Abstract
Shigella flexneri cydC, which is deficient in cytochrome bd, was rapidly cleared from the lungs of intranasally inoculated mice and was Sereny negative, yet it induced 93% protection against challenge with wild-type S. flexneri. Mice that lack immunoglobulin A (IgA) were fully protected, suggesting that IgA may not be required for adaptive immunity in this model system.
Shigella spp. are gram-negative enteric bacteria that continue to cause significant diarrhea and dysentery worldwide, especially in developing countries. Consequently, development of Shigella vaccines, including live attenuated vaccines, and analysis of the host immune response to infection have been the focus of extensive scientific research. The cytochrome bd terminal oxidase is one of two terminal oxidases required for aerobic respiration of Escherichia coli and other bacteria (1, 5). A Shigella flexneri 2a mutant (strain SSW202) that contains a nonpolar mini-Tn10 insertion in cydC lacks expression of the cytochrome bd terminal oxidase (20). Strain SSW202 also forms smaller plaques than wild-type S. flexneri on mammalian cell monolayers and demonstrates decreased survival within tissue culture cells (20). Mice do not acquire intestinal disease after oral inoculation with Shigella; consequently, a model consisting of bronchopulmonary infection following intranasal inoculation has been used (11, 12, 17–19). The lethal dose of SSW202 for intranasally inoculated mice (109 bacteria) is 100-fold higher than the lethal dose of wild-type S. flexneri (107 bacteria) (20). In the present study, we further evaluate the attenuation of strain SSW202 and the extent of protection it induces to subsequent challenge with wild-type S. flexneri. In addition, we examine the role of immunoglobulin A (IgA) in the adaptive immune response.
Clearance of S. flexneri 2a SSW202 from mouse lungs.
To evaluate whether the differences in virulence of these strains for mice was associated with differences in the clearance of the organisms from the mouse lung, the number of viable bacteria in the lungs of intranasally infected C57BL/6 mice (Jackson Laboratories, Bar Harbor, Maine) was determined at 1, 3, and 5 days after inoculation with 1 × 107 to 2 × 107 SSW202 or 1 × 107 to 2 × 107 wild-type serotype 2a 2457T (7) by plating dilutions of homogenized lung (18). Sixty-nine-fold and 190-fold fewer bacteria were recovered from mice infected with SSW202 than from mice infected with 2457T at 1 and 3 days postchallenge, respectively (P = 0.0004 and P = 0.012, respectively), and while all mice infected with 2457T had died by 5 days postchallenge, no mice infected with SSW202 had died up to 21 days postinoculation (P = 0.029) (Table 1).
TABLE 1.
Number of viable S. flexneri recovered from the lungs of mice inoculated with 1 × 107 to 2 × 107 SSW202 or 2457T
| Day post-inoculation | CFUs of S. flexneri (no. of mice)
|
P value | |
|---|---|---|---|
| SSW202 | 2457T | ||
| 1 | 1.6 × 106 (6) | 1.1 × 108 (3) | 0.0004a |
| 3 | 2.2 × 104 (4) | 4.1 × 106 (4) | 0.012a |
| 5 | 7.8 × 102 (4) | n/ab (4) | 0.029c |
P value comparing CFU counts was calculated using the independent Student’s t test.
n/a, not applicable.
P value comparing alive versus dead mice was calculated using the Fisher’s exact test.
Evaluation of strain SSW202 in the Sereny (keratoconjunctivitis) assay.
A classic assay of reactogenicity and virulence of Shigella strains is the Sereny assay, which measures the ability of a strain to cause keratoconjunctivitis in guinea pigs, rabbits, or mice (9, 14). At 24 h and up to 7 days postinoculation, the conjunctivae of BALB/c mice inoculated with SSW202 (n = 3) appeared no different from those inoculated with saline, whereas at 24 and 48 h postinoculation, the conjunctivae of all mice inoculated with 2457T (n = 3) showed mucopurulent discharge, erythema, and swelling of the eyelid.
Immunity following sublethal inoculation with strain SSW202.
Mice received two intranasal inoculations with 1 × 107 to 2 × 107 SSW202 21 days apart (12, 17, 18). Age- and sex-matched control mice were inoculated with saline alone according to an identical schedule. Challenge of mice was performed 21 days later with an intranasal dose of wild-type strain 2457T that was equivalent to the lethal dose for naive mice (1 × 107 to 2 × 107 bacteria). Ninety-one percent of mice (n = 11) that had received a single inoculation and 93% of mice (n = 15) that had received two sequential inoculations with SSW202 survived challenge; no mouse (n = 16) that had received inoculations with saline survived challenge (Table 2).
