Abstract
The production of toxin (Apx)-neutralizing antibodies during infection plays a major role in the induction of protective immunity to Actinobacillus pleuropneumoniae reinfection. In the present study, the gene encoding the ApxII-activating protein, apxIIC, was insertionally inactivated on the chromosome of a serovar 7 strain, HS93. Expression of the structural toxin, ApxIIA, and of the two genes required for its secretion, apxIB and apxID, still occurs in this strain. The resulting mutant strain, HS93C− Ampr, was found to secrete the unactivated toxin. Pigs vaccinated with live HS93C− Ampr via the intranasal route were protected against a cross-serovar challenge with a virulent serovar 1 strain of A. pleuropneumoniae. This is the first reported vaccine strain of A. pleuropneumoniae which can be delivered live to pigs and offers cross-serovar protection against porcine pleuropneumonia.
Actinobacillus pleuropneumoniae is a member of the family Pasteurellaceae and is the etiological agent of porcine pleuropneumonia, an acute or chronic infection affecting pigs of all ages. The disease, characterized by hemorrhagic, fibrinous, and necrotic lung lesions, is highly contagious and causes major losses to the swine industry (25). To date, 12 serovars have been identified worldwide (serovars 1 to 12). Within a geographical region a small number of serovars predominate; for example, in Australia serovars 1, 7, and 12 make up approximately 90% of isolates.
A number of potential virulence factors have been identified for A. pleuropneumoniae, including a family of secreted toxins (3, 5, 26, 29). These secreted toxins, or Apx toxins, are members of the RTX toxin family (11–13). The role of Apx toxins in A. pleuropneumoniae virulence was first demonstrated with spontaneous and chemically induced nonhemolytic mutants which were found to be completely or partially avirulent; this role was later confirmed by using transposon mutagenesis (1, 15, 17, 29, 30, 33, 34). At least three different Apx toxins are produced by A. pleuropneumoniae, designated ApxI, ApxII, and ApxIII. ApxI shows strong hemolytic activity, and ApxII shows relatively low hemolytic activity. Both are cytotoxic and active against a broad range of cells of different types and species (9, 19, 28). ApxIII is nonhemolytic but strongly cytotoxic, with a host range including porcine alveolar macrophages and neutrophils (19, 29). Currently, no identified serovar of A. pleuropneumoniae produces all three Apx toxins, with the majority producing only two, while a small number produce only one (8, 10–12, 19, 29).
Production and secretion of active RTX toxins requires the activity of at least four genes, apxC, -A, -B, and -D. The apxA gene encodes the structural toxin, and the apxC gene encodes a posttranslational activator which is involved in the transfer of a fatty acyl group from an acyl carrier protein to the structural toxin (18). Activation of ApxA is required for target cell binding. The apxB and apxD genes encode proteins that are required for secretion of the activated toxin (7, 36). ApxI and ApxIII are encoded by operons that consist of the four contiguous genes (-C, -A, -B, -D) expressed from a single promoter located 5′ of the apxC gene. The ApxII operon contains only the apxA and apxC genes expressed as a single RNA transcript. Secretion of ApxII is dependent on the activity of the apxIB and apxID gene products (13).
Vaccination against porcine pleuropneumonia has utilized, to date, bacterins or subunit vaccines based on various components of A. pleuropneumoniae. Results obtained with bacterin vaccines have offered, at best, homologous protection against the serovar used to prepare the vaccine material. In contrast, natural infection of pigs with any one serovar serves to prevent natural reinfection with any serovar (24). Apx’s are thought to be of particular importance for the induction of protective immunity; nonhemolytic mutants cannot induce protective immunity in animals (17), and commercial bacterin vaccines that lack Apx do not provide adequate protection (16). Previously we (26) demonstrated the ability of an A. pleuropneumoniae mutant deficient in chromosomal apxA and apxC genes to express and secrete an unactivated form of ApxI from a plasmid-encoded apxIA gene. This engineered strain was found to be attenuated in a mouse model and, when administered as a live vaccine, offered protection against homologous and heterologous challenge.
The use of a plasmid-borne protective antigen in a live vaccine strain is limited due to the potential of the plasmid to be lost during in vivo replication of the vaccine. Here we describe the construction of an A. pleuropneumoniae vaccine strain by using site-specific mutagenesis of the apxIIC gene on the chromosome. The resulting strain produces and secretes an unactivated ApxIIA by using chromosomally encoded genes, thus ensuring that the protective antigen is maintained within the vaccine strain, unlike in previous experiments, in which ApxIA was expressed from a plasmid and could therefore be lost from replicating bacteria. The potential of this modified strain to protect pigs from cross-serovar challenge with virulent A. pleuropneumoniae was investigated.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The A. pleuropneumoniae bacterial strains used in this study (serovar 1, HS25; serovar 7, HS93) were isolated from pigs with pleuropneumonia and kindly supplied by Pat Blackall (Animal Research Institute, Yeerongpilly, Queensland, Australia). Strains of A. pleuropneumoniae were grown in brain heart infusion broth (BHI), supplemented with nicotinamide adenine dinucleotide (NAD; Sigma Chemical Co., St. Louis, Mo.) to a final concentration of 10 μg/ml. Blood agar was prepared by adding 5% sterile defibrinated horse erythrocytes to the BHI agar. The antibiotics used and their final concentrations were as follows: kanamycin, 25 μg/ml; streptomycin, 50 μg/ml; and ampicillin, 5 μg/ml, unless stated otherwise. Escherichia coli DH5α was used throughout this study, by standard techniques (31).
