Abstract
Nitrogen (N) is a major limiting factor for plant growth and crop production. The use of N fertilizer in forestry production is increasing each year, but the loss is substantial. Mastering the regulatory mechanisms of N uptake and transport is a key way to improve plant nitrogen use efficiency (NUE). However, this has rarely been studied in pecans. In this study, 10 AMT and 69 NRT gene family members were identified and systematically analyzed from the whole pecan genome using a bioinformatics approach, and the expression patterns of AMT and NRT genes and the uptake characteristics of NH4+ and NO3− in pecan were analyzed by aeroponic cultivation at varying NH4+/NO3− ratios (0/0, 0/100,25/75, 50/50, 75/25,100/0 as CK, T1, T2, T3, T4, and T5). The results showed that gene duplication was the main reason for the amplification of the AMT and NRT gene families in pecan, both of which experienced purifying selection. Based on qRT-PCR results, CiAMTs were primarily expressed in roots, and CiNRTs were majorly expressed in leaves, which were consistent with the distribution of pecan NH4+ and NO3− concentrations in the organs. The expression levels of CiAMTs and CiNRTs were mainly significantly upregulated under N deficiency and T4 treatment. Meanwhile, T4 treatment significantly increased the NH4+, NO3−, and NO2− concentrations as well as the Vmax and Km values of NH4+ and NO3− in pecans, and Vmax/Km indicated that pecan seedlings preferred to absorb NH4+. In summary, considering the single N source of T5, we suggested that the NH4+/NO3− ratio of 75:25 was more beneficial to improve the NUE of pecan, thus increasing pecan yield, which provides a theoretical basis for promoting the scale development of pecan and provides a basis for further identification of the functions of AMT and NRT genes in the N uptake and transport process of pecan.
Keywords: NH4+, NO3−, AMT, NRT, pecan
1. Introduction
Ammonium nitrogen (NH4+) and nitrate nitrogen (NO3−) are the two main forms of nitrogen (N) that plants can absorb and use. The absorption, transport, and assimilation of NH4+ and NO3− in plants have been studied extensively. Physiologically, NH4+ and NO3− uptake and translocation systems were identified mainly by root absorption kinetics, which were classified into two types: high-affinity transport systems (HATs) and low-affinity transport pathways (LATs) [1]. Vmax (maximum ion uptake rate) and Km (Mie’s constant) are the two main parameters in the kinetic equation for root nutrient uptake, which can quantitatively characterize the plant uptake of nutrient ions. In general, a larger Vmax value indicates that the plant has a great uptake potential for a certain ion, and the number of the ion transport carrier protein on the cell membrane determines the size of Vmax. Km indicates the affinity between the ion absorbed by the root system and the uptake site (transport carrier), and the greater the affinity, the smaller the Km value [2]. Previous studies have found that plants have a preference for the intake and utilization of NH4+ and NO3−; when both N forms are present, plants will preferentially intake and utilize one of them [3]. At the molecular level, many members of the ammonium transporter (AMT) and nitrate transporter (NRT) gene families have been cloned and characterized, and the uptake and utilization of NH4+ and NO3− by plants is regulated by multiple genes in the N transporter protein family [4,5].
AMTs are carrier proteins that actively transport NH4+ across biological cell membranes, and they were divided into three main subfamilies: AMT/MEP/Rh (Ammonium transporter/Methylamine permease/Rhesus protein) [6]. The AMT family can usually be divided into two subfamilies, namely AMT1 and AMT2 [7]. Both AMT1 and AMT2 show high affinity for NH4+, but members of the AMT1 subfamily play a more important role in high-affinity NH4+ uptake [8]. AMT2 has a more complex gene structure and protein profile than AMT1 [9]. The AMT1 subfamily of Arabidopsis thaliana has five members, while AMT2 has low homology with the five AMT1 subfamily members and belongs to the MEP subfamily [10]. Expression of AtAMT1;1 in roots is significantly correlated with N uptake, whereas expression of AtAMT1;2 in roots is insensitive to changes in N concentration [11]. AtAMT1;4 mediate the intake of NH4+ at the pollen plasma membrane [12]. NH4+ is regulated into the cytoplasm through AtAMT1;1 and AtAMT2;1, which are primarily expressed in leaves [13]. In addition, AtAMT2;1 may play a role in the transport of NH4+ from the roots to the ground [10]. A study by Couturier et al. on AMT proteins in poplar (Populus L.) found that the expression of PtrAMT1;2 was influenced by intracellular N concentration. Most PtrAMT1s were preferentially expressed in roots, while most PtrAMT2s were majorly expressed in stems, and PtrAMT3;1 was expressed only in senescing leaves [14].
The absorption systems, corresponding genes, and regulatory mechanisms of NO3− by plants are different from those of NH4+. The uptake of NO3- by plants is the process of pumping protons out of the cell through the H+-ATPase in the plasma membrane, creating pH and electrical (ΔΨ) gradients across the plasma membrane that allows the NO3- transporter to take up NO3− into the cell [15]. Four families of transporters are known to contribute to nitrate uptake and transport in plants: the nitrate transporter protein 1 (NRT1/PTR/NPF), nitrate transporter 2 (NRT2), chloride channel (CLC), and slow anion-associated channel homolog (SLC/SLAH) family [16]. The NRT1 and NRT2 are responsible for LATS and HATS, respectively [17]. Members of the NRT1 subfamily are responsible for transporting NO3−, hormones, glucosinolates and dipeptides [18]. In Arabidopsis, AtNPF6;3/AtNRT1;1/CHL1 was the first NRT1 subfamily member to be identified and cloned, and this protein is an amphiphilic NRT [19]. Except for AtNRT1;1, most NRT1 exhibited low affinity [20]. NPF4;6/NRT1;2 and NPF2;7/NAXT1 were also shown to be involved in root NO3− uptake, with NPF4.6 acting on NO3− influx [21] and NPF2.7 involved in NO3− efflux [22]. Other NRT1s are mainly related to the internal transport of NO3− in processes such as xylem and phloem loading and transport to leaves or seeds [23]. NRT2 belongs to the major facilitator superfamily (MFS) [24], NRT3/NAR2 is a high-affinity NRT [25]. NRT2.1, NRT2.2, NRT2.4, and NRT2.5 were known to be associated with root NO3− influx, and AtNRT2.1 expression was induced at low NO3− levels and repressed at high NO3− concentrations [26]. AtNRT2.4 and AtNRT2.5 are associated with root NO3− absorbance during severe N deficiency [27]. The NRT3 subfamily plays a significant role in NO3− transport by regulating the activity of NRT2, but they are not transport proteins themselves [28].
Pecan (Carya illinoinensis (Wangenh.) K. Koch) belongs to the Juglandaceae family and is one of the world’s famous nut tree species, indigenous to the United States and Mexico [29]. Pecans have a good development prospect in China because of the high content of various nutrients and the important economic value of the kernels [30]. However, as an important economic tree species introduced for many years in China, pecan still has the problem of insufficient yield, and low nitrogen use efficiency (NUE) is an important factor leading to the underproduction of pecan. The key way to improve NUE is to master the N absorption and utilization pattern of plants and the molecular regulation mechanism, but there is no systematic and in-depth research on pecan in this subject. In this study, we determined the absorption characteristics of NH4+ and NO3− in different N forms. We identified 10 AMT and 69 NRT gene family members in pecan and classified them into different evolutionary subfamilies. Then, we analyzed their gene structure, replication events and expression patterns to explore their functions in varying N forms, and we provide a theoretical basis for improving the NUE of pecan.
2. Results
2.1. Identification and Sequence Analysis of AMT and NRT Gene Family Members in Pecan
A total of 11 AMT candidates were identified that contained AMT or AMT-like repeats, and 95 NRT candidates were identified that contained NRT or NRT-like repeats. After validation of AMT and NRT structural domains by Pfam and NCBI CD-search, 10 were identified as AMT gene family members, and 69 were identified as NRT gene family members. The AMT and NRT genes were renamed according to the Arabidopsis gene names as well as the NCBI blastp results for subsequent analysis. Table S3 provided details of AMTs and NRTs.
Sequence analysis of pecan AMT and NRT gene family members revealed that the number of exons in AMTs ranged from one to five, and in NRTs from two to nine. The CDS length of AMTs ranged from 471 to 1542 bp, and in NRTs from 1053 to 2826 bp. Most AMTs (9/10) and NRTs (56/69) were stable proteins with a low protein instability index (instability index < 40). GRAVY analysis showed that the hydration of AMT and NRT proteins in pecan was greater than 0, indicating that these proteins are hydrophobic. Subcellular localization predictions showed that most AMTs (9/10) localized to the cell membrane and most NRTs (64/69) localized to the vesicles.
2.2. Phylogenetic Analysis of AMTs and NRTs in Different Species
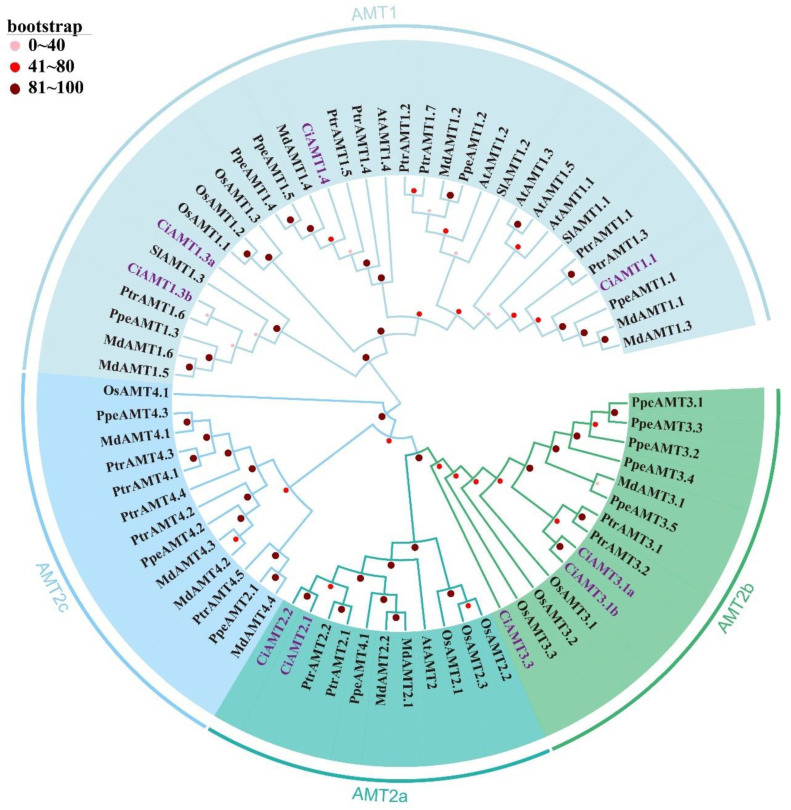
To decipher the evolutionary relationships and functional associations of pecan AMTs and NRTs, phylogenetic trees were constructed using pecan AMT and NRT proteins and other plants, respectively (Figure 1 and Figure 2). According to the phylogenetic trees, all AMT proteins were divided into two distinct evolutionary branches: AMT1 and AMT2 with strong support (Bootstrap = 100%). The AMT2 was further divided into three clusters: AMT2a, AMT2b, and AMT2c. AMT1 was the largest branch and included 34 AMTs (four CiAMTs), AMT2a included two CiAMTs, AMT2b included three CiAMTs, and AMT2c had no pecan AMT gene family members.