TABLE 2.
Survival following challenge of C57BL/6 mice vaccinated with SSW202, by size of challenge dose, number of vaccine inoculations, and time between last vaccination and challenge
| Immunization dose (CFUsa) | Challenge dose (CFUs)b | No. of vaccina-tions | Time to challenge (days)c | No. of mice | % Sur-vival | P valued |
|---|---|---|---|---|---|---|
| 1 × 107–2 × 107 | 1 × 107–2 × 107 | 2 | 21 | 15 | 93 | n/ae |
| Saline | 1 × 107–2 × 107 | 2 | 21 | 16 | 0 | <0.0001 |
| 1 × 107–2 × 107 | 1 × 107–2 × 107 | 1 | 21 | 11 | 91 | 0.82 |
| 1 × 105–2 × 105 | 1 × 107–2 × 107 | 2 | 21 | 6 | 50 | 0.053 |
| 1 × 106–2 × 106 | 1 × 107–2 × 107 | 2 | 21 | 5 | 60 | 0.14 |
| 1 × 107–2 × 107 | 5 × 107 | 2 | 21 | 5 | 40 | 0.032 |
| 1 × 107–2 × 107 | 1 × 108–2 × 108 | 2 | 21 | 5 | 40 | 0.032 |
| 1 × 107–2 × 107 | 1 × 107–2 × 107 | 2 | 45–49 | 10 | 60 | 0.12 |
Vaccination was performed by the intranasal route.
Challenge was by the intranasal route using wild-type strain 2457T.
Number of days between last vaccination and challenge.
P value was calculated using the Fisher’s exact test of percent survival versus that of mice immunized twice with 1 × 107 to 2 × 107 SSW202 and challenged with 1 × 107 to 2 × 107 2457T (first line).
n/a, not applicable.
Clearance of wild-type S. flexneri 2a from vaccinated mice.
To determine whether the protection conferred upon mice vaccinated with SSW202 was associated with increased clearance of the challenge inoculum, the number of viable bacteria within the lungs following challenge was determined. Four-fold and 180-fold fewer S. flexneri were recovered from vaccinated mice than from mock-vaccinated mice at 1 and 3 days postchallenge, respectively (P = 0.003 and P < 0.001, respectively) (Table 3). Thus, for both naive mice receiving attenuated Shigella (Table 1) and vaccinated mice challenged with wild-type Shigella (Table 3), the ability to rapidly clear the inoculum is associated with increased survival.
TABLE 3.
Number of viable bacteria recovered from the lungs of vaccinated or saline mock-vaccinated mice after challenge with wild-type S. flexneria
| Day post-challenge | No. of bacteria (CFUs) recovered from:
|
P valueb | |
|---|---|---|---|
| SSW202-vaccinated mice (no. of mice) | Mock-vaccinated mice (no. of mice) | ||
| 1 | 3.6 × 107 (8) | 1.4 × 108 (6) | 0.003 |
| 3 | 2.3 × 104 (7) | 4.1 × 106 (4) | <0.001 |
Challenge inoculum was 1 × 107 to 2 × 107 2457T.
P value was calculated using the independent Student’s t test
Specific serum antibody response in vaccinated mice.
The concentrations of serum antibody to S. flexneri serotype 2a antigens was determined by enzyme-linked immunosorbent assay using whole 2457T as antigen (19) in vaccinated and saline mock-vaccinated mice. Significant increases were observed in all antibody isotypes following vaccination except IgG3, which showed a trend in the same direction (Table 4).
TABLE 4.
Concentration of S. flexneri serotype 2a-specific serum antibody in SSW202-vaccinated and mock-vaccinated C57BL/6 micea
| Antibody isotype | Serum concn (μg/ml ± SD) in:
|
P valueb | |
|---|---|---|---|
| SSW202-vacinated mice | Mock-vaccinated mice | ||
| IgM | 14.4 ± 6.1 | 0.34 ± 0.32 | 0.004 |
| IgG1 | 5.16 ± 2.6 | 0.12 ± 0.08 | 0.008 |
| IgG2a | 0.14 ± 0.04 | 0.04 ± 0.02 | 0.004 |
| IgG2b | 1.56 ± 0.78 | 0.07 ± 0.05 | 0.009 |
| IgG3 | 0.44 ± 0.32 | 0.06 ± 0.02 | 0.05 |
| IgA | 0.08 ± 0.01 | 0.01 ± 0.00 | <0.0001 |
Sera were drawn from 3 to 5 mice per group, 20 days after two intranasal inoculations with 1 × 107 to 2 × 107 SSW202 given 21 days apart.
