Biasibetti R., Tramontina A.C., Costa A.P., Dutra M.F., Quincozes-Santos A., Nardin P., Bernardi C.L., Wartchow K.M., Lunardi P.S., Gonçalves C.A. Green tea (-)epigallocatechin-3-gallate reverses oxidative stress and reduces acetylcholinesterase activity in a streptozotocin-induced model of dementia. Behav. Brain Res. 2013;236:186–193. doi: 10.1016/j.bbr.2012.08.039. [DOI] [PubMed] [Google Scholar]
- 115.Sang S., Tian S., Wang H., Stark R.E., Rosen R.T., Yang C.S., Ho C.-T. Chemical studies of the antioxidant mechanism of tea catechins: Radical reaction products of epicatechin with peroxyl radicals. Bioorg. Med. Chem. 2003;11:3371–3378. doi: 10.1016/S0968-0896(03)00367-5. [DOI] [PubMed] [Google Scholar]
- 116.Seeram N.P., Henning S.M., Niu Y., Lee R., Scheuller H.S., Heber D. Catechin and caffeine content of green tea dietary supplements and correlation with antioxidant capacity. J. Agric. Food Chem. 2006;54:1599–1603. doi: 10.1021/jf052857r. [DOI] [PubMed] [Google Scholar]
- 117.Mandel S., Youdim M.B. Catechin polyphenols: Neurodegeneration and neuroprotection in neurodegenerative diseases. Free Radic. Biol. Med. 2004;37:304–317. doi: 10.1016/j.freeradbiomed.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 118.Weinreb O., Amit T., Mandel S., Youdim M.B. Neuroprotective molecular mechanisms of (-)-epigallocatechin-3-gallate: A reflective outcome of its antioxidant, iron chelating and neuritogenic properties. Genes Nutr. 2009;4:283–296. doi: 10.1007/s12263-009-0143-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ward R.J., Zucca F.A., Duyn J.H., Crichton R.R., Zecca L. The role of iron in brain ageing and neurodegenerative disorders. Lancet Neurol. 2014;13:1045–1060. doi: 10.1016/S1474-4422(14)70117-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Morales I., Guzmán-Martínez L., Cerda-Troncoso C., Farías G.A., Maccioni R.B. Neuroinflammation in the pathogenesis of Alzheimer’s disease. A rational framework for the search of novel therapeutic approaches. Front. Cell. Neurosci. 2014;8:112. doi: 10.3389/fncel.2014.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lee Y.J., Choi D.Y., Yun Y.P., Han S.B., Oh K.W., Hong J.T. Epigallocatechin-3-gallate prevents systemic inflammation-induced memory deficiency and amyloidogenesis via its anti-neuroinflammatory properties. J. Nutr. Biochem. 2013;24:298–310. doi: 10.1016/j.jnutbio.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 122.Wu K.J., Hsieh M.T., Wu C.R., Wood W.G., Chen Y.F. Green Tea Extract Ameliorates Learning and Memory Deficits in Ischemic Rats via Its Active Component Polyphenol Epigallocatechin-3-gallate by Modulation of Oxidative Stress and Neuroinflammation. Evid.-Based Complement. Altern. Med. eCAM. 2012;2012:163106. doi: 10.1155/2012/163106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Berra E., Municio M.M., Sanz L., Frutos S., Diaz-Meco M.T., Moscat J. Positioning atypical protein kinase C isoforms in the UV-induced apoptotic signaling cascade. Mol. Cell. Biol. 1997;17:4346–4354. doi: 10.1128/MCB.17.8.4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Alkon D.L., Sun M.K., Nelson T.J. PKC signaling deficits: A mechanistic hypothesis for the origins of Alzheimer’s disease. Trends Pharmacol. Sci. 2007;28:51–60. doi: 10.1016/j.tips.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 125.Levites Y., Amit T., Mandel S., Youdim M.B. Neuroprotection and neurorescue against Abeta toxicity and PKC-dependent release of nonamyloidogenic soluble precursor protein by green tea polyphenol (-)-epigallocatechin-3-gallate. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2003;17:952–954. doi: 10.1096/fj.02-0881fje. [DOI] [PubMed] [Google Scholar]