Figure 1.
Phylogenetic analysis of AMT gene family in pecan (Carya illinoinensis), poplar (Populus trichocarpa), apple (Malus domestica), peach (Amygdalus persica L.), tomato (Lycopersicon esculentum Miller), rice (Oryza sativa L.) and Arabidopsis (Arabidopsis thaliana). AMT proteins from seven species were divided into two subfamilies (AMT1 and AMT2). The AMT2 was further divided into three clusters (AMT2a, AMT2b, and AMT2c).
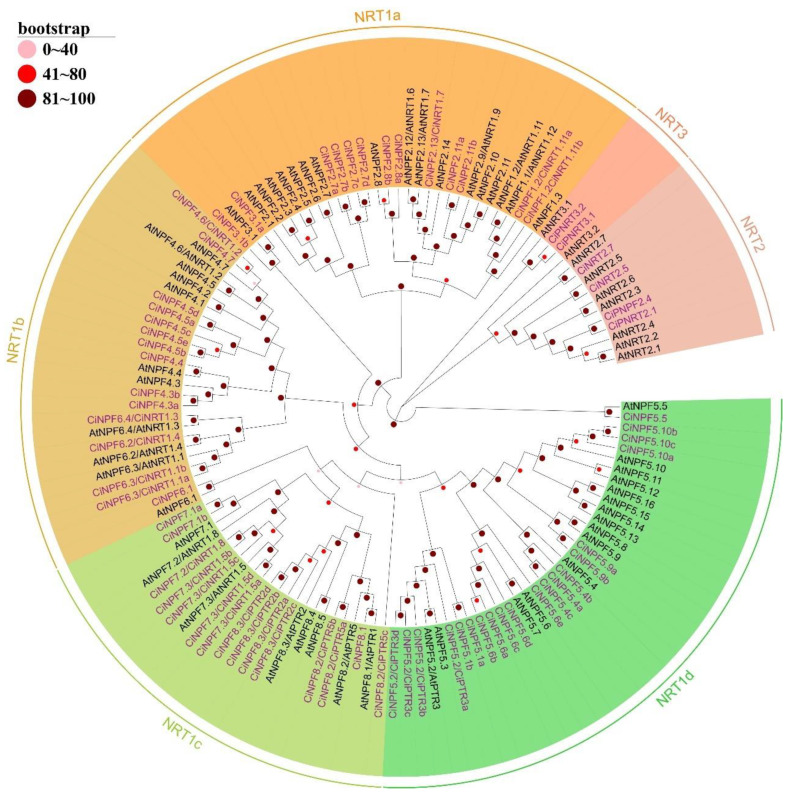
Figure 2.
Phylogenetic analysis of NRT gene family in pecan (Carya illinoinensis) and Arabidopsis (Arabidopsis thaliana). NRT proteins from two species were divided into three subfamilies (NRT1, NRT2 and NRT3). The NRT1 was further divided into four clusters (NRT1a, NRT1b, NRT1c, and NRT1d).
All NRT proteins were divided into three main clades: NRT1/PTR, NRT2, and NRT3 subfamily. The NRT1 formed four subclasses, named NRT1a, NRT1b, NRT1c, and NRT1d, and included 62 CiNRTs and 52 AtNRTs. NRT2 included five CiNRTs and seven AtNRTs, while NRT3 included two CiNRTs and two AtNRTs. The evolutionary tree had 48 sister pairs, the majority of which were paralogous proteins, 38 pairs in total (21 pairs in pecan and 17 pairs in Arabidopsis), and 10 pairs of orthologous proteins. Only the NRT3 subfamily had no orthologous proteins, while all other clades contained orthologous and paralogous proteins.
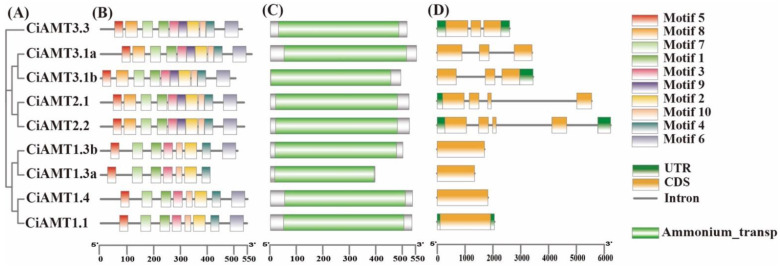
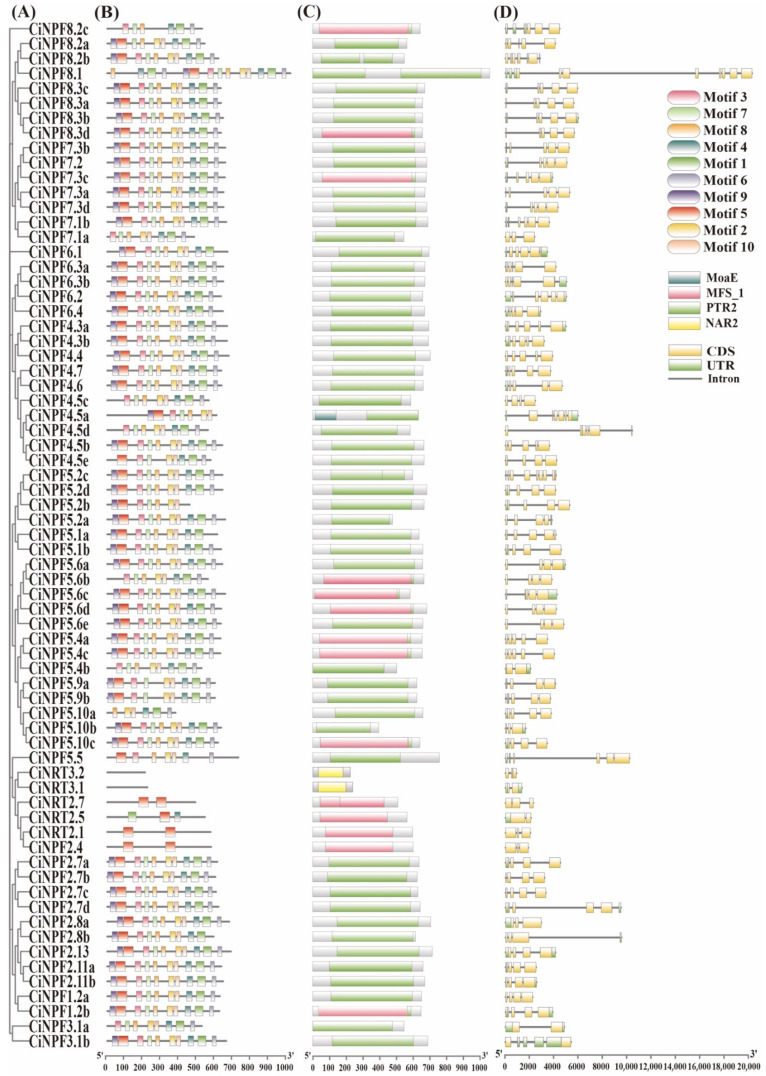
2.3. Phylogenetic Tree, Conserved Motif, Conserved Domain, and Gene Structural Analyses of CiAMTs and CiNRTs
To better understand the evolutionary relationships and functional associations of pecan AMT and NRT genes, we constructed unrooted phylogenetic trees with pecan AMT and NRT proteins, respectively (Figure 3A and Figure 4A). According to MEME analysis, we found 10 motifs in most of the pecan AMT and NRT proteins (Figure 3B and Figure 4B, Table S5). Motifs 1 to 7 were found in all AMT subfamilies, suggesting that these motifs may be characteristic motifs associated with members of the AMT gene family. Only 2–3 motifs in NRT2 were identical to the NRT1 subfamily, while NRT3 had no identical motifs to the NRT1 subfamily. We analyzed the structural domains of pecan AMT and NRT proteins using Pfam search and found that only one conserved Ammonium_trasp domain existed in all pecan AMT proteins, and they were all located at similar positions, while three conserved structural domains existed in pecan NRT proteins (Figure 3C and Figure 4C, Table S4). The NRT1 subfamily had two conserved structural domains: PTR2 and MSF_1; NRT2 had only the MSF_1 structural domain; NRT3 contained only the NAR2 structural domain.
Figure 3.
Phylogenetic analysis, conserved motif, conserved domain and gene structural of the AMT genes in pecan. Phylogenetic analysis of the AMT genes in pecan (A). The conserved motifs of the AMT genes in pecan (B). The conserved domain of the AMT genes in pecan (C). The gene structure of the AMT genes in pecan (D).
Figure 4.
Phylogenetic analysis, conserved motif, conserved domain and gene structural of the NRT genes in pecan. Phylogenetic analysis of the NRT genes in pecan (A). The conserved motifs of the NRT genes in pecan (B). The conserved domain of the NRT genes in pecan (C). The gene structure of the NRT genes in pecan (D).
