Abstract
Invasion of epithelial cells by Shigella flexneri is mediated by a set of translocated bacterial invasins, the Ipa proteins, and its dedicated type III secretion system, called Mxi-Spa. We show here that mxiM, part of the mxi-spa locus in the S. flexneri virulence plasmid, encodes an indispensable type III secretion apparatus component, required for both Ipa translocation and tissue culture cell invasion. We demonstrated that mature MxiM, first identified as a putative lipoprotein, is lipidated in vivo. Consistent with features of known lipoproteins, MxiM (i) can be labeled with [3H]palmitate and [2-3H]glycerol, (ii) is associated with the cell envelope, (iii) is secreted independently of the type III pathway, and (iv) requires an intact lipoprotein modification and processing site for full activity. The lipidated form of MxiM was detected primarily in the outer membrane, where it establishes a peripheral association with the inner leaflet. Through analysis of subcellular Ipa distribution in a mxiM null mutant background, MxiM was found to be required for the assembly and/or function of outer, but not inner, membrane regions of Mxi-Spa. This function probably requires interactions with other Mxi-Spa subunits within the periplasmic space. We discuss implications of these findings with respect to the function of MxiM and the structure of Mxi-Spa as a whole.
Pathogenic microorganisms elaborate a diverse array of secreted virulence proteins which facilitate their colonization and persistence in a variety of eukaryotic hosts and host tissues (17). In spite of this diversity, mechanisms utilized for bacterial virulence protein delivery are remarkably homologous. One such widely distributed mechanism is the virulence-specialized type III secretion system of gram-negative bacteria (28). Type III systems are complex membrane-bound structures dedicated to the delivery of virulence effectors from the bacterial cytoplasm to eukaryotic cell surfaces or intracellular environments. In general, type III secretion is referred to as contact dependent, as it can be activated in some cases by direct pathogen-host cell interaction. Additionally, expression of such systems is controlled by multicomponent regulatory pathways capable of responding to host-specific environmental signals, including temperature, osmolarity, and oxygen tension. Loci encoding homologous type III secretory apparatus components have been identified, usually within large operons, in many mammalian and plant pathogens, including enterohemorrhagic and enteropathogenic Escherichia coli and Shigella, Salmonella, Yersinia, Chlamydia, Bordetella, Pseudomonas, Xanthomonas, Ralstonia, and Erwinia spp. Several well-conserved components of type III secretion systems are also similar to proteins involved in flagellum biosynthesis, a finding which supports the theory that type III pathways arose from those responsible for flagellar subunit secretion.
Shigella spp. are the major etiologic agents of bacillary dysentery, an invasive and potentially fatal disease of the human colonic mucosa. Host-pathogen interactions characteristic of the onset of shigellosis have been well studied, particularly with regard to the process of bacterial entry into epithelial cells (36, 46). A set of bacterial gene products, called invasion plasmid antigen (Ipa) proteins, is secreted by the type III pathway of Shigella and triggers a eukaryotic membrane ruffling process responsible for mediating entry (1, 37, 38). Unlike other type III system-bearing invasive pathogens, like Salmonella spp., Shigella spp. lyse the endosomal membrane and gain access to the eukaryotic cell cytosol (52). Within the intracellular environment, shigellae multiply and begin elaborating an intercellular spread phenotype which propels the bacteria within cellular protrusions, or fireworks, into adjacent uninfected cells (9, 22). Protrusion escape establishes the infection in neighboring cells and leads to lateral bacterial spread and severe tissue damage across the colonic mucosa (2, 46).
Genetic and biochemical analyses implicate four Ipa invasins (IpaA through IpaD) and a type III secretion system consisting of up to twenty Mxi-Spa proteins as the central effectors of not only epithelial cell entry but also phagosomal escape and the induction of apoptosis in macrophages as well (28, 36). The ipa and mxi-spa loci are located within closely linked operons found on the 230-kb virulence-associated plasmid of Shigella (46). The Ipa proteins have been of particular interest and are characterized with respect to their associations with each other (39, 40), bacterial cytoplasmic chaperones (40), extracellular filamentous appendages (45), and target eukaryotic cells and their components (12, 27, 34, 37). Such studies have led to a detailed understanding of Ipa contributions to pathogenesis. The Mxi-Spa secretory system, unlike its secreted substrates, has not been the subject of such intensive study. As a result, basic questions concerning Mxi-Spa architecture within the cell envelope and the mechanisms by which Ipa proteins are translocated through it remain unanswered.
In this investigation, we extended our studies of type III Mxi-Spa pathway structure and function to the product of a locus unique to Shigella called mxiM. The mxiM locus of the mxi operon is uncharacterized with respect to its role in Shigella pathogenesis, yet it is of particular interest as mxiM likely encodes a lipid-modified protein (4). Roles for bacterial lipoproteins have been demonstrated in a diverse range of biological functions (55), in particular many pathways for transmembrane macromolecular traffic. These pathways include DNA secretion (8) and uptake systems (19), the general secretory pathway of gram-negative bacteria (25), type IV pilus assembly (49), flagellar biogenesis (53), type I protein secretion (30), and type III virulence protein secretion systems (28). This diversity of lipoprotein function warranted a detailed analysis of the putative MxiM lipoprotein with respect to its contribution to the virulent phenotype of Shigella and its role in the Mxi-Spa type III Ipa secretory pathway.
We demonstrate that MxiM exists in virulent shigellae as a lipidated protein, anchored to the inner leaflet of the outer membrane. Consistent with a requirement for MxiM in type III secretion, inactivation of mxiM blocks the Ipa invasin secretory pathway and severely attenuates Shigella virulence. Through analysis of Ipa distribution patterns in fractionated cell envelope extracts, the mxiM secretion defect was specifically attributed to a block in Ipa translocation from inner to outer membrane positions.
MATERIALS AND METHODS
Bacterial strains and growth media.
Shigella flexneri strains used in this study are described in Table 1. The following E. coli strains were used: DH5αλpir and SM10λpir (41), for construction of pGP704 derivatives and their delivery to S. flexneri; DH5α (Gibco BRL), for construction of plasmids other than pGP704 derivatives; BL21(DE3) (Novagen), for overexpression and purification of polyhistidine-tagged MxiM; and ES1578 (44), for mutagenesis of mxiM. ES1578 (mutD5) is a mutator strain that displays a high spontaneous rate of single-base substitutions.
TABLE 1.
Shigella strains and plasmids used in this study
| Strain or plasmid | Relevant genotype and/or phenotype or feature | Source or reference |
|---|---|---|
| Strains | ||
| 2457T | Wild-type S. flexneri serotype 2a | 18 |
| BS103 | Virulence plasmid-cured 2457T | 35 |
| BS260 | 2457T mxiA::lacZ | 6 |
| BS473 | 2457T streptomycin resistant | Lab stock |
| BS547 | 2457T mxiM1 (mxiM::aphA-3) | This work |
| BS548 | BS547/pRRS4 (mxiM1PBAD-mxiM+) | This work |
| BS575 | BS547/pRRS5 (mxiM1/PLAC-mxiM+) | This work |
| BS576 | BS547/pBAD18 | This work |
| BS588 | BS547/pBluescript SK(+) | This work |
| BS599 | 2457T/pRRS12 (mixiM+/PBAD-mxiM2) | This work |
| BS603 | BS547/pRRS12 (mxiM1/PBAD-mxiM2) | This work |
| Plasmids | ||
| pBAD18 | Arabinose-inducible PBAD expression vector | 23 |
| pET19b | Vector used to construct histidine-tagged MxiM | Novagen |
| pGP704 | Suicide vector used to disrupt mxiM | 41 |
| pRRS1 | 1850-bp PCR generated fragment, extending from 912 bp upstream of the mxiM start codon to 511 bp downstream of the stop codon, ligated with EcoRI-HindIII-digested pUC19 | This work |
| pRRS2 | 840-bp SmaI fragment of pUC18K (38), bearing aphA-3, ligated in the proper orientation with BspMI-digested (Klenow-treated) pRRS1 | This work |
| pRRS3 | 2690-bp HindIII (Klenow-treated)-EcoRI fragment of pRRS2, bearing the mxiM::aphA-3 allele, ligated with EcoRV-digested pGP704 | This work |
| pRRS4 | 484-bp PCR-generated fragment, extending from 19 bp upstream of the mxiM start codon to 26 bp downstream of the stop codon, ligated with EcoRI-HindIII-digested pBAD18 | This work |
| pRRS5 | 484-bp EcoRI-HindIII fragment of pRRS4, bearing mxiM, ligated with EcoRI-HindIII-digested pBluescript SK(+) | This work |
| pRRS11 | 370-bp PCR-generated fragment, extending from 67 bp downstream of the mxiM start codon (thus deleting signal sequence) to 10 bp downstream of the stop codon, ligated with NdeI-BamHI-digested pET19b | This work |
| pRRS12 | Mutagenized mxiM allele encoding the G23R substitution, ligated with EcoRI-HindIII-digested pBAD18 | This work |
Bacteria were grown in tryptic soy broth (TSB) or Luria broth (LB) with aeration at 37°C unless otherwise stated. Strains were tested for Congo red binding on TSB agar plates (1.5% agar) containing 0.025% Congo red (Sigma). Antibiotics were used at the following concentrations: ampicillin, 100 μg ml−1; kanamycin, 50 μg ml−1; and streptomycin, 200 μg ml−1. For the induction or repression of PBAD transcription by using pBAD18, growth media were supplemented with either 0.2% arabinose or 0.2% glucose, respectively (unless otherwise stated).