Isolation, amplification, and Southern blot analysis of A. pleuropneumoniae genomic DNA.
Isolation of A. pleuropneumoniae genomic DNA was performed as described by Prideaux et al. (26), using lysozyme and proteinase K digestion followed by phenol-chloroform-isoamyl alcohol extraction. Amplification of specific regions of the A. pleuropneumoniae genome was achieved by PCR, using the buffer and cycle conditions described previously (26) and a Perkin-Elmer Cetus DNA thermal cycler.
Southern blot analysis of PCR products was performed by standard techniques (31), with a final washing stringency of 0.1% sodium dodecyl sulfate (SDS)–0.1× SSPE (1× SSPE is 0.18 M NaCl, 10 mM NaH2PO4, and 1 mM EDTA [pH 7.7]) at 65°C for 10 min.
Construction of recombination plasmids.
The plasmid pEP-CAmpr was constructed for use in site-specific mutagenesis of the apxIIC gene. A 3.4-kb fragment containing the apxIIC gene was isolated by PCR. Specific oligonucleotides for use in PCR were synthesized with a Pharmacia Gene Assembler Plus DNA synthesizer (5′, CGCACCATGGTCGGGC; 3′, CTAACAGCTAGTGCA) based on published sequence (32). The fragment contained 2.0 kb of DNA 5′ and 900 bp 3′ of the apxIIC gene (480 bp). The PCR fragment was cloned into the Mycobacterium-E. coli shuttle vector pEP2 (27). The resulting plasmid, pEP2-CA, contained a unique XbaI restriction site located 180 bp downstream of the apxIIC translational start site. The Ampr gene from the 4.2-kb plasmid of Pasteurella haemolytica A1 (23) was cloned into the unique XbaI site of pEP-CA to generate the recombination plasmid pEP-CAmpr.
Mutagenesis of the apxIIC gene.
Site-specific mutagenesis of the apxIIC gene utilized the recombination plasmid pEP-CAmpr. Cesium chloride-purified pEP-CAmpr DNA was isolated from E. coli and linearized with ClaI. Following digestion, the DNA was purified by phenol-chloroform extraction and ethanol precipitated. A total of 3 μg of linearized DNA was electroporated (0.2-cm-diameter cuvettes; 400 Ω; 1.25 kV) into A. pleuropneumoniae HS93 (serovar 7, ApxII) by using the protocol described previously by Frey (7). Products of the electroporation were plated onto (BHI-NAD) blood agar plates containing ampicillin at a concentration of 1 μg/ml. Colonies that were ampicillin resistant and did not produce a zone of hemolysis on blood agar plates were selected for further characterization.
PCR was used to examine the region of the A. pleuropneumoniae chromosome containing the apxIIC gene, employing oligonucleotides that bound to the apxII operon (5′TACAGAACGTTGGTA, 3′CTAACAGCTAGTCCA) at the commencement of the apxIIC open reading frame and 900 bp after its closing.
Western blot analysis.
Western blot (immunoblot) analysis was performed as described previously by Sambrook et al. (31). Rabbit sera were produced against culture supernatants of A. pleuropneumoniae HS25 (serovar 1) by the method described previously (5). Rabbit sera were preabsorbed with an isolate of HS93 that lacks both the apxIIA and apxIIC genes, and therefore does not produce Apx (26), before use at a 1 in 50 dilution. The conjugate, used at a 1:1,000 dilution, was sheep anti-rabbit immunoglobulin affinity-isolated horseradish peroxidase-conjugated antiserum (Silenus), with tetramethylbenzidine (22) as the substrate. Bacterial samples for Western blot analysis were prepared by diluting overnight cultures 1:20 and incubating at 37°C with shaking until an optical density at 600 nm (OD600) of 0.8 was reached. At this time, culture supernatant (12,000 × g for 5 min) and cell pellet (12,000 × g for 5 min; washed in an equal volume of phosphate-buffered saline and lysed in loading buffer) samples were taken (equivalent to 20 μl of total culture) and analyzed by SDS-polyacrylamide gel electrophoresis by using the discontinuous buffer system (21). Separated proteins were transferred to nitrocellulose with a Bio-Rad transblot cell by using the protocols outlined by the manufacturer.
