Abstract
Segmented filamentous bacteria (SFB) are autochthonous bacteria inhabiting the intestinal tracts of many species, including humans. We studied the effect of SFB on the mucosal immune system by monoassociating formerly germfree C3H/HeN mice with SFB. At various time points during 190 days of colonization, fragment cultures of small intestine and Peyer’s patches (PP) were analyzed for total immunoglobulin A (IgA) and SFB-specific IgA production. Also, phenotypic changes indicating germinal center reactions (GCRs) and the activation of CD4+ T cells in PP were determined by using fluorescence-activated cell sorter analyses. A second group of SFB-monoassociated mice was colonized with a gram-negative commensal, Morganella morganii, to determine if the mucosal immune system was again stimulated and to evaluate the effect of prior colonization with SFB on the ability of M. morganii to translocate to the spleen and mesenteric lymph nodes. We found that SFB stimulated GCRs in PP from day 6 after monoassociation, that GCRs only gradually waned over the entire length of colonization, that natural IgA production was increased to levels 24 to 63% of that of conventionally reared mice, and that SFB-specific IgA was produced but accounted for less than 1.4% of total IgA. Also, the proportion of CD4+, CD45RBlow T cells, indicative of activated cells, gradually increased in the PP to the level found in conventionally reared mice. Secondary colonization with M. morganii was able to stimulate GCRs anew, leading to a specific IgA antibody response. Previous stimulation of mucosal immunity by SFB did not prevent the translocation of M. morganii in the double-colonized mice. Our findings generally indicate that SFB are one of the single most potent microbial stimuli of the gut mucosal immune system.
Segmented filamentous bacteria (SFB) are a group of autochthonous, strictly anaerobic, spore-forming, gram-positive bacteria that are identified on the basis of their morphology (10) and occur in the intestines of many vertebrate and invertebrate animal species, including humans (10, 12). Although first reported over 100 years ago by Leidy, who described the morphotype in the enteric flora of termites (16), they have never been cultured in vitro and had not been classified taxonomically (12). Using 16S rRNA sequence analysis, Snel et al. (20) have shown that SFB of mice, rats, and chickens represent a cluster within the Clostridium subphylum.
SFB can attach to small bowel epithelial cells by means of an appendage which binds to the apical plasma membrane (5). Tannock et al. (22) developed a method for harvesting SFB from the ilea of mice, based on this attachment to enterocytes. Subsequently, limiting dilution of ethanol-treated fecal samples, to remove vegetative bacteria, was used to monoassociate formerly germfree (GF) mice via spores (11), allowing studies on the relationship between SFB and mucosal immunity. It has also been suggested that SFB may competitively exclude pathogens from the intestine and contribute to colonization resistance (6). Recently, it has been reported that formerly GF mice, monoassociated with SFB, had increased numbers of lymphoid cells in the lamina propria of the ileal and cecal mucosa, increased numbers of immunoglobulin A (IgA)-secreting cells in the intestinal lamina propria, elevated IgA titers in the serum and intestinal secretions, and increased concanavalin A-induced proliferative responses of mesenteric lymph node cells compared to GF mice (12). GF mice given uncharacterized gut flora used to maintain specific-pathogen-free mice, but lacking SFB, showed similar but less pronounced changes, indicating that SFB may stimulate the immune system to a greater extent than other commensal bacteria. Also, colonization levels of SFB in the small intestine appear to be influenced by the mucosal immune system. There is a decrease in colonization levels in the small intestine of GF mice, corresponding to an increase in IgA-secreting cells (21).
It has been reported that Morganella morganii, a gram-negative murine commensal bacterium, will stimulate a self-limiting gut mucosal immune response, while permanently colonizing the intestine in monoassociated mice (19). The number of bacteria translocating to the spleen and mesenteric lymph nodes declined with the onset of a specific IgA response. In this study, we monoassociated formerly GF mice with SFB and evaluated the stimulation of germinal center reactions, total IgA production, SFB-specific IgA production, and activation of CD4+ T cells in Peyer’s patches (PP) and the small intestine. A group of these SFB-monoassociated mice were then colonized with M. morganii to determine if the mucosal immune system was again stimulated and to evaluate the effect of prior colonization with SFB on the ability of the facultative anaerobe M. morganii to translocate to the mesenteric lymph nodes and spleen. Of course the obligatively anaerobic, noncultivatible SFB have not themselves been shown to translocate.
MATERIALS AND METHODS
Mice.
The C3H/HeN mice were originally obtained from Taconic, Germantown, N.Y. These mice were rederived to be GF by using GF BALB/c mice as foster mothers. GF C.B17 scid/scid mice and GF BALB/c mice were originally obtained from the University of Wisconsin, Madison. Both the GF C.B17 scid/scid and GF C3H/HeN mice were bred and maintained within the gnotobiotic facility in the Department of Biology at the University of Pennsylvania.
Bacteria.
SFB spores in mouse fecal material were kindly provided by H. Snel, University of Nijmegen (Nijmegen, The Netherlands). GF C.B17 scid/scid mice were inoculated per os with a solution of the spores suspended in phosphate-buffered saline (PBS). GF C.B17 scid/scid mice were used to culture SFB in vivo to preclude the coating of the bacteria with IgA antibodies. Feces from the C.B17 scid/scid mice were then Gram stained to confirm the presence of SFB. Feces from these mice were then suspended in PBS and used to inoculate two large groups (40 to 50 mice/group) of GF C3H/HeN mice per os. Fecal samples were Gram stained to confirm the presence of SFB as previously described (21) each time C3H/HeN mice were sacrificed for study; however, SFB levels were not quantified. One group of mice were studied for 190 days; a second group of mice were inoculated with M. morganii on day 113 and studied for an additional 89 days until 202 days after infection with SFB. M. morganii bacteria were kindly provided by Ann Feeney, The Scripps Research Institute (La Jolla, Calif.). A 1.0-ml aliquot of the bacteria was thawed from storage at −70°C and cultured on sterile brain heart infusion agar at 37°C for 16 h. SFB-monoassociated C3H/HeN mice were inoculated per os with a 0.1-ml solution of 108 organisms. Groups of three to five mice were sacrificed for each time point studied.