- 126.Kaur T., Pathak C.M., Pandhi P., Khanduja K.L. Effects of green tea extract on learning, memory, behavior and acetylcholinesterase activity in young and old male rats. Brain Cogn. 2008;67:25–30. doi: 10.1016/j.bandc.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 127.Kim H.K., Kim M., Kim S., Kim M., Chung J.H. Effects of green tea polyphenol on cognitive and acetylcholinesterase activities. Biosci. Biotechnol. Biochem. 2004;68:1977–1979. doi: 10.1271/bbb.68.1977. [DOI] [PubMed] [Google Scholar]
- 128.Srividhya R., Gayathri R., Kalaiselvi P. Impact of epigallo catechin-3-gallate on acetylcholine-acetylcholine esterase cycle in aged rat brain. Neurochem. Int. 2012;60:517–522. doi: 10.1016/j.neuint.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 129.Ghiglieri V., Calabrese V., Calabresi P. Alpha-synuclein: From early synaptic dysfunction to neurodegeneration. Front. Neurol. 2018;9:295. doi: 10.3389/fneur.2018.00295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Burré J., Sharma M., Südhof T.C. Definition of a molecular pathway mediating α-synuclein neurotoxicity. J. Neurosci. 2015;35:5221–5232. doi: 10.1523/JNEUROSCI.4650-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Parker W.D., Jr., Boyson S.J., Parks J.K. Abnormalities of the electron transport chain in idiopathic Parkinson’s disease. Ann. Neurol. Off. J. Am. Neurol. Assoc. Child Neurol. Soc. 1989;26:719–723. doi: 10.1002/ana.410260606. [DOI] [PubMed] [Google Scholar]
- 132.Conway K.A., Harper J.D., Lansbury P.T. Accelerated in vitro fibril formation by a mutant α-synuclein linked to early-onset Parkinson disease. Nat. Med. 1998;4:1318–1320. doi: 10.1038/3311. [DOI] [PubMed] [Google Scholar]
- 133.Ehrnhoefer D.E., Bieschke J., Boeddrich A., Herbst M., Masino L., Lurz R., Engemann S., Pastore A., Wanker E. EGCG redirects amyloidogenic polypeptides into unstructured, off-pathway oligomers. Nat. Struct. Mol. Biol. 2008;15:558–566. doi: 10.1038/nsmb.1437. [DOI] [PubMed] [Google Scholar]
- 134.Ni A., Ernst C. Evidence That Substantia Nigra Pars Compacta Dopaminergic Neurons Are Selectively Vulnerable to Oxidative Stress Because They Are Highly Metabolically Active. Front. Cell. Neurosci. 2022;16:826193. doi: 10.3389/fncel.2022.826193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Barber T.R., Klein J.C., Mackay C.E., Hu M.T. Neuroimaging in pre-motor Parkinson’s disease. NeuroImage Clin. 2017;15:215–227. doi: 10.1016/j.nicl.2017.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Magrinelli F., Picelli A., Tocco P., Federico A., Roncari L., Smania N., Zanette G., Tamburin S. Pathophysiology of Motor Dysfunction in Parkinson’s Disease as the Rationale for Drug Treatment and Rehabilitation. Parkinson’s Dis. 2016;2016:9832839. doi: 10.1155/2016/9832839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Salari S., Bagheri M. In vivo, in vitro and pharmacologic models of Parkinson’s disease. Physiol. Res. 2019;68:17–24. doi: 10.33549/physiolres.933895. [DOI] [PubMed] [Google Scholar]