In addition, we analyzed the exons/introns of the pecan AMT and NRT genes to study the structural diversity. The results showed that the AMT1s contained no intron, and the AMT2s contained 2–4 introns (Figure 3D); the NRT1s had 2–9 introns, the NRT2s and NRT3s contained 1–2 introns (Figure 4D). In conclusion, the phylogenetic correlation between gene structure and prediction strongly supports a close evolutionary relationship between paired genes within the same subfamily.
2.4. Synteny Analysis of CiAMTs and CiNRTs
To determine the replication events of pecan AMT and NRT genes, we performed a synteny analysis of the pecan genome. The results showed that there were five duplicated gene pairs among the ten members of the pecan AMT gene family, two of which originated from tandem duplication and three from segmental duplication (Figure S1). There are 103 duplicated gene pairs among 69 members of the pecan NRT gene family, of which 9 originated from tandem duplication and 94 from segmental duplication. This suggested that fragment replication events played an important role in the expansion of the AMT and NRT gene families in pecan.
To examine the selection type of duplicate gene pairs in the pecan AMT and NRT gene families, the Ka/Ks ratios were analyzed for duplication events (Table S6). Ka/Ks < 1 means the gene is subjected to purifying selection, Ka/Ks > 1 means the gene underwent positive selection, and Ka/Ks = 1 means neutral evolution. The Ka/Ks values of all duplicate gene pairs were less than 1, indicating that the amplification of the pecan AMT and NRT genes was mainly influenced by purifying selection.
2.5. Effect of N Forms on Quantitative qRT-PCR Analysis of AMT and NRT Gene Expression Levels in Pecan
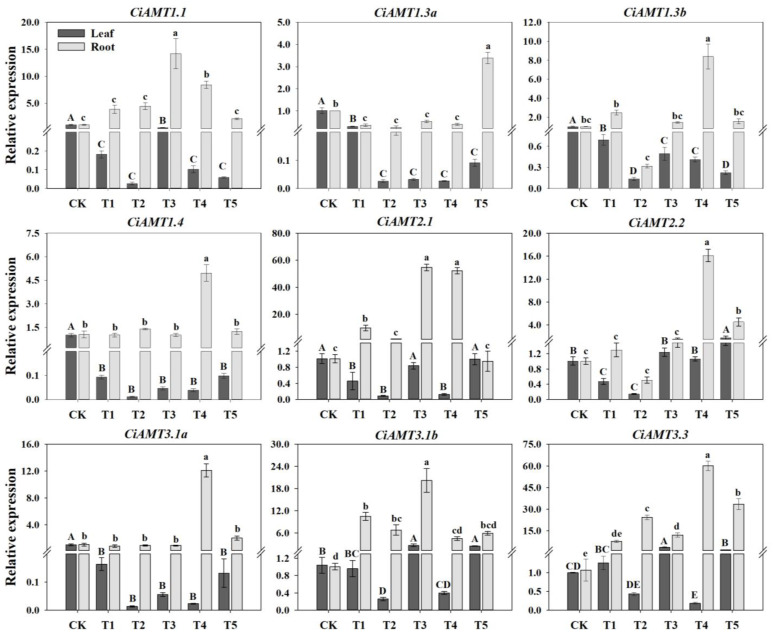
The results of qRT-PCR analysis of CiAMTs showed that the relative expression levels of CiAMTs were significantly affected by different N forms (Figure 5). The relative expression levels of almost all CiAMTs were higher in roots than in leaves, indicating that CiAMTs mainly worked in roots. In the roots, CiAMT1.1 was significantly upregulated under T3 and T4 (p < 0.05), CiAMT1.3a was significantly upregulated only under T5 (p < 0.05), and CiAMT1.3b, CiAMT1.4 and CiAMT3.1a were all significantly upregulated only under T4 (p < 0.05). CiAMT2.1 was significantly upregulated under T1, T3, and T4 (p < 0.05), CiAMT2.2 was significantly upregulated under T4 and T5 (p < 0.05), and CiAMT3.1b was significantly upregulated under T1, T2, and T3 (p < 0.05). The relative expression of CiAMT3.3 was significantly upregulated under all N form treatments, with the most significant in T4 and T5 (p < 0.05). In leaves, all of them showed significant downregulated except CiAMT2.1, CiAMT2.2, CiAMT3.1b, and CiAMT3.3, which were significantly upregulated under individual treatments (p < 0.05).
Figure 5.
Relative expression of pecan CiAMT genes under varying NH4+:NO3− ratios. The expression levels of CiAMT in pecan leaves and roots after varying NH4+:NO3− ratio treatments were quantified by qRT-PCR, with Actin as the reference gene. Different capital letters indicate significant differences in leaves (p < 0.05), and different lowercase letters indicate significant differences in roots (p < 0.05).
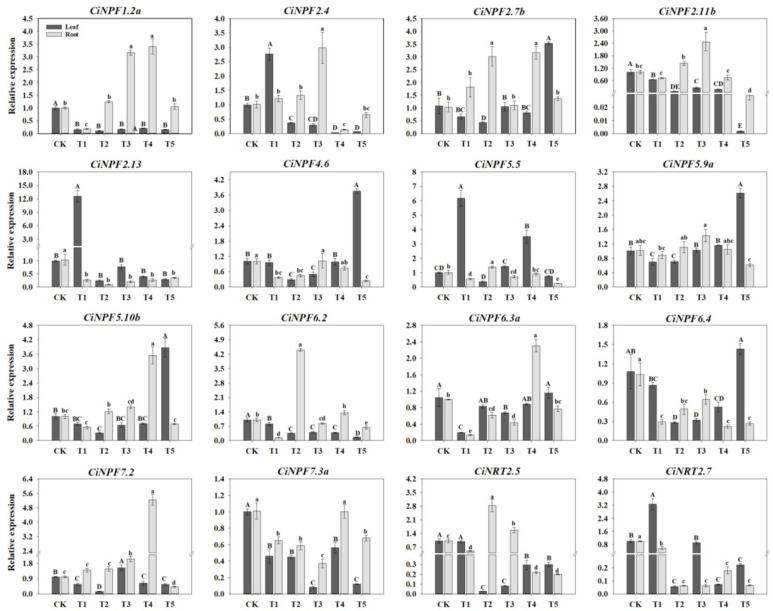
Based on the results of previous studies [16], some members of the Arabidopsis and rice NRT gene families have been shown to be associated with NO3− uptake and transport. According to the phylogenetic tree, we selected 16 pecan CiPawNRTs corresponding to them and further analyzed them using qRT-PCR (Figure 6). The qRT-PCR results showed that CiNRTs showed different expression patterns in different N forms as well as in different pecan organs. Except for CiNPF2.13, CiNPF4.6, CiNPF5.5, CiNPF6.3a, CiNPF6.4, CiNRT2.5, and CiNRT2.7, which showed relative higher expression in leaves than in roots under most N form treatments, most of the other CiNRTs showed higher expression in roots than in leaves. In leaves, CiNPF2.4 and CiNPF2.13 under T1, CiNPF5.5 under T1 and T4, CiNPF7.2 under T3, CiNRT2.7 under T1, and CiNPF2.7b, CiNPF4.6, CiNPF5.9a, CiNPF5.10b under T5 showed significantly upregulated expression (p < 0.05). In roots, CiNPF1.2a was significantly upregulated under T3 and T4 (p < 0.05), and CiNPF2.7b was significantly upregulated under T2 and T4 (p < 0.05). CiNPF2.4 and CiNPF2.11b were significantly upregulated only with T3 (p < 0.05), while CiNPF5.10b was significantly upregulated only with T4 (p < 0.05). CiNPF2.13, CiNPF6.4, and CiNRT2.7 were significantly downregulated under each N form treatment (p < 0.05), CiNPF4.6 was significantly downregulated under T1, T2, and T5 (p < 0.05), while CiNPF7.3a was significantly downregulated under T1, T2, T3, and T5 (p < 0.05). CiNPF5.5 was significantly upregulated under T2 as well as significantly downregulated under T3 (p < 0.05), CiNPF6.2 was significantly upregulated under T2 as well as significantly downregulated under T1, T3, and T5 (p < 0.05), and CiNPF6.3a was significantly upregulated under T4 as well as significantly downregulated under T1, T2, and T3 (p < 0.05). CiNPF7.2 was significantly upregulated under T3 and T4 and downregulated under T5 (p < 0.05). CiNRT2.5 was significantly upregulated under T2 and T3 and downregulated under the other treatments (p < 0.05).
Figure 6.
Relative expression of selected pecan CiNRT genes under varying NH4+:NO3− ratios. The expression levels of selected CiNRT in pecan leaves and roots after varying NH4+:NO3− ratio treatments were quantified by qRT-PCR, with Actin as the reference gene. Different capital letters indicate significant differences in leaves (p < 0.05), and different lowercase letters indicate significant differences in roots (p < 0.05).