PP and intestinal fragment cultures.
The method previously developed in our laboratory (17) was generally followed. Specifically, PP were removed from the small intestine, and 4-cm segments from the duodenum, jejunum, and ileum of each mouse were excised, opened longitudinally, and washed three to five times with Ca2+-, Mg2+-free PBS containing 0.1% gentamicin (GIBCO, Grand Island, N.Y.) and 10 mM HEPES. The tissue was then washed in Ca2+-, Mg2+-free PBS containing 0.05% EDTA, 0.1% gentamicin, and 10 mM HEPES to remove the mucin layer. Finally, the tissue fragments were washed in RPMI medium containing 10% fetal bovine serum (FBS). Tissues were cultured in a sterile 24-well plate (Costar, Cambridge, Mass.) in 1.0 ml of Kennett’s HY medium (GIBCO) containing 10% FBS, 1.0% l-glutamine, 0.01% gentamicin, and 1.0% antibiotic-antimycotic solution (100 U of penicillin per ml, 1.0% streptomycin, and 0.25 μg of amphotericin B [Fungizone] [GIBCO] per ml), for 7 days in 90% O2 and 10% CO2 at 37°C. Two approximately 3-mm-long pieces from each intestinal segment or one PP cut in half was cultured per well. Culture fluids were frozen prior to assay. Usually, replicate cultures were made from each tissue from each mouse. Tissue was sampled equally from the duodenum, jejunum, and ileum. In the first group of mice, which were monoassociated with SFB, separate cultures were made from tissues from the duodenum, jejunum, and ileum.
RIA.
The radioimmunoassay (RIA) used in our laboratory has been previously described (7, 17). To determine total IgA antibodies, plates were coated with goat anti-mouse Fab fragment (Southern Biotechnology Associates, Birmingham, Ala.). SFB sonicate was used as the coating antigen to determine the level of SFB-specific IgA. To prepare the sonicate, cecal contents from six monoassociated C.B17 scid/scid mice were suspended in 30 ml of sterile PBS and centrifuged at 33.51 × g for 10 min. Ten milliliters of the resulting supernatant was centrifuged on a discontinuous Percoll gradient (2 ml of 100% Percoll under 3 ml of 70% Percoll) at 536.16 × g for 20 min, and the material at the interface of 100 and 70% Percoll was collected and tested for bacteria. This material was then suspended in 35 ml of PBS and centrifuged at 536.16 × g for 20 min. The pellet was resuspended in 10 ml of PBS and divided into 2-ml portions which were each sonicated on ice with a sonicator cell disrupter (Misonix Inc., Farmingdale, N.Y.) three times for 20 s with 10-s interruptions. The five samples were pooled and centrifuged at 20,000 × g for 20 min. The pellets remaining after sonication were pooled in 5 ml of PBS and divided into two tubes for additional sonication and centrifugation as described above. The two preparations were pooled and passed through a 0.2-μm-pore-size sterile filter unit. Serial dilutions of this preparation were used to coat RIA plates, and that dilution was used for assays that were nonlimiting for a series of dilutions of an IgA monoclonal antibody (2A7) versus SFB (18a). A radiolabeled goat anti-mouse IgA (Southern Biotechnology Associates) was used to develop all assays.
Whole heat-killed M. morganii bacteria were prepared by first heating the bacteria for 1 h in a 70°C water bath and then preparing a suspension in sterile PBS which would register an optical density at 550 nm of 1. A 200-μl aliquot of this suspension was spun in an Eppendorf centrifuge (model 5415) at 14,000 rpm for 10 min. The pellet was washed with 500 μl of PBS containing NaN3 (PBS-Az) and again spun at 26,271.84 × g for 10 min. Either 20 μl of the sample or the blank (1.0% bovine serum albumin in PBS-Az) was mixed with the pellet, and the mixture was incubated at 4°C overnight. The mixture was centrifuged at 14,000 rpm for 10 min and then washed twice with 500 μl of PBS-Az. To determine the level of M. morganii-specific IgA, 100 μl of 125I-labeled goat anti-mouse IgA was mixed with the pellet and incubated overnight at 4°C. The sample was treated as above. After the pellet had been dried at 60°C for 30 to 60 min, the tube was cut with a hot wire apparatus, and the amount of label in bottom containing the pellet was determined in a gamma counter.
FACS analysis.
PP were incubated with an appropriate dilution of fluorochrome-coupled reagent in PBS-Az containing 5% FBS for 30 min on ice. The cells were washed and then analyzed on a fluorescence-activated cell sorter (FACS) model IV flow cytometer (Becton Dickinson, Sunnydale, Calif.). Fluorescein isothiocyanate (FLU)-labeled peanut agglutinin (PNA) (14) in conjunction with a phycoerythrin (PE)-conjugated anti-kappa chain was used to stain for germinal center B cells. The same PE-conjugated anti-kappa and FLU-labeled goat anti-mouse IgA (Southern Biotechnology Associates) was used to stain surface IgA-positive cells. PE-conjugated anti-CD4 (Pharmingen, San Diego, Calif.) and FLU-labeled anti-CD45RB (16A) (Pharmingen) were used to stain CD4+ T cells for activation.