P value was calculated using the independent Student’s t test.
Level and duration of protection.
To investigate the level of protection induced by inoculation with SSW202, mice were also challenged with inocula of wild-type strain 2457T that were fivefold or 10-fold higher than the lethal dose for naive mice. Following challenge with either of these inocula, survival was reduced significantly to 40% (n = 5 for each group) (Table 2). To determine the minimum inoculum of SSW202 that was required for the induction of significant adaptive immunity, mice were immunized with inocula that were 10-fold or 100-fold lower than that used in the experiments described above (i.e., 1 × 106 to 2 × 106 or 1 × 105 to 2 × 105 instead of 1 × 107 to 2 × 107 SSW202) and challenged with the same dose of wild-type S. flexneri as mice in experiments described above (1 × 107 to 2 × 107 2457T). Vaccination with 10-fold or 100-fold lower inocula induced 60% (n = 5) or 50% (n = 6) survival, respectively (Table 2); the differences from the survival observed following inoculation with 1 × 107 to 2 × 107 SSW202 does not reach statistical significance, although the trend of decreased survival following lower vaccine inocula suggests that the higher inoculum may be preferable (Table 2). To evaluate the duration of the adaptive immune response induced by inoculation with SSW202, vaccinated mice were challenged at 45 to 49 days, rather than at 21 days following the second SSW202 inoculation. At 45 to 49 days, survival was reduced to 60% (n = 10) (Table 2); while the difference in the survival rate observed following challenge at 21 days does not reach statistical significance, the downward trend suggests that protection may be transient. Such transience would suggest that IgM may by the protective antibody isotype, although we could not rule out IgG that may have decreased over time, as has been shown to occur in Shigella-vaccinated monkeys over approximately the same time (6). While up-regulation of nonspecific immune responses, of which major histocompatibility complex II is an indicator, during the early postvaccination period can also be important (2), it seems unlikely that this has a major effect here, since the observed protection is largely serotype specific (19).
Immunohistochemical staining of lung sections.
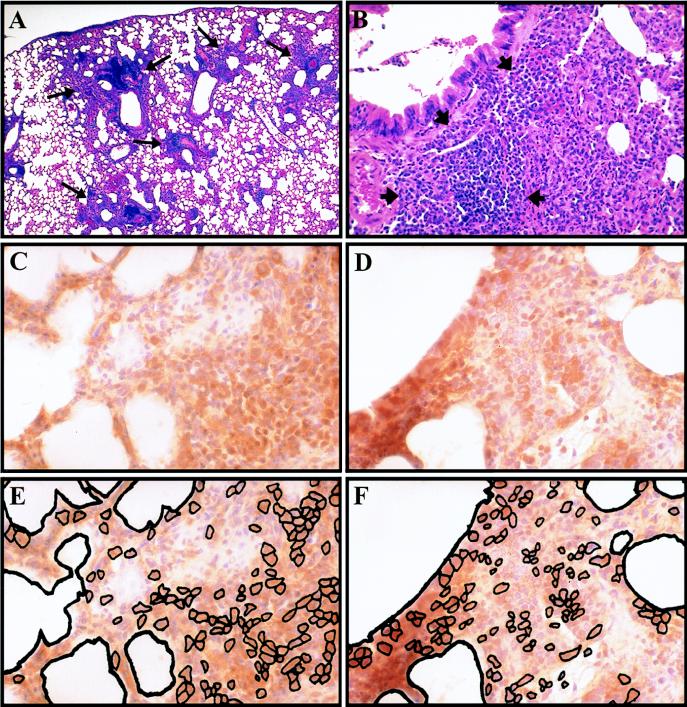
We and others have previously shown that intranasal vaccination with SSW202 induces the expansion of peribronchiolar lymphoid aggregates (11, 19), and we have shown that the presence of these aggregates is associated with protection following immunization (19). Since the cells within these aggregates could be involved in the mediation of the adaptive response, we identified the cell types in the aggregates. Immunohistochemical staining demonstrated the presence of significant numbers of both B lymphocytes (B220+ cells, stained with clone RA3-6B2; Pharmingen, San Diego, Calif.) and T lymphocytes (CD3+ cells, stained with clone 48-2B; Santa Cruz Biotechnology, Santa Cruz, Calif.) (Fig. 1).
FIG. 1.