2.6. Effect of N Forms on NH4+, NO3− and NO2− Concentration in Pecan
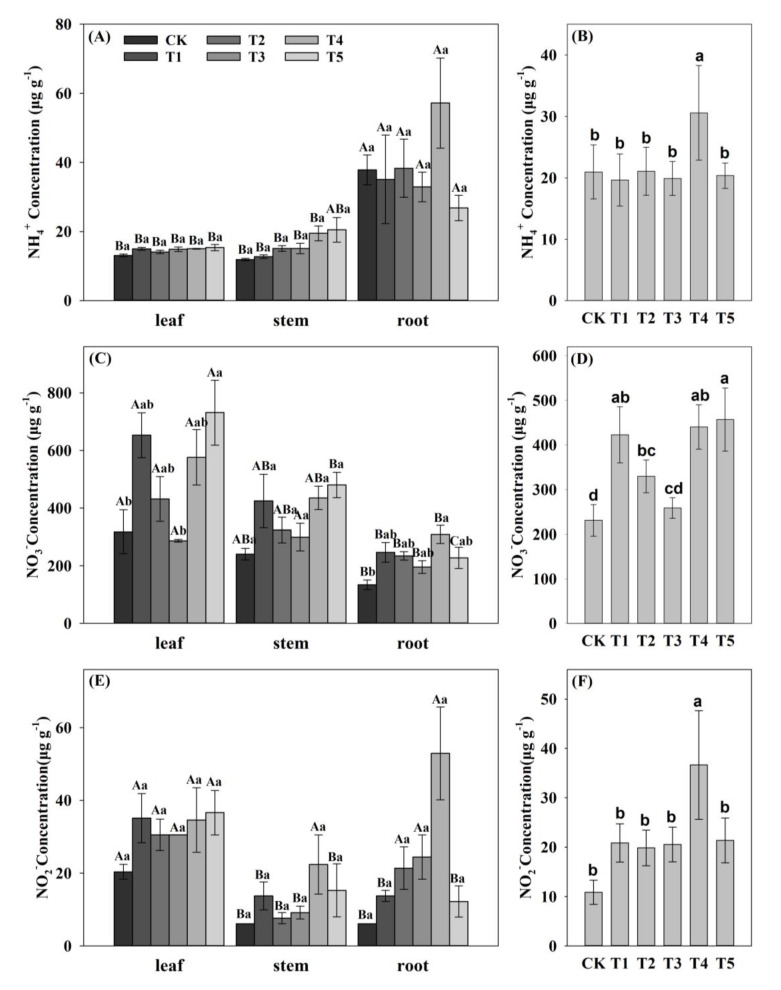
To further investigate the response mechanisms of pecan AMT and NRT genes to N forms, we measured the concentrations of NH4+, NO3−, and NO2− in pecan under different N forms (Figure 7). The results showed that there was no significant difference in the NH4+ concentrations of pecan in all organs under different N forms. Except for the T5 treatment, NH4+ concentrations under all other treatments showed greater in roots than in leaves and stem (p < 0.05) and no significant difference between leaves and stems. The variability of NO3− concentrations in each organ of pecan varied among treatments, and T5 showed significantly greater than CK and T3 in leaves (p < 0.05), and no significant differences were found between T1, T2, T4, and other treatments. In the stems, no significant differences were found between treatments. In the roots, it showed that T4 was significantly greater than CK (p < 0.05), and T4 was not significantly different from the other treatments. Except for T3 and T5 treatment, the NO3− concentrations of pecan under all other treatments showed greater leaves than roots (p < 0.05) and no significant difference between stems, leaves, and roots. There was no significant difference in NO2− concentrations in all organs of pecan under different treatments, while the variability of NO2− concentrations in different organs under each treatment varied. CK, T1, and T5 showed no significant difference between stems and roots, and both of them were significantly greater than leaves (p < 0.05), T2 and T3 showed no significant difference between leaves and roots, and both of them were significantly greater than stems (p < 0.05), while T4 showed no significant variation in different organs.
Figure 7.
Differences of NH4+, NO3−, and NO2− concentrations of pecan under varying NH4+:NO3− ratios. Differences of NH4+ concentrations in pecan leaves, stems, and roots under varying NH4+:NO3− ratios (A). The mean values of NH4+ concentrations in pecan leaves, stems, and roots under varying NH4+:NO3− ratios (B). Differences of NO3− concentrations in pecan leaves, stems, and roots under varying NH4+:NO3− ratios (C). The mean values of NO3− concentrations in pecan leaves, stems, and roots under varying NH4+:NO3− ratios (D). Differences of NO2− concentrations in pecan leaves, stems, and roots under varying NH4+:NO3− ratios (E). The mean values of NO2− concentrations in pecan leaves, stems, and roots under varying NH4+:NO3− ratios (F). Upper capital letters indicate significant differences between organs (p < 0.05), and lowercase letters indicate significant differences between varying NH4+:NO3− ratios (p < 0.05).
At the mean level, pecan NH4+ concentrations were significantly greater in the T4 than in the other treatments (p < 0.05), with no significant differences between the other treatments. The pecan NO3-concentrations revealed no significant difference between T1, T4, and T5, and both of them were significantly greater than CK, T2, and T3 (p < 0.05). There was no significant difference between T2 and T1, T3 and T4, T2 was significantly greater than CK (p < 0.05), and no significant difference between CK and T3. The variability of pecan NO2- and NH4+ concentrations was consistent, suggesting that T4 was significantly greater than the other treatments (p < 0.05), with no significant differences between the other treatments.
2.7. Effect of N Forms on the Uptake Kinetics of NH4+and NO3− in Pecan
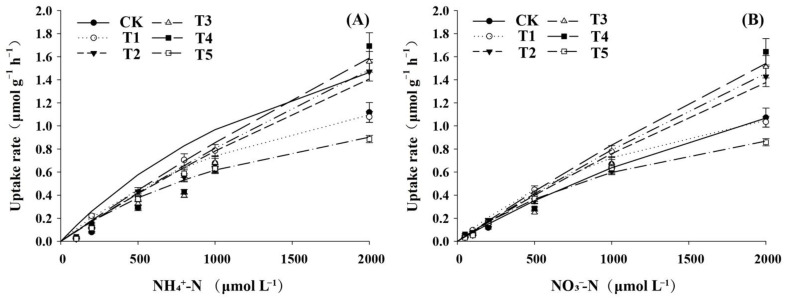
We also determined the kinetic properties of pecan NH4+ and NO3− uptake under different N forms (Figure 8). The results showed that the uptake rates of NH4+ and NO3− under T2, T3, and T4 were still in a significant upward trend at the ion concentration of 2000 μmol·L−1; the uptake rates of NH4+ and NO3− under CK, T1, and T5 leveled off at the medium ion concentration of 1000 μmol·L−1.
Figure 8.
NH4+ and NO3− uptake rates of pecan under varying NH4+:NO3− ratios. (A). NH4+ uptake rates of pecan under varying NH4+:NO3− ratios (B). NO3− uptake rates of pecan under varying NH4+:NO3− ratios.
The root uptake rates were processed data according to the Hofstee transformation equation to obtain the maximum uptake rate (Vm) and the Mee’s constant (Km) of pecan for different N forms with significant coefficients of determination R2 (Table 1). Different N forms showed significant effects on Vmax and Km of NH4+ and NO3− (p < 0.05), both showing T4 > T3 > T2 > CK > T1 > T5. This indicates that the involvement of a certain proportion of NH4+ accelerated the uptake of NH4+ and NO3− by pecans, but the affinity decreased, while NH4+ above a certain proportion decreased the uptake rate and increased the affinity. Except for the T1 treatment, the Vmax/Km of NH4+ was greater than that of NO3− under all treatments, indicating that the rate of NH4+ uptake by pecan was greater than that of NO3−.
Table 1.
N uptake kinetics of pecan roots under varying NH4+:NO3− ratios. Lowercase letters indicate significant differences between varying NH4+:NO3− ratios (p < 0.05).
| Treatment | NH4+-N | NO3−-N | ||||||
|---|---|---|---|---|---|---|---|---|
| Vmax/ (μmol·g−1·h−1) |
Km/ (mmol·L−1) |
Vmax/Km | Goodness of Fit (R2) | Vmax/ (μmol·g−1·h−1) |
Km/ (mmol·L−1) |
Vmax/Km | Goodness of Fit (R2) | |
| CK | 3.00 ± 0.21 d | 3.40 ± 0.07 d | 0.89 | 0.975 | 3.30 ± 0.01 c | 4.18 ± 0.47 c | 0.81 | 0.996 |
| T1 | 2.11 ± 0.00 d | 1.85 ± 0.18 e | 1.17 | 0.977 | 1.90 ± 0.02 d | 1.62 ± 0.14 d | 1.19 | 0.994 |
| T2 | 6.96 ± 0.42 c | 7.89 ± 0.21 c | 0.88 | 0.966 | 7.33 ± 0.52 b | 8.65 ± 0.18 b | 0.85 | 0.979 |
| T3 | 9.52 ± 0.93 b | 10.80 ± 0.43 b | 0.88 | 0.911 | 9.05 ± 0.78 a | 10.43 ± 0.36 a | 0.86 | 0.954 |
| T4 | 11.13 ± 0.93 a | 12.01 ± 0.39 a | 0.92 | 0.942 | 10.10 ± 0.55 a | 11.10 ± 0.35 a | 0.91 | 0.976 |
| T5 | 1.68 ± 0.02 d | 1.72 ± 0.10 e | 0.98 | 0.978 | 1.60 ± 0.01 d | 1.68 ± 0.12 d | 0.97 | 0.995 |
3. Discussion
3.1. Functional Differentiation of AMT and NRT Gene Family in Pecan Genome
The biological functions of AMT and NRT gene families in pecan are poorly understood. Therefore, we report for an earlier time identification of 10 AMT and 69 NRT gene family members from pecan, confirming that direct orthologs of AMT and NRT proteins should be highly conserved evolutionarily throughout the plant.
Studies on Arabidopsis AMT proteins have shown that AtAMTs had a prominent role in NH4+ assimilation at the cell membranes [10,11,31], and studies of flowering Chinese cabbage (Brassica campestris) also indicated that BcAMT2 was located on the plasma membrane [32], which was consistent with the predicted results of subcellular localization in this study (Table S3), and this is the optimal cellular structure for maintaining stable NH4+ concentrations in plants. AtNPF5.11, AtNPF5.12, and AtNPF5.16 were identified to function in the process of NO3− from the vesicle to the cytoplasm, thereby regulating NO3− distribution between roots and shoots [33]. In contrast, the predicted subcellular localization of the pecan NRT genes in this study showed that most of the NRT1s were localized to the vacuoles (Table S3), suggesting that pecan NRT1s may mostly act in the transport of NO3− between the vacuoles and the cytoplasm. There were also some NRT1s localized on the cell membrane, while all NRT2s were all localized to the cell membrane, suggesting that this fraction of NRT proteins may mediate assimilation and efflux of NO3− in plants as well as inter-subcellular transport, which was consistent with the findings of cassava (Manihot esculenta) [34]. According to the predicted results of subcellular localization, the subcellular localization of all NRT3s differed significantly from NRT1 and NRT2 in pecan, with CiNRT3.1 localized to the nucleus and CiNRT3.2 localized on the cell membrane, cell wall, chloroplast, and vacuoles (Table S3), indicating that the pecan NRT3s had more complex structures and functions.