Bacterial translocation studies for M. morganii.
The spleen or mesenteric lymph nodes were homogenized in 1.0 ml of PBS, and serial dilutions were made. A 100-μl portion of the homogenate was added to a brain heart infusion agar plate. The plates were incubated for 24 h at 37°C, and colony counts were taken.
RESULTS
Perturbations of germinal center reactions and subsets of PP cells following colonization of formerly GF mice with SFB.
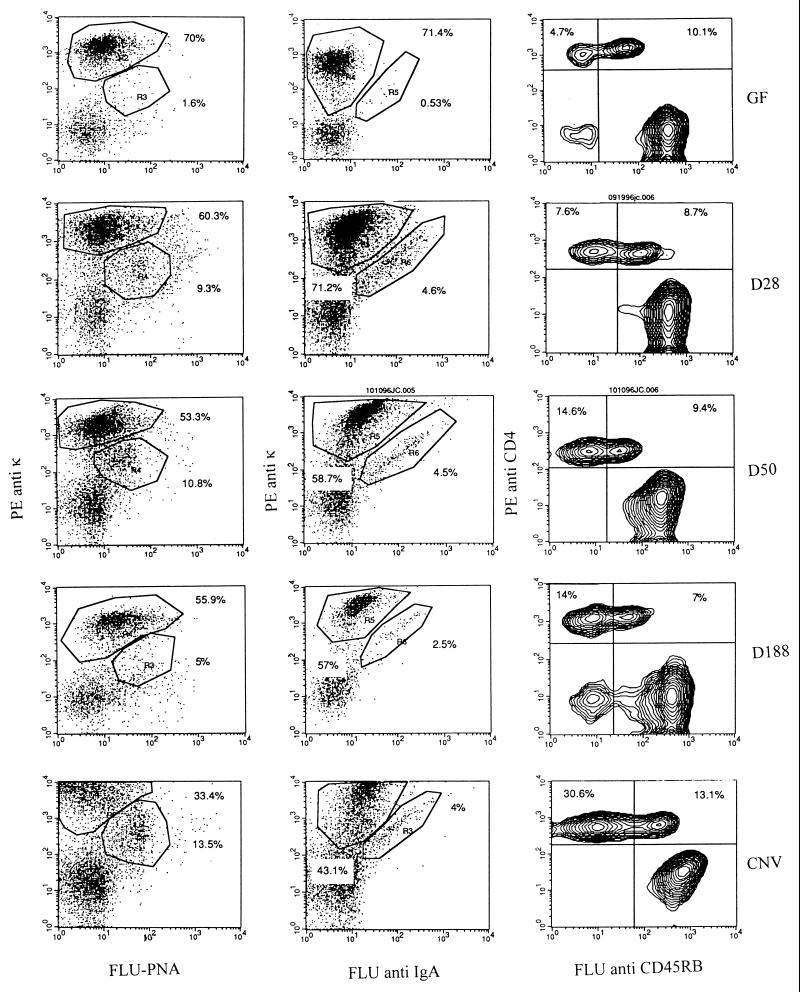
The levels of stimulation of germinal center reactions and activation of T cells in PP were determined by using flow cytometry. Previous studies by Shroff et al. (19) have shown that PNA binding by B cells can be used as a germinal center marker and that FACS analyses correspond to immunohistologic findings. Figure 1 shows the results of FACS analyses of cell suspensions from PP at various times following the colonization of formerly GF mice with SFB. The results of FACS analyses shown in Tables 1 and 2 demonstrate a prompt rise in germinal center B cells and surface IgA+ B cells within 14 to 28 days of the initial colonization of the gut with SFB. The germinal center reactions increased from the almost negligible levels found in GF mice to about one-half the levels seen in the chronically stimulated, conventionally reared mice. There was an eventual decline in germinal center reactions in both groups of mice following prolonged colonization, despite the persistence of SFB in the gut lumen. These findings are consistent with the prompt waxing of germinal center reactions in PP of formerly GF mice observed subsequent to enteric reovirus infection (24) or enteric monoassociation with M. morganii (19), followed by a somewhat more gradual waning of these reactions.
FIG. 1.
In order to assess the progress of germinal center reactions and activation of T cells in PP, cell suspensions from PP of three to five mice were analyzed by FACS analysis at various times following the colonization of formerly GF mice with SFB. These analyses were compared to those of PP from GF and conventionally reared (CNV) mice. Cells were stained with a germinal center marker, PNA, conjugated to FLU or FLU-labeled goat anti-mouse IgA, respectively, and then both were counterstained with PE-conjugated anti-kappa chain to monitor the development of germinal center reactions. Cells were also stained with the CD4 T-cell activation marker CD45RB, conjugated to FLU and counterstained with PE-conjugated anti-CD4 to monitor T-cell activation.
TABLE 1.
Stimulation of germinal center reactions and CD4+ T-cell activation in PP following colonization of GF C3H/HeN mice with SFB
| Mouse group | % of PP B lymphocytesa
|
% of PP CD4+ T cellsa CD45RBlow | |
|---|---|---|---|
| PNA+ | Surface IgA+ | ||
| GF (day 0) | |||
| Group 1 | 2.2 | 0.74 | 32 |
| Group 2 | 2.4 | 1.0 | 33 |
| Colonized with SFB | |||
| Day 14 p.i.b | 9.7 | 3.5 | 51 |
| Day 28 p.i. | 9.3 | 4.9 | 41 |
| Day 84 p.i. | 12.8 | 9.4 | 66 |
| Day 114 p.i. | 12.0 | 9.0 | 67 |
| Day 188 p.i. | 8.2 | 4.2 | 67 |
| CNVc adults | |||
| Group 1 | 32.0 | 24.0 | 61 |
| Group 2 | 22.0 | 7.3 | 55 |
Numbers are averages for groups of three to five mice.
p.i., postinfection.