- 138.Chen M., Wang T., Yue F., Li X., Wang P., Li Y., Chan P., Yu S. Tea polyphenols alleviate motor impairments, dopaminergic neuronal injury, and cerebral α-synuclein aggregation in MPTP-intoxicated parkinsonian monkeys. Neuroscience. 2015;286:383–392. doi: 10.1016/j.neuroscience.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 139.Levites Y., Weinreb O., Maor G., Youdim M.B., Mandel S. Green tea polyphenol (–)-epigallocatechin-3-gallate prevents N-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine-induced dopaminergic neurodegeneration. J. Neurochem. 2001;78:1073–1082. doi: 10.1046/j.1471-4159.2001.00490.x. [DOI] [PubMed] [Google Scholar]
- 140.Mandel S., Maor G., Youdim M.B.H. Iron and α-synuclein in the substantia nigra of MPTP-treated mice. J. Mol. Neurosci. 2004;24:401–416. doi: 10.1385/JMN:24:3:401. [DOI] [PubMed] [Google Scholar]
- 141.Kalfon L., Youdim M.B., Mandel S.A. Green tea polyphenol (–)-epigallocatechin-3-gallate promotes the rapid protein kinase C-and proteasome-mediated degradation of Bad: Implications for neuroprotection. J. Neurochem. 2007;100:992–1002. doi: 10.1111/j.1471-4159.2006.04265.x. [DOI] [PubMed] [Google Scholar]
- 142.Maher P. How protein kinase C activation protects nerve cells from oxidative stress-induced cell death. J. Neurosci. Off. J. Soc. Neurosci. 2001;21:2929–2938. doi: 10.1523/JNEUROSCI.21-09-02929.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Pan T., Fei J., Zhou X., Jankovic J., Le W. Effects of green tea polyphenols on dopamine uptake and on MPP+-induced dopamine neuron injury. Life Sci. 2003;72:1073–1083. doi: 10.1016/S0024-3205(02)02347-0. [DOI] [PubMed] [Google Scholar]
- 144.Kang K.S., Wen Y., Yamabe N., Fukui M., Bishop S.C., Zhu B.T. Dual beneficial effects of (-)-epigallocatechin-3-gallate on levodopa methylation and hippocampal neurodegeneration: In vitro and in vivo studies. PLoS ONE. 2010;5:e11951. doi: 10.1371/journal.pone.0011951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lin S.-M., Wang S.-W., Ho S.-C., Tang Y.-L. Protective effect of green tea (-)-epigallocatechin-3-gallate against the monoamine oxidase B enzyme activity increase in adult rat brains. Nutrition. 2010;26:1195–1200. doi: 10.1016/j.nut.2009.11.022. [DOI] [PubMed] [Google Scholar]
- 146.Batista-Nascimento L., Pimentel C., Andrade Menezes R., Rodrigues-Pousada C. Iron and neurodegeneration: From cellular homeostasis to disease. Oxidative Med. Cell. Longev. 2012;2012:128647. doi: 10.1155/2012/128647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Harjes P., Wanker E.E. The hunt for huntingtin function: Interaction partners tell many different stories. Trends Biochem. Sci. 2003;28:425–433. doi: 10.1016/S0968-0004(03)00168-3. [DOI] [PubMed] [Google Scholar]
- 148.Rosenblatt A. Neuropsychiatry of Huntington’s disease. Dialogues Clin. Neurosci. 2007;9:191–197. doi: 10.31887/DCNS.2007.9.2/arosenblatt. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Savani A.A., Login I.S. Tetrabenazine as antichorea therapy in Huntington disease: A randomized controlled trial. Neurology. 2007;68:797. doi: 10.1212/01.wnl.0000259143.52138.5c. [DOI] [PubMed] [Google Scholar]
- 150.Wyant K.J., Ridder A.J., Dayalu P. Huntington’s Disease-Update on Treatments. Curr. Neurol. Neurosci. Rep. 2017;17:33. doi: 10.1007/s11910-017-0739-9. [DOI] [PubMed] [Google Scholar]