3.2. Effect of N Forms on the Absorption Characteristics of NH4+and NO3− in Pecan
NH4+ and NO3− are the two main forms of N absorbed and utilized by plants, and previous studies have shown that a nutrient mixture of NH4+ and NO3− can improve crop yield and quality compared to a single source of N [35]. Therefore, understanding the best NH4+:NO3− ratios provides the possibility to improve the N utilization efficiency of plants. We investigated the effect of varying NH4+:NO3− ratios on the absorption and transport of N by measuring the concentrations of different N forms in the pecan organs (Figure 7).
This study showed that the concentrations of different N forms in pecan were tissue-specific. The NH4+ concentrations of pecan were significantly higher in roots than in leaves and stems, while NO3− and NO2− concentrations were significantly higher in leaves than in stems and roots, which was consistent with the finding that NH4+ was majorly assimilated in roots and NO3− was mostly translocated to leaves for storage or assimilation [36]. T4 significantly increased the total NH4+ concentrations of pecan, primarily in the roots, suggesting that T4 was more favorable to promote NH4+ absorbed by pecan roots, but had no effect on NH4+ transport between organs. All NH4+:NO3− ratio treatments increased the total NO3− concentrations, primarily in the leaves, indicating that the feeding of NH4+ and NO3− mainly promoted the translocation of pecan NO3− from roots to leaves, and possibly the acclimation of NO3− in the leaves. Compared to T1, T3 reduced the total pecan NO3− concentrations, which was in agreement with the results of studies in pepper (Capsicum annuum) [37]. T4 significantly increased the total NO2− concentrations of pecan, indicating that T4 promoted the conversion of NO3− to NO2−.
Previous conclusions on the effects of the simultaneous presence of NH4+ and NO3− on each other’s uptake were varied, with some suggesting that they have a facilitative or inhibitory effect [38,39], and that plant absorption of NH4+ and NO3− is limited by the maximum uptake threshold [40]. In this study, the uptake rates of NH4+ and NO3− gradually saturated with increasing substrate concentrations under a single N source, while they remained on an increasing trend under mixed N source treatments, with the T4 treatment being the most obvious, indicating that NH4+ and NO3− promoted each other’s intake in this study, as evidenced by the magnitude of Vmax values of pecan. However, single N source treatment increased the affinity of NH4+ and NO3−, and the combined application of NH4+ and NO3− decreased their affinity instead, which was generally consistent with the results of Kamminga-Van Wijk and Prins [41]. Vmax/Km is also commonly used to indicate plant preference for NH4+ and NO3− uptake [42], suggesting that pecans may be more biased toward NH4+ absorption.
3.3. Effect of N Forms on the Expression of CiAMTs and CiNRTs
The ingestion and transport of NH4+ and NO3− in plants is majorly mediated by AMT and NRT gene family members. Therefore, we investigated the expression modes of these genes in varying organs and N forms to further understand the effect of N forms on N uptake and transport in pecan.
In the present study, the relative expression levels of almost all CiAMTs were higher in roots than in leaves, indicating that the addition of NH4+ and NO3− majorly stimulated the expression of CiAMTs in roots. Studies have shown that AtAMT1.1, AtAMT1.2, AtAMT1.3, and AtAMT1.5 are the principal transporter proteins that take up high-affinity NH4+ into Arabidopsis roots, with AtAMT1.1 and AtAMT1.3 responsible for approximately two-thirds of the high-affinity NH4+ uptake capacity in the root; the CiAMT1s may have the same function [31,43]. The results of this study showed that all CiAMT1s were upregulated in roots of T4, except for CiAMT1.3a, which was upregulated in roots under T5, suggesting that T4 may have improved the absorption of high-affinity NH4+ by the pecan root. In this study, CiAMT1s were mostly upregulated in leaves under N lack, but numerous studies have shown that expression levels of AMT1s were upregulated in roots under N limited conditions [14,43,44]. This may be due to the variation caused by the longer duration of N deficiency in this study, or it is possible that the expression of CiAMTs in leaves was not only affected by N lack, but may also be involved in some other regulatory mechanisms [45]. Previous studies have shown that peach (Prunus persica) PpeAMT3;4 was primarily expressed in roots [46], and the expression pattern of CiAMT2s was similar to that of CiAMT1s, being expressed mostly in roots and almost significantly upregulated at all timepoints under T4.
Studies on Arabidopsis suggested that AtNPF6.3/AtNRT1.1 was not only an amphiphilic NO3− transport protein but might also act as a NO3− sensor under low NO3− conditions [47]. The expression level of CiNPF6.3a was significantly upregulated in roots under T4, suggesting that it may carry both translocation and signaling functions at this time. AtNPF4.6/AtNRT1.2 was not only a low-affinity NO3− transporter protein that plays a role in NO3− influx [21], but also an abscisic acid (ABA) transporter protein that positively regulated the ABA response [48]. CiNPF4.6 expression was significantly upregulated in roots under T3 and in leaves under T5, perhaps because CiNPF4.6 worked primarily on NO3− influx under T3, while it worked mostly on ABA regulation under T5. MtNPF6.8/MtNRT1.3 of Medicago truncatula was an amphipathic NO3− transport protein and was upregulated by the absence of NO3− [49]. This was the same as the results of our study, where CiNPF6.4/CiNRT1.3 expression was significantly upregulated in roots under CK and in leaves under T5. AtNPF7.3/AtNRT1.5 mediated root-to-stem transport, and the same conclusion was found for ZxNPF7.3/ZxNRT1.5 in the Zygophyllum xanthoxylum, which also contributed to the uptake of NO3− [50]. The expression levels of CiNPF7.3a/CiNRT1.3a were significantly upregulated in roots, leaves under CK, and roots under T4, indicating that both N scarcity and T4 may promote NO3− transport from roots to stems. Moreover, AtNPF7.2/AtNRT1.8 was phylogenetically similar to AtNPF7.3 [51], while CiNPF7.2/CiNRT1.8 was also significantly upregulated in roots under T4 and may function similarly to CiNPF7.3. NPF2.13/NRT1.7 and NPF1.2/NRT1.11 were proven to be involved in the transfer and redistribution of NO3− from xylem or the NO3− containing tissues to the phloem [23], CiNPF2.13 was largely induced by N limitation and T3, while CiNPF1.2a was mainly induced by N limitation, and T4. NPF2.11/NRT1.10 was identified to be involved in thioglucoside transport [52], and CiNPF2.11 expression was significantly upregulated under CK, suggesting that CiNPF2.11 may resist N deficiency stress by regulating the concentrations of thioglucosides. AtNRT2.5 was a plasma-membrane localized high-affinity NO3− transporter protein that mediated NO3− acquisition, and reactivation under N deficient conditions [27], and CiNRT2.5 in this study exhibited the same expression pattern. The transcript levels of NRT2.7 in Fraxinus mandshurica were both upregulated in leaves due to N limitation [53], whereas CiNRT2.7 in this study was significantly expressed in roots under CK and in leaves under T1, which may be caused by species differences.
4. Materials and Methods
4.1. Plant Materials and Experimental Design
In the study, aeroponic cultivation trials were carried out in the greenhouse of the campus of Nanjing Forestry University from 18 April to 9 June 2021 and from 4 May to 21 June 2022. The plant materials and experimental design refer to Chen et al. [29]. In the case of the same N supply, the five ammonia-to-nitrate ratios (NH4+:NO3−) were 100:0, 75:25, 50:50, 25:75, and 0:100, corresponding to T1, T2, T3, T4, and T5, respectively. The nutrient solution without N was used as the control (CK), and each treatment was repeated 3 times, each with 6 seedlings. Regulation of the NH4+:NO3− ratios for each treatment was achieved with specific source compounds (Table S1). Samples were taken after 45 days of treatment for further determination.
4.2. Identification of AMT and NRT Genes in Pecan
To identify the pecan AMT and NRT genes, we obtained all of the protein sequences of pecan from the Phytozome v13 database (https://phytozome-next.jgi.doe.gov/info/CillinoinensisPawnee_v1_1 (accessed on 1 November 2021)). The hidden Markov model (HMM) profiles of the AMT domain (PF00909) and NRT domain (PF07690, PF00854, PF16974), downloaded from the Pfam database (http://pfam.xfam.org/ (accessed on 1 February 2022)) [54], were used to identify the pecan AMT and NRT proteins by using HMM search through HMMER3.0 program (www.hmmer.org (accessed on 1 February 2022)) with default parameters [55]. As multiple AMT and NRT proteins were corresponding to a specific gene in several cases, only one protein sequence corresponding to each gene was retained for further detailed analysis. The Pfam database was used to confirm the presence of these conserved domains of the screened genes. The biophysical properties such as amino acid length (AA), molecular weights (MWs), theoretical isoelectric points (pIs), and grand average of hydration (GRAVY) of pecan AMT and NRT proteins were estimated by ExPASy ProtParam server (http://web.expasy.org/protparam (accessed on 1 February 2022)) [56]. Transmembrane helices (TMHs) were determined using the TMHMM tools (http://www.cbs.dtu.dk/services/TMHMM-2.0/ (accessed on 1 February 2022)), prediction of subcellular localization information for pecan AMT and NRT proteins using the Cell-PLoc 2.0 software (http://www.csbio.sjtu.edu.cn/bioinf/Cell-PLoc-2/ (accessed on 1 February 2022)) [57].
4.3. Phylogenetic Analysis
The Arabidopsis AMT and NRT protein sequences were downloaded from the TAIR database (https://www.arabidopsis.org/ (accessed on 1 November 2021)) [58]. The poplar, apple, pear, tomato, and rice AMT protein sequences were downloaded from the Phytozome database. Phylogenetic trees were constructed by MEGA 7.0 with the following settings: the neighbor-joining (NJ) method, 1000 bootstrap replicates, the Jones–Taylor–Thornton (JTT) model, and pairwise deletion [59]. The evolutionary tree was visualized using the online tool Evolview (http://evolgenius.info/ (accessed on 1 March 2022)) [60]. Because CiAMT3.1c is a partial sequence, it was not included in the phylogenetic tree.