CNV, conventionally reared.
TABLE 2.
Stimulation of germinal center reactions and CD4+ T-cell activation in PP following colonization of GF C3H/HeN mice with SFB and gram-negative M. morganii
| Mouse group | % of PP B lymphocytesa
|
% of PP CD4+ T cellsa CD45RBlow | |
|---|---|---|---|
| PNA+ | Surface IgA+ | ||
| GF (day 0) | 5.4 | 1.6 | 33 |
| Colonized with SFB | |||
| Day 6 p.i.b | 7.9 | 2.0 | 40 |
| Day 14 p.i. | 13.0 | 4.7 | 45 |
| Day 28 p.i. | 13.0 | 6.1 | 47 |
| Day 50 p.i. | 17.0 | 7.1 | 61 |
| Day 85 p.i. | 9.9 | 11.8 | |
| Day 90 p.i. | 5.0 | 2.2 | 62 |
| Colonized with M. morganii | |||
| Day 18 p.i. (day 131) | 13.0 | 5.5 | |
| Day 28 p.i. (day 141) | 13.0 | 6.7 | 59 |
| Day 63 p.i. (day 175) | 12.0 | 6.6 | 68 |
| Day 90 p.i. (day 202) | 16.0 | 3.8 | 65 |
| CNVc adults | |||
| Group 1 | 28.0 | 8.5 | 70 |
| Group 2 | 24.0 | 8.2 | 54 |
Numbers are averages for groups of three to five mice.
p.i., postinfection.
CNV, conventionally reared.
The results shown in Fig. 1 and Tables 1 and 2 also show a shift in the proportion of CD4+ PP from a CD45RBhigh phenotype to a CD45RBlow phenotype. Initially, the ratio of CD45RBlow to CD45RBhigh PP was approximately 1:2, and gradually the proportions shifted to about 2:1. The percentages of total CD4+ T cells in PP of GF and monoassociated mice remained about the same over the period of colonization (15 to 22%) and were somewhat lower than that observed in conventional mice (about 46%), as shown in Fig. 1. These observations indicate that colonization with SFB induced that level of activation of CD4+ PP normally observed in conventional mice, as judged by the proportion of CD4+ cells that are also CD45RBlow.
Perturbations of germinal center reactions and subsets of PP following secondary colonization with M. morganii in SFB-monoassociated mice.
The data in Table 2 indicate that secondary colonization with M. morganii was able to again stimulate germinal center reactions in mice that were already monoassociated with SFB, although reactions still did not reach levels observed in conventionally reared mice. Following colonization with M. morganii, there was little change in CD4+ T cells in PP, with the ratio of CD45RBlow to CD45RBhigh remaining at about 2:1, similar to that found in conventional mice.
Production of natural and SFB-specific IgA following colonization with SFB.
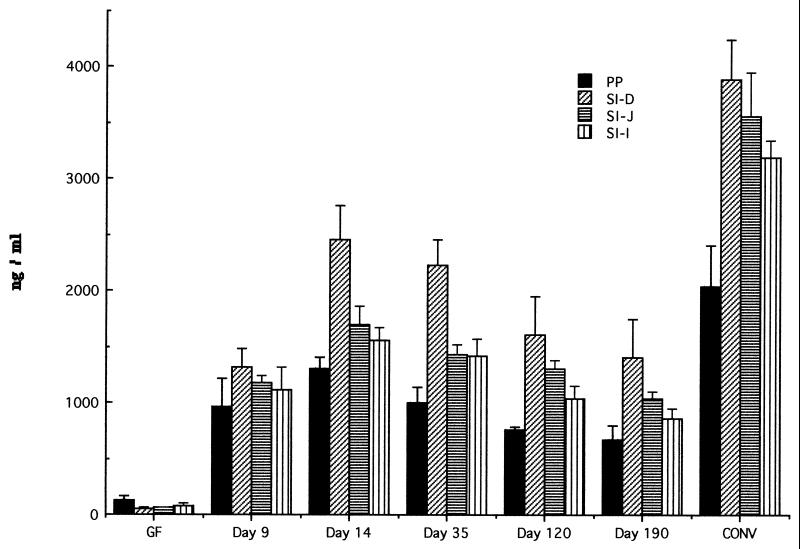
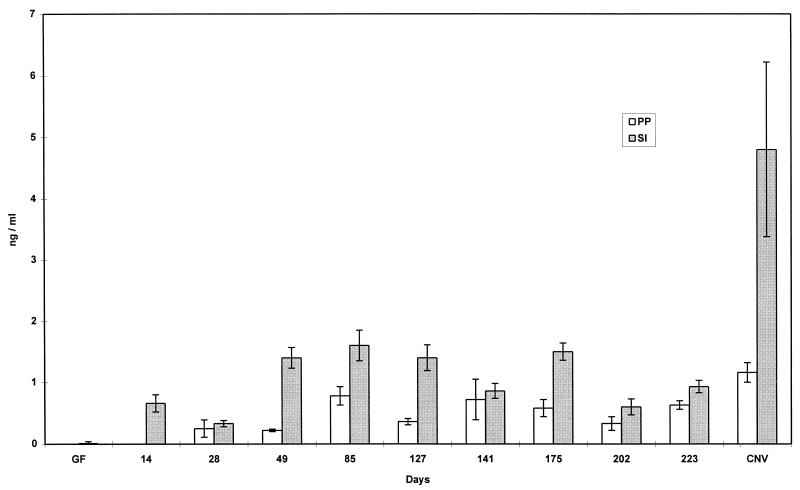
Consistent with the observations of Klaasen et al. (12) that SFB induced a dramatic increase in IgA-secreting cells in the intestinal mucosa, we found that colonization with SFB was a potent stimulator of natural IgA production (Fig. 2 and 3). While GF mice produced low levels of natural IgA, monoassociation with SFB stimulated a rise in IgA production in gut tissue to levels of 24 to 63% of that normally found in conventionally reared mice. Natural IgA production increased rapidly and then slowly decreased over 190 days of colonization.