Plasmid and strain constructions.
The plasmids used in this study are described in Table 1. Analysis of DNA, plasmid constructions, and the transformation of S. flexneri and E. coli were performed according to standard protocols. PCR amplifications for cloning and plasmid screening purposes were performed by using Pfu (Stratagene) and Hot Tub (Amersham) DNA polymerases, respectively, in a DNA Thermal Cycler 480 (Perkin-Elmer). To confirm the fidelity of PCR, all PCR-generated plasmid inserts were sequenced. Templates for DNA sequencing were prepared by using the ABI Prism Dye Terminator Cycle Sequencing Core Kit and analyzed by using an ABI Prism 377 DNA Sequencer.
After its construction, plasmid pRRS3 was transferred to S. flexneri BS473 by conjugation. Transconjugants in which a double crossover recombination event replaced the virulence plasmid-borne mxiM with the mxiM1 allele from pRRS3 were identified based on sensitivity to ampicillin and subsequent PCR analysis. The virulence plasmid-borne mxiM1 allele was then transferred by P1 transduction into 2457T, creating strain BS547. The structure of the mxiM disruption in BS547 was confirmed by PCR and Southern blot analysis.
Production of anti-MxiM antibody.
The open reading frame (ORF) encoding mature MxiM, lacking its 23 amino acid N-terminal signal sequence, was cloned into pET19b to create pRRS11 as described in Table 1. E. coli BL21(DE3), bearing pRRS11, was grown at 30°C in LB to late log phase, and induced for 4 h in the presence of 0.5 mM isopropyl-β-d-thiogalactopyranoside. Bacteria were harvested, incubated in Tris-buffered saline (pH 7.5) containing 2 mg of polymyxin B ml−1 for 1 h at 37°C, and centrifuged at 10,000 × g for 10 min. The resulting soluble fraction, bearing the 10His-MxiM fusion protein, was filtered through 0.45-μm-pore-size filters (Millipore) and further purified by metal chelate affinity chromatography by using 1-ml HisTrap chelating columns (Pharmacia Biotech) as described by the manufacturer. Protein was used to immunize New Zealand White rabbits (Hazelton Research Products) as previously described (16). Unadsorbed MxiM antiserum was used at a dilution of 1:200 for immunoblot analysis.
Protein preparation and immunoblot analysis.
For the analysis of Ipa secretion, supernatant protein samples were prepared as described (3). Protein electrophoresis was performed in sodium dodecyl sulfate (SDS)-polyacrylamide gels (either 17.5% [for the MxiM and Lpp analyses] or 10% [for the IpaB, IpaC, and IcsA analyses] polyacrylamide gels). Separated proteins were transferred to polyvinylidene difluoride membranes (Schleicher & Schuell) and treated with a blocking agent. Immunodetection was performed by using primary mouse anti-IpaB (42), anti-IpaC (42), or anti-IcsA (51) serum and primary rabbit anti-Lpp (26) or anti-MxiM (this study) serum. The activity of a mouse- or rabbit-specific alkaline phosphatase-labeled secondary antibody was then visualized by using the chemiluminescent substrate, CSPD (Boehringer Mannheim), as described by the manufacturer.
Membrane preparation and fractionation.
The labeling of proteins with either [3H]palmitate (56.5 Ci mmol−1) (NEN) or [2-3H]glycerol (200 mCi mmol−1) (NEN) and subsequent isolation of total membrane preparations were performed as described (26). Preparations made from each strain yielded roughly the same amount of total protein. Using either labeled or unlabeled total membrane extracts of strains BS548 (grown with either arabinose or glucose), 2457T, BS547, and BS260, inner and outer membrane protein-containing fractions were separated by equilibrium density gradient centrifugation. As described by Osborn et al. (43), membrane-containing pellets were resuspended in 0.5 ml of 25% sucrose–5 mM EDTA (pH 7.5) and layered on top of a sucrose density gradient consisting of 2.1 ml each of 50, 45, 40, 35, and 30% sucrose (wt/wt in 5 mM EDTA [pH 7.5]) over a 0.5-ml cushion of 55% sucrose. Gradients were centrifuged at 35,000 rpm for 20 h at 4°C in a Beckman SW40 rotor. Twelve 1-ml fractions were then collected stepwise from the top of each gradient. Fraction aliquots were analyzed to determine the refractive index and sucrose concentration (percent, on a per weight basis), protein content (using the Bio-Rad protein assay), levels of [3H] incorporation (if required), and NADH oxidase activity (as described by Osborn et al. [43]). For analysis of [3H]palmitate-labeled samples, 50-μl aliquots from each fraction were also separated using 17.5% polyacrylamide gels. Following electrophoresis, the gels were fixed, treated with Amplify (Amersham), dried, and exposed to Kodak XAR-2 film for up to 2 weeks at −80°C. For immunoblot analysis of unlabeled membrane fractions with each particular antibody, equivalent amounts of material from each fraction of every strain were separated and probed. The exception to this was the analysis of 2457T and BS260 samples with anti-MxiM serum; with each fraction, ten times more material was separated and probed than that of the BS547 and BS548 samples.
Protease accessibility experiments.
The localization of MxiM in bacteria at late exponential phase, with respect to the inner or outer face of the outer membrane, was determined by using procedures described by Loferer et al. (33). For each set of Western blots, equivalent amounts of protein were separated and probed.
Virulence assays.
The invasion assay was performed by using semiconfluent L2 fibroblast monolayers. Bacterial invasion was assessed in the manner described previously (51). Quantitation of Ipa secretion by using the suspension-labelling immunoassay (SLIM) developed by Andrews and Maurelli (7) was performed with the IpaB and IpaC antisera described above.
Generation of the mxiM2 allele.
After introduction of pRRS4 into E. coli ES1578, transformants were collected, inoculated into LB (containing twice the standard amount of yeast extract), and grown to optical density at 600 nm of ∼0.8. Mutagenized plasmid pools were then isolated and introduced into S. flexneri BS547 (mxiM1 mutant). Transformants were selected on TSB agar plates containing Congo red, ampicillin, kanamycin, and arabinose. Only those pools which yielded between 0.5 and 2.0% Congo red-negative colonies (a noncomplementing phenotype) in the BS547 background were chosen for further study. From these pools, we identified individual plasmids capable of conferring, in a wild-type (2457T) background, an arabinose-inducible and glucose-repressible dominant negative Congo red binding phenotype. Such plasmids were recovered, and their mxiM inserts were excised and ligated into a nonmutagenized pBAD18 backbone. Resulting plasmids were reintroduced back into 2457T and scored again for dominance. Those which again conferred a dominant negative phenotype (indicative of the presence of mutant mxiM alleles) were sequenced. Plasmid pRRS12 (pBAD18::mxiM2) was thus identified.