Attenuation of HS93C− Ampr in mice.
Overnight cultures of A. pleuropneumoniae were grown with vigorous shaking at 37°C in BHI broth supplemented with NAD. The following day a 1 in 20 dilution was made, and the new cultures were incubated until an OD600 of 0.8 was reached, at which point the count of viable A. pleuropneumoniae was found to be 109 CFU per ml. Various dilutions of A. pleuropneumoniae cultures were prepared so that the desired number of bacteria were contained in 200 μl of BHI broth. Six-week-old female BALB/c mice (Walter and Elisa Hall Institute of Medical Research, Parkville, Australia) were maintained in PC1 facilities with water and food ad libitum. Mice were injected intraperitoneally (i.p.) with 200 μl of A. pleuropneumoniae preparation. Control mice received 200 μl of BHI broth i.p. The number of surviving mice at 24 h postchallenge was recorded; and these mice were considered to have received a sublethal dose.
Vaccination and challenge of pigs.
Six-week-old pigs were prebled to screen for existing antibodies against A. pleuropneumoniae HS93 (serovar 7) and ApxI. Pigs found to be negative in these tests were randomly assigned to experimental groups. Nine 6-week-old pigs received 109 CFU of the A. pleuropneumoniae vaccine strain in 1 ml of growth medium, via intranasal inoculation on day 0, while nine control pigs received 1 ml of BHI. The vaccine was prepared by inoculating 10 ml of BHI-NAD (10 μg/ml) with a single colony of the vaccine strain and growing with shaking at 37°C until an OD600 of 0.8 was reached. The vaccination schedule was repeated on day 14. On day 28, the nine vaccinated and nine control pigs were divided into groups of six and three. The two groups of six pigs (i.e., vaccinated and unvaccinated) were challenged with 2 × 109 A. pleuropneumoniae HS25 (serovar 1) in 2 ml of growth medium via the intranasal route, while the groups of three were given 2 ml of BHI broth in a similar manner. The challenge strain was prepared by inoculating a single colony of HS25 into BHI-NAD (10 μg/ml) and growing until an OD600 of 0.8 was reached. At this time the viable count was 109 CFU/ml. At 5 days postchallenge, pigs were euthanized, and the number and severity of lung lesions were recorded.
RESULTS
Characterization of the apxIIC mutant.
Site-specific mutagenesis of the apxIIC gene utilized the recombination plasmid pEP-CAmpr. This plasmid contains the apxIIC open reading frame insertionally inactivated by the introduction of an Ampr gene into the unique XbaI site. The inactivated apxIIC gene is flanked by 2.0 kb of genomic DNA upstream (5′) and 900 bp downstream (3′). pEP-CAmpr was linearized and electroporated into A. pleuropneumoniae HS93, and the products of homologous recombination were selected by plating on blood agar plates containing ampicillin.
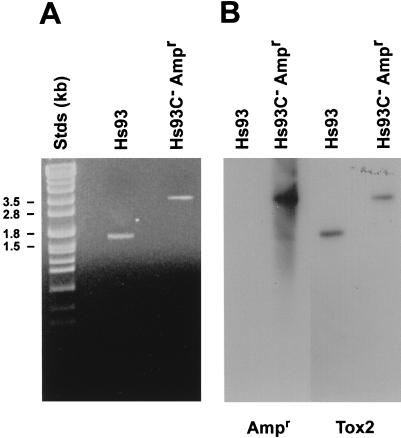
Genomic DNA was extracted from the nonzoning, ampicillin-resistant mutants designated HS93C− Ampr and the parent strain, HS93. PCR was used to examine the region of the A. pleuropneumoniae chromosome containing the apxIIC gene. The PCR product (Fig. 1) obtained by using HS93C− Ampr genomic DNA (3.5 kb) was approximately 1.8 kb larger in size than that obtained with the parent strain, HS93. This increase in size corresponded to the size of the Ampr gene. Products of the PCRs were further characterized by Southern blot hybridization with the isolated Ampr or apxC gene as a probe (Fig. 1). Hybridization of the apxC gene probe to the PCR products from HS93 and HS93C− Ampr confirmed that the region of the chromosome containing the apxIIC gene had been amplified. The Ampr gene probe hybridized to the PCR product obtained from HS93C− Ampr, confirming that this strain contained the Ampr gene associated with the apxC gene. The PCR product obtained when HS93 genomic DNA was used as template did not hybridize to the Ampr gene probe.
FIG. 1.
Characterization of the apxIIC mutant chromosome. PCR was used to amplify the region of the A. pleuropneumoniae chromosome containing the apxIIC gene from mutant (HS93C− Ampr) and wild-type (HS93) strains. (A) PCR products were resolved on gels and stained with ethidium bromide. (B) Southern blot hybridization of the PCR products with the apxIIC gene (Tox2) and the ampicillin resistance gene (Ampr).