FIG. 2.
Production of total IgA in supernatant of organ fragment cultures of PP and small intestine of GF, conventionally reared (CONV), and SFB-monoassociated C3H/HeN mice at various time points. RIA was used to detect IgA. Data are means ± standard errors of the mean. SI, small intestine; D, duodenum; J, jejunum; I, ileum. The number of fragment cultures per tissue per time point ranged from 4 to 14.
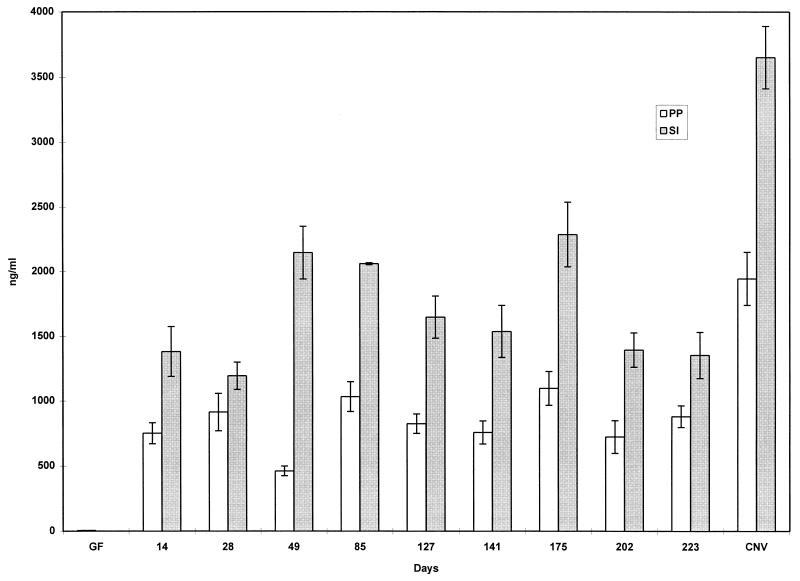
FIG. 3.
Production of total IgA in supernatant of organ fragment cultures of PP and small intestine (SI) of GF mice, conventionally reared (CNV) C3H/HeN mice, and C3H/HeN mice that had been monoassociated with SFB and then double associated (at day 113) with M. morganii. RIA was used to detect IgA. Data are means ± standard errors of the mean. The number of fragment cultures per tissue per time point ranged from 6 to 19.
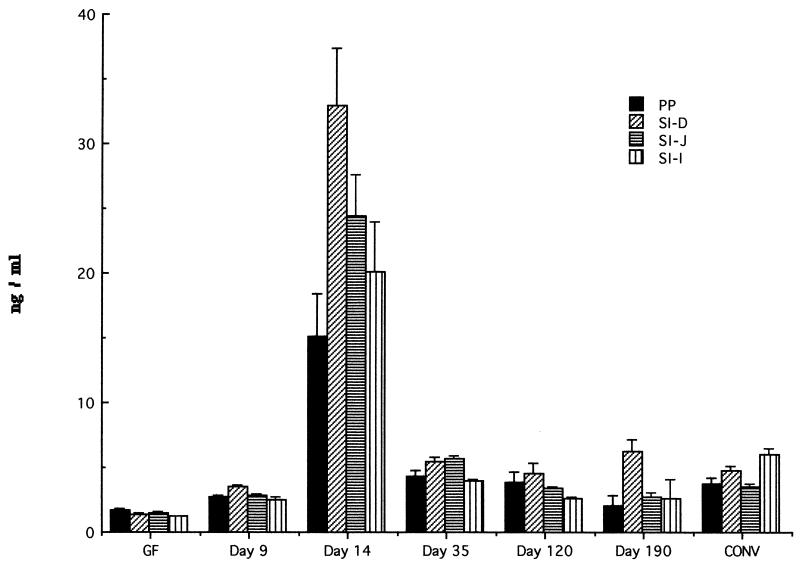
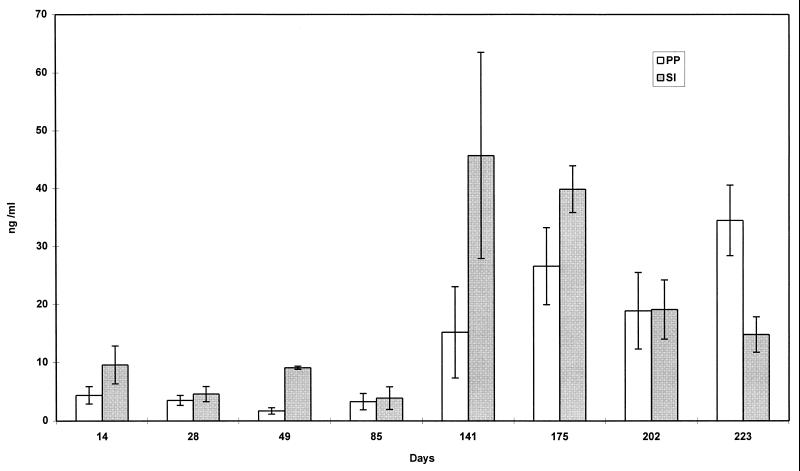
SFB-specific IgA, as seen in Fig. 4 and 5, comprised only a small proportion of total IgA (less than 1.4%). SFB-specific IgA was detected in gut tissues at steady levels for the entire length of the studies.