RESULTS
MxiM is an essential component of the Mxi-Spa secretion pathway.
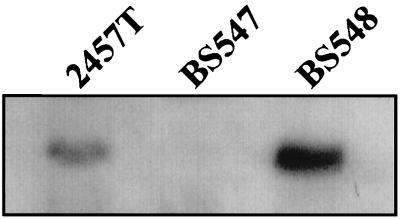
The wild-type mxiM locus in the virulence plasmid of S. flexneri 2a 2457T was insertionally inactivated by using an aphA-3 kanamycin resistance cassette (see Materials and Methods). The mxiM locus is predicted to encode a 142-residue protein with a calculated molecular mass of 15.8 kDa. Cleavage and loss of the putative 23-residue signal sequence of MxiM should yield a lipidated protein of roughly similar molecular weight. A protein of the size expected for MxiM was subsequently detected in whole-cell protein extracts from 2457T by immunoblot analysis by using anti-MxiM serum (Fig. 1). The corresponding protein was absent from a BS547 (mxiM1) whole-cell extract, thus confirming the MxiM defect.
FIG. 1.
Immunoblot analysis with MxiM antiserum of whole-cell protein extracts isolated from 2457T (wild-type), BS547 (mxiM1), and BS548 (mxiM1/PBAD-mxiM+). Bacteria were grown under standard conditions in liquid culture at 37°C. Cells were collected by centrifugation, and roughly equal numbers, as determined by culture optical density, were resuspended in SDS-PAGE sample buffer, boiled, and analyzed. MxiM-specific bands are shown.
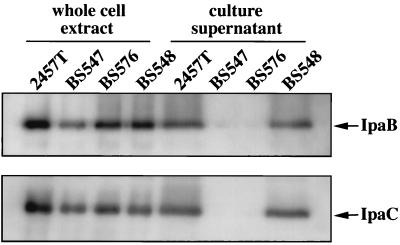
Unlike its wild-type parent strain, 2457T, strain BS547 did not bind the dye Congo red when grown on nutrient agar (Table 2). Congo red is a sulfonated azo dye that is bound by colonies of virulent shigellae but not by avirulent derivatives (35). Consistent with its inability to bind Congo red, BS547 was also unable to invade semiconfluent L2 cell monolayers (Table 2). A likely reason for the invasion defect was a block in the Ipa invasin secretory pathway. Using an anti-Ipa monoclonal antibody SLIM, we confirmed that IpaB and IpaC secretion by BS547 was, in fact, blocked (Table 2). IpaB and IpaC translocation defects were also demonstrated by immunoblot analysis of concentrated extracellular proteins isolated from 2457T and BS547 culture supernatants (Fig. 2). Extracellular forms of both IpaB and IpaC accumulated at high levels in mid-late log phase 2457T (wild-type) cultures but were absent from cultures of BS547. As noted in previous studies involving mxi and spa mutants (3–6), the block in secretion imparted by the mxiM lesion did not prevent accumulation of normal IpaB and IpaC levels in whole-cell fractions (Fig. 2).
TABLE 2.
Virulence phenotypes of wild-type strain and mxiM mutant derivativesa
| Strain (phenotype or genotype) | Crbb | Invasionc | Secretion ofd:
|
|
|---|---|---|---|---|
| IpaB | IpaC | |||
| 2457T (wild-type) | + | 85.0 ± 6.3 | 100 | 100 |
| BS547 (mxiM1) | − | 0 | 3.1 ± 1.4 | 5.8 ± 3.8 |
| BS575 (mxiM1/PLAC-mxiM+) | + | 81.5 ± 7.3 | 89.3 ± 4.3 | 95.0 ± 3.7 |
| BS588 (mxiM1/pBluescript) | − | 0 | 3.7 ± 1.6 | 1.1 ± 0.2 |
| BS548 (mxiM1/PBAD-mxiM+) | + | 78.5 ± 1.6 | NDe | ND |
| BS599 (2457T/PBAD-mxiM2) grown with: | ||||
| Glucose, 0.2% | + | 78.1 ± 7.7 | ||
| Arabinose, 0.2% | − | 20.0 ± 1.6 | ||
| Arabinose, 0.01% | − | 38.1 ± 4.9 | ||
| Arabinose, 0.005% | +/− | 56.4 ± 5.5 | ||
| Arabinose, 0.0005% | + | 75.9 ± 2.6 | ||
| BS603 (mxiM1/PBAD-mxiM2) | − | 8.5 ± 3.4 | ||
Each numerical value shown represents the average of at least three to five independent experiments ± standard deviation.
The Crb phenotype is based on a qualitative analysis of Congo red binding by bacterial colonies grown on nutrient agar. The +/− designation represents an intermediate phenotype.
Values are expressed as a percentage of 300 L2 cells in a semiconfluent monolayer which contained three or more internalized bacteria as determined by light microscopy.
Values were determined by SLIM and are expressed as a percentage of wild-type strain reactivity.
ND denotes that these values were not determined.
FIG. 2.
Effect of the mxiM1 mutation on expression and secretion of IpaB and IpaC. Proteins in whole-cell extracts and culture supernatants of 2457T (wild-type), BS547 (mxiM1), BS576 (mxiM1/pBAD18), and BS548 (mxiM1/PBAD-mxiM+) were prepared, separated by SDS-PAGE, and subjected to immunoblot analysis. The positions of proteins that reacted with either IpaB or IpaC antiserum are shown. Differences in either IpaB or IpaC signal intensity among the whole-cell extract samples isolated from different strains are likely insignificant, as subsequent analyses showed nearly identical levels among the strains.
Complementation with wild-type mxiM was used to prove that the virulence defects of BS547 were strictly attributable to the absence of MxiM. Expression of an intact copy of mxiM from pRRS5 in strain BS575 restored Congo red binding, invasion, and IpaB and IpaC secretion to the mxiM1 mutant background (Table 2). Virulence defects were also trans complemented by expression of mxiM from the PBAD promoter of pRRS4 (in strain BS548) (Table 2 and Fig. 2). Restoration of virulence phenotypes in both BS548 and BS575 coincided with the reappearance of MxiM in whole-cell protein fractions prepared from these strains (Fig. 1 and data not shown). These results demonstrated that the mxiM1 allele had no polar effects on the expression of loci with which mxiM1 is cotranscribed. MxiM is, therefore, required for virulence as a component of the Mxi-Spa type III secretion apparatus.
Lipidated MxiM associates with the outer membrane.
MxiM is predicted to be a substrate for lipidation and proteolytic processes of the bacterial lipoprotein maturation pathway, based on the findings of Allaoui et al. (4) that (i) it possesses a typical N-terminal signal sequence for Sec-dependent membrane translocation, (ii) the C-terminal end of its signal sequence bears a consensus sequence for lipoprotein modification and processing, and (iii) fusion of the MxiM signal sequence and lipidation and processing site to PhoA yields a hybrid protein that is specifically labeled with [3H]palmitate. To demonstrate rigorously whether MxiM could be detected as a lipidated protein, various S. flexneri strains were labeled in vivo with either [3H]palmitate or [2-3H]glycerol, and the resulting total cell envelope preparations were analyzed by SDS-polyacrylamide gel electrophoresis (PAGE) and fluorography. Since the lipid moieties of bacterial lipoproteins serve as hydrophobic membrane anchors (55), any radiolabeled MxiM species should copurify with envelope fractions. Strain BS548 (mxiM1/PBAD-mxiM+) produced both [3H]palmitate- and [2-3H]glycerol-labeled proteins of the appropriate molecular mass for MxiM when grown in the presence of arabinose, the inducer of the PBAD promoter (Fig. 3A and B); a MxiM-specific band was not observed in the corresponding soluble protein fractions (data not shown). In the wild-type (2457T) background, a [2-3H]glycerol-labeled MxiM species of slightly lesser intensity than observed in BS548 induced with 0.05% arabinose was observed. Additionally, MxiM was either not detected or detected at very low levels within the membrane lipoprotein profiles of BS547 (mxiM1) and BS548 (grown in the presence of glucose), respectively. These results indicate that in S. flexneri, MxiM is both lipid modified and membrane associated.