Characterization of Apx expression by HS93C− Ampr.
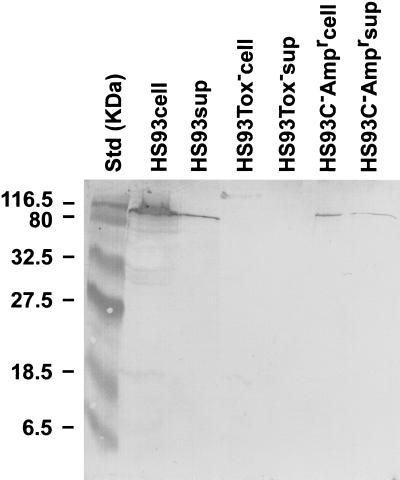
Logarithmic cultures of HS93− Ampr and HS93 were examined by Western blotting with antisera raised in rabbits against the secreted proteins of A. pleuropneumoniae HS25 (i.e., serovar 1, ApxI and ApxII). A toxin-deficient strain of HS93 resulting from deletion of the apxIIC and apxIIA genes (26) was used as a negative control. The Apx-deficient mutant (HS93 Tox−) did not react specifically with the anti-Apx antisera in the region corresponding to the ApxII molecular weight. Supernatant and cellular material from both the HS93C− Ampr mutant and the parent strain, HS93, produced a single polypeptide, corresponding in size to ApxII, that reacted with the anti-APX rabbit sera (Fig. 2). A potential high-molecular-weight HS93 Tox− polypeptide may have reacted with the antisera. The preabsorption of the sera with HS93 Tox− prior to use and the position of the band suggest that it corresponds to nonspecific cross-reaction with material remaining in the loading well.
FIG. 2.
Western blot analysis of ApxII expression. Total cells (cell) and culture supernatant (sup) samples of wild-type HS93, HS93C− Ampr, and a toxin-deficient mutant (HS93Tox−) were analyzed by Western blotting with Apx-specific antisera.
Evaluation of HS93C− Ampr virulence in mice.
To test the relative virulence of A. pleuropneumoniae HS93 and HS93C− Ampr in mice, various dilutions of each bacteria (2 × 108 to 1 × 107 CFU/mouse) were prepared in bacterial growth medium (BHI-NAD) and administered to mice i.p. The number of mice that had received a sublethal dose was determined 24 h postchallenge. Under our conditions, all mortalities occurred within the first 24 h postchallenge. A comparison of the deaths obtained with each isolate showed that 2 × 108 CFU of the parent strain, HS93, was sufficient to kill 100% of mice, while an equivalent challenge with HS93C− Ampr was sublethal (Table 1).
TABLE 1.
Virulence of A. pleuropneumoniae strains in mice
| Challenge level (106)a | % of mice dead 24 h postchallenge with indicated strain
|
|
|---|---|---|
| HS93 | HS93C− Ampr | |
| 200 | 100 | 0 |
| 20 | 15 | NT |
| 10 | 0 | NT |
Levels below those previously found to give 0% death were not tested (NT).
Vaccination and challenge of pigs.
Two groups of nine 6-week-old pigs were vaccinated with either 1 ml of BHI containing 109 CFU of HS93C− Ampr or 1 ml of sterile BHI (unvaccinated) via intranasal inoculation on days 0 and 14. Two weeks after secondary vaccination, six of the HS93C− Ampr-vaccinated and six of the unvaccinated pigs were challenged intranasally with 2 ml of growth medium containing 2 × 109 CFU of HS25. The number and severity of lung lesions present in pigs at autopsy 5 days postchallenge were recorded (Table 2). It is our experience that no additional lesions resulting from experimental challenge of pigs with this protocol develop beyond day 5 postchallenge and that at this time a number of lesions detected have commenced to resolve. The three pigs that were neither vaccinated nor challenged had no detectable lung lesions present at autopsy. Similarly, pigs (three) that were vaccinated and unchallenged showed no evidence of A. pleuropneumoniae infection. The six unvaccinated pigs that were challenged with HS25 all showed numerous lung lesions at autopsy, characterized as focal abscesses up to 3 cm in diameter, and adhesive pleuritis. Further characterization of these lesions showed them to contain high levels of A. pleuropneumoniae that had characteristics similar to those of HS25, as judged by colony morphology and zones of hemolysis on blood agar plates. In contrast, of the six pigs that had been vaccinated with HS93C− Ampr and challenged with HS25, only one had a lesion at autopsy; this was in the form of a single adhesion between the lung and the rib cage. Upon closer examination, this adhesion appeared to be older than the 5 days since challenge. Bacteria isolated from this adhesion were not NAD dependent and are therefore unlikely to have been A. pleuropneumoniae. Lung samples that were taken from vaccinated and challenged pigs, homogenized, and plated on blood agar (BHI-NAD) did not yield bacteria.