FIG. 4.
Production of anti-SFB specific IgA in supernatant of organ fragment cultures of PP and small intestine of GF, conventionally reared (CONV), and SFB-monoassociated C3H/HeN mice at various time points. RIA was used to detect IgA. Data are means ± standard errors of the mean. SI, small intestine; D, duodenum; J, jejunum; I, ileum. The number of fragment cultures per tissue per time point ranged from 4 to 14.
FIG. 5.
Production of anti-SFB specific IgA in supernatant of organ fragment cultures of PP and small intestine (SI) of GF mice, conventionally reared (CNV) C3H/HeN mice, and C3H/HeN mice that had been monoassociated with SFB and then double associated (at day 113) with M. morganii. RIA was used to detect IgA. Data are means ± standard errors of the mean. The number of fragment cultures per tissue per time point ranged from 6 to 19.
Total, SFB-specific, and M. morganii-specific IgA production following secondary colonization with M. morganii in SFB-monoassociated mice.
Figure 3 shows that there was no further perturbation of already rather high total IgA levels after a secondary colonization of the mice with M. morganii on day 113. The secondary colonization with M. morganii also did not affect the levels of SFB-specific IgA produced (Fig. 5). The data in Fig. 6 show that prior to colonization with M. morganii on day 113, the level of anti-M. morganii specific IgA that could be detected was quite low. However, following colonization, levels rose rapidly to about 3.4% of the total IgA being produced.
FIG. 6.
Production of anti-M. morganii specific IgA in supernatant of organ fragment cultures of PP and small intestine (SI) of GF mice, conventionally reared C3H/HeN mice, and C3H/HeN mice that had been monoassociated with SFB and then double associated (at day 113) with M. morganii. RIA was used to detect IgA. Data are means ± standard errors of the mean. Four fragment cultures were done per tissue per time point.
Translocation of M. morganii to the spleen and mesenteric lymph nodes in double-colonized mice.
Prior colonization and stimulation of mucosal immunity with SFB did not prevent gut colonization and subsequent translocation of M. morganii to the spleen or mesenteric lymph nodes (Table 3). In contrast to the results of studies by Shroff et al. (19), we found that translocation by M. morganii continued even though anti-M. morganii specific IgA was produced in gut tissues. By the final time point studied (63 days after colonization), the number of M. morganii bacteria that had translocated to the spleen had declined, but the number that had translocated to the mesenteric lymph nodes had increased.
TABLE 3.
Translocation of M. morganii to the spleen and mesenteric lymph nodes of C3H/HeN mice double associated with M. morganii and SFB
| Day p.i.a | No. of CFU (103) per:
|
|
|---|---|---|
| Spleen | Mesenteric lymph node | |
| 14 | ||
| Mouse 1 | 16 | 13 |
| Mouse 2 | 54 | 68 |
| Mouse 3 | 0.2 | 37 |
| Mouse 4 | 0.2 | 118 |
| 28 | ||
| Mouse 1 | 1 | 8 |
| Mouse 2 | 12 | 301 |
| Mouse 3 | 101 | 247 |
| Mouse 4 | 125 | 130 |
| Mouse 5 | 318 | 12 |
| 63 | ||
| Mouse 1 | 19 | 339 |
| Mouse 2 | 9 | 450 |
| Mouse 3 | 81 | 369 |
| Mouse 4 | 18 | 135 |
After inoculation with M. morganii in mice that previously had been monoassociated with SFB.
DISCUSSION
The monoassociation of formerly GF mice with SFB resulted in a significant stimulation of the mucosal immune system. Germinal center reactions in PP were induced, CD4+ T cells in PP were activated, SFB-specific IgA was produced, and total IgA was produced in PP and intestinal fragment cultures to a level approximately 50% of that in conventionally reared mice. It has previously been reported that SFB cause an increase in intestinal IgA cells (12). We found that SFB stimulated a large increase in the production of total IgA; however, only a small fraction was specific for SFB. Our findings indicate that SFB are one of the single most potent nonspecific microbial stimuli of the mucosal immune system.
Germinal center reactions observed in the SFB-monoassociated mice were slower in onset and more prolonged than had been reported for M. morganii-associated mice (19). In that study it was reported that germinal center reactions peaked at day 14 and subsided by day 56. In this study we report that SFB colonization resulted in germinal center reactions which peaked at day 50 for one group of mice and at day 84 for a second group. SFB colonization did not diminish the mucosal immune response to subsequent exposure to M. morganii. Inoculation of the SFB-monoassociated mice with M. morganii at day 113 was able to stimulate the mucosal immune system and cause an increase in the germinal center reactions and in IgA-bearing B cells. These reactions peaked at days 18 to 28 postinfection and remained elevated for 90 days.
The monoassociation of mice with SFB resulted in a large amount of total IgA production; however, only a small fraction was specific for SFB. In contrast, young mice given reovirus produced reovirus-specific IgA that was 10 to 20% of the total IgA (13). SFB appear to stimulate a larger amount of nonspecific IgA than has been reported for other organisms; however, there are limitations in the assay that we used to detect SFB-specific IgA. With the use of the sonicate as the coating antigen, all antigen may not be as efficiently absorbed as typical protein antigens. Also, there was some variation between the two groups of mice in background levels of natural IgA. The mice in the two groups were not age matched and likely had been exposed to some antigenic stimulation from the environment, for instance, from the sterilized standard food, even when raised in GF isolators (2).