FIG. 3.
MxiM is lipidated. Total cell envelope preparations were isolated, separated by SDS-PAGE, and visualized by fluorography. Regions extending between 7- and 32-kDa size standards are shown. (A) The mxiM1 strain BS547 (lane 1) and strain BS548 (mxiM1/PBAD-mxiM+) grown in the presence of either 0.2% arabinose (lane 2) or 0.2% glucose (lane 3) were labeled with [3H]palmitate. The signal shown is a 1-day exposure. (B) [2-3H]glycerol labeling of the wild-type strain, 2457T (lane 1), BS548 grown with either 0.05% arabinose (lane 2) or 0.2% glucose (lane 3), and BS603 grown with 0.05% arabinose (lane 4). The signal shown is an 11-day exposure.
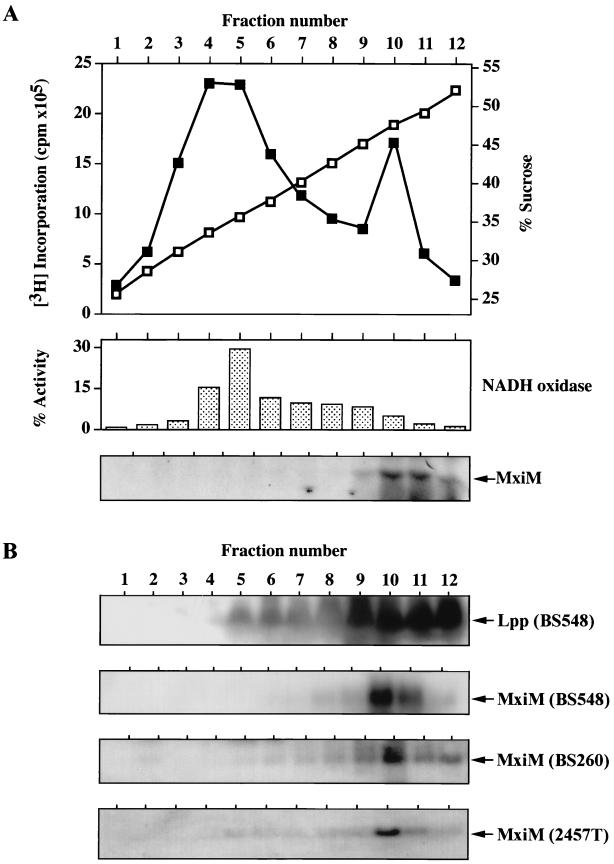
To more precisely determine the membrane position to which lipidated MxiM localizes, total cell envelope preparations isolated from both [3H]palmitate-labeled and unlabeled BS548 cultures were analyzed by sucrose density gradient centrifugation. After the sedimentation of envelope components, gradient fractions of increasing sucrose density were recovered and subjected to a variety of analyses. Two distinct peaks of [3H]palmitate incorporation were observed at ca. 35% (fractions 4 and 5) and 47.5% (fraction 10) (wt/wt) sucrose (Fig. 4A); these likely corresponded to inner and outer membrane components, respectively. NADH oxidase activity, a control for the inner membrane (43), peaked in the lower density fractions (primarily in fraction 5), showing that this section of the gradient was enriched for inner membrane proteins (Fig. 4A). Immunoblot analysis of the Lpp outer membrane lipoprotein (24) demonstrated that the high-density fractions, particularly fractions 9 through 12, were enriched for outer membrane proteins (Fig. 4B). A band of the appropriate size for MxiM was detected by [3H]palmitate labeling and by immunoblotting in the high-density outer membrane protein-containing fractions (fractions 9 through 12) (Fig. 4A and B). This band was absent from all fractions obtained from BS548 cultures grown with glucose (the repressor of PBAD) (data not shown). The outer membrane targeting of MxiM in BS548 was not an artifact of overexpression from the PBAD promoter, since the identical distribution pattern was observed in two strains, BS260 and 2457T, in which MxiM was expressed from its native promoter (Fig. 4B). Lipidated MxiM is therefore incorporated primarily into the outer membrane of the cell envelope.
FIG. 4.
Subcellular localization of MxiM determined by sucrose density gradient centrifugation. Total cell envelope preparations were fractionated and analyzed as described in Materials and Methods. (A) Distribution of [3H]palmitate-labeled MxiM in samples derived from arabinose-induced BS548 cultures. Sucrose concentration (shown as open squares) and total 3H incorporation (shown as closed squares) are plotted against fraction number; NADH oxidase activity and labeled MxiM associated with each fraction are shown. (B) Immunological analysis of fraction components derived from unlabeled BS548 (grown with arabinose), BS260, and 2457T cultures. The positions of proteins recognized by either anti-Lpp or anti-MxiM serum are shown. Inner and outer membrane markers for BS260 and 2457T samples were indistinguishable from that described for BS548.
The proper outer membrane targeting of MxiM in BS260 (an Ipa secretory mutant [6]), indicated that its modification and processing and subsequent outer membrane association occur independently of a functional type III pathway. Consistent with this, the lipidation and localization of MxiM induced from the PBAD promoter of pBAD18 were unaltered in the virulence plasmid-cured Shigella strain, BS103 (data not shown). These results show that proper interaction between MxiM and the Sec-dependent type II secretion pathway (which likely delivers all lipoproteins across the inner membrane) and the subsequent outer membrane localization of MxiM require no accessory virulence proteins.
MxiM is exposed on the inner face of the outer membrane.
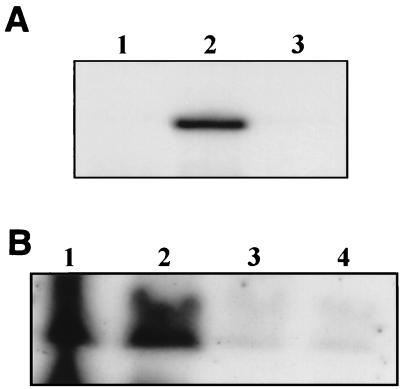
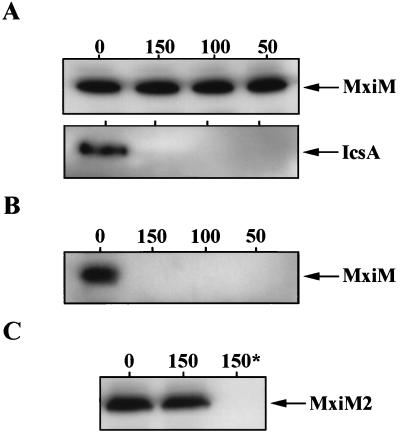
A bacterial lipoprotein may exist exposed to either the periplasmic or extracellular environments, depending on the outer membrane face into which its N-terminal acyl chains integrate (48). To probe the topology of MxiM in the outer membrane (i.e., whether it is exposed at the inner or outer face), we assessed the sensitivity of MxiM pools to extracellular protease. Culture aliquots of S. flexneri 2457T were incubated with increasing amounts of proteinase K and subsequently analyzed by Western blotting by using antiserum recognizing either MxiM or the surface-exposed protein, IcsA. Treatment of intact shigellae with proteinase K completely degraded surface-exposed IcsA but did not affect MxiM immunoblot signal intensity (Fig. 5A). Similar results were also obtained by using strain BS548 (mxiM1/PBAD-mxiM+) (data not shown). Only after permeabilization of the outer membrane by sucrose/EDTA treatment was proteolytic degradation of MxiM observed (Fig. 5B). Since denaturants were not necessary at any step to render MxiM susceptible to proteolysis, the protease resistance of MxiM is conferred by its insertion into the periplasmic face of the outer membrane.