- 151.Ehrnhoefer D.E., Duennwald M., Markovic P., Wacker J.L., Engemann S., Roark M., Legleiter J., Marsh J.L., Thompson L.M., Lindquist S., et al. Green tea (-)-epigallocatechin-gallate modulates early events in huntingtin misfolding and reduces toxicity in Huntington’s disease models. Hum. Mol. Genet. 2006;15:2743–2751. doi: 10.1093/hmg/ddl210. [DOI] [PubMed] [Google Scholar]
- 152.Khan H., Ullah H., Tundis R., Belwal T., Devkota H.P., Daglia M., Cetin Z., Saygili E.I., Campos M.d.G., Capanoglu E.J.E. Dietary flavonoids in the management of huntington’s disease: Mechanism and clinical perspective. eFood. 2020;1:38–52. doi: 10.2991/efood.k.200203.001. [DOI] [Google Scholar]
- 153.Huang W.J., Chen W.W., Zhang X. Multiple sclerosis: Pathology, diagnosis and treatments. Exp. Ther. Med. 2017;13:3163–3166. doi: 10.3892/etm.2017.4410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lublin F.D. New multiple sclerosis phenotypic classification. Eur. Neurol. 2014;72((Suppl. S1)):1–5. doi: 10.1159/000367614. [DOI] [PubMed] [Google Scholar]
- 155.Rae-Grant A., Day G.S., Marrie R.A., Rabinstein A., Cree B.A.C., Gronseth G.S., Haboubi M., Halper J., Hosey J.P., Jones D.E., et al. Practice guideline recommendations summary: Disease-modifying therapies for adults with multiple sclerosis: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2018;90:777–788. doi: 10.1212/WNL.0000000000005347. [DOI] [PubMed] [Google Scholar]
- 156.Semnani M.-R., Mashayekhi F., Azarnia M., Salehi Z. Effects of green tea epigallocatechin-3-gallate (EGCG) on proteolipid protein (PLP) and oligodendrocyte transcription factor 1 (Olig1) expression in the cerebral cortex of cuprizone induced multiple sclerosis mice; a western blot study. Casp. J. Neurol. Sci. 2016;2:1–9. doi: 10.18869/acadpub.cjns.2.6.1. [DOI] [Google Scholar]
- 157.Bellmann-Strobl J., Paul F., Wuerfel J., Dörr J., Infante-Duarte C., Heidrich E., Körtgen B., Brandt A., Pfüller C., Radbruch H., et al. Epigallocatechin Gallate in Relapsing-Remitting Multiple Sclerosis: A Randomized, Placebo-Controlled Trial. Neurol.(R) Neuroimmunol. Neuroinflamm. 2021;8:e981. doi: 10.1212/NXI.0000000000000981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Mossakowski A.A., Pohlan J., Bremer D., Lindquist R., Millward J.M., Bock M., Pollok K., Mothes R., Viohl L., Radbruch M., et al. Tracking CNS and systemic sources of oxidative stress during the course of chronic neuroinflammation. Acta Neuropathol. 2015;130:799–814. doi: 10.1007/s00401-015-1497-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Rust R., Chien C., Scheel M., Brandt A.U., Dörr J., Wuerfel J., Klumbies K., Zimmermann H., Lorenz M., Wernecke K.D., et al. Epigallocatechin Gallate in Progressive MS: A Randomized, Placebo-Controlled Trial. Neurol.(R) Neuroimmunol. Neuroinflamm. 2021;8:e964. doi: 10.1212/NXI.0000000000000964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hardiman O., Al-Chalabi A., Chio A., Corr E.M., Logroscino G., Robberecht W., Shaw P.J., Simmons Z., van den Berg L.H. Amyotrophic lateral sclerosis. Nat. Rev. Dis. Primers. 2017;3:17071. doi: 10.1038/nrdp.2017.71. [DOI] [PubMed] [Google Scholar]
- 161.Longinetti E., Regodón Wallin A., Samuelsson K., Press R., Zachau A., Ronnevi L.O., Kierkegaard M., Andersen P.M., Hillert J., Fang F., et al. The Swedish motor neuron disease quality registry. Amyotroph. Lateral Scler. Front. Degener. 2018;19:528–537. doi: 10.1080/21678421.2018.1497065. [DOI] [PubMed] [Google Scholar]