The large amount of natural IgA detected on fragment cultures supports the finding of Klaasen et al. (12) that SFB colonization stimulated a steep increase in the number of IgA-secreting cells in the small intestine. Other bacteria which have been investigated have not been as effective at stimulating an increase in IgA plasma cells (18). Klaasen et al. (12) postulated that SFB, which only colonize the intestinal tract at weaning, may be responsible for the increase in IgA-secreting cells which naturally occurs in weaning-age mice.
PP T cells were also stimulated by SFB. CD4+ T cells can be subdivided into two populations: CD45RBhigh and CD45RBlow. In newborn mice and mice raised in clean (specific-pathogen-free) conditions, splenic CD4+ T cells are predominantly CD45RBhigh, and after increased antigenic exposure, CD45RBlow cells develop (3, 15). We have found a similar predominance of CD45RBhigh, CD4+ T cells in the PP of GF mice. The percentage of activated CD4+ T cells, as indicated by the CD45RBlow phenotype, gradually increased in PP after SFB colonization to reach a level seen in conventionally reared mice. Subsequent colonization with M. morganii did not stimulate more T-cell activation, and levels remained similar to those of the conventionally reared animals for the duration of the study.
The reason why SFB may induce such a powerful stimulation of the mucosal immune system has not been determined but may be related to their interaction with intestinal epithelial cells. In vitro, some bacteria appear to stimulate intestinal epithelial cells to upregulate the expression and production of proinflammatory cytokines and also to express several cytokine receptors (9). SFB have an appendage which allows attachment to small bowel epithelial cells, and the local reorganization of actin filaments has been observed in host cells (5, 8). Colonization with SFB has also been shown to correlate with the upregulation of class II major histocompatability class antigen and asialo GM1 glycolipid expression by intestinal epithelial cells (23). Perhaps SFB may have a greater effect than many other enteric bacteria on these cells. It has recently been suggested that normal intestinal flora are essential to the pathogenesis of inflammatory bowel disease. The major inducers appear to be noncultivatible bacteria (4), and perhaps SFB may be an important microorganism in the pathogenesis of this disease.
Surprisingly, this stimulation of the mucosal immune system by SFB did not decrease the subsequent translocation of M. morganii. Previously, it has been reported that M. morganii will increasingly translocate to the spleen and mesenteric lymph nodes in monoassociated mice until about day 14, when antiphosphocholine (lipopolysaccharide) specific IgA antibody is detected (19). Later the amount of M. morganii bacteria which could be found in cultures declined, and by day 56 the bacteria were absent from the spleen and by day 107 from the mesenteric lymph nodes. In this study, mice were colonized with M. morganii 113 days after having been monoassociated with SFB, and M. morganii translocation occurred in large numbers for the 63-day period of the study. A possible explanation of this is that although colonization with SFB induced an appreciable rise in total IgA, only a small amount seemed to react with M. morganii (Fig. 6). Very low levels of antibody were detected by the M. morganii-specific RIA prior to inoculation with the bacteria. Also, the large amount of natural IgA that was induced by SFB may have eventually coated the M. morganii bacteria and actually forestalled an IgA response specific to M. morganii or the resolution of the systemic infection via antibody- and complement-mediated mechanisms for opsonization and bactericidal elimination.
Presently, we are using “back-packed” hybridomas, which have been generated to make natural IgA (1), to determine whether such semispecific IgA antibodies can forestall the development of those specific antibodies which may be more effective at excluding microbial translocation or resolving systemic infection.
ACKNOWLEDGMENTS
This work was supported by grants AI-35936 and AI-37108 from the National Institute of Allergy and Infectious Diseases.
We thank Hank Pletcher for his assistance with the FACS IV flow cytometer and the Lucille P. Markey Trust for funding the Flow Cytometry Facility of the Cancer Center at the University of Pennsylvania. We also thank Alec McKay for the preparation of the radiolabeled reagents and Ann Snyder for technical assistance.
REFERENCES
- 1.Bos N A, Bun J C A M, Popma S H, Cebra E R, Deenen G J, van der Cammen M J, Kroese F G M, Cebra J J. Monoclonal immunoglobulin A derived from peritoneal B cells is encoded by both germ line and somatically mutated VH genes and is reactive with commensal bacteria. Infect Immun. 1996;64:616–623. doi: 10.1128/iai.64.2.616-623.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bos N A, Meeuwesen C G, Wostmann B S, Pleasants J R, Brenner R. The influence of exogenous antigenic stimulation on the specificity repertoire of background immunoglobulin-secreting cells of different isotypes. Cell Immunol. 1988;112:371–380. doi: 10.1016/0008-8749(88)90306-1. [DOI] [PubMed] [Google Scholar]
- 3.Bradley L M, Atkins G G, Swain S L. Long-term CD4+ memory T cells from the spleen lack MEL-14, the lymph node homing receptor. J Immunol. 1992;148:324–331. [PubMed] [Google Scholar]