FIG. 5.
Analysis of MxiM susceptibility and resistance to extracellular protease. (A) Protease resistance of MxiM in intact 2457T. Late exponential phase 2457T culture samples were treated with the indicated concentrations (0, 150, 100, and 50 μg ml−1) of proteinase K. Samples were analyzed by immunoblotting by using antisera specific for either MxiM or the control outer leaflet protein IcsA. (B) Protease susceptibility of MxiM after outer membrane permeabilization. Late exponential phase 2457T culture samples were incubated in a sucrose/EDTA solution to permeabilize the outer membrane prior to treatment with proteinase K (0, 150, 100, and 50 μg ml−1). Samples were analyzed by immunoblotting by using anti-MxiM serum. (C) Protease susceptibility and resistance of MxiM2. Culture aliquots from the mxiM2 mutant derivative, BS603, were incubated in either the presence (150 μg ml−1) or the absence of proteinase K. One of the proteinase K-treated samples (denoted by an asterisk) was outer membrane permeabilized prior to the addition of proteinase K. Anti-MxiM serum was then used to identify MxiM2.
The lipid extensions of bacterial lipoproteins are generally considered membrane anchors for otherwise hydrophilic proteins (55), suggesting that MxiM is probably a peripheral outer membrane protein. This is supported not only by the protease sensitivity of MxiM after outer membrane permeabilization but also by the following findings: (i) the MxiM fusion protein encoded by pRRS11 (which lacks an N-terminal signal sequence and is not a substrate for lipidation) was recovered only from the soluble protein fraction of cellular extracts (data not shown), and (ii) computer analyses of MxiM secondary structure (using the SOSUI and PSORT systems) predict that the protein moiety of mature MxiM is soluble in the aqueous environment of the periplasm. The bulk of mature MxiM is, therefore, likely to exist at the interface between the outer membrane and the periplasm.
MxiM is required for IpaB association with the outer, but not inner, membrane.
As a periplasmic location would preclude direct interactions between MxiM and target host cells, it is more likely that MxiM is required either directly or indirectly as a component of the transmembrane Mxi-Spa secretion channel. Additionally, interactions of MxiM within the Mxi-Spa secretion apparatus would probably predominantly affect outer membrane components of this structure or positions proximal to it. Consequently, we exploited the mxiM mutant derivative, BS547, to study the requirement for MxiM in formation of a functional outer membrane segment of the Mxi-Spa system. The subcellular distribution pattern of IpaB was analyzed as an indicator of Mxi-Spa functional integrity.
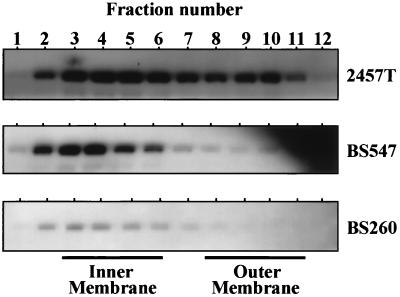
Total cell envelope preparations were isolated from strains 2457T (wild-type) and BS547 (mxiM1) and fractionated by sucrose density gradient centrifugation. As previously observed by Ménard et al. (39), IpaB was immunologically detected among both the inner and outer membrane protein-containing fractions of wild-type S. flexneri (Fig. 6). The Ipa proteins traverse both inner and outer membrane positions during the translocation process; therefore, Ipa cosedimentation with these envelope components is expected. Absence of MxiM in BS547, however, resulted in a noticeable shift in the IpaB sedimentation pattern toward the low-density inner membrane protein-containing fractions. Reductions in the accumulation of outer membrane-localized IpaB in BS547 are most consistent with a defect in the functional integrity of this region of Mxi-Spa.
FIG. 6.
Requirements for MxiM in the subcellular distribution of IpaB. Total membrane extracts from the indicated strains (2457T, BS547, and BS260) were fractionated by sucrose density gradient centrifugation. Equivalent fraction contents from each strain were separated by SDS-PAGE and analyzed by immunoblotting with anti-IpaB serum. Exposure times were exactly 2 min for all blots shown. The sucrose concentrations in each fraction (ranging from 27 to 52% in fractions 1 through 12 from each strain) as well as protein concentration and Lpp and NADH oxidase distribution were also determined. These results were nearly identical to those presented in Fig. 4 and are therefore not shown.
The MxiM-independent localization of IpaB to inner membrane positions in BS547 suggests that MxiM is required for the flow of Ipa traffic between the inner and outer membranes. As such, it is possible that inner membrane type III secretion machinery can assemble in the absence of MxiM and initiate associations with IpaB. To address this point, we examined IpaB subcellular localization in a mxiA mutant strain, BS260, which specifically lacks an inner membrane component of Mxi-Spa required for Ipa secretion (Ipa synthesis, however, is unaffected) (6, 7). A defect in mxiA resulted in a considerable decrease in both the inner membrane localization and outer membrane localization of IpaB (compared with that detected in either 2457T or BS547) (Fig. 6). MxiA and, therefore, inner membrane Mxi-Spa components mediate specific associations with IpaB and could be responsible for the inner membrane IpaB detected in BS547. The absence of IpaB in association with outer membrane fractions of the mxiM mutant would therefore reflect the formation of an incomplete Mxi-Spa pathway in this strain (i.e., one extending through the inner but not outer membrane).
MxiM interacts with other virulence proteins in the periplasm.
To define a function for MxiM, we used a genetic approach to identify mxiM alleles that, when expressed from the PBAD promoter of pBAD18, exert dominant negative effects on the Congo red binding phenotype of wild-type 2457T (see Materials and Methods). Since MxiM probably functions as a component of a larger aggregate, dominant mxiM mutants were expected. The mxiM2 allele was thus identified (expressed in strain BS599) and found to confer dominant negative effects not only on the Congo red binding phenotype but on invasion as well (Table 2). While mxiM2 was dominant over a range of inducer concentrations (0.01 to 0.2% arabinose), at levels below 0.01% the mutation was only partially dominant or recessive. The 0.01% arabinose concentration was significant since it was the inducer level which resulted in MxiM expression from PBAD that was roughly equivalent to that obtained from the native MxiM promoter (data not shown). These findings support a hypothesis that functional and defective MxiM monomers compete with each other either for direct insertion into the Mxi-Spa apparatus or for interactions with other Mxi-Spa components essential for secretion. Depending on which form is present in excess, such interactions will either suppress (with MxiM2) or enhance (with MxiM) elaboration of virulence phenotypes.
The dominant negative mxiM2 allele arose by substitution of a glycine codon at position 23 (located within the MxiM lipoprotein modification and processing sequence) with a codon for arginine. The resulting MxiM2 protein was very poorly labeled by [2-3H]glycerol (Fig. 3B) but did remain exposed to proteolytic degradation in the periplasmic environment (Fig. 5C), and it did confer very low levels of L2 cell invasion to the mxiM1 background (in strain BS603) (Table 2). These findings suggest that while MxiM2 is translocated into the periplasm, it exists there predominantly in a nonlipidated form. In the absence of efficient lipid modification, MxiM2 cannot be properly localized to the outer membrane and is, therefore, capable of manifesting only trace levels of activity. This interpretation is supported by the work of Pollitt et al. (47), which demonstrates that a similar type of alteration in the lipoprotein modification and processing site of the Lpp outer membrane-linked protein from E. coli yields an inner membrane-anchored prolipoprotein which is not a substrate for either lipidation or signal sequence cleavage. The dominant negative effects of mxiM2 expression are probably attributable to this protein mislocalization. Overtly toxic effects related to MxiM2 were ruled out, since a high level of induction in either BS599 or BS603 produced no unusual alterations in bacterial growth rate, colony morphology, or bacterial cell shape. Most likely, the presence of a mutant MxiM derivative which does not localize properly but can still establish periplasmic interactions with other Mxi-Spa subunits directly inhibits proper assembly or function of Mxi-Spa.