- 162.Greaves C.V., Rohrer J.D. An update on genetic frontotemporal dementia. J. Neurol. 2019;266:2075–2086. doi: 10.1007/s00415-019-09363-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Couratier P., Corcia P., Lautrette G., Nicol M., Marin B. ALS and frontotemporal dementia belong to a common disease spectrum. Rev. Neurol. 2017;173:273–279. doi: 10.1016/j.neurol.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 164.Koh S.H., Kwon H., Kim K.S., Kim J., Kim M.H., Yu H.J., Kim M., Lee K.W., Do B.R., Jung H.K., et al. Epigallocatechin gallate prevents oxidative-stress-induced death of mutant Cu/Zn-superoxide dismutase (G93A) motoneuron cells by alteration of cell survival and death signals. Toxicology. 2004;202:213–225. doi: 10.1016/j.tox.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 165.Koh S.H., Lee S.M., Kim H.Y., Lee K.Y., Lee Y.J., Kim H.T., Kim J., Kim M.H., Hwang M.S., Song C., et al. The effect of epigallocatechin gallate on suppressing disease progression of ALS model mice. Neurosci. Lett. 2006;395:103–107. doi: 10.1016/j.neulet.2005.10.056. [DOI] [PubMed] [Google Scholar]
- 166.Xu Z., Chen S., Li X., Luo G., Li L., Le W. Neuroprotective effects of (-)-epigallocatechin-3-gallate in a transgenic mouse model of amyotrophic lateral sclerosis. Neurochem. Res. 2006;31:1263–1269. doi: 10.1007/s11064-006-9166-z. [DOI] [PubMed] [Google Scholar]
- 167.Che F., Wang G., Yu J., Wang X., Lu Y., Fu Q., Su Q., Jiang J., Du Y. Effects of epigallocatechin-3-gallate on iron metabolism in spinal cord motor neurons. Mol. Med. Rep. 2017;16:3010–3014. doi: 10.3892/mmr.2017.6919. [DOI] [PubMed] [Google Scholar]
- 168.Taniguchi S., Suzuki N., Masuda M., Hisanaga S., Iwatsubo T., Goedert M., Hasegawa M. Inhibition of heparin-induced tau filament formation by phenothiazines, polyphenols, and porphyrins. J. Biol. Chem. 2005;280:7614–7623. doi: 10.1074/jbc.M408714200. [DOI] [PubMed] [Google Scholar]
- 169.Almeida L., Andreu-Fernández V., Navarro-Tapia E., Aras-López R., Serra-Delgado M., Martínez L., García-Algar O., Gómez-Roig M.D. Murine Models for the Study of Fetal Alcohol Spectrum Disorders: An Overview. Front. Pediatrics. 2020;8:359. doi: 10.3389/fped.2020.00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Harper C.G., Kril J.J., Holloway R.L. Brain shrinkage in chronic alcoholics: A pathological study. Br. Med. J. (Clin. Res. Ed.) 1985;290:501–504. doi: 10.1136/bmj.290.6467.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Harwood D.G., Barker W.W., Loewenstein D.A., Ownby R.L., St George-Hyslop P., Mullan M., Duara R. A cross-ethnic analysis of risk factors for AD in white Hispanics and white non-Hispanics. Neurology. 1999;52:551–556. doi: 10.1212/WNL.52.3.551. [DOI] [PubMed] [Google Scholar]
- 172.Yalcin E.B., McLean T., Tong M., de la Monte S.M. Progressive white matter atrophy with altered lipid profiles is partially reversed by short-term abstinence in an experimental model of alcohol-related neurodegeneration. Alcohol. 2017;65:51–62. doi: 10.1016/j.alcohol.2017.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hoyme H.E., Kalberg W.O., Elliott A.J., Blankenship J., Buckley D., Marais A.S., Manning M.A., Robinson L.K., Adam M.P., Abdul-Rahman O., et al. Updated Clinical Guidelines for Diagnosing Fetal Alcohol Spectrum Disorders. Pediatrics. 2016;138:e20154256. doi: 10.1542/peds.2015-4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Heaton M.B., Paiva M., Madorsky I., Mayer J., Moore D.B. Effects of ethanol on neurotrophic factors, apoptosis-related proteins, endogenous antioxidants, and reactive oxygen species in neonatal striatum: Relationship to periods of vulnerability. Dev. Brain Res. 2003;140:237–252. doi: 10.1016/S0165-3806(02)00610-7. [DOI] [PubMed] [Google Scholar]