- 4.Cong Y, Brandwein S L, McCabe R P, Laenby A, Birkenmeier E H, Sundberg J P, Elson C O. CD4+ T cells reactive to enteric bacterial antigens in spontaneously colitic C3H/HeJBir mice: increased T helper cell type 1 response and ability to transfer disease. J Exp Med. 1998;187:855–864. doi: 10.1084/jem.187.6.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis C P, Savage D C. Habitat, succession, attachment, and morphology of segmented, filamentous microbes indigenous to the murine gastrointestinal tract. Infect Immun. 1974;10:948–956. doi: 10.1128/iai.10.4.948-956.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garland C D, Lee A, Dickson M R. Segmented filamentous bacteria in the rodent small intestine: their colonization of growing animals and possible role in host resistance to Salmonella. Microb Ecol. 1982;8:181–190. doi: 10.1007/BF02010451. [DOI] [PubMed] [Google Scholar]
- 7.Hurwitz J L, Tagart V B, Schweitzer P A, Cebra J J. Patterns of isotype expression by B cell clones responding to thymus-dependent and thymus-independent antigens in vitro. Eur J Immunol. 1982;12:342–348. doi: 10.1002/eji.1830120416. [DOI] [PubMed] [Google Scholar]
- 8.Jepson M A, Clark M A, Simmons N L, Hirst B H. Actin accumulation at sites of attachment of indigenous apathogenic segmented filamentous bacteria to mouse ileal epithelial cells. Infect Immun. 1993;61:4001–4004. doi: 10.1128/iai.61.9.4001-4004.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kagnoff M F. Mucosal immunology: new frontiers. Immunol Today. 1996;17:57–59. doi: 10.1016/0167-5699(96)80579-2. [DOI] [PubMed] [Google Scholar]
- 10.Klaasen H L B M, Koopman J P, Poelma F G J, Beynen A C. Intestinal, segmented, filamentous bacteria. FEMS Microbiol Rev. 1992;88:165–180. doi: 10.1111/j.1574-6968.1992.tb04986.x. [DOI] [PubMed] [Google Scholar]
- 11.Klaasen H L B M, Koopman J P, Van den Brink M E, Van Wezel H P N, Beynen A C. Mono-association of mice with non-cultivable, intestinal, segmented filamentous bacteria. Arch Microbiol. 1991;156:148–151. doi: 10.1007/BF00290989. [DOI] [PubMed] [Google Scholar]
- 12.Klaasen H L B M, Van der Heijden P J, Stok W, Poelma F G J, Koopman J P, Van den Brink M E, Bakker M H, Eling W M C, Beynen A C. Apathogenic, intestinal, segmented, filamentous bacteria stimulate the mucosal immune system of mice. Infect Immun. 1993;61:303–306. doi: 10.1128/iai.61.1.303-306.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kramer D R, Cebra J J. Role of maternal antibody in the induction of virus specific and bystander IgA responses in Peyer’s patches of suckling mice. Int Immunol. 1995;7:911–918. doi: 10.1093/intimm/7.6.911. [DOI] [PubMed] [Google Scholar]
- 14.Lebman D A, Griffin P M, Cebra J J. Relationship between expression of IgA by Peyer’s patch cells and functional IgA memory cells. J Exp Med. 1987;166:1405–1418. doi: 10.1084/jem.166.5.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee W T, Yin X M, Vitetta E S. Functional and ontogenetic analysis of murine CD45Rhi and CD45Rlo CD4+ T cells. J Immunol. 1990;144:3288–3295. [PubMed] [Google Scholar]
- 16.Leidy J. On the existence of entophyta in healthy animals, as a natural condition. Proc Natl Acad Sci USA. 1849;4:225–233. [Google Scholar]
- 17.Logan A C, Chow K-P N, George A, Weinstein P D, Cebra J J. Use of Peyer’s patch and lymph node fragment cultures to compare local immune responses to Morganella morganii. Infect Immun. 1991;59:1024–1031. doi: 10.1128/iai.59.3.1024-1031.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moreau M C, Ducluzeau R, Guy-Grand D, Miller M C. Increase in the population of duodenal immunoglobulin A plasmocytes in axenic mice associated with different living or dead bacterial strains of intestinal origin. Infect Immun. 1978;21:532–539. doi: 10.1128/iai.21.2.532-539.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18a.Popma, S., and J. Cebra. Unpublished data.
- 19.Shroff K E, Meslin K, Cebra J J. Commensal enteric bacteria engender a self-limiting humoral mucosal immune response while permanently colonizing the gut. Infect Immun. 1995;63:3904–3913. doi: 10.1128/iai.63.10.3904-3913.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Snel J, Heinen P P, Blok H J, Carman R J, Duncan A J, Allen P C, Collins M D. Comparison of 16S rRNA sequences of segmented filamentous bacteria isolated from mice, rats, and chickens and proposal of “Candidatus arthromitus.” Int. J Syst Bacteriol. 1995;45:780–782. doi: 10.1099/00207713-45-4-780. [DOI] [PubMed] [Google Scholar]
- 21.Snel, J., C. C. Hermsen, H. J. Smits, N. A. Bos, W. M. C. Eling, J. J. Cebra, and P. J. Heidt. Interactions between gut-associated lymphoid tissue and colonization levels of indigenous, segmented, filamentous bacteria in the small intestine of mice. Can. J. Microbiol., in press. [DOI] [PubMed]
- 22.Tannock G W, Crichton C M, Savage D C. A method for harvesting non-cultivable filamentous segmented microbes inhabiting the ileum of mice. FEMS Microbiol Ecol. 1987;45:329–332. [Google Scholar]
- 23.Umesaki Y, Okada Y, Matsumoto S, Imaoka A, Setoyama H. Segmented filamentous bacteria are indigenous bacteria and induce MHC class II molecules and fucosyl asialo GM1 glycolipids on the small intestinal epithelial cells in the ex-germ-free mouse. Microbiol Immunol. 1995;39:555–562. doi: 10.1111/j.1348-0421.1995.tb02242.x. [DOI] [PubMed] [Google Scholar]
- 24.Weinstein P D, Cebra J J. The preference for switching to IgA expression by Peyer’s patch germinal center B cells is likely due to the intrinsic influence of their microenvironment. J Immunol. 1991;147:4126–4135. [PubMed] [Google Scholar]