4.4. Analysis of Gene Structure, Conservative Motifs, and Domains
The pecan genome sequence files and the General Feature Format (GFF) annotation files were downloaded from the Phytozome database. We used the online MEME tool (http://MEME-suite.org/ (accessed on 1 April 2022)) for topic prediction, keeping the maximum number of topics at 10 [61]. TBtools visualizes AMT and NRT gene structures, conservative motifs, and domains [62].
4.5. Synteny Analysis
For detecting syntenic blocks, the whole genome sequence file and the GFF annotation file of pecan were used to identify all duplication events in the pecan genome using MCScanX software [63]. Then, the AMT and NRT gene families were analyzed for synteny and visualized using TBtools.
4.6. Estimation of the Ka/Ks Values
Multiple sequence alignment of full-length coding sequences (CDS) in the AMT and NRT gene families in pecan was performed using MEGA 7 software and further used to calculate nonsynonymous (Ka) and synonymous (Ks), and the Ka/Ks ratios. Ks values were commonly used to determine the time since gene duplication, and the selection pressure of duplication event was determined by the Ka/Ks ratio.
4.7. Cis-Regulatory Elements Analysis
To investigate potential cis-regulatory elements in the promoters of the pecan AMT and NRT genes, a 1000 bp region upstream of the AMT and NRT genes was retrieved from the pecan genome sequences. Then, the cis-regulatory elements were predicted using PlantCARE software (//bioinformatics.psb.ugent.be/webtools/plantcare/html/ (accessed on 1 April 2022)) [64] and screened and visualized by TBtools software (Figures S2 and S3).
4.8. Protein–Protein Interaction Network Prediction
Protein–protein interaction networks of AMT and NRT gene families were analyzed by String (https://string-db.org/ (accessed on 1 April 2022)) (Figure S4) [65].
4.9. RNA Collection and qRT-PCR Expression Analysis
According to the manufacturer’s protocol, the total RNA was extracted from the leaves and roots of pecan using a Universal Plant Total RNA Extraction Kit (Bioteke, Beijing, China) and stored at −80 °C until further use. The purity and integrity of the isolated total RNA were analyzed by agarose gel electrophoresis and Nanodrop 2000 spectrophotometer (Thermo Scientific, Wilmington, NC, USA). First-strand cDNA was synthesized using a cDNA Synthesis Kit (HiScript ®RIII RT SuperMix for qPCR +gDNA wiper, Vazyme, Nanjing, China). The qRT-PCR was performed on a 7500 Real-Time PCR system (Applied BiosystemsTM, Foster City, CA, USA) using a Taq Pro Universal SYBR qPCR Master Mix (Vazyme, Nanjing, China). The specific primers were synthesized by Tsingke Biotechnology Ltd. (Nanjing, China), and the details of the primers were provided in Supplementary Table S2. The PCR parameters applied here were as follows: 95 °C for 30 s, followed by 40 cycles of 5 s at 95 and 30 s at 60 °C. The Actin gene was used as an internal reference gene [66], and the relative expression levels of pecan AMT and NRT genes were determined using the 2−ΔΔCt method [67]. Values represent mean calculated from three biological replicates and three technological repeats.
4.10. Measurement of NH4+, NO3− and NO2− Concentration in Pecan
The NH4+ concentrations of pecan roots, stems and leaves were determined according to the Berthelot reaction [68]. NO3− concentrations were determined according to the method suggested by Patterson et al. [69]. The NO2− concentrations were determined by the method of Ogawa et al. [70].
4.11. Kinetic Characterization of NH4+ and NO3− Uptake in Pecan
The kinetic characteristics of NH4+ and NO3− uptake in pecan seedlings were determined by the conventional depletion method, the ion concentrations in the solutions to be measured were determined after preparing different concentrations of NH4+ and NO3− for 24 h incubation of the plants, the net rate of ion uptake per unit fresh root per unit time was calculated, and the kinetic parameters of uptake were mathematically derived. The concentration of NH4+ and NO3− was determined by referring to 2.10.
4.12. Data Analysis
Before analysis of variance (ANOVA), data were checked for normality and homogeneity of variances. One-way ANOVA was performed to test the effects of different N forms on NH4+, NO3−, NO2− concentrations, absorption kinetic characteristics and relative gene expression of pecan seedlings. Differences were considered significant at p < 0.05.
The kinetic parameters of NH4+ and NO3− uptake were calculated using the Hofstee transformation of the Michaelis–Menten kinetic equation: V = C × Vmax/(Km + C), C represents the ion concentration, V indicates the net ion uptake rate, Vm indicates the maximum uptake rate, and the Km value represents the root uptake site for ion affinity. Non-linear regression fitting and graphing were performed using SPSS to obtain the Vmax and Km. The α value represents the competitive ability of the plant root system for nutrient uptake, α = Vmax/Km.
All statistical analyses were performed with SPSS 23.0 software (Version 23.0, Chicago, IL, USA). All charts were drawn with Excel (Version 2019, Redmond, WA, USA) and SigmaPlot (Version 14.0, Barcelona, Spain).
5. Conclusions
We identified 10 AMT and 69 NRT genes in the pecan genome, and the analysis showed that the biophysical properties, gene structure, and expression levels of CiAMTs and CiNRTs were strongly associated with pecan NH4+ and NO3− uptake. Combining the effects of different N form treatments on the expression levels of CiAMTs and CiNRTs, the N concentrations of pecan, and the uptake rate of NH4+and NO3−, we concluded that pecan preferred NH4+ and that the NH4+/NO3− ratio of 75:25 was more favorable to improve the N uptake capacity of pecan seedlings. This study provides a basis for further identification of the functions of AMT and NRT genes in N uptake and transport of pecan, and it provides a theoretical basis for the application of an optimal proportion of N fertilizer to improve NUE, thereby increasing pecan yield and promoting pecan industrialization.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms232113314/s1.
Author Contributions
F.P. conceived and designed the study. M.C. collected experimental data, analyzed, and wrote the manuscript. M.C. and J.L. participated in the collection of samples. M.C. and J.X. performed the experiments. P.T. and K.Z. provided help in data analysis and in improving the manuscript. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by a grant from the National Key R&D Program of China (2021YFD1000403) and the Central Financial Forestry Science and Technology Extension Demonstration Funds Program (Su (2022) TG04).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nacry P., Bouguyon E., Gojon A. Nitrogen Acquisition by Roots: Physiological and Developmental Mechanisms Ensuring Plant Adaptation to a Fluctuating Resource. Plant Soil. 2013;370:1–29. doi: 10.1007/s11104-013-1645-9. [DOI] [Google Scholar]
- 2.Van Tichelen K.K., Colpaert J.V. Kinetics of Phosphate Absorption by Mycorrhizal and Non-Mycorrhizal Scots Pine Seedlings. Physiol. Plant. 2000;110:96–103. doi: 10.1034/j.1399-3054.2000.110113.x. [DOI] [Google Scholar]
- 3.Wang L., Macko S.A. Constrained Preferences in Nitrogen Uptake across Plant Species and Environments: Plant Nitrogen Preference. Plant Cell Environ. 2011;34:525–534. doi: 10.1111/j.1365-3040.2010.02260.x. [DOI] [PubMed] [Google Scholar]
- 4.Gazzarrini S., Lejay L., Gojon A., Ninnemann O., Frommer W.B., von Wirén N. Three Functional Transporters for Constitutive, Diurnally Regulated, and Starvation-Induced Uptake of Ammonium into Arabidopsis Roots. Plant Cell. 1999;11:937–948. doi: 10.1105/tpc.11.5.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bai H., Euring D., Volmer K., Janz D., Polle A. The Nitrate Transporter (NRT) Gene Family in Poplar. PLoS ONE. 2013;8:e72126. doi: 10.1371/journal.pone.0072126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ariz I., Boeckstaens M., Gouveia C., Martins A.P., Sanz-Luque E., Fernández E., Soveral G., von Wirén N., Marini A.M., Aparicio-Tejo P.M., et al. Nitrogen Isotope Signature Evidences Ammonium Deprotonation as a Common Transport Mechanism for the AMT-Mep-Rh Protein Superfamily. Sci. Adv. 2018;4:eaar3599. doi: 10.1126/sciadv.aar3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang L., Li J., Zhang B., Hao Y., Ma F. Genome-Wide Identification and Expression Analysis of AMT Gene Family in Apple (Malus domestica Borkh.) Horticulturae. 2022;8:457. doi: 10.3390/horticulturae8050457. [DOI] [Google Scholar]