DISCUSSION
The mxiM locus of S. flexneri encodes a secreted protein with no detectable homologs among other type III secretion systems (4). Recent BLAST and FASTA searches have also failed to detect proteins with similarities to MxiM (data not shown). Since homologs have been detected for the majority of Mxi-Spa proteins, MxiM may be required for functions specific to Shigella. Of particular interest to us was the work of Allaoui et al. (4) which showed that a MxiM-PhoA hybrid protein is lipid modified (and detectable in whole-cell protein extracts). Recent work has implicated bacterial lipoproteins as essential subunits of most transmembrane traffic systems. Therefore, while it is not a component commonly found among type III secretory pathways, MxiM is likely to play an important, if not essential, role in the pathway for Ipa secretion.
In this study we characterized the contribution of MxiM to the virulent phenotype of Shigella, using a combination of genetic and biochemical analyses. By allelic replacement, the mxiM ORF was disrupted with a nonpolar kanamycin resistance cassette. The resulting mutant produced no detectable MxiM, did not translocate the Ipa invasins, and was phenotypically avirulent, indicating that MxiM is, in fact, an indispensable component of the Mxi-Spa pathway. [3H]palmitate and [2-3H]glycerol labeling studies were used to establish that, in its mature form, MxiM is both lipidated and associated with the bacterial cell envelope. Furthermore, fractionation studies identified MxiM clearly within the outer membrane of the cell envelope. Outer membrane localization of MxiM was observed by using several different Shigella backgrounds, including BS260, a strain lacking an essential inner membrane component of Mxi-Spa, and BS103, a strain lacking the virulence plasmid. The lipidation, processing, and outer membrane insertion of MxiM, therefore, require no other type III pathway components.
Based on the in vivo resistance of MxiM to treatment with extracellular protease (and susceptibility after outer membrane permeabilization), the lipid anchor of MxiM is thought to insert primarily into the inner leaflet of the outer membrane. As mature MxiM lacks predicted membrane-embedded domains and was soluble when expressed without lipid modification, the bulk of MxiM is only peripherally associated with the outer membrane. Since it is linked as such to the envelope at the interface between the outer membrane and the periplasm, several different functions can be envisioned for MxiM. MxiM could serve a structural function, via its interactions with other Mxi-Spa subunits. Furthermore, like the Lpp outer membrane lipoprotein of E. coli (10), MxiM may act as a linker between the murein sacculus and the outer membrane. In support of this association, most cell envelope-anchored lipoproteins facing the periplasmic space are believed to mediate at least noncovalent interactions with peptidoglycan (32). While motifs known to mediate noncovalent, or even covalent, bonds with peptidoglycan are absent in MxiM, other uncharacterized mechanisms for lipoprotein-peptidoglycan interaction do exist (19) and may serve to establish such a linkage involving MxiM. In this manner then, MxiM could lend structural support to the entire Mxi-Spa apparatus (stronger than that provided by simple membrane insertion of the MxiM lipid moieties). The flagellar basal body structure, which is similar to that of type III systems, interacts with peptidoglycan for structural stability (13, 15) and must support, like type III pathways, the assembly and function of extracellular filamentous appendages (21, 29, 45, 50).
Recently, a small group of outer membrane-linked lipoproteins has been assigned a chaperone-like activity in both type II and type III secretory systems. This group, which includes PulS of Klebsiella oxytoca, OutS of Erwinia chrysanthemi, PilP of Neisseria gonorrhoeae, VirG of Yersinia enterocolitica, and InvH of Salmonella typhimurium, may include MxiM as well. Work on the type II system for pullulanase secretion conducted by Hardie et al. (25) showed that PulS directs another protein, PulD, to the outer membrane and protects it from proteolytic degradation. PulD is the archetype of a family of proteins called secretins, which are related integral outer membrane proteins that have been identified in a variety of bacterial secretion systems (including the Mxi-Spa type III pathway) and which probably multimerize to form export channels (20). OutS, a type II pathway component involved in the secretion of pectate lyases, is also required for secretin (OutD) stability (54). Two secretin chaperones, VirG and InvH, have been identified among type III systems and are required at least for secretin outer membrane localization functions (14, 31). MxiM could play a role similar to that of PulS, OutS, VirG, and InvH in relation to the Mxi-Spa secretin homolog, MxiD. While the secretin chaperone sequences are poorly conserved both between each other and with MxiM, they are all (including MxiM) outer membrane lipoproteins of similar size encoded by loci closely linked to those secretins. This group of chaperones, possibly including MxiM, may comprise a group of functional homologs.
The comparison of IpaB subcellular distribution patterns in fractionated membrane extracts isolated from wild-type Shigella and the mxiM1 mutant derivative showed that MxiM is required for a high level of association of IpaB with the outer membrane. This finding is compatible with the view that MxiM either directly or indirectly helps to form a functional outer membrane secretion channel and is consistent with each of the putative MxiM functions described above. The cosedimentation of IpaB with inner membrane components of the mxiM1 mutant also suggests that MxiM is not required for formation of an inner membrane portion of the Mxi-Spa pathway. That this inner membrane-associated IpaB does interact with Mxi-Spa is supported by our findings that such localization is not observed in a background lacking an inner membrane Mxi-Spa component (MxiA). In a study of exoprotein subcellular fate in the hrp-hrc-encoded type III secretion system of Pseudomonas syringae, Charkowski et al. (11) also genetically dissected the secretory process and found that the inner membrane translocation channel was functional in the absence of a component required only for secretion across the outer membrane. These observations suggest that structural and functional similarities between the Hrp-Hrc and Mxi-Spa systems exist and imply that in type III systems, inner membrane-bound structural components assemble and sequester target proteins in the absence of outer membrane components. This situation is somewhat similar to that for type II systems, in which the inner membrane Sec system can process target proteins in the absence of outer membrane secretory elements (48).
Based on the requirements for MxiM in Ipa secretion and its location at the outer membrane-periplasm interface, MxiM likely participates in direct interactions with other Mxi-Spa subunits. The dominant nature of the mxiM2 allele accords with such a physical association. Dominance could reflect displacement of wild-type MxiM from the Mxi-Spa secretory structure or from its interactions with an essential structural subunit (displacement that is expected if, for example, MxiM is a secretin chaperone) by MxiM2. Since MxiM2 is periplasmically exposed, it should establish interactions normally involving MxiM; however, the absence of an outer membrane anchor from MxiM2 would block or hamper subsequent assembly or function of Mxi-Spa. Partial dominance or recessiveness would simply reflect the dilution of MxiM2 pools. While detailed analyses of additional dominant negative alleles are required to prove these suggestions, the results described here do support the concept that an interaction between MxiM and other components of the Mxi-Spa type III system, possibly within the periplasmic space, exists.
While the actual function for MxiM is, as yet, unknown, its absolute requirement in Shigella pathogenesis is clear. As predicted for most type III system components, MxiM is membrane associated. This association is mediated by N-terminal lipid extensions that anchor the mature protein moiety of MxiM to the inner leaflet of the outer membrane. Within the periplasmic space, MxiM probably participates in protein-protein interactions with other Mxi-Spa components. These interactions allow formation of a functional outer membrane segment of the Ipa invasin secretory channel. Future analysis of exactly which proteins MxiM interacts with during this process will be important, not only in the identification of a precise role for MxiM but also in the study of assembly and function of the Mxi-Spa system as a whole.
ACKNOWLEDGMENTS
This work was supported by grant AI24656 from the National Institute of Allergy and Infectious Diseases and grant RO7385 from the Uniformed Services University of the Health Sciences.
We thank Masaru Ohara for the gift of Lpp-specific antisera and technical assistance in membrane labeling and analysis, Michael A. Davis, Reinaldo E. Fernández, and Robin C. Sandlin for thoughtful discussion, Ed Oaks for the monoclonal IpaB and IpaC antibodies, M. G. Marinus for the gift of ES1578, and Keith A. Lampel, Darcy E. Hanes, and Mahendra Khotary for advice and technical assistance in the preparation of MxiM-specific antisera.