TABLE 2.
Vaccination and challenge of pigs
| Group | No. of pigs | Extent of lung lesions |
|---|---|---|
| Unvaccinated, unchallenged | 3 | None detected |
| Vaccinated, unchallenged | 3 | None detected |
| Vaccinated, challenged | 6 | None detected in five animals, but one pig had a small adhesion on the left diaphragmatic lobea |
| Unvaccinated, challenged | 6 | Multiple abscesses and adhesive pleuritis |
Not considered to be related to A. pleuropneumoniae challenge (see text for details).
DISCUSSION
Apx toxins are known to play a major role in both the virulence of and induction of protective immunity to A. pleuropneumoniae, the causative agent of porcine pleuropneumonia. The principal phagocytic cells of the lung and the first line of defense against bacterial invasion are the alveolar macrophages. It is possible that A. pleuropneumoniae colonizes the lung through the production of Apx toxins which lyse these cells and thus compromise the primary immune responses of the lung (37).
Targets of the Apx toxins include erythrocytes, the lysis of which leads to an increase in the availability of free iron for bacterial growth. In addition, the Apx toxins contribute to lung damage through the lysis of leukocytes, which leads to localized inflammation. Binding of Apx to target cells requires posttranslational activation of the structural toxin by the ApxIIC protein (18). In this study we utilized site-specific mutagenesis to inactivate the apxIIC gene on the A. pleuropneumoniae chromosome and examined the effect of this mutation on virulence, induction of protective immunity following infection, and potential to generate mutants for use as live vaccines.
Site-specific mutagenesis of the apxIIC gene was achieved with suicide, or nonreplicating, plasmid vectors. This method has the advantage of rapid screening of products, as theoretically only those bacteria that have undergone recombination with the plasmid vector, resulting in the transfer of the marker gene onto the bacterial chromosome, will grow under selective conditions. A number of attempts within our laboratory have failed to transform A. pleuropneumoniae with pEP2, leading to the conclusion that A. pleuropneumoniae is a nonpermissive host for this plasmid vector. A serovar 7 strain of A. pleuropneumoniae producing ApxII alone was chosen for use in this study, as the genes required for ApxII secretion are not cotranscribed with the structural and activating genes. In addition, serovar 7 is relevant as a vaccine candidate in Australia, as serovar 7 isolates are responsible for large numbers of porcine pleuropneumonia outbreaks.
Homologous recombination leading to the insertion of the Ampr gene into the apxIIC gene on the chromosome was confirmed by PCR and Southern blot hybridization. Insertion of the ampicillin resistance gene into the chromosomal copy of the apxIIC gene did not prevent transcription or translation of the apxIIA gene, as evidenced by the ability to detect ApxII in Western blots (Fig. 2). It appears that transcription initiates at the apxII promoter and continues through the ampicillin resistance gene and into the apxIIA gene. Although translation of an active apxIIC gene product is prevented by the presence of the ampicillin resistance gene (Fig. 1), translation of the apxIIA gene must recommence further downstream. Chang et al. (3) have described a potential ribosome binding site, located between the apxIIC and apxIIA genes, which may serve to reinitiate translation of ApxII. The orientation of the ampicillin resistance gene is opposite that of the apxII operon, and therefore the ampicillin resistance gene promoter cannot contribute to ApxIIA expression. Insertion of the ampicillin resistance gene into the apxIIC gene appears to have reduced the level of ApxII production, possibly due to polar effects of the ampicillin resistance gene promoter on the level of downstream apxIIA transcription. A potential solution to this possible limitation would be to clone the ampicillin resistance gene in the same orientation as the apxIIA gene, though licensing of the vaccine strain for commercial use would require the removal of any antibiotic resistance gene from the chromosome. The presence of ApxIIA in the culture supernatant would also indicate that activation of ApxIIA is not required for secretion. A similar observation has been made for both E. coli and P. haemolytica, where the RTX toxins produced by these bacteria have been shown to be secreted without activation (6, 35).
Inactivation of the apxIIC gene on the chromosome resulted in reduced virulence, as observed in a mouse model in which 2 × 108 CFU of the apxIIC-deficient mutant resulted in no mortalities compared to a mortality rate of 100% when mice were inoculated with the same level of the parent strain, HS93 (Table 1). This is in agreement with our previous observations (26) with a toxin-deficient strain of A. pleuropneumoniae expressing an unactivated form of ApxIA from a plasmid, where it was found that unless the toxin was activated, it did not contribute to bacterial pathogenesis.