- 8.Song S., He Z., Huang X., Zhong L., Liu H., Sun G., Chen R. Cloning and Characterization of the Ammonium Transporter Genes BaAMT1;1 and BaAMT1;3 from Chinese Kale. Hortic. Environ. Biotechnol. 2017;58:178–186. doi: 10.1007/s13580-017-0168-3. [DOI] [Google Scholar]
- 9.Castro-Rodríguez V., Assaf-Casals I., Pérez-Tienda J., Fan X., Avila C., Miller A., Cánovas F.M. Deciphering the Molecular Basis of Ammonium Uptake and Transport in Maritime Pine. Plant Cell Environ. 2016;39:1669–1682. doi: 10.1111/pce.12692. [DOI] [PubMed] [Google Scholar]
- 10.Giehl R.F.H., Laginha A.M., Duan F., Rentsch D., Yuan L., von Wirén N. A Critical Role of AMT2;1 in Root-To-Shoot Translocation of Ammonium in Arabidopsis. Mol. Plant. 2017;10:1449–1460. doi: 10.1016/j.molp.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 11.Shelden M.C., Dong B., de Bruxelles G.L., Trevaskis B., Whelan J., Ryan P.R., Howitt S.M., Udvardi M.K. Arabidopsis Ammonium Transporters, AtAMT1;1 and AtAMT1;2, Have Different Biochemical Properties and Functional Roles. Plant Soil. 2001;231:151–160. doi: 10.1023/A:1010303813181. [DOI] [Google Scholar]
- 12.Yuan L., Graff L., Loqué D., Kojima S., Tsuchiya Y.N., Takahashi H., von Wirén N. AtAMT1;4, a Pollen-Specific High-Affinity Ammonium Transporter of the Plasma Membrane in Arabidopsis. Plant Cell Physiol. 2009;50:13–25. doi: 10.1093/pcp/pcn186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ludewig U., Neuhäuser B., Dynowski M. Molecular Mechanisms of Ammonium Transport and Accumulation in Plants. FEBS Lett. 2007;581:2301–2308. doi: 10.1016/j.febslet.2007.03.034. [DOI] [PubMed] [Google Scholar]
- 14.Couturier J., Montanini B., Martin F., Brun A., Blaudez D., Chalot M. The Expanded Family of Ammonium Transporters in the Perennial Poplar Plant. New Phytol. 2007;174:137–150. doi: 10.1111/j.1469-8137.2007.01992.x. [DOI] [PubMed] [Google Scholar]
- 15.Tavares O.C.H., Santos L.A., Ferreira L.M., Sperandio M.V.L., da Rocha J.G., García A.C., Dobbss L.B., Berbara R.L.L., de Souza S.R., Fernandes M.S. Humic Acid Differentially Improves Nitrate Kinetics under Low- and High-Affinity Systems and Alters the Expression of Plasma Membrane H+-ATPases and Nitrate Transporters in Rice. Ann. Appl. Biol. 2017;170:89–103. doi: 10.1111/aab.12317. [DOI] [Google Scholar]
- 16.Kant S. Understanding Nitrate Uptake, Signaling and Remobilisation for Improving Plant Nitrogen Use Efficiency. Semin. Cell Dev. Biol. 2018;74:89–96. doi: 10.1016/j.semcdb.2017.08.034. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y.-Y., Hsu P.-K., Tsay Y.-F. Uptake, Allocation and Signaling of Nitrate. Trends Plant Sci. 2012;17:458–467. doi: 10.1016/j.tplants.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y.-Y., Cheng Y.-H., Chen K.-E., Tsay Y.-F. Nitrate Transport, Signaling, and Use Efficiency. Annu. Rev. Plant Biol. 2018;69:85–122. doi: 10.1146/annurev-arplant-042817-040056. [DOI] [PubMed] [Google Scholar]
- 19.Liu K.H., Huang C.Y., Tsay Y.F. CHL1 Is a Dual-Affinity Nitrate Transporter of Arabidopsis Involved in Multiple Phases of Nitrate Uptake. Plant Cell. 1999;11:865–874. doi: 10.1105/tpc.11.5.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsay Y.-F., Chiu C.-C., Tsai C.-B., Ho C.-H., Hsu P.-K. Nitrate Transporters and Peptide Transporters. FEBS Lett. 2007;581:2290–2300. doi: 10.1016/j.febslet.2007.04.047. [DOI] [PubMed] [Google Scholar]
- 21.Huang N.-C., Liu K.-H., Lo H.-J., Tsay Y.-F. Cloning and Functional Characterization of an Arabidopsis Nitrate Transporter Gene That Encodes a Constitutive Component of Low-Affinity Uptake. Plant Cell. 1999;11:1381–1392. doi: 10.1105/tpc.11.8.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Segonzac C., Boyer J.-C., Ipotesi E., Szponarski W., Tillard P., Touraine B., Sommerer N., Rossignol M., Gibrat R. Nitrate Efflux at the Root Plasma Membrane: Identification of an Arabidopsis Excretion Transporter. Plant Cell. 2007;19:3760–3777. doi: 10.1105/tpc.106.048173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iqbal A., Qiang D., Alamzeb M., Xiangru W., Huiping G., Hengheng Z., Nianchang P., Xiling Z., Meizhen S. Untangling the Molecular Mechanisms and Functions of Nitrate to Improve Nitrogen Use Efficiency. J. Sci. Food Agric. 2020;100:904–914. doi: 10.1002/jsfa.10085. [DOI] [PubMed] [Google Scholar]
- 24.Krapp A., David L.C., Chardin C., Girin T., Marmagne A., Leprince A.-S., Chaillou S., Ferrario-Méry S., Meyer C., Daniel-Vedele F. Nitrate Transport and Signalling in Arabidopsis. J. Exp. Bot. 2014;65:789–798. doi: 10.1093/jxb/eru001. [DOI] [PubMed] [Google Scholar]
- 25.Ma H., Zhao J., Feng S., Qiao K., Gong S., Wang J., Zhou A. Heterologous Expression of Nitrate Assimilation Related-Protein DsNAR2.1/NRT3.1 Affects Uptake of Nitrate and Ammonium in Nitrogen-Starved Arabidopsis. Int. J. Mol. Sci. 2020;21:4027. doi: 10.3390/ijms21114027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li W., Wang Y., Okamoto M., Crawford N.M., Siddiqi M.Y., Glass A.D.M. Dissection of the AtNRT2.1:AtNRT2.2 Inducible High-Affinity Nitrate Transporter Gene Cluster. Plant Physiol. 2007;143:425–433. doi: 10.1104/pp.106.091223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lezhneva L., Kiba T., Feria-Bourrellier A.-B., Lafouge F., Boutet-Mercey S., Zoufan P., Sakakibara H., Daniel-Vedele F., Krapp A. The Arabidopsis Nitrate Transporter NRT2.5 Plays a Role in Nitrate Acquisition and Remobilization in Nitrogen-Starved Plants. Plant J. 2014;80:230–241. doi: 10.1111/tpj.12626. [DOI] [PubMed] [Google Scholar]
- 28.Kotur Z., Mackenzie N., Ramesh S., Tyerman S.D., Kaiser B.N., Glass A.D.M. Nitrate Transport Capacity of the Arabidopsis Thaliana NRT2 Family Members and Their Interactions with AtNAR2.1. New Phytol. 2012;194:724–731. doi: 10.1111/j.1469-8137.2012.04094.x. [DOI] [PubMed] [Google Scholar]
- 29.Chen M., Zhu K., Tan P., Liu J., Xie J., Yao X., Chu G., Peng F. Ammonia–Nitrate Mixture Dominated by NH4+–N Promoted Growth, Photosynthesis and Nutrient Accumulation in Pecan (Carya illinoinensis) Forests. 2021;12:1808. doi: 10.3390/f12121808. [DOI] [Google Scholar]
- 30.Zhu K., Fan P., Liu H., Tan P., Ma W., Mo Z., Zhao J., Chu G., Peng F. Insight into the CBL and CIPK Gene Families in Pecan (Carya illinoinensis): Identification, Evolution and Expression Patterns in Drought Response. BMC Plant Biol. 2022;22:221. doi: 10.1186/s12870-022-03601-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yuan L., Loqué D., Kojima S., Rauch S., Ishiyama K., Inoue E., Takahashi H., von Wirén N. The Organization of High-Affinity Ammonium Uptake in Arabidopsis Roots Depends on the Spatial Arrangement and Biochemical Properties of AMT1-Type Transporters. Plant Cell. 2007;19:2636–2652. doi: 10.1105/tpc.107.052134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu Y., Hao Y., Liu H., Sun G., Chen R., Song S. Identification and Characterization of Two Ammonium Transporter Genes in Flowering Chinese Cabbage (Brassica campestris) Plant Biotechnol. 2018;35:59–70. doi: 10.5511/plantbiotechnology.18.0202a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.He Y.-N., Peng J.-S., Cai Y., Liu D.-F., Guan Y., Yi H.-Y., Gong J.-M. Tonoplast-Localized Nitrate Uptake Transporters Involved in Vacuolar Nitrate Efflux and Reallocation in Arabidopsis. Sci. Rep. 2017;7:6417. doi: 10.1038/s41598-017-06744-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.You L., Wang Y., Zhang T., Zhu Y., Ren N., Jiang X., Zhou Y. Genome-Wide Identification of Nitrate Transporter 2 (NRT2) Gene Family and Functional Analysis of MeNRT2.2 in Cassava (Manihot esculenta Crantz) Gene. 2022;809:146038. doi: 10.1016/j.gene.2021.146038. [DOI] [PubMed] [Google Scholar]
- 35.Zhu Y., Qi B., Hao Y., Liu H., Sun G., Chen R., Song S. Appropriate NH4+/NO3− Ratio Triggers Plant Growth and Nutrient Uptake of Flowering Chinese Cabbage by Optimizing the PH Value of Nutrient Solution. Front. Plant Sci. 2021;12:724–739. doi: 10.3389/fpls.2021.656144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oh K., Kato T., Xu H.L. Transport of Nitrogen Assimilation in Xylem Vessels of Green Tea Plants Fed with NH4+-N and NO3−-N. Pedosphere. 2008;18:222–226. doi: 10.1016/S1002-0160(08)60010-7. [DOI] [Google Scholar]
- 37.Zhang J., Lv J., Dawuda M.M., Xie J., Yu J., Li J., Zhang X., Tang C., Wang C., Gan Y. Appropriate Ammonium-Nitrate Ratio Improves Nutrient Accumulation and Fruit Quality in Pepper (Capsicum annuum L.) Agronomy. 2019;9:683. doi: 10.3390/agronomy9110683. [DOI] [Google Scholar]