REFERENCES
- 1.Adam T, Arpin M, Prévost M-C, Gounon P, Sansonetti P J. Cytoskeletal rearrangements and the functional role of T-plastin during entry of Shigella flexneri into HeLa cells. J Cell Biol. 1995;129:367–381. doi: 10.1083/jcb.129.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allaoui A, Mounier J, Prévost M-C, Sansonetti P J, Parsot C. icsB: a Shigella flexneri virulence gene necessary for the lysis of protrusions during intercellular spread. Mol Microbiol. 1992;6:1605–1616. doi: 10.1111/j.1365-2958.1992.tb00885.x. [DOI] [PubMed] [Google Scholar]
- 3.Allaoui A, Sansonetti P J, Ménard R, Barzu S, Mounier J, Phalipon A, Parsot C. MxiG, a membrane protein required for secretion of Shigella spp. Ipa invasins: involvement in entry into epithelial cells and in intercellular dissemination. Mol Microbiol. 1995;17:461–470. doi: 10.1111/j.1365-2958.1995.mmi_17030461.x. [DOI] [PubMed] [Google Scholar]
- 4.Allaoui A, Sansonetti P J, Parsot C. MxiJ, a lipoprotein involved in secretion of Shigella Ipa invasins, is homologous to YscJ, a secretion factor of the Yersinia Yop proteins. J Bacteriol. 1992;174:7661–7669. doi: 10.1128/jb.174.23.7661-7669.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allaoui A, Sansonetti P J, Parsot C. MxiD: an outer membrane protein necessary for the secretion of the Shigella flexneri Ipa invasins. Mol Microbiol. 1993;7:59–68. doi: 10.1111/j.1365-2958.1993.tb01097.x. [DOI] [PubMed] [Google Scholar]
- 6.Andrews G P, Hromockyj A E, Coker C, Maurelli A T. Two novel virulence loci, mxiA and mxiB, in Shigella flexneri 2a facilitate excretion of invasion plasmid antigens. Infect Immun. 1991;59:1997–2005. doi: 10.1128/iai.59.6.1997-2005.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andrews G P, Maurelli A T. mxiA of Shigella flexneri 2a, which facilitates export of invasion plasmid antigens, encodes a homolog of the low-calcium-response protein, LcrD, of Yersinia pestis. Infect Immun. 1992;60:3287–3295. doi: 10.1128/iai.60.8.3287-3295.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baron C, Thorstenson Y R, Zambryski P C. The lipoprotein VirB7 interacts with VirB9 in the membranes of Agrobacterium tumefaciens. J Bacteriol. 1997;179:1211–1218. doi: 10.1128/jb.179.4.1211-1218.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bernardini M L, Mounier J, d’Hauteville H, Coquis-Rondon M, Sansonetti P J. Identification of icsA, a plasmid locus of Shigella flexneri that governs bacterial intra- and intercellular spread through interaction with F-actin. Proc Natl Acad Sci USA. 1989;86:3867–3871. doi: 10.1073/pnas.86.10.3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Braun V, Wolff H. The murein-lipoprotein linkage in the cell wall of Escherichia coli. Eur J Biochem. 1970;14:387–391. doi: 10.1111/j.1432-1033.1970.tb00301.x. [DOI] [PubMed] [Google Scholar]
- 11.Charkowski A O, Huang H-C, Collmer A. Altered localization of HrpZ in Pseudomonas syringae pv. syringae hrp mutants suggests that different components of the type III secretion pathway control protein translocation across the inner and outer membranes of gram-negative bacteria. J Bacteriol. 1997;179:3866–3874. doi: 10.1128/jb.179.12.3866-3874.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen Y, Smith M R, Thirumalai K, Zychlinsky A. A bacterial invasin induces macrophage apoptosis by binding directly to ICE. EMBO J. 1996;15:3853–3860. [PMC free article] [PubMed] [Google Scholar]
- 13.Chun S Y, Parkinson J S. Bacterial motility: membrane topology of the Escherichia coli MotB protein. Science. 1988;239:276–278. doi: 10.1126/science.2447650. [DOI] [PubMed] [Google Scholar]
- 14.Daefler S, Russel M. The Salmonella typhimurium InvH protein is an outer membrane lipoprotein required for the proper localization of InvG. Mol Microbiol. 1998;28:1367–1380. doi: 10.1046/j.1365-2958.1998.00908.x. [DOI] [PubMed] [Google Scholar]
- 15.DePamphilis M L, Adler J. Attachment of flagellar basal bodies to the cell envelope: specific attachment to the outer lipopolysaccharide membrane and the cytoplasmic membrane. J Bacteriol. 1971;105:396–407. doi: 10.1128/jb.105.1.396-407.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evans D G, Evans D J, Jr, Tjoa W. Hemagglutination of human group A erythrocytes by enterotoxigenic Escherichia coli isolated from adults with diarrhea: correlation with colonization factor. Infect Immun. 1977;18:330–337. doi: 10.1128/iai.18.2.330-337.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Finlay B B, Falkow S. Common themes in microbial pathogenicity revisited. Microbiol Mol Biol Rev. 1997;61:136–169. doi: 10.1128/mmbr.61.2.136-169.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Formal S B, Dammin G J, LaBrec E H, Schneider H. Experimental Shigella infections: characteristics of a fatal infection produced in guinea pigs. J Bacteriol. 1958;75:604–610. doi: 10.1128/jb.75.5.604-610.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fussenegger M, Facius D, Meier J, Meyer T F. A novel peptidoglycan-linked lipoprotein (ComI) that functions in natural transformation competence of Neisseria gonorrhoeae. Mol Microbiol. 1996;19:1095–1105. doi: 10.1046/j.1365-2958.1996.457984.x. [DOI] [PubMed] [Google Scholar]
- 20.Genin S, Boucher C A. A superfamily of proteins involved in different secretion pathways in Gram-negative bacteria: modular structure and specificity of the N-terminal domain. Mol Gen Genet. 1994;243:112–118. doi: 10.1007/BF00283883. [DOI] [PubMed] [Google Scholar]
- 21.Ginocchio C C, Olmsted S B, Wells C L, Galán J E. Contact with epithelial cells induces the formation of surface appendages on Salmonella typhimurium. Cell. 1994;76:717–724. doi: 10.1016/0092-8674(94)90510-x. [DOI] [PubMed] [Google Scholar]
- 22.Goldberg M B, Parsot C, Barzu O, Sansonetti P J. Unipolar localization and ATPase activity of IcsA, a Shigella flexneri protein involved in intracellular movement. J Bacteriol. 1993;175:2189–2196. doi: 10.1128/jb.175.8.2189-2196.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guzman L-M, Belin D, Carson M J, Beckwith J. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J Bacteriol. 1995;177:4121–4130. doi: 10.1128/jb.177.14.4121-4130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hankte K, Braun V. Covalent binding of lipid to protein. Diglyceride and amide-linked fatty acid at the N-terminal end of the murein-lipoprotein of the Escherichia coli outer membrane. Eur J Biochem. 1973;34:284–296. doi: 10.1111/j.1432-1033.1973.tb02757.x. [DOI] [PubMed] [Google Scholar]
- 25.Hardie K R, Seydel A, Guilvout I, Pugsley A P. The secretin-specific, chaperone-like protein of the general secretory pathway: separation of proteolytic protection and piloting functions. Mol Microbiol. 1996;22:967–976. doi: 10.1046/j.1365-2958.1996.01539.x. [DOI] [PubMed] [Google Scholar]
- 26.Hayashi S, Wu H C. Identification and characterization of lipid-modified proteins in bacteria. In: Hooper N M, Turner A J, editors. Lipid modification of proteins: a practical approach. New York, N.Y: Oxford University Press; 1992. pp. 261–285. [Google Scholar]