- 175.Joya X., Garcia-Algar O., Salat-Batlle J., Pujades C., Vall O. Advances in the development of novel antioxidant therapies as an approach for fetal alcohol syndrome prevention. Birth Defects Res. Part A Clin. Mol. Teratol. 2015;103:163–177. doi: 10.1002/bdra.23290. [DOI] [PubMed] [Google Scholar]
- 176.Pei J., Baugh L., Andrew G., Rasmussen C. Intervention recommendations and subsequent access to services following clinical assessment for fetal alcohol spectrum disorders. Res. Dev. Disabil. 2017;60:176–186. doi: 10.1016/j.ridd.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 177.Chu K.O., Wang C.C., Chu C.Y., Choy K.W., Pang C.P., Rogers M.S. Uptake and distribution of catechins in fetal organs following in utero exposure in rats. Hum. Reprod. 2007;22:280–287. doi: 10.1093/humrep/del353. [DOI] [PubMed] [Google Scholar]
- 178.Tiwari V., Kuhad A., Chopra K. Epigallocatechin-3-gallate ameliorates alcohol-induced cognitive dysfunctions and apoptotic neurodegeneration in the developing rat brain. Int. J. Neuropsychopharmacol. 2010;13:1053–1066. doi: 10.1017/S146114571000060X. [DOI] [PubMed] [Google Scholar]
- 179.Long L., Li Y., Wang Y.D., He Q.Y., Li M., Cai X.D., Peng K., Li X.P., Xie D., Wen Y.L., et al. The preventive effect of oral EGCG in a fetal alcohol spectrum disorder mouse model. Alcohol. Clin. Exp. Res. 2010;34:1929–1936. doi: 10.1111/j.1530-0277.2010.01282.x. [DOI] [PubMed] [Google Scholar]
- 180.Almeida-Toledano L., Andreu-Fernández V., Aras-López R., García-Algar Ó., Martínez L., Gómez-Roig M.D. Epigallocatechin Gallate Ameliorates the Effects of Prenatal Alcohol Exposure in a Fetal Alcohol Spectrum Disorder-Like Mouse Model. Int. J. Mol. Sci. 2021;22:715. doi: 10.3390/ijms22020715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Antonio A.M., Druse M.J. Antioxidants prevent ethanol-associated apoptosis in fetal rhombencephalic neurons. Brain Res. 2008;1204:16–23. doi: 10.1016/j.brainres.2008.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Lee W.J., Shim J.Y., Zhu B.T. Mechanisms for the inhibition of DNA methyltransferases by tea catechins and bioflavonoids. Mol. Pharmacol. 2005;68:1018–1030. doi: 10.1124/mol.104.008367. [DOI] [PubMed] [Google Scholar]
- 183.Dierssen M. Down syndrome: The brain in trisomic mode. Nat. Rev. Neurosci. 2012;13:844–858. doi: 10.1038/nrn3314. [DOI] [PubMed] [Google Scholar]
- 184.Lott I.T., Dierssen M. Cognitive deficits and associated neurological complications in individuals with Down’s syndrome. Lancet Neurol. 2010;9:623–633. doi: 10.1016/S1474-4422(10)70112-5. [DOI] [PubMed] [Google Scholar]