Lymphocytes in peribronchiolar lymphoid aggregates. Representative hematoxylin-and-eosin-stained sections (A and B) and sections immunohistochemically stained with hematoxylin counterstain (C to F) at 1 (A) and 3 (B to F) days postchallenge. (A) Lung of vaccinated mouse following challenge showing peribronchiolar lymphoid aggregates (long arrows); (B) higher magnification of peribronchiolar lymphoid aggregates (short arrows). Staining of adjacent sections for T lymphocytes (CD3+ cells) (C) and B lymphocytes (B220+ cells) (D) with corresponding schematic representation of stained cells within each section (E and F, respectively). Magnification: (A) ×25; (B) ×50; (C to F) ×100.
Adaptive immune response in mice that lack IgA.
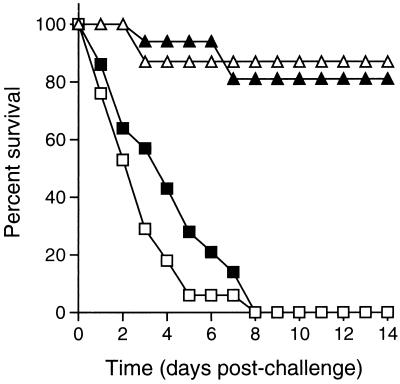
Shigella-specific secretory IgA antibodies have been observed in secretions following natural infection in humans (4, 15) and experimental infection in monkeys (6) and rabbits (10). Phalipon et al. (16) have shown that Shigella lipopolysaccharide-specific IgA antibodies, either delivered via “back pack” hybridoma tumors or mixed with the bacterial inoculum, are able to confer serotype-specific protection to intranasally inoculated mice. However, humans with congenital IgA deficiency, a frequent abnormality (1 in 500 individuals [13]), have an increased incidence of respiratory and inner ear infections but not of Shigella infection (3, 13). The role of IgA was therefore addressed by analysis of the adaptive immune response in mice that lack IgA. Vaccinated IgA−/− mice (129Sv × C57BL/6 [8]) were as protected against challenge as vaccinated congenic IgA+/+ mice (87 and 81% survival, respectively) (Fig. 2). The high-level protection attained by vaccinated IgA−/− mice in the present study suggests that secretory IgA at the mucosal surfaces is not required for adaptive immunity, although we cannot rule out an effect of alterations in other immune factors in the IgA−/− mouse. In sum, the data presented here suggest that in this model system IgA is not required for adaptive immunity and further suggest that IgM or IgG that decreases over time may be the protective antibody isotype.
FIG. 2.
Effect of IgA on survival of vaccinated mice upon challenge. Survival of IgA−/− versus congenic IgA+/+ mice following challenge. Vaccinated IgA−/− mice (open triangles, n = 23), vaccinated IgA+/+ mice (solid triangles; n = 16), mock-vaccinated IgA−/− mice (open squares; n = 17), and mock-vaccinated IgA+/+ mice (solid squares; n = 14).
Acknowledgments
The authors are indebted to C. J. Chang for statistical analysis; to I. N. Mbawuike for the gift of IgA−/− mice; to M. D. Scharff, B. Diamond, and A. Casadevall for helpful discussions; and to D. Caroll and R. Dominitz for technical assistance.
This work was supported by NIH grants T32 GM07288 (S.S.W.) and AI35817 (M.B.G.), a Pew Scholars Award in the Biomedical Sciences (M.B.G.), and Established Investigator (M.B.G.) and Grant-in-Aid (M.B.G.) Awards from the American Heart Association.