To test the protective efficacy of the vaccine in the target species, we vaccinated pigs with HS93C− Ampr, a serovar 7 strain, via the intranasal route and challenged with HS25 (Table 2), which belongs to serovar 1 and produces both ApxI and ApxII. This combination of Apx production is associated with the most severe outbreaks of pleuropneumonia (13, 20). Prior to vaccination, pigs were determined to be free of both HS93- and ApxI-specific antibodies, therefore ensuring their naive status for both vaccination (HS93) and challenge (HS25: ApxI) strains. Vaccination and challenge were both via the intranasal route. This method of delivery was chosen because it best mimics the natural route of exposure of pigs to A. pleuropneumoniae. The three pigs that were vaccinated and not challenged had no lung lesions present at autopsy, indicating that the vaccine strain does not cause lesions in pigs that are evident at 3 weeks postvaccination. Previously we had administered the toxin-deficient strain HS93 Tox− to pigs at doses similar to that of the challenge used in this experiment and autopsied the pigs at day 5 but observed no lesions. Apx-deficient mutants of APP produced by either chemical or transposon mutagenesis have previously been shown to have a reduced ability to induce lung lesions (1, 15, 17, 29, 30, 33, 34). The six unvaccinated pigs challenged with HS25 showed numerous lung lesions that were visible on autopsy, indicating that the level of challenge used was sufficient to induce lesions in unprotected animals. In contrast, only one of the six vaccinated pigs showed any sign of infection, in the form of a single lung adhesion, which was unlikely to be a result of the challenge. The ability to achieve cross-serovar protection following live vaccination, but not after vaccination with bacterin preparations, suggests that cross-serovar protection may be dependent on the presentation of in vivo-regulated proteins to the immune system. In addition, the route of vaccination may also play a role in the level of cross-protection obtained. Intranasal vaccination was chosen because it best mimics the natural route of A. pleuropneumoniae infection, which is known to induce an immune response that is cross-protective. In contrast, bacterin vaccines are delivered by subcutaneous or intramuscular injection. It has been demonstrated previously that the immune responses induced by a commercial vaccine are very different from those induced following aerosol exposure of pigs to A. pleuropneumoniae (14). Sera obtained from animals postvaccination and prior to challenge responded weakly to ApxIIA by enzyme-linked immunosorbent assay (results not shown). Additional work is ongoing to further characterize the immune responses obtained through vaccination with HS93C− Ampr and to compare them with those obtained during natural infection.
The findings of this protection study demonstrate the potential of HS93C− Ampr to be delivered via the nasal route as a vaccine to protect pigs against porcine pleuropneumonia. Activation of ApxIIA was found to be necessary for virulence in a mouse model but not for secretion. The ability of HS93C− Ampr to protect pigs from virulent A. pleuropneumoniae challenge, together with the central role of Apx immunity in protecting pigs from A. pleuropneumoniae infection, suggests that activation of the toxin is not required to induce protective immunity. This is the first report of a live vaccine strain of A. pleuropneumoniae that is suitable for use in pigs and offers cross-serovar protection.
ACKNOWLEDGMENT
We gratefully acknowledge the financial support of the Australian Pig Research and Development Corporation.
REFERENCES
- 1.Anderson C, Potter A A, Gerlach G-F. Isolation and molecular characterization of spontaneously occurring cytolysin-negative mutants of Actinobacillus pleuropneumoniae serotype 7. Infect Immun. 1991;59:4110–4116. doi: 10.1128/iai.59.11.4110-4116.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhatia B, Mittal K R, Frey J. Factors involved in the immunity against Actinobacillus pleuropneumoniae in mice. Vet Microbiol. 1991;28:147–158. doi: 10.1016/0378-1135(91)90122-v. [DOI] [PubMed] [Google Scholar]
- 3.Chang Y-F, Young R Y, Struck D K. Cloning and characterization of a hemolysin gene from Actinobacillus (Haemophilus) pleuropneumoniae. DNA. 1989;8:635–647. doi: 10.1089/dna.1.1989.8.635. [DOI] [PubMed] [Google Scholar]