- 185.Singh B.N., Shankar S., Srivastava R.K. Green tea catechin, epigallocatechin-3-gallate (EGCG): Mechanisms, perspectives and clinical applications. Biochem. Pharmacol. 2011;82:1807–1821. doi: 10.1016/j.bcp.2011.07.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Wyganowska-Świątkowska M., Matthews-Kozanecka M., Matthews-Brzozowska T., Skrzypczak-Jankun E., Jankun J. Can EGCG Alleviate Symptoms of Down Syndrome by Altering Proteolytic Activity? Int. J. Mol. Sci. 2018;19:248. doi: 10.3390/ijms19010248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Souchet B., Guedj F., Penke-Verdier Z., Daubigney F., Duchon A., Herault Y., Bizot J.C., Janel N., Créau N., Delatour B., et al. Pharmacological correction of excitation/inhibition imbalance in Down syndrome mouse models. Front. Behav. Neurosci. 2015;9:267. doi: 10.3389/fnbeh.2015.00267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Abunofal O., Mohan C. Salubrious Effects of Green Tea Catechins on Fatty Liver Disease: A Systematic Review. Medicines. 2022;9:20. doi: 10.3390/medicines9030020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Catuara-Solarz S., Espinosa-Carrasco J., Erb I., Langohr K., Gonzalez J.R., Notredame C., Dierssen M. Combined Treatment with Environmental Enrichment and (-)-Epigallocatechin-3-Gallate Ameliorates Learning Deficits and Hippocampal Alterations in a Mouse Model of Down Syndrome. eNeuro. 2016;3:e0103. doi: 10.1523/ENEURO.0103-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Catuara-Solarz S., Espinosa-Carrasco J., Erb I., Langohr K., Notredame C., Gonzalez J.R., Dierssen M. Principal Component Analysis of the Effects of Environmental Enrichment and (-)-epigallocatechin-3-gallate on Age-Associated Learning Deficits in a Mouse Model of Down Syndrome. Front. Behav. Neurosci. 2015;9:330. doi: 10.3389/fnbeh.2015.00330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.De Toma I., Ortega M., Aloy P., Sabidó E., Dierssen M. DYRK1A Overexpression Alters Cognition and Neural-Related Proteomic Pathways in the Hippocampus That Are Rescued by Green Tea Extract and/or Environmental Enrichment. Front. Mol. Neurosci. 2019;12:272. doi: 10.3389/fnmol.2019.00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.De Toma I., Ortega M., Catuara-Solarz S., Sierra C., Sabidó E., Dierssen M. Re-establishment of the epigenetic state and rescue of kinome deregulation in Ts65Dn mice upon treatment with green tea extract and environmental enrichment. Sci. Rep. 2020;10:16023. doi: 10.1038/s41598-020-72625-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Valenti D., De Rasmo D., Signorile A., Rossi L., de Bari L., Scala I., Granese B., Papa S., Vacca R.A. Epigallocatechin-3-gallate prevents oxidative phosphorylation deficit and promotes mitochondrial biogenesis in human cells from subjects with Down’s syndrome. Biochim. Biophys. Acta. 2013;1832:542–552. doi: 10.1016/j.bbadis.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 194.Valenti D., de Bari L., de Rasmo D., Signorile A., Henrion-Caude A., Contestabile A., Vacca R.A. The polyphenols resveratrol and epigallocatechin-3-gallate restore the severe impairment of mitochondria in hippocampal progenitor cells from a Down syndrome mouse model. Biochim. Biophys. Acta. 2016;1862:1093–1104. doi: 10.1016/j.bbadis.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 195.Stagni F., Giacomini A., Emili M., Trazzi S., Guidi S., Sassi M., Ciani E., Rimondini R., Bartesaghi R. Short- and long-term effects of neonatal pharmacotherapy with epigallocatechin-3-gallate on hippocampal development in the Ts65Dn mouse model of Down syndrome. Neuroscience. 2016;333:277–301. doi: 10.1016/j.neuroscience.2016.07.031. [DOI] [PubMed] [Google Scholar]