- 38.Ruan L., Wei K., Wang L., Cheng H., Zhang F., Wu L., Bai P., Zhang C. Characteristics of NH4+ and NO3− Fluxes in Tea (Camellia Sinensis) Roots Measured by Scanning Ion-Selective Electrode Technique. Sci. Rep. 2016;6:38370. doi: 10.1038/srep38370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu S., Hua J., Lu Y., Zhang R., Yin Y. Characteristics of NH4+ and NO3− Fluxes in Taxodium Roots under Different Nitrogen Treatments. Plants. 2022;11:894. doi: 10.3390/plants11070894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tang Y., Gao F., Yu Q., Guo S., Li F. The Uptake Kinetics of NH4+ and NO3− by Lettuce Seedlings under Hypobaric and Hypoxic Conditions. Sci. Hortic. 2015;197:236–243. doi: 10.1016/j.scienta.2015.09.043. [DOI] [Google Scholar]
- 41.Kamminga-Van Wijk C., Prins H.B.A. The Kinetics of NH4+ and NO3− Uptake by Douglas Fir from Single N-Solutions and from Solutions Containing Both NH4+ and NO3−. Plant Soil. 1993;151:91. doi: 10.1007/BF00010789. [DOI] [Google Scholar]
- 42.Nishikawa T., Tarutani K., Yamamoto T. Nitrate and Phosphate Uptake Kinetics of the Harmful Diatom Coscinodiscus Wailesii, a Causative organism in the Bleaching of Aquacultured Porphyra thalli. Harmful Algae. 2010;9:563–567. doi: 10.1016/j.hal.2010.04.007. [DOI] [Google Scholar]
- 43.Loqué D., Yuan L., Kojima S., Gojon A., Wirth J., Gazzarrini S., Ishiyama K., Takahashi H., Von Wirén N. Additive Contribution of AMT1;1 and AMT1;3 to High-Affinity Ammonium Uptake across the Plasma Membrane of Nitrogen-Deficient Arabidopsis Roots. Plant J. 2006;48:522–534. doi: 10.1111/j.1365-313X.2006.02887.x. [DOI] [PubMed] [Google Scholar]
- 44.Kumar A., Silim S.N., Okamoto M., Siddiqi M.Y., Glass A.D.M. Differential Expression of Three Members of the AMT1 Gene Family Encoding Putative High-Affinity NH4+ Transporters in Roots of Oryza sativa Subspecies Indica. Plant Cell Environ. 2003;26:907–914. doi: 10.1046/j.1365-3040.2003.01023.x. [DOI] [PubMed] [Google Scholar]
- 45.Camañes G., Cerezo M., Primo-Millo E., Gojon A., García-Agustín P. Ammonium Transport and CitAMT1 Expression Are Regulated by N in Citrus Plants. Planta. 2008;229:331. doi: 10.1007/s00425-008-0833-y. [DOI] [PubMed] [Google Scholar]
- 46.You S., Wang Y., Li Y., Li Y., Tan P., Wu Z., Shi W., Song Z. Cloning and Functional Determination of Ammonium Transporter PpeAMT3;4 in Peach. BioMed Res. Int. 2020;2020:e2147367. doi: 10.1155/2020/2147367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wei W., Hu B., Li A., Chu C. NRT1.1 in Plants: Functions beyond Nitrate Transport [Cover Story] J. Exp. Bot. 2020;71:4373–4379. doi: 10.1093/jxb/erz554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang L., Yu Z., Xu Y., Yu M., Ren Y., Zhang S., Yang G., Huang J., Yan K., Zheng C., et al. Regulation of the Stability and ABA Import Activity of NRT1.2/NPF4.6 by CEPR2-Mediated Phosphorylation in Arabidopsis. Mol. Plant. 2021;14:633–646. doi: 10.1016/j.molp.2021.01.009. [DOI] [PubMed] [Google Scholar]
- 49.Morère-Le Paven M.-C., Viau L., Hamon A., Vandecasteele C., Pellizzaro A., Bourdin C., Laffont C., Lapied B., Lepetit M., Frugier F., et al. Characterization of a Dual-Affinity Nitrate Transporter MtNRT1.3 in the Model Legume Medicago Truncatula. J. Exp. Bot. 2011;62:5595–5605. doi: 10.1093/jxb/err243. [DOI] [PubMed] [Google Scholar]
- 50.Yuan J.-Z., Cui Y.-N., Li X.-T., Wang F.-Z., He Z.-H., Li X.-Y., Bao A.-K., Wang S.-M., Ma Q. ZxNPF7.3/NRT1.5 from the Xerophyte Zygophyllum Xanthoxylum Modulates Salt and Drought Tolerance by Regulating NO3−, Na+ and K+ Transport. Environ. Exp. Bot. 2020;177:104123. doi: 10.1016/j.envexpbot.2020.104123. [DOI] [Google Scholar]
- 51.Li J.-Y., Fu Y.-L., Pike S.M., Bao J., Tian W., Zhang Y., Chen C.-Z., Zhang Y., Li H.-M., Huang J., et al. The Arabidopsis Nitrate Transporter NRT1.8 Functions in Nitrate Removal from the Xylem Sap and Mediates Cadmium Tolerance. Plant Cell. 2010;22:1633–1646. doi: 10.1105/tpc.110.075242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nour-Eldin H.H., Andersen T.G., Burow M., Madsen S.R., Jørgensen M.E., Olsen C.E., Dreyer I., Hedrich R., Geiger D., Halkier B.A. NRT/PTR Transporters Are Essential for Translocation of Glucosinolate Defence Compounds to Seeds. Nature. 2012;488:531–534. doi: 10.1038/nature11285. [DOI] [PubMed] [Google Scholar]
- 53.Zhao X., Zhang X., Liu Z., Lv Y., Song T., Cui J., Chen T., Li J., Zeng F., Zhan Y. Comparing the Effects of N and P Deficiency on Physiology and Growth for Fast- and Slow-Growing Provenances of Fraxinus Mandshurica. Forests. 2021;12:1760. doi: 10.3390/f12121760. [DOI] [Google Scholar]
- 54.El-Gebali S., Mistry J., Bateman A., Eddy S.R., Luciani A., Potter S.C., Qureshi M., Richardson L.J., Salazar G.A., Smart A., et al. The Pfam Protein Families Database in 2019. Nucleic Acids Res. 2019;47:D427–D432. doi: 10.1093/nar/gky995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Finn R.D., Clements J., Eddy S.R. HMMER Web Server: Interactive Sequence Similarity Searching. Nucleic Acids Res. 2011;39:W29–W37. doi: 10.1093/nar/gkr367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Artimo P., Jonnalagedda M., Arnold K., Baratin D., Csardi G., de Castro E., Duvaud S., Flegel V., Fortier A., Gasteiger E., et al. ExPASy: SIB Bioinformatics Resource Portal. Nucleic Acids Res. 2012;40:W597–W603. doi: 10.1093/nar/gks400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chou K.-C., Shen H.-B. Cell-PLoc 2.0: An Improved Package of Web-Servers for Predicting Subcellular Localization of Proteins in Various Organisms. Nat. Sci. 2010;2:1090. doi: 10.4236/ns.2010.210136. [DOI] [PubMed] [Google Scholar]
- 58.Rhee S.Y., Beavis W., Berardini T.Z., Chen G., Dixon D., Doyle A., Garcia-Hernandez M., Huala E., Lander G., Montoya M., et al. The Arabidopsis Information Resource (TAIR): A Model Organism Database Providing a Centralized, Curated Gateway to Arabidopsis Biology, Research Materials and Community. Nucleic Acids Res. 2003;31:224–228. doi: 10.1093/nar/gkg076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kumar S., Stecher G., Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Subramanian B., Gao S., Lercher M.J., Hu S., Chen W.-H. Evolview v3: A Webserver for Visualization, Annotation, and Management of Phylogenetic Trees. Nucleic Acids Res. 2019;47:W270–W275. doi: 10.1093/nar/gkz357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bailey T.L., Boden M., Buske F.A., Frith M., Grant C.E., Clementi L., Ren J., Li W.W., Noble W.S. MEME Suite: Tools for Motif Discovery and Searching. Nucleic Acids Res. 2009;37:W202–W208. doi: 10.1093/nar/gkp335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen C., Chen H., Zhang Y., Thomas H.R., Frank M.H., He Y., Xia R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant. 2020;13:1194–1202. doi: 10.1016/j.molp.2020.06.009. [DOI] [PubMed] [Google Scholar]
- 63.Wang Y., Li J., Paterson A.H. MCScanX-Transposed: Detecting Transposed Gene Duplications Based on Multiple Colinearity Scans. Bioinformatics. 2013;29:1458–1460. doi: 10.1093/bioinformatics/btt150. [DOI] [PubMed] [Google Scholar]
- 64.Lescot M., Déhais P., Thijs G., Marchal K., Moreau Y., Van de Peer Y., Rouzé P., Rombauts S. PlantCARE, a Database of Plant Cis-Acting Regulatory Elements and a Portal to Tools for in Silico Analysis of Promoter Sequences. Nucleic Acids Res. 2002;30:325–327. doi: 10.1093/nar/30.1.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Szklarczyk D., Morris J.H., Cook H., Kuhn M., Wyder S., Simonovic M., Santos A., Doncheva N.T., Roth A., Bork P., et al. The STRING Database in 2017: Quality-Controlled Protein–Protein Association Networks, Made Broadly Accessible. Nucleic Acids Res. 2017;45:D362–D368. doi: 10.1093/nar/gkw937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhu K., Fan P., Mo Z., Tan P., Feng G., Li F., Peng F. Identification, Eexpression and Co-Expression Analysis of R2R3-MYB Family Genes Involved in Graft Union Formation in Pecan (Carya illinoinensis) Forests. 2020;11:917. doi: 10.3390/f11090917. [DOI] [Google Scholar]
- 67.Livak K.J., Schmittgen T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 68.Bräutigam A., Gagneul D., Weber A.P.M. High-Throughput Colorimetric Method for the Parallel Assay of Glyoxylic Acid and Ammonium in a Single Extract. Anal. Biochem. 2007;362:151–153. doi: 10.1016/j.ab.2006.12.033. [DOI] [PubMed] [Google Scholar]
- 69.Patterson K., Cakmak T., Cooper A., Lager I., Rasmusson A.G., Escobar M.A. Distinct Signalling Pathways and Transcriptome Response Signatures Differentiate Ammonium- and Nitrate-Supplied Plants. Plant Cell Environ. 2010;33:1486–1501. doi: 10.1111/j.1365-3040.2010.02158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ogawa T., Fukuoka H., Yano H., Ohkawa Y. Relationships between Nitrite Reductase Activity and Genotype-Dependent Callus Growth in Rice Cell Cultures. Plant Cell Rep. 1999;18:576–581. doi: 10.1007/s002990050625. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.