- 4.Fedorka-Cray P J, Huether M J, Stine D L, Anderson G A. Efficacy of a cell extract from Actinobacillus (Haemophilus) pleuropneumoniae serotype 1 against disease in swine. Infect Immun. 1990;58:358–365. doi: 10.1128/iai.58.2.358-365.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fedorova N D, Highlander S K. Generation of targeted nonpolar insertions and operon fusions in Pasteurella haemolytica and creation of a strain that produces and secretes inactive leukotoxin. Infect Immun. 1997;65:2593–2598. doi: 10.1128/iai.65.7.2593-2598.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Felmlee T, Pellett S, Lee E Y, Welch R A. Escherichia coli hemolysin is released extracellularly without cleavage of a single peptide. J Bacteriol. 1985;163:88–93. doi: 10.1128/jb.163.1.88-93.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frey J. Construction of a broad host range shuttle vector for gene cloning and expression in Actinobacillus pleuropneumoniae and other Pasteurellaceae. Res Microbiol. 1992;143:263–268. doi: 10.1016/0923-2508(92)90018-j. [DOI] [PubMed] [Google Scholar]
- 8.Frey J, Nicolet J. Regulation of hemolysin expression in Actinobacillus pleuropneumoniae serotype 1 by Ca2+ Infect Immun. 1988;56:2570–2575. doi: 10.1128/iai.56.10.2570-2575.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frey J, Nicolet J. Hemolysin patterns of Actinobacillus pleuropneumoniae. J Clin Microbiol. 1990;28:232–236. doi: 10.1128/jcm.28.2.232-236.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frey J, van den Bosch H, Segers R, Nicolet J. Identification of a second hemolysis (HlyII) in Actinobacillus pleuropneumoniae serotype 1 and expression of the gene in Escherichia coli. Infect Immun. 1992;60:1671–1676. doi: 10.1128/iai.60.4.1671-1676.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frey J, Beck M, Stucki U, Nicolet J. Analysis of hemolysin operons in Actinobacillus pleuropneumoniae. Gene. 1993;123:51–58. doi: 10.1016/0378-1119(93)90538-e. [DOI] [PubMed] [Google Scholar]
- 12.Frey J, Bosse J T, Chang Y-F, Cullen J M, Fenwick B, Gerlach G F, Gygi D, Haesebrouck F, Inzana T J, Jansen R, Kamp E M, Macdonald J, MacInnes J L, Mittal K R, Nicolet J, Rycroft A N, Segers R P A M, Smits M A, Stenbaek E, Struck D K, van den Bosch J F, Willson P J, Young R. Actinobacillus pleuropneumoniae RTX-toxins: uniform designation of haemolysins, cytolysins, pleurotoxins and their genes. J Gen Microbiol. 1993;139:1723–1728. doi: 10.1099/00221287-139-8-1723. [DOI] [PubMed] [Google Scholar]
- 13.Frey J, Beck M, Nicolet J. RTX-toxins of Actinobacillus pleuropneumoniae. In: Freer J, Aitken R, Alouf J E, Boulnois G, Falmagne P, Fehrenbach F, Montecucco C, Piemont Y, Rappuoli R, Wadstrom T, Witholt B, editors. Bacterial protein toxins. Stuttgart, Germany: Gustav Fischer Verlag; 1994. pp. 322–332. [Google Scholar]
- 14.Furesz S E, Mallard B A, Bossé J T, Rosendal S, Wilkie B N, Macinnes J I. Antibody- and cell-mediated immune responses of Actinobacillus pleuropneumoniae-infected and bacterin-vaccinated pigs. Infect Immun. 1997;65:358–365. doi: 10.1128/iai.65.2.358-365.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gerlach G-F, Anderson C, Rossi-Campos A, Potter A A. Proceedings of the 12th International Pig Veterinary Society Congress. 1992. The role of the 103 kd Actinobacillus pleuropneumoniae cytolysin in virulence and the association of the gene encoding the insertion sequence-like elements; p. 199. The Hague, The Netherlands. [Google Scholar]
- 16.Higgins R, Lariviere S, Mittal K R, Martinaue G P, Rousseau P, Cameron J. Evaluation of a killed vaccine against porcine pleuropneumonia due to Haemophilus pleuropneumoniae. Can Vet J. 1985;26:86–89. [PMC free article] [PubMed] [Google Scholar]
- 17.Inzana T J, Todd J, Ma J, Veit H. Characterisation of a nonhemolytic mutant of Actinobacillus pleuropneumoniae serotype 5: role of the 110 kilodalton hemolysin in virulence and immunoprotection. Microb Pathog. 1991;10:281–296. doi: 10.1016/0882-4010(91)90012-y. [DOI] [PubMed] [Google Scholar]
- 18.Issartel J P, Koronakis V, Hughes C. Activation of Escherichia coli prohaemolysin to the mature toxin by acyl carrier protein-dependent fatty acylation. Nature (London) 1991;351:759–761. doi: 10.1038/351759a0. [DOI] [PubMed] [Google Scholar]