REFERENCES
- 1.Anraku Y, Gennis R B. The aerobic respiratory chain of Escherichia coli. Trends Biochem Sci. 1987;12:262–266. [Google Scholar]
- 2.Bancroft G J, Bosma M J, Bosma G C, Unanue E R. Regulation of macrophage Ia expression in mice with severe combined immunodeficiency: induction of Ia expression by a T cell-independent mechanism. J Immunol. 1986;137:4–9. [PubMed] [Google Scholar]
- 3.Beard L J, Ferrante A, Oxelius V-A, Maxwell G M. IgG subclass deficiency in children with IgA deficiency presenting with recurrent or severe respiratory infections. Pediatr Res. 1986;20:937–942. doi: 10.1203/00006450-198610000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Cleary T G, Winsor D K, Reich D, Ruiz-Palacios G, Calve J J. Human milk immunoglobulin A antibodies to Shigella virulence determinants. Infect Immun. 1989;57:1675–1679. doi: 10.1128/iai.57.6.1675-1679.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cotter P A, Chepuri V, Gennis R B, Gunsalus R P. Cytochrome o (cyoABCDE) and d (cydAB) oxidase gene expression in Escherichia coli is regulated by oxygen, pH, and the fnr gene product. J Bacteriol. 1990;172:6333–6338. doi: 10.1128/jb.172.11.6333-6338.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dinari G, Hale T L, Austin S W, Formal S B. Local and systemic antibody responses to Shigella infection in rhesus monkeys. J Infect Dis. 1987;155:1065–1069. doi: 10.1093/infdis/155.5.1065. [DOI] [PubMed] [Google Scholar]
- 7.Formal S B, Dammin G J, LaBrec E H, Schneider H. Experimental Shigella infections: characteristics of a fatal infection produced in guinea pigs. J Bacteriol. 1958;75:604–610. doi: 10.1128/jb.75.5.604-610.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harriman G R, Bogue M, Rogers P, Finegold M, Pacheco S, Bradley A, Zhang Y-X, Mbawuike I N. Targeted deletion of the IgA constant region in mice leads to IgA deficiency with alterations in expression of other immunoglobulin isotypes. J Immunol. 1999;162:2521–2529. [PubMed] [Google Scholar]
- 9.Hong M, Payne S M. Effect of mutations in Shigella flexneri chromosomal and plasmid-encoded lipopolysaccharide gene on invasion and serum resistance. Mol Microbiol. 1997;24:779–791. doi: 10.1046/j.1365-2958.1997.3731744.x. [DOI] [PubMed] [Google Scholar]
- 10.Keren D, Kern S, Bauer D, Scott P, Porter P. Direct demonstration in intestinal secretions of an IgA memory response to orally administered Shigella flexneri antigens. J Immunol. 1982;128:475–479. [PubMed] [Google Scholar]
- 11.Mallett C P, Hale T L, Kaminski R W, Larsen T, Orr N, Cohen D, Lowell G H. Intranasal or intragastric immunization with proteosome-Shigella lipopolysaccharide vaccines protects against lethal pneumonia in a murine model of Shigella infection. Infect Immun. 1995;63:2382–2386. doi: 10.1128/iai.63.6.2382-2386.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mallett C P, VanDeVerg L, Collins H H, Hale T L. Evaluation of Shigella vaccine safety and efficacy in an intranasal challenged mouse model. Vaccine. 1993;11:190–196. doi: 10.1016/0264-410x(93)90016-q. [DOI] [PubMed] [Google Scholar]
- 13.Morgan G, Levinsky R J. Clinical significance of IgA deficiency. Arch Dis Childhood. 1988;63:579–581. doi: 10.1136/adc.63.6.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murayama S Y, Sakai T, Makino S, Kurata T, Sasakawa C, Yoshikawa M. The use of mice in the Sereny test as a virulence assay of shigellae and enteroinvasive Escherichia coli. Infect Immun. 1986;51:696–698. doi: 10.1128/iai.51.2.696-698.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oberhelman R, Kopecko D, Salazar-Lindo E, Gotuzzo E, Buysee J, Venkatesan M, Yi A, Fernandez-Prada C, Guzman M, Leon-Barua R, Sack R. Prospective study of systemic and mucosal immune responses in dysenteric patients to specific Shigella invasion plasmid antigens and lipopolysaccharides. Infect Immun. 1991;59:2341–2350. doi: 10.1128/iai.59.7.2341-2350.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phalipon A, Kaufmann M, Michetti P, Cavaillon J-M, Huerre M, Sansonetti P, Kraehenbuhl J-P. Monoclonal immunoglobulin A antibody directed against serotype-specific epitope of Shigella flexneri lipopolysaccharide protects against murine experimental shigellosis. J Exp Med. 1995;182:769–778. doi: 10.1084/jem.182.3.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.VanDeVerg L L, Mallett C P, Collins H H, Larsen T, Hammack C, Hale T L. Antibody and cytokine responses in a mouse pulmonary model of Shigella flexneri serotype 2a infection. Infect Immun. 1995;63:1947–1954. doi: 10.1128/iai.63.5.1947-1954.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Way S S, Borczuk A C, Dominitz R, Goldberg M B. An essential role for gamma interferon in innate resistance to Shigella flexneri infection. Infect Immun. 1998;66:1342–1348. doi: 10.1128/iai.66.4.1342-1348.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Way, S. S., A. C. Borczuk, and M. B. Goldberg. Protection against the intracellular pathogen Shigella flexneri is thymic-independent. Submitted for publication.
- 20.Way, S. S., S. Sallustio, R. S. Magliozzo, and M. B. Goldberg. The impact of either elevated or decreased levels of cytochrome bd expression on Shigella flexneri virulence. J. Bacteriol. 181:1229–1237. [DOI] [PMC free article] [PubMed]