- 27.High N, Mounier J, Prévost M-C, Sansonetti P J. IpaB of Shigella flexneri causes entry into epithelial cells and escape from the phagocytic vacuole. EMBO J. 1992;11:1991–1999. doi: 10.1002/j.1460-2075.1992.tb05253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hueck C J. Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol Mol Biol Rev. 1998;62:379–433. doi: 10.1128/mmbr.62.2.379-433.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knutton S, Rosenshine I, Pallen M J, Nisan I, Neves B C, Bain C, Wolff C, Dougan G, Frankel G. A novel EspA-associated surface organelle of enteropathogenic Escherichia coli involved in protein translocation into epithelial cells. EMBO J. 1998;17:2166–2176. doi: 10.1093/emboj/17.8.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kornacki J A, Oliver D B. Lyme disease-causing Borrelia species encode multiple lipoproteins homologous to peptide-binding proteins of ABC-type transporters. Infect Immun. 1998;66:4115–4122. doi: 10.1128/iai.66.9.4115-4122.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koster M, Bitter W, de Cock H, Allaoui A, Cornelis G R, Tommassen J. The outer membrane component, YscC, of the Yop secretion machinery of Yersinia enterocolitica forms a ring-shaped multimeric complex. Mol Microbiol. 1997;26:789–797. doi: 10.1046/j.1365-2958.1997.6141981.x. [DOI] [PubMed] [Google Scholar]
- 32.Lecuc M, Ishidate K, Shakibai N, Rothfield L. Interactions of Escherichia coli membrane lipoproteins with the murein sacculus. J Bacteriol. 1992;174:7982–7988. doi: 10.1128/jb.174.24.7982-7988.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Loferer H, Hammar M, Normark S. Availability of the fibre subunit CsgA and the nucleator protein CsgB during assembly of fibronectin-binding curli is limited by the intracellular concentration of the novel lipoprotein CsgG. Mol Microbiol. 1997;26:11–23. doi: 10.1046/j.1365-2958.1997.5231883.x. [DOI] [PubMed] [Google Scholar]
- 34.Marquart M E, Picking W L, Picking W D. Soluble invasion plasmid antigen C (IpaC) from Shigella flexneri elicits epithelial cell responses related to pathogen invasion. Infect Immun. 1996;64:4182–4187. doi: 10.1128/iai.64.10.4182-4187.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maurelli A T, Blackmon B, Curtiss R., III Loss of pigmentation in Shigella flexneri 2a is correlated with loss of virulence and virulence-associated plasmid. Infect Immun. 1984;43:397–401. doi: 10.1128/iai.43.1.397-401.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ménard R, Dehio C, Sansonetti P J. Bacterial entry into epithelial cells: the paradigm of Shigella. Trends Microbiol. 1996;4:220–225. doi: 10.1016/0966-842X(96)10039-1. [DOI] [PubMed] [Google Scholar]
- 37.Ménard R, Prévost M-C, Gounon P, Sansonetti P J, Dehio C. The secreted Ipa complex of Shigella flexneri promotes entry into mammalian cells. Proc Natl Acad Sci USA. 1996;93:1254–1258. doi: 10.1073/pnas.93.3.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ménard R, Sansonetti P J, Parsot C. Nonpolar mutagenesis of the ipa genes defines IpaB, IpaC, and IpaD as effectors of Shigella flexneri entry into epithelial cells. J Bacteriol. 1993;175:5899–5906. doi: 10.1128/jb.175.18.5899-5906.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ménard R, Sansonetti P J, Parsot C. The secretion of the Shigella flexneri Ipa invasins is induced by the epithelial cell and controlled by IpaB and IpaD. EMBO J. 1994;13:5293–5302. doi: 10.1002/j.1460-2075.1994.tb06863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ménard R, Sansonetti P J, Parsot C, Vasselon T. Extracellular association and cytoplasmic partitioning of the IpaB and IpaC invasins of S. flexneri. Cell. 1994;79:515–525. doi: 10.1016/0092-8674(94)90260-7. [DOI] [PubMed] [Google Scholar]
- 41.Miller V L, Mekalanos J J. A novel suicide vector and its use in construction of insertion mutations; osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires ToxR. J Bacteriol. 1988;170:2575–2583. doi: 10.1128/jb.170.6.2575-2583.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mills J A, Buysse J M, Oaks E V. Shigella flexneri invasion plasmid antigens B and C: epitope location and characterization with monoclonal antibodies. Infect Immun. 1988;56:2933–2941. doi: 10.1128/iai.56.11.2933-2941.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Osborn M J, Gander J E, Parisi E, Carson J. Mechanism of assembly of the outer membrane of Salmonella typhimurium. J Biol Chem. 1972;247:3962–3972. [PubMed] [Google Scholar]
- 44.Palmer B R, Marinus M G. DNA methylation alters the pattern of spontaneous mutation in Escherichia coli cells (mutD) defective in DNA polymerase III proofreading. Mutat Res. 1991;264:15–23. doi: 10.1016/0165-7992(91)90040-b. [DOI] [PubMed] [Google Scholar]
- 45.Parsot C, Ménard R, Gounon P, Sansonetti P J. Enhanced secretion through the Shigella flexneri Mxi-Spa translocon leads to assembly of extracellular proteins into macromolecular structures. Mol Microbiol. 1995;16:291–300. doi: 10.1111/j.1365-2958.1995.tb02301.x. [DOI] [PubMed] [Google Scholar]
- 46.Parsot C, Sansonetti P J. Invasion and pathogenesis of Shigella infections. In: Miller V L, editor. Bacterial invasiveness. New York, N.Y: Springer-Verlag; 1996. pp. 25–42. [DOI] [PubMed] [Google Scholar]
- 47.Pollitt S, Inouye S, Inouye M. Effect of amino acid substitutions at the signal peptide cleavage site of the Escherichia coli major outer membrane lipoprotein. J Biol Chem. 1986;261:1835–1837. [PubMed] [Google Scholar]
- 48.Pugsley A P. The complete general secretory pathway in gram-negative bacteria. Microbiol Rev. 1993;57:50–108. doi: 10.1128/mr.57.1.50-108.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ramer S W, Bieber D, Schoolnik G. BfpB, an outer membrane lipoprotein required for the biogenesis of bundle-forming pili in enteropathogenic Escherichia coli. J Bacteriol. 1996;178:6555–6563. doi: 10.1128/jb.178.22.6555-6563.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roine E, Wei W, Yuan J, Nurmiaho-Lassila E-L, Kalkkinen N, Romantschuk M, He S-Y. Hrp pilus: an hrp-dependent bacterial surface appendage produced by Pseudomonas syringae pv. tomato DC3000. Proc Natl Acad Sci USA. 1997;94:3459–3464. doi: 10.1073/pnas.94.7.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sandlin R C, Goldberg M B, Maurelli A T. Effect of O side-chain length and composition on the virulence of Shigella flexneri 2a. Mol Microbiol. 1996;22:63–73. doi: 10.1111/j.1365-2958.1996.tb02656.x. [DOI] [PubMed] [Google Scholar]
- 52.Sansonetti P J, Ryter A, Clerc P, Maurelli A T, Mounier J. Multiplication of Shigella flexneri within HeLa cells: lysis of the phagocytic vacuole and plasmid-mediated contact hemolysis. Infect Immun. 1986;51:461–469. doi: 10.1128/iai.51.2.461-469.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schoenhals G J, Macnab R M. Physiological and biochemical analyses of FlgH, a lipoprotein forming the outer membrane L ring of the flagellar basal body of Salmonella typhimurium. J Bacteriol. 1996;178:4200–4207. doi: 10.1128/jb.178.14.4200-4207.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shevchik V E, Robert-Baudouy J, Condemine G. Specific in teraction between OutD, an Erwinia chrysanthemi outer membrane protein of the general secretory pathway, and secreted proteins. EMBO J. 1997;16:3007–3016. doi: 10.1093/emboj/16.11.3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu H C. Biosynthesis of lipoproteins. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C: American Society for Microbiology Press; 1996. pp. 1005–1014. [Google Scholar]