- 19.Kamp E M, Popma J K, Anakotta J, Smits M A. Identification of hemolytic and cytotoxic proteins of Actinobacillus pleuropneumoniae by use of monoclonal antibodies. Infect Immun. 1991;59:3079–3085. doi: 10.1128/iai.59.9.3079-3085.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Komal J P S, Mittal K R. Grouping of Actinobacillus pleuropneumoniae strains of serotypes 1-12 on the basis of their virulence in mice. Vet Microbiol. 1990;25:229–240. doi: 10.1016/0378-1135(90)90080-f. [DOI] [PubMed] [Google Scholar]
- 21.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 22.McKimm-Breschkin J L. The use of teramethylbenzidine for solid phase immunoassays. J Immunol Methods. 1990;135:277–280. doi: 10.1016/0022-1759(90)90282-z. [DOI] [PubMed] [Google Scholar]
- 23.Newman P R, Corstvet R E, Panciera R J. Distribution of Pasteurella haemolytica and Pasteurella multocida in the bovine lung following vaccination and challenge exposure as an indicator of lung resistance. Am J Vet Res. 1982;43:417–422. [PubMed] [Google Scholar]
- 24.Nielson R. Seroepidemiology of Actinobacillus pleuropneumoniae. Can J Vet Res. 1988;29:580–582. [PMC free article] [PubMed] [Google Scholar]
- 25.Pohl S, Berstchinger H U, Frederiksen W, Mannheim W. Transfer of Haemophilus pleuropneumoniae and the Pasteurella haemolytica-like organism causing porcine necrotic pleuropneumonia to the genus Actinobacillus (Actinobacillus pleuropneumoniae comb. nov.) on the basis of phenotypic and deoxyribonucleic acid relatedness. Int J Syst Bacteriol. 1983;33:510–514. [Google Scholar]
- 26.Prideaux C T, Pierce L, Krywult J, Hodgson A L M. Protection of mice against challenge with homologous and heterologous serovars of Actinobacillus pleuropneumoniae, following live vaccination. Curr Microbiol. 1998;37:324–332. doi: 10.1007/s002849900386. [DOI] [PubMed] [Google Scholar]
- 27.Radford A J, Hodgson A L M. Construction and characterisation of a Mycobacterium-Escherichia coli shuttle vector. Plasmid. 1991;25:149–153. doi: 10.1016/0147-619x(91)90029-v. [DOI] [PubMed] [Google Scholar]
- 28.Rosendale S, Devenish J, MacInnes J L, Lumsden J H, Watson S, Xun H. Evaluation of heat-sensitive, neutrophil-toxic, and hemolytic activity of Haemophilus (Actinobacillus) pleuropneumoniae. Am J Vet Res. 1988;49:1053–1058. [PubMed] [Google Scholar]
- 29.Rycroft A N, Williams D, Cullen J M, Macdonald J. The cytotoxin of Actinobacillus pleuropneumoniae (pleurotoxin) is distinct from the haemolysin and is associated with a 120 kDa polypeptide. J Gen Microbiol. 1991;137:561–568. doi: 10.1099/00221287-137-3-561. [DOI] [PubMed] [Google Scholar]
- 30.Rycroft A N, Williams D, McCandlish I A P, Taylor D J. Experimental reproduction of acute lesions of porcine pleuropneumonia with a haemolysin-deficient mutant of Actinobacillus pleuropneumoniae. Vet Res. 1991;16:441–443. doi: 10.1136/vr.129.20.441. [DOI] [PubMed] [Google Scholar]
- 31.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 32.Smits M A, Briaire J, Jansen R, Smith H, Kamp E M, Gielkens A. Cytolysins of Actinobacillus pleuropneumoniae serotype 9. Infect Immun. 1991;59:4497–4504. doi: 10.1128/iai.59.12.4497-4504.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tascon R I, Rodriguez-Ferri E F, Gutierrez-Martin C B, Rodriguez-Barbosa I, Berche P, Vazquez-Boland J A. Transposon mutagenesis in Actinobacillus pleuropneumoniae with a Tn10 derivative. J Bacteriol. 1993;175:5717–5722. doi: 10.1128/jb.175.17.5717-5722.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tascón R I, Vázquez-Boland J A, Gutiérrez-Martin C B, Rodríguez-Barbosa I, Rodríguez-Ferri E F. The RTX haemolysins ApxI and ApxII are major virulence factors of the swine pathogen Actinobacillus pleuropneumoniae: evidence from mutational analysis. Mol Microbiol. 1994;14:207–216. doi: 10.1111/j.1365-2958.1994.tb01282.x. [DOI] [PubMed] [Google Scholar]
- 35.Wagner W, Kuhn M, Goebel W. Active and inactive forms of hemolysin (HlyA) from Escherichia coli. Mol Microbiol. 1988;369:39–46. doi: 10.1515/bchm3.1988.369.1.39. [DOI] [PubMed] [Google Scholar]
- 36.Welch R A. Pore-forming cytolysins of Gram-negative bacteria. Mol Microbiol. 1991;5:521–528. doi: 10.1111/j.1365-2958.1991.tb00723.x. [DOI] [PubMed] [Google Scholar]
- 37.Welch R A, Bauer M E, Kent A D, Leeds J A, Moayeri M, Regassa L B, Swenson D L. Battling against host phagocytes: the wherefore of the RTX family of toxins? Infect Agents Dis. 1995;4:254–272. [PubMed] [Google Scholar]